Abstract
Teosinte, wild maize relatives originating from Mexico and Central America, emerged as a noxious agricultural weed in France and Spain. In 2016, the European Food Safety Authority (EFSA) issued a technical report that assessed the available scientific information on teosinte for its relevance for the environmental risk assessment (ERA) and risk management (RM) of genetically modified (GM) maize MON810, Bt11, 1507 and GA21 for cultivation. It was concluded that the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny (potentially acquired through hybridisation between GM maize and teosinte) on target and non‐target organisms, the abiotic environment and biogeochemical cycles would be very low under EU conditions. Following a request of the European Commission, EFSA evaluated whether the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, or require revision in light of new scientific evidence on teosinte that has become available since the publication of EFSA (2016). A protocol was developed to clarify the interpretation of the terms of reference of the mandate and make them operational. The assessment relied on evidence retrieved via an extensive literature search and from reports of the Competent Authorities of France and Spain, and on hearing expert testimonies. A limited collection of 18 publications of varying relevance and quality was retrieved and assessed. Based on this evidence, it is concluded that the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, except those pertaining to the use of glyphosate‐based herbicides on maize GA21 which should be considered under Regulation (EC) No 1107/2009. In infested agricultural areas (especially in regions where maize MON810 is widely grown), weed management measures implemented to monitor, control and/or eradicate teosinte must remain in place, as they will contribute to further reduce the low vertical gene flow potential between GM maize and EU teosinte.
Keywords: Bt‐maize, evidence appraisal, genetically modified maize, hybridisation, invasiveness, pathway to harm, persistence, non‐target organisms, target organisms, teosinte
Summary
Teosinte, a group of wild species related to maize (Zea mays subsp. mays) originating from Mexico and Central America, has emerged as a new weed in maize fields in two European countries, France (FR) and Spain (ES). In these regions, teosinte is considered a noxious agricultural weed that is subject to control and/or eradication measures and monitoring.
Risk concerns have been expressed that genetically modified (GM) maize may hybridise with EU teosinte in regions where they co‐occur, leading to the development of more persistent and invasive weeds that may pose unconsidered risks to the environment, including target organisms (TOs) and non‐target organisms (NTOs).
In 2016, the European Food Safety Authority (EFSA) issued a technical report that assessed the available scientific information on teosinte for its relevance for the environmental risk assessment (ERA) and risk management (RM) of genetically modified (GM) maize MON810, Bt11, 1507 and GA21 for cultivation. Based on the available evidence, it was concluded that the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny (potentially acquired through hybridisation between GM maize and teosinte) on TOs, NTOs, the abiotic environment and biogeochemical cycles would be very low under EU conditions.
Since the publication of EFSA (2016), new scientific evidence on teosinte that is relevant for the ERA and RM of maize MON810, Bt11, 1507 and GA21 has become available. Following a request of the European Commission, EFSA evaluated whether the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, or require revision in light of new scientific evidence on teosinte.
A protocol, consisting of a problem formulation and an analysis plan, was developed to clarify the interpretation of the terms of reference of the mandate and make them operational. A pathway to harm approach (consisting of a causal chain of events) was followed as a conceptual model for the definition of assessment questions and subquestions and clarifying their relationship. The pathway to harm proposed for the cultivation of GM maize in EU areas infested with teosinte is constructed around risk concerns typically considered in the ERA of GM plants. These risk concerns include: (1) persistence and invasiveness of the GM plant, including vertical gene flow; (2) interactions of the GM plant with NTOs; and (3) interactions of the GM plant with TOs. For each step in the pathway to harm, a risk hypothesis (RH) was formulated as a negative statement to be tested (corroborated or falsified/rejected) using the new evidence that has become available since the publication of EFSA (2016). The assessment and testing of RHs relied on the evidence retrieved via an extensive literature search, from reports supplied to the European Commission by the ES/FR Competent Authorities, and on hearing expert testimonies.
The literature search consisted of an all‐encompassing approach to capture all assessment questions and subquestions. The search string was designed using teosinte as the key element of the review question. The search string included a wide range of free‐text terms and where available, controlled vocabulary. The literature searches identified 1444 records (= publications) in BIOSIS Citation Index, CAB Abstracts, Current Contents Connect, FSTA, Medline and SciELO (n = 575), Dialnet (n = 34), Scopus (n = 405) and Web of Science Core Collection (n = 423), and reports of the ES/FR Competent Authorities (n = 7). Of these 1444 records, 840 were removed after deduplication. Six hundred and four records were screened against eligibility criteria, of which 574 were excluded in Step 1 (i.e. rapid assessment based on title and abstract) and 12 in Step 2 (i.e. a detailed assessment based on full‐text documents).
A total of 18 relevant publications on teosinte were retrieved after detailed assessment of the full‐text documents, of which:
Eight are relevant for RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’;
Ten for RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’;
None for RH1c ‘GM teosinte hybrid progeny is not more persistent and invasive than non‐GM teosinte hybrid progeny under EU field conditions’;
Eight for RH1d ‘Non‐target organism (NTO) does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’;
None for RH1e ‘NTO is not adversely affected by exposure to GM teosinte hybrid progeny under EU field conditions’;
Eight for RH1f ‘Target organism (TO) of Bt‐maize does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’;
Six for RH2a ‘Transgene product in GM teosinte hybrid progeny is high dose under EU field conditions’;
Four for RH2b ‘GM teosinte hybrid progeny does not occur in non‐Bt‐maize refuge areas, nor in Bt‐maize fields in the EU’.
For the answering of subquestions, a narrative approach based on expert judgement was followed. Owing to the limited availability of evidence to answer RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’ and RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’, a narrative approach was followed to appraise the relevant evidence to test RH1a and RH1b, instead of a more structured approach.
The 18 publications retained for evidence appraisal following the literature screening/selection process are of varying relevance and quality, and represent a limited evidence base. For example, 12 out of the 18 publications focused on EU teosinte and two of those used maize MON810 as test material, in contrast to native teosinte and conventional maize. Moreover, some of the experimental designs implemented most likely resulted in an overestimation or underestimation of the true intervention effect under real‐life conditions. For some publications, insufficient details were reported about the materials, methods and results, hampering the assessment of the quality of the evidence reported. Therefore, some of ERA and RM assumptions previously made in EFSA (2016) could not be confirmed or rejected by the newly available evidence.
For the completion of the mandate, EFSA relied on the expertise of the CompERA expert Working Group of the GMO Panel and hearing expert testimonies (including those of some of the experts suggested by the ES/FR Competent Authorities). The expertise covered included: agronomy, integrated pest management, the assessment of the persistence and invasiveness potential of plants, the ERA of GM plants, vertical gene flow, resistance evolution in target organisms and entomology.
The new relevant evidence that has become available since the publication of EFSA (2016) is not sufficient to corroborate all risk hypotheses along the pathway to harm proposed for the cultivation of GM maize in EU areas infested with teosinte, neither to show that the pathway is blocked at any step. However, at each step in the pathway to harm, a hypothesis that the event is rare can be corroborated to a greater or lesser extent. Therefore, it can be concluded that completion of the pathway to harm requires a succession of rare events, of which the combined probabilities are very low. Consequently, it is unlikely that environmental harm will be realised through the postulated pathway to harm.
The new evidence retrieved confirms that where maize and EU teosinte plants co‐occur and flower synchronously, maize alleles (transgenic or not), can move into teosinte populations at rates that depend on different factors. Hence, the possible introgression of transgenes from maize MON810, Bt11, 1507 and GA21 into EU teosinte may only provide a selective advantage to GM teosinte hybrid progeny under high infestation of target pests and/or when glufosinate‐ammonium‐ and/or glyphosate‐based herbicides are applied. However, this fitness advantage will not allow GM teosinte hybrid progeny to overcome other biological and abiotic factors limiting their persistence and invasiveness. Therefore, EFSA considers that the growth habits of EU teosinte plants and teosinte hybrid progeny are such that the acquisition of insect resistance and/or herbicide tolerance is unlikely to change their relative persistence and invasive characteristics under EU conditions.
It is noted that the overall environmental exposure to GM teosinte hybrid plants, bearing either the insect resistance or herbicide tolerance trait or both, will remain low compared to exposure to GM maize, provided that measures continue to be employed to monitor, control and/or eradicate EU teosinte in infested agricultural areas. Therefore, in line with EFSA (2016) and if the measures employed to monitor, control and/or eradicate teosinte in infested agricultural areas remain in place, it is assumed that the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny (potentially acquired through hybridisation between GM maize and teosinte) on TOs, NTOs, the abiotic environment and biogeochemical cycles will be very low under EU conditions.
EFSA encourages the ES/FR Competent Authorities to continue employing comprehensive weed management measures to monitor, control and/or eradicate teosinte in infested agricultural areas, and restrict the cultivation of maize MON810 in fields where the incidence of teosinte plants exceeds regional infestation thresholds. The monitoring, control and eradication measures put in place in ES (especially in Aragón and Cataluña where maize MON810 is widely grown) contribute to further reduce the low potential of vertical gene flow between GM maize and ES teosinte, and thus the likelihood of environmental harm to occur through the postulated pathway to harm.
For future annual PMEM reports on the cultivation of maize MON810, it is recommended that:
The consent holder explicitly considers all new scientific evidence on teosinte relevant for the ERA and RM of maize MON810;
The consent holder revises farmer questionnaires to include the reporting of both the occurrence of ES teosinte and corresponding levels of infestation;
The consent holder and the Competent Authorities share relevant information on teosinte for regions where maize MON810 cultivation may co‐occur with teosinte.
Moreover, it is encouraged that the research/monitoring activities pertaining to teosinte performed/commissioned by the ES/FR Competent Authorities be continued and expanded. This will be critical for the generation of empirical data on EU teosinte that could be used to further test specific risk hypotheses of the devised pathway to harm, and confirm previously made ERA and RM assumptions.
Overall, it is concluded that the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, except those pertaining to the use of glyphosate‐based herbicides on maize GA21 which should be considered under Regulation (EC) No 1107/2009.1
1. Introduction
Teosinte is the common name for a group of annual and perennial species of the genus Zea which are native to Mexico and Central America. Teosinte comprises seven taxa that are divided into two sections and five species. The five species in the genus are Zea diploperennis, Zea perennis, Zea luxurians, Zea nicaraguensis and Zea mays. The last species is further divided into four subspecies, comprising several geographic races: Z. mays subsp. huehuetenangensis, Z. mays subsp. mexicana, Z. mays subsp. parviglumis and maize (Z. mays subsp. mays). The currently most accepted hypothesis is that maize was domesticated ~ 9,000 years ago from the annual teosinte Z. mays subsp. parviglumis in southern Mexico.
Teosinte is not indigenous outside its centres of origin, but has become naturalised/established elsewhere (e.g. in Australia, Brazil, Egypt, Malaysia, the Philippines, Sri Lanka and the United States) (Silva et al., 2015; Pardo et al., 2016). In these regions, teosinte does not represent an environmental entity of concern requiring protection. Instead, it is occasionally cultivated for forage purposes, or considered a noxious weed that can compete with maize in agricultural fields, thereby reducing yield and compromising harvest quality (Balbuena et al., 2011). Teosinte can produce 3.3 times more seed than maize, most of which are shed before or during harvest (Chavez et al., 2012). Depending on their dormancy potential (López et al., 2011), teosinte seeds can remain viable in the soil for a few years. Seeds can germinate, establish seedlings and lead to plants that flower and set seed in subsequent years. Seeds can be dispersed in forage, and by field machinery and livestock. In infested regions, teosinte is subject to control and/or eradication measures and monitoring.
Since 1990, teosinte has been detected in France (FR) in the region of Poitou‐Charentes (the north of the Nouvelle Aquitaine region) (reviewed by EFSA, 2016; Le Corre et al., 2020, and references therein). In Spain (ES), the presence of teosinte has been reported formally for the first time in Aragón (e.g. Ebro valley) and Cataluña in 2014 (reviewed by EFSA, 2016; Montull et al., 2020; Lohn et al., 2021, and references therein). Le Corre et al. (2020) established that ES/FR teosinte originated from Z. mays subsp. mexicana, which is a weedy teosinte from the Mexican highlands, suggesting a single geographical origin for teosinte found in Europe. Moreover, the authors demonstrated that FR teosinte adapted to European temperate latitude growing conditions (i.e. early flowering), compared to Z. mays subsp. mexicana. Díaz et al. (2020) found that ES teosinte has a complex origin being related to both commercial maize and wild teosinte (Z. mays subsp. mexicana and Z. mays subsp. parviglumis), while Trtikova et al. (2017) suggested that ES teosinte would be of admixed origin, most likely involving Z. mays subsp. mexicana as parental taxon, and an unidentified cultivated maize variety as the other.
Genetically modified (GM) maize event MON810 is currently the only GM crop approved for cultivation in the EU. In recent years, maize MON810 has been grown mainly in ES and to a lesser extent in Portugal (PT) (Camargo et al., 2018; EFSA, 2021; Álvarez‐Alfageme et al., 2022). In 2019, maize MON810 represented approximately 35% of ES’s total maize area and less than 10% in PT (Álvarez‐Alfageme et al., 2022).
The renewal of authorisation of the cultivation of maize MON810, and the authorisation of the cultivation of the maize events Bt11, 1507 and GA21 are pending at EU level. Maize MON810 and Bt11 express a Cry1Ab insecticidal protein derived from Bacillus thuringiensis subsp. kurstaki, and maize 1507 expresses a truncated Cry1F protein from B. thuringiensis subsp. aizawai, both conferring protection against lepidopteran target pests such as the European corn borer (ECB, Ostrinia nubilalis) and species belonging to the genus Sesamia. Maize Bt11 and 1507 also express phosphinothricin‐N‐acetyltransferase (PAT) from Streptomyces viridochromogenes, providing tolerance to the herbicidal active substance glufosinate‐ammonium, but are not intended to be marketed as herbicide‐tolerant crops. Maize GA21 expresses a modified version of 5‐enolpyruvylshikimate‐3‐phosphate synthase (mEPSPS), conferring tolerance to the herbicidal active substance glyphosate.
Risk concerns have been expressed that GM maize may hybridise with EU teosinte in regions where they co‐occur, leading to the development of more persistent and invasive weeds that may pose unconsidered risks to the environment, including target organisms (TOs) and non‐target organisms (NTOs) (Testbiotech, 2016a,b). In 2016, the European Food Safety Authority (EFSA) issued a technical report that assessed the plausibility of the above‐mentioned risk concerns through a pathway to harm approach and evaluates their relevance for the environmental risk assessment (ERA) and risk management (RM) of maize MON810, Bt11, 1507 and GA21 cultivation (EFSA, 2016). It was concluded that ‘the possible introgression of transgenes from maize MON810, Bt11, 1507 and GA21 into weedy teosinte may provide a selective advantage to hybridising teosinte progeny only under high infestation of target pests and/or when glufosinate‐ammonium‐ and/or glyphosate‐containing herbicides are applied’. EFSA (2016) considered that ‘the overall environmental exposure to GM maize × teosinte hybrids [termed hereafter as GM teosinte hybrid progeny], bearing either the insect resistance or herbicide tolerance trait or both, would remain low compared to exposure to GM maize, provided that measures are employed to control and/or eradicate weedy teosinte and their progeny in infested agricultural areas’. Based on the available evidence, the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny on TOs, NTOs, the abiotic environment and biogeochemical cycles was considered by EFSA (2016) ‘very low under EU conditions’ at that time.
EFSA (2016) also concluded that ‘the use of glyphosate‐containing herbicides on maize GA21 may enhance the fitness of glyphosate tolerant teosinte hybrid progeny, should they occur within the confines of a managed field environment where glyphosate is applied’. To ensure effective long‐term management of weedy teosinte and its hybrid progeny that may have acquired glyphosate tolerance through vertical gene flow from maize GA21, and avoid exacerbating weed problems, EFSA (2016) recommended that ‘integrated weed management reliant on multiple tactics (e.g. alternative chemistry mixtures, mechanical, rotational) are deployed, should maize GA21 be grown in areas where weedy teosinte occurs’.
Since the publication of EFSA (2016), new scientific evidence on teosinte that is relevant for the ERA and RM of maize MON810, Bt11, 1507 and GA21 has become available (e.g. Devos et al., 2018; Bauer‐Panskus et al., 2020; Díaz et al., 2020; Le Corre et al., 2020; Lohn et al., 2021). Some of these publications have been considered by EFSA and the CompERA expert Working Group of EFSA’s GMO Panel, to determine whether the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, or require revision.2
In April 2021, the European Commission (Directorate‐General for Health and Food Safety (DG SANTE)) requested EFSA to assess whether, based on the evidence supplied by the ES/FR Competent Authorities, existing scientific literature and any other pertinent evidence, there is a need to update: (1) the post‐market environmental monitoring (PMEM) plan for maize MON810; and (2) the EFSA (2016) technical report on teosinte. Following the request of the European Commission, in May 2021, EFSA recommended: (1) the consent holder to put more emphasis on ES teosinte in the annual PMEM reports on the cultivation of maize MON810 (see also EFSA, 2021); and (2) to update EFSA (2016) to integrate and report the most recent and relevant evidence on EU teosinte for the ERA and RM of maize MON810, Bt11, 1507 and GA21.2
1.1. Background and Terms of Reference as provided by the requestor
Following a request of the European Commission (EC) (dated 29 September 2021), EFSA was mandated to provide scientific and technical assistance on teosinte under Article 31 of Regulation (EC) No 178/2002. The mandate was formally acknowledged by EFSA on 14 October 2021 (mandate number M‐2021‐00086 and question number EFSA‐Q‐2021‐00557). Further details about the mandate are available on the OpenEFSA portal.3
In the mandate, DG SANTE requested EFSA to ‘update EFSA (2016) conclusions and recommendations on teosinte, notably by:
Integrating the most recent and relevant scientific evidence;
Proposing insect risk management measures for the cultivation of maize MON810, if needed’.
In view of acquiring more details on the data reported by the ES/FR Competent Authorities, EFSA was also invited to ‘directly contact the Competent Authorities of France and Spain’ for the completion of the mandate.
1.2. Interpretation of the Terms of Reference
The Terms of Reference (ToRs) of the mandate were interpreted by EFSA as follows:
ToR(1): To update and revise the ERA conclusions of EFSA (2016) in light of new and relevant scientific information on teosinte that has become available after the publication of EFSA (2016);
ToR(2): To update and revise the RM recommendations of EFSA (2016) in light of new and relevant scientific information on teosinte that has become available after the publication of EFSA (2016).
In line with the Commission Directive (EU) 2018/350, the ERA of the use of a plant protection product, including its use on a GM plant, falls within the scope of Regulation (EC) No 1107/2009 and is carried out at a Member State level to account for specific agricultural conditions. Therefore, risk concerns associated with the use of glyphosate‐based herbicides on maize GA21 are not addressed here. According to Commission Directive (EU) 2018/350, the ERA of a GM plant that is made tolerant to a herbicide should be consistent with the scope of Directive 2001/18/EC.
2. Data and methodologies
Data used and methodologies followed are described in the protocol, which was endorsed by an expert of the CompERA Working Group of EFSA’s GMO Panel on 18 December 2021 (see Appendix A). The protocol has been developed following the principles and process for dealing with data and evidence in scientific assessments (EFSA, 2015), and is based on the recommendations for protocol development for non‐application mandates (EFSA, 2020). Feedback received from DG SANTE (E3) representatives and an expert of the CompERA Working Group of EFSA’s GMO Panel were considered during the development of the protocol.
In line with EFSA (2020), the protocol consists of a problem formulation that outlines what the assessment aims to address and thus the objectives of the assessment, and an analysis plan that outlines which methods will be used to address the problem (i.e. how the assessment will be carried out).
A pathway to harm approach (consisting of a causal chain of events) was followed as a conceptual model for the definition of assessment questions (AQs) and subquestions and clarifying their relationship. The pathway to harm proposed for the cultivation of GM maize in EU areas infested with teosinte is constructed around risk concerns typically considered in the ERA of GM plants. These risk concerns include: (1) persistence and invasiveness of the GM plant, including vertical gene flow; (2) interactions of the GM plant with NTOs; and (3) interactions of the GM plant with TOs.
For each step in the pathway to harm, a risk hypothesis (RH) was formulated as a negative statement to be tested (corroborated or falsified/rejected) using the new evidence that has become available since the publication of EFSA (2016). The following RHs were tested:
RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’;
RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’;
RH1c ‘GM teosinte hybrid progeny is not more persistent and invasive than non‐GM teosinte hybrid progeny under EU field conditions’;
RH1d ‘Non‐target organism (NTO) does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’;
RH1e ‘NTO is not adversely affected by exposure to GM teosinte hybrid progeny under EU field conditions’;
RH1f ‘Target organism (TO) of Bt‐maize does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’;
RH2a ‘Transgene product in GM teosinte hybrid progeny is high dose under EU field conditions’;
RH2b ‘GM teosinte hybrid progeny does not occur in non‐Bt‐maize refuge areas, nor in Bt‐maize fields in the EU’.
The assessment and testing of RHs relied on the evidence retrieved via an extensive literature search (EFSA, 2010, 2019), from reports supplied to the European Commission by the ES/FR Competent Authorities, and on hearing expert testimonies.
3. Assessment
3.1. Extensive literature search
3.1.1. Outcomes of the literature search
The literature searches, which are reported in the protocol (see Appendix A), identified 1444 records (= publications) in BIOSIS Citation Index, CAB Abstracts, Current Contents Connect, FSTA, Medline, SciELO (n = 575), Dialnet (n = 34), Scopus (n = 405) and Web of Science Core Collection (n = 423), and reports of the ES/FR Competent Authorities (n = 7) (see Figure 1). Of these 1,444 records, 840 were removed after deduplication.
Figure 1.
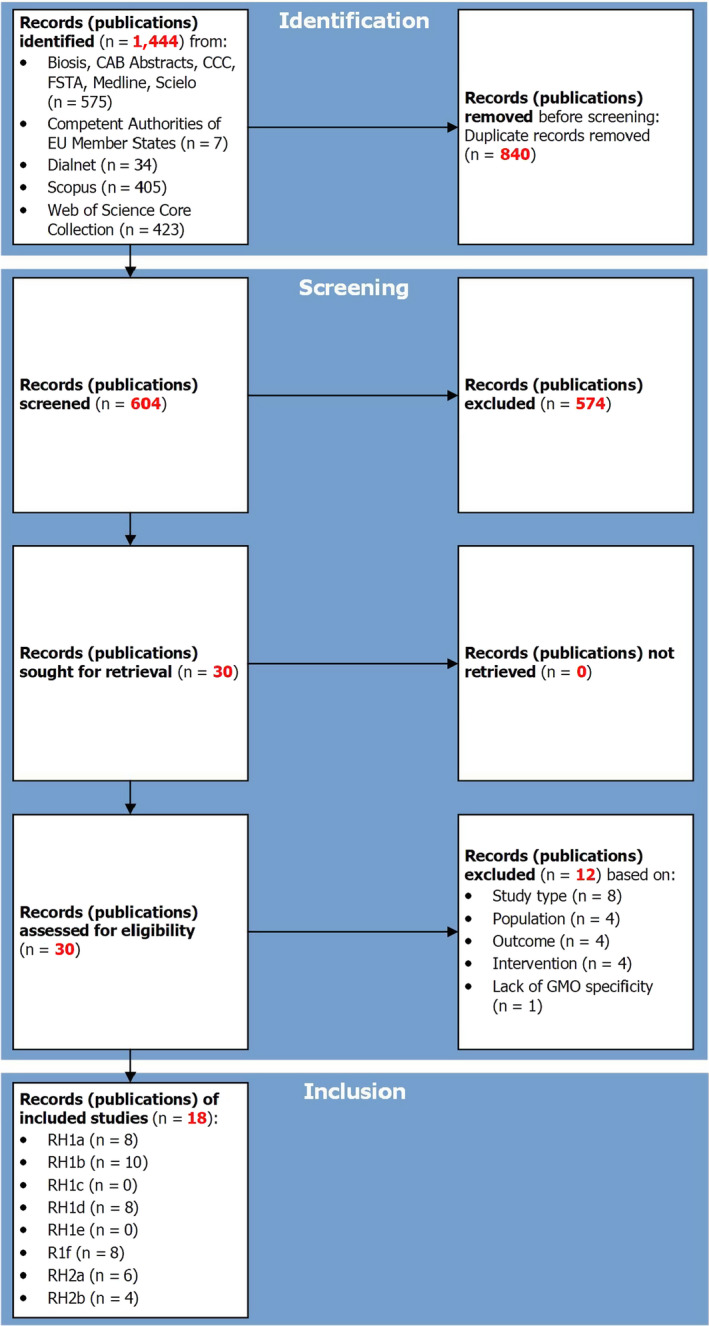
Results of the publication selection process
3.1.2. Results of the selection process
Six hundred and four records were screened against eligibility criteria (reported in Tables A.3 and A.4 of Appendix A), of which 574 were excluded in Step 1 (i.e. rapid assessment based on title and abstract) and 12 in Step 2 (i.e. a detailed assessment based on full‐text documents) (see Figure 1). The bibliographic details regarding the 12 publications excluded based on full‐text screening are reported in Table B.1 (see Appendix B).
Table A.3.
Eligibility criteria to establish the relevance of evidence pertaining to study characteristics for each of the assessment questions (AQs) and subquestions (i.e. risk hypotheses (RHs))
| AQs/RHs | Key elements of AQs (concepts) | Eligibility criteria for evidence inclusion |
|---|---|---|
| 1a (teosinte occurrence) | Study type/design | Observational studies (such as field surveys, field monitoring, case reports) covering the European Union (EU) |
| Population | Teosinte and its progeny | |
| Outcome | Teosinte occurrence in the EU | |
| 1b | Study type/design | Observational studies (such as population genetic analyses, transgene/transgene product detection) and experimental studies (such as hybridisation experiments performed under laboratory, greenhouse, semi‐field and field conditions) |
| Population |
– Teosinte occurring in the EU, GM maize and conventional maize8 – For experimental studies, maize as pollen donor9 |
|
| Outcome | Hybridisation potential (see Table A.1 for examples of relevant endpoints) between maize and teosinte and gene introgression | |
| 1c | Study type/design | Observational studies (such as field surveys, field monitoring, case reports) and experimental studies (such as experiments performed under field conditions to assess the persistence and invasiveness potential of GM teosinte hybrid progeny) |
| Population | GM teosinte hybrid progeny | |
| Outcome | Persistence and invasiveness potential (see Table 1 for examples of relevant endpoints) of GM teosinte hybrid progeny | |
| 1d | Study type/design | Observational studies (such as field observations) and experimental studies (such as host plant specificity experiments performed under laboratory, greenhouse, semi‐field and field conditions) |
| Population | Non‐target organisms (NTOs) of GM maize | |
| Outcome | Host plant suitability of teosinte for NTOs | |
| 1e | Study type/design | Experimental studies (such as laboratory bioassays to assess adverse effects of the transgene product or plant material of GM teosinte hybrid progeny on susceptible NTOs, and greenhouse studies) |
| Population | Susceptible NTOs (mainly Lepidoptera)10 of Bt‐maize | |
| Intervention | Transgene product and plant material/plants of GM teosinte hybrid progeny | |
| Comparator | Negative and positive control, and conventional teosinte hybrid progeny | |
| Outcome | Potential adverse effects on susceptible NTOs (see Table A.1 for examples of relevant endpoints) following exposure to transgene product at concentrations present in the field and/or GM teosinte hybrid progeny plant material/plants | |
| 1f | Study type/design | Observational studies (such as field observations) and experimental studies (such as host plant specificity experiments performed under laboratory, greenhouse, semi‐field and field conditions) |
| Population | Target organisms (TOs) of Bt‐maize (mainly European and Mediterranean corn borers)11 | |
| Outcome | Host plant suitability of teosinte for TOs of Bt‐maize | |
| 2a | Study type/design |
– Observational and experimental studies designed to quantify transgene expression levels in relevant plant parts12 of GM teosinte hybrid progeny using ELISA – Laboratory assays to assess TO mortality |
| Population |
– GM teosinte hybrid progeny – TO |
|
| Outcome |
– Transgene product concentrations in relevant plant parts of GM teosinte hybrid progeny – TO mortality following exposure to transgene product at concentrations present in the field and/or GM teosinte hybrid progeny plant material/plants |
|
| 2b | Study type/design | Observational studies |
| Population | GM teosinte hybrid progeny | |
| Outcome | Plant/population density of GM teosinte hybrid progeny in non‐Bt‐maize refuge areas planted near or adjacent to, or within Bt‐maize fields |
Table A.4.
Eligibility criteria to establish the relevance of evidence pertaining to record characteristics
| Key elements (concepts) | Eligibility criteria | Rationale | |
|---|---|---|---|
| Time | In | Study is published since 2016 | Focus on new evidence that became available after the publication of EFSA (2016) |
| Language | In | Study is reported in English (EN), French (FR) or Spanish (ES) | Include evidence reported in local case reports written in ES or FR to cover languages of regions where teosinte has been reported to occur |
| Publication type | In |
– Primary research studies (i.e.. studies generating new data) – Conference abstracts or posters if they contain primary data – Reports of the Competent Authorities of EU Member States – Reviews (reviews will be used as sources of further references and to assess the appropriateness of the search strategy applied) |
– Cover new data or assess the appropriateness of the search strategy applied – Include reports of the Competent Authorities of EU Member States to address the mandate, as not necessarily reported elsewhere or accessible – Exclude opinions/statements, as they do not report primary data – Exclude PhD theses and dissertations, as primary data reported are assumed to have been published in primary research studies |
| Out |
– Letters to the editor and editorials – Expert opinions – PhD theses and dissertations |
A total of 18 relevant publications were retrieved after detailed assessment of the full text documents, of which:
Eight are relevant for RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’;
Ten for RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’;
None for RH1c ‘GM teosinte hybrid progeny is not more persistent and invasive than non‐GM teosinte hybrid progeny under EU field conditions’;
Eight for RH1d ‘Non‐target organism (NTO) does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’;
None for RH1e ‘NTO is not adversely affected by exposure to GM teosinte hybrid progeny under EU field conditions’;
Eight for RH1f ‘Target organism (TO) of Bt‐maize does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’;
Six for RH2a ‘Transgene product in GM teosinte hybrid progeny is high dose under EU field conditions’;
Four for RH2b ‘GM teosinte hybrid progeny does not occur in non‐Bt‐maize refuge areas, nor in Bt‐maize fields in the EU’.
The bibliographic details regarding the 18 publications are reported in Table 1.
Table 1.
List of relevant publications retrieved and their relevance for subquestions (i.e. risk hypotheses)
| # | Publication references | Relevance for risk hypotheses |
|---|---|---|
| 1 | Government of the Autonomous Community of Aragon. Department for Agriculture, Livestock and the Environment, 2021. REPORT ON THE TEOSINTE (Zea mays subspp.) SITUATION IN THE AUTONOMOUS COMMUNITY OF ARAGON [January]. | 1a, 1b, 1d, 1f, 2a, 2b |
| 2 | Government of the Autonomous Community of Aragon. Department for Agriculture, Livestock and the Environment, 2021. REPORT ON THE TEOSINTE (Zea mays subspp.) SITUATION IN THE AUTONOMOUS COMMUNITY OF ARAGON [October]. | 1a, 1f, 2a, 2b |
| 3 | Government of the Autonomous Community of Catalonia. Catalonian Department of Agriculture, Livestock, Fisheries and Food. Directorate General for Agriculture and Livestock. 2021. REPLY TO REQUEST FOR INFORMATION ON THE TEOSINTE SITUATION IN CATALONIA [January]. | 1a, 1d, 1f, 2a, 2b |
| 4 | Government of the Autonomous Community of Catalonia. Catalonian Department of Agriculture, Livestock, Fisheries and Food. Directorate General for Agriculture and Livestock 2021. REPLY TO REQUEST FOR INFORMATION ON THE TEOSINTE SITUATION IN CATALONIA [October]. | 1a, 1f, 2b |
| 5 | Montull JM, Pardo G, Aibar J, Llenes JM, Marí AI, Taberner A and Cirujeda A, 2020. Aspects of the dispersion and viability of the teosinte seeds (Zea mays ssp.) in the Ebro valley. Informacion Tecnica Economica Agraria, 116, 227–240. | 1a |
| 6 | Republic of France, 2021. NOTE FROM THE FRENCH AUTHORITIES: Request for information from the Commission on the situation of teosinte. Paris, 12 March 2021. | 1a, 1b, 1f |
| 7 | Spanish Ministry for Environmental Transition and the Demograhpic Challenge. Directorate General for Environmental Quality and Assessment, 2021. REPORT OF THE NATIONAL BIOSAFETY COMMITTEE (CNB) ON THE PRESENCE OF TEOSINTE IN EUROPE [January]. | 1a, 1b, 1d, 1f, 2a |
| 8 | Spanish Ministry for Environmental Transition and the Demograhpic Challenge. Directorate General for Environmental Quality and Assessment, 2021. REPORT OF THE NATIONAL BIOSAFETY COMMITTEE (CNB) ON THE PRESENCE OF TEOSINTE IN EUROPE [October]. | 1a, 1b, 1d, 1f, 2a |
| 9 | Calfee E, Gates D, Lorant A, Perkins MT, Coop G and Ross‐Ibarra J, 2021. Selective sorting of ancestral introgression in maize and teosinte along an elevational cline. PLoS Genetics, 17, e1009810. | 1b |
| 10 | Díaz A, Taberner A and Vilaplana L, 2020. The emergence of a new weed in maize plantations: characterization and genetic structure using microsatellite markers. Genetic Resources and Crop Evolution, 67, 225–239. | 1b |
| 11 | Le Corre V, Siol M, Vigouroux Y, Tenaillon MI and Délye C, 2020. Adaptive introgression from maize has facilitated the establishment of teosinte as a noxious weed in Europe. Proceedings of the National Academy of Sciences of the United States of America, 117, 25618–25627. | 1b |
| 12 | Lohn AF, Trtikova M, Chapela I, Binimelis R and Hilbeck A, 2021. Transgene behavior in genetically modified teosinte hybrid plants: transcriptome expression, insecticidal protein production and bioactivity against a target insect pest. Environmental Sciences Europe, 33, 67. | 1b, 2a |
| 13 | Lu Y, Hokin SA, Kermicle JL, Hartwig T and Evans MMS, 2019. A pistil‐expressed pectin methylesterase confers cross‐incompatibility between strains of Zea mays . Nature Communications, 10, 2304. | 1b |
| 14 | Trtikova M, Lohn A, Binimelis R, Chapela I, Oehen B, Zemp N, Widmer A and Hilbeck A, 2017. Teosinte in Europe ‐ Searching for the origin of a novel weed. Scientific Reports, 7, 1560. | 1b |
| 15 | Bellota E, Dávila‐Flores A and Bernal JS, 2018. A bird in the hand versus two in the bush? The specialist leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) does not discriminate against sub‐optimal host plants (Zea spp.). Neotropical Entomology, 47, 171–180. | 1d |
| 16 | Gaillard MDP, Glauser G, Robert CAM and Turlings TCJ, 2018. Fine‐tuning the ‘plant domestication‐reduced defense’ hypothesis: specialist vs generalist herbivores. New Phytologist, 217, 355–366. | 1d |
| 17 | Moya‐Raygoza G, Cuevas‐Guzmán R, Pinedo‐Escatel JA and Morales‐Arias JG, 2019. Comparison of leafhopper (Hemiptera: Cicadellidae) diversity in maize and its wild ancestor teosinte, and plant diversity in the teosinte habitat. Annals of the Entomological Society of America, 112, 99–106. | 1d |
| 18 | Naranjo‐Guevara N, Peñaflor MFGV, Silva DB and Bento JMS, 2021. A comparison of the direct and indirect defence abilities of cultivated maize versus perennial and annual teosintes. Chemoecology, 31, 63–74. | 1d |
3.2. Assessment questions and subquestions
The evidence retrieved through an extensive literature search and evidence supplied by the Competent Authorities of EU Member States are reported and appraised below for each AQ and subquestion (i.e. RHs).
For the answering of subquestions, a narrative approach based on expert judgement was followed. Owing to the limited availability of evidence to answer RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’ and RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’, a narrative approach was followed to appraise the relevant evidence to test RH1a and RH1b, instead of a more structured approach.
3.2.1. RH1a – Teosinte does not occur in EU areas where genetically modified (GM) maize is grown
3.2.1.1. Evidence description/summary
Maize MON810 cultivation
The new evidence retrieved (ES/FR Competent Authority reports, relevant websites and annual PMEM reports on the cultivation of maize MON810) indicates that maize MON810 has been grown in the EU mainly in ES and to a lesser extent in PT in recent years (Table 2).
Table 2.
Total area (ha) devoted to the cultivation of maize MON810 in the EU since 2016 (see Table B.2 for data before 2016; adapted from EFSA (2021) and Álvarez‐Alfageme et al. (2022))4
| Growing season | CZ | DE | ES | FR | PL | PT | RO | SK | Total |
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 75 | 0 | 129,081 | 0 | 0 | 7,056 | 0 | 122 | 136,335 |
| 2017 | 0 | 0 | 124,227 | 0 | 0 | 7,308 | 0 | 0 | 131,535 |
| 2018 | 0 | 0 | 115,246 | 0 | 0 | 5,733 | 0 | 0 | 120,979 |
| 2019 | 0 | 0 | 107,127 | 0 | 0 | 4,718 | 0 | 0 | 111,845 |
| 2020 | 0 | 0 | 98,152 | 0 | 0 | 4,216 | 0 | 0 | 102,368 |
| 2021 | 0 | 0 | 96,606 | 0 | 0 | 4,228 | 0 | 0 | 100,834 |
CZ: Czech Republic; DE: Germany; ES: Spain; FR: France; PL: Poland; PT: Portugal; RO: Romania; SK: Slovakia.
In ES, maize MON810 is mostly grown in North‐Eastern ES (Aragón, Cataluña and Navarra) and less in South‐Western ES (Andalucía and Extremadura) and Central ES (Albacete) (Table 3).
Table 3.
Total area (ha) devoted to the cultivation of maize MON810 in Spain since 2016 (adapted from EFSA (2021) and Álvarez‐Alfageme et al. (2022))4
| Autonomous communities | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Aragón* | 46,546 | 49,608 | 44,932 | 42,646 | 40,995 | 40,663 |
| Cataluña* | 41,567 | 39,092 | 38,752 | 36,430 | 31,833 | 32,538 |
| Comunidad Foral de Navarra | 8,066 | 7,778 | 8,101 | 8,253 | 8,310 | 9,074 |
| Extremadura | 15,039 | 13,976 | 14,138 | 12,255 | 10,718 | 8,894 |
| Castilla la Mancha | 5,932 | 5,069 | 3,805 | 3,101 | 2,601 | 2,958 |
| Andalucía | 10,919 | 8,013 | 4,972 | 3,795 | 2,724 | 1,774 |
| Castilla León | 169 | 17 | 9 | 287 | 347 | 399 |
| Islas Baleares | 128 | 106 | 163 | 156 | 160 | 169 |
| Comunidad Valenciana | 302 | 292 | 238 | 90 | 335 | 95 |
| Comunidad de Madrid | 402 | 271 | 135 | 91 | 79 | 21 |
| Región de Murcia | 0 | 0 | 2 | 0 | 26 | 19 |
| La Rioja | 10 | 4 | 0 | 23 | 23 | 1 |
| Islas Canarias | 0 | 1 | 0 | 0 | 0 | 0 |
| País Vasco | 1 | 0 | 0 | 0 | 0 | 0 |
| Total Spain | 129,081 | 124,227 | 115,246 | 107,127 | 98,152 | 96,605 |
Teosinte occurrence reported in the autonomous community.
In 2021, the total area cropped to maize was 359,188 ha in ES (with 61,190 ha in Aragón and 41,711 ha in Cataluña).5
Teosinte occurrence
In the new evidence retrieved, data on teosinte occurrence in the EU are reported in the ES/FR Competent Authority reports and Montull et al. (2020). Overall, the evidence confirms the presence of teosinte in ES and FR.
In ES, teosinte has been found in Aragón (in the Huesca and Zaragoza provinces) and Cataluña (in the Lleida province);
In FR, teosinte has been found in the region Poitou‐Charentes (now part of Nouvelle Aquitaine), mainly in the departments of Charente, Charente‐Maritime and Deux Sèvres.
The arable land infested by teosinte was estimated to be around 428 ha in Aragón in 2021 (ES), around 111 ha in Cataluña in 2021 (ES), and around 300 ha in Nouvelle Aquitaine over the period of 2017–2020 (FR).
Teosinte occurrence in ES
The observations on teosinte reported in ES are based on field surveys conducted in Aragón and Cataluña between 2014–2021 and 2015–2021, respectively. These field surveys are ongoing in both regions;
Both in Aragón and Cataluña, teosinte is mostly found in maize fields;
While maize MON810 is widely grown in Aragón and Cataluña, teosinte has been found mostly in conventional maize fields so far;
Teosinte is not found in field margins or further away, suggesting that the potential of teosinte to establish and survive (as feral plant) outside the confines of a managed field environment is very limited, except if irrigated;
Overall, the occurrence of teosinte seems to be stable or even lower since it was first detected, most likely due to the weed management practices implemented.
Teosinte occurrence in FR
The observations reported on the occurrence of teosinte in FR are based on field surveys conducted by Arvalis in 2012, FREDON de Nouvelle Aquitaine in 2019 and INRAE between 2017 and 2020;
Teosinte is mostly found in maize fields (more than 80% of the observations), and less in soybean fields (ca. 10%) and other fields (sunflower, cereal stubble or sorghum);
There is no indication that the occurrence of teosinte has expanded to new areas since 2012;
Teosinte is rarely found in field margins or off‐field (two plants were observed). No teosinte populations were observed in non‐cropped land.
Teosinte occurrence in other EU Member States
No information on teosinte occurrence was provided for other EU Member States, except for the Competent Authorities of Belgium (BE). The BE Competent Authorities indicated to DG SANTE that they have consulted their regional Agricultural Research Centres (CRA‐W in Wallonia and ILVO in Flanders), and stated that ‘the presence of Teosinte in fields does not seem to be an issue for the time being, the plant being sensitive to winter frost. Also, Teosinte is not present in the Belgian production of maize’.
3.2.1.2. Evidence appraisal
There are insufficient details on materials and methods used to gather and analyse teosinte occurrence data in the evidence considered. Details about surveillance methods and efforts were provided via hearing expert testimonies. Overall, the evidence is considered adequate to test RH1a and confirms the presence of teosinte in certain locations of ES and FR.
3.2.1.3. Conclusion
The new evidence retrieved confirms that teosinte occurs in regions in ES (mostly in Aragón and, to a lesser extent, in Cataluña) where maize MON810 is widely grown, and in FR (Poitou‐Charentes) where there is no maize MON810 cultivation. EFSA is not aware – based on available evidence – of teosinte occurrence in other EU Member States. Considering that maize MON810 and ES teosinte co‐occur in Aragón and Cataluña, RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’ is rejected for these regions, which is consistent with the ERA assumptions made previously in EFSA (2016).
3.2.2. RH1b – Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions
3.2.2.1. Evidence description/summary
Calfee et al. (2021)
Calfee et al. (2021) investigated through genomic analyses how vertical gene flow from native highland Z. mays subsp. mexicana facilitated the range expansion of maize from the valleys where it was domesticated to sites over 1,500 m in the mountains of Mexico. Genomic signatures of admixture and selection in paired sympatric maize and Z. mays subsp. mexicana populations, sampled from 14 locations across an elevational gradient in Mexico, were investigated to test for adaptive introgression and identify likely source populations. The authors found loci where Z. mays subsp. mexicana ancestry has been repeatedly favoured in highland maize populations, and where Z. mays subsp. mexicana ancestry increased steeply with elevation. Moreover, the selection against Z. mays subsp. mexicana ancestry, especially near domestication genes was shown.
Díaz et al. (2020)
Díaz et al. (2020) investigated the genetic variability of ES teosinte, and its genetic relationship with (commercial) maize varieties, maize‐like weeds, putative hybrids with maize and wild teosinte – both Z. mays subsp. mexicana and Z. mays subsp. parviglumis – using 17 microsatellites. The evidence reported suggests that ES teosinte represents an unidentified and genetically distinct group that has a complex origin being related to both commercial maize and wild teosinte, Z. mays subsp. mexicana and Z. mays subsp. parviglumis.
ES Competent Authority reports
The ES Competent Authority reports refer to a preliminary hybridisation study performed under greenhouse conditions in 2016 and repeated in 2017, in which the Cry1Ab protein was not detected in the progeny (grains) derived from the artificial crosses between maize MON810 and ES teosinte. No further details about the study are reported in the ES Competent Authority reports.
Reference is also made to ongoing research conducted at INIA and CSIC that aims to detect the Cry1Ab protein in (GM) teosinte hybrid progeny and determine Cry1Ab protein expression levels in such hybrids, but without providing more details.
FR Competent Authority report
The FR Competent Authority report refers to a preliminary hybridisation experiment carried out by INRAE. Based on unpublished data, the FR Competent Authority report mentions the spontaneous hybridisation rate of maize by teosinte pollen is an order of magnitude higher than that of teosinte by maize pollen (less than 0.1%). However, no primary data about the study are reported in the FR Competent Authority report.
The FR Competent Authority report also mentions that INRAE has continued molecular genetics work in 2021 and 2022 to further characterise introgression between maize and FR teosinte, but without reporting results.
Le Corre et al. (2020)
Le Corre et al. (2020) performed a phenotypic comparison of FR and Mexican (MX) teosinte under European conditions and characterised patterns of genetic variation in ES, FR and MX teosinte as well as in maize germplasm using single‐nucleotide polymorphisms (SNPs). The authors indicated that some characteristics/phenotypic traits of conventional maize (i.e. high latitude dent maize) varieties, including early flowering and herbicide tolerance, have been transferred successfully to FR teosinte. The introgression of the mutant herbicide target ACC1 gene from herbicide tolerant maize varieties commercialised after 2000 in FR indicates that maize to teosinte gene flow has occurred after the introduction of teosinte to maize fields in FR.
Lohn et al. (2021)
Lohn et al. (2021) assessed the rate of hybridisation between GM maize and ES teosinte under controlled conditions. In the summer of 2015, ES teosinte seeds were collected from the field in the province of Zaragoza (Aragón). The plants derived from the seeds were hand‐pollinated (in both directions, with teosinte as either pollen donor or pollen recipient). The authors reported that ES teosinte plants (#9) hand‐pollinated by maize MON810 (variety LG30490YG) pollen under controlled conditions (i.e. climate chambers, with removal of immature tassels from female plants) produced 2.7% viable hybrid seeds (in contrast to 92.8% when maize MON810 plants (#6 plants) were hand‐pollinated by ES teosinte pollen).
Lu et al. (2019)
Lu et al. (2019) investigated the genetic basis and mechanism of the teosinte crossing barrier1‐s (Tcb1‐s) leading to cross‐incompatibility in native Z. mays subsp. mexicana. The authors observed that in silk carrying the Tcb1‐s, pollen tubes had clustered callose plugs and their growth was slower in comparison to pollen tubes of compatible crosses. Lu et al. (2019) concluded that the modification of the pollen tube cell wall by the pistil (female) is likely a key mechanism to prevent continued pollen tube growth and delivery of the sperm cells in plant populations of maize and some teosintes.
Trtikova et al. (2017)
Trtikova et al. (2017) performed a genome‐wide analysis of SNP data to identity and define the origin of ES teosinte. ES teosinte and hybrid‐like seeds (autumn 2014 and 2015) and leaf samples of ES teosinte and cultivated maize (summer 2015) in the region of Aragón were gathered and genotyped, together with teosinte reference plants. The authors revealed that ES teosinte does not group with any of the currently recognised teosinte taxa. Based on Bayesian clustering analysis and hybridisation simulations, Trtikova et al. (2017) inferred that ES teosinte is of admixed origin, most likely involving Z. mays subsp. mexicana as one parental taxon, and an unidentified cultivated maize variety as the other.
Trtikova et al. (2017) also conducted experimental crosses with six ES maize MON810 varieties (LG30490YG) and 14 ES teosinte plants (grown from seeds collected in ES) under controlled conditions in a climate chamber. Based on these experimental crosses, the authors observed that maize plants hand‐pollinated by ES teosinte pollen failed to yield viable seeds. Referring to unpublished data derived from exploratory crossing experiments previously conducted by the authors, Trtikova et al. (2017) suggested that hand pollination of ES teosinte with maize pollen results only rarely in viable seeds.
3.2.2.2. Evidence appraisal
Some of the evidence retrieved is not considered relevant for the assessment of the hybridisation potential between maize and EU teosinte, as it focuses on the domestication process of maize (i.e. Calfee et al., 2021), or mechanisms of reproductive barriers leading to cross‐incompatibility in native teosinte populations of Z. mays subsp. mexicana (Lu et al., 2019). Since the publications reporting this evidence do not meet the eligibility criteria used to establish the relevance of evidence, they should have been excluded during the study screening/selection process. Moreover, in other cases, the entire data set or part of it mainly provides indirect evidence on vertical gene flow by investigating the genetic origin of ES/FR teosinte (e.g. Trtikova et al., 2017; Díaz et al., 2020). This evidence will not be appraised and considered further.
While reference is made to hybridisation studies in the ES/FR Competent Authority reports, no primary data are reported. Moreover, some of the studies referred to in the ES/FR Competent Authority reports have not been completed or published, so no final results are available at present. It is also noted that insufficient details are reported on materials and methods used to gather and analyse hybridisation data. It is therefore not possible to appraise the quality of evidence on hybridisation between maize and teosinte mentioned in the ES/FR Competent Authority reports.
The experimental crosses reported by Trtikova et al. (2017) and Lohn et al. (2021) provide new direct evidence on the potential of hybridisation between maize and EU teosinte. Similar type of evidence was reported before in the scientific literature, but for native teosinte mainly (reviewed by EFSA (2016)). Moreover, Trtikova et al. (2017) and Lohn et al. (2021) used maize MON810 as test material, in contrast to conventional maize. Yet, the experimental design of the hybridisation studies reported by Trtikova et al. (2017) and Lohn et al. (2021) was tailored to maximise hybridisation rates by overcoming various barriers to hybridisation (such as lack of proximity of maize and teosinte plants/plots, temporal flowering asynchrony, self‐pollination), so the approach followed may have overestimated true hybridisation rates under real‐life conditions. Moreover, the rate of hybridisation at the field level, and the fitness of any such hybrids under field conditions were not assessed by Trtikova et al. (2017) and Lohn et al. (2021). Even though Trtikova et al. (2017) indicated that their exploratory hand pollination experiments of ES teosinte with maize pollen yielded only rarely viable seeds, those data were unpublished at that time, and thus not available for appraisal.
Le Corre et al. (2020) investigated gene introgression of a maize gene (the mutant herbicide target ACC1) in FR teosinte, demonstrating that vertical gene flow from maize to FR teosinte has occurred in maize fields in FR.
3.2.2.3. Conclusion
The new evidence retrieved confirms that where maize and EU teosinte plants co‐occur and flower synchronously, maize alleles (transgenic or not), can move into teosinte populations at rates that depend on different factors. While it is challenging to compare the probability of hybridisation between teosinte and maize plants across available scientific publications due to differences in experimental design and test materials used, the experimental design implemented by Trtikova et al. (2017) and Lohn et al. (2021) was tailored to maximise hybridisation rates by overcoming various barriers to hybridisation (such as lack of proximity of maize and teosinte plants/plots, temporal asynchrony, self‐pollination), so the approach followed may have overestimated true hybridisation rates under real‐life conditions. However, the observation that hybridisation between maize (MON810) and teosinte is possible (Le Corre et al., 2020; Lohn et al., 2021) is consistent with previous literature (reviewed by EFSA (2016)) and does not represent a new result. Therefore, based on the available evidence, RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’ is rejected, which is consistent with the ERA assumptions made previously in EFSA (2016).
3.2.3. RH1c – GM teosinte hybrid progeny is not more persistent and invasive than non‐GM teosinte hybrid progeny under EU field conditions
3.2.3.1. Evidence description
Not applicable, as no new evidence relevant for RH1c was retrieved.
3.2.3.2. Evidence appraisal
Not applicable, as no new evidence relevant for RH1c was retrieved.
3.2.3.3. Conclusion
In EFSA (2016), it was concluded very unlikely that the establishment, spread and survival of potential GM teosinte hybrid progeny would be increased by insect resistance and/or herbicide tolerance. These traits can only be regarded as providing a potential selective advantage to GM teosinte hybrid progeny under high infestation of target pests and/or when glufosinate‐ammonium‐ and/or glyphosate‐based herbicides are applied. However, this fitness advantage will not allow GM teosinte hybrid progeny to overcome other biological and abiotic factors limiting their persistence and invasiveness. For example, ES teosinte seeds have no potential to survive in the soil after 2 years of burial at 2, 10 or 18 cm depth (Pardo et al., 2016; personal communication by Alicia Cirujeda). Therefore, EFSA considers that the growth habits of EU teosinte plants and teosinte hybrid progeny are such that the acquisition of insect resistance and/or herbicide tolerance is unlikely to change their relative persistence and invasive characteristics under EU conditions. Considering that no new evidence relevant for RH1c ‘GM teosinte hybrid progeny is not more persistent and invasive than non‐GM teosinte hybrid progeny under EU field conditions’ was retrieved, the ERA assumptions previously made in EFSA (2016) remain applicable.
3.2.4. RH1d – NTO does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions
3.2.4.1. Evidence description
Bellota et al. (2018)
Bellota et al. (2018) investigated whether host acceptance by female corn leafhoppers (Dalbulus maidis) varies among Zea hosts, and correlates with variation in defensive levels across such hosts. Host acceptance (in terms of feeding and oviposition) by corn leafhopper of Zea diploperennis, Z. mays subsp. parviglumis and landrace and commercial maize varieties was studied in no‐choice assays under confined conditions. Results showed no differences in host acceptance for oviposition or feeding among hosts. Moreover, oviposition frequency per plant by females did not seem to correlate with the performance of offspring.
ES Competent Authority reports
The ES Competent Authority reports refer to anecdotal evidence from Central America and Mexico to indicate that maize and teosinte may host similar insects, but do no report primary data, nor provide more details.
Gaillard et al. (2018)
Gaillard et al. (2018) performed feeding assays to assess the performance of eight species of insect herbivores (i.e. Spodoptera frugiperda, Spodoptera littoralis, Spodoptera exigua, Diabrotica virgifera virgifera, Diabrotica undecimpunctata howardi, Diabrotica balteata, D. maidis and Zyginidia scutellaris) on six European maize lines and six teosinte populations (of which five Z. mays subsp. parviglumis and one Z. mays subsp. mexicana). The effect of reduced defences in cultivated maize was most evident for generalist herbivores and significantly less pronounced for specialist herbivores. A metabolomics approach was used in an attempt to identify compounds responsible for observed differences in insect performance. Insects consistently performed better on maize than on teosinte. Differences in defence metabolites among the different genotypes were found, but none that consistently correlated with differences in insect performance.
Moya‐Raygoza et al. (2019)
Moya‐Raygoza et al. (2019) compared leafhopper diversity in maize and Z. mays subsp. parviglumis under field conditions, and assessed the potential influence of plant species diversity in the teosinte habitat on the diversity of leafhoppers. Leafhopper adults were collected in Jalisco province in Mexico from seven maize field sites and seven Z. mays subsp. parviglumis sites during the wet season of 2016 and 2017, whereas teosinte and teosinte‐associated plants were collected during the wet season of 2017 only. A higher level of leafhopper diversity was observed in the teosinte habitats than in the maize fields, with a 50% reduction in leafhopper species diversity seen in the maize sites compared with the teosinte sites. The authors reported a high plant diversity in the teosinte sites, and found a significant correlation between some leafhopper subfamilies and plant families in these teosinte sites.
Naranjo‐Guevara et al. (2021)
Naranjo‐Guevara et al. (2021) compared the direct and indirect defence abilities of cultivated maize, Z. diploperennis and Z. mays subsp. mexicana against S. frugiperda. The authors measured larval survival, and used indices related to food intake/utilisation as proxies for the direct defences of teosinte and maize, as well as the olfactory preference of the night‐active predatory earwig (Doru luteipes) for emissions of nocturnal herbivore‐induced plant volatiles for indirect defences. Results indicated that teosinte is better defended than maize in terms of direct and indirect defences, while Z. diploperennis has stronger direct defences against the fall armyworm than Z. mays subsp. mexicana.
3.2.4.2. Evidence appraisal
While the ES Competent Authority reports mention that maize NTOs may use teosinte and its progeny as host plant/food source under EU field conditions, no primary data are reported to substantiate the assumption. Moreover, the ES Competent Authority reports indicate that the suitability of teosinte and its progeny for maize NTOs has not been investigated/monitored systematically in ES. It is therefore not possible to appraise the quality of evidence on the suitability of teosinte and its progeny for maize NTOs mentioned in the ES Competent Authority reports.
Overall, the evidence reported by Bellota et al. (2018), Gaillard et al. (2018), Moya‐Raygoza et al. (2019) and Naranjo‐Guevara et al. (2021) is considered adequate. However, it is noted that the studies focus on native teosintes and a subset of NTOs (mainly insect herbivores that are not considered to have conservation value, i.e. maize pest species). Moreover, the experiments performed by Bellota et al. (2018), Gaillard et al. (2018) and Naranjo‐Guevara et al. (2021) were mostly carried under controlled conditions (e.g. feeding assays), which may not necessarily be representative of real‐life conditions. While Moya‐Raygoza et al. (2019) performed experiments under field conditions in the Jalisco province in Mexico, it is unclear whether these receiving environments are representative of those found in the EU.
3.2.4.3. Conclusion
Even though the new evidence retrieved focuses on native teosintes, a subset of NTOs (mainly maize pests), controlled conditions and non‐EU receiving environments, it adds to the weight of scientific evidence suggesting that maize NTOs may be using teosinte and its progeny as host plant/food source. Since teosinte is closely related to maize, EFSA assumes that a similar insect fauna occurs on maize and teosinte. Therefore, based on the available evidence, RH1d ‘Non‐target organism (NTO) does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’ is rejected, which is consistent with the ERA assumptions made previously in EFSA (2016).
3.2.5. RH1e – NTO is not adversely affected by exposure to GM teosinte hybrid progeny under EU field conditions
3.2.5.1. Evidence description
Not applicable, as no new evidence relevant for RH1e was retrieved.
3.2.5.2. Evidence appraisal
Not applicable, as no new evidence relevant for RH1e was retrieved.
3.2.5.3. Conclusion
In EFSA (2016), it was assumed that the overall environmental exposure to GM teosinte hybrid progeny, bearing either the insect resistance or herbicide tolerance trait or both, would remain low compared to exposure to GM maize, provided that measures are employed to monitor, control and/or eradicate EU teosinte in infested agricultural areas. Therefore, the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny on NTOs was assumed to be very low under EU conditions. Considering that no new evidence relevant for RH1e ‘NTO is not adversely affected by exposure to GM teosinte hybrid progeny under EU field conditions’ was retrieved, the ERA assumptions previously made in EFSA (2016) remain applicable.
3.2.6. RH1f – TO of Bt‐maize does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions
3.2.6.1. Evidence description
ES/FR Competent Authority reports
The ES/FR Competent Authority reports indicate that the suitability of teosinte and its progeny for the European and Mediterranean corn borers (ECB and MCB, respectively) has not been investigated/monitored systematically in ES and FR. Anecdotal evidence mentioned in the ES/FR Competent Authority reports suggests that:
Larvae of both corn borer species have been observed at various larval stages in teosinte plants;
During visits of infested sites over the course of all the years (2014–2020), corn borer infestations of teosinte have been observed;
In plots sampled by CITA and INRAE, the presence of larvae was occasionally observed on teosinte plants and/or in their ears or stem;
At present, it is not known whether corn borer larvae are able to complete their development cycle on teosinte plants. Research efforts are ongoing to address the matter.
3.2.6.2. Evidence appraisal
While reference is made to use of teosinte and its progeny as host plant/food source by ECB/MCB in the ES/FR Competent Authority reports, no primary data are reported. Moreover, no details on materials and methods are reported. Therefore, it is not possible to appraise the quality of evidence on the suitability of teosinte and its progeny for ECB/MCB mentioned in the ES/FR Competent Authority reports.
3.2.6.3. Conclusion
Even though the new evidence retrieved is scarce and of unclear quality, it adds to the weight of scientific evidence suggesting that ECB/MCB may be using teosinte and its progeny as host plant/food source. Since teosinte is closely related to maize, EFSA assumes that a similar insect fauna occurs on maize and teosinte. Therefore, based on the available evidence, RH1f ‘Target organism (TO) of Bt‐maize does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions’ is rejected, which is consistent with the ERA assumptions previously made in EFSA (2016).
3.2.7. RH2a – Transgene product in GM teosinte hybrid progeny is high dose under EU field conditions
3.2.7.1. Evidence description
ES Competent Authority reports
The ES Competent Authority reports mention that activities have been implemented in Aragón since 2021 to determine the potential of hybridisation between maize MON810 and teosinte. In this context, samples are taken in fields where maize MON810 is grown to determine both the presence of the Cry1Ab protein and levels of Cry1Ab protein expression in (GM) teosinte hybrid plants. Preliminary yet incomplete results are reported on Cry1Ab presence, but without providing further details.
Lohn et al. (2021)
Lohn et al. (2021) investigated whether GM teosinte hybrid progeny had comparable cry1Ab transcription activity and Cry1Ab expression levels to maize MON810 plants and TO bioactivity. The latter was investigated in insect bioassays in which ECB larvae were fed leaf samples (discs) of maize MON810, Cry1Ab‐expressing teosinte hybrids and ‘parental’ teosinte. The cry1Ab transgene was stably expressed as mRNA in all crossings and backgrounds. Toxicity on second‐instar larvae of ECB, presumably due to Cry1Ab protein, was consistently expressed in GM teosinte hybrid progeny, with mortality rates 95% or higher after only 4‐day exposure, similar to rates on maize MON810 plants, while the mean larval mortality rate of ECB fed plant material from teosinte was 27%. The authors observed no strong correlations between cry1Ab transcription levels and Cry1Ab concentrations, nor between Cry1Ab concentrations and insect mortality rates across all of the different genetic backgrounds.
3.2.7.2. Evidence appraisal
While reference is made to some preliminary results on lack of Cry1Ab presence in teosinte and/or teosinte hybrid progeny in Aragón in the ES Competent Authority reports, insufficient details are reported on materials and methods used to gather and analyse the preliminary data reported. It is therefore not possible to appraise the quality of evidence on Cry1Ab expression level in possible GM teosinte hybrid progeny mentioned in the ES Competent Authority reports.
Lohn et al. (2021) measured bioactivity against ECB using methodology different from what is widely published and accepted within the scientific literature. ECB susceptibility to Cry proteins has been shown to decrease with larval instar size/development, so the choice of second‐instar larvae for bioassays will tend to underestimate impacts of the plant lines on ECB. Moreover, exposure in the field will be overwhelmingly at the first‐instar stage.
A short duration assay (4 days) was conducted, in which larval survival was the only endpoint measured. Because larvae will be able to survive the assay without feeding on leaf disks, the short duration of the bioassay together with the measurement endpoint selected will underestimate effects of the Cry1Ab exposure. Typically, in such bioassays, mortality is considered along with other more sensitive and thus informative measurement endpoints such as larval weight. Therefore, the less than 100% mortality observed may have been due to the insect bioassay design.
The larval mortality of ECB fed parental teosinte leaf discs was substantial (ca. 27%). This could be attributed to the shift from artificial diet (perfect feed) to plant material which is a less suitable feed. To investigate potential effects associated with host plant suitability, additional treatments should have been included in the insects bioassay, whereby ECB larvae would have been continuously kept on artificial diet and the conventional (non‐GM) maize counterpart. Moreover, the inclusion of a control based on artificial diet only would also have allowed for the confirmation of insect quality across the insect bioassays, as presumably different batches of ECB eggs were used in the insect bioassays that were conducted from January to June 2018.
Finally, the observed lack of correlation between Cry1Ab level and bioactivity is unsurprising because there is minimal variability in the measured TO mortality, so hence there is no ability to observe a correlation, if present.
3.2.7.3. Conclusion
The new evidence retrieved indicates that teosinte hybrids that have acquired the cry1Ab transgene through hybridisation can express the Cry1Ab protein at sufficient concentration in leaves to kill 95% or more of second ECB instars. The less than 100% mortality observed by Lohn et al. (2021) may have been due to the insect bioassay design, and the authors’ comment about incomplete control and resistance selection is therefore not supported fully by the data. However, uncertainties remain about whether GM teosinte hybrid progeny expressing the Cry1A protein can be considered high dose. Therefore, RH2a ‘Transgene product in GM teosinte hybrid progeny is high dose under EU field conditions’ is rejected, which is consistent with the RM assumptions previously made in EFSA (2016).
The conclusion by Lohn et al. (2021) that there is no correlation between Cry1Ab expression levels and ECB response, and that this is a concern for risk assessment, is not supported by the data.
3.2.8. RH2b – GM teosinte hybrid progeny does not occur in non‐Bt‐maize refuge areas, nor in Bt‐maize fields in the EU
3.2.8.1. Evidence description
ES Competent Authority reports
The ES Competent Authority reports indicate that the incidence/occurrence of GM teosinte hybrid progeny expressing the Cry1Ab protein in non‐Bt‐maize refuge areas planted near or adjacent to, or within maize MON810 fields has not been investigated/monitored systematically in ES. The ES Competent Authority reports refer to anecdotal evidence to suggest that no teosinte has been found in non‐Bt‐maize refuges in Aragón and Cataluña.
3.2.8.2. Evidence appraisal
Not applicable, as no new evidence to test RH2b is reported in the ES Competent Authority reports.
3.2.8.3. Conclusion
In EFSA (2016), it was assumed that the overall environmental exposure to GM teosinte hybrid progeny, bearing the insect resistance trait, would remain low compared to exposure to GM maize, provided that measures are employed to control and/or eradicate teosinte and its progeny in infested agricultural areas. Therefore, the impact of insect resistance in GM teosinte hybrid progeny on TOs was assumed to be very low under EU conditions. Considering that no new evidence relevant for RH2b ‘GM teosinte hybrid progeny does not occur in non‐Bt‐maize refuge areas, nor in Bt‐maize fields in the EU’ is reported in the ES Competent Authority reports, the ERA assumptions previously made in EFSA (2016) remain applicable.
3.3. Risk assessment and risk management implications
The 18 publications retained for evidence appraisal following the literature screening/selection process are of varying relevance and quality, and represent a limited evidence base. For example, 12 out of the 18 publications focused on EU teosinte and two of those used maize MON810 as test material, in contrast to native teosinte and conventional maize. Moreover, some of the experimental designs implemented most likely resulted in an overestimation or underestimation of the true intervention effect under real‐life conditions. For some publications, insufficient details were reported about the materials, methods and results, hampering the assessment of the quality of the evidence reported. Therefore, some of ERA and RM assumptions previously made in EFSA (2016) could not be confirmed or rejected by the newly available evidence.
The new relevant evidence that has become available since the publication of EFSA (2016) is not sufficient to corroborate all risk hypotheses along the pathway to harm proposed for the cultivation of GM maize in EU areas infested with teosinte, neither to show that the pathway is blocked at any step. However, at each step in the pathway to harm, a hypothesis that the event is rare can be corroborated to a greater or lesser extent. Therefore, it can be concluded that completion of the pathway to harm requires a succession of rare events, of which the combined probabilities are very low. Consequently, it is unlikely that environmental harm will be realised through the postulated pathway to harm.
The new evidence retrieved confirms that where maize and EU teosinte plants co‐occur and flower synchronously, maize alleles (transgenic or not), can move into teosinte populations at rates that depend on different factors. Hence, the possible introgression of transgenes from maize MON810, Bt11, 1507 and GA21 into EU teosinte may only provide a selective advantage to GM teosinte hybrid progeny under high infestation of target pests and/or when glufosinate‐ammonium‐ and/or glyphosate‐based herbicides are applied. However, this fitness advantage will not allow GM teosinte hybrid progeny to overcome other biological and abiotic factors limiting their persistence and invasiveness. Therefore, EFSA considers that the growth habits of EU teosinte plants and teosinte hybrid progeny are such that the acquisition of insect resistance and/or herbicide tolerance is unlikely to change their relative persistence and invasive characteristics under EU conditions.
It is noted that the overall environmental exposure to GM teosinte hybrid plants, bearing either the insect resistance or herbicide tolerance trait or both, will remain low compared to exposure to GM maize, provided that measures continue to be employed to monitor, control and/or eradicate EU teosinte in infested agricultural areas. Therefore, in line with EFSA (2016) and if the measures employed to monitor, control and/or eradicate teosinte in infested agricultural areas remain in place, it is assumed that the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny (potentially acquired through hybridisation between GM maize and teosinte) on TOs, NTOs, the abiotic environment and biogeochemical cycles will be very low under EU conditions.
In infested agricultural areas in Aragón and Cataluña, a set of measures across many fields have been implemented since 2014 and 2015 to monitor, control and/or eradicate teosinte. These measures include (see also Pardo et al., 2016; Cirujeda et al., 2019):
-
Conduct of systematic field inspection surveys at different crop development stages, to identify, map and monitor the presence and evolution of teosinte, and assess the level of infestation at a field level;
-
○
In Aragón, two infestation levels are considered: ‘low’ (= scattered, isolated teosinte plants) and ‘high’ (= teosinte plants occurring in patches or being spread out throughout the entire field);
-
○
In Cataluña, three infestation levels are considered: ‘low’ (= scattered, isolated teosinte plants), ‘medium’ (= and few teosinte plants occurring in patches) and ‘high’ (= teosinte plants being spread out throughout the entire field);
-
○
-
Implement control and/or eradication measures that are proportionate to the level of infestation. Depending on the level of infestation, such measures may include:
-
○
Removal of teosinte plants close to sprinklers, pivots and other structures as soon as they are detected and always prior to seed ripening;
-
○
Removal of teosinte plants potentially appearing in field margins;
-
○
Clean field machinery to avoid dispersal of teosinte seed between agricultural fields;
-
○
Prohibit/avoid the growing of maize or sorghum on severely infested agricultural fields during the next two to three spring/summer sowing seasons;
-
○
Use stale or false seedbeds to promote teosinte seed germination and their control prior to planting of a crop through chemical and/or mechanical means;
-
○
Use shallow tilling practices to control teosinte that emerged between maize rows;
-
○
Rotate crops by growing a summer, broadleaf crop (such as sunflower, alfalfa and beans) and winter cereals (e.g. wheat, barley) in up to three subsequent years, instead of maize or sorghum;
-
○
Apply graminicides (grass herbicides) and/or mechanical weed control in the subsequent broadleaved crops in the rotation;
-
○
Avoid off‐field removal of forage and any harvest remains;
-
○
Prohibit grazing on highly infested agricultural fields as long as teosinte is not fully eradicated;
-
○
Check compliance with measures to implement;
Assess the efficacy of the measures implemented;
Raise farmer awareness about monitoring, control and/or eradication measures, and ensure farmer compliance with the mandatory requirements;
Notify teosinte occurrence to the Competent Authorities in a timely manner;
Maintain a close liaison between maize farmers and technicians operating at a regional scale to ensure the notification of any potential new teosinte appearances.
Evidence suggests that high level of farmers’ compliance to the monitoring, control and eradication measures have contributed to reduce the abundance of teosinte plants and limit their spread in the infested agricultural areas in ES. The monitoring, control and eradication measures put in place in ES (especially in Aragón and Cataluña where maize MON810 is widely grown) therefore contribute to further reduce the low potential of vertical gene flow between GM maize and ES teosinte, and thus the likelihood of environmental harm to occur through the postulated pathway to harm.
4. Conclusions
Following a request of the European Commission, EFSA evaluated whether the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, or require revision in light of new scientific evidence on teosinte that has become available since the publication of EFSA (2016). A limited collection of 18 publications of varying relevance and quality was retrieved and assessed. Based on the newly available scientific information on teosinte, it is concluded that the ERA conclusions and RM recommendations of EFSA (2016) remain applicable, except those pertaining to the use of glyphosate‐based herbicides on maize GA21 which should be considered under Regulation (EC) No 1107/2009.1 If the measures employed to monitor, control and/or eradicate EU teosinte in infested agricultural areas (especially in regions where maize MON810 is widely grown) remain in place, the impact of insect resistance and/or herbicide tolerance in GM teosinte hybrid progeny (potentially acquired through hybridisation between GM maize and teosinte) on TOs, NTOs, the abiotic environment and biogeochemical cycles will be very low under EU conditions.
5. Recommendations
EFSA encourages the ES/FR Competent Authorities to continue employing comprehensive weed management measures (summarised in Section 3.3, above) to monitor, control and/or eradicate teosinte in infested agricultural areas, and restrict the cultivation of maize MON810 in fields where the incidence of teosinte plants exceeds regional infestation thresholds. The monitoring, control and eradication measures put in place in ES (especially in Aragón and Cataluña where maize MON810 is widely grown) contribute to further reduce the low potential of vertical gene flow between GM maize and ES teosinte, and thus the likelihood of environmental harm to occur through the postulated pathway to harm.
For future annual PMEM reports on the cultivation of maize MON810, it is recommended that:
The consent holder explicitly considers all new scientific evidence on teosinte relevant for the ERA and RM of maize MON810;
The consent holder revises farmer questionnaires to include the reporting of both the occurrence of ES teosinte and corresponding levels of infestation;
The consent holder and the Competent Authorities share relevant information on teosinte for regions where maize MON810 cultivation may co‐occur with teosinte.
Moreover, it is encouraged that the research/monitoring activities pertaining to teosinte performed/commissioned by the ES/FR Competent Authorities be continued and expanded. This will be critical for the generation of empirical data on EU teosinte that could be used to further test specific risk hypotheses of the devised pathway to harm, and confirm previously made ERA and RM assumptions.
6. Documentation as provided to EFSA
Request for scientific assistance on teosinte. September 2021. Submitted by the European Commission (Directorate‐General for Health and Food Safety);
Acknowledgement of receipt of the mandate. October 2021. Submitted by the European Food Safety Authority.
Appendix A – Protocol supporting the update of the environmental risk assessment conclusions and risk management recommendations of EFSA (2016) on EU teosinte
| Prepared by: |
– Yann DEVOS (EFSA, SCER) – Elisa AIASSA (EFSA, AMU) – Irene MUNOZ‐GUAJARDO (EFSA, AMU) |
|---|---|
| Reviewed by: |
– Antoine MESSEAN (EFSA GMO CompERA Working Group expert) – Ewen MULLINS (EFSA GMO CompERA Working Group chair and GMO Panel chair) |
| Endorsed by: | Leslie FIRBANK (EFSA GMO CompERA Working Group expert and GMO Panel member) |
A.1 Scope and structure of the protocol
This document outlines the protocol for the scientific assessment of new evidence on teosinte, which will be used as input for the update of the environmental risk assessment (ERA) conclusions and risk management (RM) recommendations of EFSA (2016). This protocol has been developed with the aim of defining the methods for collecting data, appraising the relevant evidence, and analysing and integrating the evidence in light of the identified uncertainties.
The protocol has been developed following the principles and process for dealing with data and evidence in scientific assessments (EFSA, 2015), and is based on the recommendations for protocol development for non‐application mandates (EFSA, 2020). The draft protocol has been reviewed by representatives of DG SANTE (E3) and CompERA expert Working Group of EFSA’s GMO Panel, and revised based on the feedback received.
In line with EFSA (2020) on protocol development for non‐application mandates, EFSA developed this protocol to clarify the interpretation of the Terms of Reference (ToRs) of the mandate and make them operational.
The protocol consists of:
A problem formulation that outlines what the assessment aims to address and thus the objectives of the assessment;
An analysis plan that outlines which methods will be used to address the problem (i.e. how the assessment will be carried out).
A.2 Problem formulation (Step 1 of EFSA (2020))
In line with EFSA (2020), the ToRs of the mandate were translated into two scientifically answerable assessment questions (AQs) and subquestions, respectively. In addition, relationships between AQs and subquestions were clarified through a conceptual model that follows a pathway to harm approach. Moreover, an approach to take to answer AQs and sub‐questions was selected.
A.2.1 Objectives of the assessment literature search
The overall objectives of the assessment are to establish the following:
What new relevant scientific and technical evidence on teosinte is now available since the publication of EFSA (2016) that should be considered for the update and possible revision of the ERA conclusions of EFSA (2016);
What new relevant scientific and technical evidence on teosinte is now available since the publication of EFSA (2016) that should be considered for the update and possible revision of the RM recommendations of EFSA (2016).
A.2.2 Assessment questions
The ToRs were translated into the two AQs below:
AQ1: Is the acquisition of transgenes (i.e. cry1Ab, cry1F and/or mepsp s) from GM maize (i.e. events MON810; Bt11, 1507 and GA21) by teosinte likely to occur through vertical gene flow under EU field conditions when genetically modified (GM) maize is grown in EU areas infested with teosinte, and would it lead to environmental harms that have not been previously considered in EFSA (2016), thereby requiring the update and revision of former ERA conclusions?
AQ2: Will the occurrence of teosinte hybrid progeny that have acquired the cry1Ab or Cry1F gene from Bt‐maize (i.e. MON810, Bt11 and 1507) through vertical gene flow in non‐Bt‐maize refuge areas planted near or adjacent to, or within Bt‐maize fields increase the likelihood of Cry1Ab/Cry1F resistance to evolve in European and Mediterranean corn borers, hence requiring the update and revision of risk management recommendations in EFSA (2016)?
A.2.3 Subquestions and conceptual model
When devising subquestions for each of the two AQs and clarifying their relationship, a pathway to harm approach (consisting of a causal chain of events) was followed as a conceptual model. The reason is that the pathway to harm approach enables to describe and hypothesise how a proposed action (i.e. the cultivation of GM maize in EU areas infested with teosinte) could be harmful to specific protection goals, through a causal chain of events. For each individual event (i.e. step) in the pathway a risk hypothesis (RH) is formulated that can then be tested. This approach enables the characterisation of risk against well‐defined criteria of hypothesis corroboration or falsification. If the testing of a RH concludes that a step in a pathway is unlikely to occur, then the likelihood of that particular harm occurring through that particular pathway is also unlikely. By contrast, falsification of all of the RHs in a pathway would indicate high probability of harm, and high risk if the harm was severe enough.
The pathway to harm proposed for the cultivation of GM maize in EU areas infested with teosinte consists of three interconnected pathways that share some of the same steps (Figure A.1). For simplicity, only the main events of the aggregated pathway are represented, focusing on risk concerns typically considered in the ERA of GM plants. These risk concerns include: (1) persistence and invasiveness of the GM plant, including vertical gene flow; (2) interactions of the GM plant with non‐target organisms (NTOs); and (3) interactions of the GM plant with target organisms (TOs).
A first risk concern addressed by the pathway is that the acquisition of transgenes from GM maize through vertical gene flow by teosinte would increase the persistence and invasiveness of GM teosinte hybrid progeny compared with vertical gene flow from conventional maize. If GM teosinte hybrid progeny becomes more persistent in arable land, it may exacerbate weed problems. If a transgene acquired by teosinte results in increased invasiveness of teosinte hybrid progeny outside cultivated land and beyond the geographical range of its conventional counterpart, such plants may cause environmental harm by locally displacing valued species;
A second environmental risk concern covered by the pathway is that NTOs would be adversely affected by exposure to teosinte hybrid progeny that have acquired a transgene from GM maize through vertical gene flow;
A final risk concern included in the pathway is that teosinte hybrid progeny that have acquired a transgene from GM maize through vertical gene flow may speed up resistance evolution to the transgene product by the TOs of GM maize, exacerbating pest control issues.
Figure A.1.
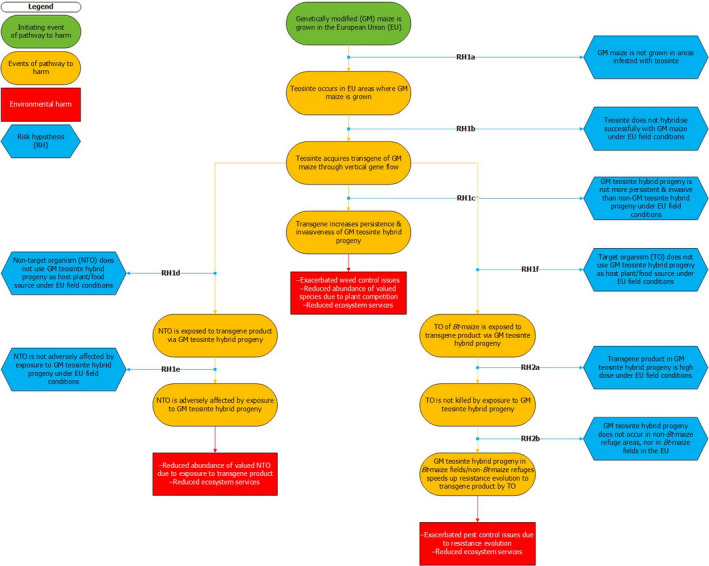
Conceptual model that is based on the pathway to harm approach for the cultivation of genetically modified (GM) maize in EU areas infested with teosinte, and that interlinks the assessment questions, subquestions (i.e. risk hypotheses (RHs)) and relevant events of the pathway
The subquestions devised for the pathway to harm for the cultivation of GM maize in EU areas infested with teosinte are reported in Table A.1. They represent the RHs formulated for each step in the pathway. RHs were formulated as negative statements that will be tested (corroborated or falsified/rejected) using the new evidence that has become available since the publication of EFSA (2016).
Table A.1.
Summary of subquestions (i.e. risk hypotheses (RHs)) for each assessment question (AQ) that will be answered in this scientific assessment, and relevant endpoints to consider for testing RHs
| AQs | RHs | Relevant endpoints (information) to test (corroborate or falsify/reject) RHs | |
|---|---|---|---|
| 1 | 1a | Teosinte does not occur in EU areas where genetically modified (GM) maize is grown | Spatial overlap between GM maize cultivation and teosinte occurrence |
| 1b | Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions |
– Hybridisation rate (e.g. number of (viable and fertile) hybrid seeds/plants relative to the number of non‐hybrid ones) – Gene introgression6 |
|
| 1c | GM teosinte hybrid progeny is not more persistent and invasive than non‐GM teosinte hybrid progeny under EU field conditions |
– Vegetative vigour – Reproductive fitness and seed production – Abundance and geographical distribution of GM teosinte hybrid progeny (that has acquired the transgene from GM maize through vertical gene flow) |
|
| 1d | Non‐target organism (NTO) does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions | Host plant suitability | |
| 1e | NTO is not adversely affected by exposure to GM teosinte hybrid progeny under EU field conditions | NTO mortality, development time, growth, weight, fecundity, fertility, number of progeny and progeny survival | |
| 1f | Target organism (TO) of Bt‐maize does not use GM teosinte hybrid progeny as host plant/food source under EU field conditions | Host plant suitability | |
| 2 | 2a | Transgene product in GM teosinte hybrid progeny is high dose under EU field conditions |
– Concentration of transgene product in relevant plant parts of GM teosinte hybrid progeny – TO mortality |
| 2b | GM teosinte hybrid progeny does not occur in non‐Bt‐maize refuge areas, nor in Bt‐maize fields in the EU | Plant/population density of GM teosinte hybrid progeny in non‐Bt‐maize refuge areas planted near or adjacent to, or within Bt‐maize fields | |
A.2.4 Approach to take
In regard to the approach to take to answer the AQs and subquestions, EFSA (2020) offers the option to prioritise subquestions over others, based on criteria such as the anticipated impact of subquestions on the conceptual model. Therefore, a relative priority (either high or low) was assigned to each of the RHs of the pathway to harm for the cultivation of GM maize in EU areas infested with teosinte, based on the anticipated relative impact of the RH on the conceptual model (e.g. relevance of the RH in the pathway to harm).
Both RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’ and RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’ were assigned a relative high priority because they represent the common steps to the three interconnected pathways to harm considered in the generic pathway, while the remaining RHs were assigned a relative priority.
Based on the relative priority assigned to the subquestions, preliminary considerations and proposals on the approaches to take to answer them were made (Table A.2). For RHs deemed of low priority, narrative approaches based on expert judgement will be applied, while for RHs identified as of high priority, a more structured approach will be followed, provided that sufficient evidence exists and will be available.
Table A.2.
Prioritisation of the assessment questions (AQs) and subquestions (i.e. risk hypotheses (RHs)), and outline of the methods for assessing them
| Relative priority | AQs/RHs | Approach |
|---|---|---|
| High | 1a |
Maize MON810 cultivation Data collection: assess by summarising relevant information reported by the Competent Authorities of EU Member States7, and submitted by the consent holder to the European Commission (DG SANTE)/EFSA in the frame of the annual post‐market environmental monitoring (PMEM) reports on the cultivation of maize MON810 – Evidence appraisal: narrative – Evidence synthesis: qualitative Teosinte occurrence – Data collection: extensive literature search and reports of Competent Authorities of EU Member States submitted to EFSA by DG SANTE – Study selection process: structured study selection process based on pre‐defined eligibility criteria (which will also be used for selecting the reports of the Competent Authorities of EU Member States submitted to EFSA by DG SANTE) – Evidence appraisal: structured, using critical appraisal tool(s) – Evidence synthesis: qualitative |
| 1b | Same as for teosinte occurrence (second part of 1a, above) | |
| Low | 1c, 1d, 1e, 1f, 2a, 2b |
–Data collection: extensive literature search and reports of Competent Authorities of EU Member States submitted to EFSA by DG SANTE – Study selection process: structured study selection process based on predefined eligibility criteria (which will also be used for selecting the reports of the Competent Authorities of EU Member States submitted to EFSA by DG SANTE) – Evidence appraisal: narrative – Evidence synthesis: qualitative |
A.3 Methods foreseen for undertaking the assessment (Step 2 of EFSA (2020))
The assessment will be based on the evidence retrieved via an extensive literature search (following the general principles and stepwise approach described in EFSA (2010, 2019)), and from reports supplied to DG SANTE by the Competent Authorities of EU Member States.
A.3.1 Eligibility criteria to establish the relevance of evidence
Eligibility criteria will be used to assess the relevance of evidence for inclusion in the review (see Tables A.3 and A.4). Such criteria will be applied for selecting the evidence retrieved from the literature as well as that provided by the Competent Authorities of EU Member States. These criteria will be tested on a subset of publications and refined if found to be prone to misinterpretation.
A.3.2 Evidence sources
Two main sources of evidence – electronic bibliographic databases and reports of the Competent Authorities of EU Member States – will be used to identify the necessary evidence for the review.
A.3.2.1 Electronic bibliographic databases
For the review of scientific literature, electronic bibliographic databases listed in Table A.5 will be searched to identify relevant studies. The databases selected have been identified in line with the defined scope of the review.
Table A.5.
Electronic bibliographic databases searched for relevant evidence
| Source of information | Platform |
|---|---|
| BIOSIS Citation Index | Web of Science |
| CAB Abstracts | |
| Current Contents Connect | |
| FSTA | |
| Medline | |
| SciELO | |
|
Web of Science Core Collection:
| |
| Dialnet | Dialnet |
| Scopus | Scopus.com |
A.3.2.2 Reports of the Competent Authorities of EU Member States
In December 2020, DG SANTE requested the Competent Authorities of France (FR) and Spain (ES) to provide them with: (1) an update on the current situation of teosinte in the affected areas in their territory; and (2) any relevant data on teosinte that they would have gathered through research and monitoring projects.
Following the DG SANTE request, the ES/FR Competent Authorities supplied the following reports to that were subsequently translated in English (EN):
-
Documents provided by the FR Competent Authority (original documents in French):
-
a
Note on the teosinte situation in FR from the authorities;
-
b
Annex – Teosinte: A weed that requires special vigilance.
-
a
-
Documents provided by the ES Competent Authority (original documents in Spanish):
-
a
Report of the National Biosafety Committee (CNB) on the presence of teosinte in Europe;
-
b
Reply to the request for information on the teosinte situation in Catalonia;
-
c
Report on the teosinte (Zea mays subsp.) situation in the autonomous community of Aragón, 2020.
-
a
At the meeting of the Standing Committee on Plants, Animals, Food and Feed – Section Genetically Modified Food and Feed of 20 September 2021, DG SANTE invited the Competent Authorities of EU Member States to supply any relevant evidence on teosinte that they may have gathered through research and monitoring projects in their territory.
A.3.3 Search strategy to identify relevant evidence
The search strategy for identifying evidence from the scientific literature has been designed to retrieve as many relevant studies as possible to address the two AQs and sub‐questions reported in Table A.1.
An EFSA information specialist has been involved in the design and conduct of the search strategy.
The approach used to develop the search strategy follows a lumping method using an all‐encompassing approach that captures all assessment questions and sub‐questions. The search string has been designed using teosinte as the key element of the review question (concept). The search string includes a wide range of free‐text terms and where available, controlled vocabulary that defines search terms (Table A.6). Search terms have been selected with the support of consulting thesaurus (e.g. CAB Thesaurus), dictionaries and previous publications relevant to answer the questions of interest, such as EFSA (2016) and relevant publications cited therein, as well as the feedback of EFSA scientific officers and experts.
Table A.6.
Translation of key elements of the review question into search terms
| Key elements of the assessment question (concepts) | Candidate search terms | |
|---|---|---|
| Population (teosinte) | Free‐text terms |
– Teosinte; téosinte; teocintle – Zea diploperennis; Z diploperennis – Zea luxurians; Z luxurians – Zea nicaraguensis; Z nicaraguensis – Zea perennis; Z perennis – Zea mays subspecies huehuetenangensis; Zea mays subsp huehuetenangensis; Zea mays spp huehuetenangensis; Zea mays huehuetenangensis; Zea huehuetenangensis; Z mays subsp huehuetenangensis; Z mays spp huehuetenangensis; Z mays huehuetenangensis; Z huehuetenangensis – Zea mays subspecies mexicana; Zea mays subsp mexicana; Zea mays spp mexicana; Zea mays mexicana; Zea mexicana; Z mays subsp mexicana; Z mays spp mexicana; Z mays mexicana; Z mexicana – Zea mays subspecies parviglumis; Zea mays subsp parviglumis; Zea mays spp parviglumis; Zea mays parviglumis; Zea parviglumis; Z mays subsp parviglumis; Z mays spp parviglumis; Z mays parviglumis; Zea parviglumis |
| Controlled vocabulary (CAB Abstracts) |
– Zea diploperennis – Zea luxurians – Zea mexicana |
|
The search terms and their combinations have been established in EN, ES and FR dependent on the characteristics of the source of information. The language of publications to be considered as described in the eligibility criteria will be EN, ES and FR (Tables A.3 and A.4).
The search strings as will be run in the sources of information to retrieve relevant studies are reported in Tables A.7, A.8–A.9. They combine search terms using Boolean and proximity operators.
Table A.7.
Search strings. Dialnet
| Set | Search string | Key elements of the assessment question (concepts) |
|---|---|---|
| #1 | teosinte OR téosinte OR teocintle | Teosinte |
| #2 | "Z diploperennis" OR "Z huehuetenangensis" OR "Z luxurians" OR "Z mexicana" OR "Z nicaraguensis" OR "Z parviglumis" OR "Z perennis" | |
| #3 | "Zea diploperennis" OR "Zea huehuetenangensis " OR "Zea luxurians" OR "Zea mexicana" OR "Zea nicaraguensis" OR "Zea parviglumis" OR "Zea perennis" | |
| #4 | (("Z mays" OR "zea mays") AND (huehuetenangensis OR mexicana OR parviglumis)) | |
| #5 | Filtro: articulo de revista [Filter: journal article] | Publication type limit |
| #6 | (#1 OR #2 OR #3 OR #4) AND #5 | Teosinte AND Publication type limit |
Table A.8.
Search strings. Scopus
| Set | Search string | Key elements of the assessment question (concepts) |
|---|---|---|
| #1 | TITLE‐ABS‐KEY (teosinte OR téosinte OR teocintle OR "Z diploperennis" OR "Z huehuetenangensis" OR "Z luxurians" OR "Z mexicana" OR "Z nicaraguensis" OR "Z parviglumis" OR "Z perennis" OR (zea W/3 (diploperennis OR huehuetenangensis OR luxurians OR mexicana OR nicaraguensis OR parviglumis OR perennis)) OR ("Zea mays" W/3 (huehuetenangensis OR mexicana OR parviglumis)) OR ("Z mays" W/3 (huehuetenangensis OR mexicana OR parviglumis))) | Teosinte |
| #2 | (PUBYEAR > 2015) AND (LIMIT‐TO (LANGUAGE, "English") OR LIMIT‐TO (LANGUAGE, "Spanish") OR LIMIT‐TO (LANGUAGE, "French")) | Time/Publication language limits |
| #3 | #1 AND #2 | Teosinte AND Time/Publication language limits |
Table A.9.
Search strings. Web of Science Platform
| Set | Search string | Key elements of the assessment question (concepts) |
|---|---|---|
| #1 | TS=(Teosinte OR téosinte OR Teocintle OR "Z diploperennis" OR "Z huehuetenangensis" OR "Z luxurians" OR "Z mexicana" OR "Z nicaraguensis" OR "Z parviglumis" OR "Z perennis" OR (Zea NEAR/3 (diploperennis OR huehuetenangensis OR luxurians OR mexicana OR nicaraguensis OR parviglumis OR perennis)) OR (("Z mays" OR "zea mays") NEAR/3 (huehuetenangensis OR mexicana OR parviglumis))) | Teosinte |
| #2 | Timespan: 2016‐01‐01 to 2022‐12‐31 (Publication Date) AND English OR French OR Spanish (Languages) | Time/Language of publication limits |
| #3 | #1 AND #2 | Teosinte AND Time/Publication language limits |
The search is limited by the approval year of EFSA (2016), i.e. 2016, and thus only consider studies that have been published in and after 2016. Thus, the extensive literature search will cover the time span of 2016 till the end of December 2021. Studies that were already addressed in EFSA (2016) will be excluded from the review.
Reference publications that are relevant to answer the two AQs and sub‐questions are within the scope of the review will be used for identifying search terms as well as validating the search strategy. A list of reference publications, complying with the above criteria and used in validating the search strategy as part of the protocol development, is provided in Table A.10.
Table A.10.
Reference publications
| Publication year | Authors | Title | Journal | Volume | Pages |
|---|---|---|---|---|---|
| 2020 | Le Corre V, Siol M, Vigouroux Y, Tenaillon MI and Délye C | Adaptive introgression from maize has facilitated the establishment of teosinte as a noxious weed in Europe | Proceedings of the National Academy of Sciences | 117 | 25618–25627 |
| 2021 | Lohn AF, Trtikova M, Chapela I, Binimelis R and Hilbeck A | Transgene behavior in genetically modified teosinte hybrid plants: transcriptome expression, insecticidal protein production and bioactivity against a target insect pest | Environmental Sciences Europe | 33 | 67 |
The output of the searches will be loaded into Endnote bibliographic management software (Clarivate Analytics). Duplicate references will be removed by a combination of automatic and manual detection of duplicates. Additional deduplication might be performed using the review management software DistillerSR (Evidence Partners).
The final search processes and strategies will be documented and reported in the EFSA Statement, i.e. the date of the search, sources of information, search string for each bibliographic database and additional sources, and the number of records before and after deduplication.
A.3.4 Study selection process
The evidence retrieved through the literature searches and supplied by the Competent Authorities of EU Member States will be screened for its relevance – against the eligibility criteria illustrated above (Tables A.3 and A.4). Relevant evidence will need to comply with all the eligibility criteria of the review.
The study selection process will be undertaken in two steps:
Step 1: A rapid assessment based on title and abstract to exclude records that obviously are irrelevant;
Step 2: A detailed assessment of full‐text documents.
Records that appear to be relevant and that of unclear relevance in Step 1 will be analysed further in Step 2, using the full‐text document.
If a full‐text document is not immediately accessible, other attempts to retrieve it will be made, including contact with the authors. Records that cannot be obtained will be reported in a table of unobtainable evidence.
During the selection process, studies reported in multiple records will be identified and duplicates removed. In addition, during this step of the process each study will be classified according to its relevance to the sub‐questions (i.e. RHs), having in mind that one study can be relevant to multiple sub‐questions.
Each record will be screened independently by two reviewers (i.e. EFSA scientist and topic expert for Step 1, and topic experts for Step 2) to minimise the risk of error. Results of the independent screenings will be compared. Any ambiguities between reviewers will be discussed to reach a consensus. If no consensus is reached, an additional independent opinion will be sought, from another member of the review team, in order to resolve differences of opinion.
The screening process will be undertaken in the review management software DistillerSR.
The results of the different phases of the record selection process will be reported in a flowchart as recommended in the PRISMA statement on preferred reporting items for systematic reviews and meta‐analyses.13
A.3.5 Evidence appraisal
Studies selected for the assessment will be appraised by looking at their risk of bias (internal and external bias) and, if possible, imprecision.
The approach will vary dependent on the relative priority of the sub‐question. A structured approach will be followed for studies underpinning the sub‐questions RH1a ‘Teosinte does not occur in EU areas where genetically modified (GM) maize is grown’ and RH1b ‘Teosinte (occurring in the EU) does not hybridise successfully with GM maize under EU field conditions’, while a narrative approach will be followed for the studies underpinning the remaining sub‐questions.
Overall, the presence of bias (internal or external) affects the validity of a study. Internal bias refers to any error in the conduct of the study that results in a conclusion which is different from the truth. For instance, experimental designs tailored to maximise hybridisation rates (e.g. hand‐pollination, studies performed under confined conditions) may overestimate actual real‐life hybridisation rates. External bias affects the extent to which the study results are generalisable to the assessment question, e.g. when the study settings are not representative of the reference population, conditions, or landscape settings. An example of a source of external bias is a study site that is not representative of EU receiving environments.
Imprecision pertains to random error and indicates the ability of a study to provide similar results when repeated under the same conditions. Such aspect is mainly related to sample size, which may not be sufficient to provide a precise estimate of the outcome of interest.
A.3.5.1 Appraisal of studies relevant to sub‐questions RH1a and RH1b
The studies relevant to sub‐questions RH1a and RH1b will be appraised using standardised critical appraisal tools (CATs), containing a predefined list of study design‐specific sources of bias (and, if possible, imprecision) and guidance for judging the risk of bias due to each of them.
Ideally, this judgement should attempt to quantify the impact of each source of bias on the likely direction and magnitude of the study estimated parameters. Moreover, it should be based on empirical evidence for a scientifically defendable method. However, owing to the expected lack of empirical evidence on the impact of bias in the research field relevant to RH1a and RH1b, it is foreseen that RoB judgements will be mostly based on expert judgement, when possible and without attempting to guess.
The scale that will be used for expressing the judgement on RoB is illustrated in Table A.11 (based on OHAT/NTP RoB assessment tool (NTP, 2015)). The judgement will be accompanied by a narrative description of the basis for that rating. The rating scale for assessing imprecision will be defined throughout the process, if such assessment is possible.
Table A.11.
Rating scale for risk of bias (RoB) judgements for the studies underpinning sub‐question RH1b
| Rating scale | Explanation |
|---|---|
| Definitely Low RoB (DLRoB) | There is direct evidence (i.e. it is clearly indicated in the study) of low RoB practices |
| Probably Low RoB (PLRoB) |
There is indirect evidence (i.e. it can be reasonably inferred) of low RoB practices OR It is deemed that deviations from low RoB practices for these criteria during the study would not appreciably bias results, including consideration of direction and magnitude of bias |
| Probably High RoB/NR (PHRoB/NR) |
There is indirect evidence of high RoB practices OR There is insufficient information (e.g. not reported or ‘NR’) provided about relevant RoB practices |
| Definitely High RoB (DHRoB) | There is direct evidence of high RoB practices |
The appraisal will be done by endpoint (or group of endpoints) as within the same study unit as RoB (and precision) may change dependent on the endpoint(s). To this end, study appraisal will be preceded by the identification and list of all relevant endpoints for each study unit.
CATs will be incorporated into DistillerSR to allow a web‐based appraisal of studies. Each study will be appraised by at least one (EFSA/non‐EFSA) topic expert. In case of doubt, additional evaluators will be involved to discuss together and collectively determine the reliability of the evidence.
A.3.5.2 Presentation of the results of evidence appraisal for the studies underpinning sub‐questions RH1a and RH1b, by endpoint(s)
The results of evidence appraisal for each study by endpoint will be presented by source of bias and without an attempt to summarise the overall risk of bias across sources. They will be presented in tabular format both for individual studies and at the level of the body of evidence (see Table A.12 for an example on internal validity; a similar table will be used for external validity). When presenting the results at individual study level, the rationale for the rating of each source of bias will be reported. Elements related to precision could also be illustrated.
Table A.12.
Example of heat map for presenting the results of evidence appraisal across the body of evidence for internal validity
| Study number | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | n | ||
| Endpoint(s) | … | … | … | … | … | … | … | … | … | |
| Result(s) | … | … | … | … | … | … | … | … | … | |
| Source of bias | 1 | PHRoB/NR | PLRoB | DLRoB | PHRoB/NR | DLRoB | PLRoB | DHRoB | PHRoB/NR | … |
| 2 | DHRoB | DLRoB | PHRoB/NR | DLRoB | PHRoB/NR | DLRoB | DLRoB | PHRoB/NR | … | |
| 3 | PLRoB | PLRoB | DHRoB | PHRoB/NR | DLRoB | PLRoB | DLRoB | PLRoB | … | |
| 4 | DLRoB | PHRoB/NR | DLRoB | PLRoB | DHRoB | PHRoB/NR | PLRoB | DLRoB | … | |
| 5 | DLRoB | DLRoB | PHRoB/NR | PLRoB | DLRoB | DLRoB | PHRoB/NR | PLRoB | … | |
| 6 | PHRoB/NR | DHRoB | DLRoB | DLRoB | PHRoB/NR | DLRoB | DLRoB | DLRoB | … | |
| 7 | PHRoB/NR | DLRoB | PHRoB/NR | PHRoB/NR | DLRoB | PLRoB | PHRoB/NR | DHRoB | … | |
| n | … | … | … | … | … | … | … | … | … | |
DHRoB: Definitively High Risk of Bias; DLRoB: Definitively Low Risk of Bias; PHRoB: Probably High Risk of Bias/Not Reported; PLRoB: Probably Low Risk of Bias.
A.3.6 Evidence synthesis and integration accounting for uncertainties
It is foreseen that the synthesis and integration will be carried out using a qualitative approach. EFSA will summarise the results of the studies narratively discussing uncertainties and their potential impact. Examples of sources of uncertainty that will be considered are those arising from the individual studies (risk of bias, imprecision) and at the level of the body of evidence (e.g. publication bias, unexplained inconsistency). Considerations will also be made on consistency across different types of evidence (e.g. observational and experimental studies).
For those studies underpinning sub‐questions and RH1a and RH1b, more structured considerations regarding their validity and, if possible, precision, will be made based on the outcomes of the approach to evidence appraisal.
Other uncertainties may arise from the overall methods applied for conducting the assessment (e.g. the way the conceptual model was built, the approach to searching, the methods for appraising the evidence, the lack of empirical evidence for bias in this research field).
Depending on the volume, quality and diversity of the evidence that will be retrieved, there is a possibility that no meaningful synthesis/integration will be possible both at the level of the individual sub‐questions as well as for the full conceptual model. In this case, the data will be considered as such, without any further elaboration.
A.3.7 Experts
EFSA will involve and liaise with some experts of the CompERA expert Working Group of the GMO Panel. Experts with expertise in agronomy, integrated pest management, the assessment of the persistence and invasiveness potential of plants, the ERA of GM plants, vertical gene flow, resistance evolution in target organisms and entomology will be selected.
To further support its work, EFSA will invite topic experts (i.e. hearing experts) with particular and relevant knowledge to contribute to one or more meetings of the CompERA expert Working Group by providing additional data, reports and publications and answering questions. In this respect, representatives of the ES/FR Competent Authorities will be consulted to identify potential ES/FR topic experts that could provide relevant support for the accomplishment of the project.
A.3.8 Reporting
Both the protocol and assessment will be reported in an EFSA Statement for publication in the EFSA Journal. The protocol will be annexed to the EFSA Statement. Potential deviations to the protocol will be reported directly in the relevant sections of the protocol.
A.4 References
EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 45 pp. https://doi.org/10.2903/j.efsa.2010.1637
EFSA (European Food Safety Authority), 2015. Scientific report on Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(6):4121, 45 pp. https://doi.org/10.2903/j.efsa.2015.4121
EFSA (European Food Safety Authority), 2016. Relevance of new scientific evidence on the occurrence of teosinte in maize fields in Spain and France for previous environmental risk assessment conclusions and risk management recommendations on the cultivation of maize events MON810, Bt11, 1507 and GA21. EFSA supporting publication 2016;13(1):EN‐1094, 55 pp. https://doi.org/10.2903/sp.efsa.2016.EN‐1094
EFSA (European Food Safety Authority), Devos Y, Guajardo IM, Álvarez F and Glanville J, 2019. Explanatory note on literature searching conducted in the context of GMO applications for (renewed) market authorisation and annual post‐market environmental monitoring reports on GMOs authorised in the EU market. EFSA supporting publication 2019;16(1):EN‐1614, 55 pp. https://doi.org/10.2903/sp.efsa.2019.EN‐1614
EFSA (European Food Safety Authority), Martino L, Aiassa E, Halldórsson TI, Koutsoumanis PK, Naegeli H, Baert K, Baldinelli F, Devos Y, Lodi F, Lostia A, Manini P, Merten C, Messens W, Rizzi V, Tarazona J, Titz A and Vos S, 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA supporting publication 2020;17(3):EN‐1843, 30 pp. https://doi.org/10.2903/sp.efsa.2020.EN‐1843
NTP (National Toxicology Program), 2015. OHAT risk of bias rating tool for human and animal studies. Office of Health Assessment and Translation (OHAT), 37 pp. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf
Appendix B – Supplementary tables
Table B.1.
List of relevant publications excluded based on full text screening
| Exclusion criteria | # | Publication references |
|---|---|---|
| Publication type | 1 | Agapito‐Tenfen SZ and Wickson F, 2018. Challenges for transgene detection in landraces and wild relatives: Learning from 15 years of debate over GM maize in Mexico. Biodiversity and Conservation, 27, 539–566. |
| 2 | EFSA (European Food Safety Authority), Álvarez F, Messéan A and Streissl F, 2021. Scientific Opinion on the assessment of the 2019 post‐market environmental monitoring report on the cultivation of genetically modified maize MON 810 in the EU. EFSA Journal, 19(7), 6683. | |
| 3 | Bauer‐Panskus A, Miyazaki J, Kawall K and Then C, 2020. Risk assessment of genetically engineered plants that can persist and propagate in the environment. Environmental Sciences Europe, 32, 32–32. | |
| 4 | Bhatta U and Smith SM, 2019. Phenotypic and genotypic characterization of resistance to Ustilago maydis from teosinte and maize‐teosinte introgression lines. Phytopathology, 109, 88. | |
| 5 | Devos Y, Ortiz‐García S, Hokanson KE and Raybould A, 2018. Teosinte and maize × teosinte hybrid plants in Europe−Environmental risk assessment and management implications for genetically modified maize. Agriculture, Ecosystems and Environment, 259, 19–27. | |
| 6 | EFSA (European Food Safety Authority), 2016. Relevance of new scientific evidence on the occurrence of teosinte in maize fields in Spain and France for previous environmental risk assessment conclusions and risk management recommendations on the cultivation of maize events MON810, Bt11, 1507 and GA21. EFSA Supporting Publications 2016;13(1):EN‐1094. | |
| 7 | Llenes JM, Cónsola S, Montull JM and Taberner A, 2020. Experience in the control of invasive weeds in catalonia from the point of view of its management. Informacion Tecnica Economica Agraria, 116, 256–275. | |
| 8 | Pardo Sanclemente G, Cirujeda Ranzenberger A, Marí León AI, Aibar Lete J, Fuertes Lázaro SJ and Taberner Palou A, 2016. El teosinte: Descripción, situación actual en el valle del Ebro y resultados de los primeros ensayos. Vida Rural, 408, 42–48. | |
| Population | 9 | De Lange ES, Farnier K, Gaudillat B and Turlings TCJ, 2016. Comparing the attraction of two parasitoids to herbivore‐induced volatiles of maize and its wild ancestors, the teosintes. Chemoecology, 26, 33–44. |
| 10 | Moya‐Raygoza G, 2020. Diversity and density‐dependence relationship between hymenopteran egg parasitoids and the corn leafhopper (Hemiptera: Cicadellidae) in maize agroecosystem vs. teosinte wild habitat. Florida Entomologist, 103, 48–53. | |
| 11 | Moya‐Raygoza G, 2021. Efficacy and emergence of parasitic wasps that attack herbivorous insects on maize and its relatives in their region of origin. Arthropod‐Plant Interactions, 15, 409–415. | |
| 12 | Moya‐Raygoza G and Triapitsyn SV, 2017. Egg parasitoids of Dalbulus maidis on wild teosintes in Mexico. Southwestern Entomologist, 42, 691–700. |
Table B.2.
Total area (ha) devoted to the cultivation of genetically modified (Bt) maize (including maize MON810) in the European Union since 1998 (adapted from EFSA (2021) and Álvarez‐Alfageme et al. (2022))14
| Growing season | CZ | DE | ES | FR | PL | PT | RO | SK | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1998 | 0 | 0 | 22,317 | 1,500 | 0 | 0 | 0 | 0 | 23,817 |
| 1999 | 0 | 0 | 24,952 | 0 | 0 | 180 | 0 | 0 | 25,132 |
| 2000 | 0 | 0 | 25,816 | 0 | 0 | 0 | 0 | 0 | 25,816 |
| 2001 | 0 | 0 | 11,550 | 0 | 0 | 0 | 0 | 0 | 11,550 |
| 2002 | 0 | 0 | 23,280 | 0 | 0 | 0 | 0 | 0 | 23,280 |
| 2003 | 0 | 0 | 32,249 | 0 | 0 | 0 | 0 | 0 | 32,249 |
| 2004 | 0 | 0 | 58,219 | 0 | 0 | 0 | 0 | 0 | 58,219 |
| 2005 | 270 | 270 | 53,226 | 500 | 0 | 780 | 0 | 0 | 55,046 |
| 2006 | 1,290 | 950 | 53,667 | 5,200 | 30 | 1,250 | 0 | 30 | 62,417 |
| 2007 | 5,000 | 2,685 | 75,148 | 22,135 | 327 | 4,263 | 350 | 900 | 110,808 |
| 2008 | 8,380 | 3,173 | 79,269 | 0 | 3,000 | 4,851 | 7,146 | 1,900 | 107,719 |
| 2009 | 6,480 | 0 | 76,057 | 0 | 3,000 | 5,094 | 3,344 | 875 | 94,850 |
| 2010 | 4,675 | 0 | 76,574 | 0 | 3,000 | 4,868 | 823 | 1,248 | 91,188 |
| 2011 | 5,090 | 0 | 97,346 | 0 | 3,000 | 7,723 | 588 | 760 | 114,507 |
| 2012 | 3,052 | 0 | 116,306 | 0 | 0 | 9,278 | 217 | 189 | 129,042 |
| 2013 | 2,560 | 0 | 136,962 | 0 | 0 | 8,202 | 835 | 100 | 148,659 |
| 2014 | 1,754 | 0 | 131,537 | 0 | 0 | 8,542 | 711 | 411 | 142,955 |
| 2015 | 997 | 0 | 107,749 | 0 | 0 | 8,017 | 2.5 | 104 | 116,870 |
| 2016 | 75 | 0 | 129,081 | 0 | 0 | 7,056 | 0 | 122 | 136,335 |
| 2017 | 0 | 0 | 124,227 | 0 | 0 | 7,308 | 0 | 0 | 131,535 |
| 2018 | 0 | 0 | 115,246 | 0 | 0 | 5,733 | 0 | 0 | 120,979 |
| 2019 | 0 | 0 | 107,127 | 0 | 0 | 4,718 | 0 | 0 | 111,845 |
| 2020 | 0 | 0 | 98,152 | 0 | 0 | 4,216 | 0 | 0 | 102,368 |
| 2021 | 0 | 0 | 96,606 | 0 | 0 | 4,228 | 0 | 0 | 100,834 |
CZ: Czech Republic; DE: Germany; ES: Spain; FR: France; PL: Poland; PT: Portugal; RO: Romania; SK: Slovakia.
Suggested citation: EFSA (European Food Safety Authority) , Devos Y, Aiassa E, Muñoz‐Guajardo I, Messéan A and Mullins E, 2022. Statement on the update of environmental risk assessment conclusions and risk management recommendations of EFSA (2016) on EU teosinte. EFSA Journal 2022;20(4):7228, 40 pp. 10.2903/j.efsa.2022.7228
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00557
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank the following for the support provided to this scientific output: Ana Afonso, Fernando Álvarez‐Alfageme, Alicia Cirujeda, Giuseppe Condorelli, Tewodros Firdissa Duressa, Leslie Firbank, Graham Head, Andrea Gennaro, Paschalina Grammatikou, Valérie Le Corre, Yustina Olshevska‐Grigorov, Anastasia Pagida, Pilar Eugenia Ramon Pons, Duska Stojsin, Eve Veromann and Fabio Veronesi. EFSA wishes to acknowledge the Competent Authorities of France and Spain for providing data for this scientific output.
Reproduction of the data presented in Tables 2, 3 and B.2 is prohibited and permission must be sought directly from the copyright holders, as EFSA is not the copyright holder of those data.
Adopted: 17 March 2022
Notes
In line with the Commission Directive (EU) 2018/350, the ERA of the use of a plant protection product, including its use on a GM plant, falls within the scope of Regulation (EC) No 1107/2009 and is carried out at a Member State level to account for specific agricultural conditions. Therefore, risk concerns associated with the use of glyphosate‐based herbicides on maize GA21 are not addressed here.
https://www.mapa.gob.es/es/agricultura/temas/biotecnologia/omg/registro-publico-omg/superficie_cultivada.aspx and https://www.dgav.pt/wp-content/uploads/2022/03/DADOS-NACIONAIS-2021-atualizado.pdf
Stable incorporation of genetic material in a population, generally through the repeated backcrossing of an interspecific hybrid with one of its parent species.
https://www.miteco.gob.es/es/calidad‐y‐evaluacion‐ambiental/temas/biotecnologia/organismos‐modificados‐geneticamente‐omg‐/consejo‐interministerial‐de‐ogms/superficie.aspx and https://www.mapa.gob.es/es/estadistica/temas/estadisticas‐agrarias/agricultura/avances‐superficies‐producciones‐agricolas/
Rationale: Transgenes of maize MON810, Bt11, 1507 and GA21 do not alter the hybridisation potential of maize, hence both GM and conventional maize will be considered.
Rationale: Maize progeny (including that derived from cross‐fertilisation by teosinte) is unlikely to serve as stepping stone for vertical gene flow. Grains on maize do not easily shatter and will be harvested with the maize crop so largely removed from the field. The potential of occasional maize volunteer plants to cross‐fertilise other maize plants would be extremely limited. Therefore, relevant experimental studies should use maize as pollen donor. Experimental studies that use maize as pollen recipient will not be considered further.
Rationale: The focus is on NTOs that are susceptible to the transgene product of GM maize. The insecticidal proteins Cry1Ab and Cry1F expressed in maize MON810, Bt11 and 1507 are lepidopteran active.
Rationale: The main TOs of maize MON810, Bt11 and 1507 are the European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae), and the Mediterranean corn borer, Sesamia nonagrioides (Lepidoptera: Noctuidae) in the EU. Maize GA21 has no TOs.
Rationale: Plant parts consumed by TOs.
References
- Álvarez‐Alfageme F, Devos Y, Camargo AM, Arpaia S and Messéan A, 2022. Managing resistance evolution to transgenic Bt maize in corn borers in Spain. Critical Reviews in Biotechnology, 42, 201–219. [DOI] [PubMed] [Google Scholar]
- Avendaño López AN, de Jesús Sánchez González J, Ruíz Corral JA, De La Cruz Larios L, Santacruz‐Ruvalcaba F, Sánchez Hernández CV and Holland JB, 2011. Seed dormancy in Mexican teosinte. Crop Science, 51, 2056–2066. [Google Scholar]
- Balbuena MA, Rosales E, Valencia JC, González A, Pérez D, Sánchez S, Franco ALY and Vences CC, 2011. Competencia entre maíz y teocintle: efecto en el rendimiento y sus componentes. Centro Agrícola, 38, 5–12. [Google Scholar]
- Bauer‐Panskus A, Miyazaki J, Kawall K and Then C, 2020. Risk assessment of genetically engineered plants that can persist and propagate in the environment. Environmental Sciences Europe, 32, 32. [Google Scholar]
- Bellota E, Dávila‐Flores A and Bernal JS, 2018. A bird in the hand versus two in the bush? The specialist leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) does not discriminate against sub‐optimal host plants (Zea spp.). Neotropical Entomology, 47, 171–180. [DOI] [PubMed] [Google Scholar]
- Calfee E, Gates D, Lorant A, Perkins MT, Coop G and Ross‐Ibarra J, 2021. Selective sorting of ancestral introgression in maize and teosinte along an elevational cline. PLoS Genetics, 17, e1009810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo AM, Andow DA, Castanera P and Farinos GP, 2018. First detection of a Sesamia nonagrioides resistance allele to Bt maize in Europe. Scientific Reports, 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez NB, Flores JJ, Martin J, Ellstrand NC, Guadagnuolo R, Heredia S and Welles SR, 2012. Maize × teosinte hybrid cobs do not prevent crop gene introgression. Economic Botany, 66, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirujeda A, Pardo G, Marí AI, Joy M and Casasús I, 2019. Emergence and viability of teosinte seeds (Zea mays ssp. mexicana ad int.) subjected to sheep digestion. Weed Research, 59, 145–154. [Google Scholar]
- Devos Y, Ortiz‐Garcia S, Hokanson KE and Raybould A, 2018. Teosinte and maize × teosinte hybrid plants in Europe — Environmental risk assessment and management implications for genetically modified maize. Agriculture, Ecosystems & Environment, 259, 19–27. [Google Scholar]
- Díaz A, Taberner A and Vilaplana L, 2020. The emergence of a new weed in maize plantations: characterization and genetic structure using microsatellite markers. Genetic Resources and Crop Evolution, 67, 225–239. [Google Scholar]
- EFSA (European Food Safety Authority) , 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal, 2010;8(6):1637, 45 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. Scientific report on Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(6):4121, 36 pp. 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2016. Relevance of new scientific evidence on the occurrence of teosinte in maize fields in Spain and France for previous environmental risk assessment conclusions and risk management recommendations on the cultivation of maize events MON810, Bt11, 1507 and GA21. EFSA Supporting Publication 2016;13(9):1094, 45 pp. 10.2903/sp.efsa.2016.EN-1094 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Álvarez F, Messéan A and Streissl F, 2021. Scientific Opinion on the assessment of the 2019 post‐market environmental monitoring report on the cultivation of genetically modified maize MON 810 in the EU. EFSA Journal, 2021;19(7):6683, 55 pp. 10.2903/j.efsa.2021.6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Devos Y, Guajardo IM, Álvarez F and Glanville J, 2019. Explanatory note on literature searching conducted in the context of GMO applications for (renewed) market authorisation and annual post‐market environmental monitoring reports on GMOs authorised in the EU market. EFSA Supporting Publication, 2019;16(4):1614, 62 pp. 10.2903/sp.efsa.2019.EN-1614 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Martino L, Aiassa E, Halldórsson TI, Koutsoumanis PK, Naegeli H, Baert K, Baldinelli F, Devos Y, Lodi F, Lostia A, Manini P, Merten C, Messens W, Rizzi V, Tarazona J, Titz A and Vos S, 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA Supporting Publication 2020;EN‐1843, 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI] [Google Scholar]
- Gaillard MDP, Glauser G, Robert CAM and Turlings TCJ, 2018. Fine‐tuning the ‘plant domestication‐reduced defense’ hypothesis: specialist vs generalist herbivores. New Phytologist, 217, 355–366. [DOI] [PubMed] [Google Scholar]
- Le Corre V, Siol M, Vigouroux Y, Tenaillon MI and Délye C, 2020. Adaptive introgression from maize has facilitated the establishment of teosinte as a noxious weed in Europe. Proceedings of the National Academy of Sciences, 117, 25618–25627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohn AF, Trtikova M, Chapela I, Binimelis R and Hilbeck A, 2021. Transgene behavior in genetically modified teosinte hybrid plants: transcriptome expression, insecticidal protein production and bioactivity against a target insect pest. Environmental Sciences Europe, 33, 67. [Google Scholar]
- Lu Y, Hokin SA, Kermicle JL, Hartwig T and Evans MMS, 2019. A pistil‐expressed pectin methylesterase confers cross‐incompatibility between strains of Zea mays . Nature Communications, 10, 2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montull JM, Pardo G, Aibar J, Llenes JM, Marí AI, Taberner A and Cirujeda A, 2020. Aspects of the dispersion and viability of the teosinte seeds (Zea mays ssp.) in the Ebro valley. Informacion Tecnica Economica Agraria, 116, 227–240. [Google Scholar]
- Moya‐Raygoza G, Cuevas‐Guzmán R, Pinedo‐Escatel JA and Morales‐Arias JG, 2019. Comparison of leafhopper (Hemiptera: Cicadellidae) diversity in maize and its wild ancestor teosinte, and plant diversity in the teosinte habitat. Annals of the Entomological Society of America, 112, 99–106. [Google Scholar]
- Naranjo‐Guevara N, Peñaflor MFGV, Silva DB and Bento JMS, 2021. A comparison of the direct and indirect defence abilities of cultivated maize versus perennial and annual teosintes. Chemoecology, 31, 63–74. [Google Scholar]
- Pardo G, Cirujeda A, Marí AI, Aibar J, Fuertes S and Taberner A, 2016. El teosinte: descripción, situación actual en el valle del Ebro y resultados de los primeros ensayos. Vida Rural, 408, 42–48. [Google Scholar]
- Silva NCdA, Vidal R, Costa FM, Vaio M and Ogliari JB, 2015. Presence of Zea luxurians (Durieu and Ascherson) bird in southern Brazil: Implications for the conservation of wild relatives of maize. PLoS One, 10, e0139034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testbiotech , 2016. Genetically Engineered Maize can Give Rise to Superweeds. Available online: https://www.testbiotech.org/en/press‐superweed‐genetically‐engineered‐maize [Google Scholar]
- Testbiotech , 2016. Genetically Engineered Plants: Reinforce Precaution! Available online: https://www.testbiotech.org/en/node/1694 [Google Scholar]
- Trtikova M, Lohn A, Binimelis R, Chapela I, Oehen B, Zemp N, Widmer A and Hilbeck A, 2017. Teosinte in Europe ‐ searching for the origin of a novel weed. Scientific Reports, 7, 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]


