Abstract
Primary CNS germ cell tumors (GCTs) are rare neoplasms predominantly observed in the pediatric and young adult populations. In line with the hypothesis that the primordial germ cell is the cell-of-origin, histopathological examinations for this pathology involve a diverse range of components mirroring the embryogenic developmental dimensions. Chemotherapy and radiotherapy are the mainstays of treatment, with surgery having a limited role for diagnosis and debulking of residual tissue after treatment. While better management has been achieved over recent decades by modifying radiation coverage and selecting appropriate chemotherapy, standardization of treatment remains challenging, partly due to the low volume of cases encountered in each institution. As the incidence is higher in East Asia, including Japan, the Japan Society for Neuro-Oncology established a multidisciplinary task force to create an evidence-based guideline for CNS GCTs. This guideline provides recommendations for multiple dimensions of clinical management for CNS GCTs, with particular focus on diagnostic measures including serum markers, treatment algorithms including surgery, radiotherapy, and chemotherapy, and under-investigated but important areas such as treatment for recurrent cases, long-term follow-up protocols, and long-term sequelae. This guideline serves the purpose of helping healthcare professionals keep up to date with current knowledge and standards of management for patients with this rare disease in daily clinical practice, as well as driving future translational and clinical research by recognizing unmet needs concerning this tumor.
Keywords: CNS germ cell tumor, diagnosis, guideline, prognosis, treatment
Primary CNS germ cell tumors (GCTs) are rare neoplasms mostly affecting pediatric, adolescent, and young adult populations. Five histological classifications are recognized by the WHO: germinoma; teratoma (mature or immature); yolk sac tumor; choriocarcinoma; and embryonal carcinoma.1 Tumors involving more than one histological subtype exist as mixed GCTs. Germinoma is the predominant form, accounting for 60–70% of cases, followed by mixed GCT, while each of the non-germinomatous GCTs (NGGCTs) other than teratoma is extremely rare.2–4 The midline structures, namely the neurohypophysis (hypothalamus/posterior pituitary) and pineal gland are preferential sites of occurrence. Simultaneous lesions at both sites are called “bifocal” tumors, mostly in the form of germinoma when tumor markers are negative, although exceptions are recognized.5,6 Presenting symptoms depend greatly on the site of occurrence; pineal GCTs are often accompanied by intracranial hypertension due to obstructive hydrocephalus at the cerebral aqueduct and upward gaze palsy caused by compression or infiltration of the quadrigeminal body in the midbrain.7,8 Neurohypophyseal GCTs cause symptoms such as visual field defects and hormonal disorders including diabetes insipidus in most cases.9–11 Paralysis, sensory deficits, and psychiatric compromises are observed with GCTs at the basal ganglia or thalamus.12 A locoregional sex predominance is evident, with most pineal GCTs occurring in male patients, and neurohypophyseal GCTs showing either an even distribution or slight female predominance.7,9
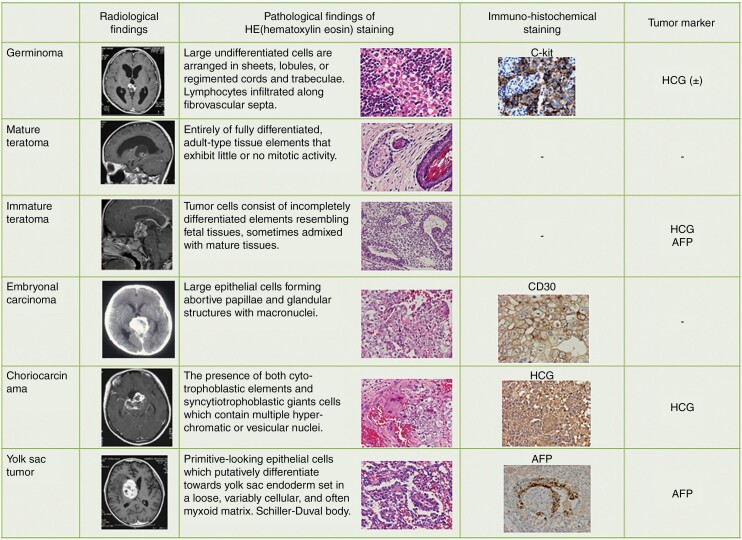
Imaging, serum/cerebrospinal fluid (CSF) tumor markers (α-fetoprotein (AFP), human chorionic gonadotropin (HCG), and the β-subunit of HCG (β-HCG)) and histopathological characteristics of any surgical specimens are employed for diagnosis. Each of these diagnostic methods is crucial, so standardization of diagnostic algorithms is of pivotal importance to forestall misdiagnosis leading to under- or over-treatment (Figure 1).
Fig. 1.
Imaging, histopathological findings, immunohistochemical findings, and tumor markers summarized according to the WHO histological classification.
Distinct geographical differences are seen in the incidence of GCT, with 3–8 times higher incidences in East Asia than in Western countries.9 According to the brain tumor registry in Japan, GCT makes up 2.7% of primary brain tumors, and 15.3% of pediatric brain tumors.13 In contrast, this entity represents only 1–3% of pediatric CNS tumors in Western countries.14
From a biological perspective, the cell-of-origin for GCT is hypothesized to be the primordial germ cell (PGC), surviving as ectopic embryonic cells following mis-migration into CNS structures, and undergoing tumorigenesis.15 This tumorigenesis is explained by genetic abnormalities including mutations in the MAPK and PI3K pathways and chromosomal instability represented by 12p gain.16–19 Global DNA hypomethylation in germinoma is considered to reflect the same situation in PGCs.20 Germinoma is characterized by expression of genes related to stem cells and pluripotency, whereas NGGCTs display features associated with expression of neural development, the WNT/beta-catenin pathway, invasion, and epithelial-mesenchymal transition.21,22 Characteristic miRNA expressions have been recognized, including of the miR-371–373 and miR-302/367 clusters, and have potential clinical utility.23
The 5-year overall survival (OS) rate varies according to the histological type, but is approximately 90–95% for germinoma,4,24–26 so health-related quality-of-life (HR-QOL) becomes an important issue for survivors who have undergone chemo- and radiotherapy (CRT). The prognosis of NGGCT has improved over decades, with 5-year progression-free survival (PFS) improving to >70%.4,27–29 Clinical trials are ongoing in North America through the Children’s Oncology Group (COG), in Europe through the International Society of Pediatric Oncology (SIOP), in Korea through the Korean Society for Pediatric Neuro-Oncology (KSPNO) and in Japan through the Japan Children's Cancer Group, for each of the major histological categorizations (mostly germinoma and NGGCTs). The goal of these trials is to achieve better tumor control with fewer long-term treatment-related sequelae. Notably, the classification into two broad treatment groups of germinoma and NGGCT is now employed in Western countries, whereas Japan has traditionally leveraged a three-class histopathology-based classification system to better reflect differences in tumorigenic potential.3
International collaborations to set standards of clinical management have proven fruitful, with five international GCT symposia held worldwide and consensus processes undertaken, resulting in the recognition of similar clinical practices.30 Concurrently, many fields continue to show variance in clinical practice, partly due to the rarity of clinical encounters and the absence of documented standards.
The Japan Society for Neuro-Oncology (JSNO) launched a task force to develop a guideline aimed at clinical practitioners caring for patients with CNS GCTs. Defining the standard of care and answering outstanding clinical questions are the purposes of this review. As many uncertain areas remain in the management of CNS GCTs due to the scarcity of clinical trials, this guideline was created to provide the best in up-to-date evidence and knowledge that experts can share to define the current standards for disease management.
Methods
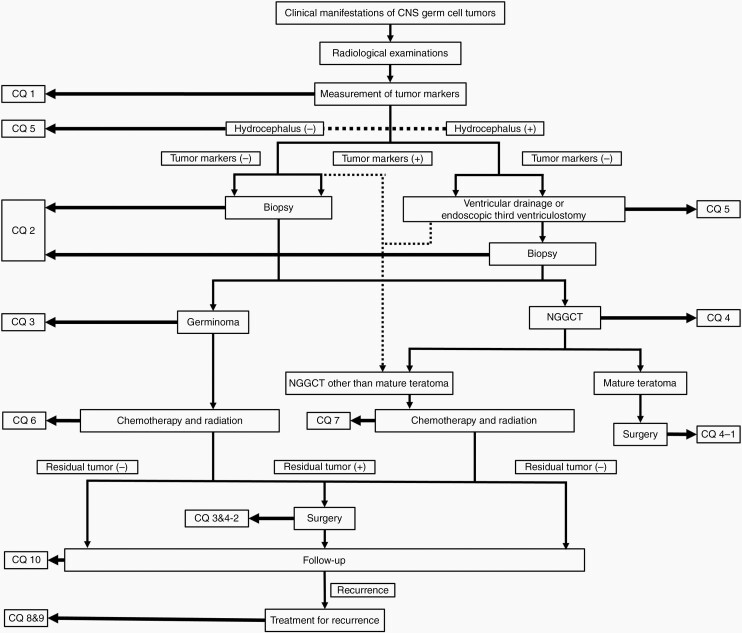
A multidisciplinary task force was launched by the JSNO, with contributions from medical experts in multidisciplinary fields including neurosurgery, pediatric oncology, radiation oncology, and neuropathology. This guideline is aimed at aiding healthcare professionals involved in the management of patients with primary CNS GCTs as a source of knowledge on current standards of care. We assessed the available English/Japanese literature up to December 31, 2018, extracted mainly from the MEDLINE database (http://www.ncbi.nlm.nih.gov), but including the Cochrane Library and Web of Science. Sensitive and specific keywords, as well as various combinations of keywords, were used to search the database, including “germ cell tumor,” “germinoma,” “non-germinomatous,” “intracranial,” “central nervous system,” “radiotherapy,” “chemotherapy,” and “surgery.” The Medical Information Network Distribution Service (Minds) guideline was referred to and utilized in creating this guideline (https://minds.jcqhc.or.jp/docs/minds/guideline/pdf/MindsHB2014.pdf). We chose 6 topics and 10 clinical questions (CQs), as shown in Table 1. An algorithm map was created to facilitate navigation of these CQs (Figure 2). Recommendations are outlined along with strength of evidence (Grades A–D) and level of recommendation (1: strong or 2: weak [suggestion]). Finally, the guideline was reviewed externally by a wide range of qualified individuals, including medical professionals from other fields, legal experts, and patient representatives, with revisions to the guideline made according to the suggestions provided. This article also encompasses recent information published after the above systematic review, as needed; such modifications are highlighted as * in the reference list. Nevertheless, recommendations for each clinical question are maintained as per the original statement.
Table 1.
Subjects, Clinical Questions, Recommendations, and Level of Recommendations
| Clinical Questions and Recommendations | Recommendation Level | |
|---|---|---|
| Subject 1 | Diagnosis and Classification | |
| CQ1 | Is measurement of tumor markers (AFP and HCG) useful in the management of CNS GCTs? | |
| Recommendation 1 | When primary CNS GCT is suspected, measurement of concentrations of tumor markers (AFP and HCG) in the blood and cerebrospinal fluid is recommended, unless medically contraindicated | 1A |
| CQ2 | Is histopathological diagnosis necessary in CNS GCTs? | |
| Recommendation 2 | When GCT is suspected, histopathological diagnosis is recommended to achieve definitive diagnosis | 2C |
| Subject 2 | Role of Surgery | |
| CQ3 | Is aggressive resection of germinoma necessary? | |
| Recommendation 3 | We strongly recommend against aggressive resection of germinoma | 1B |
| CQ4 | Is surgical resection of NGGCT necessary? | |
| Recommendation 4-1 | For mature teratomas, resection is strongly recommended | 1B |
| Recommendation 4-2 | For NGGCT other than mature teratoma, resection of residual tumor following chemo- (and radiotherapy) is strongly recommended | 1C |
| CQ5 | Is surgical intervention necessary for CNS GCT-associated hydrocephalus? | |
| Recommendation 5 | Surgical intervention, such as endoscopic third ventriculostomy, is strongly recommended to treat hydrocephalus | 1B |
| Subject 3 | Treatment for Germinoma | |
| CQ6 | Are chemo- and radiotherapy necessary for germinoma? | |
| Recommendation 6-1 | Radiation therapy covering the whole ventricle system together with chemotherapy is strongly recommended for germinoma without spinal dissemination | 1B |
| Recommendation 6-2 | Prophylactic spinal radiotherapy is not recommended for germinoma without spinal dissemination | 1C |
| Recommendation 6-3 | Treating germinoma with chemotherapy alone is strongly recommended against | 1B |
| Subject 4 | Treatment for NGGCT | |
| CQ7 | Are chemo- and radiotherapy effective for NGGCTs? | |
| Recommendation 7 | Chemo- and radiotherapy are strongly recommended for NGGCT excluding mature teratoma | 1B |
| Subject 5 | Treatment Strategy for Recurrence | |
| CQ8 | Is salvage treatment necessary for recurrent germinoma cases? | |
| Recommendation 8 | Treatment aimed at disease cure is strongly recommended | 1B |
| CQ9 | Is salvage treatment effective for recurrent NGGCTs? | |
| Recommendation 9 | Remission-oriented treatment is recommended, but palliative treatment is also recommended when treatment response is unfavorable | 2C |
| Subject 6 | Long-term Prognosis | |
| CQ10 | Is follow-up necessary for CNS GCT cases? | |
| Recommendation 10 | Follow-up for as long as possible is strongly recommended | 1B |
Abbreviations: AFP, α-fetoprotein; GCT, germ cell tumor; HCG, human chorionic gonadotropin; NGGCT, non-germinomatous germ cell tumor.
Fig. 2.
Flow chart of the management algorithm for CNS GCTs, and position of each clinical question in the chart.
Subject 1. Diagnosis and Classification
Significance of Tumor Markers (AFP and HCG)
CQ1: Is measurement of tumor markers (AFP and HCG) useful in the management of CNS GCTs?
When primary CNS GCT is suspected, measurement of concentrations of tumor markers (AFP and HCG) in the blood and CSF is recommended, unless medically contraindicated (Grade 1A)
For the diagnosis of primary CNS GCTs, measurement of tumor markers is the first evaluation conducted when typical imaging and clinical findings are present. Mild-to-moderate elevations in HCG can be seen in germinoma, while highly elevated HCG is a sign of choriocarcinoma. Elevated AFP is particularly seen in yolk sac tumors, while immature teratomas may show elevated HCG and AFP.31Table 2 shows the criteria for using tumor markers to differentiate between germinoma and NGGCT in clinical trials around the globe. Of note, no established values of HCG and AFP have been able to allow omission of histological diagnosis when identifying NGGCTs with malignant histological components. Furthermore, HCG elevation can also be observed in Langerhans cell histiocytosis, so caution is required, especially in cases showing mild elevation.32 As criteria for NGGCTs in clinical trials by the COG and SIOP have encompassed HCG-secreting germinomas, which seem to show clinical behaviors distinct from those of NGGCTs in general, caution is needed when comparing their results with those from the Japanese group using histopathological diagnosis as the principle in the treatment algorithm.
Table 2.
Diagnostic criteria of germinoma and NGGCT based on tumor markers in Japan, North America, Europe, Korea, and Taiwan
| SIOP-CNS-GCT-96 (Calminus et al.)28 |
COG ACNS1123 (Fangusaro et al.)29 |
Japan (Matsutani et al.)3 |
KSPNO-S053 (Baek et al.)33 |
Taiwan (Wong et al.)34 |
|
|---|---|---|---|---|---|
| Germinoma | Pathological confirmation of the component of germinoma and AFP ≦ 25 ng/ml and HCG ≦ 50 IU/L | Pathological confirmation of the component of germinoma and AFP ≦ 10 ng/ml, and β-HCG ≦ 100 IU/L |
Good prognosis group
Pathological confirmation of the component of only germinoma or germinoma with syncytiotrophoblastic giant cell |
Pathological confirmation of the component of germinoma and AFP ≦ 25 ng/ml, And β-HCG ≦ 50 mIU/ml | Pathological confirmation of the component of germinoma And AFP ≦ 5 ng/dl and β-HCG ≦ 100 mIU/ml |
| NGGCT | Pathological confirmation of the component of choriocarcinoma, yolk sac tumor, embryonal carcinoma or AFP > 25 ng/ml, or HCG > 50 IU/L | Pathological confirmation of the component of choriocarcinoma, yolk sac tumor, embryonal carcinoma or AFP > 10 ng/ml, or β-HCG > 100 IU/L |
Intermediate prognosis group
a) immature teratoma b) teratoma with malignant transformation c) germinoma + teratoma which the main component of tumor is germinoma or teratoma with a small component of malignant tumor in mixed tumor Poor prognosis group a) Choriocarcinoma b) Yolk sac tumor c) Embryonal carcinoma d) Mixed tumor in which main component are a) or b) or c) e) AFP ≧ 2000 ng/ml, or HCG ≧ 2000 mIU/ml |
Pathological confirmation of the component of choriocarcinoma, yolk sac tumor, embryonal carcinoma or AFP > 25 ng/ml, or β-HCG > 50 mIU/ml | Pathological confirmation of the component of choriocarcinoma, yolk sac tumor, embryonal carcinoma or AFP > 5 ng/dl, or β-HCG > 100 mIU/ml |
Abbreviations: AFP, α-fetoprotein; COG, Children's Oncology Group; HCG, human chorionic gonadotropin; KSPNO, Korean Society of Pediatric Neuro-Oncology; SIOP, International Society of Paediatric Oncology.
Tumor markers are also useful to differentiate between tumor progression and “growing teratoma syndrome” during CRT, as the latter is characterized by normalization of tumor markers despite tumor growth.34,35 In addition, monitoring of tumor markers plays a role in evaluating treatment response,36 and provides a highly sensitive method of detecting relapse.37
Necessity of Histopathological Diagnosis
CQ2: Is histopathological diagnosis necessary in CNS GCTs?
When GCT is suspected, histopathological diagnosis is recommended to achieve definitive diagnosis (Grade 2C)
While imaging and tumor markers are indispensable for diagnosis, these noninvasive measures show diagnostic limitations, as partly discussed in the previous section. For example, 1 of 85 GCT cases diagnosed on T1-weighted imaging with/without contrast and the apparent diffusion coefficient level of MRI was found on histopathological examination to be non-GCT.38 Regarding bifocal tumors, 3 of 10 cases were histopathologically NGGCTs with normal β-HCG and normal or mildly elevated AFP.5 Furthermore, 3 of 89 bifocal tumor cases with diabetes insipidus and negative tumor markers (3.4%) were histopathologically NGGCTs.6 Those reports advocate proactive histopathological diagnosis for bifocal tumors.6 In the SIOP-CNS-GCT-96 trial, 10 of 149 cases (6.7%) diagnosed as NGGCT (and not mature teratoma) did not meet the criteria of either HCG > 50 IU/L or AFP > 25 ng/ml. All 5 histopathologically confirmed teratomas showed elevated AFP.28 These findings reiterate that marker-negative NGGCT, marker-positive germinoma, and non-GCT can be encountered, and diagnoses based solely on tumor markers, imaging, or clinical findings display limitations. The potential for over-treatment of patients with germinoma and elevated HCG levels is a concern, as is under-treatment in cases of immature teratoma, embryonal carcinoma, or mixed tumors including these components when tumor markers are negative (although most such cases would undergo histopathological evaluation). That said, controversy remains regarding the necessity of surgical biopsy when NGGCT is strongly suspected. Notably, surgical exploration can place the patient at risk of tumor dissemination, as well as surgery-related complications such as hemorrhage and infection, and inconclusive histopathological results. Considering these issues, the guideline basically recommends conducting histopathological diagnosis, but concurrent CRT may be considered without histopathological diagnosis when markedly high tumor markers (see CQ1, Table 2, HCG ≧ 2000 mIU/ml or AFP ≧ 2000 ng/ml) are observed and both the clinical course and imaging findings are typical.
Subject 2. Role of Surgery
Germinoma
CQ3: Is aggressive resection of germinoma necessary?
We strongly recommend against aggressive resection of germinoma (Grade 1B)
As CRT is effective against germinoma, the aim of surgical intervention is to obtain a tumor specimen allowing histopathological diagnosis. The prognosis of germinoma is known to be noninferior when resection is confined to biopsy rather than debulking. Considering that attempts at aggressive resection carry certain procedural risks, surgical resection is not recommended. Biopsy procedures include craniotomy, transsphenoidal, stereotactic, and endoscopic surgery, selected depending on tumor location.
As biopsy specimens are usually tiny, the specimen may not adequately reflect the entire tumor, potentially leading to under-diagnosis. One in 6 cases diagnosed with germinoma reportedly showed elevated β-HCG during follow-up after CRT, suggesting the existence of NGGCT at presentation.39 Another report found that 1 in 16 germinoma cases diagnosed from endoscopically obtained specimens showed elevated AFP as well as non-complete response (non-CR) following CRT, resulting in surgical resection of residual tumors subsequently diagnosed as immature teratoma. Such reports remind us of the potential risk of under-treatment in biopsied cases due to underestimation of the entire specimen.40 While the guideline holds that biopsy, not aggressive resection, is recommended in cases that germinoma is suspected, collection and integration of clinical presentation, tumor markers, and histopathology are thus important to minimize the risk of dismissing NGGCT components, and the more malignant findings point to the appropriate diagnosis and treatment when discrepancies are found.
NGGCT
CQ4: Is surgical resection of NGGCT necessary?
1. For mature teratomas, resection is strongly recommended (Grade 1B)
2. For NGGCT other than mature teratoma, resection of residual tumor following chemo- (and radiotherapy) is strongly recommended (Grade 1C)
Several reports have described long recurrence-free survival for patients with mature teratoma after surgical resection alone without adjuvant treatment.3,4,41,42 Reflecting such findings, the Delphi consensus identified total resection as the decisive treatment for mature teratoma.30
As described previously in CQ1, CRT may be considered without histopathological diagnosis when tumor markers are extremely elevated, but otherwise histopathological diagnosis is recommended initially to guide the appropriate treatment. In the COG ACNS0122 study, radiotherapy was conducted if CR was achieved after upfront chemotherapy. If not CR, second-look surgery was performed followed by radiotherapy or high-dose chemotherapy (HDC). Tissues after chemotherapy are usually teratomas or other nonneoplastic tissues.43–45 Tumor growth during CRT with normalization of tumor marker levels is called “growing teratoma syndrome,” and necessitates histopathological confirmation and resection.34,35 As many as 21% of NGGCT cases show growing teratoma syndrome.34 Considering that a certain proportion of NGGCT cases are clinically diagnosed without histopathological confirmation before undergoing chemotherapy, and that growing teratoma syndrome can be diagnosed based on histopathological evaluation, resection of residual tumor following CRT is of significance in deciphering the following management scheme. Furthermore, residual tumors have shown higher relapse rates, corroborating the significance of surgical intervention for residual tumor.28 Of note, residual tumor for germinoma does not have an unfavorable indication.26 Usually any enhancing lesion can be recognized as residual tumor, although size cutoffs in decision-making regarding intervention remain unexplored. Traditionally, surgical resection of residual tumor is performed after CRT in Japan, but after chemotherapy alone in clinical trials by the COG and SIOP. The optimal timepoint for second-look surgery should be discussed in the future.
Surgery for Hydrocephalus
CQ5: Is surgical intervention necessary for CNS GCT-associated hydrocephalus?
Surgical intervention, such as endoscopic third ventriculostomy, is strongly recommended to treat hydrocephalus (Grade 1B)
Endoscopic third ventriculostomy (ETV) and concurrent tumor biopsy at the suprasellar and pineal regions are regarded as safe and effective measures to address obstructive hydrocephalus and simultaneously reach a histopathological diagnosis. Surgical complications are usually minimal,41 obviating the need for ventriculo-peritoneal shunt (VPS) or external ventricular drainage (EVD).7 ETV was reported to not increase the risk of cerebrospinal dissemination associated with the procedure, although this was based on a relatively small number of publications.46,47 In cases where ETV is unfeasible for anatomical reasons such as a large suprasellar lesion or aberrant position of the basilar artery, EVD can provide an alternative means of addressing hydrocephalus. Of note, tumor shrinkage can be expected in response to CRT, leading to resolution of hydrocephalus. Regarding the potential risk of extra-CNS dissemination caused by VPS, systematic appraisal of extraneural metastases associated with shunting for primary CNS tumors revealed that 25 of 106 shunt-related extra-CNS metastases were germinoma, all within the abdominal cavity.47 ETV is thus recommended to treat hydrocephalus associated with CNS GCT.
Subject 3. Treatment for Germinoma
Roles of Chemo- and Radiotherapy for Germinoma
CQ6: Are chemo- and radiotherapy necessary for germinoma?
1. Radiation therapy covering the whole ventricular system together with chemotherapy is strongly recommended for germinoma without spinal dissemination (Grade 1B)
2. Prophylactic spinal radiotherapy is not recommended for germinoma without spinal dissemination (Grade 1C)
3. Treating germinoma with chemotherapy alone is strongly recommended against (Grade 1B)
Germinoma is known to induce tumor progression along the ventricle wall, and local radiotherapy alone is unable to sufficiently prevent recurrence outside the radiotherapy field.9,26,48–50 In the SFOP TGM-TC-90 study, local radiotherapy with 40-Gy was applied to the tumor area plus a 2-cm margin after chemotherapy with carboplatin, etoposide, and ifosfamide for nondisseminated cases, and recurrence was observed in 10 of 60 cases, 8 of which were around the ventricles. Local radiotherapy alone was concluded to carry a high risk of recurrence, and whole-ventricular irradiation (WVI) was thus necessary.51 Similarly, the SIOP CNS GCT-96 study compared craniospinal irradiation (CSI) of 24-Gy plus local radiotherapy of 16-Gy vs local radiotherapy of 40-Gy plus chemotherapy for nondisseminated germinomas. Local tumor recurrence was observed in 4 of 125 patients in the former, while recurrence was observed in 7 of 65 patients in the latter group, of whom 6 patients developed intraventricular recurrences outside the radiotherapy field. WVI was again recognized as necessary when combined with chemotherapy.26
Regarding the dose of radiation, the SIOP CNS GCTII clinical trial and the completed Japanese clinical trial both used 24-Gy WVI as standard, as this is considered the maximum tolerable dose for the hypothalamic-pituitary system.52 In contrast, the current COG clinical trial ACNS1123 is using 18-Gy WVI with 12-Gy local radiotherapy for cases with CR after chemotherapy.29 Those that did not achieve CR underwent 24-Gy WVI plus 12-Gy local radiotherapy.
CSI has been shown to be effective, and the 5-year disease-free survival (DFS) rate of germinoma exceeds 90%.40,41,53,54 However, since very few reports have described spinal cord recurrence without prophylactic spinal radiotherapy, progression of germinoma into the spinal cord is considered almost absent. In addition, based on a retrospective study of 180 germinoma cases, spinal radiotherapy proved to be of no additional benefit in survival over local or whole-brain irradiation (WBI).55 Furthermore, WBI and CSI have tremendous repercussions in terms of growth and development in children and young adults who are expected to show long-term survival. Problems such as cognitive dysfunction and endocrine deficiency can be lifelong burdens for survivors.56–59 WBI and CSI should thus be avoided in patients with germinoma at typical sites (neurohypophysis, pineal gland, and ventricles), who are expected to experience long-term survival. Regarding the treatment of germinomas in the basal ganglia and thalamus, regimens may be differentiated. As germinomas in the basal ganglia and thalamus are likely to infiltrate the brain parenchyma, WBI (20–24-Gy) is preferable over WVI in addition to local radiotherapy up to 40–45-Gy.60
Regarding chemotherapy for germinoma, clinical trials usually employ platinum-based combination therapies with etoposide and/or additional alkylating drugs such as ifosfamide. On the grounds of the paucity of evidence regarding the superiority of either cisplatin or carboplatin and of either platinum-based agent plus etoposide or these two agents plus alkylating agent so far, considering therapeutic toxicity, we recommend combination therapy with a platinum-based agent and etoposide for germinoma. In a clinical trial by the Japanese group, 3 courses of carboplatin and etoposide (CARE), concomitant with 24-Gy of extended local radiotherapy were used for 75 germinoma cases without spinal dissemination.52 CR was achieved in 92%, and recurrence was observed in 12% during a median observation period of 2.9 years. The 10-year OS rate was 97.5%. This “extended local radiotherapy” is the same as WVI, except the lower half of the fourth ventricle is not covered. Likewise, a retrospective study of 20 germinoma cases that underwent chemotherapy with carboplatin and etoposide and subsequent 21.6-Gy WVI and local boost (up to 30–30.6-Gy) resulted in a favorable prognosis, with a 3-year recurrence-free survival rate of 89% and a 3-year OS rate of 100%.61 An ongoing clinical study (jRCTs031180223) in Japan is investigating reducing the dose of WVI to 23.4-Gy with the CARE regimen. Further attempts at reducing radiation doses may be considered in future clinical trials.
A recently conducted COG clinical trial (ACNS1123 stratum 2) is also employing the combination of carboplatin and etoposide for localized germinoma, followed by WVI and local boost with a response-based radiation dose (results not yet published).62 Clinical trials by the SIOP have alternated administration of carboplatin and etoposide with ifosfamide and etoposide (CarboPEI). As favorable treatment response has been observed from both regimens, evaluating which regimen is better is difficult.
Attempts have been made to treat germinoma with strong chemotherapy alone to eliminate the toxicity of radiotherapy. However, approximately half of the cases in several studies developed recurrence.63–65 Chemotherapy alone is therefore not recommended.
Subject 4. Treatment for NGGCT
CQ7: Are chemo- and radiotherapy effective for NGGCTs?
Chemo- and radiotherapy are strongly recommended for NGGCT excluding mature teratoma (Grade 1B)
In Japan, NGGCT encompasses all GCTs except pure germinoma. In contrast, the same terminology is used in a slightly different fashion by the COG and SIOP; clinical trials define NGGCTs as lesions harboring non-germinomatous components or those with elevated tumor markers. Japanese clinical trials have historically treated GCTs other than germinoma differentially, as either intermediate-risk or high-risk. Intermediate risk is defined as: a) immature teratoma; b) teratoma with malignant transformation; or c) mixed GCT with dominant germinoma/teratoma components. High risk is defined as choriocarcinoma, yolk sac tumor, embryonal carcinoma, mixed GCT with dominance of these malignant components, or cases with excessively high tumor marker levels (see CQ2). This difference in the classification of NGGCT across continents can obviously result in confusion when comparing treatment results. Furthermore, NGGCTs range from those with dominant histological subtypes of yolk sac tumors, embryonal carcinomas, and choriocarcinomas, which are often resistant to standard treatment and fare poorly, to mature teratomas that are cured with surgical resection alone. This diversity in the NGGCT category contributes to the complex nature of NGGCT treatment.
In Japan, the “University of Tokyo Series” (1963–1994) and a multicenter prospective study (1995–2003) by the Japanese Pediatric Brain Tumor Study Group funded by the former Ministry of Health and Welfare Cancer Research Grant classified NGGCT cases into intermediate- and high-risk groups as described above, leading to different treatment outcomes. According to the interim report at the median observation period of 3.7 years (the final report is expected soon), the intermediate-risk group showed a PFS rate of 89%, with extended local irradiation/WVI (23.4-Gy) plus local radiotherapy (total, 50.4-Gy) and combination chemotherapy with CARE.52 For high-risk NGGCT, CSI and combination chemotherapy with ifosfamide, cisplatin, and etoposide (ICE) were conducted, obtaining a moderate survival benefit. The current ongoing clinical study in Japan fundamentally adheres to the previous protocol, with germinoma showing elevated HCG (generally ≦50 mIU/ml or less than the institution standard level) transitioning to the good prognosis group, and the extended local field of radiotherapy changed to WVI.
In COG, ACNS0122 used alternating cycles of carboplatin and etoposide with ifosfamide and etoposide with 36-Gy CSI and 18-Gy local boost. This resulted in 5-year event-free survival and OS rates of 84 ± 4% and 93 ± 3%, respectively.27 In the COG ACNS1123 trial, the same chemotherapy regimen was used, but radiation was limited to 30.6-Gy WVI and local boost to 50.4-Gy for localized NGGCTs. Comparable results were obtained, with 3-year PFS and OS rates of 88 ± 4% and 92 ± 3%, respectively.29 SIOP CNS GCT-96 used ICE with 54-Gy local irradiation for localized disease and 30-Gy CSI with local boost to 54-Gy for disseminated cases. The former group showed 5-year PFS and OS rates of 72 ± 4% and 82 ± 4%, respectively, and the latter group showed 5-year PFS and OS rates of 68 ± 9% and 75 ± 8%, respectively. That study also identified AFP > 1000 ng/ml and residual disease following treatment as unfavorable prognostic factors.28
The radiation field in the treatment of NGGCT without spinal dissemination remains controversial. The SIOP study suggested omitting CSI was sufficient.28 CSI with focal boost has been the standard treatment in clinical trials in North America (COG ACNS0122) and Japan, and currently, the COG and SIOP defer CSI for patients with metastatic disease only, while treating localized NGGCT using local radiotherapy (SIOP28) or WVI + local boost (COG).29
For intermediate-risk NGGCTs, which often represent mixed GCTs with a germinomatous component, whether local radiotherapy is sufficient or WVI is necessary (current protocol) requires further investigation. Treatment results for high-risk NGGCT remain unsatisfactory, with treatment resistance at an early stage and early recurrence or dissemination.
Unlike germinoma, NGGCT may occur in very young children less than 3–4 years old. Different treatment strategies may be necessary for this category of patients, who are more vulnerable to intense CRT. Such patients should preferably receive treatment in more-dedicated and specialized facilities capable of handling surgery and systematic CRT, potentially on a clinical trial basis.
Subject 5. Treatment Strategy for Recurrence
Recurrent Germinoma
CQ8: Is salvage treatment necessary for recurrent germinoma cases?
Treatment aimed at disease cure is strongly recommended (Grade 1B)
Recurrent germinoma is reportedly curable with salvage therapies, according to descriptive studies based on case reports, case series, or retrospective analyses.66 While such reports have shown the significance of conducting salvage treatment during recurrence, standardizing treatments has been difficult. In a report of treatment for 25 recurrent cases, 17 (68%) were salvaged by treatment. While all 13 cases that received CRT (CSI in 8, local radiotherapy in 4, and WVI in 1) survived, 7 of 11 cases treated with radiotherapy alone (local radiotherapy) and 1 case treated with chemotherapy alone succumbed. All 7 failure cases treated with radiotherapy alone underwent local radiotherapy. In contrast, of the 4 survivors, 2 received CSI and the other 2 local radiotherapy. The study concluded that local radiotherapy alone may be insufficient.33 Another report analyzing 11 recurrent cases advocated CSI as a prognostic factor.67 The effectiveness of thiotepa-based HDC with autologous stem-cell rescue was reported for 9 recurrent cases, with 7 cases (78%) achieving DFS (median, 48 months). Of these, four did not undergo radiotherapy, two underwent WBI and one underwent CSI.68 The KSPNO S-530 clinical trial used HDC for 9 recurrent cases. While 4 of 7 cases treated with HDC alone survived, both cases treated with radiotherapy following HDC survived.69
One report suggested that HDC did not cause serious adverse events or drastically impair QOL,69 but the number of reported cases was small, so standardization of treatment for recurrent germinoma has yet to be achieved.
Recurrent NGGCT
CQ9: Is salvage treatment effective for recurrent NGGCTs?
Remission-oriented treatment is recommended, but palliative treatment is also recommended when treatment response is unfavorable (Grade 2C)
The prognosis of recurrent NGGCT is undoubtedly severe. Compared with recurrent germinoma cases, even fewer reports have described salvage of recurrent NGGCT cases.
In a report examining the effectiveness of thiotepa-based HDC with autologous stem-cell rescue, 4 of 12 cases (33%) achieved DFS (median, 35 months). HDC was administered with additional radiotherapy given only to those cases that had not undergone radiotherapy during initial treatment.68 In the KSPNO S-530 clinical trial using HDC for 11 treatment-resistant or recurrent cases, 4 cases achieved DFS, and all such cases had seen CR following chemotherapy. These studies concluded that CR following chemotherapy was most important for prognosis. Of note, 3 of those 4 cases received 23.4–39.6-Gy of CSI at recurrence, totaling 75.6-Gy (initial 45-Gy plus 30.6-Gy during recurrence) to the initial site (pineal gland and hypothalamus) in one case. The remaining case did not undergo additional radiotherapy. For 32 recurrent cases enrolled in the SIOP CNS GCT-96 clinical trial, the authors compared 5-year OS in 22 cases that received HDC and 10 cases that received standard chemotherapy (carboplatin or cisplatin, ifosfamide, etoposide, etc.).67 The 5-year OS was zero for the latter group, and 3 of 22 patients in the former group were surviving at 5 years. Radiotherapy was administered for only one of those surviving cases. Based on this result, radiotherapy at the time of recurrence did not appear to impact prognosis.
Salvage treatment for NGGCT at the time of recurrence can be affected by the initial treatment, particularly the radiotherapy regimen. Even with HDC, remission is achieved only infrequently. In addition, little knowledge has been accumulated regarding posttreatment disorders and effects on QOL. Although cases of successful HDC or combination high-dose chemo- and radiotherapy have been reported, survival rates remain modest and new therapies are needed.
Subject 6. Long-Term Prognosis
CQ10: Is follow-up necessary for CNS GCT cases?
Follow-up for as long as possible is strongly recommended (Grade 1B)
In most reports, follow-up periods for CNS GCT have extended as far as roughly 10 years. A survival plateau seems to be reached in 5–10 years.4,27–29,70 Nonetheless, from a much longer perspective, the Kaplan–Meier survival curve almost consistently declined at a much faster rate than that of the general demographics, according to a report on 405 germinoma and 94 NGGCT cases in the SEER database (1973–2005).71 Disease-related deaths accounted for only 16% of the 46 mortalities from germinoma over 5 years (about half of which were due to recurrence), and median survival after relapse was 9.1 years. In other words, death related to the original disease appears to occur within the first 20 years. Correspondingly, causes of mortality other than those directly related to the disease are responsible for the constant decline in survival in later periods of life. For example, the incidence of mortality associated with stroke was reported as approximately 59 times higher than the demographic norm, with a median survival of 23.8 years.71 The cumulative overall risk of cerebrovascular events was estimated as 12–20% over the first 20 years postirradiation.72 Glioma is the most common secondary neoplasm in postirradiation settings, and meningioma is predominantly a late-presenting secondary tumor with long latency (18.7 ± 10.2 years).73,74 Cavernous malformation and melanoma in the irradiated field are also concerns.45,75 Accordingly, recognizing that follow-up of CNS GCT has no end appears reasonable, and lifelong management and care directed at the disease, treatment-related complications, QOL, and social welfare are warranted.
Specific matters in follow-up, particularly regarding neurological symptoms, cognitive function, social life, anterior and posterior pituitary functions, fertility, brain and spine MRI and tumor markers, are discussed separately in Supplementary Document 1.
Conclusions
This guideline represents the current state of knowledge at the time of writing. The guideline will be updated according to ongoing and future clinical trials and retrospective clinical studies sequentially. A multidisciplinary team approach is necessary for this predominantly pediatric and young adult neoplasm, which involves not just disease management using surgery, chemotherapy, and radiotherapy, but also ophthalmological, endocrinological, and reproductive care together with long-term follow-up focusing on disease relapse, treatment-related side-effects, cognitive function, and social life. This guideline illuminates the pathway of care for patients, as well as clarifies unmet needs where further translational and clinical studies are required to better inform our understanding of this complex, multifaceted disease and facilitate improvements in management.
Supplementary Material
Acknowledgments
We are grateful to the members of: The Japan Society for Neuro-Oncology, The Japan Neurosurgical Society, Japan Society of Clinical Oncology, Japanese Society of Pediatric Neurosurgery, The Japanese Society of Pediatric Hematology/Oncology, Japanese Society for Radiation Oncology, Japanese Society of Medical Oncology, The Japanese Society of Child Neurology, Children’s Cancer Association of Japan, Japan Brain Tumor Alliance, for their helpful comments and to Rumi Ito (Kurume University) for secretarial assistances.
Contributor Information
Hideo Nakamura, Department of Neurosurgery, Kurume University School of Medicine, Fukuoka, Japan.
Hirokazu Takami, Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan.
Takaaki Yanagisawa, Department of Neurosurgery, Jikei University School of Medicine, Tokyo, Japan.
Toshihiro Kumabe, Department of Neurosurgery, Kitasato University School of Medicine, Kanagawa, Japan.
Takamitsu Fujimaki, Department of Neurosurgery, Saitama Medical University Hospital, Saitama, Japan.
Yoshiki Arakawa, Department of Neurosurgery, Kyoto University Graduate School of Medicine, Kyoto, Japan.
Katsuyuki Karasawa, Division of Radiation Oncology, Department of Radiology, Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital, Tokyo, Japan.
Keita Terashima, Division of Neuro-Oncology, National Center for Child Health and Development, Tokyo, Japan.
Hideaki Yokoo, Department of Human Pathology, Gunma University Graduate School of Medicine, Gunma, Japan.
Kohei Fukuoka, Department of Hematology and Oncology, Saitama Children’s Medical Center, Saitama, Japan.
Yukihiko Sonoda, Department of Neurosurgery, Yamagata University Hospital, Yamagata, Japan.
Kaori Sakurada, Department of Neurosurgery, Yamagata University Hospital, Yamagata, Japan.
Yohei Mineharu, Department of Neurosurgery, Kyoto University Graduate School of Medicine, Kyoto, Japan.
Toshinori Soejima, Department of Radiation Oncology, Kobe Proton Center, Kobe, Japan.
Motoaki Fujii, Department of Radiation Therapy, Mitsui Memorial Hospital, Tokyo, Japan.
Naoki Shinojima, Department of Neurosurgery, Kumamoto University School of Medicine, Kumamoto, Japan.
Junichi Hara, Department of Pediatric Hematology and Oncology, Osaka City General Hospital, Osaka, Japan.
Kai Yamasaki, Department of Pediatric Hematology and Oncology, Osaka City General Hospital, Osaka, Japan.
Junya Fujimura, Department of Pediatrics, Juntendo University Faculty of Medicine, Tokyo, Japan.
Fumiyuki Yamasaki, Department of Neurosurgery, Hiroshima University Hospital, Hiroshima, Japan.
Mayu Takahashi, Department of Neurosurgery, University of Occupational and Environmental Health, Fukuoka, Japan.
Tomonari Suzuki, Department of Neuro-Oncology/Neurosurgery, Saitama Medical University International Medical Center, Saitama, Japan.
Iori Sato, Department of Family Nursing, School of Health Sciences and Nursing, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
Ryo Nishikawa, Department of Neuro-Oncology/Neurosurgery, Saitama Medical University International Medical Center, Saitama, Japan.
Kazuhiko Sugiyama, Department of Clinical Oncology and Neuro-Oncology Program, Hiroshima University Hospital, Hiroshima, Japan.
Funding
This work was supported by The Japan Society for Neuro-Oncology and Health and Labor Sciences Research Grant/Research for Promotion of Cancer Control Programs (No. H29-013).
Conflict of interest statement. None declared.
Authorship statement. Conceptualization: R.N. and H.N. Each author contributed to and approved the final version of the manuscript.
References
- 1. Louis D, Ohgaki H, Wiestler O, Cavenee W. WHO Classification of Tumours of the Central Nervous System (Revised 4th Edition). Lyon: International Agency for Research on Cancer (IARC); 2016. [Google Scholar]
- 2. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985; 63(2):155–167. [DOI] [PubMed] [Google Scholar]
- 3. Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997; 86(3):446–455. [DOI] [PubMed] [Google Scholar]
- 4. Takami H, Fukuoka K, Fukushima S, et al. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019; 21(12):1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aizer AA, Sethi RV, Hedley-Whyte ET, et al. Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro Oncol. 2013; 15(7):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanamori M, Takami H, Yamaguchi S, et al. So-called bifocal tumors with diabetes insipidus and negative tumor markers: are they all germinoma? Neuro Oncol. 2021; 23(2):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takami H, Graffeo CS, Perry A, Giannini C, Daniels DJ. The third eye sees double: cohort study of clinical presentation, histology, surgical approaches, and ophthalmic outcomes in pineal region germ cell tumors. World Neurosurg. 2021; 150:e482–e490. [DOI] [PubMed] [Google Scholar]
- 8. Hankinson EV, Lyons CJ, Hukin J, Cochrane DD. Ophthalmological outcomes of patients treated for pineal region tumors. J Neurosurg Pediatr. 2016; 17(5):558–563. [DOI] [PubMed] [Google Scholar]
- 9. Takami H, Graffeo CS, Perry A, Giannini C, Daniels DJ. Epidemiology, natural history, and optimal management of neurohypophyseal germ cell tumors. J. Neurosurg. 2020; 1(aop):1–9. [DOI] [PubMed] [Google Scholar]
- 10. Jorsal T, Rørth M. Intracranial germ cell tumours. A review with special reference to endocrine manifestations. Acta Oncol. 2012; 51(1):3–9. [DOI] [PubMed] [Google Scholar]
- 11. Sethi RV, Marino R, Niemierko A, Tarbell NJ, Yock TI, MacDonald SM. Delayed diagnosis in children with intracranial germ cell tumors. J Pediatr. 2013; 163(5):1448–1453. [DOI] [PubMed] [Google Scholar]
- 12. Ozelame RV, Shroff M, Wood B, et al. Basal ganglia germinoma in children with associated ipsilateral cerebral and brain stem hemiatrophy. Pediatr Radiol. 2006; 36(4):325–330. [DOI] [PubMed] [Google Scholar]
- 13. Brain tumor registry of Japan (2005–2008). Neurol Med Chir (Tokyo). 2017; 57(Supplement-1):9–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zapotocky M, Ramaswamy V, Lassaletta A, Bouffet E. Adolescents and young adults with brain tumors in the context of molecular advances in neuro-oncology. Pediatr Blood Cancer. 2018; 65(2):e26861. [DOI] [PubMed] [Google Scholar]
- 15. Teilum G. Classification of endodermal sinus tumour (mesoblatoma vitellinum) and so-called “embryonal carcinoma” of the ovary. Acta Pathol Microbiol Scand. 1965; 64(4):407–429. [DOI] [PubMed] [Google Scholar]
- 16. Ichimura K, Fukushima S, Totoki Y, et al. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016; 131(6):889–901. [DOI] [PubMed] [Google Scholar]
- 17. Fukushima S, Otsuka A, Suzuki T, et al. Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014; 127(6):911–925. [DOI] [PubMed] [Google Scholar]
- 18. Schulte SL, Waha A, Steiger B, et al. CNS germinomas are characterized by global demethylation, chromosomal instability and mutational activation of the Kit-, Ras/Raf/Erk- and Akt-pathways. Oncotarget. 2016; 7(34):55026–55042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Yamaguchi S, Burstein MD, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 2014; 511(7508):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukushima S, Yamashita S, Kobayashi H, et al. Genome-wide methylation profiles in primary intracranial germ cell tumors indicate a primordial germ cell origin for germinomas. Acta Neuropathol. 2017; 133(3):445–462. [DOI] [PubMed] [Google Scholar]
- 21. Hoei-Hansen CE, Sehested A, Juhler M, et al. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: expression of pluripotency and cell differentiation markers. J Pathol. 2006; 209(1):25–33. [DOI] [PubMed] [Google Scholar]
- 22. Wang HW, Wu YH, Hsieh JY, et al. Pediatric primary central nervous system germ cell tumors of different prognosis groups show characteristic miRNome traits and chromosome copy number variations. BMC Genomics. 2010; 11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray MJ, Ajithkumar T, Harris F, et al. Clinical utility of circulating miR-371a-3p for the management of patients with intracranial malignant germ cell tumors. Neuro-Oncol Adv. 2020; 2(1):vdaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong KT, Lee DH, Kim BK, et al. Treatment outcome and long-term follow-up of central nervous system germ cell tumor using upfront chemotherapy with subsequent photon or proton radiation therapy: a single tertiary center experience of 127 patients. BMC Cancer. 2020; 20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo AC, Hodgson D, Dang J, et al. Intracranial germ cell tumors in adolescents and young adults: a 40-year multi-institutional review of outcomes. Int J Radiat Oncol Biol Phys. 2020; 106(2):269–278. [DOI] [PubMed] [Google Scholar]
- 26. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013; 15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldman S, Bouffet E, Fisher PG, et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: a children’s oncology group study. J Clin Oncol. 2015; 33(22):2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol. 2017; 19(12):1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fangusaro J, Wu S, MacDonald S, et al. Phase II trial of response-based radiation therapy for patients with localized CNS nongerminomatous germ cell tumors: a children’s oncology group study. J Clin Oncol. 2019; 37(34):3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015; 16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 31. Kim A, Ji L, Balmaceda C, et al. The prognostic value of tumor markers in newly diagnosed patients with primary central nervous system germ cell tumors. Pediatr Blood Cancer. 2008; 51(6):768–773. [DOI] [PubMed] [Google Scholar]
- 32. Kinoshita Y, Yamasaki F, Usui S, et al. Solitary Langerhans cell histiocytosis located in the neurohypophysis with a positive titer HCG-β in the cerebrospinal fluid. Childs Nerv Syst. 2016; 32(5):901–904. [DOI] [PubMed] [Google Scholar]
- 33. Kamoshima Y, Sawamura Y, Ikeda J, Shirato H, Aoyama H. Late recurrence and salvage therapy of CNS germinomas. J Neurooncol. 2008; 90(2):205–211. [DOI] [PubMed] [Google Scholar]
- 34. Kim CY, Choi JW, Lee JY, et al. Intracranial growing teratoma syndrome: clinical characteristics and treatment strategy. J Neurooncol. 2011; 101(1):109–115. [DOI] [PubMed] [Google Scholar]
- 35. Michaiel G, Strother D, Gottardo N, et al. Intracranial growing teratoma syndrome (iGTS): an international case series and review of the literature. J Neurooncol. 2020; 147(3):721–730. [DOI] [PubMed] [Google Scholar]
- 36. Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, Wharam M. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007; 48(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheung V, Segal D, Gardner SL, et al. Utility of MRI versus tumor markers for post-treatment surveillance of marker-positive CNS germ cell tumors. J Neurooncol. 2016; 129(3):541–544 [DOI] [PubMed] [Google Scholar]
- 38. Wu CC, Guo WY, Chang FC, et al. MRI features of pediatric intracranial germ cell tumor subtypes. J Neurooncol. 2017; 134(1):221–230. [DOI] [PubMed] [Google Scholar]
- 39. Luther N, Edgar MA, Dunkel IJ, Souweidane MM. Correlation of endoscopic biopsy with tumor marker status in primary intracranial germ cell tumors. J Neurooncol. 2006; 79(1):45–50. [DOI] [PubMed] [Google Scholar]
- 40. Kinoshita Y, Yamasaki F, Tominaga A, et al. Pitfalls of neuroendoscopic biopsy of intraventricular germ cell tumors. World Neurosurg. 2017; 106:430–434. [DOI] [PubMed] [Google Scholar]
- 41. Noudel R, Vinchon M, Dhellemmes P, Litré CF, Rousseaux P. Intracranial teratomas in children: the role and timing of surgical removal. J Neurosurg Pediatr. 2008; 2(5):331–338. [DOI] [PubMed] [Google Scholar]
- 42. Kageyama N, Kobayashi T, Kida Y, Yoshida J, Kato K. Intracranial germinal tumors. Prog Exp Tumor Res. 1987; 30:255–267. [DOI] [PubMed] [Google Scholar]
- 43. Nakamura H, Makino K, Kochi M, Ushio Y, Kuratsu J. Evaluation of neoadjuvant therapy in patients with nongerminomatous malignant germ cell tumors. J Neurosurg Pediatr. 2011; 7(4):431–438. [DOI] [PubMed] [Google Scholar]
- 44. Ogiwara H, Kiyotani C, Terashima K, Morota N. Second-look surgery for intracranial germ cell tumors. Neurosurgery. 2015; 76(6):658–661; discussion 661-652. [DOI] [PubMed] [Google Scholar]
- 45. Takami H, Perry A, Graffeo CS, Giannini C, Daniels DJ. Novel diagnostic methods and posttreatment clinical phenotypes among intracranial germ cell tumors. Neurosurgery. 2020; 87(3):563–572. [DOI] [PubMed] [Google Scholar]
- 46. Luther N, Stetler WR Jr, Dunkel IJ, Christos PJ, Wellons JC 3rd, Souweidane MM. Subarachnoid dissemination of intraventricular tumors following simultaneous endoscopic biopsy and third ventriculostomy. J Neurosurg Pediatr. 2010; 5(1):61–67. [DOI] [PubMed] [Google Scholar]
- 47. Xu K, Khine KT, Ooi YC, Quinsey CS. A systematic review of shunt-related extraneural metastases of primary central nervous system tumors. Clin Neurol Neurosurg. 2018; 174:239–243. [DOI] [PubMed] [Google Scholar]
- 48. Eom KY, Kim IH, Park CI, et al. Upfront chemotherapy and involved-field radiotherapy results in more relapses than extended radiotherapy for intracranial germinomas: modification in radiotherapy volume might be needed. Int J Radiat Oncol Biol Phys. 2008; 71(3):667–671. [DOI] [PubMed] [Google Scholar]
- 49. Uematsu Y, Tsuura Y, Miyamoto K, Itakura T, Hayashi S, Komai N. The recurrence of primary intracranial germinomas. Special reference to germinoma with STGC (syncytiotrophoblastic giant cell). J Neurooncol. 1992; 13(3):247–256. [DOI] [PubMed] [Google Scholar]
- 50. Jensen AW, Laack NN, Buckner JC, Schomberg PJ, Wetmore CJ, Brown PD. Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys. 2010; 77(5):1449–1456. [DOI] [PubMed] [Google Scholar]
- 51. Alapetite C, Brisse H, Patte C, et al. Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol. 2010; 12(12):1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsutani M, Group JPBTS. Combined chemotherapy and radiation therapy for CNS germ cell tumors–the Japanese experience. J Neurooncol. 2001; 54(3):311–316. [DOI] [PubMed] [Google Scholar]
- 53. Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999; 17(8):2585–2592. [DOI] [PubMed] [Google Scholar]
- 54. Ogawa K, Shikama N, Toita T, et al. Long-term results of radiotherapy for intracranial germinoma: a multi-institutional retrospective review of 126 patients. Int J Radiat Oncol Biol Phys. 2004; 58(3):705–713. [DOI] [PubMed] [Google Scholar]
- 55. Shikama N, Ogawa K, Tanaka S, et al. Lack of benefit of spinal irradiation in the primary treatment of intracranial germinoma: a multiinstitutional, retrospective review of 180 patients. Cancer. 2005; 104(1):126–134. [DOI] [PubMed] [Google Scholar]
- 56. Liang SY, Yang TF, Chen YW, et al. Neuropsychological functions and quality of life in survived patients with intracranial germ cell tumors after treatment. Neuro Oncol. 2013; 15(11):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: from the First International CNS Germ-Cell Tumor Study. Neuro Oncol. 2001; 3(3):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martens T, Rotermund R, Zu Eulenburg C, Westphal M, Flitsch J. Long-term follow-up and quality of life in patients with intracranial germinoma. Neurosurg Rev. 2014; 37(3):445–50; discussion 451. [DOI] [PubMed] [Google Scholar]
- 59. Park Y, Yu ES, Ha B, Park HJ, Kim JH, Kim JY. Neurocognitive and psychological functioning of children with an intracranial germ cell tumor. Cancer Res Treat. 2017; 49(4):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang M, Zhou P, Zhang S, et al. Clinical features, radiologic findings, and treatment of pediatric germ cell tumors involving the basal ganglia and thalamus: a retrospective series of 15 cases at a single center. Childs Nerv Syst. 2018; 34(3):423–430. [DOI] [PubMed] [Google Scholar]
- 61. Khatua S, Dhall G, O’Neil S, et al. Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr Blood Cancer. 2010; 55(1):42–46. [DOI] [PubMed] [Google Scholar]
- 62. Bartels U, Fangusaro J, Shaw D, et al. GCT-41. Response-based radiation therapy in patients with newly diagnosed central nervous system localized germinoma: a children’s oncology group (COG) prospective phase 2 clinical trial. Neuro-Oncology. 2020; 22(Suppl 3):iii336. [Google Scholar]
- 63. Kellie SJ, Boyce H, Dunkel IJ, et al. Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: failure of a primary chemotherapy approach. Pediatr Blood Cancer. 2004; 43(2):126–133. [DOI] [PubMed] [Google Scholar]
- 64. da Silva NS, Cappellano AM, Diez B, et al. Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer. 2010; 54(3):377–383. [DOI] [PubMed] [Google Scholar]
- 65. Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996; 14(11):2908–2915. [DOI] [PubMed] [Google Scholar]
- 66. Janjetovic S, Bokemeyer C, Fiedler W, Frenzel T, Calaminus G, Honecker F. Late recurrence of a pineal germinoma 14 years after radiation and chemotherapy: a case report and review of the literature. Onkologie. 2013; 36(6):371–373. [DOI] [PubMed] [Google Scholar]
- 67. Hu YW, Huang PI, Wong TT, et al. Salvage treatment for recurrent intracranial germinoma after reduced-volume radiotherapy: a single-institution experience and review of the literature. Int J Radiat Oncol Biol Phys. 2012; 84(3):639–647. [DOI] [PubMed] [Google Scholar]
- 68. Modak S, Gardner S, Dunkel IJ, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol. 2004; 22(10):1934–1943. [DOI] [PubMed] [Google Scholar]
- 69. Baek HJ, Park HJ, Sung KW, et al. Myeloablative chemotherapy and autologous stem cell transplantation in patients with relapsed or progressed central nervous system germ cell tumors: results of Korean Society of Pediatric Neuro-Oncology (KSPNO) S-053 study. J Neurooncol. 2013; 114(3):329–338. [DOI] [PubMed] [Google Scholar]
- 70. Calaminus G, Bamberg M, Harms D, et al. AFP/beta-HCG secreting CNS germ cell tumors: long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics. 2005; 36(2):71–77. [DOI] [PubMed] [Google Scholar]
- 71. Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. 2015; 17(5):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Twitchell S, Karsy M, Guan J, Couldwell WT, Taussky P. Sequelae and management of radiation vasculopathy in neurosurgical patients. J Neurosurg. 2019; 130:1889–1897. [DOI] [PubMed] [Google Scholar]
- 73. Choudhary A, Pradhan S, Huda MF, Mohanty S, Kumar M. Radiation induced meningioma with a short latent period following high dose cranial irradiation - case report and literature review. J Neurooncol. 2006; 77(1):73–77. [DOI] [PubMed] [Google Scholar]
- 74. Pettorini BL, Park YS, Caldarelli M, Massimi L, Tamburrini G, Di Rocco C. Radiation-induced brain tumours after central nervous system irradiation in childhood: a review. Childs Nerv Syst. 2008; 24(7):793–805. [DOI] [PubMed] [Google Scholar]
- 75. Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst. 2011; 27(3):445–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.