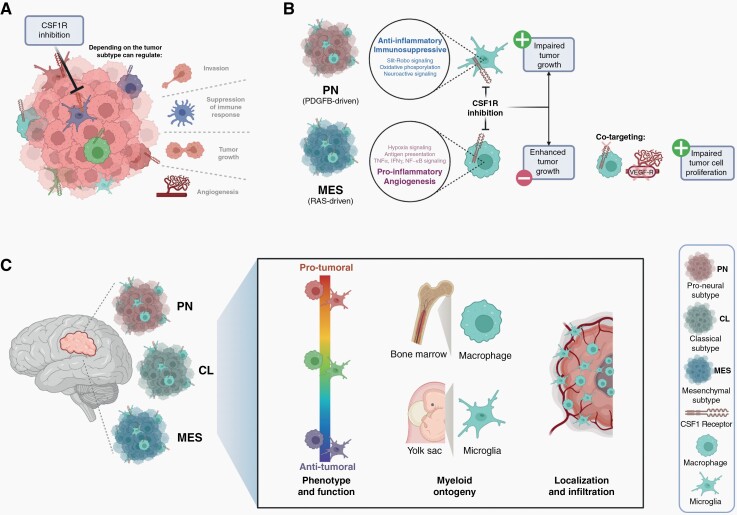
Advances in the molecular classification of glioblastoma (GBM) going beyond histology have provided a wealth of information on tumor biology but have led to no treatment breakthroughs for most patients. Intra- and intertumoral heterogeneity is justifiably invoked as being an impediment to successful outcomes in clinical trials based on broad patient cohorts, which can include gliomas with different transcriptional profiles, namely mesenchymal, proneural/neural, and classical subtypes. This subtyping has prognostic value and mechanistic relevance because many of the core genes are linked to pathways driving gliomagenesis, which has led to the development of mouse models recapitulating these subtypes. These models offer an opportunity to address the impact of transcriptional subtype on the tumor microenvironment (TME) and immune cell infiltration. Tumor-associated macrophages (TAMs), derived either from bone marrow/blood monocytes or from brain resident microglia, are the main immune cell of the TME and can promote tumor growth.1 These TAM populations are phenotypically and functionally diverse, and are in constant dialogue with glioma cells, the consequences of which can depend upon the GBM subtype.1,2 A highly promising TAM-targeting approach in GBM is the depletion or reprogramming of myeloid cells by colony-stimulating factor 1 receptor (CSF1R) inhibitors. Despite promising results in mouse GBM models,3,4 CSFR1 inhibition failed to demonstrate effectiveness in clinical trials.5 In mice, resistance to CSF1R inhibition was driven by insulin-like growth factor 1 released by reprogrammed TAMs,6 but other factors within the TME such as vasculature, heterogeneous microglia/macrophage populations, and the dynamics of their interactions with different subtypes of glioma cells also warrant exploration (Figure 1A).
Fig. 1.
GBM subtype-specific tumor microenvironment can influence TAM function and responsiveness to CSF1R inhibition. (A) Depending on the tumor subtype, targeting TAMs with CSF1R inhibition can regulate tumor growth, vascular networks, invasion, and immunosuppression. (B) CSF1R inhibition blocks PDGFB-driven (PN: proneural-like) glioma growth, in contrast to accelerated growth of RAS-driven (MES: mesenchymal-like) gliomas. Transcriptome analysis showed predominantly pro-tumoral microglia in PDGFB-driven gliomas and predominantly pro-inflammatory and angiogenic macrophages in RAS-driven gliomas. Co-targeting of TAMs and angiogenesis decreased cell proliferation and inhibited tumor growth. (C) GBM subtype-specific tumor microenvironment influences the function (activation state), the source, and the type of infiltrating myeloid cells as well as their localization. Taken together, these factors will likely dictate different sensitives to TAM-targeting agents and determine the clinical outcome. Abbreviations: CSF1R, colony-stimulating factor 1 receptor; GBM, glioblastoma; PDGFB, platelet derived growth factor subunit B; RAS, rat sarcoma virus; TAM, tumor-associated macrophages.
In this issue of Neuro-Oncology,7 Rao et al describe the identification of distinct TAM subpopulations in different RAS- and PDGF-driven mouse GBM models resembling mesenchymal-like and proneural-like gliomas, respectively. The authors observed that CSF1R inhibitor PLX3397 was either ineffective or promoted the growth of mesenchymal-like gliomas, whereas the growth of proneural-like gliomas was inhibited. These outcomes were recapitulated in xenografted mesenchymal models, but not in proneural glioma xenografted mice, which should encourage caution in interpreting treatments depending upon mouse-human cellular interactions. Although previous studies using CSF1R inhibitor BLZ945 demonstrated that its activity was through TAMs and not directly on tumor cells,4 PLX3397 is a more broadly acting tyrosine kinase inhibitor; nevertheless, it was also inactive on the glioma cells used in the present study.7 In view of this strict TAM targeting of CSF1R inhibitors in GBM, TAM profiling becomes an indispensable task to elucidate CSF1R inhibitor responsiveness. This has been pursued by transcriptional analyses using publicly available datasets, which showed distinct proportions of microglia and macrophages according to the GBM subtype,8 as also noted by Rao et al, although some differences in proportions of each cell type were reported, potentially reflecting different definitions of macrophages and microglia.7 In proneural and mesenchymal mouse models, a similar hierarchy of macrophage and microglia proportions was observed as in human GBM, however, more microglia were detected in mice than in humans, which might reflect the limited timeframe (a few weeks) for macrophage accumulation from the periphery in mouse GBM. This may also explain why GBM growth and PLX3397 responsiveness in Ccr2-deficient mice (with inefficient recruitment of blood monocytes) were comparable to wild-type mice.7
Understanding TAM functions in addition to their abundance can shed light on responsiveness to CSF1R inhibition, moreover, spatiotemporal aspects should also be considered. In this regard, the outcome of CSFR1 inhibition in GBM can depend on the timing and duration of treatment. Acute treatment with CSFR1 inhibitor did not influence TAM reprogramming, nor survival, in proneural GBM models.1 In the RAS-driven (mesenchymal-like) GBM model used by Rao et al,7 PLX3397 administration early after tumor grafting accelerated tumor progression and shortened survival. This correlated with decreased TAM infiltration (especially macrophages), in contrast to maintenance of these cells in similarly treated proneural-like GBM. Single-cell transcriptomics revealed an enrichment of TAMs with a pro-inflammatory and angiogenic signature in the RAS-driven GBM model, which was associated with higher tumor vessel density compared to PDGFB-driven GBM. Considering these TME characteristics, co-targeting angiogenesis with VEGFR (vascular endothelial growth factor receptor) inhibitor and TAMs with CSF1R inhibitor is a rational approach to consider for certain GBM subtypes; indeed, this combination impacted RAS-driven GBM growth characteristics, with decreased cell proliferation and vessel density (not observed in PDGF-driven glioma), but with no major effects on survival (Figure 1B). Modulation of the TME in solid tumors will not only impact the cells expressing the targeted structures, but also other cells important for host-cancer cell interactions, such as T cells, as observed in this study.7 The plasticity of myeloid cells and their myriad potential functions has encouraged a simplified nomenclature correlating with pro-inflammatory/anti-tumoral (M1) or anti-inflammatory/pro-tumoral (M2) functions, both potentially derived from M0 cells.1 This taxonomy can be helpful, as long as M1- or M2-like activation states are considered as part of a spectrum. Indeed, therapeutic efficacy after CSF1R inhibition in mouse GBM models was correlated with reduced M2 marker expression by TAMs.4 Moreover, such TAM-typing in human GBM allowed delineation of distinct populations in different tumor regions, with tumor core being dominated by M0-M1 TAMs (predominately macrophages) and peripheral regions by M0-M2 TAMs (predominately microglia).9
The study by Rao et al highlights TAM heterogeneity in GBM (Figure 1C), its link to GBM genetic drivers, and how this can dictate response to certain therapeutics.7 However, few mouse GBM models are fully characterized in terms of their tumor characteristics (molecular subtype, histology) in addition to their immune infiltrate (TAMs and T cells), but all these factors will influence tumor growth and mouse survival. Moreover, the localization of TAM (eg, in hypoxic, perivascular, necrotic, and border zones)10 and their dynamics (eg, early in gliomagenesis, during tumor progression, or at recurrence) should guide additional treatment modalities, such as those impacting angiogenesis, hypoxia, or T cells. Arguably, it may be impractical to incorporate all aspects of human glioma biology and interactions with all immune cells in a single model; valid clinical translation will therefore depend upon the judicious interpretation of multiple preclinical models.
Acknowledgments
The text is the sole product of the authors and no third party had input or gave support to its writing. The figure was created with Biorender (biorender.com).
Contributor Information
Felipe I Espinoza, Translational Research Centre in Oncohaematology, Geneva University Hospitals and University of Geneva, Geneva, Switzerland.
Paul R Walker, Translational Research Centre in Oncohaematology, Geneva University Hospitals and University of Geneva, Geneva, Switzerland.
Funding
This work is supported by the Fonds Lionel Perrier (to P.R.W.)
Conflict of interest statement. None declared.
References
- 1. Wei J, Chen P, Gupta P, et al. Immune biology of glioma-associated macrophages and microglia: functional and therapeutic implications. Neuro Oncol. 2020;22(2):180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hara T, Chanoch-Myers R, Mathewson ND, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell. 2021;39(6):779–792.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akkari L, Bowman RL, Tessier J, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12(552). doi: 10.1126/scitranslmed.aaw7843. [DOI] [PubMed] [Google Scholar]
- 4. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butowski N, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an ivy foundation early phase clinical trials consortium phase II study. Neuro Oncol. 2016;18(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288):aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rao R, Han R, Ogurek S, et al. Glioblastoma genetic drivers dictate the function of tumor-associated macrophages/microglia and responses to CSF1R inhibition [published online ahead of print September 25, 2021]. Neuro Oncol. 2022;24(4):584–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui X, Wang Q, Zhou J, et al. Single-cell transcriptomics of glioblastoma reveals a unique tumor microenvironment and potential immunotherapeutic target against tumor-associated macrophage. Front Oncol. 2021;11:710695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landry AP, Balas M, Alli S, Spears J, Zador Z. Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma. Sci Rep. 2020;10(1):19542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muller S, Kohanbash G, Liu SJ, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]