Abstract
Human communication is remarkably versatile, enabling teachers to share highly abstracted and novel information with their students. What neural processes enable such transfer of information across brains during naturalistic teaching and learning? Here, a teacher was scanned in functional magnetic resonance imaging while giving an oral lecture with slides on a scientific topic followed by a review lecture. Students were then scanned while watching either the intact Lecture and Review (N = 20) or a temporally scrambled version of the lecture (N = 20). Using intersubject correlation, we observed widespread Teacher–Student neural coupling spanning sensory cortex and language regions along the superior temporal sulcus as well as higher-level regions including posterior medial cortex (PMC), superior parietal lobule, and dorsolateral and dorsomedial prefrontal cortex. Teacher–student alignment in higher-level areas was not observed when learning was disrupted by temporally scrambling the lecture. Moreover, teacher–student coupling in PMC was significantly correlated with learning: the more closely the student’s brain mirrored the teacher’s brain, the more the student improved their learning score. Together, these results suggest that the alignment of neural responses between teacher and students may reflect effective communication of complex information across brains in classroom settings.
Keywords: learning, communication, naturalistic, fMRI, ISC
Introduction
Humans have a unique ability to share knowledge of the world with each other via communication. Often, communication consists of sharing narratives or recalling memories in social contexts where interlocutors have a common background and shared knowledge. However, in other contexts, such as in teaching and learning, human communication is often asymmetric and involves the transfer of novel, non-social information from an expert (teacher) to a novice (student). What neural processes enable transfer of information across brains during naturalistic teaching and learning of complex, abstract information?
Verbal communication of social narratives elicits correlated neural responses between speakers and listeners in regions overlapping with the default mode network (DMN; Stephens et al., 2010; Dikker et al., 2014; Silbert et al., 2014; Zadbood et al., 2017). This ‘speaker-listener coupling’ is thought to be driven by shared understanding of the narrative: speaker–listener coupling in DMN is observed only during comprehensible communication and is correlated with listener comprehension (Stephens et al., 2010; Silbert et al., 2014). Moreover, shared DMN responses among listeners are sensitive to background context or knowledge that enables similar comprehension of interpretation of the narrative. Subjects who receive contextualizing background information show more strongly correlated neural responses in DMN in response to ambiguous narratives than subjects lacking this information (van Kesteren et al., 2010; Ames et al., 2014; Chen et al., 2015; Oren et al., 2017). Together, these findings point to a central role of the DMN in integrating information from the past with the present in order to dynamically ‘make sense’ of situations as they unfold over time (Hasson et al., 2015; Yeshurun et al., 2021).
However, during communication of non-narrative, technical information, individuals may lack a common context or background for communicating effectively. Teaching is therefore a means for establishing a shared common ground for understanding new information. Here, we suggest that the process of establishing this shared knowledge via teaching is reflected in coupled neural processes between the teacher and students in regions of the DMN. This work therefore builds on previous functional magnetic resonance imaging (fMRI) studies that identified speaker–listener alignment in the communication of narratives to show that speaker–listener alignment in DMN reflects shared understanding of information broadly, whether narrative or technical in content. Several recent studies have also investigated non-narrative communication using electroencephalogram (EEG) and functional near-infrared spectroscopy (fNIRS; Holper et al., 2013; Zheng et al., 2018; Bevilacqua et al., 2019; Pan et al., 2020). These imaging modalities can capture shared brain activity in real-time during naturalistic, interactive learning and can be readily deployed across multiple subjects simultaneously—both of which are prohibitively difficult with fMRI. The current study, however, leverages the high-resolution whole-brain coverage of fMRI to capture shared signals in medial cortical areas that are largely inaccessible to EEG and fNIRS. These include key regions of interest in the DMN, including posterior medial cortex and medial prefrontal cortex. Our previous work (Hasson et al., 2015; Yeshurun et al., 2021) significantly implicate these regions in the processing and comprehension of complex, temporally extended information, and EEG in particular cannot capture neural responses in these areas. In addition, to our knowledge this work is the first to use fMRI to measure teacher–student alignment during an extended, naturalistic lesson that mimics the length and complexity of a college-level lecture.
Here, we extend prior research on the neural basis of real-life learning by using fMRI to investigate teacher–student alignment during teaching and learning of complex scientific material. We scanned a teacher giving an extended, 32-min lecture on a scientific topic followed by a 6-min review of the material. Students were then scanned while watching the video lessons. Based on previous work, we predicted that watching video lessons would elicit shared, or correlated, neural responses among students in regions ranging from early sensory cortex to high-level regions of the DMN. We additionally predicted that the shared neural response among students in high-level regions will be coupled to the teacher’s brain activity and will correlate with learning outcomes, particularly doing the review session. Finally, because the main lesson serves to create a shared knowledge between teachers and students, we predicted that teacher–student coupling will be greater during the review than the lesson.
Methods
Subjects
One teacher (author M.N.) was scanned using fMRI while giving a verbal lecture and review with accompanying slides. One of the authors served as the teacher in order to facilitate data collection and to have full control over the lesson materials. The teacher had college-level teaching experience at three different universities, received consistently high evaluations for the quality of her lectures and teaching, and was previously recognized for excellence in teaching.
Forty-eight subjects with normal hearing and normal or corrected-to-normal vision participated in the experiment as students. Data from four subjects were excluded from analysis for excessive motion during scanning (>3 mm), two for falling asleep during scanning, and two for significant pre-existing knowledge of the lesson material (scored ≥80% on the pre-lesson test). This left 20 subjects (ages 19–34, mean = 22.2 years; 13 female) in the Intact Learning condition and 20 subjects (ages 18–26, mean = 19.65 years; 10 female) in the Scrambled control condition. The majority of participants had graduated high school and completed at least some college (70%), while the remaining had Bachelor’s degrees or higher. Only four participants reported studying psychology or neuroscience in college. All experimental procedures were approved by Princeton University’s Internal Review Board, and all subjects provided written, informed consent.
Experimental design
Stimuli
The teacher developed a 32-min Lesson and a 6-min Review, both with slides (Figure 1A). In order to engage the subjects with the lesson and utilize the teacher’s expertise, we selected fMRI itself as the lesson topic (Figure 1B). All lesson materials were developed by the teacher through extensive piloting using Amazon’s Mechanical Turk (6 pilots; 237 subjects, 82 female, mean age 34.5 years). For each pilot, the teacher recorded a version of the Lesson and Review, which were then viewed by online subjects. The subjects took a quiz before and after watching the videos and provided feedback on their experiences. Based on this feedback, the teacher modified and optimized the lesson materials. For the main experiment, we selected the version of the Lesson, Review and quiz questions that were rated as highly engaging and effective by participants. The teacher then memorized the script and timing of both lectures.
Fig. 1.
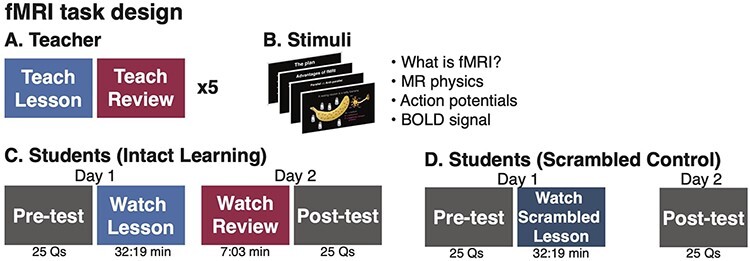
fMRI task design. (A) A teacher was scanned in fMRI while giving a 32-min Lesson and a 7-min Review with slides, repeating each five times in the scanner while her voice was recorded. (B) The Lecture and Review introduced fMRI concepts. (C) Students in the Intact Learning condition took a Pre-test quiz and watched the Lesson in the scanner. On the following day, they were scanned watching the Review and then completed a Post-test quiz. (D) Subjects in the Scrambled condition took the same Pre- and Post-test quizzes, but watched a scrambled version of the Lesson.
The teacher was then scanned in fMRI while giving the selected Lesson and Review. The teacher repeated the same Lesson and Review five times across three different scanning sessions (Lesson duration: mean = 32:20 min, range = 31:29–34:29 min; Review duration: mean = 5:58 min, range = 5:54–6:13 min). During teaching, slides were presented using PsychoPy2 (Peirce et al., 2019) and presented via a liquid crystal display projector on a rear-projection screen mounted in the back of the scanner bore. The teacher viewed slides through a mirror mounted on the head coil and advanced slides using a button box. The teacher’s speech was recorded using an MRI-compatible microphone with online sound canceling (FOMRI III; Optoacoustics Ltd).
Following the teacher scans, the audio recordings were denoised using Adobe Audition, transcribed and timestamped, and aligned with slide presentation. The highest quality recordings were selected to present to students (Lesson length: 32:19 min, Review: 6:13 min). To minimize transient, non-selective responses that occur at the abrupt onset of a stimulus, the lectures were preceded by an unrelated 37-s movie clip followed by a 6-s fixation cross. Videos ended with another 6 s of fixation cross. The unrelated movie clip and fixation periods were cropped from analyses.
Finally, the teacher developed a 25-question, multiple choice quiz to assess learning. The same quiz was administered before and after learning (pre-test and post-test). To help minimize students using the quiz questions as attentional cues during the lessons, students were not informed that the same questions would be administered before and after learning. In addition, the quiz questions were designed to require integrating information over many sentences and sometimes sections of the lecture, as in a typical college-level course.
Student sessions: intact learning
Subjects completed the main experiment on two consecutive days. In the Intact Learning condition, on Day 1, subjects (N = 20) took the multiple choice quiz (Pre-test) assessing their pre-existing knowledge of the lesson topic. They were then scanned in fMRI watching the 32-min Intact Lesson that was previously recorded by the teacher. On Day 2, subjects watched the 6-min Review, again previously recorded by the teacher, completed additional scans that were not analyzed here and then answered the same 25-question quiz (Post-test). Stimuli were presented and viewed in an identical setup as during the teacher sessions. Audio was played through MRI-compatible insert earphones (Sensimetrics models S14 and S15). Following scanning, students were asked to rate how closely they attended to the video on a scale of 1–5 (1 = not at all attentive and 5 = extremely attentive).
Student sessions: scrambled lesson control
To test that neural coupling between the teacher and students reflected shared processing of lesson content, rather than shared perception of low-level stimulus features, a second sample of subjects (N = 20) was scanned while watching a Scrambled Lesson control. In this control, the 32-min Intact Lesson was scrambled at the sentence level, while the text on the slides was scrambled at the word level. In the Scrambled Lesson control, on Day 1, students took the Pre-test, were scanned watching the Scrambled Lesson and then rated their attention. On Day 2, they took the Post-test outside of the scanner.
MRI acquisition
Subjects were scanned in a 3T Siemens Magnetom Skyra scanner at the Princeton Neuroscience Institute using a 64-channel head/neck coil (Siemens). During functional scans, volumes were acquired using a T2*-weighted gradient-echo echo-planar pulse sequence (TR 2000 ms; echo time (TE) 28 ms; voxel size 3 × 3 × 3mm with 38 slices; flip angle 80°; FOV 192 × 192 mm2; matrix size 64 × 64; slice orientation axial; anterior-to-posterior phase encoding; interleaved slice acquisition; iPAT GRAPPA 2) with whole-brain coverage. A high-resolution anatomical image was collected using a T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE) pulse sequence (repetition time (TR) 2300 ms; TE 2.98 ms; TI 900 ms; voxel size 1 × 1 × 1mm with 176 slices; flip angle 9°; field of view (FOV) 256 × 256 mm2; slice orientation axial; no fat suppression).
MRI data analysis
Preprocessing
MRI data were preprocessed using FSL 5.0 and FEAT 6.0 [FMRIB, Oxford; (Jenkinson et al., 2012)] including motion correction, linear trend removal, high-pass filtering (cutoff: 140s or ∼.007 Hz) and spatial smoothing with a 6 mm Full width half max (FWHM) Gaussian kernel. Motion correction was performed using FSL’s MCFLIRT with six degrees of freedom. Subjects with excessive head motion (>3 mm absolute displacement) were discarded from the analysis. Functional data were registered to high-resolution structural images and then to 3 mm Montreal Neurological Institute (MNI) standard space using FSL’s FLIRT with 12 degrees of freedom. Preprocessed data were z-scored over time. All analyses were conducted in volume space using custom MATLAB (R2018a) and Python 3.0 scripts and visualized using nilearn (https://nilearn.github.io; Abraham et al., 2014) and NeuroElf v1.1 (https://neuroelf.net).
Temporal interpolation procedure for teacher scans
Teacher scans of the Lesson and Review were separately averaged to provide a more reliable measure of the Teacher’s neural responses. The Teacher was extensively trained to reproduce the same Lesson and Review with high fidelity in timing and word use. To account for natural and minor variation in timing, the recorded Lessons and Reviews were timestamped at the approximate sentence level (137 segments, mean length = 13.78 s, s.d. = 4.11 s). The teacher’s neural response was then linearly interpolated within each timestamped sentence in order to align stimulus content over time relative to the actual recordings presented to the student subjects. The interpolated teacher’s neural responses were then averaged and used in subsequent analyses.
Unscrambling procedure for scrambled lesson control
The order of sentences in the Scrambled Lesson were randomized, so in order to compute coupled neural responses between the Teacher and Student Scrambled Lesson, the Scrambled Lesson response time series were reordered to match the temporal order of the Intact Lesson. Following (Lerner et al., 2011), sentence-level timestamps were shifted by 6 s (3 TRs) to account for the hemodynamic response function and then the response time series were segmented by sentence and reordered. Sentences less than 6 s long were removed from analysis (original stimulus length: 945 TRs; reordered scrambled stimulus length: 658 TRs). The resulting reordered time series was used in the Teacher–Student ‘Unscrambled’ Lesson analyses described below. The full, original scrambled time series was used in Student–Student Scrambled analyses.
Intersubject correlation
Shared neural responses among subjects were measured using intersubject correlation (ISC; Hasson et al., 2004; Nastase et al., 2019). Four ISC analyses were conducted: (i) among students in the Intact Lesson and Review (Student–Student), (ii) among the scrambled students in the Scrambled Lesson control (Student–Student Scrambled), (iii) between the teacher and the students in the Intact Lesson and Review (Teacher–Student) and (iv) between the teacher and the students in the Scrambled Lesson control (Teacher–Student Unscrambled). ISC was calculated at each voxel within an anatomically defined gray matter mask. For within-group analyses among students (Comparisons 1 and 2), ISC was calculated using the leave-one-out approach: each student’s response time course was correlated to the average response time course of the remaining (N − 1) students at each voxel. ISC was separately calculated among the students in the Intact Learning condition for both Lesson and Review and among the students in the Scrambled Control condition for the Scrambled Lesson only. The same analysis was repeated for the between-group Student and Teacher analyses (Comparisons 3 and 4). In these analyses, ISC was calculated by correlating each student’s response time series to the average Teacher response time series.
Statistical significance of ISC was assessed using a bootstrap hypothesis test (Chen et al., 2016; Nastase et al. 2019). In each iteration, we randomly sampled N = 20 subject ISC values with replacement and then computed the mean of the sample. We repeated this procedure 10 000 times, producing a bootstrapped distribution around the mean ISC value across subjects. We then subtracted the observed mean ISC value from the bootstrap distribution to create a null distribution and used this null distribution to calculate P-values. We corrected for multiple comparisons across voxels by controlling the false discovery rate (FDR) (Benjamini and Hochberg, 1995) at q = 0.05.
ISC in lesson vs review
We predicted that Teacher–Student coupling during the review will be greater than during the lesson as the initial lesson serves to build a shared knowledge background. To test this prediction, we used paired t-tests (two-tailed) to compare Teacher–Student ISC during the Intact Lesson condition vs the Review condition in every region of a 61-region parcellation comprising regions of the brain that respond reliably to naturalistic audiovisual stimuli (Regev et al., 2018). We also directly compared Student–Student ISC in the two conditions. Statistical significance was assessed using a sign test: in each permutation, the sign of the difference in the two conditions was randomly flipped, and the t-value was calculated under the null hypothesis of no systematic difference between conditions. This procedure was repeated 10 000 times to produce a permutation-based null distribution, which was used to calculate P-values. We corrected for multiple comparisons using FDR (q = 0.05).
Correlation with learning
To test the prediction that the level of shared neural responses will be correlated with learning, we first calculated a normalized measure of learning outcome: normalized score = (Post-test − Pre-test) × mean(Post-test + Pre-test). This measure incorporates both improvement in score and total score. Two students may improve by the same amount, but a student who has a higher total score, indicating greater understanding of the material, will have a higher normalized score.
We correlated both Student–Student ISC and Teacher–Student ISC in the Intact Lesson and Review in each of the 61 regions of interest (ROIs) with the normalized score. Statistical significance of the ISC-normalized score correlation was assessed using a permutation test. In each permutation, the ISC values were randomly shuffled across subjects and correlated with the normalized scores. This procedure was repeated 10 000 times, producing a permutation-based null distribution (under the null hypothesis of no systematic relationship between ISC values and learning scores across subjects). We corrected for multiple comparisons using FDR (q = 0.05).
Lagged correlation
In prior research of verbal communication, the listener’s neural response follows the speaker’s neural response with a several second lag in several high-level DMN regions, including temporoparietal junction (TPJ) and posterior medial cortex (PMC; Stephens et al., 2010; Silbert et al., 2014; Zadbood et al., 2017). In early sensory areas, however, there is no lag between speaker and listener, suggesting that the lag in higher-order areas reflects the flow of information from the speaker’s brain to the listener’s brain. We therefore tested for a lag between student and teacher neural responses during the Intact Lesson posterior cingulate cortex (PCC), by shifting the Students’ time series by −10 to +10 TRs relative to the teacher’s time series and calculating ISC at each lag. We also conducted the same analysis in V1+ as a control. The same analysis was also repeated for Student–Student ISC in the Intact Lesson by shifting each subject relative to the average of others.
Results
Behavioral results
Subjects in the Intact Learning condition improved significantly from the Pre-test to the Post-test Quiz [t(19) = 10.35, P < 0.001], scoring on average 48.8% (s.d. = 12.2%) on the Pre-test and 87.2% (s.d. = 17.5%) on the Post-test (Figure 2A). All subjects in the Intact Learning condition improved their score. Subjects in the Scrambled Lesson control condition also significantly improved their scores [Pre-test: mean = 47.4%, s.d. = 1 3.3%; Post-test: 59.4%, s.d. = 14.1%; t(19) = 3.47, P = 0.003], but significantly less than the subjects in the Intact Learning condition [t(38) = 5.21, P < 0.001]. The two groups did not differ significantly in Pre-test scores [t(38) = 0.345, P = 0.73], indicating a similar baseline of prior knowledge.
Fig. 2.
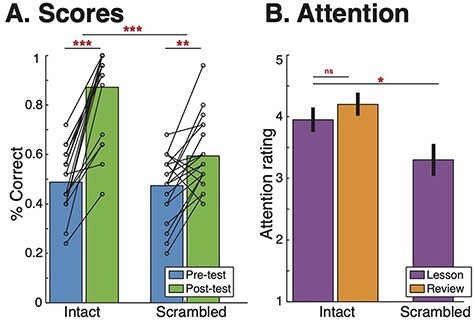
Behavior. (A) Students in both the Intact and Scrambled conditions significantly improved their scores from Pre-test to Post-test [Intact: t(19) = 10.35, P < 0.00001; Scrambled: [t(19) = 3.47, P = 0.0026], but Students learned significantly more in the Intact condition [t(38) = 5.21, P < 0.00001]. (B) On average, students were very attentive during the videos, but students in the Intact Lesson were more attentive than students in the Scrambled lesson [t(38) = 2.27, P = 0.029]. ***P < 0.00001, **P < 0.01, *P < 0.05.
Subjects were attentive throughout the video lessons (Figure 2B). On a five-point scale (1 = not at all attentive and 5 = extremely attentive), subjects in the Intact Learning condition rated their attention during the Lesson on average to be 3.95 (s.d. = 0.76) and during the Review to be 4.20 (s.d. = 0.70). There was no significant difference between attention during the Lesson and the Review [t(19) = 1.56, P = 0.14]. During the Scrambled Lesson control, subjects rated their attention as 3.30 (s.d. = 1.03), which is significantly lower than the subjects in the Intact Lesson [t(38) = 2.27, P = 0.029].
fMRI results
Aligned neural responses among students during learning
We first identified regions of the brain that are significantly correlated responses across students during the Lesson and Review in the Intact Learning condition. Consistent with previous work using audiovisual narrative stimuli, we observe widespread significant ISC during both the Lesson and Review (q < 0.05, FDR corrected voxelwise; Figure 3A) throughout much of visual cortex, auditory and linguistic regions from early auditory cortex (A1+) to superior temporal gyrus (STG) and middle temporal gyrus (MTG), and extending into higher-order DMN regions including PMC, bilateral dorsolateral prefrontal cortex (dlPFC) and right medial prefrontal cortex (mPFC). However, unlike previous work on narrative processing, we also observe strong ISC in bilateral superior parietal lobule (SPL). There were no significant differences between Student–Student ISC during the Lesson vs Review.
Fig. 3.
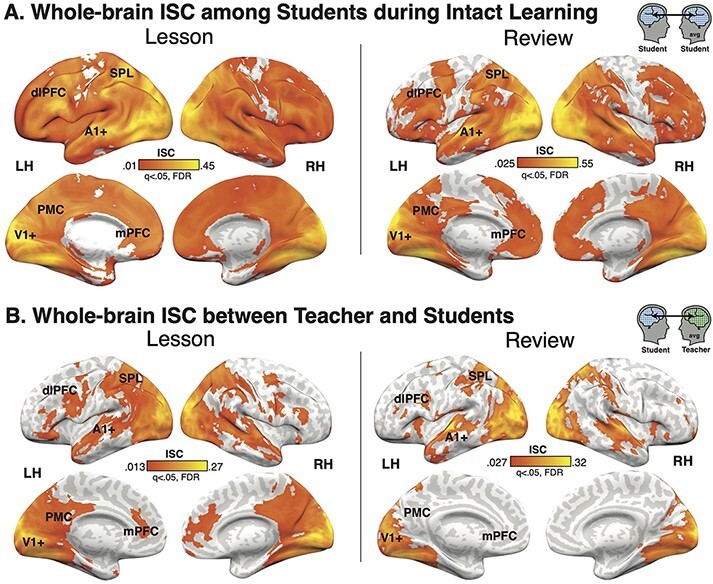
ISC. (A) Students in the Intact Learning condition have significant ISC throughout the cortex in both the Lesson and Review, with the strongest ISC in visual and auditory cortex, superior and middle temporal gyrus, bilateral SPL, bilateral dlPFC, and right mPFC. (B) Student neural responses are coupled to the teacher’s response in similar regions. Non-parametric bootstrap hypothesis test; q < 0.05, FDR corrected.
Teacher–student neural coupling during teaching and learning
We next calculated ISC between the Teacher and Student neural responses in the Intact Learning condition to identify regions of the brain that are coupled during teaching and learning (Figure 3B). Similar to speaker–listener coupling during narrative storytelling, significant Teacher–Student ISC was observed in early sensory cortices; linguistic and extralinguistic regions including STG, MTG and temporal pole; and DMN regions including PMC, dlPFC and mPFC. Unlike during narrative storytelling, we additionally observe significant ISC in bilateral SPL and notably weak ISC in bilateral TPJ.
No significant differences were observed between Teacher–Student ISC during the Intact Lesson vs Review. However, the substantially different durations of the Lesson and Review may undermine this comparison or obscure differences in Teacher–Student ISC from the start vs end of the Lesson compared to the Review. We therefore compared Teacher–Student ISC from the first and last 6 min of the Lesson to Teacher–Student ISC in the Review (Supplementary Figure S1). We observed that Teacher–Student ISC in the first 6 min of the Lesson was significantly larger than during the Review in medial visual areas and precuneus (Supplementary Figure S1A). In contrast, Teacher–Student ISC was larger during the Review than the last 6 min of the Lesson in STG, MTG and SPL (Supplementary Figure S1B).
The lagged correlation analysis does not indicate a lag between the Teacher and Students’ neural responses. In both V1 and PCC, Student–Student and Teacher–Student ISC peaks at lag = 0.
Teacher–Student ISC emerges in DMN only during intact learning
Shared neural responses between the Teacher and Students could be driven by shared sensory input rather than shared high-level understanding of the lesson content as the Teacher and Students view and hear the same stimuli. To dissociate content-related shared responses from shared sensory responses, we scanned an additional group of students who watched a temporally scrambled version of the Lesson. This manipulation preserves the low-level visual and auditory properties of the stimulus while disrupting conceptual understanding and reducing learning (Figure 2A).
Consistent with the hypothesis that Teacher–Student coupling in high-order areas reflects shared understanding, not just shared stimulus processing, we observed significant Teacher–Student ISC in DMN regions only in the Intact Learning conditions (Figure 4). These regions include PMC, bilateral angular gyrus, bilateral temporal poles, mPFC and lateral PFC. Teacher–Student Unscrambled ISC largely overlapped with the ISC in the Intact Lesson in visual areas, extending from V1+ to ventral-temporal cortex and dorsal occipital regions. In addition, however, there was significant Teacher–Student Unscrambled ISC in attention-related regions of dorsal PFC that did not overlap with the Intact Lesson ISC map.
Fig. 4.
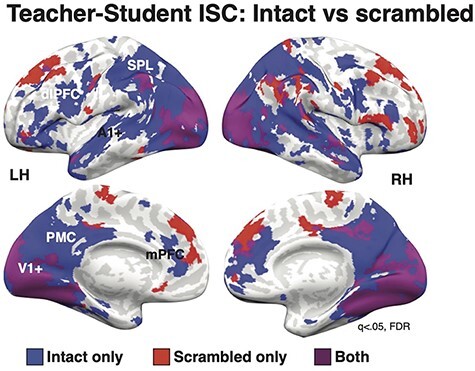
Scrambled vs intact ISC. ISC in both the Teacher–Intact Student and Teacher–Scrambled Student conditions were significant in visual cortex. However, only the Intact condition showed significant ISC in high-level DMN regions, including PMC, MPFC and dlPFC. Surprisingly, the Scrambled condition also showed significant ISC in dorsal PFC. q < 0.05, FDR corrected.
Finally, there were no differences in mean framewise displacement between the students in the Intact condition and the Scrambled Lesson [t(38) = 1.48, P = 0.146], suggesting that the differences in Teacher–Student coupling cannot be attributed to group-level differences in motion during the scans.
Teacher–Student ISC correlates with learning
If Teacher–Student coupling reflects shared understanding of novel information, we also expect that coupling in high-level regions, but not low-level sensory regions, will be related to learning outcomes. We therefore correlated both Student–Student and Teacher–Student ISC with a measure of learning outcomes (improvement in score weighted by total score) across each of 61 areas from an independent parcellation (Regev et al., 2018). The analysis revealed seven regions with a significant correlation between Teacher–Student ISC during the lesson and learning outcomes (Figure 5, FDR corrected). Five regions were located primarily in posterior medial cortex: PCC (r = 0.663, P = 0.002), right precuneus (rPCUN; r = 0.626, P = 0.003), left precuneus (lPCUN; r = 0.751, P < 0.001), superior occipital gyrus (SOG; r = 0.634, P = 0.003) and dorsal precuneus (dPCUN; r = 0.755, P < 0.001). Two regions were in high-order visual cortex: left lateral occipital complex (lLOC; r = 0.633, P = 0.003) and right human V4 (rhV4; r = 0.619, P = 0.004). Notably, these areas largely overlap with regions that only show significant Teacher–Student coupling in the Intact Learning condition. There were no significant correlations between Teacher–Student coupling and learning outcomes in the Scrambled Control condition (P > 0.05) in any of the ROIs. Across ROIs, the correlation between Teacher–Student coupling and learning was significantly greater in the Intact Learning condition than the Scrambled Control condition [t(120) = 5.56, P < 0.0001].
Fig. 5.
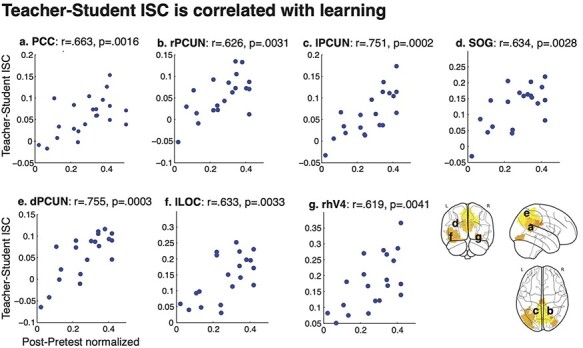
ISC correlation with behavior. Teacher–student ISC is correlated with normalized improvement in quiz score [Post-Pre/(1/avg(Post + Pre)] in five ROIs, primarily in posterior medical cortex (q < 0.05, FDR corrected). ROIs from 61 ROI parcellation (Regev et al., 2018).
While the correlation between Student–Student ISC and improvement in quiz score showed a similar pattern of effects, no region passed FDR correction. There was no relationship between learning and either Student–Student or Teacher–Student ISC during the review.
Discussion
Teaching and learning are key processes by which humans build shared knowledge (Hasson et al., 2012; Hasson and Frith, 2016). Here, we report the first fMRI study to identify regions of the DMN that are coupled between teachers and students during naturalistic teaching and learning of novel information and that predict learning outcomes. fMRI in particular allows for full-brain coverage with high spatial resolution and, unlike previously used methods such as EEG, allows for measurements from medial regions of interest in the DMN. Moreover, we employ an extended lecture on a single scientific topic, more closely mimicking the typical college-level lecture than the short lessons on unrelated topics that have previously been used. Finally, we recorded the teacher’s brain as she delivered the lessons over multiple sessions, increasing the measurement reliability of the teacher’s neural responses.
We observe teacher–student coupling across the brain, extending from sensory cortices to high-level areas including DMN. While teacher–student alignment in early sensory regions is likely driven by shared stimulus features, our results suggest that alignment in higher-order regions is driven by shared understanding of lesson content. First, when learning was disrupted by temporally scrambling the lesson, teacher–student coupling was only observed in early visual and linguistic regions, consistent with shared processing of stimulus features on short timescales of a few seconds (Hasson et al., 2008; Lerner et al., 2011; Chen et al., 2015). Teacher–student alignment in DMN and bilateral SPL only emerged during intact learning, consistent with these regions supporting integration of information over longer timescales of many minutes (Hasson et al., 2008; Lerner et al., 2011; Honey et al., 2012a; Stephens et al., 2013) and high-level context-dependent understanding (Honey et al., 2012b; Regev et al., 2013 p. 2; Ames et al., 2014; Yeshurun et al., 2017; Nguyen et al., 2019). Second, teacher–student coupling in PMC during the intact lesson significantly predicted learning outcomes, suggesting that students who learn the most are the ones who are most aligned with the teacher. Notably, PMC was also among the regions that only showed teacher–student alignment during intact learning. Our findings are consistent with several recent teacher–student hyperscanning studies: in EEG, teacher–student synchrony in the alpha band (8–12 Hz) is correlated with delayed learning outcomes (Davidesco et al., 2019), while fNIRS have found an association between inferior frontal regions and superior temporal regions (Pan et al., 2018, 2020; Zheng et al., 2018).
Teacher–student coupling was observed in many of the same regions that show coupled responses between speaker and listener during narrative storytelling (Stephens et al., 2010; Dikker et al., 2014; Silbert et al., 2014). These regions include linguistic regions STS and STG extending to the temporal poles and DMN regions including PMC, mPFC and dlPFC. However, unlike during narrative storytelling, and in accordance with the scientific nature of our lecture, we also observed teacher–student alignment in bilateral SPL, which has been implicated in mental rotation, geometry and mathematical symbols (Gogos et al., 2010; Prescott et al., 2010; Zhang et al., 2012; Harvey et al., 2013), as well as the manipulation and rearrangement of information in working memory (Koenigs et al., 2009). We also do not observe teacher–student coupling in bilateral TPJ, a region of the brain that is strongly implicated in mentalizing and theory of mind (Saxe and Kanwisher, 2003; Schilbach et al., 2008; Mars et al., 2012), suggesting that aspects of speaker–listener coupling are content-specific.
Interestingly, we also observe several regions in which teacher–student coupling is observed during the scrambled condition but not the intact learning condition, notably in dorsal prefrontal cortex. Subjects in the scrambled condition may have needed to exert greater attention and cognitive control in order to attempt to make sense of the confusing scrambled lesson than the subjects in the intact learning condition, resulting in greater recruitment and alignment of these regions. Indeed, these regions overlap with the frontoparietal control network that is implicated in the executive control and attention and notably shows strong functional connectivity with the DMN during difficult tasks requiring visuospatial attention (Dixon et al., 2018).
Unlike previous studies (Stephens et al., 2010; Silbert et al., 2014; Zadbood et al., 2017; Davidesco et al., 2019), we did not observe a reliable temporal lag between the teacher’s and the listener’s neural responses. The time-locked visual stimulus viewed by both the teacher and the students may have exerted a strong bottom-up visual signal that was absent in narrative storytelling studies that used auditory-only stimuli. In addition, the low temporal resolution of fMRI and the temporal interpolation procedure for averaging teacher sessions may obscure a temporal lag. Indeed, a recent EEG study with both visual and auditory processing identified a short, 200-ms lag between the teacher and student neural responses (Davidesco et al., 2019).
We also did not observe significant differences in the level of neural coupling between the first Lesson video and the second Review video. Based on previous research suggesting that context increases neural coupling, we had hypothesized that the initial lesson would serve to create a shared knowledge base, which would then be reflected in greater student–student and teacher–student alignment during the review than during the lesson. The lack of significant differences between two scans could be due to countervailing differences in the two contexts. For example, this result may reflect the nature of teaching: a teacher gradually builds common ground with students by connecting novel ideas to basic concepts. Using this ‘scaffolding’ approach, the teacher should effectively guide the student’s learning process to minimize gaps in understanding, thus reducing the difference in coupling between learning and review.
Overall, these findings suggest that neural coupling between teachers and students can be used as an index of learning, or shared understanding. While the precise biological processes that give rise to neural alignment has not yet been elucidated, in a recent review (Yeshurun et al., 2021), we propose that neural alignment in the DMN reflects shared representation of semantic knowledge, shaped by both the external stimuli and internal representations that may vary substantially across individuals. On the one hand, here we show that the DMN is robustly driven by the Lesson and Review, two exogenous, naturalistic stimuli. This finding is consistent with converging evidence that synchronized neural activity across individuals in the DMN is locked to the content of the stimulus (Hasson et al., 2008; Simony et al., 2016; Nastase et al., 2019; Finn et al., 2020). On the other hand, we also show that activity in the DMN is modulated by internal representations, or the individual student’s level of understanding. Indeed, several recent studies have shown that DMN is modulated by internal representations (context, background, memory, interpretation and personality traits) that may vary significantly across individuals (Yeshurun et al., 2017; Finn et al., 2018; Cetron et al., 2019; Nguyen et al., 2019). By taking into account an individual’s personal background and societal context, we suggest that activity in the DMN reflects both shared and idiosyncratic components of making sense of the external world. In other words, shared understanding of a stimulus across individuals is reflected in shared neural responses in the DMN.
Teaching is therefore a process of building or creating this shared semantic knowledge and aligning representations between students (novices) and teachers (experts). For some concepts (e.g. ‘net magnetization’ or ‘action potential’), students may be starting from scratch, requiring teachers to build up new concepts from their existing semantic or conceptual knowledge. In other cases, however, teaching may require shifting existing concepts to new contexts. For example, the word ‘BOLD’ in everyday life describes a fearless or daring person or idea. In fMRI, however, ‘BOLD’ refers to the blood-oxygen-level-dependent response. Teachers (or experts) may be able to flexibly shift between conceptual representations of the word ‘BOLD’ while students (or novices) will need to more laboriously shift representations from ‘fearless’ to ‘fMRI’. Alignment of conceptual knowledge and shared understanding of terms is then reflected in shared neural responses between teachers and students.
Joint attention also likely has a role in synchronizing neural responses among individuals (Zion Golumbic et al., 2013; Dikker et al., 2021; Schmälzle et al., 2015; Cohen et al., 2018; Regev et al., 2018). For example, students who learn most effectively likely attend to the same aspects of the lesson that the teacher is. This joint attention may facilitate learning. Indeed, some have suggested that joint attention to stimulus features during processing of naturalistic stimuli may underlie coupled neural responses (Cohen et al., 2018; Dikker et al., 2021). Several studies show that subjects who are more engaged and pay greater attention to the stimulus show better comprehension and greater alignment of neural responses in the DMN (Cohen and Parra, 2016; Ki et al., 2016; Cohen et al., 2018), suggesting that shared attention to stimulus content may facilitate shared understanding and shared neural processing. Moreover, an intriguing new study suggests that attention can modulate the processing and propagation of information from early sensory areas up to the DMN (Regev et al., 2018). This study presented two unrelated narratives at the same time, but in different modalities: written and spoken. Subjects were directed to attend to one narrative only, and the researchers found that neural alignment emerged in the DMN only among participants attending the same narrative. Processing of the unattended narrative was primarily restricted to sensory cortices. Joint attention may therefore play a critical role in facilitating shared understanding and eliciting shared neural responses in the DMN during learning.
Limitations and future directions
Due to limitations in the study design, subjects in the control study only watched the scrambled lesson and did not also see a scrambled version of the review. The difference in learning time, even disrupted by the scrambling, may have contributed to the lower post-test scores in this group. Similarly, decreased attention during the scrambled lesson may have also contributed to decreased learning. Decreased attention may also partially explain the decreased neural coupling between the teacher and students in the scrambled control condition compared to the intact learning. Indeed, several studies have previously found that increased attention is associated with increased alignment (Cohen and Parra, 2016; Ki et al., 2016; Cohen et al., 2018). However, we also observe that Teacher–Student coupling in sub-regions of the DMN is correlated with learning outcomes. Thus, while shared attention is necessary for shared understanding, both likely play a role in inducing neural coupling.
Future studies are needed to further explore the building and shifting of shared representations of concepts over time. While we do not observe differences in teacher–student alignment between lesson and review, studies over longer time periods (e.g. over a semester of classes) or using higher temporal resolution imaging coupled with more frequent measures of learning may be able to detect changes in neural alignment reflecting changes in conceptual alignment. Work with higher temporal resolution could also using sliding window correlations to identify changes in coupling within a lesson. These studies and others could also dissect the content of learning by taking advantage of semantic embeddings (e.g. Mikolov et al., 2013). In addition, future studies are needed to further explore the effects of joint attention during interactive learning and teaching using hyperscanning. During in-person teaching, teachers may be able to adjust their style and explanation following real-time student feedback, enabling better teacher–student alignment and hence learning in contrast to no-feedback conditions. Finally, future studies may consider applying computational modeling approaches to these kinds of inter-subject neuroimaging studies of communication. As described in a recent commentary (Bolis and Schilbach, 2017), such an approach could enable researchers to better disentangle and understand the contributions of the environment, the individuals and their interactions to neural responses during social communication.
Conclusions
This work provides evidence that shared understanding of technical, non-narrative information during teaching and learning is reflected in the coupled neural responses between teachers and students in the DMN and other high-level regions. These findings speak to the flexibility and importance of the DMN in integrating novel information over long time periods and provide insight into how our brains build a shared understanding via communication.
Supplementary Material
Contributor Information
Mai Nguyen, Department of Psychology, Princeton University, Princeton, NJ 08540, USA.
Ashley Chang, Department of Psychology, Princeton University, Princeton, NJ 08540, USA.
Emily Micciche, Department of Psychology, Vanderbilt University, Nashville, TN 37240, USA.
Meir Meshulam, Princeton Neuroscience Institute, Princeton University, Princeton, NJ 08540, USA.
Samuel A Nastase, Princeton Neuroscience Institute, Princeton University, Princeton, NJ 08540, USA.
Uri Hasson, Department of Psychology, Princeton University, Princeton, NJ 08540, USA; Princeton Neuroscience Institute, Princeton University, Princeton, NJ 08540, USA.
Acknolwedgements
We thank the entire Hasson Lab for helpful feedback on the analysis and paper.
Funding
This work was supported by the National Institute of Health [R01MH112566-01 to M.N., E.M. and S.N.; 5DP1HD091948-92 to M.M. and U.H.] and Princeton ReMatch+ Summer Funding [to A.C.].
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
Author contributions
M.N. and U.H. designed research; M.N., A.C. and E.M. performed the research; M.N., M.M. and S.N. contributed analytic tools; and all authors wrote the paper.
Supplementary data
Supplementary data are available at SCAN online.
References
- Abraham A., Pedregosa F., Eickenberg M., et al. (2014). Machine learning for neuroimaging with scikit-learn. Frontiers inNeuroinformatics, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames D.L., Honey C.J., Chow M.A., Todorov A., Hasson U. (2014). Contextual alignment of cognitive and neural dynamics. Journal of Cognitive Neuroscience, 27, 655–64. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B: Statistical Methodology, 57, 289–300. [Google Scholar]
- Bevilacqua D., et al. (2019). Brain-to-brain synchrony and learning outcomes vary by Student—Teacher dynamics: evidence from a real-world classroom electroencephalography study. Journal of Cognitive Neuroscience, 31, 401–11. [DOI] [PubMed] [Google Scholar]
- Bolis D., Schilbach L. (2017). Beyond one bayesian brain: modeling intra- and inter-personal processes during social interaction: commentary on “Mentalizing homeostasis: The social origins of interoceptive inference” by Fotopoulou & Tsakiris. Neuropsychoanalysis, 19, 35–8. [Google Scholar]
- Cetron J.S., Connolly A.C., Diamond S.G., May V.V., Haxby J.V., Kraemer D.J.M. (2019). Decoding individual differences in STEM learning from functional MRI data. Nature Communications, 10, 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Shin Y.-W., Taylor P.A., et al. (2016). Untangling the relatedness among correlations, part I: nonparametric approaches to inter-subject correlation analysis at the group level. NeuroImage, 142, 248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Honey C.J., Simony E., Arcaro M.J., Norman K.A., Hasson U. (2015). Accessing real-life episodic information from minutes versus hours earlier modulates hippocampal and high-order cortical dynamics. CerebralCortex, 26, 3428–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.S., Madsen J., Touchan G., et al. (2018). Neural engagement with online educational videos predicts learning performance for individual students. Neurobiology of Learning and Memory, 155, 60–4. [DOI] [PubMed] [Google Scholar]
- Cohen S.S., Parra L.C. (2016). Memorable audiovisual narratives synchronize sensory and supramodal neural responses. eNeuro, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidesco I., Laurent E., Valk H., et al. (2019). Brain-to-brain synchrony between students and teachers predicts learning outcomes. bioRxiv, 644047. [DOI] [PubMed] [Google Scholar]
- Dikker S., Silbert L.J., Hasson U., Zevin J.D. (2014). On the same wavelength: predictable language enhances speaker-listener brain-to-brain synchrony in posterior superior temporal gyrus. Journal of Neuroscience, 34, 6267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikker S., Michalareas G., Oostrik M., et al. (2021). Crowdsourcing neuroscience: inter-brain coupling during face-to-face interactions outside the laboratory. Neuroscience, 227, 117436. [DOI] [PubMed] [Google Scholar]
- Dixon M. L., et al. (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences, 201715766.doi: 10.1073/pnas.1715766115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Corlett P.R., Chen G., Bandettini P.A., Constable R.T. (2018). Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. Nature Communications, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., et al. (2020). Idiosynchrony: from shared responses to individual differences during naturalistic neuroimaging. NeuroImage, 215, 116828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A., Gavrilescu M., Davison S., et al. (2010). Greater superior than inferior parietal lobule activation with increasing rotation angle during mental rotation: an fMRI study. Neuropsychologia, 48, 529–35. [DOI] [PubMed] [Google Scholar]
- Harvey B.M., Klein B.P., Petridou N., Dumoulin S.O. (2013). Topographic representation of numerosity in the human parietal cortex. Science, 341, 1123–6. [DOI] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303, 1634–40. [DOI] [PubMed] [Google Scholar]
- Hasson U., Yang E., Vallines I., Heeger D.J., Rubin N. (2008). A hierarchy of temporal receptive windows in human cortex. Journal of Neuroscience, 28, 2539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A.A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16, 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Chen J., Honey C.J. (2015). Hierarchical process memory: memory as an integral component of information processing. Trends in Cognitive Sciences, 19, 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Frith C.D. (2016). Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philosophical Transactions of the Royal SocietyB: Biological Sciences, 371, 20150366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holper L., et al. (2013). The teaching and the learning brain: a cortical hemodynamic marker of teacher—student interactions in the Socratic dialog. International Journal of Educational Research, 59, 1–10. [Google Scholar]
- Honey C.J., Thesen T., Donner T.H., et al. (2012a). Slow cortical dynamics and the accumulation of information over long timescales. Neuron, 76, 423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C.J., Thompson C.R., Lerner Y., Hasson U. (2012b). Not lost in translation: neural responses shared across languages. Journal of Neuroscience, 32, 15277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. (2012). FSL. NeuroImage, 62, 782–90. [DOI] [PubMed] [Google Scholar]
- Ki J.J., Kelly S.P., Parra L.C. (2016). Attention strongly modulates reliability of neural responses to naturalistic Narrative Stimuli. Journal of Neuroscience, 36, 3092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Barbey A.K., Postle B.R., Grafman J. (2009). Superior parietal cortex is critical for the manipulation of information in working memory. Journal of Neuroscience, 29, 14980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y., Honey C.J., Silbert L.J., Hasson U. (2011). Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci Off J Soc Neurosci, 31, 2906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R.B., Neubert F.-X., Noonan M.P., Sallet J., Toni I., Rushworth M.F.S. (2012). On the relationship between the ‘default mode network’ and the ‘social brain’. Frontiers in Human Neuroscience, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolov T., Sutskever I., Chen K., Corrado G.S., Dean J. (2013). Distributed representations of words and phrases and their compositionality. In: Burges, C.J.C., Bottou, L., Welling, M., Ghahramani, Z., Weinberger, K.Q., editors. Advances in Neural Information Processing Systems. Vol. 26, Red Hook, NY: Curran Associates, Inc, 3111–9. [Google Scholar]
- Nastase S.A., Gazzola V., Hasson U., Keysers C. (2019). Measuring shared responses across subjects using intersubject correlation. Social Cognitive and Affective Neuroscience, 14, 667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastase S.A., Liu Y.-F., Hillman H., Norman K.A., Hasson U. (2020). Leveraging shared connectivity to aggregate heterogeneous datasets into a common response space. NeuroImage, 217, 116865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M., Vanderwal T., Hasson U. (2019). Shared understanding of narratives is correlated with shared neural responses. NeuroImage, 184, 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren N., Shapira-Lichter I., Lerner Y., et al. (2017). Schema benefit vs. proactive interference: contradicting behavioral outcomes and coexisting neural patterns. NeuroImage, 158, 271–81. [DOI] [PubMed] [Google Scholar]
- Pan Y., Novembre G., Song B., Li X., Hu Y. (2018). Interpersonal synchronization of inferior frontal cortices tracks social interactive learning of a song. NeuroImage, 183, 280–90. [DOI] [PubMed] [Google Scholar]
- Pan Y., Dikker S., Goldstein P., Zhu Y., Yang C., Hu Y. (2020). Instructor-learner brain coupling discriminates between instructional approaches and predicts learning. NeuroImage, 211, 116657. [DOI] [PubMed] [Google Scholar]
- Peirce J., Gray J.R., Simpson S., et al. (2019). PsychoPy2: experiments in behavior made easy. Behavior ResearchMethods, 51, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J., Gavrilescu M., Cunnington R., O’Boyle M.W., Egan G.F. (2010). Enhanced brain connectivity in math-gifted adolescents: an fMRI study using mental rotation. Cognitive Neuroscience, 1, 277–88. [DOI] [PubMed] [Google Scholar]
- Regev M., Honey C.J., Simony E., Hasson U. (2013). Selective and invariant neural responses to spoken and written narratives. Journal of Neuroscience, 33, 15978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev M., Simony E., Lee K., Tan K.M., Chen J., Hasson U. (2018). Propagation of information along the cortical hierarchy as a function of attention while reading and listening to stories. Cerebral Cortex (New York, N.Y.: 1991), 29, 4017–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people: the role of the temporo-parietal junction in ‘theory of mind’. NeuroImage, 19, 1835–42. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S.B., Rotarska-Jagiela A., Fink G.R., Vogeley K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the ‘default system’ of the brain. Consciousness and Cognition, 17, 457–67. [DOI] [PubMed] [Google Scholar]
- Schmälzle R., Häcker F.E.K., Honey C.J., Hasson U. (2015). Engaged listeners: shared neural processing of powerful political speeches. Social Cognitive and Affective Neuroscience, 10, 1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert L.J., Honey C.J., Simony E., Poeppel D., Hasson U. (2014). Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proceedings of the National Academy of Sciences, 111, E4687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E., Honey C.J., Chen J., et al. (2016). Dynamic reconfiguration of the default mode network during narrative comprehension. Nature Communications, 7, 12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens G.J., Silbert L.J., Hasson U. (2010). Speaker-listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences, 107, 14425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens G.J., Honey C.J., Hasson U. (2013). A place for time: the spatiotemporal structure of neural dynamics during natural audition. Journal of Neurophysiology, 110, 2019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M.T.R.V., Fernández G., Norris D.G., Hermans E.J. (2010). Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences, 107, 7550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodrahalli K., Chen P.-H., Liang Y., et al. (2018). Mapping between fMRI responses to movies and their natural language annotations. NeuroImage, 180, 223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Swanson S., Simony E., et al. (2017). Same story, different story: the neural representation of interpretive frameworks. Psychological Science, 28, 307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Nguyen M., Hasson U. (2021). The default mode network: where the idiosyncratic self meets the shared social world. Nature Reviews Neuroscience, 22, 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadbood A., Chen J., Leong Y.C., Norman K.A., Hasson U. (2017). How we transmit memories to other brains: constructing shared neural representations via communication. Cerebral Cortex (New York, N.Y.: 1991), 27, 4988–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen C., Zhou X. (2012). Neural correlates of numbers and mathematical terms. NeuroImage, 60, 230–40. [DOI] [PubMed] [Google Scholar]
- Zheng L., Chen C., Liu W., et al. (2018). Enhancement of teaching outcome through neural prediction of the students’ knowledge state. HumanBrain Mapping, 39, 3046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion Golumbic E.M., Ding N., Bickel S., et al. (2013). Mechanisms underlying selective neuronal tracking of attended speech at a ‘cocktail party’. Neuron, 77, 980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


