Abstract
Background
Though a single nonmedical switch from the originator infliximab (IFX) to a biosimilar is considered effective and safe for most patients with inflammatory bowel disease (IBD), very limited data are available on multiple successive switches.
Methods
We performed a prospective multicenter cohort study of adult IBD patients who underwent 2 switches from the originator IFX to CT-P13 to SB2 (group 1), 1 switch from CT-P13 to SB2 (group 2), and 1 switch from the originator IFX to CT-P13 (group 3). Patients were assessed at 4 and 12 months since the most recent switch for remission using clinical (physician’s assessment) and biochemical (C-reactive protein [CRP], and fecal calprotectin [FC]) measures. Patients discontinuing treatment for ineffectiveness or adverse events before month 12 were imputed as nonremitters.
Results
One hundred seventy-six patients (Crohn’s disease 71%, ulcerative colitis 27.8%, IBD unclassified 1.2%; group 1, 69; group 2, 80; group 3, 27) were included. At 12 months after the most recent switch 76.9% (40 of 52, group 1), 65.7% (46 of 70, group 2) and 76.9% (20 of 26, group 3) of patients were in clinical remission. Treatment persistence at 12 months was 85.0%, 87.0%, and 70.1%, respectively. There were no significant differences in the rate of clinical, CRP, FC remission, or treatment persistence at 12 months between the 3 groups. Infusion reactions occurred in 1.7% of patients (3/176), all in patients with antidrug antibodies from group 2.
Conclusions
Multiple successive switching and switching between biosimilars of IFX seemed to be effective and safe.
Keywords: SB2, CT-P13, multiple switches
Introduction
In recent years, biosimilar tumor necrosis factor (TNF) antagonists have become available, and their use in treating inflammatory bowel diseases (IBD), Crohn’s disease (CD), and ulcerative colitis (UC) is increasingly common.1 The first infliximab (IFX) biosimilar to receive approval was CT-P13 based on data from rheumatoid arthritis2 and ankylosing spondylitis,3 followed by extrapolation to other indications of originator IFX. The second IFX biosimilar, SB2, received authorization based on a pharmacokinetic study in healthy volunteers4 and a study in rheumatoid arthritis.5
The introduction of biosimilars in clinical practice has raised a number of concerns, namely the extrapolation from other indications to IBD and the potential for subtle differences in attributes and tertiary and quaternary structure to result in increased immunogenicity.6 Randomized trials7, 8 and prospective cohort studies9–11 have not found convincing evidence for any of these reservations with single switches from originator to biosimilar. This is reflected in the position statement of the European Crohn’s and Colitis Organization, which describes switching from an originator to a biosimilar as acceptable.12 Biosimilars are cost-effective and may expand patient access to biologics.1 The increasing number of available IFX biosimilars and changes in reimbursement policies inevitably lead to switching between biosimilars or multiple successive switches from originator to different biosimilars. Outcomes for these 2 groups of patients were only reported for small cohorts of no more than 43 patients.13, 14 It is unlikely that an adequately powered randomized controlled trial on multiple switches will ever be performed due to high cost and marginal commercial benefit.
The aim of our study was to evaluate the effectiveness and safety of multiple successive switching from originator to a second IFX biosimilar compared with a single switch from originator to CT-P13 and a single switch between CT-P13 and SB2 in IBD patients.
Materials and Methods
Patients
This was a multicenter prospective cohort study. In the 2 participating nonacademic hospitals (Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands and Martini Ziekenhuis, Groningen, the Netherlands), adult IBD patients underwent a switch from originator IFX to CT-P13 from August 2015 onward as part of routine care. This was followed by a routine switch for economic reasons from CT-P13 to SB2 from July 2018 onward. The dose and interval remained unchanged after the switch unless clinical need dictated otherwise—trough concentrations <3 mg/L in the absence of antidrug antibodies prompted dosing adjustment. The switching procedures yielded 3 groups: patients who successively switched from originator IFX to CT-P13 and finally to SB2, patients who switched from originator IFX to CT-P13, and patients who switched from CT-P13 to SB2. Patients starting treatment with a different form of IFX more than 4 months after the last exposure to the previous form (eg, upon disease flare after treatment discontinuation for stable remission) were excluded from the study.
Study Design and Outcomes
The most recent switch was regarded as the index switch for this study (ie, the switch from CT-P13 to SB2 in patients undergoing multiple successive switching). Patient demographics, disease phenotype, and concomitant IBD medication were recorded at index switch. Patients were followed according to protocolized switch pathways with C-reactive protein (CRP) and fecal calprotectin (FC) measurements before the index switch and at 4 and 12 months after the index switch. Measurements of IFX serum concentrations and antibodies to IFX were performed at the discretion of the treating physician, using a drug-sensitive assay (Sanquin, Amsterdam, the Netherlands).15 Clinical disease activity was assessed at baseline, at 4 months, and at 12 months per the physician’s global assessment. Infusion-related adverse events were prospectively recorded in infusion unit protocols. Information about IBD-related hospitalizations and other adverse events was also recorded prospectively.
The primary efficacy outcome was clinical remission per physician’s assessment without concomitant steroid therapy 12 months since the index switch. Secondary efficacy end points included CRP remission defined as CRP < 5 mg/L, FC remission defined as FC < 250 mg/kg, and time to treatment discontinuation for reasons other than long-term sustained remission. The primary safety end point was the occurrence of infusion reactions, with IBD-related hospitalization and other adverse events as secondary end points. Treatment discontinuation and switchbacks were recorded together with the reason for discontinuation. Patients with missing biochemical measurements at a given time point, and patients discontinuing treatment for long-term sustained remission were censored. Patients discontinuing treatment due to adverse events, nonresponse, or the presence of antidrug antibodies before completing 12 months of follow-up were imputed as nonremitters at subsequent time points.
Statistical Analysis
Continuous variables were presented as medians with interquartile ranges (IQRs) and categorical values as frequency and percentages. The Kruskal-Wallis test was used to compare medians in the 3 treatment groups, and the χ 2 test or Fisher exact test were used for categorical variables. Binary logistic regression was used to identify associations between baseline characteristics and clinical remission at 12 months after the index switch patients who discontinued treatment before that time point for adverse events or inefficacy were imputed as nonremitters. Kaplan-Meier estimates were used to plot treatment persistence for the 3 groups, which was compared using log-rank tests. Cox proportional hazard models were used to evaluate the association between baseline characteristics and time to treatment discontinuation for inefficacy or adverse events. Patients discontinuing treatment for sustained remission were censored in survival analyses, as this was indicative of treatment efficacy. Variables with P < 0.1 in univariable analysis were subjected to multivariable regression. Median serum concentrations of IFX between time baseline and 12 months were compared using the Wilcoxon signed-rank test. Statistical analyses were performed using SPSS, version 26.0 (Armonk, NY, USA), and a 2-tailed P value < 0.05 was considered statistically significant.
Ethical Considerations
All patients provided consent for the switches and the collection of routine clinical and biochemical data; the study was approved by the local ethical committees at both hospitals.
Results
Patients
A total of 193 patients underwent switching, 16 were excluded because they had started a biosimilar after a drug holiday, and 1 was younger than 18 years, which yielded a final cohort of 176 patients (Table 1). Patients undergoing multiple successive switching had a longer disease duration and longer duration of exposure to IFX before the index switch. For the majority of patients (156 of 176; 88.6%), IFX was the first biological drug. At the time of the index switch, patients switching from CT-P13 to SB2 had a lower rate of clinical remission than patients from the other 2 groups.
Table 1.
Patient Characteristics at Index Switch (Most Recent Switch for Patients Undergoing Multiple Successive Switches)
| Variable | Originator to CT-P13 to SB2 (n = 69) | CT-P13 to SB2 (n = 80) | Originator to CT-P13 (n = 27) | P |
|---|---|---|---|---|
| Female, n (%) | 37 (53.6) | 43 (53.8) | 13 (48.1) | 0.87 |
| Age (years), median (IQR) | 44 (32–56) | 39.5 (30–55) | 34 (29–56) | 0.22 |
| Disease type, n (%) | 0.60 | |||
| CD | 49 (71) | 54 (67.5) | 22 (81.5) | |
| UC | 19 (28) | 25 (31.3) | 5 (18.5) | |
| IBD-U | 1 (1.4) | 1 (1.3) | ||
| Disease duration (years), median (IQR) | 13 (8–23) | 5 (2–9) | 8 (6–17) | <0.001 |
| Disease extent, n (%) | ||||
| CD | <0.001 | |||
| Ileal | 12 (24) | 26 (48) | 5 (23) | |
| Colonic | 18 (37) | 10 (19) | 6 (27) | |
| Ileocolonic | 19 (39) | 18 (33) | 11 (50) | |
| Upper GI | 3 (6) | 1 (2) | 1 (5) | |
| Perianal | 19 (39) | 18 (33) | 7 (32) | 0.827 |
| UC | 0.224 | |||
| Proctitis | 0 | 1 (20) | ||
| Left-sided | 9 (47) | 10 (40) | 1 (20) | |
| Extensive | 10 (53) | 15 (60) | 3 (60) | |
| CD behavior, n (%) | 0.51 | |||
| Inflammatory | 34 (68) | 36 (67) | 16 (72) | |
| Stricturing | 12 (24) | 10 (19) | 5 (23) | |
| Penetrating | 3 (6) | 8 (14) | 1 (5) | |
| History of extraintestinal manifestations, n (%) | 10 (14) | 8 (10) | 7 (26) | 0.12 |
| Previous IBD-related surgery, n (%) | 18 (26) | 14 (18) | 6 (22) | 0.436 |
| Combination therapy with immunosuppressant at most recent switch, n (%) | 25 (36) | 45 (56) | 6 (22) | 0.003 |
| Systemic steroids at most recent switch, n (%) | 0 | 1 (1.3) | 0 | NA |
| Previous exposure to biologics other than IFX, n (%) | 7 (10) | 10 (13) | 3 (11) | 0.99 |
| Duration of IFX exposure before index switch (years), median (IQR) | 6.8 (4.1–10.2) | 1.9 (0.9–2.6) | 3.2 (1.3–6.1) | <0.001 |
| Serum infliximab concentration at index switch (mg/L), median (IQR) | 4.2 (1.9–6.5) | 5.0 (1.7–7.1) | 3.4 (1.8–6.5) | 0.911 |
| Clinical remission at index switch, n (%) | 58 (84) | 55 (69) | 25 (93) | 0.026 |
| CRP at index switch (mg/L), median (IQR) | 1.7 (0.6–5.4) | 2.6 (0.7–6.1) | 0.9 (0.6–3.2) | 0.038 |
| FC at index switch (mg/kg), median (IQR) | 35 (15–150) | 108 (41–381) | 41 (10–198) | 0.008 |
The total follow-up time for patients successively switching from the originator to CT-P13 to SB2 was 54.6 patient-years (PYs), 66.7 PYs for patients switching from CT-P13 to SB2, and 21.8 PYs for patients switching from the originator to CT-P13.
Effectiveness
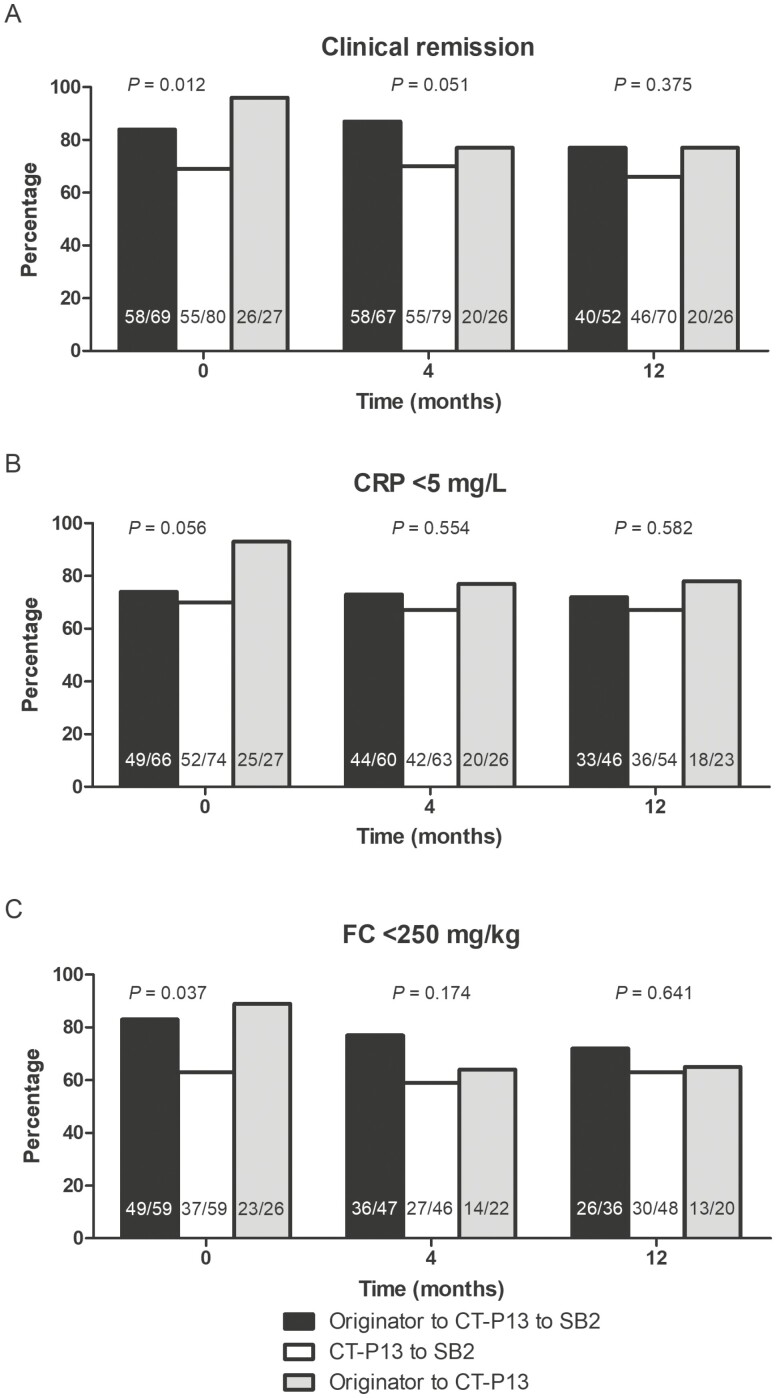
At 12 months after the index switch, 76.9% (40 of 52) of patients successively switching from the originator to CT-P13 and then to SB2, 65.7% (46 of 70) of patients switching from CT-P13 to SB2, and 76.9% (20 of 26) of patients switching from the originator to CT-P13 were in clinical remission. There were no significant differences in the rate of clinical, CRP, or FC remission at 12 months, although rates were numerically lower in patients switching from CT-P13 to SB2 (Fig. 1). There were no significant differences in need for dosing escalation between the 3 groups (2.9% [2 of 69] vs 3.8% [3 of 80] vs 3.7% [1 of 27]; P = 0.956). On univariable logistic regression, only clinical remission at the index switch was associated with clinical remission at 12 months (Table 2). In a sensitivity analysis with a patient group forced into a multivariable model together with clinical remission at index switch, the switching group was not significantly associated with clinical remission at 12 months (originator to CT-P13 as reference; odds ratio [OR] for CT-P13 to SB2, 0.861; 95% confidence interval [CI], 0.298–2.764; OR for originator to CT-P13 to SB2, 0.880; 95% CI, 0.288–2.690). In a further sensitivity analysis including only patients in clinical remission at the most recent switch, the switching group was not associated with clinical remission at 12 months (originator to CT-P13 as reference; OR for CT-P13 to SB2, 1.281; 95% CI, 0.371–4.422; OR for originator to CT-P13 to SB2, 0.788; 95% CI, 0.247–2.519).
Figure 1.
Rates of clinical remission (A), C-reactive protein (CRP) < 5 mg/L (B), and fecal calprotectin <250 mg/kg (C) across the treatment groups at switch, 4, and 12 months following the most recent switch. Patients discontinuing treatment for inefficacy, appearance of antidrug antibodies, or adverse events were imputed as nonremitters at subsequent time points. Patients with missing biochemical measurements were censored.
Table 2.
Variables Associated with Clinical Remission at 12 Months After Index Switch
| Variable | Univariable logistic regression | ||
|---|---|---|---|
| Odds’ ratio | 95% CI | P | |
| Disease duration | 1.005 | 0.938–1.078 | 0.881 |
| Duration of IFX exposure before index switch | 1.044 | 0.957–1.140 | 0.330 |
| Clinical remission at index switch | 7.846 | 3.47–17.8 | <0.001 |
| Immunomodulator at index switch | 1.376 | 0.705–2.681 | 0.350 |
| UC versus CD | 1.481 | 0.692–3.170 | 0.312 |
| Patient group | |||
| Originator to CT-P13 | 1 | ||
| Originator to CT-P13 to SB2 | 0.730 | 0.253–2.104 | 0.560 |
| CT-P13 to SB2 | 0.516 | 0.185–1.441 | 0.207 |
Safety
Infusion reactions occurred in 3.8% (3 of 80) of patients switching from CT-P13 to SB2, and 6.3% (5 of 80) of patients from this group had an IBD-related hospitalization; all hospitalizations were associated with disease flares. All 3 patients with infusion reactions had antidrug antibodies; IFX was discontinued in these patients. No infusion reactions or IBD-related hospitalizations were recorded in the other 2 groups. Other adverse events included worsening of eczema in 1 patient with previous exposure to the originator upon switching from CT-P13 to SB2, worsening headache in 3 patients switching from originator to CT-P13, and musculoskeletal pain in 1 patient from the same group. All these patients were switched back to the previously effective formulation of IFX, which led to the resolution of side effects with maintained efficacy. In 1 patient exposed to the originator and successively switching to CTP13 and SB2, malignant melanoma was diagnosed during treatment, which was subsequently interrupted.
Treatment Persistence
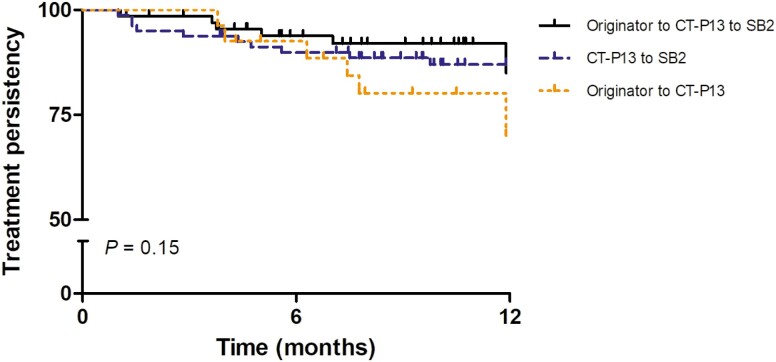
At 12 months, the estimated treatment persistence was 85.0% for patients successively switching from originator to CT-P13 to SB2, 87.0% for patients switching from CT-P13 to SB2, and 70.1% for patients switching from the originator to CT-P13 (log rank P = 0.153; Fig. 2). On univariable Cox regression, clinical remission at the most recent switch and patient group (originator to CT-P13 to SB2 and CT-P13 to SB2) were associated with a reduced hazard of treatment discontinuation for inefficacy or adverse events (Table 3). The same variables were significant on multivariable Cox regression. In the group of patients successively switching from the originator to CT-P13 to SB2, 8.7% (6 of 69) discontinued treatment for active disease, 8.7% (6 of 69) for long-term sustained remission, and 2.9% (2 of 69) for adverse events. In the group of patients switching from CT-P13 to SB2, 8.8% (7 of 80) discontinued treatment for active disease, 3.8% (3 of 80) for adverse events, and 1.3% (1 of 80) for long-term sustained remission. Among patients switching from the originator to CT-P13, 14.8% (4 of 27) discontinued treatment for active disease, 22.2% (6 of 27) for long-term sustained remission, and 14.8% (4 of 27) for adverse events.
Figure 2.
Kaplan-Meier curves for treatment persistence in the 3 treatment groups. Patients discontinuing treatment for long-term sustained remission were censored at discontinuation.
Table 3.
Variables Associated With Treatment Discontinuation for Inefficacy or Adverse Event
| Variable | Univariable Cox Regression | Multivariable Cox Regression | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Disease duration | 1.01 | 0.97–1.05 | 0.638 | |||
| Duration of prior IFX exposure | 0.99 | 0.90–1.09 | 0.884 | |||
| Clinical remission at index switch | 0.33 | 0.15–0.75 | 0.008 | 0.51 | 0.33–0.78 | 0.002 |
| Immunomodulator at index switch | 0.61 | 0.26–1.42 | 0.250 | |||
| UC versus CD | 1.27 | 0.78–2.09 | 0.339 | |||
| Patient group | ||||||
| Originator to CT-P13 | 1 | 1 | ||||
| Originator to CT-P13 to SB2 | 0.39 | 0.14–1.11 | 0.077 | 0.33 | 0.11–0.94 | 0.038 |
| CT-P13 to SB2 | 0.42 | 0.16–1.12 | 0.083 | 0.27 | 0.10–0.78 | 0.015 |
Immunogenicity and Pharmacokinetics
At the moment of the index switch, antidrug antibodies were detected in 5.8% (4 of 69) of patients successively switching from the originator to CT-P13 and SB2, in 8.8% (7 of 80) of patients switching from CT-P13 to SB2, and in no patients switching from originator IFX to CT-P13. De novo antidrug antibodies were not detected in any of the patients successively switching from originator IFX to CT-P13 and SB2, in 3.8% (3 of 80) of patients switching from CT-P13 and SB2, and in 3.7% (1 of 27) of patients switching from originator IFX to CT-P13. Infliximab was discontinued in these 4 patients.
In the group of patients successively switching from the originator to CT-P13 to SB2, median IFX serum concentrations increased nonsignificantly from 4.2 mg/L (IQR, 1.9–6.5) to 5.0 mg/L (IQR, 2.1–6.3) at 4 months and 5.3 mg/L (IQR, 3.6–7.6) at 12 months (P = 0.429). In patients switching from CT-P13 to SB2, median IFX serum concentrations decreased nonsignificantly from 5.0 mg/L (IQR, 1.7–7.1) to 3.8 mg/L (IQR, 2.2–6.5) at 4 months and 3.3 mg/L (IQR, 1.9–5.8) at 12 months (P = 0.084). In patients switching from the originator to CT-P13, median IFX serum concentrations first increased nonsignificantly from 3.4 mg/L (IQR, 1.8–6.5) to 4.0 mg/L (IQR, 0.7–7.3) at 4 months and then decreased to 2 mg/L (IQR, 1.6–4.7) at 12 months (P = 0.593).
Discussion
This is the largest study evaluating the efficacy and safety of multiple successive switches between IFX originator and biosimilars in a real-world cohort of patients with IBD to date. We observed similar rates of clinical and biochemical remission at 12 months in patients undergoing multiple successive switches, a single switch between biosimilars, or a single switch from the originator to CT-P13. No unexpected adverse events or de novo immunogenicity were observed in patients after multiple successive switches.
Virtually all studies on biosimilars in IBD have focused on a single switch between originator IFX and CT-P13. Available randomized controlled trials were not ideally designed to address this question from a daily clinical practice viewpoint for IBD patients. The NOR-SWITCH study demonstrated noninferiority of switching to CT-P13 compared with ongoing maintenance treatment with originator IFX as measured by the end point of disease worsening but was not powered to show this for each individual indication.7 The difference in disease worsening for CD was only just within the prespecified noninferiority margin of 15%. A subsequent randomized controlled trial in CD showed noninferiority of CT-P13 to originator IFX for induction and maintenance until week 30 but was not powered to assess noninferiority beyond week 30 when a quarter of patients switched from the originator to CT-P13—although clinical outcomes were numerically similar.8 Randomized controlled trials with multiple switching have only been conducted in psoriasis.16 These observations underscore the difficulty and limited interest in performing randomized controlled trials on this subject, which nonetheless remains highly relevant to clinical practice.
In the absence of data from randomized controlled trials, clinicians have relied on real-world cohort studies to support single switches from the originator to CT-P13. Recognizing the limitations of uncontrolled studies mostly reporting clinical well-being, a single nonmedical switch from originator IFX to CT-P13 seems to be safe and effective in most patients.17 Shifting reimbursement policies and the availability of several IFX biosimilars have resulted in a situation where practice precedes the accumulation of evidence: multiple successive switches from the originator to different biosimilars and switches between biosimilars. The efficacy of multiple successive switches was evaluated in a prospective study of 24 patients with 16.2 PYs of follow-up, where the treatment persistence was 82.4% at 48 weeks.14 In an additional study published in abstract form, the treatment persistence in 58 patients at week 48 was 86%, and 92% of patients were in clinical remission.18 These observations are broadly in line with our findings; the lower rate of clinical remission in our cohort may be a result of nonresponder imputation, which was not explicitly mentioned in the study cited previously. Our findings are further corroborated by objective biochemical markers of disease activity, which also support the efficacy of multiple successive switches.
Switching from CT-P13 to SB2 was assessed in a study with 43 patients with 28 PYs of follow-up,14 and an additional study of 133 patients followed for 16 weeks.19 Treatment persistence at 48 weeks was 75.3%14 and 86.5% at 16 weeks, which is somewhat lower than the treatment persistence of 87.0% at 52 weeks in our study. Our study was not powered or designed to compare differences in the rates of remission between different types of single switches and multiple successive switches, nor were any adjustments made for baseline differences between the 3 study groups. Nonetheless, neither remission rates nor treatment persistence at 12 months differed significantly between the 3 switching groups in our study. Although direct comparisons in treatment persistence with other real-world studies examining the efficacy of switching from the originator to CT-P13 are confounded by differences in baseline characteristics, treatment persistence at 12 months for multiple successive switches and switches between biosimilars is within the range observed for a single switch from the originator (57%–85%).9,11,20–22 Importantly, our study re-emphasizes the strong association between remission at the time of switching and subsequent maintenance of remission. Despite differences between groups in the duration of exposure to IFX at the time of the most recent switch, this factor was not associated with subsequent outcomes, suggesting that switching may also be considered in patients in remission who started IFX more recently.
The incidence of infusion reactions in our study was 4.5 per 100 PYs (3.8% of patients) in the group of patients switching from CT-P13 to SB2, as none were recorded in the other 2 groups. The pooled rate for the entire study was 2.1 per 100 PYs (1.7% of patients). The observed rate of infusion reactions is within the range reported in real-world studies on switching from the originator to CT-P13.10, 11, 20, 22–24 The adverse events profile was consistent with previous reports, no serious infections were recorded. Switchbacks to the previous formulation of IFX resulted both in maintaining effectiveness and the disappearance of side effects. The emergence of side effects after switching and their resolution after reverting to the formulation of IFX used previously may have been a result of the nocebo effect,25 although at least a subset of emerging adverse effects, such as hemolytic anemia,26 may also reflect incompletely understood immune-mediated mechanisms.
The potential of increased immunogenicity is one of the key concerns about multiple successive switches. Results of studies evaluating the development of antidrug antibodies should be interpreted with attention for and knowledge of the characteristics of the assay used for their detection. Studies on patients undergoing multiple successive switches, including ours, used drug-sensitive assays, which may have resulted in the underestimation of antibody development. In a French study of patients with immune-mediated inflammatory diseases including IBD, the rate of antidrug antibody formation with multiple successive switches regardless of diagnosis was 3 per 100 PYs compared with a single switch, and multiple switches were not found to be a risk factor for immunogenicity.13 No antidrug antibodies developed de novo in patients with multiple successive switches in our study; the rate was 2.5 per 100 PYs in patients switching from CT-P13 to SB2. These estimates should only be regarded as the lower limit of immunogenicity because antidrug antibodies were not sought consistently and systematically in all our patients. An in vitro study using sera of patients who had developed antibodies to IFX after treatment with originator IFX, CT-P13, or both demonstrated full cross-reactivity with SB2.27 This observation, however, cannot be taken as evidence of interchangeability or equal rates of immunogenicity between biosimilars; it merely confirms that the negative consequences of antidrug antibodies cannot be overcome by switching to a different biosimilar. No statistically significant differences in serum IFX concentrations were observed in any of the switching groups in our study. This is in line with previous work that showed the noninferiority of switching from the originator to CT-P13 for the stability of serum concentrations.28
Our study is the first and largest to primarily focus on IBD patients undergoing multiple successive switches from the originator to biosimilars with clinical and biochemical end points at 12 months, together with the first report on pharmacokinetics in this particular patient group to date. Nonetheless, our findings should be interpreted within the context of the limitations of our study. It was an observational study based on routine clinical care, which may have resulted in the underreporting of adverse events that were perceived as less serious by the patient or the treating physician. Inevitably, the characteristics of patients within each group were affected by the timing of switches, with patients undergoing multiple successive switches having a longer disease duration than patients who switched from CT-P13 to SB2. Patients undergoing a single switch from the originator to CT-P13 either discontinued treatment or were lost to follow-up beyond 12 months, as they would otherwise have undergone multiple successive switches due to mandatory switching after July 2018.
Glossary
Abbreviations
- CD
Crohn’s disease
- CI
confidence interval
- CRP
C-reactive protein
- FC
fecal calprotectin
- IBD
inflammatory bowel disease
- IFX
infliximab
- IQR
interquartile range
- PY
patient-year
- UC
ulcerative colitis
Conflicts of Interest: JH has received speaker’s fees from Biogen, Janssen, Pfizer, and Takeda. JMJ has served on advisory boards, or as speaker or consultant for Abbvie, Amgen, Ferring, Fresenius, Janssen, MSD, Pfizer, and Takeda. RWFS has nothing to declare. KBG has received consultancy fees and/or speaker’s honoraria fromfrom AbbVie, Boehringer Ingelheim, Celltrion, Ferring, Gilead, Immunic Therapeutics, Janssen, Pfizer, Sandoz, Samsung Bioepis, Takeda, and Tillotts. GRD has served as advisor for Abbvie, Ablynx, Alimentiv, Allergan, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Covidien/Medtronics, Ferring, DrFALK Pharma, Eli Lilly, Engene, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Hospira/Pfizer, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millennium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prometheus laboratories/Nestle, Progenity, Protagonist, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor; received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dohme, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts, and Vifor.
Conclusion
Our findings suggest that multiple successive switches from originator IFX to biosimilars are effective and safe, particularly if patients are in remission at the time of the switch. The treatment persistence, remission rates, and adverse event profile were consistent with reports from studies of a single switch from the originator to CT-P13.
References
- 1. Kim H, Alten R, Avedano L, et al. The future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs. 2020;80:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shin D, Kim Y, Kim YS, et al. A Randomized, Phase I pharmacokinetic study comparing SB2 and infliximab reference product (Remicade(®)) in healthy subjects. Biodrugs. 2015;29:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reinisch W, Gecse K, Halfvarson J, et al. Clinical practice of adalimumab and infliximab biosimilar treatment in adult patients with Crohn’s disease. Inflamm Bowel Dis. 2021;27:106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jørgensen KK, Olsen IC, Goll GL, et al. ; NOR-SWITCH study group . Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389:2304–2316. [DOI] [PubMed] [Google Scholar]
- 8. Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 noninferiority study. Lancet. 2019;393:1699–1707. [DOI] [PubMed] [Google Scholar]
- 9. Bergqvist V, Kadivar M, Molin D, et al. Switching from originator infliximab to the biosimilar CT-P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018;11:1756284818801244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bronswijk M, Moens A, Lenfant M, et al. Evaluating efficacy, safety, and pharmacokinetics after switching from infliximab originator to biosimilar CT-P13: experience from a large tertiary referral center. Inflamm Bowel Dis. 2020;26:628–634. [DOI] [PubMed] [Google Scholar]
- 11. Chaparro M, Garre A, Guerra Veloz MF, et al. Effectiveness and safety of the switch from Remicade(R) to CT-P13 in patients with inflammatory Bowel disease. J Crohn’s Colitis. 2019;13:1380–1386. [DOI] [PubMed] [Google Scholar]
- 12. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory Bowel disease-an update. J Crohns Colitis. 2017;11:26–34. [DOI] [PubMed] [Google Scholar]
- 13. Lauret A, Moltó A, Abitbol V, et al. Effects of successive switches to different biosimilars infliximab on immunogenicity in chronic inflammatory diseases in daily clinical practice. Semin Arthritis Rheum. 2020;50:1449–1456. [DOI] [PubMed] [Google Scholar]
- 14. Macaluso FS, Fries W, Viola A, et al. The SPOSIB SB2 sicilian cohort: safety and effectiveness of infliximab biosimilar SB2 in inflammatory bowel diseases, including multiple switches. Inflamm Bowel Dis. 2021;27:182–189. [DOI] [PubMed] [Google Scholar]
- 15. Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:711–715. [DOI] [PubMed] [Google Scholar]
- 16. Griffiths CEM, Thaçi D, Gerdes S, et al. ; EGALITY study group . The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176:928–938. [DOI] [PubMed] [Google Scholar]
- 17. Bernard EJ, Fedorak RN, Jairath V. Systematic review: nonmedical switching of infliximab to CT-P13 in inflammatory Bowel disease. Dig Dis Sci. 2020;65:2354–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazza S, Fasci A, Casini V, et al. Safety and clinical efficacy of double switch from originator infliximab to biosimilars CT-P13 and SB2 in patients with inflammatory bowel diseases (SCESICS): a multicentre study. J Crohn’s Colitis. 2020;14:S342tre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris C, Harris R, Young D, et al. IBD biosimilar to biosimilar infliximab switching study: preliminary results. United European Gastroenterol J. 2019;7:P0419. [Google Scholar]
- 20. Schmitz EMH, Boekema PJ, Straathof JWA, et al. Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: a 12-month multicentre observational prospective cohort study. Aliment Pharmacol Ther. 2018;47:356–363. [DOI] [PubMed] [Google Scholar]
- 21. Razanskaite V, Bettey M, Downey L, et al. Biosimilar infliximab in inflammatory Bowel disease: outcomes of a managed switching programme. J Crohns Colitis. 2017;11:690–696. [DOI] [PubMed] [Google Scholar]
- 22. Plevris N, Jones GR, Jenkinson PW, et al. Implementation of CT-P13 via a managed switch programme in Crohn’s disease: 12-month real-world outcomes. Dig Dis Sci. 2019;64:1660–1667. [DOI] [PubMed] [Google Scholar]
- 23. Smits LJ, Derikx LA, de Jong DJ, et al. Clinical outcomes following a switch from Remicade(R) to the Biosimilar CT-P13 in inflammatory Bowel disease patients: a prospective observational cohort study. J Crohn’s Colitis. 2016;10:1287–1293. [DOI] [PubMed] [Google Scholar]
- 24. Fiorino G, Manetti N, Armuzzi A, et al. ; PROSIT-BIO Cohort . The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory Bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017;23:233–243. [DOI] [PubMed] [Google Scholar]
- 25. Pouillon L, Danese S, Hart A, et al. Consensus report: clinical recommendations for the prevention and management of the nocebo effect in biosimilar-treated IBD patients. Aliment Pharmacol Ther. 2019;49:1181–1187. [DOI] [PubMed] [Google Scholar]
- 26. Strik AS, D’Haens GR, Löwenberg M. Hemolytic anemia after switching from infliximab originator to biosimilar CT-P13 in a patient with inflammatory bowel disease: A case report. Clin Case Rep. 2019;7:2049–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fiorino G, Ruiz-Argüello MB, Maguregui A, et al. Full interchangeability in regard to immunogenicity between the infliximab reference biologic and biosimilars CT-P13 and SB2 in inflammatory Bowel disease. Inflamm Bowel Dis. 2018;24:601–606. [DOI] [PubMed] [Google Scholar]
- 28. Strik AS, van de Vrie W, Bloemsaat-Minekus JPJ, et al. ; SECURE study group . Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 noninferiority trial. Lancet Gastroenterol Hepatol. 2018;3:404–412. [DOI] [PubMed] [Google Scholar]