Abstract
The therapeutic efficacy of long-circulating polyethylene glycol-coated liposomal amphotericin B (AMB) (PEG-AMB-LIP) was compared with that of AMB desoxycholate (Fungizone) in a model of severe invasive pulmonary aspergillosis in persistently leukopenic rats as well as in temporarily leukopenic rats. PEG-AMB-LIP treatment (intravenous administration) consisted of a single, or double (every 72 h), or triple (every 72 h) dose of 10 mg of AMB/kg of body weight, a double dose (every 72 h) of 14 mg of AMB/kg, or a 5-day treatment (every 24 h) with 6 mg/kg/dose. AMB desoxycholate was administered for 10 consecutive days at 1 mg of AMB/kg/dose. Treatment was started 30 h after fungal inoculation, at which time mycelial growth was firmly established. Both persistently and temporarily leukopenic rats died between 4 and 9 days after Aspergillus fumigatus inoculation when they were left untreated or after treatment with a placebo. In persistently leukopenic rats, a single dose of PEG-AMB-LIP (10 mg/kg) was as effective as the 10-day treatment with AMB desoxycholate (at 1 mg/kg/dose) in significantly prolonging the survival of rats infected with A. fumigatus and in reducing the dissemination of A. fumigatus to the liver. Prolongation of PEG-AMB-LIP treatment (double or triple dose or 5-day treatment) did not further improve efficacy. For temporarily leukopenic rats no major advances in efficacy were achieved compared to those for persistently leukopenic rats, probably because the leukocyte numbers in blood were restored too late in the course of infection.
Because a high rate of mortality from pulmonary aspergillosis continues to be seen for leukopenic patients, despite aggressive antifungal therapy, there is an urgent need for therapeutic advances (10). At present three lipid formulations of amphotericin B (AMB) are industrially produced: Abelcet (The Liposome Company, Inc.), Amphocil or Amphotec (SEQUUS Pharmaceuticals, Inc.), and AmBisome (NeXstar Pharmaceuticals, Inc.). Although it is clear from a number of clinical studies that all three AMB-lipid formulations are substantially less toxic than AMB desoxycholate (Fungizone), it is still too early to draw sharp conclusions on the optimal dosing of these AMB-lipid formulations in the treatment of invasive pulmonary aspergillosis (4, 7, 11, 13, 14, 16–18, 21, 29–34).
Experimental studies on the efficacy of systemic treatment with these three AMB-lipid formulations have been performed with different animal models of invasive aspergillosis (2, 9, 12, 13, 15, 19, 20, 23, 28). In only two of these models, one with rabbits (2, 12, 28) and another with rats (15), the respiratory route of infection and profound and persistent granulocytopenia were part of the experimental design. In these two models improved survival of animals after multidose treatment with Amphocil (2, 28) or AmBisome (12, 15) compared to that after treatment with AMB desoxycholate has been reported.
It is evident that the three AMB-lipid formulations have quite different structural and pharmacokinetic characteristics (13). Abelcet and Amphocil are rapidly taken up from the blood by phagocytic cells of the mononuclear phagocyte system (MPS) in the liver and spleen, resulting in relatively low AMB concentrations in blood compared to those of AMB desoxycholate when the two formulations are given at equivalent doses. AmBisome consists of small rigid liposomes that remain in the circulation for a relatively prolonged period of time. With AmBisome, the concentrations of AMB in blood are increased compared to those achieved with AMB desoxycholate given at an equivalent dose. For AmBisome, the blood residence time is primarily dependent on the dose administered (12, 24). At a dose of 7 mg of AMB/kg of body weight the elimination half-life of AmBisome in mice is about 8 h (24).
At our laboratory a new type of liposomal AMB, in which AMB is complexed to a hydrophilic phospholipid derivative of polyethylene glycol 1900 (PEG), was prepared and is designated PEG-AMB-LIP (25). Incorporation of PEG-derivatized distearoylphosphatidylethanolamine (PEG-DSPE) results in a hydrophilic PEG coating on the surface of the liposomes, to which binding of blood proteins is substantially reduced. As a result, uptake of liposomes by the MPS is substantially avoided and a relatively long blood residence time of intact liposomes is achieved, with the residence time being independent of the dose administered (35). For PEG-AMB-LIP the elimination half-life in mice was shown to be approximately 20 h for a dose range of 0.5 to 9 mg of AMB/kg (25, 26).
This prolonged blood residence time of PEG-AMB-LIP may be important to obtain increased concentrations of liposomal AMB at sites of fungal infection outside the MPS, such as the kidneys and lungs. The superior efficacy of PEG-AMB-LIP compared with that of AmBisome after treatment with a single dose was previously demonstrated in our model of severe invasive Candida albicans infection (26, 27), in which the kidney is the most severely infected organ. With respect to the treatment of pulmonary infections, it has been shown in our laboratory that PEG-containing liposomes selectively localize at the site of Klebsiella pneumoniae-infected lung tissue (5), resulting in an improved efficacy of gentamicin or ceftazidime entrapped in such long-circulating liposomes (6). An improved efficacy of PEG-containing AMB liposomes (19) having a lipid composition different from that of PEG-AMB-LIP was recently demonstrated against invasive pulmonary aspergillosis in mice when efficacy was compared to that of AmBisome. Further improvement of efficacy was observed when monoclonal antibodies directed to the luminal surface of the pulmonary vessel wall in the mouse lung were attached to these PEG-containing AMB liposomes. In that study (19) only temporary immunosuppression was applied, and treatment was started very early (2 h) after fungal inoculation.
In the present study the efficacies of single- or multiple-dose treatment with PEG-AMB-LIP were compared with that of AMB desoxycholate in the treatment of severe invasive pulmonary aspergillosis in persistently as well as temporarily leukopenic rats, in which treatment was started at the time that fungal infection was firmly established (30 h after fungal inoculation).
MATERIALS AND METHODS
Animals.
Female R strain albino rats (specified pathogen free; age, 18 to 25 weeks; weight, 185 to 225 g) were obtained from Harlan CPB (Austerlitz, The Netherlands).
Materials.
Sabouraud dextrose agar (SDA) was from Unipath Ltd. (Basingstoke, England). AMB and AMB desoxycholate were kindly provided by Bristol Myers-Squibb, Woerden, The Netherlands. Hydrogenated soybean phosphatidylcholine (HSPC) and PEG-DSPE were obtained from Avanti Polar Lipids, Inc. (Alabaster, Ala.). Dimethyl sulfoxide (DMSO) was from Janssen Chimica (Tilburg, The Netherlands). Cyclophosphamide and cholesterol (Chol) were from Sigma (St. Louis, Mo.). Chloroform and methanol were from Merck (Darmstadt, Germany).
Liposome preparation.
PEG-DSPE–HSPC–Chol–AMB in a molar ratio of 0.21:1.79:1:0.32 (PEG-AMB-LIP) and placebo liposomes (liposomes devoid of AMB) were prepared as described previously (25, 26). Briefly, AMB was complexed to PEG-DSPE in chloroform-methanol (1:1; vol/vol) at 65°C, followed by the addition of HSPC and Chol. This lipid mixture was evaporated to dryness and was subsequently hydrated by vortex mixing in a buffer solution containing 10 mM sodium succinate and 10% (wt/vol) sucrose (pH 5.5) at 65°C. The phospholipid concentration was determined by a phosphate assay (3). The AMB concentration was determined spectrophotometrically at 405 nm, after destruction of the liposomes in DMSO-methanol (1/1; vol/vol).
Aspergillus strain.
A clinical isolate of Aspergillus fumigatus from an immunocompromised patient with invasive pulmonary aspergillosis was used. The MIC and minimal fungicidal concentration of AMB for the isolate are 0.4 and 0.8 mg/liter, respectively (15). This strain was stored under oil on SDA. At least once every 2 months the strain was passed through a rat to maintain its virulence. For inoculation a suspension of A. fumigatus conidia in sterile saline was prepared as described previously (15).
Immunosuppression and supportive care.
The first series of experiments was performed with persistently leukopenic rats. Granulocytopenia (<0.5 × 109 granulocytes/liter) from the time of A. fumigatus inoculation to termination of the study was obtained by intraperitoneal administration of cyclophosphamide at 90 mg/kg starting 5 days before A. fumigatus inoculation (day −5), followed by administration of additional doses of 60 mg/kg on the day before inoculation (day −1) and at 4-day intervals thereafter (day 3 and day 7) (15). In a second series of experiments cyclophosphamide was given up to day 3 in order to obtain temporary leukopenia. To prevent bacterial superinfection, strict hygienic care was applied, and rats received ciprofloxacin (660 mg/liter) and polymyxin E (100 mg/liter) in their drinking water during the whole experiment. From the day before inoculation, intramuscularly administered amoxicillin (40 mg/kg/dose) was added to this regimen daily for the remainder of the experiment. Shortly before and after inoculation, gentamicin (40 mg/kg/dose) was administered intramuscularly.
Experimental lung infection.
Experimental pulmonary aspergillosis was obtained as described previously (15). Briefly, rats were anesthetized, after which the left main bronchus was intubated and the left lung was inoculated with 0.02 ml of a suspension containing 2 × 104 conidia.
Efficacies of PEG-AMB-LIP and AMB desoxycholate in leukopenic rats infected with A. fumigatus.
The left lobes of the lungs of leukopenic rats were inoculated with A. fumigatus at time zero. Groups of 15 animals each were treated intravenously with PEG-AMB-LIP or AMB desoxycholate. PEG-AMB-LIP treatment consisted of a single or double (every 72 h) dose of 10 mg of AMB/kg or a double dose (every 72 h) of 14 mg of AMB/kg. AMB desoxycholate was administered for 10 consecutive days (every 24 h) at 1 mg of AMB/kg/dose. In two additional groups of five rats each, treatment with PEG-AMB-LIP three times (every 72 h) with 10 mg of AMB/kg/dose or for 5 consecutive days (every 24 h) with 6 mg/kg/dose was investigated. No toxicity in terms of acute death directly after treatment was observed for any of the treatment regimens. Controls were treated with placebo liposomes or 5% glucose or were left untreated. Treatment was started 30 h after fungal inoculation, at which time mycelial growth was firmly established by histopathologic examination (periodic acid-Schiff staining). Survival was recorded daily for each rat. At the postmortem examination, the left and right lungs and the liver were removed and were processed for the determination of viable A. fumigatus counts, as described previously for C. albicans (26). The following criteria were used to assess the efficacy of treatment: survival of rats up to 12 days after A. fumigatus inoculation, the presence of viable A. fumigatus organisms in the left lung, and the presence of dissemination to the right lung and liver at the time of death.
Determination of leukocyte counts in blood of leukopenic rats infected with A. fumigatus.
Two separate groups of 15 rats each were immunosuppressed with cyclophosphamide up to either day 3 or day 7 after A. fumigatus inoculation, as described above, and were treated twice (every 72 h) with PEG-AMB-LIP at 10 mg of AMB/kg/dose. The total numbers of leukocytes in the blood of the surviving rats were determined with an automatic blood cell counter (COBAS MINOS STEX; ABX Hematology, Montpeiller, France). Blood samples were obtained by orbital puncture while the rats were under light CO2 anesthesia. The percentages of granulocytes were derived from differential counts of leukocytes in cytospin preparations of buffy coats.
Statistical analysis.
Statistical evaluation of differences in survival (Kaplan-Meier plot) was performed by the log rank test. This test examines the decrease in survival with time as well as the final percentage of surviving rats. Differences in the numbers of rats with A. fumigatus in the left lung and the numbers of rats with A. fumigatus dissemination to the right lung and liver at the time of death were examined by Fisher's exact test. P values of ≤0.05 were considered significant in these analyses.
RESULTS
Efficacy of treatment in persistently leukopenic rats.
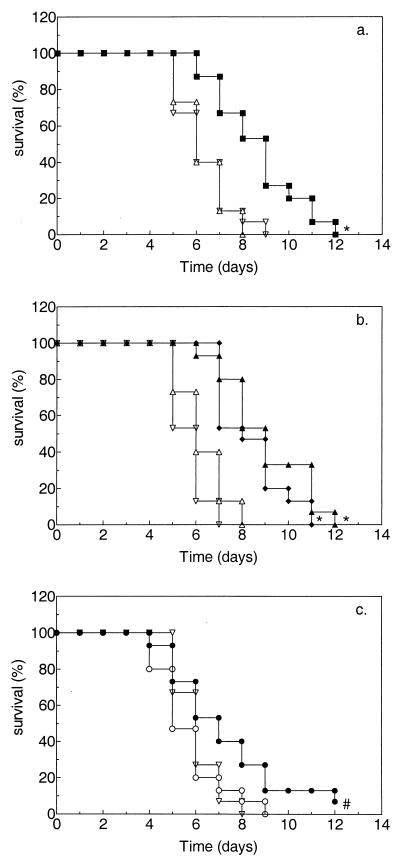
The effects of treatment on survival of persistently leukopenic rats with pulmonary aspergillosis and on the presence of viable A. fumigatus in the left lung, right lung, and liver at the time of death are shown in Fig. 1 and Table 1, respectively. In this model of pulmonary aspergillosis, untreated rats as well as placebo-treated rats died between 4 and 9 days after inoculation. Postmortem cultures of samples from these rats revealed that at the time of death the infection had disseminated to the right lung and liver in the majority of rats. Treatment with a single dose of PEG-AMB-LIP (10 mg of AMB/kg) resulted in significantly prolonged (P ≤ 0.005) survival (Fig. 1a) and significantly less (P ≤ 0.005) dissemination to the liver (Table 1) compared to those that resulted from placebo treatment. Similar results were obtained after two treatments with either 10 or 14 mg of AMB/kg/dose (Fig. 1b and Table 1, respectively) or after more prolonged treatment (three 10-mg/kg doses, with doses given every 72 h) or more intensive treatment early in the course of infection (five 6-mg/kg doses, with doses given every 24 h) (data not shown). Treatment with AMB desoxycholate for 10 consecutive days at 1 mg of AMB/kg/dose resulted in significantly prolonged (P ≤ 0.05) survival (Fig. 1c) and significantly less dissemination to the right lung (P ≤ 0.05) as well as to the liver (P ≤ 0.005) (Table 1) compared to those that resulted from placebo treatment. When the different treatment regimens were compared with each other, no significant differences in efficacy were observed.
FIG. 1.
Effects of a single dose (a) or double doses (b) of PEG-AMB-LIP versus multiple doses of AMB desoxycholate (c) on survival of persistently leukopenic rats with pulmonary aspergillosis (Kaplan-Meier plot). Leukopenic rats were inoculated in the left lobe of the lung with 2 × 104 A. fumigatus conidia at time zero. Groups of 15 animals each were treated intravenously with PEG-AMB-LIP as a single dose of 10 mg of AMB/kg (■), a double dose (given every 72 h) of 10 mg of AMB/kg/dose (⧫) or a double dose (given every 72 h) of 14 mg of AMB/kg/dose (▴); AMB desoxycholate was administered for 10 consecutive days at 1 mg of AMB/kg/dose (●). Controls were treated with placebo liposomes (▵) or 5% glucose (○) or were left untreated (▿). Treatment was started 30 h after fungal inoculation, at which time mycelial growth was firmly established. ⧣, P ≤ 0.05; and ∗, P ≤ 0.005, versus untreated or placebo-treated rats.
TABLE 1.
Effects of a single dose or double doses of PEG-AMB-LIP versus multiple doses of AMB desoxycholate on presence of viable A. fumigatus in the left lung and dissemination to the right lung and liver at the time of death in persistently or temporarily leukopenic ratsa
| Treatmentb | Daily dose of AMB (mg/kg) | No. of doses (dosing interval [h]) | No. of culture-positive organsc/total no. of ratsd
|
|||||
|---|---|---|---|---|---|---|---|---|
| Left lung
|
Right lung
|
Liver
|
||||||
| P | T | P | T | P | T | |||
| Untreated | 15/15 | 15/15 | 14/15 | 12/15 | 10/15 | 9/15 | ||
| PEG-AMB-LIP | 10 | 1 | 15/15 | 15/15 | 13/15 | 12/15 | 1/15e | 2/15f |
| PEG-AMB-LIP | 10 | 2 (72) | 15/15 | 15/15 | 9/15 | 8/15 | 0/15e | 0/15e |
| PEG-AMB-LIP | 14 | 2 (72) | 15/15 | 15/15 | 9/15 | 8/15 | 0/15e | 0/15e |
| AMB desoxycholate | 1 | 10 (24) | 15/15 | 15/15 | 9/15f | 6/15e | 1/15e | 1/15e |
| Placebo liposomes | 2 (72) | 15/15 | 15/15 | 12/15 | 12/15 | 11/15 | 8/15 | |
| Placebo (glucose) | 10 (24) | 15/15 | 15/15 | 15/15 | 14/15 | 10/15 | 8/15 | |
Leukopenic rats were inoculated in the left lung at time zero with 2 × 104 A. fumigatus conidia.
PEG-AMB-LIP, AMB desoxycholate, or placebo treatment was administered intravenously.
Effect of treatment was determined by postmortem cultures.
P, persistent leukopenia; T, temporary leukopenia.
P ≤ 0.005 versus placebo treatment.
P ≤ 0.05 versus placebo treatment.
Toxicity in terms of death or renal or hepatic dysfunction was not observed after treatment with PEG-AMB-LIP (2 doses of 14 mg of AMB/kg) or AMB desoxycholate (10 doses of 1 mg of AMB/kg) in uninfected rats that were rendered leukopenic with cyclophosphamide and that received antibiotics for the prevention of bacterial superinfections (data not shown).
Efficacy of treatment in temporarily leukopenic rats.
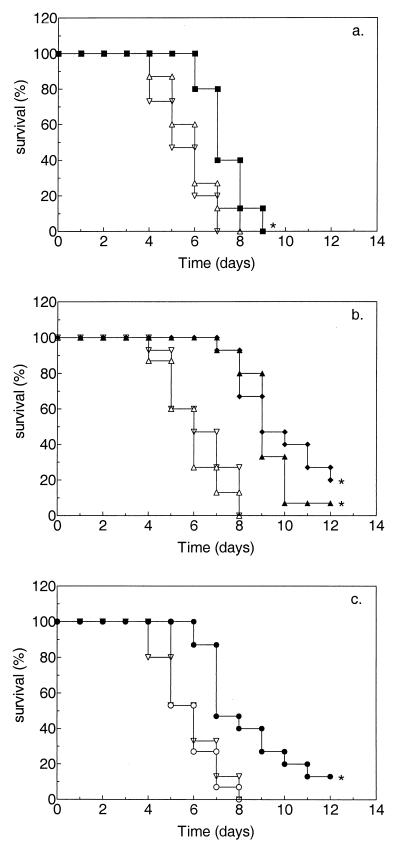
The effects of treatment on survival of temporarily leukopenic rats with pulmonary aspergillosis and on the presence of viable A. fumigatus organisms in the left lung, right lung, and liver at the time of death are shown in Fig. 2 and Table 1, respectively. Untreated rats as well as placebo-treated rats still died between 4 and 9 days after inoculation, with dissemination to the right lung and liver in the majority of rats. Treatment with a single dose of PEG-AMB-LIP (10 mg of AMB/kg) resulted in significantly prolonged (P ≤ 0.005) survival (Fig. 2a) and significantly less (P ≤ 0.05) dissemination to the liver (Table 1) compared to those that resulted from placebo treatment. For PEG-AMB-LIP similar results were obtained after two treatments with either 10 or 14 mg of AMB/kg/dose (Fig. 2b and Table 1, respectively) or after more prolonged treatment (three 10-mg/kg doses, with doses given every 72 h) or more intensive treatment early in the course of infection (five 6-mg/kg doses, with doses given every 24 h) (data not shown). After two treatments with PEG-AMB-LIP at 10 mg of AMB/kg/dose, survival was significantly more prolonged in temporarily leukopenic rats than in persistently leukopenic rats (P ≤ 0.05). Treatment with AMB desoxycholate for 10 consecutive days at 1 mg of AMB/kg/dose resulted in significantly prolonged (P ≤ 0.005) survival (Fig. 2c) and significantly less dissemination to the right lung (P ≤ 0.005) as well as to the liver (P ≤ 0.005) (Table 1) compared to those that resulted from placebo treatment.
FIG. 2.
Effects of a single dose (a) or double doses (b) PEG-AMB-LIP versus multiple doses of AMB desoxycholate (c) on survival of temporarily leukopenic rats with pulmonary aspergillosis (Kaplan-Meier plot). For explanation of symbols and panels, see the legend to Fig. 1.
Number of leukocytes in blood of leukopenic rats infected with A. fumigatus.
Persistently leukopenic rats immunosuppressed with cyclophosphamide up to day 7 after A. fumigatus inoculation and treated with a double dose of PEG-AMB-LIP at 10 mg of AMB/kg/dose are profoundly leukopenic from the time of A. fumigatus inoculation (time zero) up to 12 days after inoculation, during which time the mean leukocyte count is <0.6 × 109/liter (data not shown). Differential counts showed that less than 10% of the leukocytes consisted of granulocytes. The total numbers of leukocytes in the blood of temporarily leukopenic rats immunosuppressed with cyclophosphamide up to day 3 after A. fumigatus inoculation were determined in rats that were treated with a double dose of PEG-AMB-LIP at 10 mg of AMB/kg/dose. Surviving rats are profoundly leukopenic from the time of A. fumigatus inoculation (time zero) up to 7 days after inoculation. From day 9 after inoculation a substantial rise in leukocyte numbers is observed, with the mean numbers of leukocytes being 0.7 × 109/liter at day 9 (with 5 of 15 rats surviving), 8.0 × 109/liter at day 11 (with 3 of 15 rats surviving), and 13.7 × 109/liter at day 13 (with 3 of 15 rats surviving), which is a twofold increased level compared to normal values (mean value, 6.5 × 109/liter).
DISCUSSION
The importance of invasive aspergillosis has progressively increased, and as a result it is now a major direct or contributory cause of death at leukemia treatment centers and bone marrow transplantation and solid-organ transplantation centers (10). In the present study the efficacy of PEG-AMB-LIP, which has a prolonged circulation in blood, was compared to that of AMB desoxycholate in a rat model of severe pulmonary aspergillosis. The same animal model was previously used to investigate the efficacy of a lipid formulation of AMB (AmBisome) versus that of AMB desoxycholate (15). Clinically relevant issues are addressed with this animal model, such as profound and persistent leukopenia, with inoculation of A. fumigatus via the respiratory route resulting in a one-sided pulmonary infection with dissemination to the right lung and to the liver during the course of infection and with infiltration of pulmonary tissue by hyphae with angioinvasion. In the present study treatment was started when hyphal growth was firmly established. It is known from clinical experience that persistent leukopenia, histological evidence of angioinvasion, and delayed therapy are important factors that predict a poor response to treatment (10). The experimental setup in the present study was deliberately chosen, as we wanted to investigate the potential of PEG-AMB-LIP under very severe circumstances.
In persistently leukopenic rats survival was significantly prolonged after administration of only a single dose of PEG-AMB-LIP. Similar results were obtained after the 10-day treatment with AMB desoxycholate. It should be stated that although the survival of the rats was significantly prolonged, treatment did not result in eradication of the infection, and eventually, a high rate of mortality was still observed at day 12 after A. fumigatus inoculation.
The results for AMB desoxycholate with respect to the survival of rats confirm previous results (15). The survival of rats after treatment with a single dose of PEG-AMB-LIP is also very similar to the survival after a 10-day treatment with AmBisome (10 mg/kg/dose) in the same animal model (15).
In the present study the dissemination of A. fumigatus to the right lung and liver was reduced after AMB desoxycholate treatment, whereas this was not observed in the previous study (15). In our view daily administration of AMB desoxycholate may help to prevent hematological fungal dissemination, and therefore, the conclusion from Leenders et al. (15) that AmBisome was more effective than AMB desoxycholate in reducing the degree of dissemination of unilateral pulmonary aspergillosis should now be reconsidered. The degree of dissemination of A. fumigatus to the liver was also significantly reduced by PEG-AMB-LIP treatment, even after only a single dose. Evidently, mortality did not seem to directly correlate with the degree of dissemination of infection, as was also remarked by Leenders et al. (15). In fact, we still do not know the exact cause of death in this animal model. It is presently under investigation whether the animals die from respiratory insufficiency resulting from vascular involvement of the left lung or from toxins produced by the fungus.
To investigate whether the efficacy of PEG-AMB-LIP could be further improved, treatment with a double or a triple dose of 10 mg/kg/day was examined. The dosing interval of 72 h was chosen because of the prolonged elimination half-life of PEG-AMB-LIP (approximately 20 h) (25, 26) in comparison to that of AmBisome (about 8 h) (24), for which a dosing interval of 24 h was previously chosen by Leenders et al. (15). PEG-AMB-LIP was also administered at a double dose of 14 mg/kg/day, being the maximum dose tolerated by these infected rats (acute death immediately after administration was observed with higher dosages). As untreated rats had already died by 4 days after A. fumigatus inoculation, a more intensive treatment early in the course of infection was also investigated. When PEG-AMB-LIP was administered for 5 consecutive days, acute toxicity was observed during treatment with 10 mg/kg/day. The daily dose was reduced to 6 mg/kg, which was the maximum tolerated dose for this intensive treatment regimen.
In persistently leukopenic rats prolongation of PEG-AMB-LIP treatment (double or triple doses or 5-day treatment) did not further improve its efficacy. Apparently, the initial delay in the progression of infection that was obtained after treatment with a single dose of PEG-AMB-LIP could not be further extended. These results cannot readily be explained, mainly because of our lack of understanding of the exact means of pathogenesis and the cause of death in this animal model. Another issue is that endpoints based on survival and culture-positive organs at the time of death may be too austere to allow detection of differences in response to antifungal therapy. In a model of severe pulmonary aspergillosis in profoundly leukopenic rabbits (2, 12, 28), markers of fungus-mediated tissue injury (pulmonary lesion scores and lung weight scores) and levels of galactomannan in serum or d-mannitol in bronchoalveolar lavage fluid were also used as parameters for determination of antifungal efficacy. Whether these markers can also be applied in our rat model of pulmonary aspergillosis is under investigation.
From clinical experience it is known that bone marrow recovery and increasing numbers of leukocytes in blood are crucial factors in the outcome of treatment (10). In the second part of the study it was investigated whether the prolongation of survival observed after antifungal treatment in persistently leukopenic rats was sufficient enough to keep the animals alive when the numbers of leukocytes in blood were restored late in the course of infection (temporarily leukopenic rats). Unfortunately, no major advances in efficacy were achieved in temporarily leukopenic rats compared to that achieved with persistently leukopenic rats. The total numbers of leukocytes in blood were determined for a separate group of temporarily immunosuppressed rats infected with A. fumigatus and treated with the optimal treatment regimen (double dose of PEG-AMB-LIP). Untreated rats could not be used for this purpose, as all animals had ultimately died at day 9. In the treated rats a substantial rise in leukocyte numbers was observed from day 9. At that time point only 33% (5 of 15) of the animals were still alive. It was remarkable that from these 5 rats only the rats with the highest leukocyte counts (3 of 15) eventually survived. From these data it is concluded that the leukocyte numbers in blood were restored too late in the course of the infection to have a significant effect on the outcome of treatment. It seems to be very worthwhile to investigate the effect of treatment when the numbers of leukocytes are rising from day 5 after A. fumigatus inoculation, at which time point the majority of rats are still alive.
In our opinion the therapeutic options of PEG-AMB-LIP in pulmonary aspergillosis need to be further explored, e.g., the effect of earlier (empirical) treatment. Another option might be local administration of PEG-AMB-LIP via aerosol inhalation. Two different experimental studies have been published on the effect of prophylaxis of pulmonary aspergillosis with aerosolized AmBisome (1) or Abelcet (8), both of which were found to be highly protective. Whether this route of administration of AMB-lipid formulations will be well tolerated and will be effective in human patients is not known, as only one report on the use of nebulized AmBisome in a single patient has been published (22).
In summary, a single dose of PEG-AMB-LIP (10 mg/kg) is as effective as a 10-day treatment with AMB desoxycholate (1 mg/kg/dose) in significantly prolonging the survival of persistently leukopenic rats infected with A. fumigatus. Unfortunately, this observed delay in the progression of the infection after antifungal treatment was not sufficient to keep the animals alive when the numbers of leukocytes in blood were restored late in the course of infection.
ACKNOWLEDGMENTS
This study was financially supported by the Jan Dekker Stichting & Dr. Ludgardina Bouwman Stichting and by the Dr. Saal van Zwanenberg Stichting.
REFERENCES
- 1.Allen S D, Sorensen K N, Nejdl M J, Durrant C, Proffitt R T. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-liposomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J Antimicrob Chemother. 1994;34:1001–1013. doi: 10.1093/jac/34.6.1001. [DOI] [PubMed] [Google Scholar]
- 2.Allende M C, Lee J W, Francis P, Garrett K, Dollenberg H, Berenguer J, Lyman C A, Pizzo P A, Walsh T J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1994;38:518–522. doi: 10.1128/aac.38.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames B N, Dubin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 4.Anaissie E J, Mattiuzzi G N, Miller C B, Noskin G A, Gurwith M J, Mamelok R D, Pietrelli L A. Treatment of invasive fungal infections in renally impaired patients with amphotericin B colloidal dispersion. Antimicrob Agents Chemother. 1998;42:606–611. doi: 10.1128/aac.42.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker-Woudenberg I A, Lokerse A F, ten Kate M T, Mouton J W, Woodle M C, Storm G. Liposomes with prolonged blood circulation and selective localization in Klebsiella pneumoniae-infected lung tissue. J Infect Dis. 1993;168:164–171. doi: 10.1093/infdis/168.1.164. [DOI] [PubMed] [Google Scholar]
- 6.Bakker-Woudenberg I A, ten Kate M T, Stearne-Cullen L E, Woodle M C. Efficacy of gentamicin or ceftazidime entrapped in liposomes with prolonged blood circulation and enhanced localization in Klebsiella pneumoniae infected lung tissue. J Infect Dis. 1995;171:938–947. doi: 10.1093/infdis/171.4.938. [DOI] [PubMed] [Google Scholar]
- 7.Bowden R A, Cays M, Gooley T, Mamelok R D, van Burik J A. Phase I study of amphotericin B colloidal dispersion for the treatment of invasive fungal infections after marrow transplant. J Infect Dis. 1996;173:1208–1215. doi: 10.1093/infdis/173.5.1208. [DOI] [PubMed] [Google Scholar]
- 8.Cicogna C E, White M H, Bernard E M, Ishimura T, Sun M, Tong W P, Armstrong D. Efficacy of prophylactic aerosol amphotericin B lipid complex in a rat model of pulmonary aspergillosis. Antimicrob Agents Chemother. 1997;41:259–261. doi: 10.1128/aac.41.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark J M, Whitney R R, Olsen S J, George R J, Swerdel M R, Kunselman R, Bonner D P. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob Agents Chemother. 1991;35:615–621. doi: 10.1128/aac.35.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 11.Ellis M, Spence D, de Pauw B, Meunier F, Marinus A, Collette L, Sylvester R, Meis J, Boogaerts M, Selleslag D, Krcmery V, von Sinner W, MacDonald P, Doyen C, van der Cam B. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin Infect Dis. 1998;27:1406–1412. doi: 10.1086/515033. [DOI] [PubMed] [Google Scholar]
- 12.Francis P, Lee J W, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P A, Walsh T J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 13.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(Suppl. 2):S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 14.Krüger W, Stockschläder M, Rüssmann B, Berger C, Hoffknecht M, Sobottka I, Kohlschütter B, Kroschke G, Kröger N, Horstmann M, Kabisch H, Zander A R. Experience with liposomal amphotericin B in 60 patients undergoing high-dose therapy and bone marrow or peripheral blood stem cell transplantation. Br J Haematol. 1995;91:684–690. doi: 10.1111/j.1365-2141.1995.tb05369.x. [DOI] [PubMed] [Google Scholar]
- 15.Leenders A C, de Marie S, ten Kate M T, Bakker-Woudenberg I A, Verbrugh H A. Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared to amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary aspergillosis. J Antimicrob Chemother. 1996;38:215–225. doi: 10.1093/jac/38.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Leenders A C, Daenen S, Jansen R L H, Hop W C J, Lowenberg B, Wijermans P W, Cornelissen J, Herbrecht R, van der Lelie H, Hoogsteden H C, Verbrugh H A, de Marie S. AmBisome compared with amphotericin B deoxycholate in the treatment of neutropenia-associated invasive fungal infections. Br J Haematol. 1998;103:205–212. doi: 10.1046/j.1365-2141.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehta J, Kelsey S, Chu P, Powles R, Hazel D, Riley U, Evans C, Newland A, Treleaven J, Singhal S. Amphotericin B lipid complex (ABLC) for the treatment of confirmed or presumed fungal infections in immunocompromised patients with hematologic malignancies. Bone Marrow Transplant. 1997;20:39–43. doi: 10.1038/sj.bmt.1700842. [DOI] [PubMed] [Google Scholar]
- 18.Myint H, Kyi A A, Winn R M. An open, non-comparative evaluation of the efficacy and safety of amphotericin B lipid complex as treatment of neutropenic patients with presumed or confirmed pulmonary fungal infections. J Antimicrob Chemother. 1998;41:424–426. doi: 10.1093/oxfordjournals.jac.a020867. [DOI] [PubMed] [Google Scholar]
- 19.Otsubo T, Maruyama K, Maesaki S, Miyazaki Y, Tanaka E, Takizawa T, Moribe K, Tomono K, Tashiro T, Kohno S. Long-circulating immunoliposomal amphotericin B against invasive pulmonary aspergillosis in mice. Antimicrob Agents Chemother. 1998;42:40–44. doi: 10.1128/aac.42.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson T F, Miniter P, Dijkstra J, Szoka F C, Ryan J L, Andriole V T. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol sulfate complexes. J Infect Dis. 1989;159:717–724. doi: 10.1093/infdis/159.4.717. [DOI] [PubMed] [Google Scholar]
- 21.Prentice H G, Hann I M, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, Pinkerton C R, Schey S A, Jacobs F, Oakhill A, Stevens R F, Darbyshire P J, Gibson B E. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol. 1997;98:711–718. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 22.Purcell I F, Corris P A. Use of nebulized liposomal amphotericin B in the treatment of Aspergillus fumigatus empyema. Thorax. 1995;50:1321–1323. doi: 10.1136/thx.50.12.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swenson C E, Perkins W R, Roberts P, Ahmad I, Stevens R, Stevens D A, Janoff A S. In vitro and in vivo antifungal activity of amphotericin B lipid complex: are phospholipases important? Antimicrob Agents Chemother. 1998;42:767–771. doi: 10.1128/aac.42.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Etten E W, Otte-Lambillion M, van Vianen W, ten Kate M T, Bakker-Woudenberg I A. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B-desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother. 1995;35:509–519. doi: 10.1093/jac/35.4.509. [DOI] [PubMed] [Google Scholar]
- 25.Van Etten E W, van Vianen W, Tijhuis R H, Storm G, Bakker-Woudenberg I A. Sterically stabilized amphotericin B-liposomes: toxicity and biodistribution in mice. J Control Release. 1995;37:123–129. [Google Scholar]
- 26.Van Etten E W, ten Kate M T, Stearne L E, Bakker-Woudenberg I A. Amphotericin B liposomes with prolonged circulation in blood: in vitro antifungal activity, toxicity, and efficacy in systemic candidiasis in leukopenic mice. Antimicrob Agents Chemother. 1995;39:1954–1958. doi: 10.1128/aac.39.9.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Etten E W, Snijders S V, van Vianen W, Bakker-Woudenberg I A. Superior efficacy of liposomal amphotericin B with prolonged circulation in blood in the treatment of severe candidiasis in leukopenic mice. Antimicrob Agents Chemother. 1998;42:2431–2433. doi: 10.1128/aac.42.9.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh T J, Garrett K, Feuerstein E, Girton M, Allende M, Bacher J, Francesconi A, Schaufele R, Pizzo P A. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob Agents Chemother. 1995;39:1065–1069. doi: 10.1128/aac.39.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh T J, Hiemenz J W, Seibel N L, Perfect J R, Horwith G, Lee L, Silber J L, DiNubile M J, Reboli A, Bow E, Lister J, Anaissie E J. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–1396. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 30.Walsh T J, Yeldandi V, McEvoy M, Gonzalez C, Chanock S, Freifeld A, Seibel N I, Whitcomb P O, Jarosinski P, Boswell G, Bekersky I, Alak A, Buell D, Barret J, Wilson W. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother. 1998;42:2391–2398. doi: 10.1128/aac.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh T J, Finberg R W, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg R N, Dummer S, Schuster M, Holcenberg J S. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T J, Seibel N L, Arndt C, Harris R E, Dinubile M J, Reboli A, Hiemenz J, Chanock S J. Amphotericin B lipid complex in pediatric patients with invasive fungal infections. Pediatr Infect Dis J. 1999;18:702–708. doi: 10.1097/00006454-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 33.White M H, Anaissie E J, Kusne S, Wingard J R, Hiemenz J W, Cantor A, Gurwith M, Du Mond C, Mamelok R D, Bowden R A. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis. Clin Infect Dis. 1997;24:635–642. [PubMed] [Google Scholar]
- 34.Wingard J R. Efficacy of amphotericin lipid complex injection (ABLC) in bone marrow transplant recipients with life-threatening systemic mycoses. Bone Marrow Transplant. 1997;19:343–347. doi: 10.1038/sj.bmt.1700664. [DOI] [PubMed] [Google Scholar]
- 35.Woodle M C, Newman M S, Cohen J A. Sterically stabilized liposomes: physical and biological properties. J Drug Target. 1994;2:397–403. doi: 10.3109/10611869408996815. [DOI] [PubMed] [Google Scholar]