Abstract
OBJECTIVES
Most strokes associated with atrial fibrillation (AF) result from left atrial appendage thrombi. Oral anticoagulation can reduce stroke risk but is limited by complication risk and non-compliance. Left atrial appendage exclusion (LAAE) is a new surgical option to reduce stroke risk in AF. The study objective was to evaluate the safety and feasibility of standalone thoracoscopic LAAE in high stroke risk AF patients.
METHODS
This was a retrospective, multicentre study of high stroke risk AF patients who had oral anticoagulation contraindications and were not candidates for ablation nor other cardiac surgery. Standalone thoracoscopic LAAE was performed using 3 unilateral ports access and epicardial clip. Periprocedural adverse events, long-term observational clinical outcomes and stroke rate were evaluated.
RESULTS
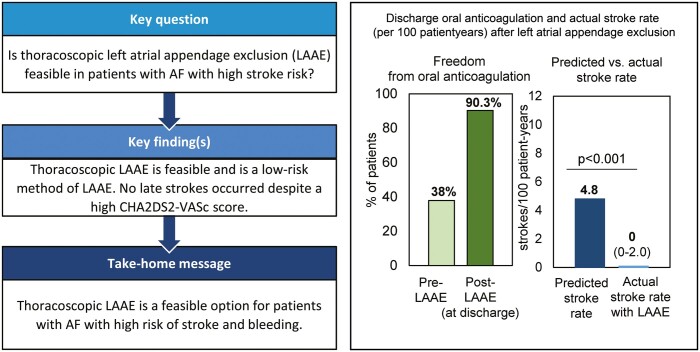
Procedural success was 99.4% (174/175 patients). Pleural effusion occurred in 4 (2.3%) patients; other periprocedural complications were <1% each. One perioperative haemorrhagic stroke occurred (0.6%). No phrenic nerve palsy or cardiac tamponade occurred. Predicted annual ischaemic stroke rate of 4.8/100 patient-years (based on median CHA2DS2-VASc score of 4.0) was significantly higher than stroke risk observed in follow-up after LAAE. No ischaemic strokes occurred (median follow-up: 12.5 months), resulting in observed rate of 0 (95% CI 0–2.0)/100 patient-years (P < 0.001 versus predicted). Six all-cause (non-device-related) deaths occurred during follow-up.
CONCLUSIONS
Study proved that a new surgical option, standalone thoracoscopic LAAE, is feasible and safe. With this method, long-term stroke rate may be reduced compared to predicted for high-risk AF population.
Keywords: Left atrial appendage, Stroke, Thoracoscopic, Atrial fibrillation, Clip, Anticoagulation
Atrial fibrillation (AF) remains a significant cause of morbidity and mortality worldwide and is associated with increased thromboembolic stroke risk [1, 2].
INTRODUCTION
Atrial fibrillation (AF) remains a significant cause of morbidity and mortality worldwide and is associated with increased thromboembolic stroke risk [1, 2]. The major origin of thrombus formation in patients with AF is the left atrial appendage (LAA) [3]. Prophylactic oral anticoagulation (OAC) remains a class I indication for thromboembolism in AF patients [4]. However, anticoagulation effectiveness is limited by therapy discontinuation due to bleeding complications, patients’ non-compliance and present contraindications. Mechanical LAA occlusion (LAAO) has recently emerged for this patient population who often had no other option. In such cases, recent guidelines recommend LAAO [4, 5]. Several transcatheter and surgical devices are either approved for LAA management or being studied in clinical trials [6–9]. Long-term efficacy of standalone surgical LAA exclusion (LAAE) has not been evaluated. Surgical epicardial exclusion using an LAAE clip (AtriClip LAA Exclusion Device, AtriCure, Mason, OH, USA) has demonstrated exceptional safety and highly durable exclusion rates [10]. The LAAE clip is mostly used concomitantly with cardiac surgical procedures including open chest and thoracoscopic. There are emerging data analysing the safety and efficacy, including stroke prevention, of LAAE in a standalone thoracoscopic procedure [11–13]. We previously reported early single-centre data on 4 patients who had standalone thoracoscopic LAAE [14]. The study aim was to assess feasibility, safety and long-term outcomes of thoracoscopic standalone LAAE in a large population of nonvalvular AF patients with a retrospective analysis of 3 centres.
PATIENTS AND METHODS
Ethical statement
This retrospective study was deemed exempt by local ethics committees; thus, patient consent was waived.
Study population
The study included patients with non-valvular AF who underwent isolated thoracoscopic LAAE at 3 US and European centres. All patients who had the procedure during the specified time period at each centre (BRRH: January 2015–April 2018; MIW: July 2016–November 2018; CCHMI: June 2016–March 2019) were included. Thoracoscopic standalone LAAE was offered for the prevention of AF-induced thromboembolism for patients with high risk of bleeding, those who had had thromboembolic events even on OAC or those with intolerance, and low chance for successful AF ablation due to advanced age, left atrial enlargement or AF duration. If AF was expected to be responsive to ablation, patients underwent thoracoscopic ablation with LAAE and were not included in the study. There were several common contraindications for standalone LAAE across all study sites: history of left-sided pleural cavity surgery or severe disease like left lung cancer. Presence of a thrombus in the LAA confirmed in transoesophageal echocardiography (TEE) a day before surgery was a contraindication. We considered redo cardiac surgery a relative contraindication for the procedure because for an individual patient it is possible that risks or complications with OAC and leaving the LAA open could be greater than the standalone LAAE procedure after prior cardiac surgery and potential adhesions. Other anatomical features that would preclude the thoracoscopic approach were also considered. The decision for standalone LAAE involved internists, clinical cardiologists, electrophysiologists and cardiac surgeons (Atrial Fibrillation Heart Team).
Surgical procedure
The surgical procedure involved totally thoracoscopic epicardial placement of the LAAE clip at the LAA base. The clip is a rectangular, self-closing epicardial LAAE device constructed with 2 nitinol springs joined with 2 titanium members covered with a knit-braided polyester fabric and attached to a dedicated guiding system. The parallel compression planes symmetrically exert a pressure with effect verified by both preclinical and clinical data [15–17].
Prior to the day of surgery, patients underwent TEE to exclude LAA thrombus. The totally thoracoscopic standalone LAAE procedure has been previously described [14] and was performed off-pump. Patients were extubated at the operating room or the intensive care unit directly after transfer from the operating room. Chest drainage was removed during the first 24 h after surgery and patients were transferred to general ward or discharged. Post-procedure, chest X-ray was performed to ensure the lack of pneumothorax.
Study end points
Perioperative outcomes
Perioperative safety end points included freedom from procedure-related mortality, conversion to sternotomy, bleeding requiring reoperation, stroke or transient ischaemic attack (TIA), phrenic nerve palsy or cardiac tamponade. Acute procedural effectiveness was determined by the completeness of LAAE assessed with intraoperative TEE. Echocardiographic criteria of successful LAAE were the absence of blood flow in LAA and LAA remnant stump <10 mm. Procedure duration and need for blood transfusions were also recorded.
Long-term clinical outcomes
All attempts were made to bring patients for in-person follow-up visit. Patients unable to come for an office visit were contacted by phone. Long-term clinical outcomes included freedom from device/procedure-related mortality, thromboembolic events, major bleeding (defined as requiring hospitalization or blood product transfusion) or recurrent minor bleeding and any intervention as a result of the primary procedure. TEE or CT to confirm LAAE post-procedurally was performed on a subset of patients per physicians’ discretion. According to patient and physician choice, follow-up CT or TEE for LAAE durability was planned however not obligatory due to tests’ inconvenience.
Statistical analysis
Categorical variables are reported as n/N and percentage, with 95% exact binomial confidence interval as indicated. Continuous variables are reported as median [interquartile range (IQR)] due to non-normality of data. For all variables, only patients with data were included; missing data were not imputed. For the confidence interval of observed stroke rate in patients with follow-up available, a 95% exact Poisson confidence interval was used. If the specific day of last follow-up was unknown, follow-up was considered to the first day of the month. SAS 9.4 (SAS Institute Inc.) was used.
RESULTS
Clinical characteristics of patient population
There were 175 patients included in the study. The most common indications for standalone LAAE were history of bleeding on OAC (67/173; 38.7%), lifestyle factors (including frailty, non-compliance and fall risk, 86/173; 49.7%), prior thromboembolism despite OAC (7/173, 4.0%) and OAC intolerance (5/173, 2.9%). Other specific indications were dialysis fistula (1/173; 0.6%), disqualification from percutaneous LAAO (3/173; 1.8%), meningioma requiring immediate OAC withdrawal (1/173, 0.6%), prior/recurring thromboembolism (7/173; 4.0%) and recurrent thrombus in the LAA despite OAC (4/173; 2.7%). Detailed patient characteristics are shown in Table 1. The median CHA2DS2-VASc score was 4.0 [IQR 3.0, 5.0]. The median HAS-BLED score was 4.0 [IQR 3.0, 5.0]. Forty-seven percentage (83/175) had long-standing persistent AF, 9.1% (16/175) had persistent AF and 43% (76/175) had paroxysmal AF. Twenty-six percentage (43/165) had prior stroke, TIA and/or thromboembolism and 77.7% (136/175) had a history of or predisposition to major bleeding. Prior to LAAE, 62% (108/174) were on OAC.
Table 1:
Baseline characteristics
| Characteristic | Median [IQR]a or n/N (%) |
|---|---|
| Age | |
| ≤64 | 22/175 (12.6) |
| 65–74 | 59/175 (33.7) |
| ≥75 | 94/175 (53.7) |
| Gender | |
| Male | 89/175 (50.9) |
| Female | 86/175 (49.1) |
| CHA2DS2-VASc | 4.0 [3.0, 5.0]; N = 172 |
| ≤1 | 11/172 (6.4) |
| 2–3 | 58/172 (33.7) |
| ≥4 | 103/172 (59.9) |
| HAS-BLED | 4.0 [3.0, 5.0], N = 172 |
| <3 | 46/172 (26.7) |
| ≥3 | 126/172 (73.3) |
| AF | |
| Long standing persistent | 83/175 (47.4) |
| Paroxysmal | 76/175 (43.4) |
| Persistent | 16/175 (9.1) |
| History of hypertension | 148/173 (85.5) |
| History of congestive heart failure | 30/173 (17.3) |
| History of diabetes | 33/173 (19.1) |
| Prior stroke/TIA or thromboembolism | 43/165 (26.1) |
| Prior bleeding | 136/175 (77.7) |
| History of vascular disease | 50/173 (28.9) |
| History of renal disease | 33/173 (19.1) |
AF: atrial fibrillation; IQR: interquartile range; TIA: transient ischaemic attack.
Median and interquartile range (IQR) reported due to non-normality of data.
Perioperative period outcomes
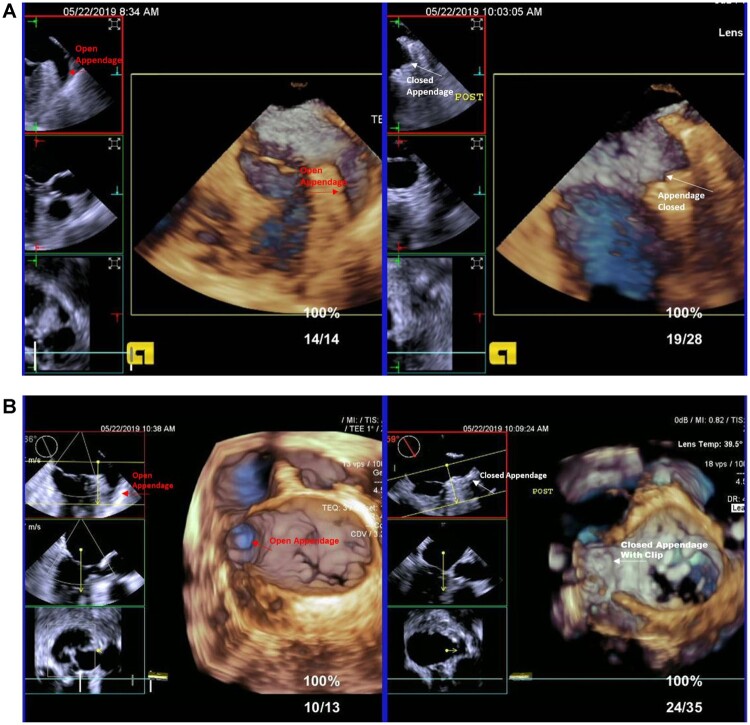
Complete LAAE was achieved intraoperatively in 174 of 175 (99.4%) patients (Fig. 1), with 1 residual stump of 10 mm. The median skin-to-skin procedural duration was 38 [IQR: 22.0–60.0] min (N = 169). In 174 (99.4%) patients, the procedure was totally thoracoscopic. There were no periprocedural complications in 92% (159/173) of patients (Table 2). One patient (0.6%) required conversion to sternotomy due to LAA bleeding. One patient with severe pleural adhesions from a previous cardiac surgery experienced bleeding from apex of the lung; mini-thoracotomy was performed to address this. Four patients (2.3%) had pleural effusions treated with thoracentesis and resolved; no chest tubes were required in these cases. No patients required a blood transfusion. No significant device-related perioperative bleeding, phrenic nerve palsy or cardiac tamponade were observed. One patient (0.6%) with a history of previous ischaemic and haemorrhagic strokes experienced a haemorrhagic stroke 3 days after the LAAE procedure. After discharge, 1 patient received an excessive dose of unfractionated heparin and developed a pulmonary haematoma, confirmed by exploratory thoracotomy.
Figure 1:
Postoperative left atrial appendage exclusion. (A) Representative transoesophageal echocardiograms before (intraoperative, left panel) and after (immediate post-procedure, right panel) clip deployment. (B) Three-dimensional reconstructions from representative transoesophageal echocardiograms before and after clip deployment, showing complete left atrial appendage exclusion immediately post-procedure. Red and white arrows indicate the open and excluded left atrial appendage, respectively.
Table 2:
Procedural complications
| Complication | n/N (%) |
|---|---|
| Acute heart failure | 1/173 (0.6) |
| Bleeding from pre-existing pleural adhesionsa | 1/173 (0.6) |
| Chest revisionb | 1/173 (0.6) |
| Conversion to sternotomy | 1/173 (0.6) |
| Haematuria | 1/173 (0.6) |
| Haemorraghic stroke | 1/173 (0.6) |
| Ileus | 1/173 (0.6) |
| Other bleeding complication | 1/173 (0.6) |
| Pleural effusion | 4/173 (2.3) |
| Pneumonia | 1/173 (0.6) |
| Pneumothorax | 1/173 (0.6) |
Minithoracotomy was performed to address bleeding from existing pleural adhesion from previous cardiac surgery.
Patient received excessive dose of unfractionated heparin and had an exploratory thoracotomy that confirmed pulmonary haematoma.
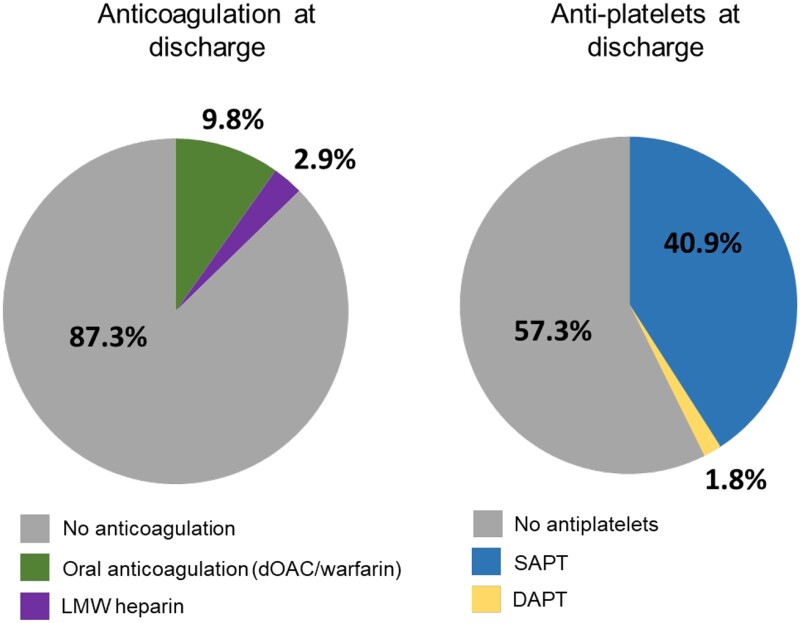
OAC was stopped in 90.3% (158/175) at discharge (Table 3). Post-LAAE, vitamin K antagonists or direct OAC were administered in 17 (10%) patients, while low molecular weight heparin was administered in 5 (3%) patients (Table 3 and Fig. 2). All 17 patients discharged with OAC were taking OAC prior to LAAE, and 3 out of the 5 patients discharged with low molecular weight heparin were taking it prior to LAAE. Fifty-seven percentage (98/171) of patients were discharged without anti-platelet therapy, with the remaining 41% (70/171) discharged on single antiplatelet therapy and 2% (3/171) on dual antiplatelet therapy.
Table 3:
Pre- and post-implant (at discharge) medication use
| Parameter | n/N (%) | Exact 95% confidence interval |
|---|---|---|
| Pre-implant medications | ||
| Freedom from OAC | 66/174 (37.9) | (30.7%, 45.6%) |
| Freedom from LMW heparin | 168/174 (96.6) | (92.6%, 98.7%) |
| Freedom from anticoagulation (OAC or LMW heparin) | 60/174 (34.5) | (27.5%, 42.1%) |
| Post-implant (at discharge) medications | ||
| Freedom from OAC use post-implant | 158/175 (90.3) | (84.9%, 94.2%) |
| Freedom from LMW heparin use post-implant | 168/173 (97.1) | (93.4%, 99.1%) |
| Freedom from anticoagulation (OAC or LMW heparin) use post-implant | 151/173 (87.3) | (81.4%, 91.9%) |
| Freedom from antiplatelet use post-implant | 98/171 (57.3) | (49.5%, 64.8%) |
| Single antiplatelet use | 70/171 (40.9) | (33.5%, 48.7%) |
| Dual antiplatelet use | 3/171 (1.8) | (0.4%, 5.0%) |
LMW: low molecular weight; OAC: oral anticoagulation.
Figure 2:
Medication use at discharge after left atrial appendage clip exclusion. Left panel: post-implant use of oral anticoagulation (direct oral anticoagulation or warfarin) at discharge (green), low molecular weight heparin (purple) or none (grey). Right panel: post-implant use of single antiplatelet therapy (blue), dual antiplatelet therapy (yellow) or none (grey).
Long-term clinical outcomes
Follow-up was available for 165/175 (92%) patients (median 12.5 [IQR 5.9, 17.1] months). There were 6 all-cause deaths (3.6%; 95% CI 1.3%, 7.8%) but no device related or procedure related (0%; 95% CI 0%, 2.2%). In patients with successful LAAE, no ischaemic strokes occurred during follow-up. Based on the CHA2DS2-VASc score (median 4.0 [IQR 3.0, 5.0]), predicted ischaemic stroke rate for this population was 4.8/100 patient-years [18, 19]. The actual stroke rate (95% CI 0–2.0/100 patient-years) was significantly reduced from predicted level (P < 0.001 by Poisson exact test, Fig. 3). During follow-up, no major bleeding was reported.
Figure 3:
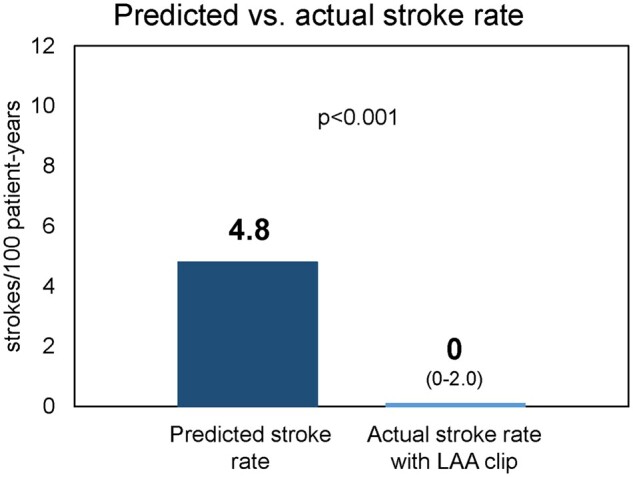
Actual stroke rate with left atrial appendage clip exclusion compared to prognosis. The bar chart depicts the predicted stroke rate per 100-patient years based on CHA2DS2-VASc score compared to actual stroke rate during the study period (with 95% CI). P-value reflects Poisson exact test.
Verification of durability of LAAE
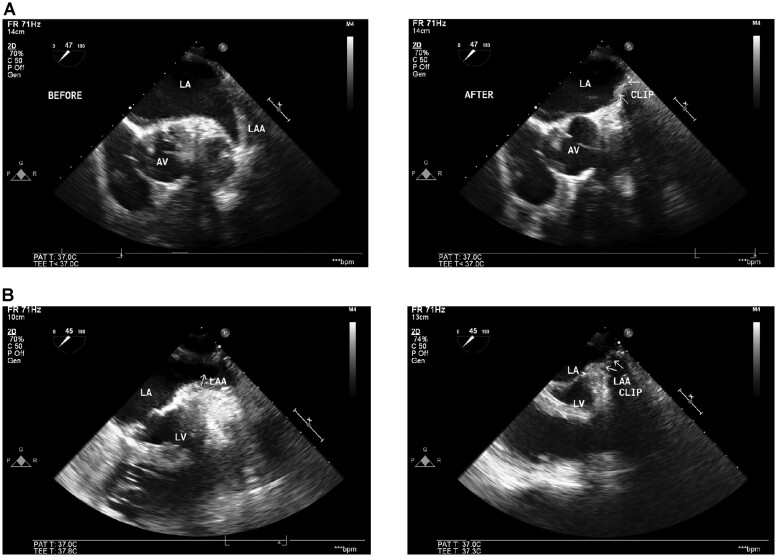
At registry closing, 36 (21%) patients received a computed tomography (CT) or TEE (Fig. 4). The median time from index procedure to follow-up imaging was 9.7 [IQR 4.6, 19.9] months. No LAA leaks were detected. Two patients (5.6%) had residual LAA stumps of 10 mm; however, device migration had not occurred. No intracardiac thrombi in the area of occluder were reported (0%; 95% CI 0, 9.7%).
Figure 4:
Durability of left atrial appendage exclusion. Representative follow-up transoesophageal echocardiograms from 2 patients (A and B) with continued exclusion at least 6 months from index procedure. Left panels are pre-left atrial appendage exclusion; right panels are follow-up transoesophageal echocardiograms. AV: aortic valve; LA: left atrium; LV: left ventricle.
DISCUSSION
Clinical relevance
Typically, 2 major complications are reported in AF patients treated with OAC: (i) inadequate level of anticoagulation leading to increased stroke risk and (ii) occurrence of major bleeding that necessitates OAC discontinuation. Complications on chronic OAC and risks associated with discontinuation have been reported. A real-world study revealed that ∼30% of patients with nonvalvular AF discontinued OAC in first 2 years after therapy admission [20]. The FibStroke study showed significant underuse of anticoagulants in patients with previously diagnosed AF suffering from stroke [21]. In that cohort, only 55.1% of high-risk AF patients (CHA2DS2-Vasc ≥2) were on OAC prior to stroke. Primary reasons for discontinuing anticoagulation were bleeding (13%) and infrequent AF paroxysms (14%). A US MarketScan analysis (12 129 patients) revealed over a mean duration of 120 days, up to 47% AF patients independently discontinued anticoagulation therapy, as documented by no OAC prescription claims ≥60 days after prior prescription expiration [22]. Patients with bleeding were significantly more likely to discontinue OAC. There is an unmet need for a reliable therapeutic option to reduce stroke risk in high-risk AF patients.
Current role of mechanical devices for stroke prevention in non-valvular AF
Because most thrombi causing strokes in AF patients arise in the LAA, it is occluded in an effort to mitigate stroke risk. Currently, 2 LAAO devices are approved in the USA and/or Europe for thromboembolic prevention [8, 9]. However, potential concerns with transcatheter LAAO include observed acute complication rates, need for bridging anticoagulation, mid- and long-term leaks or device-related thromboembolism [23].
Favourable long-term safety, efficacy and durability have been reported for the LAAE clip when implanted during concomitant open heart surgical or thoracoscopic ablation procedures [10]. Standalone thoracoscopic LAAE clip represents a new therapeutic solution that combines the efficacy of epicardial LAAE through a minimally invasive method. Standalone LAAE is primarily suited for selected patients who are unable to receive OAC and are disqualified from transcatheter LAAO, including patients with a high bleeding risk (usually intermittent) requiring immediate cessation of anticoagulants due to clinical status [12–14, 24]. In contrast with percutaneous devices, epithelialization of the external LAA clip is not required; thus, bridging anticoagulation is not mandatory. Individuals whose LAA anatomy is not suitable for transcatheter LAAO may also benefit from thoracoscopic epicardial clipping. Some patients in this cohort were referred for thoracoscopic procedure due to disqualification from transcatheter LAAO for these reasons.
LAAE is a safe and effective therapy for stroke reduction in non-valvular AF
Our results demonstrated the feasibility and safety of standalone totally thoracoscopic LAAE. Complete intraoperative LAAE was obtained in all but 1 patient who had a 10-mm residual stump. We hypothesize that the high acute efficacy of LAAE is due to the ease of optimizing device placement by reopening and repositioning it until proper seating is obtained. As such, a close collaboration between surgeon and echocardiographer is critical. In 36 patients who had follow-up LAA imaging (median 9.7 months from index procedure), 94.4% of LAAs did not have residual stumps, 100% were leak free and no device migration was observed. There are currently no standard methods of measuring the LAA stump, so the 2 residual stumps observed during follow-up were likely due to variations with measurements made on intraprocedural and follow-up imaging, as previously discussed [25]. In these 2 patients, the difference between intraoperative and follow-up remnant stump length measurement crossed the arbitrarily adopted 10 mm cut-off. It is highly probable that this resulted from different techniques of echocardiographic calculations of remnant stump, which has not been standardized yet. It should be underscored that the 10-mm borderline for remnant stump is used as intraoperative end point but has never been found as clinically relevant risk factor. Thus, the usage of second parameter, which is blood flow in LAA, is crucial. Other studies have confirmed a lack of clip migration using follow-up imaging [12, 26]. Previously published data on 43 patients with LAA clip implanted during concomitant open heart surgery demonstrated complete LAAE with no blood flow and only one residual stump >10 mm at a mean 7.1 (0.8) years post-procedure [25]. Because of reported LAAE durability, strict imaging follow-up to verify continued exclusion is not necessitated in routine clinical practice.
Although transcatheter LAAO and thoracoscopic LAAE have not been compared in a randomized study, our data suggest that totally thoracoscopic LAAE may potentially be considered a safe alternative to transcatheter LAAO. Many perioperative adverse events associated with transcatheter LAAO devices were absent with LAAE. No device-related thrombi were observed with LAAE. Due to visualization to rule out bleeding and pericardial incision, thoracoscopic LAAE has a low risk of cardiac tamponade. Other advantages include an off-pump and short (median 38 min) procedure, requiring short general anaesthesia with mechanical ventilation. Tissue puncture is not required, keeping periprocedural bleeding risk low. The device can be repositioned before final deployment and is generally associated with a lower risk of bleeding and residual pouch than surgical excision. Fluoroscopy, atrial septal puncture (left to right residual shunting) or intravascular contrast (thus suitable for renal failure patients) are not required. Pericardial sac incision during epicardial access prevents high intrapericardial pressure formation by allowing communication with the left pleural cavity. Lastly, surgical epicardial LAAE has the benefit of electrically isolating the LAA [27], a favourable finding for positive antiarrhythmic effect [28].
This study evaluated the stroke rate in treated patients. One patient had a haemorrhagic stroke 3 days postoperatively. This patient had a previous haemorrhagic stroke 2 years prior to LAAE and the periprocedural stroke occurred in the same location; a previous ischaemic stroke was noted 6 years prior to LAAE. The median CHA2DS2-VASc score of the study population was 4.0 [IQR 3.0, 5.0], corresponding to an expected annual stroke rate of 4.8% [18, 19]. However, with the median follow-up of 12.5 months in this study population, the effective ischaemic stroke rate was 0%, suggesting that LAAE may have reduced the risk of stroke in this population. Six all-cause deaths occurred during follow-up; however, no procedural- or device-related deaths occurred. All-cause deaths were due to pre-existing bladder tumour, resulting in kidney failure, urosepsis and death; myocardial infarction; respiratory failure 2 years after the procedure in a patient who developed pneumonia and had pre-existing end-stage renal disease; and 2 age-related deaths, one of whom had other comorbidities leading to sepsis.
With transcatheter LAAO devices, OAC is typically taken for at least 45 days. In this study with LAAE, 151 out of 173 patients (87%) were able to discontinue anticoagulation therapy post-procedure. While 38% of patients were free from OAC pre-procedure, 90.3% of patients were free from OAC at discharge. This ability to safely discontinue OAC represents a benefit of epicardial LAAE compared to endocardial LAAO. While we found this procedure particularly effective in the subgroup of patients suffering from bleeding on anticoagulation, it is reasonable to continue anticoagulation post-implant according to HRS/ESC guidelines for patients who can tolerate OAC [4, 5]. LAAOS III results suggested that surgical LAAO may have an additive effect to OAC for stroke prevention [29]. In our study, there were no cases in which anticoagulation was reinitiated due to LAA thrombus or persistent LAA communication with the left atrium. One reason for remaining on OAC at discharge was prior stroke history, but some patients were able to reduce dosage after LAAE. Overall, individual risk–benefit of OAC continuation versus discontinuation was evaluated by the physician for each patient with consideration to guideline recommendations.
Limitations
Limitations associated with thoracoscopic intervention include the need for short induction and intubation. Individual risk factors should be considered. Patients with severely lowered lung function, previous cardiac surgery or surgery with left pleural cavity opening should be assessed individually and treated at experienced centres only. One study design limitation was that patients who were unable to return for in-person clinical visits self-reported their outcomes. Minor strokes, TIAs and thromboembolic events could have been underreported. However, patient-reported outcomes were confirmed by medical chart review. This was a retrospective observational study without a control arm; therefore, stroke rate was compared to the risk factor-predicted rate. Anti-platelet and OAC use at discharge were reported; long-term discontinuation was not confirmed, thus limiting assessment of OAC impact on long-term stroke risk. An expected study limitation was that the standalone LAA procedure is new and not yet standardized. Participating centres used their own standards for qualification, anticoagulation and follow-up. However, these variations were unlikely to detract from demonstrating procedural feasibility and safety.
CONCLUSION
Standalone totally thoracoscopic LAAE is feasible, safe and durable option for AF patients with contraindications/intolerance for routine OAC. AF-related thromboembolism risk including stroke may be potentially reduced with this new method.
Funding
No funding was provided for this study.
Conflict of interest: Piotr Suwalski is a consultant for AtriCure, Inc. Richard Cartledge has ownership interest/partnership/principal with SMISSON-CARTLEDGE (uncompensated).
Data availability
Data are available upon reasonable request to the corresponding author.
Author contributions
Richard Cartledge: Investigation; Project administration; Supervision; Writing—review & editing. Grzegorz Suwalski: Data curation; Writing—review & editing. Anna Witkowska: Data curation; Investigation. Gary Gottlieb: Conceptualization; Investigation; Project administration; Supervision; Writing—review & editing. Anthony Cioci: Conceptualization; Formal analysis; Investigation; Methodology; Writing—original draft. Gilbert Chidiac: Data curation; Methodology. Burak Ilsin: Data curation; Methodology; Project administration. Barry Merrill: Investigation; Validation; Writing—original draft. Piotr Suwalski: Formal analysis; Investigation; Project administration; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Martin Misfeld, Christoph Schmitz, Jian Ye and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AF
Atrial fibrillation
- IQR
Interquartile range
- LAA
Left atrial appendage
- LAAE
Left atrial appendage exclusion
- LAAO
Left atrial appendage occlusion
- OAC
Oral anticoagulation
- TEE
Transoesophageal echocardiography
Presented at the virtual American College of Cardiology meeting, Chicago, USA, 28-30 March, 2020.
REFERENCES
- 1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD. et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB.. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 3. Blackshear JL, Odell JA.. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–9. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016;50:e1–e88. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 6. Emmert MY, Puippe G, Baumuller S, Alkadhi H, Landmesser U, Plass A. et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg 2014;45:126–31. [DOI] [PubMed] [Google Scholar]
- 7. Lee RJ, Lakkireddy D, Mittal S, Ellis C, Connor JT, Saville BR. et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): rationale and design. Am Heart J 2015;170:1184–94. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K. et al. ; PREVAIL and PROTECT AF Investigators. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017;70:2964–75. [DOI] [PubMed] [Google Scholar]
- 9. Hildick-Smith D, Landmesser U, Camm AJ, Diener HC, Paul V, Schmidt B. et al. Left atrial appendage occlusion with the Amplatzer Amulet device: full results of the prospective global observational study. Eur Heart J 2020;41:2894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toale C, Fitzmaurice GJ, Eaton D, Lyne J, Redmond KC.. Outcomes of left atrial appendage occlusion using the AtriClip device: a systematic review. Interact CardioVasc Thorac Surg 2019;29:655–62. [DOI] [PubMed] [Google Scholar]
- 11. Smith NE, Joseph J, Morgan J, Masroor S.. Initial experience with minimally invasive surgical exclusion of the left atrial appendage with an epicardial clip. Innovations (Phila) 2017;12:28–32. [DOI] [PubMed] [Google Scholar]
- 12. Branzoli S, Marini M, Guarracini F, Pederzolli C, Pomarolli C, D'Onghia G. et al. Epicardial standalone left atrial appendage clipping for prevention of ischemic stroke in patients with atrial fibrillation contraindicated for oral anticaogulation. J Cardiovasc Electrophysiol 2020;31:2187–91. [DOI] [PubMed] [Google Scholar]
- 13. Franciulli M, De Martino G, Librera M, Desoky A, Mariniello A, Iavazzo A. et al. Stand-alone thoracoscopic left atrial appendage closure in nonvalvular atrial fibrillation patients at high bleeding risk. Innovations (Phila) 2020;15:541–6. [DOI] [PubMed] [Google Scholar]
- 14. Suwalski P, Witkowska A, Drobiński D, Rozbicka J, Sypuła S, Liszka I. et al. Stand-alone totally thoracoscopic left atrial appendage exclusion using a novel clipping system in patients with high risk of stroke—initial experience and literature review. Kardiochir Torakochirurgia Pol 2015;12:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fumoto H, Gillinov AM, Ootaki Y, Akiyama M, Saeed D, Horai T. et al. A novel device for left atrial appendage exclusion: the third-generation atrial exclusion device. J Thorac Cardiovasc Surg 2008;136:1019–27. [DOI] [PubMed] [Google Scholar]
- 16. Salzberg SP, Gillinov AM, Anyanwu A, Castillo J, Filsoufi F, Adams DH.. Surgical left atrial appendage occlusion: evaluation of a novel device with magnetic resonance imaging. Eur J Cardiothorac Surg 2008;34:766–70. [DOI] [PubMed] [Google Scholar]
- 17. Salzberg SP, Plass A, Emmert MY, Desbiolles L, Alkadhi H, Grunenfelder J. et al. Left atrial appendage clip occlusion: early clinical results. J Thorac Cardiovasc Surg 2010;139:1269–74. [DOI] [PubMed] [Google Scholar]
- 18. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 19. Friberg L, Rosenqvist M, Lip GY.. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–10. [DOI] [PubMed] [Google Scholar]
- 20. Johnson ME, Lefevre C, Collings SL, Evans D, Kloss S, Ridha E. et al. Early real-world evidence of persistence on oral anticoagulants for stroke prevention in non-valvular atrial fibrillation: a cohort study in UK primary care. BMJ Open 2016;6:e011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palomaki A, Mustonen P, Hartikainen JE, Nuotio I, Kiviniemi T, Ylitalo A. et al. Underuse of anticoagulation in stroke patients with atrial fibrillation—the FibStroke Study. Eur J Neurol 2016;23:133–9. [DOI] [PubMed] [Google Scholar]
- 22. Kachroo S, Hamilton M, Liu X, Pan X, Brixner D, Marrouche N. et al. Oral anticoagulant discontinuation in patients with nonvalvular atrial fibrillation. Am J Manag Care 2016;22:e1–e8. [PubMed] [Google Scholar]
- 23. Badheka AO, Chothani A, Mehta K, Patel NJ, Deshmukh A, Hoosien M. et al. Utilization and adverse outcomes of percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation in the United States: influence of hospital volume. Circ Arrhythm Electrophysiol 2015;8:42–8. [DOI] [PubMed] [Google Scholar]
- 24. Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW. et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2017;70:3042–67. [DOI] [PubMed] [Google Scholar]
- 25. Caliskan E, Eberhard M, Falk V, Alkadhi H, Emmert MY.. Incidence and characteristics of left atrial appendage stumps after device-enabled epicardial closure. Interact CardioVasc Thorac Surg 2019;29:663–669. [DOI] [PubMed] [Google Scholar]
- 26. Kurfirst V, Mokracek A, Canadyova J, Frana R, Zeman P.. Epicardial clip occlusion of the left atrial appendage during cardiac surgery provides optimal surgical results and long-term stability. Interact CardioVasc Thorac Surg 2017;25:37–40. [DOI] [PubMed] [Google Scholar]
- 27. Starck CT, Steffel J, Emmert MY, Plass A, Mahapatra S, Falk V. et al. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact CardioVasc Thorac Surg 2012;15:416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedman DJ, Black-Maier EW, Barnett AS, Pokorney SD, Al-Khatib SM, Jackson KP. et al. Left atrial appendage electrical isolation for treatment of recurrent atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2018;4:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M. et al. ; LAAOS III Investigators. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.