Abstract
The incidence of intracranial germ cell tumors (iGCT) is much lower in European and North American (E&NA) than in Asian population. However, E&NA cooperative groups have simultaneously developed with success treatment strategies with specific attention paid to long-term sequelae. Neurological sequelae may be reduced by establishing a diagnosis with an endoscopic biopsy and/or cerebrospinal fluid (CSF) and/or serum analysis, deferring the need to perform a radical surgery. Depending on markers and/or histological characteristics, patients are treated as either germinoma or non-germinomatous germ cell tumors (NGGCT). Metastatic disease is defined by a positive CSF cytology and/or distant drops in craniospinal MRI. The combination of surgery and/or chemotherapy and radiation therapy is tailored according to grouping and staging. With more than 90% 5-year event-free survival (EFS), localized germinomas can be managed without aggressive surgery, and benefit from chemotherapy followed by whole ventricular irradiation with local boost. Bifocal germinomas are treated as non-metastatic entities. Metastatic germinomas may be cured with craniospinal irradiation. With a 5-year EFS over 70%, NGGCT benefit from chemotherapy followed by delayed surgery in case of residual disease, and some form of radiotherapy. Future strategies will aim at decreasing long-term side effects while preserving high cure rates.
Keywords: adolescents and young adults, brain tumors, germ cell tumor, germinoma, non-germinomatous germ cell tumor
Most publications on intracranial germ cell tumors (iGCT) begin with an introduction that points out the 5- to 8-fold greater incidence between the Asian and non-Asian populations. Another major difference particularly concerns the management of this condition, which is primarily under the supervision of neurosurgical teams in Asian countries, whereas it is primarily coordinated by pediatric or medical oncologists in E&NA. This has contributed to differences in the risk classification and in the surgical management of iGCT. The aim of this review from Society for Neuro-Oncology (SNO), European Association for Neuro-Oncology (EANO), and EUropean reference network for Rare Adult solid CANcers (Euracan) is to describe how Western cooperative groups have developed a specific consensus on most aspects of the diagnosis and management of this complex entity.
Epidemiology and Symptoms
iGCT are rare CNS neoplasms that mainly affect adolescents and young adults (AYA), with a peak incidence between 10 and 19 years.1,2 In E&NA, iGCTs make up 1%-3% of brain tumors, in contrast to 8%-15% in East Asia.2 High incidence persists in East Asiatic migrants, suggesting a genetic background.3 No specific genetic origin has been identified in the E&NA populations. The incidence of iGCT is lower in the African American population. The incidence of iGCT has significantly increased in the United States over the recent decades. Most iGCT arise in the midline pineal (40%-60%) or suprasellar (30%-40%) regions, simultaneously in both locations (bifocal) (2%-26%) and rarely in basal ganglia and other sites.1,4 AYA iGCT predominate in males in whom the majority (50%) are germinomas in the pineal region, where the male:female ratio is roughly 13-15:1 while germinomas in the suprasellar region show a slight female predominance.1,5
Pathology and Markers
According to 2016 WHO classification, iGCT include 5 subtypes: germinoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratoma (mature and immature) (Table 1). iGCT are divided into germinoma (55%-65%) and non-germinomatous germ cell tumors (NGGCT) (35%-45%).1 Mixed iGCTs are composed of two or more of the subtypes, including most often germinoma and teratoma6,7 The immature teratomatous component may be graded following the Gonzalez-Crussi classification; however, its prognostic value remains unclear.8 Residual masses after chemotherapy mostly show mature teratoma.9 Teratomas with somatic-type malignancy are rare with the malignant components being mostly sarcomas.10
Table 1.
Histopathological and Immunohistochemical Features of Germ Cell Tumors in the Central Nervous System
| Main Histopathological Features | Typical Pattern of Immunoreactivity | |
|---|---|---|
| Germinoma | - Large monomorphous round cells with PAS (periodic acid Schiff)-positive clear cytoplasm - Atypical vesicular nuclei with prominent nucleolus - Association with an inflammatory population of lymphocytes (T cells > B cells) - Prominent granulomatous reaction may be observed and should not be misinterpreted as sarcoidosis or tuberculosis - Isolated syncytiotrophoblastic cells may be found and should not lead to the diagnosis of choriocarcinoma. It explains why low level (<50 IU/L) of β-HCG may be detected in serum or CSF |
SALL4+; OCT3/4+; CD30− CD117+ (membranous +++ and golgian) D2-40 (membranous) |
| Embryonal carcinoma | - Poorly differentiated epithelial cells arranged in solid sheets, glandular structures, and/or papillae - Marked nuclear atypia - High mitotic activity - Necrosis |
SALL4+; OCT3/4+; CD30+ Cytokeratins+ |
| Yolk sac tumor | - Neoplasm composed of atypical epithelial cells arranged in various architectural patterns - Reticular pattern: the most common, consisting of an anastomosing network of vacuolated tumoral cells lying in a myxoid stroma and delineating microcysts - Endodermal sinus pattern: the most typical, characterized by Schiller-Duval bodies (fibrovascular core surrounded by cuboidal to columnar neoplastic cells, the whole structure lying in a cystic space) - PAS-positive hyaline globules (especially found in hepatoid areas) - AFP may be detected in serum and/or CSF |
SALL4+; OCT3/4−; CD30− Cytokeratins+; alpha-fetoprotein+; glypican 3+ |
| Choriocarcinoma | - Neoplastic mononucleated cytotrophoblastic cells and neoplastic multinucleated syncytiotrophoblastic cells - Large blood lakes - Necrotic and hemorrhagic areas - High level ≥50 IU/L of β-HCG may be found in serum and/or CSF |
SALL4 ± (cytotrophoblast); OCT3/4−; CD30− Cytokeratins+; β-HCG+ |
| Teratoma | - Mature: admixture of adult-type tissue derived from the 3 main embryonic layers (ectoderm, endoderm, mesoderm) - Immature: admixture of fetal/embryonic tissues derived from the 3 main embryonic layers |
Immunophenotype according to the tissue SALL4 immunopositivity may be focally observed in some tissues (eg, primitive neuroepithelial tissue, mature-appearing enteric-type glands) |
iGCT may secrete HCG (the beta subunit of human chorionic gonadotrophin) and/or alpha-fetoprotein (AFP) in serum and/or cerebrospinal fluid (CSF): AFP is mainly produced by yolk sac tumors, though low levels may be detected from embryonal carcinomas and immature teratomas. HCG is secreted by choriocarcinoma (that contains both cyto- and syncytiotrophoblastic cells) and embryonal carcinomas. Germinoma through their syncytiotrophoblastic component can also secrete small amounts of HCG. In E&NA, there is no consensus regarding the cutoff of HCG value between germinoma and choriocarcinoma: patients with a similar HCG level may be treated as a germinoma in some protocols and as non-germinomatous germ cell tumors in others. Levels of HCG tend to be higher in the CSF, especially in ventricular compared to lumbar samples, while AFP values tend to be more elevated in the serum.
Biology
iGCT may arise from primordial germ cells (PGCs) that did not migrate properly to the genital ridge in the first weeks of gestation due to altered expression of additional mediators.11 Germinoma cells recapitulate the features of pluripotent human embryonic stem cells by upregulation of genes responsible for self-renewal such as OCT4, NANOG, and KLF4. In contrast, NGGCT are characterized by the expression of genes associated with neuronal differentiation, epithelial-mesenchymal transition, or the Wnt/β-catenin pathway. The latter is important for PGC proliferation and differentiation of pluripotent embryonal carcinoma.11,12 While chromosomal instability is a characteristic of all iGCT,13–15 global DNA hypomethylation is exclusively present in germinoma.16 Somatic KIT/RAS15,16 and PI3K/AKT15–17 mutations have been described in all iGCT, but more frequently in germinoma. Germinomas are further characterized by abundant expression of genes linked to immune response such as CCL18, CD72, and IL6R, consistent with lymphocyte infiltration.18,19 Germinoma cells highly express PD-L1, whereas tumor-infiltrating leukocytes show PD-1 expression,19 which may suppress the antitumoral immune response and lead to subsequent tumor growth.
Clinical Presentation and Imaging
Most pineal iGCTs present with obstructive hydrocephalus increased intracranial pressure and/or Parinaud syndrome.5,20 Suprasellar iGCT present with hypothalamic-pituitary axis insufficiency and/or decreased visual acuity and/or bi-temporal field deficits from compression or involvement of the optic chiasm. Diabetes insipidus (DI) is especially common, and other pituitary hormone deficits occur in 40%-60% of cases.21,22 HCG secreting tumors may present with precocious puberty and gynecomastia. Basal ganglia iGCT often present with gradual hemiparesis and cognitive decline. They are exceptional in the Western population.23
Standard craniospinal MRI is the primary imaging method for evaluating iGCTs (Table 2).
Table 2.
Brain and Spine MRI Protocol
| Essential MRI Study | |||
|---|---|---|---|
| Sequence | Slice thickness (mm) | Gap | Comment |
| Pre-contrast brain sequences | |||
| Axial DWI (b = 0.1000) with ADC | ≤4 | ≤0.4 | Or axial DTI |
| Axial T2 TSE/FSE | ≤4 | ≤0.4 | |
| Axial T1 SE/TSE/FSE | ≤4 | ≤0.4 | Or 3D T1 (≤1-mm slice thickness) |
| Axial 2D FLAIR (or post-contrast) | ≤4 | ≤0.4 | Or 3D FLAIR |
| Post-contrast brain sequences | |||
| Axial T1 SE/TSE/FSE | ≤4 | ≤0.4 | Or 3D T1 (≤1-mm slice thickness) |
| Coronal T1 SE/TSE/FSE | ≤4 | ≤0.4 | |
| Sagittal T1 SE/TSE/FSE | ≤3 | ≤0.3 | |
| Spine sequences | |||
| Post-contrast sagittal T1 SE (whole thecal sac) | 3 | ≤0.3 | If necessary, add post-contrast axial T1 |
| Complementary Brain Sequences | |||
| Axial SWI | Or axial T2* GRE (4-mm slice thickness) | ||
| Sagittal CISS, FIESTA or DRIVE | ≤0.6 | ||
| Single voxel MRS | TE = 135-144 ms at 1.5T; TE = 288 ms at 3T (due to j-coupling at 135-144 ms) |
Abbreviations: ADC, apparent diffusion coefficient; CISS, constructive interference in steady state; DRIVE, driven equilibrium; DTI, diffusion tensor imaging; DWI, diffusion-weighted imaging; FIESTA, fast imaging employing steady-state acquisition; FLAIR, fluid-attenuated inversion recovery; FSE, fast spin echo; GRE, gradient echo; MRS, magnetic resonance spectroscopy; SE, spin echo; SWI, susceptibility-weighted imaging; TE, echo time; TSE, turbo spin echo.
iGCTs are iso- or hypointense on T1- and T2-weighted sequences, with moderate to marked enhancement, with multiple small cyst-like areas without necrosis, and with a drop in apparent diffusion coefficient. Teratomas may be suggested when the 3 components (parenchymal, fat, and calcifications) are present. Basal ganglia germinomas show ipsilateral atrophy of the cerebral peduncle and/or basal ganglia and/or cerebral hemisphere; contrast enhancement is inconstant and patchy.
Among additional sequences, heavily T2-weighted sequences (driven equilibrium (DRIVE), constructive interference in steady state [CISS], or fast imaging employing steady-state acquisition [FIESTA] sequences) can be useful in cases of suprasellar involvement.24 A T2*-based MR sequence (conventional T2* gradient echo [GRE] or susceptibility-weighted imaging [SWI]) is very sensitive for the detection of basal ganglia involvement.25 Magnetic resonance spectroscopy (MRS) typically shows a prominent lipid peak.26
Disappearance of the spontaneously bright spot of the posterior pituitary on T1 is correlated to DI. DI with isolated pituitary stalk thickening can precede the diagnosis of iGCT by years. Algorithms have been suggested for the monitoring of these patients.27
The assessment of response is not homogeneous in E&NA protocols (volumetric vs bi-dimensional tumor measurements, volumetric changes defining response or progression, evaluation of complete response [CR]). Several E&NA protocols are proposing a response-based radiation strategy, real-time central review is becoming part of many trials. A consensus is highly desirable to facilitate data comparability.
Initial Staging
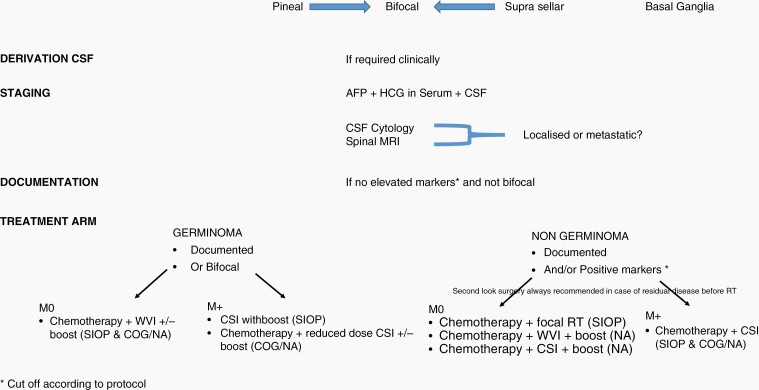
The diagnosis of iGCT is based on clinical signs, neuroimaging, markers in serum and CSF, and on histology (Figure 1). In the International Society of Pediatric Oncology (SIOP) trials, serum and/or CSF AFP 25 ng/mL or higher and/or HCG ≥ 50 IU/L define a secreting NGGCT whereas the cutoffs in Children’s Oncology Group (COG) studies is 10 ng/mL for AFP and 100 IU/L for HCG.28,29 SIOP trials have suggested an inferior outcome for patients whose AFP ≥ 1000 ng/mL.30 But these have not been replicated in 2 COG protocols. In E&NA grouping systems, similarly to other pediatric brain tumors, metastases (M+) are defined by a positive CSF cytology and/or drop metastasis: around 20% of iGCT have metastatic disease at diagnosis. However, interpretation of CSF cytology is still a matter of debate in E&NA. If CSF sample obtained from the third ventricle prior to biopsy shows tumor cells, a lumbar CSF cytology is advised with a 2 weeks delay: if negative, the tumor is considered localized. Deposits found on the walls of the ventricle during endoscopic procedure do not qualify the patient to have metastatic disease, unless they are visible on MRI. Bifocal lesions defined as lesions occurring within the pineal and suprasellar region are considered non-disseminated.31 Bifocal lesions and negative tumor markers are considered by consensus as germinomas and treated as such if imaging features are compatible. Loco-regional extension may not portend an inferior prognosis.32
Fig. 1.
Flow chart of management.
Surgery
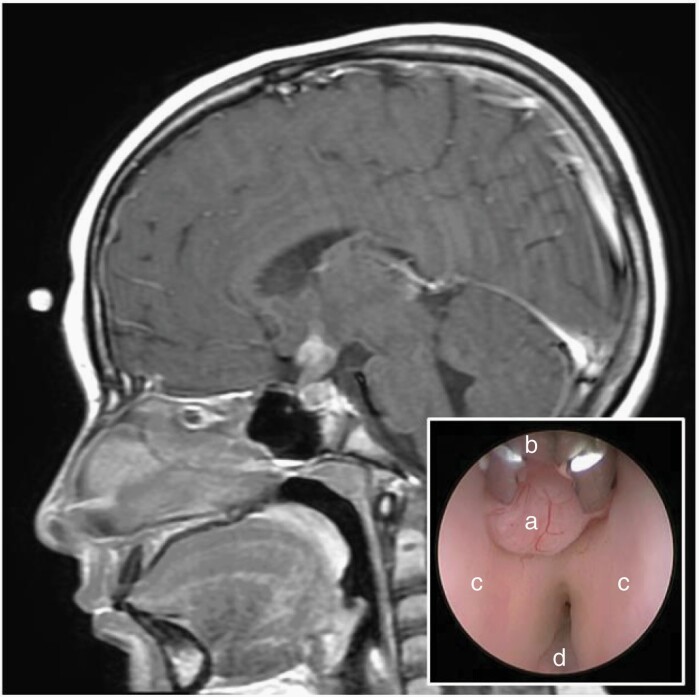
In E&NA countries, surgery has a limited role at the time of diagnosis. The benefit/risk of a systematic documentation argues in favor of selective indications for biopsy at the time of diagnosis though this will prevent the development of biological studies. A biopsy restricted to those who relapse may guide targeted therapy. Only marker-negative tumors should be biopsied with a preference for endoscopic techniques (Figure 2). Preferred management for pineal tumors and obstructive hydrocephalus is endoscopic third ventriculostomy (ETV) with or without tumor biopsy. If unsuitable, externalized ventricular drainage is an acceptable alternative. Ventriculoperitoneal shunt (VPS) should be avoided in most cases. For large supra or parasellar masses causing compartmentalized hydrocephalus simultaneous VPS with septal fenestration is favored. Sampling for cytology and markers is recommended in any form of CSF diversion prior to any biopsy.
Fig. 2.
Sagittal post-contrast MRI of the brain demonstrating a hypothalamic and infundibular mass in a 10-year-old female presenting with diabetes insipidus. Given normal serum and CSF tumor markers, a right frontal endoscopic tumor biopsy was performed which confirmed pure germinoma. Endoscopic view of the third ventricle (inset) revealing the exophytic tumor mass (a), 1 mm endoscopic biopsy forceps (b), and the bilateral hypothalami (c).
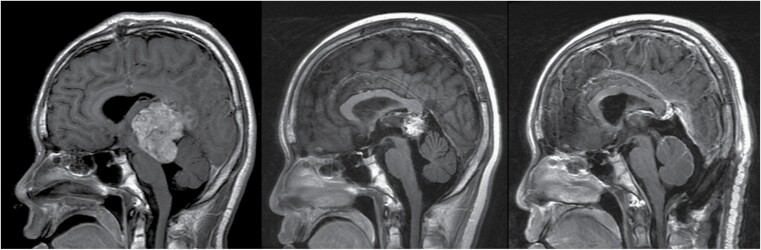
Surgery of the residual mass is highly recommended in NGGCT30 (Figure 3). In germinoma, surgery is recommended when an associated teratoma component is suspected based on failure of the tumor to shrink during chemotherapy. The intent of second-look surgery is a safe maximal resection. When tumor progression occurs during induction chemotherapy while markers are decreasing, this usually represents growing teratoma syndrome unresponsive to chemo/radiation therapy.33 It warrants a surgical excision The prognosis of these patients is usually excellent.34
Fig. 3.
This adolescent male presenting with obstructive hydrocephalus underwent an endoscopic third ventriculostomy (ETV) with simultaneous CSF sampling that confirmed a non-germinomatous germ cell tumor (NGGCT). Sagittal post-contrast MRI scans at diagnosis (left), after induction chemotherapy with normalization of tumor markers (center), and after second-look surgery via a supracerebellar infratentorial approach.
Radiotherapy
Radiotherapy is an integral component in the curative treatment of iGCT. A major aim of modern treatment technologies (volumetric-modulated arc therapy [VMAT], intensity-modulated radiotherapy [IMRT], proton beam radiation therapy [PBRT]) is to reduce the risk for long-term sequelae, in particular neurocognitive dysfunction.35,36 Chemo-radiotherapy approaches for adults are essentially based on data from retrospective and prospective pediatric and adolescent series.37 Treatment volumes can comprise craniospinal irradiation (CSI), whole-brain irradiation (WBI), whole ventricular irradiation (WVI), or involved-field radiotherapy (IF-RT) of tumor site. Selection of treatment volumes and dose prescriptions are tailored according to histological subtypes, extent of disease, the combination with and the response to chemotherapy. Pre-irradiation chemotherapy has allowed reduction of treatment volumes (eg, from CSI to WVI in germinoma) and dose prescriptions to tumor site and/or adjuvant regions. Given the heterogeneity of data, it is difficult to define a standard (Table 3).29,38,39 Increasingly, PBRT is being used,38 allowing a dose reduction to the nontarget brain.
Table 3.
European and North American Concepts for Radiotherapy
| Europe (SIOP) | North America (COG) | |
|---|---|---|
| Pure germinoma |
M0
Chx, WVI24Gy, F-RT/boost 16Gy; if CR no boost (currently under evaluation) M+ RT CSI alone 24Gy, IF-RT/boost 16Gy42,43 |
M0
ACNS1123: Chx WVI 18Gy, IF-RT/boost 12Gy for CR; WVI 24Gy, IF-RT/boost 12Gy if no CR is currently under evaluation |
| NGGCT |
M0
Chx, IF-RT 54Gy (surgery if not CR before RT) M+ Chx, CSI 30Gy, IF-RT/boost 24Gy29 |
M0
ACNS0122:Chx, CSI 36Gy, boost 18Gy ACNS1123: Chx, WVI/30.6Gy, boost 23.4Gy M+ ACNS0122: CSI 36Gy, boost 18Gy28,39 |
| Teratoma | No detailed recommendations (register) | No detailed recommendations |
Abbreviations: Chx: chemotherapy; CSI: craniospinal irradiation; F-RT: focal irradiation; NGGCT: non-germinomatous germ cell tumors; WVI, whole ventricular irradiation.
Because of their rarity and histological heterogeneity, no detailed recommendations can be given for the radiation treatment of teratoma. After incomplete resection IF-RT with 54Gy dose is an option40; The role of radiosurgery remains unclear.41
Chemotherapy
The chemosensitivity of iGCT is well recognized. Cisplatin or carboplatin have become the backbone of E&NA protocols, which have also included etoposide plus either cyclophosphamide or ifosfamide in most regimens.,30,39,43 The use of bleomycin has been progressively abandoned.44 There is no clear demonstration of survival benefit of cisplatin compared to carboplatin in iGCT. Rather, there is some evidence that cisplatin and other agents such as ifosfamide that require hyper-hydration are associated with a higher risk of toxicity (renal and/or neurological) among patients with DI.45 High-dose, marrow-ablative chemotherapy (HDC) regimens are used for poor responders or patients with recurrence. Most high-dose regimens include high-dose thiotepa combined with etoposide and/or carboplatin.46,47 Newer chemotherapeutic agents such as the combination of gemcitabine, oxaliplatin, or paclitaxel (GemPOx) and topotecan-containing regimens are currently being assessed in the recurrent setting.48
Strategy for Germinomas
Survival rates over 90% are reported in both E&NA trials. Current strategies have focused on treatment reduction to minimize long-term sequelae of treatment. The standard treatment comprises of combination of chemotherapy and radiotherapy, with surgery playing a limited role. Germinomas can be successfully treated with less chemotherapy and radiotherapy than NGGCT.
Since the 1950s, germinomas have been treated successfully with radiotherapy. Modern radiotherapy-only regimens can achieve high cure rates.49 CSI, followed by a tumor bed boost, was considered the gold standard treatment until the 1990s. It resulted in a 97% 5-year event-free survival (EFS) in the SIOP CNS GCT 96 trial, but this may amount to overtreatment for localized cases,50 The introduction of chemotherapy into treatment regimens aimed to reduce both the dose and volume of radiotherapy. Attempts to treat germinoma with chemotherapy alone resulted in less than 50% of cases being cured.51 Adjuvant chemotherapy followed by reduced dose and field radiotherapy, even for patients in CR after chemotherapy, is now considered standard of care, at least for localized germinoma.4
The focus over the last 30 years has been to identify the optimum combination of chemotherapy and radiotherapy. Early studies in the 1980s demonstrated excellent radiological responses to single-agent cyclophosphamide or carboplatin, with reduction of tumor bed boost from 50Gy to 30Gy without affecting overall survival (OS) for localized germinoma.52,53 International prospective trials in the late 1990s from SIOP and COG evaluated combinations of chemotherapy followed by IF-RT for localized germinoma. The COG trial used a combination of cisplatin, etoposide, cyclophosphamide, and vincristine followed by 30.4Gy focal radiotherapy and achieved a 3-year EFS of 92% ± 8% in 12 evaluable patients.42 The SIOP CNS GCT 96 trial compared 24Gy CSI plus 16Gy tumor bed boost with a combined approach using alternating courses of carboplatin-etoposide with ifosfamide-etoposide (“CarboPEI”) followed by 40Gy IF-RT. At 5 years, the EFS in the combined treatment group (n = 65) was 88% ± 4%.43 An excess of recurrences in the ventricles was identified.43,45,54 Thus WVI was adopted in place of IF-RT. The SIOP group utilized chemotherapy followed by 24Gy WVI with 16Gy boost to the tumor bed as the standard arm in their recently closed SIOP CNS GCT II trial, with the boost dose omitted for those with CR to “CarboPEI.” Other groups have successfully employed only carboplatin and etoposide before radiotherapy, thus avoiding the need for hyper-hydration; these regimens have been followed successfully with WVI to a total of 24Gy without boost.55 The COG conducted a response-based trial with a WVI dose of 18Gy and 12Gy tumor bed boost for patients with localized germinoma achieving CR after 4 courses of carboplatin-etoposide. Of 137 patients enrolled, 74 achieved a CR with an estimated 3-year progression-free survival (PFS) of 94.4% ± 2.7%.56
There is a general consensus that bifocal germinomas can be safely treated as localized tumors (at 2 sites) rather than as metastatic tumors,57 although the need for a biopsy in radiologically typical cases is a subject of ongoing debate,4 particularly given the report of non-secreting bifocal tumors that were confirmed as NGGCT.31,58,59
Successful treatment of metastatic germinoma requires CSI. While the European standard of care is 24Gy CSI with 16Gy boost to primary and metastatic sites, without additional chemotherapy,43 there is evidence supporting treatment without boost if chemotherapy is delivered before radiotherapy.55
Residual of germinoma following a good response to treatment can be left without negative impact on outcome.43 The presence of teratoma in cases initially treated as germinoma may only become apparent later, when reassessment imaging demonstrates less than expected response to chemotherapy; In such cases, where imaging indicates stable disease or poor response, surgical removal of residual tumor should be attempted before radiotherapy.
Strategy for NGGCT
One major specific change over the past decade in the E&NA experience is the reliance on tumor markers in serum and CSF alone for diagnosis, thereby avoiding the need for a biopsy or the risks associated with surgical debulking. Multi-modality treatment includes a timely combination of chemotherapy, radiation therapy, and selected neurosurgical resection. The optimum goal is to obtain a CR before initiating radiotherapy. Four to six courses of multi-agent chemotherapy are usually administered. For patients not experiencing CR, additional radical surgery or HDC is considered followed by some form of radiotherapy. Although there is some consistency and overlap in chemotherapy regimens used by E&NA cooperative groups, the doses and volumes of radiotherapy have been variable, with a persistent trend to consider CSI as the standard of care in North America, while the European approach was to deliver IF-RT to localized tumors and CSI to patients with metastatic disease only. As the diagnostic criteria for NGGCT are becoming more reliant on tumor markers in serum ± CSF, it is challenging to compare current outcomes to prior historical and cooperative group experiences where the diagnosis was histologically based.
In the SIOP CNS GCT 96, patients received 4 courses of chemotherapy with cisplatin, ifosfamide, and etoposide followed by IF-RT to 54Gy in case of localized disease (n = 116) or CSI to 30Gy along with a boost to a total dose of 54Gy to the primary and metastatic sites (n = 37). The 5-year PFS was 72% ± 4% and OS was 82% ± 4% for patients with localized disease and 68% ± 9% and 75% ± 8%, respectively, for patients with metastatic disease. AFP ≥ 1000 ng/mL in serum and/or CSF (n = 19) and the presence of residual disease at the end of treatment (n = 52) were adverse prognostic factors (5-year PFS of 32% ± 12% and 48% ± 7%, respectively).30 The SIOP study CNS GCTII now recommends upfront HDC in patients with AFP ≥ 1000 ng/mL and advises surgical resection in case of residual disease following completion of chemotherapy.
COG ACNS0122 utilized full-dose CSI (36Gy) along with a 18Gy tumor bed boost following 6 alternating cycles of carboplatin and etoposide with ifosfamide and etoposide. The 5-year EFS and OS for 102 eligible patients was 84% ± 4% and 93% ± 3%, respectively. There was no impact of serum or CSF elevations of HCG ≥ 1000 mIU/mL on survival; however, there was a trend toward inferior survival for patients with AFP elevations in serum and/or CSF (AFP ≥ 10 ng/L, P = .063). The 3-year PFS and OS for patients with CR + PR were 92% and 98%, respectively.39 In the successor COG study ACNS1123, only patients with localized NGGCT were included. The same induction chemotherapy regimen was used, but for patients with CR/PR, the dose was reduced to 30.6Gy WVI along with a boost to a total dose of 54Gy to the tumor bed. Of 107 eligible/enrolled patients on the study, the 4-year PFS and OS for 66 patients with CR/PR to induction was 88% ± 4% and 92% ± 3%, respectively.29 Twenty-four patients underwent second-look surgery, of whom 17 had mature teratoma or fibrosis/scar tissues and continued to receive reduced radiation, illustrating the fact that second-look surgery has a significant impact on treatment and should be strongly considered for patients with radiographic residual post-induction. The 3-year PFS and OS rates (±SD) for the 41 patients who did not qualify for CR/PR were 60.2% ± 7.8% and 81.7% ± 6.4%, respectively. There was no significant difference in survival for NGGCT patients with localized disease and CR/PR to induction chemotherapy in the 2 COG studies ACNS0122 and ACNS1123. However, the predominant site of relapse for patients in ACNS1123 was outside the WVI field, that is, in the spine (Table 4). Although it is tempting to speculate that the increase in spinal relapses was secondary to elimination of spinal irradiation, it is difficult to reconcile this observation when a similar trend was not observed on SIOP CNS 96. Based on the results of ACNS1123, COG recently launched a phase II trial of chemotherapy followed by WVI and spinal irradiation, with the aim of reducing the incidence of spinal relapses. In future studies, it may be reasonable to continue to validate the safety of dose and/or volume of radiotherapy reductions in patients experiencing a CR to pre-radiotherapy chemotherapy. The optimum radiotherapy treatment plan, however, is yet to be defined. There is an emerging consensus about the value of second-look surgery for persistent residual disease, post-induction chemotherapy, in the absence of tumor marker positivity. One of the confounding challenges when comparing outcomes is the recent reliance on tumor markers alone for diagnosis and enrollment on clinical trials in E&NA. Variation in observed levels of β-HCG may arise from differences in the timing (pre- vs post-op) and anatomic site of CSF acquisition (ventricular vs lumbar) in the E&NA studies, as the timing and site of fluid acquisition is not always apparent.
Table 4.
Strategy for Non-Germinomatous Intracranial Germ Cell Tumors
| Patterns of Relapse | |||||||
|---|---|---|---|---|---|---|---|
| Study | Treatment | PFS (5 y) | OS (5 y) | Local | Distant | Combined | Markers Alone |
| ACNS0122 (N = 48) | Induction (6 cycles) Carboplatin/Etoposide alternating with ifosfamide/etoposide Followed by CSI (36Gy) plus tumor bed boost (54Gy) |
92% | 98% | 9 | 4 | 0 | 2 |
| SIOP’96(N = 116) | Induction (4 cycles) With cisplatin, etoposide, and ifosfamide Followed by IF-RT (54Gy) |
72% | 82% | 14 | 9 | 6 | 0 |
| ACNS1123 (N = 66 CR/PR) |
Induction (6 cycles) Carboplatin/etoposide alternating with ifosfamide/etoposide Followed by WVI (30.6Gy) plus tumor bed boost (54Gy) |
88% (4 y) | 92% (4 y) | 0 | 7 | 1 | 0 |
Abbreviations: CR, complete response; CSI, craniospinal irradiation; IF, involved field; M0, localized disease; OS, overall survival; PFS, progression-free survival; PR, partial response; WVI, whole ventricular irradiation.
Relapses
Available data in the literature are limited due to the paucity of these events, and most are derived from E&NA experience. The median delay to relapse is longer in germinoma than in NGGCT. As some patients with positive markers may relapse without markers and vice versa, palliative situations excluded, complete staging should be performed prior to initiation of relapse treatment. Second-line treatments for relapse comprise chemotherapy, surgery, and re-irradiation.46,60–62 A prominent role of HDC for chemosensitive tumors has been suggested,46,63 with etoposide-thiotepa (with or without carboplatin) conditioning being the most commonly used regimen46,64; however, exact indications still remain to be defined. These outcomes are far better in germinoma, with post-relapse 5-year OS ranging from 55% to 88.9%60,61 vs 9% to 60% in NGGCT.4,47,60 Pure teratomas require exclusive surgical treatment. Other patients should be rechallenged with standard-dose chemotherapy (SDC) containing platinum-salt compounds, such as oxaliplatin, carboplatin, or cisplatin. In germinoma, HDC or re-irradiation are both valid options60 after SDC. Their combination may represent overtreatment with significant risk of toxicity. In NGCCT, both HDC and irradiation should be used after SDC in patients with at least good partial responses,4,60 Whether single or tandem transplants are superior in this setting remains unproven, but the former is likely ineffective in the absence of CR to reinduction. In refractory/relapsed disease, molecular profiling is encouraged and may identify therapeutic targets. This is particularly important as most relapsed patients are those with NGGCTs rather than germinomas, and by definition, many would not have an upfront biopsy as they will have had serum and/or CSF AFP and HCG marker levels above the thresholds required for biopsy at original diagnosis. Checkpoint inhibitors65 and brentuximab vedotin66 warrant study for PD-L1- and CD30-positive non-chemosensitive tumors, respectively.
Side Effects and Long-Term Patient Care
Due to their primary locations, iGCT can cause early neurological and endocrine symptoms.67 Given the favorable prognosis of iGCT,22,30 long-term tumor or treatment-related toxicities are therefore especially relevant.39
Radiotherapy plays a central role in the development of long-term sequelae such as second malignant neoplasms (SMN), strokes, neurocognitive deficits, and decline of health-related quality of life.55,68,69 Chemotherapy has long-term toxicity, mainly related to platinum agents such as permanent kidney impairment, hearing loss or peripheral neuropathy, hypogonadism, and infertility.70
Endocrine deficiencies occur in roughly half of the patients.71 Although there is always a risk of endocrine deficit associated with aggressive surgery and/or radiotherapy to the hypothalamic/pituitary region, endocrinopathies in iGCTs are generally related to the tumor itself rather than its management. DI, central hypothyroidism, central hypoadrenalism, growth hormone deficiency (GHD), hypogonadism, and hypothalamic obesity (HO) are the main endocrine complications.72 Management of these deficits requires specific attention: adrenocorticotropic hormone deficiency requires adequate hydrocortisone replacement and stress dose cover when needed. Growth hormone treatment should be initiated usually more than 1 year after completion of therapy.73 In hypogonadic patients, replacement therapy is needed. Patients should be referred for fertility preservation prior to treatment and later on for assisted reproductive technologies. Annual monitoring of thyroid and adrenal function and search for thyroid nodules is required. HO is one of the most challenging sequelae. Lifestyle intervention and psychological support should be initiated early as they have a limited success once HO is established.74 The cumulative incidence for SMN at 25 years is 6.1% in germinoma and 4.1% in NGGCT. The most common non-cancer-related cause of death is stroke.69 A recent report on 499 long-term survivors of iGCT from the SEER database identified a 59-fold increase in the risk of death from stroke at 25 years.68 Neurocognitive impairment is related to tumor site, age at diagnosis, fields and doses of irradiation. Irradiation may negatively impact intellectual functions, concept crystallization, executive function, working memory, quality of life, and adaptive skills, particularly in psychosocial domains. Patients treated with WVI had better outcomes than those with whole-brain radiotherapy or with CSI.75 Patients with pineal tumors showed early and stable deficits, whereas patients with suprasellar and bifocal tumors showed more protracted declines from initial average functioning.
Younger patients are at increased risk for psychosocial and physical problems. Quality of life at follow-up was better for patients ≥19 years (average range) than for those ≤18 years (low average borderline).
Perspectives and Future Directions in Diagnostics and Therapy
Five International CNS GCT Symposia have been held from 2003 to 2017 in Japan, the United States, and Europe. Since the third one,20 a formal international consensus process was undertaken, and many similar areas of practice were identified.4 Future directions in diagnostics include working toward a common AFP/HCG marker threshold definition. Consensus on which cases require diagnostic biopsy is also required. In future iGCT trials, the incorporation of prospective biological studies is critical. Reports of the mutational landscape of these tumors13,15,76 have improved our molecular understanding. However, limited tissue specimens are available to study, particularly for NGGCT cases.29,30 If high-risk groups can be identified upfront, then biopsy with molecular interrogation would become an attractive option. Future collection of biospecimens such as CSF and serum/plasma may allow minimally invasive diagnosis and disease monitoring using microRNA expression levels77 and also identification of the presence of tumor mutations through circulating tumor DNA analysis,78 which may inform prognosis and/or novel treatment strategies. It will be important to successfully exploit dysregulated molecular pathways. Mutually exclusive KIT/KRAS mutations occur in germinomas,15 similar to their testicular counterparts, but only rarely in NGGCT.15 However, when tyrosine kinase inhibitors have been employed in germinoma to target KIT, eg, imatinib79 or dasatinib,80 no partial/complete remissions were reported due to the predominance of exon 17 mutations present in germinoma which are less sensitive to early generations of KIT inhibitors. mTOR pathway alterations have also been described in iGCT,17 but monotherapy with the mTOR inhibitor everolimus had minimal success for testicular disease. Combinations of novel targeted agents will be required to overcome treatment resistance in such tumors, such as erlotinib and rapamycin which target EGFR and mTOR pathways, respectively.81 Unfortunately, poor accrual resulted in early closure of a trial that was testing erlotinib and sirolimus for this purpose. Brentuximab vedotin was successfully used in a patient with embryonal carcinoma and Down syndrome82 as was palbociclib in a patient with unresectable growing teratoma syndrome.83 Other potential novel treatment options include exploring BMP/SMAD pathway dysregulation, targeting CD30 (expressed by embryonal carcinoma), and/or altering immune regulation.84 In the future, the role of immunotherapy in the treatment of iGCT is another avenue to be explored.
Summary
Though much less frequent in E&NA as compared to Eastern Asia, the success story of iGCT management over the last 50 years is a paradigm. Both E&NA cooperative groups have successfully developed large networks and conducted clinical trials with a significant impact on clinical practice. Avoiding initial surgery when CSF and/or serum markers are positive, and using CSF cytology with spinal MRI to define metastatic disease is a hallmark of these trials. The combination of surgery, chemotherapy, and radiation therapy is tailored accordingly. With more than 90% 5-year EFS, most patients with localized or bifocal germinomas avoid aggressive surgery and benefit from chemotherapy followed by WVI with/without local boost. Only metastatic germinomas patients are treated with CSI. NGGCT are essentially diagnosed by positive markers and less often by pathology. With a 5-year EFS over 70%, they benefit from chemotherapy followed by aggressive surgery in case of residue. There are still ongoing debates regarding the optimal radiation management. Future strategies will aim at decreasing long-term side effects while preserving these high cure rates.
Acknowledgments
The authors would like to thank Kathy Oliver for reviewing the manuscript.
Conflict of interest statement. None declared.
Contributor Information
Didier Frappaz, Institut d’Hématologie Oncologie Pédiatrique, Lyon, France.
Girish Dhall, University of Alabama at Birmingham (UAB), Birmingham, Alabama, USA.
Matthew J Murray, Department of Pathology, University of Cambridge, Cambridge, UK; Department of Paediatric Haematology and Oncology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Stuart Goldman, Phoenix Children’s Hospital University of Arizona, Phoenix, Arizona, USA.
Cecile Faure Conter, Institut d’Hématologie Oncologie Pédiatrique, Lyon, France.
Jeffrey Allen, NYU Grossman School, New York, New York, USA.
Rolf Dieter Kortmann, University of Leipzig Medical Center, Leipzig, Germany.
Daphne Haas-Kogen, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Giovanni Morana, University of Turin, Turin, Italy.
Jonathan Finlay, Nationwide Children’s Hospital, Columbus, Ohio, USA.
James C Nicholson, Department of Paediatric Haematology and Oncology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Ute Bartels, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada.
Mark Souweidane, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Stefan Schönberger, Department of Pediatric Hematology and Oncology, University Hospital Essen, Essen, Germany.
Alexandre Vasiljevic, Centre de Pathologie et Neuropathologie Est, Hospices Civils de Lyon, Lyon, France.
Patricia Robertson, Mott Children’s Hospital, Ann Arbor, Michigan, USA.
Assunta Albanese, Royal Marsden Hospital, London, UK.
Claire Alapetite, Institut Curie, Paris, France.
Thomas Czech, Medical University of Vienna, Vienna, Austria.
Chin C Lau, Connecticut Children’s Medical Center, Hartford, Connecticut, USA.
Patrick Wen, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
David Schiff, University of Virginia School of Medicine, Charlottesville, Virginia, USA.
Dennis Shaw, Seattle Children’s Hospital and University of Washington, Seattle, Washington, USA.
Gabriele Calaminus, University of Bonn, Bonn, Germany.
Eric Bouffet, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada.
References
- 1. Goodwin TL, Sainani K, Fisher PG. Incidence patterns of central nervous system germ cell tumors: a SEER study. J Pediatr Hematol Oncol. 2009;31(8):541–544. [DOI] [PubMed] [Google Scholar]
- 2. Zapotocky M, Ramaswamy V, Lassaletta A, Bouffet E. Adolescents and young adults with brain tumors in the context of molecular advances in neuro-oncology. Pediatr Blood Cancer. 2018;65(2):e26861. [DOI] [PubMed] [Google Scholar]
- 3. Keene D, Johnston D, Strother D, et al. ; Canadian Pediatric Brain Tumor Consortium . Epidemiological survey of central nervous system germ cell tumors in Canadian children. J Neurooncol. 2007;82(3):289–295. [DOI] [PubMed] [Google Scholar]
- 4. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 5. Villano JL, Propp JM, Porter KR, et al. Malignant pineal germ-cell tumors: an analysis of cases from three tumor registries. Neuro Oncol. 2008;10(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takami H, Fukuoka K, Fukushima S, et al. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019;21(12):1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takami H, Perry A, Graffeo CS, Giannini C, Daniels DJ. Novel diagnostic methods and posttreatment clinical phenotypes among intracranial germ cell tumors. Neurosurgery. 2020;87(3):563–572. [DOI] [PubMed] [Google Scholar]
- 8. Gonzales-Crussi F. Extragonadal teratomas. In: Hartmann WH, ed. Atlas of Tumor Pathology. Second Series, Fascicle 18. Washington (DC): Armed Forces Institute of Pathology; 1982:1–49. [Google Scholar]
- 9. Takami H, Perry A, Graffeo CS, et al. Comparison on epidemiology, tumor location, histology, and prognosis of intracranial germ cell tumors between Mayo Clinic and Japanese consortium cohorts. J Neurosurg. 2020;134(2):446–456. [DOI] [PubMed] [Google Scholar]
- 10. Sato K, Takeuchi H, Kubota T. Pathology of intracranial germ cell tumors. Prog Neurol Surg. 2009;23:59–75. [DOI] [PubMed] [Google Scholar]
- 11. Oosterhuis JW, Looijenga LHJ. Human germ cell tumours from a developmental perspective. Nat Rev Cancer. 2019;19(9):522–537. [DOI] [PubMed] [Google Scholar]
- 12. Atlasi Y, van Dorsten RT, Sacchetti A, et al. Ectopic activation of WNT signaling in human embryonal carcinoma cells and its effects in short- and long-term in vitro culture. Sci Rep. 2019;9(1):11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takami H, Fukuoka K, Fukushima S, et al. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019;21(12):1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang HW, Wu YH, Hsieh JY, et al. Pediatric primary central nervous system germ cell tumors of different prognosis groups show characteristic miRNome traits and chromosome copy number variations. BMC Genomics. 2010;11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukushima S, Otsuka A, Suzuki T, et al. ; Intracranial Germ Cell Tumor Genome Analysis Consortium (iGCT Consortium) . Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014;127(6):911–925. [DOI] [PubMed] [Google Scholar]
- 16. Schulte SL, Waha A, Steiger B, et al. CNS germinomas are characterized by global demethylation, chromosomal instability and mutational activation of the Kit-, Ras/Raf/Erk- and Akt-pathways. Oncotarget. 2016;7(34):55026–55042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ichimura K, Fukushima S, Totoki Y, et al. ; Intracranial Germ Cell Tumor Genome Analysis Consortium . Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016;131(6):889–901. [DOI] [PubMed] [Google Scholar]
- 18. Zapka P, Dörner E, Dreschmann V, et al. Type, frequency, and spatial distribution of immune cell infiltrates in CNS germinomas: evidence for inflammatory and immunosuppressive mechanisms. J Neuropathol Exp Neurol. 2018;77(2):119–127. [DOI] [PubMed] [Google Scholar]
- 19. Liu B, Arakawa Y, Yokogawa R, et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One. 2018;13(4):e0194594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray MJ, Horan G, Lowis S, Nicholson JC. Highlights from the Third International Central Nervous System Germ Cell Tumour symposium: laying the foundations for future consensus. Ecancermedicalscience. 2013;7:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crawford JR, Santi MR, Vezina G, et al. CNS germ cell tumor (CNSGCT) of childhood: presentation and delayed diagnosis. Neurology. 2007;6:1668–73. [DOI] [PubMed] [Google Scholar]
- 22. Jorsal T, Rørth M. Intracranial germ cell tumours. A review with special reference to endocrine manifestations. Acta Oncol. 2012;51(1):3–9. [DOI] [PubMed] [Google Scholar]
- 23. Ozelame RV, Shroff M, Wood B, et al. Basal ganglia germinoma in children with associated ipsilateral cerebral and brain stem hemiatrophy. Pediatr Radiol. 2006;36(4):325–330. [DOI] [PubMed] [Google Scholar]
- 24. Godano E, Morana G, Di Iorgi N, et al. Role of MRI T2-DRIVE in the assessment of pituitary stalk abnormalities without gadolinium in pituitary diseases. Eur J Endocrinol. 2018;178(6):613–622. [DOI] [PubMed] [Google Scholar]
- 25. Morana G, Alves CA, Tortora D, et al. T2*-based MR imaging (gradient echo or susceptibility-weighted imaging) in midline and off-midline intracranial germ cell tumors: a pilot study. Neuroradiology. 2018;60(1):89–99. [DOI] [PubMed] [Google Scholar]
- 26. Yamasaki F, Kinoshita Y, Takayasu T, et al. Proton magnetic resonance spectroscopy detection of high lipid levels and low apparent diffusion coefficient is characteristic of germinomas. World Neurosurg. 2018;112:e84–e94. [DOI] [PubMed] [Google Scholar]
- 27. Robison NJ, Prabhu SP, Sun P, et al. Predictors of neoplastic disease in children with isolated pituitary stalk thickening. Pediatr Blood Cancer. 2013;60(10):1630–1635. [DOI] [PubMed] [Google Scholar]
- 28. PDQ Pediatric Treatment Editorial Board. Childhood Brain and Spinal Cord Tumors Treatment Overview (PDQ®): Health Professional Version; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389453. Accessed 2021.
- 29. Fangusaro J, Wu S, MacDonald S, et al. Phase II trial of response-based radiation therapy for patients with localized CNS nongerminomatous germ cell tumors: a Children’s Oncology Group study. J Clin Oncol. 2019;37(34):3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of patients with intracranial non-germinomatous germ cell tumors – lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol. 2017;19(12):1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cuccia V, Alderete D. Suprasellar/pineal bifocal germ cell tumors. Childs Nerv Syst. 2010;26(8):1043–1049. [DOI] [PubMed] [Google Scholar]
- 32. Duron L, Sadones F, Thiesse P, et al. Loco-regional extensions of central nervous system germ cell tumors: a retrospective radiological analysis of 100 patients. Neuroradiology. 2018;60(1):27–34. [DOI] [PubMed] [Google Scholar]
- 33. Kim CY, Choi JW, Lee JY, et al. Intracranial growing teratoma syndrome: clinical characteristics and treatment strategy. J Neurooncol. 2011;101(1):109–115. [DOI] [PubMed] [Google Scholar]
- 34. Michaiel G, Strother D, Gottardo N, et al. Intracranial growing teratoma syndrome (iGTS): an international case series and review of the literature. J Neurooncol. 2020;147(3):721–730. [DOI] [PubMed] [Google Scholar]
- 35. Park J, Park Y, Lee SU, Kim T, Choi YK, Kim JY. Differential dosimetric benefit of proton beam therapy over intensity modulated radiotherapy for a variety of targets in patients with intracranial germ cell tumors. Radiat Oncol. 2015;10(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Correia D, Terribilini D, Zepter S, et al. Whole-ventricular irradiation for intracranial germ cell tumors: dosimetric comparison of pencil beam scanned protons, intensity-modulated radiotherapy and volumetric-modulated arc therapy. Clin Transl Radiat Oncol. 2019;15:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lo AC, Hodgson D, Dang J, et al. Intracranial germ cell tumors in adolescents and young adults: a 40-year multi-institutional review of outcomes. Int J Radiat Oncol Biol Phys. 2020;106(2):269–278. [DOI] [PubMed] [Google Scholar]
- 38. Kortmann RD. Current concepts and future strategies in the management of intracranial germinoma. Expert Rev Anticancer Ther. 2014;14(1):105–119. [DOI] [PubMed] [Google Scholar]
- 39. Goldman S, Bouffet E, Fisher PG, et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: a Children’s Oncology Group study. J Clin Oncol. 2015;33(22):2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee YH, Park EK, Park YS, Shim KW, Choi JU, Kim DS. Treatment and outcomes of primary intracranial teratoma. Childs Nerv Syst. 2009;25(12):1581–1587. [DOI] [PubMed] [Google Scholar]
- 41. Chiu CD, Chung WY, Pan DH, Wong TT, Shih YH, Lee LS. Gamma knife radiosurgery for intracranial mature teratoma – long-term results and review of literature. Surg Neurol. 2006;65(4):343–351. [DOI] [PubMed] [Google Scholar]
- 42. Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, Wharam M. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;48(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Donelli MG, Zucchetti M, D’Incalci M. Do anticancer agents reach the tumor target in the human brain? Cancer Chemother Pharmacol. 1992;30(4):251–260. [DOI] [PubMed] [Google Scholar]
- 45. Afzal S, Wherrett D, Bartels U, et al. Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol. 2010;97(3):393–399. [DOI] [PubMed] [Google Scholar]
- 46. Modak S, Gardner S, Dunkel IJ, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol. 2004;22(10):1934–1943. [DOI] [PubMed] [Google Scholar]
- 47. Callec L, Lardy-Cleaud A, Guerrini-Rousseau L, et al. Relapsing intracranial germ cell tumours warrant retreatment. Eur J Cancer. 2020;136:186–194. [DOI] [PubMed] [Google Scholar]
- 48. Perez-Somarriba M, Moreno-Tejero ML, Rozas MI, Pelaez I, Madero L, Lassaletta A. Gemcitabine, paclitaxel, and oxaliplatin (GEMPOX) in the treatment of relapsed/refractory intracranial nongerminomatous germ cell tumors. Pediatr Blood Cancer. 2020;67(2):e28089. [DOI] [PubMed] [Google Scholar]
- 49. Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17(8):2585–2592. [DOI] [PubMed] [Google Scholar]
- 50. Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol. 2005;6(7):509–519. [DOI] [PubMed] [Google Scholar]
- 51. da Silva NS, Cappellano AM, Diez B, et al. Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer. 2010;54(3):377–383. [DOI] [PubMed] [Google Scholar]
- 52. Allen JC, Kim JH, Packer RJ. Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J Neurosurg. 1987;67(1):65–70. [DOI] [PubMed] [Google Scholar]
- 53. Allen JC, DaRosso RC, Donahue B, Nirenberg A. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74(3):940–944. [DOI] [PubMed] [Google Scholar]
- 54. Alapetite C, Brisse H, Patte C, et al. Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol. 2010;12(12):1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng S, Kilday JP, Laperriere N, et al. Outcomes of children with central nervous system germinoma treated with multi-agent chemotherapy followed by reduced radiation. J Neurooncol. 2016;127(1):173–180. [DOI] [PubMed] [Google Scholar]
- 56. Bartels U, Fangusaro J, Shaw D, et al. GCT-41. Response-based radiation therapy in patients with newly diagnosed central nervous system localized germinoma: a Children’s Oncology Group (COG) prospective phase 2 clinical trial. Neuro Oncol. 2020;22(Supplement_3):iii336–iii336. [Google Scholar]
- 57. Lafay-Cousin L, Millar BA, Mabbott D, et al. Limited-field radiation for bifocal germinoma. Int J Radiat Oncol Biol Phys. 2006;65(2):486–492. [DOI] [PubMed] [Google Scholar]
- 58. Aizer AA, Sethi RV, Hedley-Whyte ET, et al. Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro Oncol. 2013;15(7):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Esfahani DR, Alden T, Dipatri A, Xi G, Goldman S, Tomita T. Pediatric suprasellar germ cell tumors: a clinical and radiographic review of solitary vs. bifocal tumors and its therapeutic implications. Cancers (Basel). 2020;12(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Murray MJ, Bailey S, Heinemann K, et al. Treatment and outcomes of UK and German patients with relapsed intracranial germ cell tumors following uniform first-line therapy. Int J Cancer. 2017;141(3):621–635. [DOI] [PubMed] [Google Scholar]
- 61. Baek HJ, Park HJ, Sung KW, et al. Myeloablative chemotherapy and autologous stem cell transplantation in patients with relapsed or progressed central nervous system germ cell tumors: results of Korean Society of Pediatric Neuro-Oncology (KSPNO) S-053 study. J Neurooncol. 2013;114(3):329–338. [DOI] [PubMed] [Google Scholar]
- 62. Kubota H, Umeda K, Kagehiro K, et al. High-dose chemotherapy with autologous stem cell transplantation spares re-irradiation for recurrent intracranial germinoma. Pediatr Blood Cancer. 2018;65(8):e27104. [DOI] [PubMed] [Google Scholar]
- 63. Bouffet E. The role of myeloablative chemotherapy with autologous hematopoietic cell rescue in central nervous system germ cell tumors. Pediatr Blood Cancer. 2010;54(4):644–646. [DOI] [PubMed] [Google Scholar]
- 64. Finlay JL, Goldman S, Wong MC, et al. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors. The Children’s Cancer Group. J Clin Oncol. 1996;14(9):2495–2503. [DOI] [PubMed] [Google Scholar]
- 65. Zschäbitz S, Lasitschka F, Jäger D, Grüllich C. Activity of immune checkpoint inhibition in platinum refractory germ-cell tumors. Ann Oncol. 2016;27(7):1356–1360. [DOI] [PubMed] [Google Scholar]
- 66. Yeste-Velasco M, Guo T, Mao X, et al. The potential of brentuximab vedotin, alone or in combination with current clinical therapies, in the treatment of testicular germ cell tumors. Am J Cancer Res. 2019;9(5):855–871. [PMC free article] [PubMed] [Google Scholar]
- 67. Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86(3):446–455. [DOI] [PubMed] [Google Scholar]
- 68. Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. 2015;17(5):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perkins SM, Fei W, Mitra N, Shinohara ET. Late causes of death in children treated for CNS malignancies. J Neurooncol. 2013;115(1):79–85. [DOI] [PubMed] [Google Scholar]
- 70. Chovanec M, Abu Zaid M, Hanna N, El-Kouri N, Einhorn LH, Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol. 2017;28(11):2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hale GA, Marina NM, Jones-Wallace D, et al. Late effects of treatment for germ cell tumors during childhood and adolescence. J Pediatr Hematol Oncol. 1999;21(2):115–122. [DOI] [PubMed] [Google Scholar]
- 72. Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sklar CA, Antal Z, Chemaitilly W, et al. Hypothalamic-pituitary and growth disorders in survivors of childhood cancer: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(8):2761–2784. [DOI] [PubMed] [Google Scholar]
- 74. Abuzzahab MJ, Roth CL, Shoemaker AH. Hypothalamic obesity: prologue and promise. Horm Res Paediatr. 2019;91(2):128–136. [DOI] [PubMed] [Google Scholar]
- 75. Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: from the First International CNS Germ-Cell Tumor Study. Neuro Oncol. 2001;3(3):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang L, Yamaguchi S, Burstein MD, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 2014;511(7508):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murray MJ, Ajithkumar T, Harris F, et al. Clinical utility of circulating miR-371a-3p for the management of patients with intracranial malignant germ cell tumors. Neurooncol Adv. 2020;2(1):vdaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Einhorn LH, Brames MJ, Heinrich MC, Corless CL, Madani A. Phase II study of imatinib mesylate in chemotherapy refractory germ cell tumors expressing KIT. Am J Clin Oncol. 2006;29(1):12–13. [DOI] [PubMed] [Google Scholar]
- 80. Osorio DS, Finlay JL, Dhall G, Goldman S, Eisenstat D, Brown RJ. Feasibility of dasatinib in children and adolescents with new or recurrent central nervous system germinoma. Pediatr Blood Cancer. 2013;60(9):E100–E102. [DOI] [PubMed] [Google Scholar]
- 81. Chen KS, Fustino NJ, Shukla AA, et al. EGF receptor and mTORC1 are novel therapeutic targets in nonseminomatous germ cell tumors. Mol Cancer Ther. 2018;17(5):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abu Arja MH, Conley SE, Salceda V, Al-Sufiani F, Boué DR, Finlay JL. Brentuximab-vedotin maintenance following chemotherapy without irradiation for primary intracranial embryonal carcinoma in down syndrome. Childs Nerv Syst. 2018;34(4):777–780. [DOI] [PubMed] [Google Scholar]
- 83. Schultz KA, Petronio J, Bendel A, Patterson R, Vaughn DJ. PD0332991 (palbociclib) for treatment of pediatric intracranial growing teratoma syndrome. Pediatr Blood Cancer. 2015;62(6):1072–1074. [DOI] [PubMed] [Google Scholar]
- 84. Abu Arja MH, Bouffet E, Finlay JL, AbdelBaki MS. Critical review of the management of primary central nervous nongerminomatous germ cell tumors. Pediatr Blood Cancer. 2019;66(6):e27658. [DOI] [PubMed] [Google Scholar]