Abstract
A number of psychiatric disorders, including body dysmorphic disorder (BDD), anorexia nervosa, bulimia nervosa, and social anxiety disorder, are characterized by heightened appearance concerns and increased cognitive and perceptual biases toward one’s own physical appearance. In the present study, we examined individual differences in self-reported appearance anxiety and symptoms of BDD in relation to the late positive potential (LPP)—an index of stimulus significance—in response to pictures of oneself, strangers and objects among 83 female college students. The results indicated that the LPP was larger for pictures of oneself compared to pictures of strangers and objects. Further, the Yale–Brown Obsessive-Compulsive Scale Modified for Body Dysmorphic Disorder and Appearance Anxiety Inventory scales both related to an increased LPP to pictures of oneself but not to strangers or objects. The findings suggest that the LPP elicited by pictures of oneself may function as a neural marker of appearance concerns, which could be leveraged to study the development and maintenance of a range of psychiatric disorders characterized by increased appearance concerns.
Keywords: ERP, LPP, appearance concerns, attention bias, self-images
Physical appearance concerns are a primary feature of several psychiatric disorders. Body dysmorphic disorder (BDD), for example, is characterized by excessive concern with perceived defects or flaws in physical appearance that are slight or unnoticeable to others (American Psychiatric Association [APA], 2013). This preoccupation typically relates to the individual’s facial features, skin, hair, or weight and shape. Disturbed body images and concerns related to weight and shape are also considered to be central features of eating disorders, such as anorexia nervosa and bulimia nervosa (APA, 2013). Individuals with eating disorders are characterized by extreme fear of gaining weight and overvaluation of weight and shape in relation to self-worth (American Psychiatric Association, 2013; Linardon et al., 2018). Further, preoccupation with physical appearance is also common among individuals with social anxiety disorder (SAD), which is evident in terms of excessive self-consciousness and an intense fear of judgment or negative evaluation (American Psychiatric Association, 2013).
Collectively, these disorders are not only associated with significant distress, psychosocial impairment and morbidity but also highly comorbid (Fang and Hofmann, 2010; American Psychiatric Association, 2013; Kelly et al., 2013). Ruffolo et al. (2006) found that out of 200 individuals with BDD, nearly a third also met criteria for a lifetime eating disorder. Previous work has shown that 36–42% of individuals with an eating disorder also have a co-occurring SAD diagnosis in their lifetime (Godart et al., 2003; Swinbourne et al., 2012). For those with SAD, the lifetime prevalence of comorbid BDD is a bit lower, with estimates just over 11%—although these estimates are based on relatively small samples (Brawman-Mintzer et al., 1995; Wilhelm et al., 1997; Kelly et al., 2013).
Overall then, disorders characterized by appearance concerns co-occur much more frequently than would be expected by chance, suggesting possible shared vulnerability factors (Levinson et al., 2013). These high rates of comorbidity are also particularly concerning, as those with comorbid disorders are less likely to seek treatment (Goodwin and Fitzgibbon, 2002) and are at an increased risk for negative outcomes over and above the risks incurred by a single disorder—including greater psychosocial impairment, poorer treatment outcome, greater risk of concurrent substance or alcohol use disorder and increased risk of suicide (Gunstad and Phillips, 2003; Ruffolo et al., 2006; Fang and Hofmann, 2010; Kelly et al., 2013).
Due to the transdiagnostic nature of appearance concerns and the heavy burden of associated psychiatric disorders, there is a critical need and opportunity to investigate mechanisms and measures that may be useful for better understanding the manifestation and development of appearance concerns and appearance-related psychopathology. To this end, some researchers have employed psychophysiological assessments to measure attentional biases related to appearance concerns; these appearance-related attentional biases are thought to play an important etiological role in body image disturbances (Cash and Labarge, 1996; Veale, 2004; Williamson et al., 2004; Rodgers and DuBois, 2016). A review by Rodgers and DuBois (2016) noted a relative dearth of research in this area but found robust evidence supporting the presence of attentional biases toward appearance-related stimuli among those with high levels of body dissatisfaction compared to those with lower levels. Specifically, they found that individuals in an eye-tracking study with higher levels of body dissatisfaction fixated most on their own self-identified unattractive body parts and to a control body’s most attractive body parts (Roefs et al., 2008; Rodgers and DuBois, 2016). Another eye-tracking study demonstrated that men high in muscle dysmorphia (i.e. a specifier of BDD related to muscularity) displayed a significant bias toward parts of their own bodies that they felt negatively about (Waldorf et al., 2019). Together, these studies suggest that elevated appearance concerns may be characterized by increased salience of self-relevant stimuli.
Research using event-related brain potentials (ERPs) has examined stimulus salience using the late positive potential (LPP; Cuthbert et al., 2000; Schupp et al., 2000, 2004; Hajcak et al., 2009; Hajcak and Foti, 2020). The LPP is a sustained positive deflection in the ERP waveform with a central-parietal scalp distribution that begins as early as 200 ms following stimulus presentation, persists throughout stimulus duration (Schupp et al., 2000, 2004; Codispoti et al., 2006; Foti et al., 2009) and is larger following the presentation of more salient stimuli (Hajcak and Foti, 2020). The LPP is potentiated to stimuli that contain people compared to stimuli without people (Ferri et al., 2012) and to ideographic stimuli compared to stimuli low in personal relevance (Speed et al., 2017). Indeed, one study found that the LPP was larger following the presentation of one’s own name and face compared to others’ names and faces (Tacikowski and Nowicka, 2010). It seems then the LPP may be an ideal neural measure to index possible information processing abnormalities related to elevated appearance concerns.
To our knowledge, very few studies to date have examined the LPP elicited by self-relevant stimuli in individuals with elevated appearance concerns. A study by Uusberg et al. (2018) found that women who endorsed higher preoccupation with body images exhibited increased LPP amplitude to images of their bodies that had been artificially enlarged or reduced—the LPP was not sensitive to size modifications of peer images; on the other hand, women with lower body image preoccupation displayed very similar responses to images of the self and others. Generally, this finding supports the notion that the LPP might be sensitive to the preferential processing of self-related stimuli in individuals with elevated appearance concerns; however, more research is needed to replicate and extend these findings.
The present study aimed to further examine the LPP in relation to the transdiagnostic construct of appearance concerns—as assessed in a normative sample of female college students. The LPP was measured during a passive viewing task wherein participants viewed pictures of an object, pictures of a stranger and pictures of themselves. Consistent with previous research, we hypothesized that LPP amplitude would be increased to pictures that contained people (Ferri et al., 2012); however, we further expected pictures of oneself to elicit a larger LPP than pictures of a stranger. We also examined the internal consistency of the LPP to determine if it has psychometric properties sufficient to study individual differences (e.g. Hajcak et al., 2017, 2019). Related to individual differences, we hypothesized that higher self-reported appearance concerns would predict a larger LPP to pictures of oneself. Finally, we examined the specificity of this relationship by testing whether depressive symptoms would similarly relate to an increased LPP to pictures of oneself.
Methods
Participants
Self-report and EEG data were collected from 83 female participants who were part of a larger study on appearance-related concerns. Participants were recruited from an undergraduate subject pool at a large southeastern university and received course credit for their participation. The mean age of the sample was 19.45 (s.d. = 2.6) years; 24 (28.9%) identified as Hispanic or Latino; 65 (78.3%) were Caucasian, 9 (10.8%) were Black or African American, 6 (7.2%) were Asian, and 3 (3.6%) identified as more than one race. There was no specific screening or exclusionary criteria other than gender.
Measures
Appearance Anxiety Inventory (AAI; Veale et al., 2014)
The AAI is a 10-item measure of appearance anxiety symptoms related to BDD as described by the DSM-5 (American Psychiatric Association, 2013), including obsessive thoughts and repeated behaviors with respect to appearance (e.g. ‘I avoid situations or people because of my appearance’). Each item is scored on a 5-point Likert scale ranging from 0 (‘not at all’) to 4 (‘all the time’), with a possible total score ranging from 0 to 40. A higher summed score reflects more severe symptoms. None of the items are reverse scored. The AAI demonstrated excellent reliability in the current sample (α = 0.92).
Yale–Brown Obsessive-Compulsive Scale modified for BDD (BDD-YBOCS: Phillips et al., 1997)
The original BDD-YBOCS is a 12-item, semistructured, clinician-rated measure of current BDD severity. The first five items assess obsessional preoccupations about perceived appearance defects, the next five items assess BDD-related repetitive behaviors, item 11 assesses insight into appearance beliefs and item 12 assesses avoidance due to BDD symptoms. Consistent with prior work, this measure was modified for self-report in the current study by removing the final two items assessing insight and avoidance (e.g. Marques et al., 2011; Summers and Cougle, 2018; Wilver et al., 2020). The score of each item ranges from 0 (‘no symptoms’) to 4 (‘extreme symptoms’), with a possible total score for the modified 10-item version ranging from 0 to 40. A higher summed score reflects more severe symptoms. In the current sample, the self-report version of the BDD-YBOCS demonstrated good-to-excellent reliability (α = 0.88).
Center for epidemiologic studies depression scale (CES-D; Radloff, 1977)
The CES-D is a 20-item self-report scale designed to measure depressive symptomatology in general populations over the previous week (e.g. ‘I had crying spells’ or ‘I felt sad.’). Each item is scored on a 4-point scale ranging from 0 for ‘rarely or none of the time (less than 1 day)’ to 4 for ‘all the time (5–7 days)’, with a possible total score ranging from 0 to 60. A higher summed score reflects more severe symptoms. Four of the items are reverse scored. The CES-D demonstrated acceptable reliability in the current sample (α = 0.71).1
LPP task
Participants completed a passive viewing task that included three different picture categories: pictures of a cabinet, pictures of a stranger and pictures of the participant (from this point on, the three categories will be referred to as ‘Object’, ‘Stranger’ and ‘Self’, respectively). For each picture category, there were five different variations of the subject’s position. One picture was a close-up taken 50 cm away from the subject and encompassed the upper third of the subject. The other four pictures were taken 100 cm away from the subject and encompassed the upper two-thirds of the subject and only varied by the following angles relative to a front-facing position of 0°: 0° (front-facing), −45° (slightly left-facing), 45° (slightly right-facing) and 90° (fully right-facing). Figure 1 presents exemplar stimuli of the stranger condition. All picture categories used identical composition and the same Stranger and Object pictures were used for every participant. Other than the specified variations, all pictures were taken in the same lab location and had identical background, lighting, angles, dimension, resolution and distance from the camera.
Fig. 1.

Example stranger stimuli.
For the experiment, stimuli were displayed at 456 × 800 pixels on a 24.5-inch monitor with a resolution of 1920 × 1080 pixels and a 60 Hz refresh rate. Each of the 15 pictures was presented to the participant 10 times in a randomized order. Each stimulus was presented for 1500 ms followed by a fixation cross that was displayed for 1500–2500 ms to refocus the participant’s attention. The total length of the task was approximately 9 min.
EEG processing and recording
Electroencephalogram (EEG) data were recorded during the passive viewing task using an ActiCHamp active electrode system (Brain Products GmbH, Gilching, Germany) with 32 ActiCap slim (Ag/AgCl) electrodes arranged in accordance with the extended international 10/20 system. The electrooculogram (EOG) was recorded with two electrodes placed above and below the left eye vertical electrooculogram (VEOG) and two electrodes placed near the outer canthi of the left and right eyes horizontal electrooculogram (HEOG). Continuous EEG signals were recorded at a sampling rate of 1000 Hz using an online band-pass filter of 0.01–100 Hz that were online referenced to Cz. Impedance was kept at or below 15 kΩ throughout recording.
All offline analyses were performed using BrainVision Analyzer version 2.1 (Brain Products GmbH, Gilching, Germany). Data were re-referenced to the average of the mastoid electrodes and then band-pass filtered from 0.01 to 30 Hz using a fourth-order Butterworth filter. The continuous EEG data were segmented to create stimulus-locked epochs for Self, Stranger and Object using a −200 to 1500 ms time window. Ocular correction was then performed using a regression-based approach (Gratton et al., 1983). Epochs that contained voltage steps greater than 50 µV, a voltage difference of 175 µV within a 400 ms interval, or a maximum voltage difference of less than 0.50 µV within 100 ms were eliminated channel wise using an automated artifact rejection process. Additional artifacts not automatically rejected were visually inspected and subsequently discarded.
To define ERP measurement parameters, a collapsed localizer approach was used, wherein the waveforms for each ERP was first collapsed across conditions and then the timing and scalp distribution that showed the largest activity of interest was selected for analysis (Luck and Gaspelin, 2017). Based on this approach, the LPP was measured as the mean amplitude in a 300–700 ms time window at a parieto-occipital region-of-interest electrode pool (P3, Pz, P4, O1, Oz and O2; Figure 2). The internal consistency of the LPP was computed using split-half reliability analyses examining correlations between odd and even trials for all picture types. The Spearman–Brown coefficients were the following: 0.78 for LPP to Self, 0.82 for LPP to Stranger and 0.79 for LPP to Object.2,3
Fig. 2.
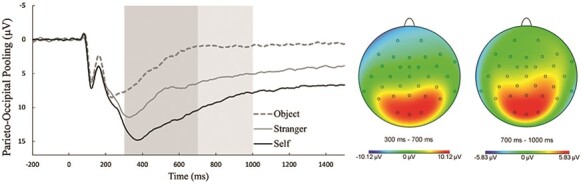
Grand-average LPP parent waveforms by picture type at a parieto-occipital electrode site pooling (left) and topographies collapsed across picture types (right).
Note: On the left, the darker shaded box reflects the 300–700 ms time window used to measure the early LPP; the lighter shaded box reflects the 700–1000 ms time window used to measure the late LPP. On the right, the topographic maps depict neural activity during the time windows used for the early (left headmap) and late (right headmap) LPPs. The early and late LPPs were scored at a parieto-occipital electrode site pooling consisting of P3, Pz, P4, O1, Oz, and O2. µV = microvolt; ms = millisecond.
Procedures
The University Institutional Review Board approved all study procedures prior to data collection. Potential participants were scheduled to come into the lab for an in-person appointment. After reviewing the protocol and providing informed consent, participants were first measured for an EEG cap. Next, the participant was instructed to stand in a relaxed pose while the experimenter took five images of the participant (as described above) for later use in the LPP task. Participants were then asked to complete a battery of self-report questionnaires administered using Qualtrics as well as other behavioral tasks reported elsewhere. Afterward, participants were escorted down the hall to complete the EEG portion of the study. Upon completion, participants were debriefed, granted course credit and thanked for their participation.
Statistical analysis
All statistical analyses were conducted using SPSS Statistics version 25 (IBM Corp., Armonk, NY) with a two-tailed, familywise error rate of α = 0.05. All variables were initially screened for normality by examining their skewness (S) and kurtosis (K) and were considered to be within an acceptable normal score distribution if the measures had S and K values between –2 and 2. Repeated-measures analyses of variance (RM-ANOVA) were used to test whether the LPP differed by picture type (Object, Stranger and Self). If the sphericity assumption was violated, the Greenhouse–Geisser correction was used. Post hoc paired-sample t-tests were conducted to decompose the main effect using the Bonferroni adjustment.
In addition to the RM-ANOVAs, additional repeated-measures analyses of covariance (RM-ANCOVAs) were conducted to examine the potential moderating effects of the self-report measures on the ERPs. In one analysis, AAI was included as a continuous covariate; separate analyses included the BDD-YBOCS and CES-D to test for convergent and discriminant validity of any main effects and interactions. Given that the self-report measures were continuous variables in the RM-ANCOVAs, significant interactions were decomposed using follow-up simple slope analyses, which were conducted using the MEMORE macro for SPSS (version 2.1; Montoya, 2019). These analyses assessed the relationship between the moderator (mean-centered) at each level of the outcome (Object, Stranger and Self).
Sensitivity power analysis
Due to the exploratory nature of the study, the sample size was not estimated prior to data collection. However, we conducted a sensitivity power analysis using the SPSS effect size specification in G*Power software (version 3.1.9.7; Faul et al., 2007) to report the effect sizes that could be reliably detected with 80% power. With a sample size of 83, the current study had sensitivity to detect medium-sized effects (f ≥ 0.26) using α = 0.05, three measurements (LPP to Self, LPP to Stranger and LPP to Object), and a non-sphericity correction (ε = 0.80).
Results
Preliminary analyses
Grand-average stimulus-locked parent ERP waveforms and topographic maps are presented in Figure 2. S and K values indicated normal score distributions for all questionnaires and ERP variables (Table 1 for the full descriptive statistics).
Table 1.
Descriptive statistics
| Variable | M | s.d. | Skewness | Kurtosis | N |
|---|---|---|---|---|---|
| Age | 19.45 | 2.60 | 83 | ||
| AAI | 17.28 | 9.39 | 0.21 | −0.77 | 83 |
| BDD-YBOCS | 13.42 | 6.11 | 0.04 | −0.59 | 83 |
| CES-D | 16.04 | 10.12 | 0.65 | −0.58 | 80 |
| LPP to Self | 12.58 | 4.91 | 0.90 | 0.81 | 83 |
| LPP to Stranger | 8.44 | 3.96 | 0.86 | 1.72 | 83 |
| LPP to Object | 3.85 | 3.05 | −0.02 | 0.44 | 83 |
Note: AAI = Appearance Anxiety Inventory; BDD-YBOCS = Yale–Brown Obsessive-Compulsive Scale, modified for BDD; CES-D = Center for Epidemiologic Studies Depression Scale.
Zero-order correlations indicated that age was unrelated to all self-report and ERP measures (all P values >0.160). AAI scores were highly correlated with BDD-YBOCS scores, r(81) = 0.67, P < 0.001, and CES-D, r(78) = 0.59, P < 0.001; BDD-YBOCS and CES-D scores were also highly correlated, r(78) = 0.67, P < 0.001. In terms of relationships between self-report and ERP measures, increased AAI and BDD-YBOCS scores were related to a larger LPP to Self.4 There were no other relationships between self-report and ERP measures. Zero-order correlations between self-report and ERP measures are reported in Table 2.
Table 2.
Correlations among questionnaire sum scores and ERPs
| Variable | AAI | BDD-YBOCS | CES-Da |
| LPP to Self | 0.22* | 0.26* | 0.13 |
| LPP to Stranger | 0.03 | 0.10 | 0.00 |
| LPP to Object | 0.002 | 0.06 | −0.001 |
Note: AAI = Appearance Anxiety Inventory, BDD-YBOCS = Yale–Brown Obsessive-Compulsive Scale modified for BDD, CES-D = Center for Epidemiologic Studies Depression Scale.
P < 0.05.
Analyses with the CES-D included 80 participants due to missing data from three participants.
ERPs
As suggested by Figure 2, there was a main effect of picture type on the LPP, F(1.64,134.50) = 253.89, P < 0.001, η2p = 0.76, ε = 0.82. Post hoc comparisons (adjusted-α = 0.05/3 = 0.017) indicated a larger LPP to Self compared to both Stranger, t(82) = 11.70, P < 0.001, d = 1.28, and Object, t(82) = 18.63, P < 0.001, d = 2.05; the LPP to Stranger was also larger than the LPP to Object, t(82) = 14.12, P < 0.001, d = 1.55.
When AAI was included as a covariate, the main effect of picture type on the LPP remained significant, F(1.66,134.47) = 37.07, P < 0.001, η2p = 0.31, ε = 0.83; there was a significant Picture Type × AAI interaction, F(1.66,134.47) = 4.77, P = 0.014, η2p = 0.06, ε = 0.83. Follow-up analyses indicated that increased AAI scores were related to a larger LPP to Self [b = 0.11, SE = 0.06, t(81) = 2.02, P = 0.047; Figure 3] but not LPP to Stranger [b = 0.01, SE = 0.05, t(81) = 0.25, P = 0.801] or Object [b = 0.001, SE = 0.04, t(81) = 0.01, P = 0.989].
Fig. 3.
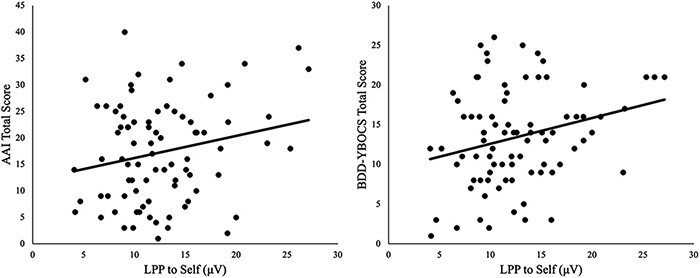
Scatterplots between LPP to Self and AAI (left) and BDD-YBOCS (right).
Note: AAI = Appearance Anxiety Inventory; BDD-YBOCS = Yale–Brown Obsessive-Compulsive Scale, modified for BDD; µV = microvolt.
When BDD-YBOCS was included as a covariate, the main effect of picture type on the LPP remained significant, F(1.67,135.23) = 24.05, P < 0.001, η2p = 0.23, ε = 0.84; there was a significant Picture Type × BDD-YBOCS interaction, F(1.67,135.23) = 4.75, P = 0.015, η2p = 0.06, ε = 0.84. Follow-up analyses indicated that increased BDD-YBOCS scores were related to a larger LPP to Self [b = 0.21, SE = 0.09, t(81) = 2.44, P = 0.017; Figure 3] but not LPP to Stranger [b = 0.07, SE = 0.07, t(81) = 0.92, P = 0.363] or Object [b = 0.03, SE = 0.06, t(81) = 0.51, P = 0.615].
When the CES-D was included as a covariate, the main effect of picture type on the LPP remained significant, F(1.65,129) = 54.15, P < 0.001, η2p = 0.41, ε = 0.83; there was no Picture Type × CES-D interaction, F(1.65,129) = 1.67, P = 0.196, η2p = 0.02. 5,6
Discussion
The current study examined the LPP elicited by Object, Stranger and Self pictures in the context of individual differences in appearance concerns. Consistent with previous work (Ferri et al., 2012), we found that stimuli that contained people elicited a larger LPP than stimuli without people. Moreover, the LPP was further potentiated by Self compared to Stranger pictures. Finally, we found that increased appearance concerns (i.e. self-reported appearance anxiety and BDD symptoms) predicted an increased LPP to Self pictures—an effect that was not evident when we examined the LPP elicited by Stranger or Object pictures. The relationship between appearance concerns and LPP to Self pictures did not appear to reflect individual differences in more general distress—as self-reported depressive symptoms was unrelated to an increased LPP to Self pictures.
The current finding, which demonstrates that pictures of oneself elicit a potentiated LPP relative to pictures of a stranger and object, is in line with other studies that have found that self-relevant information elicit a larger LPP compared to other stimuli. Stimuli containing people are thought to potentiate the LPP due to the critical information they can convey related to both survival and social processes (Haxby et al., 2002; LoBue and DeLoache, 2010; Ferri et al., 2012). Other studies have found that self-relevant stimuli elicit an increased LPP relative to self-irrelevant stimuli, which might be due to familiarity; in addition, it is possible that self-relevant information has preferential access to attention (e.g. cocktail party effect) due to increased relevance and adaptive value (Speed et al., 2017). Thus, one’s own image (i.e. which includes both people and self-relevant stimuli) would reflect both effects and elicit a potentiated neural response (Tanaka et al., 2006; Tacikowski and Nowicka, 2010; Tacikowski et al., 2011). Our findings buttress the notion that one’s own body/face may naturally capture attention and have increased salience relative to other images of people.
Further, the current findings indicate that an increased LPP to pictures of oneself is predicted by individual differences in self-reported appearance concerns, as measured by both the AAI and BDD-YBOCS. These results are in line with previous research on attentional biases and appearance concerns using eye-tracking paradigms (Roefs et al., 2008; Rodgers and DuBois, 2016; Waldorf et al., 2019) and ERPs (Uusberg et al., 2018) and support the notion that self-relevant stimuli are preferentially processed among individuals with elevated appearance concerns. Additionally, LPP amplitude to pictures of oneself was unrelated to self-reported depressive symptoms, suggesting that variability in the LPP to pictures of oneself was not related to more broad individual differences in distress or negative affect. Overall, these data are consistent with the possibility that the LPP elicited by pictures of oneself may represent a transdiagnostic neural marker that characterizes increased appearance concerns that are typical across multiple disorders, including BDD, eating disorders and SAD. In this way, the LPP elicited by pictures of oneself might be leveraged to study treatment-related changes in appearance concerns and to help identify those at elevated risk of developing appearance-related psychopathology.
As the present study represents a preliminary investigation of the LPP in relation to appearance concerns, several limitations could be addressed in future research. First, although the LPP indexes stimulus salience, it does not discern stimulus valence (i.e. it is larger for both pleasant and unpleasant emotional pictures compared to neutral pictures). Future studies could include psychophysiological measures that are sensitive to valence (e.g. the startle reflex) and could collect subjective ratings to integrate subjective experience in response to pictures of oneself. Another limitation of the current study is that participants were never asked if they knew the person in the stranger pictures—thus we did not ensure unfamiliarity. Additionally, the current sample was composed of undergraduates; it is unclear whether our findings would generalize to individuals with more clinically severe appearance concerns. Further, the current data are cross-sectional, and additional research is needed to investigate temporal relationships between variables. Future studies could leverage longitudinal designs to determine whether the LPP elicited by pictures of oneself represents a possible neural indicator of vulnerability for appearance-related psychopathology, whether the LPP changes concomitantly with appearance-related symptoms and whether the LPP is predictive of the prospective course of the disorder or treatment outcome (Hajcak et al., 2019; Perkins et al., 2020). Future studies might also extend data collection to male samples to elucidate possible sex differences in those with elevated appearance concerns. Finally, future studies could also include measures of self-esteem to examine its relation to LPP and appearance concerns.
Overall, the current study provides initial evidence regarding the utility and specificity of the LPP elicited by pictures of oneself as it relates to the transdiagnostic construct of appearance concerns. These data suggest that the LPP elicited by pictures of oneself could be utilized as a neurophysiological index of appearance concerns that could be relevant to studies on the development and maintenance of psychiatric disorders characterized by a preoccupation with appearance. LPP amplitude to pictures of oneself could be leveraged for clinically meaningful insight into studies of risk, treatment development and treatment outcome. A more in-depth understanding of these possibilities might pave the way for specific interventions that target the LPP to images of oneself to reduce appearance concerns and appearance-related symptomatology.
Supplementary Material
Acknowledgements
We would like to acknowledge both the Risk for Anxiety and Depression (RAD) lab and Cougle lab research staff who aided in data collection as well as our participants.
Footnotes
CES-D data from three participants were excluded from analyses due to missing data; therefore, 80 participants were included in analyses using the CES-D.
Additionally, we scored LPP as the mean amplitude in a later time window from 700 to 1000 ms using the same parieto-occipital electrode pool (P3, Pz, P4, O1, Oz, O2; Figure 2). We refer to this LPP as the late LPP hereafter. The Spearman–Brown coefficients for the late LPP were the following: 0.70 for Self, 0.81 for Stranger, and 0.66 for Object.
The P1 was also scored, and details regarding its measurement and internal consistency are reported in the Supplementary Material.
S and K values for late LPP to Self (S = 0.68, K = 0.89) and LPP to Object (S = −0.64, K = 1.60) values indicated normal distribution; however, late LPP to Stranger (S = −0.30, K = 2.49) values were outside the acceptable range and winsorized to the median ± 2 times the interquartile range resulting in improved S and K of 0.18 and 0.30, respectively. Additionally, late LPP to Self (700–1000 ms) correlated with BDD-YBOCS (r = 0.22, P = 0.05) and no other self-report measures. Late LPP to Stranger and Object did not correlate with any measures.
We also performed the same analyses on the late LPP time window. The RM-ANOVA indicated a significant main effect of picture type on the late LPP, F(1.67,136.56) = 150.24, P < 0.001, η2p = 0.65, ε = 0.83. Post hoc comparisons (adjusted-α = 0.05/3 = 0.017) confirmed a larger late LPP to Self (M = 8.65, s.d. = 4.49) compared to both Stranger (M = 5.61, s.d. = 3.64), t(82) = 7.87, P < 0.001, d = 0.86, and Object (M = 1.04, s.d. = 2.94), t(82) = 14.31, P < 0.001, d = 1.57; the late LPP to Stranger was also larger than the late LPP to Object, t(82) = 11.65, P < 0.001, d = 1.28. When self-report measures were included as covariates in separate RM-ANCOVAs, the picture type main effects remained significant (all P values <0.001); however, there were no significant interactions between picture type and the self-report measures (all P values >0.183).
All analyses including the P1 component can be found in the Supplementary Material.
Contributor Information
Carson D Jordan, Department of Psychology, Florida State University, Tallahassee, FL 32306, USA; Department of Biomedical Sciences, Florida State University, Tallahassee, FL 32306, USA.
Rochelle A Stewart, Department of Psychology, Florida State University, Tallahassee, FL 32306, USA.
C J Brush, Department of Psychology, Florida State University, Tallahassee, FL 32306, USA.
Jesse R Cougle, Department of Psychology, Florida State University, Tallahassee, FL 32306, USA.
Greg Hajcak, Department of Psychology, Florida State University, Tallahassee, FL 32306, USA; Department of Biomedical Sciences, Florida State University, Tallahassee, FL 32306, USA.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
Conflict of interest
The author(s) declare they have no competing or potential conflicts of interest.
Supplementary data
Supplementary data are available at SCAN online.
References
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th edn, Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Brawman-Mintzer O., Lydiard B., Phillips K.A., et al. (1995). Body dysmorphic disorder in patients with anxiety disorders and major depression: a comorbidity study. American Journal of Psychiatry, 152(11), 1665–7. [DOI] [PubMed] [Google Scholar]
- Cash T.F., Labarge A.S. (1996). Development of the appearance schemas inventory: a new cognitive body-image assessment. Cognitive Therapy and Research, 20(1), 37–50. [Google Scholar]
- Codispoti M., Ferrari V., Bradley M.M. (2006). Repetitive picture processing: autonomic and cortical correlates. Brain Research, 1068(1), 213–20. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Fang A., Hofmann S.G. (2010). Relationship between social anxiety disorder and body dysmorphic disorder. Clinical Psychology Review, 30(8), 1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–91. [DOI] [PubMed] [Google Scholar]
- Ferri J., Weinberg A., Hajcak G. (2012). I see people: the presence of human faces impacts the processing of complex emotional stimuli. Social Neuroscience, 7(4), 436–43. [DOI] [PubMed] [Google Scholar]
- Foti D., Hajcak G., Dien J. (2009). Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–30. [DOI] [PubMed] [Google Scholar]
- Godart N.T., Flament M.F., Curt F., et al. (2003). Anxiety disorders in subjects seeking treatment for eating disorders: a DSM-IV controlled study. Psychiatry Research, 117(3), 245–58. [DOI] [PubMed] [Google Scholar]
- Goodwin R.D., Fitzgibbon M.L. (2002). Social anxiety as a barrier to treatment for eating disorders. International Journal of Eating Disorders, 32(1), 103–6. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–84. [DOI] [PubMed] [Google Scholar]
- Gunstad J., Phillips K.A. (2003). Axis I comorbidity in body dysmorphic disorder. Comprehensive Psychiatry, 44(4), 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Dunning J.P., Foti D. (2009). Motivated and controlled attention to emotion: time-course of the late positive potential. Clinical Neurophysiology, 120(3), 505–10. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Meyer A., Kotov R. (2017). Psychometrics and the neuroscience of individual differences: internal consistency limits between-subjects effects. Journal of Abnormal Psychology, 126(6), 823–34. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Klawohn J., Meyer A. (2019). The utility of event-related potentials in clinical psychology. Annual Review of Clinical Psychology, 15(1), 71–95. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Foti D. (2020). Significance? Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: an integrative review. Psychophysiology, 57(7), e13570. doi: 10.1111/psyp.13570 [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51(1), 59–67. [DOI] [PubMed] [Google Scholar]
- Kelly M.M., Dalrymple K., Zimmerman M., Phillips K.A. (2013). A comparison study of body dysmorphic disorder versus social phobia. Psychiatry Research, 205(1–2), 109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson C.A., Rodebaugh T.L., White E.K., et al. (2013). Social appearance anxiety, perfectionism, and fear of negative evaluation. Distinct or shared risk factors for social anxiety and eating disorders? Appetite, 67, 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardon J., Phillipou A., Castle D., et al. (2018). Feeling fat in eating disorders: Testing the unique relationships between feeling fat and measures of disordered eating in anorexia nervosa and bulimia nervosa. Body image, 25, 163–7. [DOI] [PubMed] [Google Scholar]
- LoBue V., DeLoache J.S. (2010). Superior detection of threat-relevant stimuli in infancy: threat detection in infancy. Developmental Science, 13(1), 221–8. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Gaspelin N. (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t): how to get significant effects. Psychophysiology, 54(1), 146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques L., Weingarden H.M., LeBlanc N.J., Wilhelm S. (2011). Treatment utilization and barriers to treatment engagement among people with body dysmorphic symptoms. Journal of Psychosomatic Research, 70(3), 286–93. [DOI] [PubMed] [Google Scholar]
- Montoya A.K. (2019). Moderation analysis in two-instance repeated measures designs: probing methods and multiple moderator models. Behavior Research Methods, 51(1), 61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins E.R., Joyner K.J., Patrick C.J., et al. (2020). Neurobiology and the hierarchical taxonomy of psychopathology: progress toward ontogenetically informed and clinically useful nosology. Dialogues in Clinical Neuroscience, 22(1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K.A., Hollander E., Rasmussen S.A., Aronowitz B.R., DeCaria C., Goodman W.K. (1997). A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown obsessive compulsive scale. Psychopharmacology Bulletin, 33(1), 17–22. [PubMed] [Google Scholar]
- Radloff L.S. (1977). The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Rodgers R.F., DuBois R.H. (2016). Cognitive biases to appearance-related stimuli in body dissatisfaction: a systematic review. Clinical Psychology Review, 46, 1–11. [DOI] [PubMed] [Google Scholar]
- Roefs A., Jansen A., Moresi S., Willems P., van Grootel S., van der Borgh A. (2008). Looking good. BMI, attractiveness bias and visual attention. Appetite, 51(3), 552–5. [DOI] [PubMed] [Google Scholar]
- Ruffolo J.S., Phillips K.A., Menard W., Fay C., Weisberg R.B. (2006). Comorbidity of body dysmorphic disorder and eating disorders: severity of psychopathology and body image disturbance. International Journal of Eating Disorders, 39(1), 11–9. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–61. [PubMed] [Google Scholar]
- Schupp H.T., Öhman A., Junghöfer M., Weike A.I., Stockburger J., Hamm A.O. (2004). The facilitated processing of threatening faces: an ERP analysis. Emotion, 4(2), 189–200. [DOI] [PubMed] [Google Scholar]
- Speed B.C., Levinson A.R., Gross J.J., Kiosses D.N., Hajcak G. (2017). Emotion regulation to idiographic stimuli: testing the autobiographical emotion regulation task. Neuropsychologia, 145, 106346. doi: 10.1016/j.neuropsychologia.2017.04.032 [DOI] [PubMed] [Google Scholar]
- Summers B.J., Cougle J.R. (2018). An experimental test of the role of appearance-related safety behaviors in body dysmorphic disorder, social anxiety, and body dissatisfaction. Journal of Abnormal Psychology, 127(8), 770–80. [DOI] [PubMed] [Google Scholar]
- Swinbourne J., Hunt C., Abbott M., Russell J., St Clare T., Touyz S. (2012). The comorbidity between eating disorders and anxiety disorders: prevalence in an eating disorder sample and anxiety disorder sample. AustralianandNew Zealand Journal of Psychiatry, 46(2), 118–31. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Jednoróg K., Marchewka A., Nowicka A. (2011). How multiple repetitions influence the processing of self-, famous and unknown names and faces: an ERP study. International Journal of Psychophysiology, 79(2), 219–30. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Nowicka A. (2010). Allocation of attention to self-name and self-face: an ERP study. Biological Psychology, 84(2), 318–24. [DOI] [PubMed] [Google Scholar]
- Tanaka J.W., Curran T., Porterfield A.L., Collins D. (2006). Activation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarity. Journal of Cognitive Neuroscience, 18(9), 1488–97. [DOI] [PubMed] [Google Scholar]
- Uusberg H., Peet K., Uusberg A., Akkermann K. (2018). Attention biases in preoccupation with body image: an ERP study of the role of social comparison and automaticity when processing body size. Biological Psychology, 135, 136–48. [DOI] [PubMed] [Google Scholar]
- Veale D. (2004). Advances in a cognitive behavioural model of body dysmorphic disorder. Body Image, 1(1), 113–25. [DOI] [PubMed] [Google Scholar]
- Veale D., Eshkevari E., Kanakam N., Ellison N., Costa A., Werner T. (2014). The appearance anxiety inventory: validation of a process measure in the treatment of body dysmorphic disorder. Behavioural and Cognitive Psychotherapy, 42(5), 605–16. [DOI] [PubMed] [Google Scholar]
- Waldorf M., Vocks S., Düsing R., Bauer A., Cordes M. (2019). Body-oriented gaze behaviors in men with muscle dysmorphia diagnoses. Journal of Abnormal Psychology, 128(2), 140–50. [DOI] [PubMed] [Google Scholar]
- Wilhelm S., Otto M.W., Zucker B.G., Pollack M.H. (1997). Prevalence of body dysmorphic disorder in patients with anxiety disorders. Journal of Anxiety Disorders, 11(5), 499–502. [DOI] [PubMed] [Google Scholar]
- Williamson D.A., White M.A., York-Crowe E., Stewart T.M. (2004). Cognitive-behavioral theories of eating disorders. Behavior Modification, 28(6), 711–38. [DOI] [PubMed] [Google Scholar]
- Wilver N.L., Summers B.J., Cougle J.R. (2020). Effects of safety behavior fading on appearance concerns and related symptoms. Journal of Consulting and Clinical Psychology, 88(1), 65–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


