Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease that mainly targets the synovial membrane, thus causing stiffness, deformity and dysfunction of joints. To date, no effective anti-inflammatory treatments are available for RA. Piceatannol (PIC) is a natural derivative of resveratrol, which has been reported to attenuate the inflammatory response. To evaluate the effect of PIC on RA and to determine the underlying molecular target of PIC, both in vitro and in vivo experiments were performed in the present study. A CIA rat model was established to evaluate the therapeutic effects of PIC. TNF-α, IL-1β and IL-6 levels in blood were measured by ELISA. Western blotting, immunofluorescence analysis and reverse transcription-quantitative PCR (RT-qPCR) were used to analyze the expression levels of protein and mRNA. In vitro, RA-fibroblast-like synoviocytes (FLSs) were pretreated with PIC and subsequently stimulated with TNF-α. The results revealed that PIC significantly upregulated the expression levels of proapoptotic proteins such as Bax and cleaved caspase-3. PIC also significantly reduced the production of proinflammatory cytokines, including PGE2, IL-6 and IL-1β, and significantly downregulated the expression of cyclooxygenase-2 at both the mRNA and protein expression levels. Furthermore, PIC downregulated the expression of MMP-3 and MMP-13, which have been found to be highly expressed in the synovium of patients with RA. Mechanistically, PIC was capable of significantly downregulating the expression levels of proteins involved in the NF-κB and MAPK signaling pathways. The results of the in vivo experiments using a rat collagen-induced arthritis model demonstrated that PIC decreased the arthritis score and exerted beneficial effects in cartilage and significantly reduced the expression of MMP-13. In conclusion, the findings of the present study revealed that PIC could suppress the inflammatory response, promote apoptosis, and exert a significant regulatory effect on the NF-κB and MAPK signaling pathways in RA-FLSs. Therefore, PIC may represent a potential drug for the future treatment of RA.
Keywords: fibroblast-like synoviocytes, MAPK, NF-κB, piceatannol, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic and complex inflammatory disorder that mainly affects the synovial membrane. RA results in the consequential damage of articular cartilage and bone, as well as in joint swelling and space narrowing, which leads to joint stiffness, deformity and dysfunction (1,2). RA is characterized by synovitis and synovial hyperplasia with the infiltration of immune cells. Synovial tissues express numerous cytokines, such as IL-6, and the dysregulation of pro- and anti-inflammatory cytokine levels has been directly implicated in the pathogenesis of multiple pathological processes, including induction of neovascularization, infiltration of inflammatory cells and synovial hyperplasia (3). The drug treatment of rheumatoid arthritis mainly includes nonsteroidal anti-inflammatory drugs (NSAIDs), anti-rheumatic drugs, immunosuppressants, immune and biological agents, and botanical drugs. NSAIDs show adverse effects in gastrointestinal, cardiovascular, hepatic, renal, cerebral, and pulmonary complications (4). Methotrexate can lead to hepatic fibrosis (5). Therefore, novel therapeutic targets are required.
Accumulating evidence has revealed that fibroblast-like synoviocytes (FLSs) are crucial in RA, as they produce proteases and cytokines that perpetuate inflammation (3,6). Furthermore, TNF-α, a proinflammatory cytokine produced by monocytes or macrophages, serves a disease-promoting role in RA (7,8). Selectively inhibiting TNF-α alleviates the progression of RA symptoms (9). Previous studies have reported that TNF-α is highly expressed in the synovium of patients with RA, where it is required to initiate subsequent inflammatory responses (9,10). In the inflamed synovium, RA-FLSs are also found to contribute to RA pathogenesis by producing matrix-degrading enzymes, such as MMP-3 and MMP-13, which are involved in articular cartilage dysfunction and destruction (11,12). Therefore, targeting TNF-α-induced inflammation and proliferation may be a novel therapeutic strategy for preventing and treating RA (8).
Piceatannol (PIC) is a hydroxylated resveratrol derivative (13,14) that exerts multiple biological activities, including antiproliferative, anti-adipogenic and anti-inflammatory effects (15,16). PIC was also found to suppress lipopolysaccharide-induced proinflammatory responses by blocking NF-κB in RAW264.7 macrophages (17–19). Furthermore, PIC inhibits TNF-α-mediated inflammation by blocking IκBα phosphorylation and NF-κB activation via the suppression of the nuclear translocation of p65 and JNK/MAPK activation (20). However, to the best of our knowledge, the effect of PIC on RA remains unclear. Collagen-induced arthritis (CIA) models are widely used as RA models. The pathological features of a rat CIA model are consistent with typical chronic proliferative synovitis, resembling adjuvant arthritis, which is observed in patients with RA (21). Therefore, the present study aimed to establish an in vivo CIA model using Wistar rats, and to investigate the anti-inflammatory and antiproliferative effects of PIC in RA. The present study also investigated the effect of PIC on TNF-α-stimulated RA-FLSs, and explored the underlying mechanism in vitro and in vivo.
Materials and methods
Reagents and antibodies
PIC (>98% pure) was purchased from Nantong FeiYu Biological Technology Co., Ltd. Cell Counting Kit-8 (CCK-8) assay was purchased from Dojindo Molecular Technologies, Inc. Rat TNF-α (cat. no. RTA00), IL-1β (cat. no. PRLB00), PGE2(KGE004B) and IL-6 (cat. no. R6000B) ELISA kits were purchased from R&D Systems, Inc. DAPI nuclear stain was purchased from Beyotime Institute of Biotechnology. DMEM/F12, FBS and BSA were purchased from HyClone (Cytiva). TRIzol® reagent was purchased from Invitrogen (Thermo Fisher Scientific, Inc.). SYBR-Green Master Mix was purchased from Bio-Rad Laboratories, Inc., while QuantiTect Reverse Transcription kit was purchased from Qiagen, Inc.
The following primary antibodies were used in the present study: Anti-GAPDH (cat. no. ab8245; Abcam), anti-cyclooxygenase-2 (COX-2; cat. no. ab179800; Abcam), anti-Bax (cat. no. ab182733; Abcam), anti-cleaved caspase-3 (cat. no. 9661; Cell Signaling Technology, Inc.), anti-caspase-3 (cat. no. 9662; Cell Signaling Technology, Inc.), anti-MMP-3 (cat. no. ab52915; Abcam), anti-MMP-13 (cat. no. 18165-1-AP; Wuhan Sanying Biotechnology), anti-phosphorylated (p)-p65 (cat. no. 3033; Cell Signaling Technology, Inc.), anti-p65 (cat. no. 3034; Cell Signaling Technology, Inc.), anti-p-IκBα (cat. no. 2859; Cell Signaling Technology, Inc.), anti-IκBα (cat. no. 4812; Cell Signaling Technology, Inc.), anti-p-JNK (cat. no. ab76572; Abcam), anti-JNK (cat. no. ab179461; Abcam), anti-p-ERK (cat. no. ab184699; Abcam), anti-ERK (cat. no. ab201015; Abcam), anti-p38 (cat. no. ab170099; Abcam) and anti-p-p38 (cat. no. ab195049; Abcam). HRP-conjugated goat anti-rabbit (cat. no. HAF017) and goat anti-mouse IgG (cat. no. HAF007) antibodies were acquired from R&D Systems, Inc. Alexa Fluor 488- and Alexa Fluor 594-conjugated goat anti-rabbit IgG (heavy + light chain) secondary antibodies(111-546-003; 115-585-006) were purchased from Jackson ImmunoResearch Laboratories, Inc.
Cell coutning kit (CCK)-8 assay
The effect of PIC on cell viability were detected via CCK-8 (Dojindo Co.) according to the manufacturer's protocol. RA-FLSs were transferred to 12-well plates at a density of 3×105 cells/ml and incubated with PIC (0, 2, 5, 10 µM) for 24 h in 37°C. Cells were washed with PBS and 10 µl CCK-8 solution was added to each well for 2 h at 37°C. The absorbance of the wells was then measured at 450 nm using a microplate reader. All experiments were performed in triplicate.
Establishment of a CIA rat model and effect of PIC treatment
A total of 24 male Wistar rats (age 8 weeks; weight,180±10 g) were purchased from the Animal Laboratory Center of Wenzhou Medical University. Rats were housed at a stable temperature of 22–24°C, 60–65% humidity, under a 12-h dark/light cycle and access to standard laboratory food and water at all times. CIA was induced in Wistar rats using bovine type II collagen (CII; MilliporeSigma) emulsified in complete Freund's adjuvant (CFA; MilliporeSigma). The present study was performed according to the recommendations outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (22). All animal treatments, surgical interventions and postoperative care procedures were approved by the Animal Care and Use Committee of Wenzhou Medical University (Wenzhou, China; approval no. wydw2019-0129).
Briefly, male rats received an injection of 150 µl emulsion at the base of the tail. The day of the first injection was considered as day 0. On day 14, the rats were injected with the same volume of CII emulsified in CFA through a booster injection to ensure induction of CIA. Control rats were injected in the same manner, but with saline or CFA only. Following injection, the rats were evaluated every 3 days for arthritis incidence. Each paw was evaluated and scored individually on a scale of 0–4, with 4 indicating the most severe inflammation. The clinical arthritis score for each limb was then scored using the following scoring system ranging from 0 to 4: 0, normal; 1, presence of erythema and mild swelling confined to the ankle joint and toes; 2, presence of erythema and mild swelling extending from the ankle to the midfoot; 3, presence of erythema and severe swelling extending from the ankle to the metatarsal joints; and 4, presence of severe swelling, erythema and joint rigidity of the ankle, foot and digits (23).
To evaluate the therapeutic effects of PIC, 8 rats were used as negative controls in a non-arthritis group, while arthritic rats (those with an arthritis score of ~6 on day 14) were randomly divided into a PIC-treated group (CIA + PIC group) and a vehicle-treated group (CIA group) (n=8 rats/group). In each group, 50 mg/kg PIC dissolved in 0.5% Carboxymethylcellulose (CMC) or vehicle (0.5% CMC alone) was intragastricly administered once/day from day 14 to day 41. The arthritis score for each rat was calculated as the sum of the scores for the four limbs. The rats were sacrificed by inhalation of 5% isoflurane maintained for 2 min. All the rats involved in the experiment exhibited a weight of 350±30 g at the time of sacrifice, and animal death was verified by judging factors such as breathing, heartbeat and body temperature for 10 min. After the rats were euthanized on day 41, 200 µl blood was taken from tail vein of rats to detect the levels of IL-1β, IL-6 and TNF-α via ELISA.
Preparation and culture of RA-FLSs
RA-FLSs from the synovial tissues of CIA model rats were isolated using the tissue explant cultivation method, as previously described (24). Briefly, CIA model rats were anesthetized with 2% pentobarbital sodium solution (45 mg/kg; intraperitoneal injection) and sacrificed by exsanguination by collecting 1 ml blood from the abdominal aorta on day 41 following the primary immunization. Fresh synovial tissue was removed from each rat under sterile conditions, which was subsequently placed into culture dishes and washed by PBS, and all fat and connective tissues were then removed. The CIA rat synovial membranes were cut into small pieces (~2 mm), incubated and cultured in DMEM/F12 supplemented with 20% FBS at 37°C with 5% CO2. The complete medium was replaced every 2 days. As RA-FLSs proliferated from synovial tissue, small pieces of synovial tissue were discarded. Adherent cells were trypsinized, and the cells were routinely split at 1:2/1:3 ratios and cultured in medium. The RA-FLSs obtained from the 3rd and 4th passages were used for the subsequent experiments.
ELISA
After rats were sacrificed, 1 ml of blood was immediately collected from five randomly selected rats from each group, and ELISA kits were used to determine the levels of IL-6, IL-1β PGE2 and TNF-α in blood, according to the manufacturer's instructions. All assays were performed in triplicate.
Western blotting
RA-FLSs were seeded in DMEM/F12 in 6-well plates at a density of 3×105 cells/ml and incubated for 24 h at 37°C. Following pretreatment with 0, 2, 5 or 10 µM PIC for 12 h and stimulation with after pre-treatment with 10 ng/ml TNF-α for 24 h at 37°C. Total protein was extracted from RA-FLSs by adding RIPA lysis buffer (MilliporeSigma) supplemented with 1 mM PMSF, placing the cells on ice for 10 min and then centrifuging for 15 min at 12,800 g at 4°C. Protein concentration was quantified using a BCA protein assay kit (Beyotime Institute of Biotechnology), and 40 µg protein/well was separated via 6–12% SDS-PAGE. The separated proteins were subsequently transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.) and blocked with 5% skimmed milk for 2 h at room temperature. The membranes were then incubated with the following primary antibodies overnight at 4°C: Anti-Caspase-3 (1:1,000), anti-cleaved caspase-3(1:1,000), anti-Bax(1:1,000), anti-COX-2 (1:1,000), anti-GADPH (1:5,000), anti-p-IκBα (1:1,000), anti-IκBα (1:1,000), anti-MMP-3 (1:1,000), anti-MMP-13 (1:1,000), anti-p65 (1:1,000), anti-p-p65 (1:1,000), anti-p-ERK (1:1,000), anti-ERK (1:1,000), anti-JNK (1:1,000) and anti-p-JNK (1:1,000). Following the primary antibody incubation and washing with TBST (0.1% Tween-20; cat no. P0231; Beyotime Institute of Biotechnology), the membranes were incubated with secondary antibodies (1:1,000) for 2 h at room temperature. Protein bands were visualized using an ECL solution (MilliporeSigma) and were semi-quantified using Image Lab 3.0 software (Bio-Rad Laboratories, Inc.). GAPDH was used as the loading control.
Immunofluorescence analysis
Samples were fixed with 4% paraformaldehyde for 2 h at 4°C, incubated with 0.5–2% Triton X-100, blocked with 10% FBS for 1 h at 37°C. RA-FLSs were subsequently incubated overnight with an anti-MMP-13 primary antibody (1:200) at 4°C. Following the primary antibody incubation, cells were washed with PBS and incubated with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies (1:400) in the dark for 1 h at room temperature. Finally, the sections were counterstained with DAPI (cat. no. P0131; Beyotime Institute of Biotechnology) for 5 min at room temperature. Three fields of each slides were chosen randomly for observation with a fluorescence microscope (Olympus Inc.; magnification ×100), and fluorescence intensity was measured using ImageJ software 2.1 (National Institutes of Health) by observers who were blinded to the experimental groups.
Reverse transcription-quantitative PCR (RT-qPCR)
RA-FLSs were seeded in DMEM/F12 in 6-well plates at a density of 3×105 cells/ml and incubated for 24 h at 37°C. Following pretreatment with 0, 2, 5 or 10 µM PIC for 12 h and 10 ng/ml TNF-α for 24 h at 37°C, total RNA was extracted from the monolayer of cultured RA-FLSs using TRIzol reagent. RT-qPCR was performed using SYBR-Green (Bio-Rad Laboratories, Inc.). The RNA concentration was determined using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc.) at a wavelength of 260 nm, and the quality and purity of RNA were determined using the A260/A280 ratio. Total RNA (1,000 ng) was reverse transcribed into cDNA using a QuantiTect Reverse Transcription kit according to the manufacturer's instructions. qPCR was subsequently performed using a CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc.). The following thermocycling conditions were used: Initial denaturation for 10 min at 95°C; followed by 40 cycles of 15 sec at 95°C, annealing at 60°C for 30 sec and elongation at 72°C for 1 min, and a final extension at 72°C for 5 min. The following primer sequences were used for qPCR: COX-2 forward (F), 5′-AACCCAGGGGATCGAGTGT-3′ and reverse (R), 5′-CGCAGCTCAGTGTTTGGGAT-3′; and GAPDH F, 5′-GCAAGTTCAACGGCACAG-3′ and R, 5′-CGCCAGTAGACTCCACGAC-3′. mRNA expression levels were quantified using the 2−∆∆Cq method (25) and normalized to GAPDH expression levels. Experiments were performed in triplicate.
Immunohistochemistry
Collected joint tissues were fixed in 4% paraformaldehyde for 24 h at 4°C and subsequently decalcified in 10% EDTA solution (MilliporeSigma) at 4°C for 2 weeks. The tissues were subsequently embedded in paraffin blocks and cut into 5-µm-thick sections. The sections were deparaffinized in xylene, rehydrated in alcohol gradient and then rinsed in PBS. Then the sections were incubated with 0.4% pepsin (Sangon Biotech Co., Ltd.) in 5 mM HCl at 37°C for 15 min for antigen retrieval. The sections were then incubated with 5% BSA for 30 min at room temperature. Following 12 h of incubation at 4°C with an anti-MMP-13 primary antibody (1:100), the histological sections were incubated with an appropriate HRP-conjugated secondary antibodies (Beyotime Institute of Biotechnology) for 2 h at room temperature. The slides were then stained with a DAB substrate system (OriGene Technologies, Inc.) for 5 min at room temperature, and nuclei were stained with hematoxylin for 10 min at room temperature. The slides were observed and captured observed with inverted light microscope (×200; Leica). At least three sections from each sample were used to analyze the expression of these proteins. The number of positive cells in each area was counted by observers blinded to the experimental groups, and was semi-quantified using Image J software version 2.1 (National Institutes of Health). The area of positive and negative cells in each section was calculated and shown as a percentage of the total number of cells.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc.). Data are presented as the mean ± SD of three independent experiments. Statistical differences between groups were determined using one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of PIC on arthritic progression
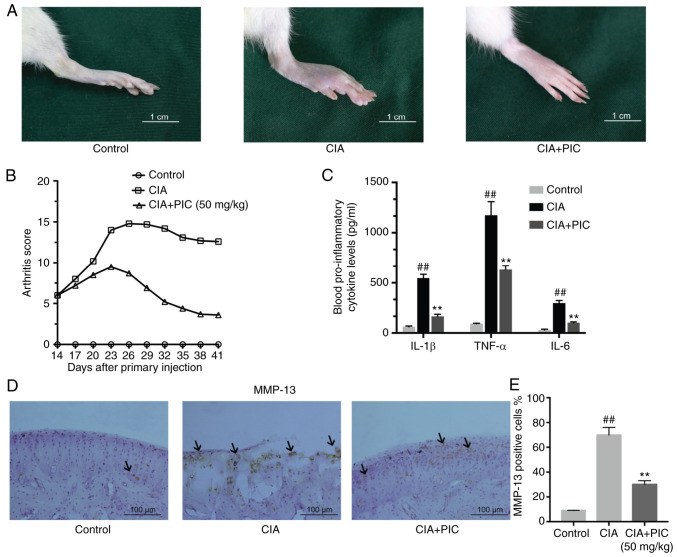
To determine if PIC treatment reduced the RA arthritis scores, a CIA model was established in Wistar rats (Fig. 1A). PIC or vehicle solution was administered every day following first injection, and the arthritis scores were recorded every 3 days. The levels of the proinflammatory cytokines IL-1β, IL-6 and TNF-α in the blood were analyzed via ELISA on day 41. The development of CIA was inhibited by treatment with PIC, which was demonstrated by differences in the arthritis scores between the CIA + PIC-treated and CIA groups on day 41 (Fig. 1B). Notably, the PIC group decreased from day 21 with regards to the arthritis scores compared with the CIA group (Fig. 1B). These results were also demonstrated in the changes in serum concentrations of proinflammatory cytokines in the control, CIA and CIA + PIC-treated rats. The levels of IL-1β, IL-6 and TNF-α were significantly increased in CIA rats compared with those in control rats. However, the inflammatory cytokine levels were significantly decreased in PIC-treated rats compared with those in CIA group (Fig. 1C). The expression levels of MMP-13 in the cartilage matrix were further investigated using immunohistochemistry. The results demonstrated that the CIA group exhibited an increased number of MMP-13-positive cells compared with that of the control group (Fig. 1D). However, treatment with PIC decreased the number of MMP-13-positive cells compared with that of the CIA group.
Figure 1.
PIC inhibits the progression and reduces the arthritis scores of rheumatoid arthritis. (A) Development of CIA was inhibited by treatment with PIC. Scale bar, 1 cm. (B) Differences in arthritis scores between CIA + PIC-treated, CIA and control rats. (C) Levels of IL-1β, IL6 and TNF-α. (D) Immunohistochemical staining of joint tissue sections of MMP13 (arrow). (E) Expression of MMP-13 in cartilage tissue. Scale bar, 100 µm. (F) MMP-13-positive cell rate. n=8 rats/group. ##P<0.01 vs. control; and **P<0.01 vs. CIA. PIC, piceatannol; CIA, collagen-induced arthritis.
PIC treatment induces the apoptosis and decreases the viability of RA-FLSs
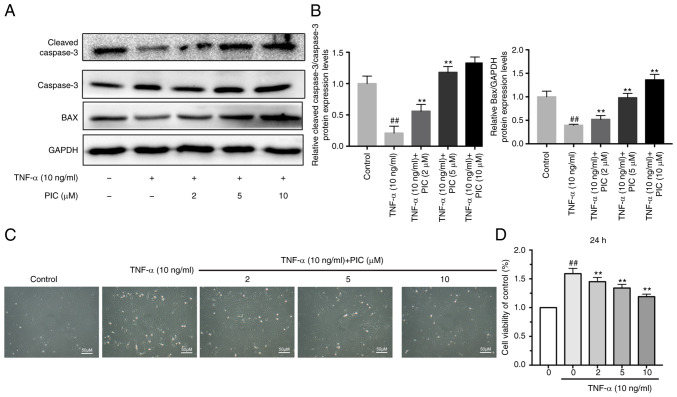
To evaluate the effect of PIC on apoptosis, the effect of PIC on Bax upregulation and cleaved caspase-3 protein expression was determined via western blotting. The results of western blot analysis revealed that PIC treatment significantly upregulated the TNF-α-induced downregulation of cleaved caspase-3 protein expression compared with the TNF-α only group. Furthermore, Bax expression was significantly upregulated in a dose-dependent manner in RA-FLSs treated with TNF-α and PIC compared with that observed in the group subjected to TNF-α treatment only (Fig. 2A and B).
Figure 2.
PIC increases the apoptosis and decreases the viability of rheumatoid arthritis-fibroblast-like synoviocytes. (A) Protein expression levels of Bax and cleaved caspase-3 were analyzed using western blotting. (B) Semi-quantification of cleaved caspase-3 and Bax protein expression levels. (C) Activity of different groups of cells under a microscope. Scale bar, 10 µm. (D) Cell viability was assessed via Cell Counting Kit-8 assay. n=3. ##P<0.01 vs. control; and **P<0.01 vs. TNF-α. PIC, piceatannol.
CCK-8 assay was used to determine the effects of PIC on RA-FLS cell viability. RA-FLSs were pretreated with different concentrations of PIC (0, 2, 5 and 10 µM) for 12 h followed by stimulation with 10 ng/ml TNF-α for 24 h. Treatment with TNF-α only for 24 h significantly increased cell viability compared with that of the control group. However, following treatment with PIC, the viability of RA-FLSs significantly decreased in a dose-dependent manner compared with that of cells subjected to TNF-α treatment only (Fig. 2D). The morphology of RA-FLSs was also observed via microscopy under the same conditions and grouping as those described for the aforementioned CCK-8 assay (Fig. 2C).
Effect of PIC on the expression levels of COX-2, an inflammatory mediator
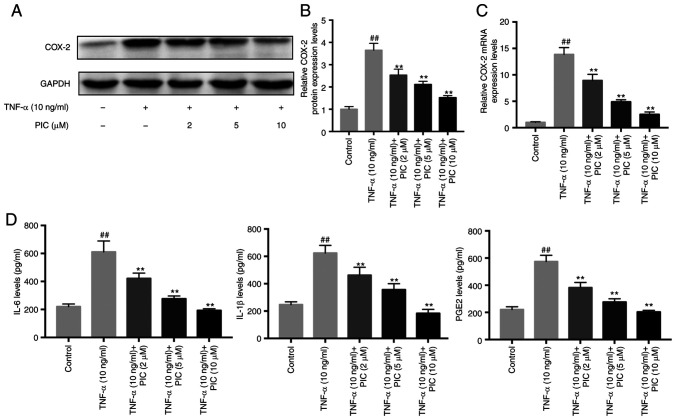
To evaluate the anti-inflammatory effects of PIC, the expression levels of COX-2 following PIC treatment were analyzed using western blotting and RT-qPCR. RA-FLSs were pretreated with different concentrations of PIC (0, 2, 5 or 10 µM) for 12 h followed by stimulation with 10 ng/ml TNF-α for 24 h. Western blotting demonstrated that PIC significantly downregulated the TNF-α-induced upregulation of COX-2 expression levels in a dose-dependent manner in RA-FLSs compared with those exhibited by the cells subjected to TNF-α treatment only (Fig. 3A and B). Furthermore, RT-qPCR analysis demonstrated that TNF-α treatment significantly upregulated the mRNA expression levels of COX-2 in RA-FLSs compared with the those of the control, while PIC treatment significantly reduced these effects compared with the findings in the group subjected to TNF-α treatment only (Fig. 3C). Subsequently, the effects of PIC on IL-6 and IL-1β production in TNF-α-stimulated RA-FLSs were investigated. The results demonstrated that TNF-α only treatment significantly induced the production of IL-6 compared with that of the control, whereas pretreatment with PIC significantly inhibited TNF-α-induced IL-6 production in RA-FLSs compared with that exhibited by the cells subjected to TNF-α treatment only (Fig. 3D). Furthermore, pretreatment with PIC also significantly suppressed TNF-α-induced IL-1β and prostaglandin E2 (PGE2) production in RA-FLSs compared with the results obtained in cells subjected to TNF-α treatment only.
Figure 3.
Effect of PIC on COX-2 expression levels. (A) Protein expression levels of COX-2 were analyzed via western blotting. (B) Semi-quantification of COX-2 protein expression levels. (C) COX-2 mRNA expression levels were analyzed using reverse transcription-quantitative PCR. (D) IL-6, IL-1β and PGE2 levels were quantified via ELISA. n=3. ##P<0.01 vs. control; and **P<0.01 vs. TNF-α. PIC, piceatannol; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2.
PIC downregulates the TNF-α-induced upregulation of MMP expression in RA-FLSs
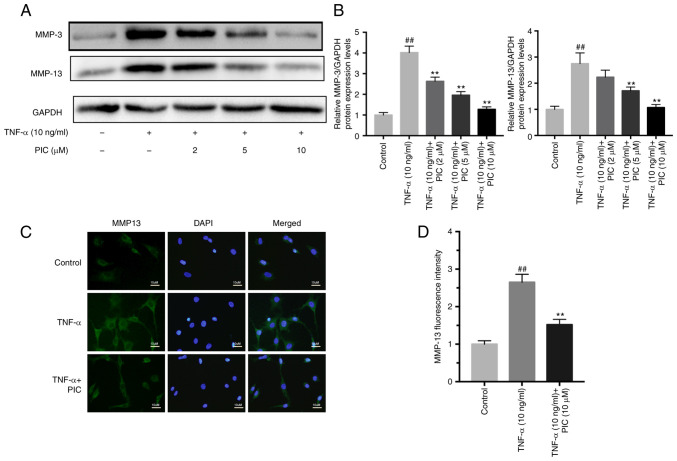
MMPs are considered to be crucial factors involved in the development of RA. Western blotting demonstrated that TNF-α significantly upregulated the protein expression levels of MMP-3 and MMP-13 compared with those of the control (Fig. 4A and B). However, PIC treatment significantly reduced the MMP-3 and MMP-13 protein expression levels in a dose-dependent manner compared with those exhibited by cells subjected to TNF-α treatment only. Furthermore, the results of immunofluorescence staining of MMP-13 expression were consistent with the western blotting data. PIC inhibited the TNF-α-induced upregulation of mmp-13 fluorescence staining intensity. (Fig. 4C and D).
Figure 4.
PIC downregulates the TNF-α-induced upregulation of MMP expression in rheumatoid arthritis-fibroblast-like synoviocytes. (A) MMP-3 and MMP-13 protein expression levels were analyzed using western blotting. (B) Semi-quantification of MMP-3 and MMP-13 protein expression levels. (C) MMP-13 Protein expression levels of MMP-13 were analyzed using immunofluorescence. Scale bar, 10 µm. (D) Fluorescence intensity of MMP-13. n=3. ##P<0.01 vs. control; and **P<0.01 vs. TNF-α. PIC, piceatannol.
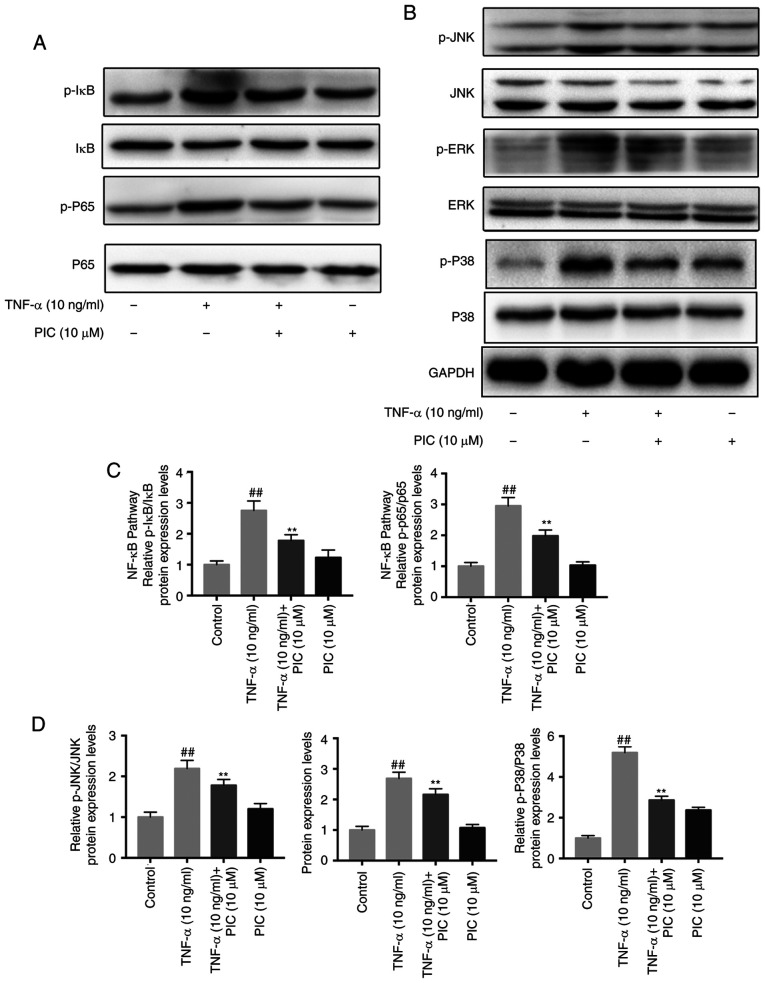
Effect of PIC treatment on the TNF-α-induced increase in NF-κB and MAPK signaling pathway activation in RA-FLSs
To further determine the underlying mechanisms of PIC treatment, the roles of the NF-κB and MAPK signaling pathways following PIC treatment were analyzed. RA-FLSs were pretreated with or without PIC (10 µM) for 12 h, then stimulated with 10 ng/ml TNF-α for 24 h. The phosphorylation levels of p65 and IκBα in the NF-κB signaling pathway, and those of JNK, ERK and p38 in the MAPK signaling pathway were determined using western blotting. The results revealed that the expression levels of all the aforementioned phosphorylated biomarkers were significantly upregulated following TNF-α induction compared with those of the control. Furthermore, the expression levels of all the phosphorylated molecules analyzed were significantly downregulated in the PIC-treated groups compared with those observed in the TNF-α + PIC treatment (Fig. 5A and B). Semi-quantitative analyses also identified that the suppressive effects of PIC were positively associated with the concentration of PIC in the treatment groups (Fig. 5C and D).
Figure 5.
Effect of PIC on TNF-α-induced NF-κB and MAPK signaling pathway activation in rheumatoid arthritis-fibroblast-like synoviocytes. (A) NF-κB signaling pathway protein expression levels were analyzed via western blotting. (B) MAPK signaling pathway protein expression levels were analyzed via western blotting. (C) Semi-quantification of NF-κB signaling pathway protein expression levels. (D) Semi-quantification of MAPK signaling pathway protein expression levels. n=3. ##P<0.01 vs. control; and **P<0.01 vs. TNF-α. PIC, piceatannol; p, phosphorylated.
Discussion
Numerous studies have focused on the potential of key molecules derived from natural compounds in treating RA, due to their low toxicity profiles and side effects (26,27). Piceatannol (PIC) is a compound with a chemical structure similar to resveratrol that is widely found in fruit, such as grapes and blueberries. The potential of PIC as a treatment option in several diseases has been widely reported. PIC was found to exert anti-inflammatory effects and suppress LPS-induced NO synthase induction in macrophages. PIC also was shown antioxidant activity in leukemia cell lines. and it had reported to suppressed the proliferation and induced apoptosis of osteosarcoma cells through PI3K/AKT/mTOR pathway (17,28,29). And a previous study reported that resveratrol inhibited inflammation in RA-FLSs (30). Notably, Piceatannol had shown more potent than resveratrol in free radical scavenging in association with antiarrhythmic and cardioprotective activities in ischaemic-reperfused rat hearts (31). RA is identified as chronic arthritic inflammation accompanied by hyperplasia of the synovial joint lining, which invades and degrades cartilage, eventually leads to severe joint pain and functional impairment. Previous studies have identified an apoptosis-resistant RA-FLS phenotype, which may be related to cleaved-caspase-3 (32). Thus, the present study aimed to investigate the effects of PIC in RA. TNF-α is considered to play a central role in RA pathogenesis, and is used to stimulate synovial fibroblasts to model RA (33). TNF-α not only stimulates FLS proliferation, but also causes the secretion of pro-inflammatory cytokines and induces the activation of inflammation-related pathways such as the MAPK signaling pathway (34). The present study demonstrated that PIC treatment significantly suppressed the inflammatory response and decreased cell viability in vitro by possibly blocking the activation of the NF-κB and MAPK signaling pathways. Furthermore, the present data indicated that PIC exerted a therapeutic effect in a CIA rat model.
Cleaved caspase-3 and Bax proteins are closely associated with apoptosis, and the upregulation of the expression of Bax induces apoptosis in RA-FLSs (35). The present study demonstrated that PIC may decrease abnormal cell proliferation by significantly upregulating the expression of Bax and cleaved caspase-3. The inflammatory mediator PGE2 is generated from endogenous arachidonic acid via COX-2 (36). The present study demonstrated that PIC treatment significantly inhibited the production of PGE2 and the upregulation of COX-2 at the mRNA and protein expression levels. The current data also demonstrated that PIC inhibited TNF-α-related inflammation in RA-FLSs.
MMPs are also known to serve important roles in the development and progression of RA (37). A previous study reported that the expression levels of MMP-3 and MMP-13 were upregulated in the synovial tissues and fluid of patients with RA (38). Furthermore, it was reported that TNF-α influenced the expression of MMPs in RA-FLSs (39). RA-FLSs secrete MMPs that drive cartilage degradation and contribute to joint destruction (40). In the present study, consistent with the findings of these previous studies, TNF-α treatment was revealed to significantly upregulate the protein expression levels of MMP-3 and MMP-13. However, pretreatment with PIC significantly attenuated the TNF-α-induced upregulation of MMP protein expression levels in RA-FLSs. These results suggested that PIC may downregulate the expression of MMP-3 and MMP-13 in RA-FLSs.
The NF-κB and MAPK signaling pathways, which are known to serve important roles in the pathogenesis of RA, regulate numerous proinflammatory genes that contribute to joint inflammation, particularly TNF-α-stimulated inflammation (41,42). The present study demonstrated that the phosphorylation levels of IκBα and p65 were significantly increased in TNF-α-induced RA-FLSs, whereas PIC pretreatment significantly suppressed this phosphorylation. These data indicated that PIC may inhibit the activation of the NF-κB signaling pathway by affecting the phosphorylation of IκBα and p65. The MAPK signaling pathway also serves an important role in the regulation of the production of inflammatory mediators (43). In previous studies, ERK, c-JNK and p38 were found to be expressed and highly phosphorylated in tissues from patients with RA (44,45). In the present study, the phosphorylation of JNK, ERK and p38 was significantly inhibited by PIC treatment. The findings of the present study suggest that PIC may inhibit the TNF-α-induced inflammatory response and may increase apoptosis by suppressing the activation of the NF-κB and MAPK signaling pathways. Further studies are required to elucidate the precise mechanism by which PIC acts on the inflammatory process in RA-FLSs.
The CIA rat model is the most commonly used autoimmune model, as it shares several pathological features with RA (46). Previous studies have reported different methods of alleviating CIA symptoms in mouse models and decreasing the serum levels of TNF-α, IL-1β and IL-6 cytokines (21,47). Therefore, inhibiting the production of inflammatory factors may be the key to delaying the progression of RA. The present study first analyzed the effect of PIC treatment on CIA progression in CIA model rats. The results revealed that PIC treatment reduced the symptoms of CIA, decreased cartilage erosion, significantly reduced MMP-13 expression, and significantly downregulated the serum levels of TNF-α, IL-1β and IL-6. These findings indicated that PIC may serve a role in promoting apoptosis in overproliferated RA synovial fibroblasts and as an anti-inflammatory drug in the treatment of RA. In addition, there are some limitations in the present study. For example, MMP-3 is also a crucial protein in the RA. Current research only detected MMP-3 at the cellular level, but for tissue slice immunohistochemistry, only MMP-13 was detected. Moreover, further study of how the PIC activates the upstream transcription factor to make the downstream NF-κB and MAPK signaling pathways work will also require further research in future experiments.
In conclusion, the results of the present study suggested the potential of PIC in attenuating the inflammatory response in RA in vitro and in vivo. Thus, PIC may serve as a novel anti-inflammatory agent for the treatment of RA.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by grants from the Zhejiang Provincial Medical Science and Technology Project (no. 2018KY525), the Natural Science Foundation of Zhejiang Province (no. LY20H060004) and the Wenzhou Science and Technology Project (no. Y20170393).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XG, EX and JM conceived and designed the research. XG, XK, HL and RC collected, analyzed and interpreted data. JP participated in the analysis and interpretation of data. XG, EX and XK edited the manuscript. All authors have read and approved the final manuscript. JM and XK confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was performed following the recommendations outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal treatments, surgical interventions and postoperative care procedures were approved by the Animal Care and Use Committee of Wenzhou Medical University (Wenzhou, China; approval no. wydw2019-0129).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartok B, Firestein GS. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 2019;86:301–307. doi: 10.1016/j.jbspin.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 7.Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC, Furst DE, Mease PJ, et al. Etanercept therapy in rheumatoid arthritis: A randomized, controlled trial. Ann Intern Med. 1999;130:478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 9.Brennan FM, Maini RN, Feldmann M. TNFα-a pivotal role in rheumatoid arthritis? Rheumatology. 1992;31:293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- 10.Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: A disease specific process or a common response to chronic inflammation? Autoimmun Rev. 2011;10:595–598. doi: 10.1016/j.autrev.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat Rev Rheumatol. 2015;11:606–615. doi: 10.1038/nrrheum.2015.95. [DOI] [PubMed] [Google Scholar]
- 12.Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: A key player in inflammation and joint destruction. Clin Immunol. 2005;115:118–128. doi: 10.1016/j.clim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Szekeres T, Fritzer-Szekeres M, Saiko P, Jäger W. Resveratrol and resveratrol analogues-structure-activity relationship. Pharm Res. 2010;27:1042–1048. doi: 10.1007/s11095-010-0090-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Kwon HS, Kim DH, Cho HJ, Lee HS, Jun JG, Park JH, Kim JK. Piceatannol, a stilbene present in grapes, attenuates dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2008;8:1695–1702. doi: 10.1016/j.intimp.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Kita Y, Miura Y, Yagasaki K. Antiproliferative and anti-invasive effect of piceatannol, a polyphenol present in grapes and wine, against hepatoma AH109A cells. J Biomed Biotechnol. 2012;2012:672416. doi: 10.1155/2012/672416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon JY, Seo SG, Heo YS, Yue S, Cheng JX, Lee KW, Kim KH. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J Biol Chem. 2012;287:11566–11578. doi: 10.1074/jbc.M111.259721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol. 2002;169:6490–6497. doi: 10.4049/jimmunol.169.11.6490. [DOI] [PubMed] [Google Scholar]
- 18.Djoko B, Chiou R YY, Shee JJ, Liu YW. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW 264.7 macrophages. J Agric Food Chem. 2007;55:2376–2383. doi: 10.1021/jf062741a. [DOI] [PubMed] [Google Scholar]
- 19.Son Y, Chung HT, Pae HO. Differential effects of resveratrol and its natural analogs, piceatannol and 3,5,4′-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in RAW264.7 macrophages. Biofactors. 2014;40:138–145. doi: 10.1002/biof.1108. [DOI] [PubMed] [Google Scholar]
- 20.Jin CY, Moon DO, Lee KJ, Kim MO, Lee JD, Choi YH, Park YM, Kim GY. Piceatannol attenuates lipopolysaccharide-induced NF-kappaB activation and NF-kappaB-related proinflammatory mediators in BV2 microglia. Pharmacol Res. 2006;54:461–467. doi: 10.1016/j.phrs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health, corp-author. National Academies; 1985. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 23.Zhu L, Zhu L. Sophocarpine suppress inflammatory response in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Eur Cytokine Netw. 2017;28:120–126. doi: 10.1684/ecn.2017.0400. [DOI] [PubMed] [Google Scholar]
- 24.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M, Sur B, Villa T, Yun J, Nah SY, Oh S. Gintonin regulates inflammation in human IL-1β-stimulated fibroblast-like synoviocytes and carrageenan/kaolin-induced arthritis in rats through LPAR2. J Ginseng Res. 2021;45:575–582. doi: 10.1016/j.jgr.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Xu Q, Liu Q, Pan D, Jiang Y, Liu M, Liu M, Xu H, Lin C. Leonurine attenuates fibroblast-like synoviocyte-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Rheumatology. 2017;56:1417–1427. doi: 10.1093/rheumatology/kex142. [DOI] [PubMed] [Google Scholar]
- 28.Ovesná Z, Kozics K, Bader Y, Saiko P, Handler N, Erker T, Szekeres T. Antioxidant activity of resveratrol, piceatannol and 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol Rep. 2006;16:617–624. [PubMed] [Google Scholar]
- 29.Wang B, Li J. Piceatannol suppresses the proliferation and induced apoptosis of osteosarcoma cells through PI3K/AKT/mTOR pathway. Cancer Manag Res. 2020;12:2631–2640. doi: 10.2147/CMAR.S238173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao L, Wan Y, Xiao J, Tang Q, Deng H, Chen L. A study of Sirt1 regulation and the effect of resveratrol on synoviocyte invasion and associated joint destruction in rheumatoid arthritis. Mol Med Rep. 2017;16:5099–5106. doi: 10.3892/mmr.2017.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WP, Hung LM, Hsueh CH, Lai LP, Su MJ. Piceatannol, a derivative of resveratrol, moderately slows I(Na) inactivation and exerts antiarrhythmic action in ischaemia-reperfused rat hearts. Br J Pharmacol. 2010;157:381–391. doi: 10.1111/j.1476-5381.2008.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MD, Weedon H, Papangelis V, Walker J, Roberts-Thomson PJ, Ahern MJ. Apoptosis in the rheumatoid arthritis synovial membrane: Modulation by disease-modifying anti-rheumatic drug treatment. Rheumatology (Oxford) 2010;49:862–875. doi: 10.1093/rheumatology/kep467. [DOI] [PubMed] [Google Scholar]
- 33.Cai L, Zong P, Zhou MY, Liu FY, Meng B, Liu MM, Li Z, Li R. 7-Hydroxycoumarin mitigates the severity of collagen-induced arthritis in rats by inhibiting proliferation and inducing apoptosis of fibroblast-like synoviocytes via suppression of Wnt/β-catenin signaling pathway. Phytomedicine. 2022;94:153841. doi: 10.1016/j.phymed.2021.153841. [DOI] [PubMed] [Google Scholar]
- 34.Namba S, Nakano R, Kitanaka T, Kitanaka N, Nakayama T, Sugiya H. ERK2 and JNK1 contribute to TNF-α-induced IL-8 expression in synovial fibroblasts. PLoS One. 2017;12:e0182923. doi: 10.1371/journal.pone.0182923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013;80:274–279. doi: 10.1016/j.jbspin.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Itoh Y. Metalloproteinases: Potential therapeutic targets for rheumatoid arthritis. Endocr Metab Immune Disord Drug Targets. 2015;15:216–222. doi: 10.2174/1871530315666150316122335. [DOI] [PubMed] [Google Scholar]
- 38.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennan FM, Browne KA, Green PA, Jaspar JM, Maini RN, Feldmann M. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-alpha (cA2) therapy. Br J Rheumatol. 1997;36:643–650. doi: 10.1093/rheumatology/36.6.643. [DOI] [PubMed] [Google Scholar]
- 40.Zhai KF, Duan H, Luo L, Cao WG, Han FK, Shan LL, Fang XM. Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology. 2017;25:523–532. doi: 10.1007/s10787-017-0385-5. [DOI] [PubMed] [Google Scholar]
- 41.Brown KD, Claudio E, Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res Ther. 2008;10:212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke J, Long X, Liu Y, Zhang YF, Li J, Fang W, Meng QG. Role of NF-kappaB in TNF-alpha-induced COX-2 expression in synovial fibroblasts from human TMJ. J Dent Res. 2007;86:363–367. doi: 10.1177/154405910708600412. [DOI] [PubMed] [Google Scholar]
- 43.Culbert AA, Skaper SD, Howlett DR, Evans NA, Facci L, Soden PE, Seymour ZM, Guillot F, Gaestel M, Richardson JC. MAPK-activated protein kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity. Relevance to neuroinflammation in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2006;281:23658–23667. doi: 10.1074/jbc.M513646200. [DOI] [PubMed] [Google Scholar]
- 44.Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, Zenz P, Redlich K, Xu Q, Steiner G. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43:2501–2512. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 45.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joe B, Wilder RL. Animal models of rheumatoid arthritis. Mol Med Today. 1999;5:367–369. doi: 10.1016/S1357-4310(99)01528-2. [DOI] [PubMed] [Google Scholar]
- 47.Marinova-Mutafchieva L, Williams RO, Mason LJ, Mauri C, Feldmann M, Maini RN. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107:507–512. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.