Abstract
OBJECTIVES
This study aims to systematically review published literature on male–female differences in presentation, management and outcomes in patients diagnosed with acute thoracic aortic dissection (AD).
METHODS
A systematic literature search was conducted for studies published between 1 January 1999 and 19 October 2020 investigating mortality and morbidity in adult patients diagnosed with AD. Patient and treatment characteristics were compared with odds ratios (ORs) and standardized mean differences and a meta-analysis using a random-effects model was performed for early mortality. Overall survival and reoperation were visualized by pooled Kaplan–Meier curves.
RESULTS
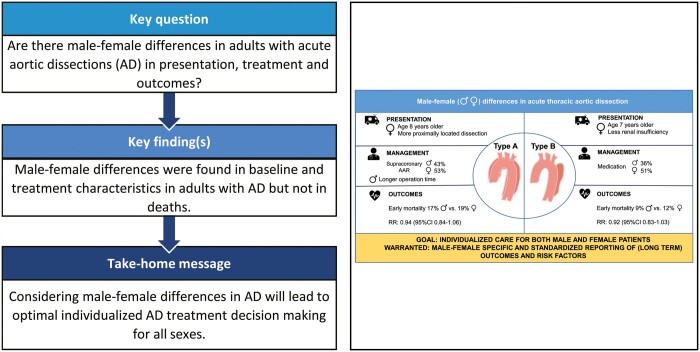
Nine studies investigating type A dissections (AD-A), 1 investigating type B dissections (AD-B) and 3 investigating both AD-A and AD-B were included encompassing 18 659 patients. Males were younger in both AD-A (P < 0.001) and AD-B (P < 0.001), and in AD-A patients males had more distally extended dissections [OR 0.57, 95% confidence interval (CI) 0.46–0.70; P < 0.001]. Longer operation times were observed for males in AD-A (standardized mean difference 0.29, 95% CI 0.17–0.41; P < 0.001) while male patients were less often treated conservatively in AD-B (OR 0.65, 95% CI 0.58–0.72; P < 0.001). The pooled early mortality risk ratio for males versus females was 0.94 (95% CI 0.84–1.06, P = 0.308) in AD-A and 0.92 (95% CI 0.83–1.03, P = 0.143) in AD-B. Pooled overall mortality in AD-A showed no male–female difference, whereas male patients had more reinterventions during follow-up.
CONCLUSIONS
This systematic review shows male–female differences in AD patient and treatment characteristics, comparable early and overall mortality and inconsistent outcome reporting. As published literature is scarce and heterogeneous, large prospective studies with standardized reporting of male–female characteristics and outcomes are clearly warranted. Improved knowledge of male–female differences in AD will help shape optimal individualized care for both males and females.
Clinical registration number
PROSPERO, ID number: CRD42020155926.
Keywords: Thoracic aorta, Acute aortic dissection, Sex and gender
Acute thoracic aortic dissection (AD) is a cardiovascular emergency with in-hospital mortality of 26–58% for type A dissections (AD-A) and 11–31% for type B dissections (AD-B) [1].
INTRODUCTION
Acute thoracic aortic dissection (AD) is a cardiovascular emergency with in-hospital mortality of 26–58% for type A dissections (AD-A) and 11–31% for type B dissections (AD-B) [1]. AD has an estimated annual incidence of 4.6–7.2 per 100 000 inhabitants [2–4]. Although male–female differences in cardiovascular disease are gaining attention, the disparities between males and females in AD have not been extensively studied. As AD is a potentially fatal disease, accurate diagnosis and patient-tailored treatment are essential to improve the survival. In this respect, it is important to elucidate male–female differences in AD.
Unfortunately, published evidence on male–female differences in AD concerns mainly single-centre retrospective series with limited sample size and follow-up, and therefore limited value in advancing knowledge on male–female differences. Previous research from the International Registry of Aortic Dissections (IRAD) found differences in clinical profiles between male and female patients for AD-A and AD-B [5]. In addition, surgically managed females with AD-A had higher in-hospital mortality compared to males [5]. On the contrary, Fukui et al. [6] concluded that there were no differences in early and late outcomes between male and female patients undergoing surgery for AD-A.
As it remains unclear whether male–female differences exist in AD, and current published evidence is fragmented, the aim of this study was to systematically review published literature conducted in adult AD patients investigating male–female differences in presentation, treatment, and mortality and morbidity.
PATIENTS AND METHODS
Protocol and registration
A systematic review and meta-analysis were conducted according to PRISMA guidelines [7]. The protocol was registered on PROSPERO (ID number: CRD42020155926). A systematic literature search was conducted by the Erasmus University Medical Centre librarian on 19 October 2020 in the scientific databases Embase, PubMed, Web of Science and the Cochrane Library. Studies published between 1 January 1999 and 19 October 2020 were eligible for inclusion. The complete search strings are available in Supplementary Material, Appendix SI.
Study selection
Original research papers investigating patient characteristics, and/or treatment characteristics, and/or outcome after Stanford type A and/or type B acute aortic dissection [8] were eligible for inclusion if male–female differences were mentioned in the title and/or abstract. Studies needed to be written in English, conducted in adult human patients receiving any treatment for AD and describe a study population of at least 30 AD patients. Studies describing a specific study population, such as connective tissue disease, specific age group, obstetric population, reoperations, previous cardiac surgery, malperfusion syndromes, specific operative techniques and traumatic dissections were excluded. Furthermore, studies focusing on non-acute dissections (≥14 days) were excluded. Two researchers independently screened the eligible studies using pre-specified inclusion and exclusion criteria (F.M. and A.L.G.). In case of disagreement, an agreement was negotiated and where necessary, a third researcher was consulted (J.W.R.-H.). The reference lists of included studies were cross-checked for relevant studies.
Endpoints and definitions
Aortic dissections were defined as acute when patients were diagnosed within 14 days after symptom onset [9]. The primary outcomes included male–female differences in early and overall mortality, secondary outcomes were male–female differences in presentation, management, early morbidity (including stroke, acute renal failure and re-exploration for bleeding) and late reoperation. Early mortality and morbidity were defined as in-hospital or within 30 days. Early mortality was defined differently in included studies; therefore, in-hospital mortality and 30-day mortality were summarized as ‘early mortality’.
Data collection
The extracted data were collected in Microsoft Excel 2016 (v16.0, Microsoft Corporation, 2016) independently by F.M. and checked by A.L.G. The patient and treatment characteristics, and early and late events were extracted from the included studies. The complete list of extracted variables and definitions is available in Supplementary Material, Appendix SII.
Risk of bias in individual studies
The quality of the included studies was assessed independently by 2 reviewers according to the ‘Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies’ [10].
Synthesis of results
The patient and treatment characteristics were pooled using an inverse-variance weighted approach. Categorical data were presented as percentages and continuous data as the mean or median, both including the corresponding 95% confidence intervals (CIs). In order to compare baseline and treatment characteristics between males and females, odds ratios (ORs) were used for categorical data, and standardized mean differences for continuous data. When the standard deviation was not reported in studies, it was estimated [11]. For early mortality and morbidity, risk ratios and the corresponding 95% CI comparing males to females were computed. A random-effects model was used based on the DerSimonian–Laird method [12] for all meta-analyses. All meta-analyses were performed using statistical and computing programme R (R Foundation for Statistical Computing, Vienna, Austria. Version 3.5) using the ‘Metafor’ package [13]. P-values <0.05 were considered statistically significant.
Between study heterogeneity was assessed using the Cochrane Q statistic and the I2 test [14]. For the Cochrane Q statistic, a P-value of <0.05 was considered statistically significant.
If reported, survival and reoperation during follow-up were depicted in pooled Kaplan–Meier (KM) curves for males and females separately, derived from the originally published KM curves using the method described by Guyot et al. [15]. The Engauge Digitizer v10.0 [16] was used to produce a list of coordinates of the KM curve, and an algorithm written in R was used to reconstruct the original patient data.
Publication bias was assessed with funnel plots and a trim-and-fill analysis, when the number of studies included in the analysis reached the minimum requirement of 10 studies.
Three sensitivity analyses on early mortality in AD-A were performed: (i)based on region by exclusion of studies performed in Asia; (ii)including studies that encompassed only surgical patients; and (iii) including good quality studies according to our scoring method.
RESULTS
Study selection
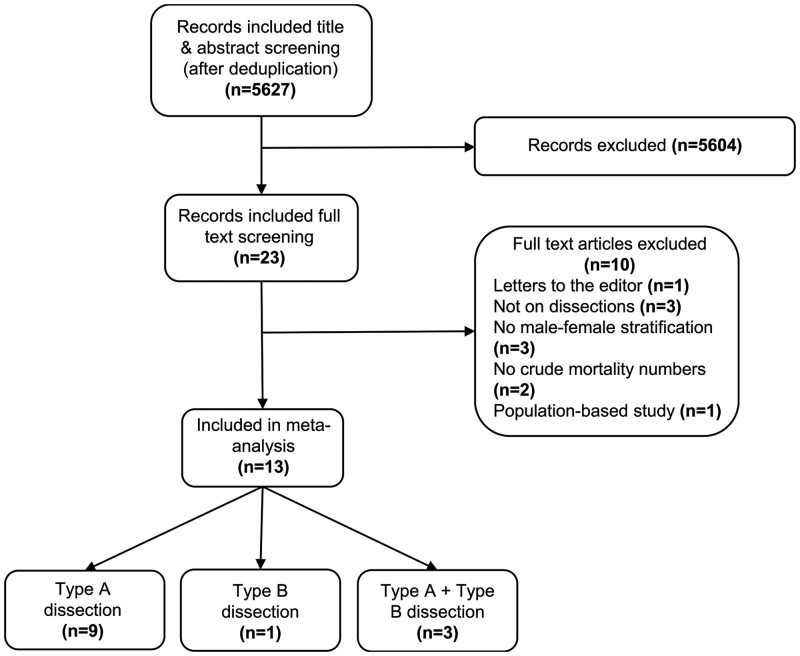
The flowchart of study selection is shown in Fig. 1. The studies comprised 13 papers [5, 6, 17–27]: 9 on AD-A, 1 on AD-B and 3 on type A and type B dissections combined, encompassing a total of 18 659 patients. All included studies were retrospective cohort studies and the publication years ranged from 2004 to 2019. The studies including AD-A and AD-B [5, 26, 27] presented patient characteristics on the whole population; however, treatment strategy and mortality were reported for AD-A and AD-B separately. In 8/12 studies on AD-A, only surgical patients were included [6, 17–19, 21–24], whereas in 4/4 studies on AD-B, all diagnosed patients were included [5, 25–27]. Two studies were not included in the meta-analysis for early mortality: Liu et al. [26] as the mortality numbers were not clearly reported and Sabashnikov et al. [22] since mortality was only reported for the matched population. The study quality was graded as ‘good’ for 6/13 studies [5, 6, 18, 19, 21, 23], as ‘fair’ for 6/13 studies [17, 20, 22, 24, 25, 27] and ‘poor’ for 1 study [26]. An overview of the individual study characteristics and quality assessment is presented in Supplementary Material, Appendices SIII and SIV.
Figure 1:
Flowchart of study selection.
Patient characteristics and presentation
Pooled patient characteristics and presentation are shown in Tables 1 and 2.
Table 1:
Pooled estimates of baseline characteristics of AD-A and AD-B patients
| Variables | AD-A |
AD-A + AD-B |
AD-B |
Significant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males, pooled estimate (95% CI) | Females, pooled estimate (95% CI) | OR/SMD (95% CI) | P-value | Studies | I 2 (%) | Males, pooled estimate (95% CI) | Females, pooled estimate (95% CI) | OR/SMD (95% CI) | P-value | Studies | I 2 (%) | Males | Females | ||
| Age (mean) | 59.6 | 67.7 | −0.62 | <0.001 | 9 | 80.4 | 53.6 | 58.5 | −0.39 | <0.001 | 3 | 15.2 | 62.8 | 69.8 | [25] (1/1) |
| (58.5–60.7) | (65.4–70.0) | (−0.77 to −0.48)a | (47.4–60.6) | (50.5–67.7) | (−0.49 to 0.30)a | ||||||||||
| Mean BSA (m2) | 1.99 | 1.59 | 0.79 | 0.016 | 3 | 96.3 | - | - | - | - | - | - | - | - | - |
| (1.83–2.15) | (1.37–1.84) | (0.15–1.44)a | |||||||||||||
| Mean BMI (kg/m2) | 26.0 | 25.1 | 0.23 | 0.040 | 5 | 80.0 | 25.9 | 25.3 | - | - | 1 | - | - | - | - |
| (25.1–26.9) | (23.4–26.8) | (0.01–0.45)a | |||||||||||||
| History of hypertension (%) | 69.6 | 76.0 | 0.78 | 0.052 | 7 | 44.5 | 74.5 | 75.6 | 0.93 | 0.693 | 3 | 55.6 | 78.9 | 79.2 | [25] (0/1) |
| (58.7–82.5) | (68.5–84.3) | (0.60–1.00)b | (67.3–82.5) | (72.4–78.9) | (0.67–1.31)b | ||||||||||
| Diabetes mellitus (%) | 5.35 | 6.30 | 0.89 | 0.700 | 7 | 60.3 | 7.10 | 7.24 | 0.98 | 0.958 | 3 | 60.7 | 14.8 | 18.0 | [25] (1/1) |
| (3.15–9.08) | (4.33–9.16) | (0.50–1.60)b | (2.17–23.2) | (4.32–12.2) | (0.54–1.79)b | ||||||||||
| Hyperlipidaemia (%) | 14.4 | 16.0 | 0.94 | 0.615 | 5 | 11.0 | - | - | - | - | - | - | - | - | - |
| (9.50–21.7) | (12.0–21.4) | (0.73–1.20)b | |||||||||||||
| Smoking (%) | 39.5 | 14.4 | 4.27 | 0.077 | 4 | 97.1 | 61.6 | 9.22 | 15.1 | <0.001 | 2 | 74.5 | - | - | - |
| (23.5–66.3) | (6.39–32.5) | (0.85–21.4)b | (51.5–73.7) | (2.27–37.4) | (5.18–43.8)b | ||||||||||
| COPD (%) | 6.24 | 7.98 | 0.74 | 0.138 | 5 | 18.8 | - | - | - | - | - | - | 21.4 | 26.2 | [25] (1/1) |
| (4.00–9.75) | (5.61–11.4) | (0.49–1.10)b | |||||||||||||
| History of cerebrovascular disease (%) | 6.88 | 9.30 | 0.85 | 0.559 | 3 | 33.7 | - | - | - | - | - | - | - | - | - |
| (2.65–17.8) | (4.17–20.8) | (0.49–1.47)b | |||||||||||||
| Chronic kidney disease (%) | 8.26 | 7.24 | 1.38 | 0.224 | 5 | 46.1 | - | - | - | - | - | - | - | - | - |
| (2.84–24.0) | (3.35–15.7) | (0.82–2.33)b | |||||||||||||
| Congestive heart failure (%) | - | - | - | - | - | - | 3.39 | 4.26 | 0.70 | 0.199 | 3 | 15.1 | 17.9 | 20.4 | [25] (1/1) |
| (2.12–5.45) | (1.60–11.3) | (0.41–1.20)b | |||||||||||||
| Marfan syndrome (%) | 4.12 | 2.67 | 1.57 | 0.007 | 5 | 2.11 | 3.48 | 2.88 | 1.37 | 0.300 | 2 | 0.0 | - | - | - |
| (3.34–5.07) | (1.87–3.82) | (1.13–2.19)b | (1.33–9.11) | (0.91–9.12) | (0.75–2.50)b | ||||||||||
| Bicuspid aortic valve (%) | - | - | - | - | - | - | 3.07 | 3.92 | 0.80 | 0.615 | 2 | 10.0 | - | - | - |
| (2.03–4.63) | (2.06–7.47) | (0.34–1.88)b | |||||||||||||
| Previous aortic aneurysm (%) | - | - | - | - | - | - | 18.9 | 14.5 | 1.23 | 0.256 | 2 | 18.5 | - | - | - |
| (10.4–34.4) | (10.9–19.3) | (0.86–1.77)b | |||||||||||||
| Previous aortic dissection (%) | - | - | - | - | - | - | 3.26 | 2.94 | 1.33 | 0.341 | 2 | 0.0 | - | - | - |
| (0.99–10.7) | (0.85–10.2) | (0.74–2.38)b | |||||||||||||
‘-’ indicates the variable was not reported in any of the included studies.
SMD.
OR.
AD-A: acute type A aortic dissection; AD-B: acute type B aortic dissection; BMI: body mass index; BSA: body surface area; CI: confidence interval; COPD: chronic obstructive pulmonary disease; OR: odds ratio; SMD: standardized mean difference.
Table 2:
Pooled estimates of patient presentation of AD-A and AD-B patients
| Variables | AD-A |
AD-A + AD-B |
AD-B |
Significant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males, pooled estimate (95% CI) | Females, pooled estimate (95% CI) | OR/SMD (95% CI) | P-value | Studies | I 2 (%) | Males, pooled estimate (95% CI) | Females, pooled estimate (95% CI) | OR/SMD (95% CI) | P-value | Studies | I 2 (%) | Males | Females | ||
| Hypotension/shock at presentation (%) | 17.6 | 19.6 | 0.86 | 0.136 | 5 | 0.0 | 6.07 | 3.97 | 1.24 | 0.685 | 2 | 33.6 | 4.93 | 4.31 | [25] (0/1) |
| (11.7–26.6) | (13.7–28.0) | (0.7–1.05)a | (2.38–15.5) | (0.46–34.3) | (0.44–3.44)a | ||||||||||
| Tamponade (%) | 19.4 | 21.9 | 0.87 | 0.042 | 4 | 0.0 | - | - | - | - | - | - | - | - | - |
| (15.2–24.8) | (19.2–25.0) | (0.75–0.99)a | |||||||||||||
| Pericardial effusion (%) | 33.0 | 42.5 | 0.70 | 0.436 | 2 | 78.7 | 20.8 | 30.8 | 0.61 | <0.001 | 3 | 0.0 | - | - | - |
| (16.9–64.3) | (35.4–50.9) | (0.29–1.71)a | (15.3–28.4) | (22.6–42.0) | (0.50–0.75)a | ||||||||||
| Cerebral ischaemia (%) | 8.87 | 11.3 | 0.91 | 0.350 | 3 | 0.87 | - | - | - | - | - | - | - | - | - |
| (5.33–14.8) | (7.94–16.2) | (0.74–1.11)a | |||||||||||||
| Presentation ≥24 h (%) | - | - | - | - | - | - | 39.4 | 45.7 | 0.74 | 0.024 | 2 | 3.94 | - | - | - |
| (13.9–112.1) | (20.5–101.9) | (0.58–0.96)a | |||||||||||||
| Abrupt onset of pain (%) | - | - | - | - | - | - | 87.5 | 86.4 | 0.78 | 0.791 | 2 | 88.8 | - | - | - |
| (84.1–91.0) | (68.9–108.4) | (0.12–4.97)a | |||||||||||||
| Chest pain (%) | - | - | - | - | - | - | 78.3 | 77.9 | 1.07 | 0.646 | 3 | 32.8 | - | - | - |
| (73.3–83.5) | (71.3–85.2) | (0.81–1.41)a | |||||||||||||
| Back pain (%) | - | - | - | - | - | - | 57.5 | 58.6 | 0.95 | 0.760 | 3 | 61.8 | - | - | - |
| (52.9–62.6) | (48.6–70.8) | (0.69–1.31)a | |||||||||||||
| Abdominal pain (%) | - | - | - | - | - | - | 18.4 | 15.8 | 1.22 | 0.462 | 2 | 51.7 | - | - | - |
| (13.9–24.4) | (7.65–32.8) | (0.72–2.05)a | |||||||||||||
| Any focal neurological deficits (%) | - | - | - | - | - | - | 11.4 | 6.31 | 1.74 | 0.481 | 2 | 77.4 | - | - | - |
| (7.38–17.7) | (0.92–43.1) | (0.37–8.19)a | |||||||||||||
| Syncope (%) | - | - | - | - | - | - | 3.06 | 4.54 | 0.86 | 0.440 | 2 | 0.0 | - | - | - |
| (0.18–52.5) | (0.38–54.8) | (0.59–1.26)a | |||||||||||||
| Altered consciousness/ coma (%) | - | - | - | - | - | - | 8.07 | 11.9 | 0.66 | 0.012 | 2 | 0.0 | - | - | - |
| (6.76–9.63) | (9.47–14.9) | (0.48–0.91)a | |||||||||||||
| Pulse deficit (%) | - | - | - | - | - | - | 22.4 | 12.6 | 1.99 | <0.001 | 2 | 0.0 | - | - | - |
| (14.6–34.5) | (6.57–24.2) | (1.47–2.69)a | |||||||||||||
| Mean creatinine (mg/dl) | 1.23 | 0.97 | 0.38 | <0.001 | 5 | 73.4 | - | - | - | - | - | - | - | - | - |
| (1.12–1.34) | (0.87–1.07) | (0.20–0.56)b | |||||||||||||
| Renal insufficiency (%) | – | – | – | – | – | – | – | – | – | 23.6 | 19.9 | [25] (1/1) | |||
| DeBakey type II (%) | 14.3 | 23.3 | 0.57 | <0.001 | 4 | 0.0 | - | - | - | - | - | - | - | - | - |
| (9.18–22.2) | (16.7–32.5) | (0.46–0.70)a | |||||||||||||
| Max. diameter ascending aorta (mm), mean | 54.4 | 53.4 | 0.06 | 0.654 | 2 | 0.0 | - | - | - | - | - | - | - | - | - |
| (51.4–57.5) | (51.1–55.8) | (−0.20 to 0.32)b | |||||||||||||
| Aortic regurgitation (%) | 27.7 | 25.6 | 1.09 | 0.211 | 5 | 0.0 | - | - | - | - | - | - | - | - | - |
| (18.4–41.6) | (15.9–41.2) | (0.95–1.24)a | |||||||||||||
‘-’ indicates the variable was not reported in any of the included studies.
OR.
SMD.
AD-A: acute type A aortic dissection; AD-B: acute type B aortic dissection; CI: confidence interval; OR: odds ratio; SMD: standardized mean difference.
Treatment strategy
Figure 2 shows pooled proportions of males and females by treatment strategy for AD-A based on 4 studies [5, 20, 26, 27] (Fig. 2A) and AD-B patients based on 4 studies [5, 25–27] (Fig. 2B). A sensitivity analysis for AD-A was performed by excluding 1 study as outlier [27], which resulted in an OR of 1.70 (95% CI 0.94–3.07; P = 0.080) for surgical repair, 0.25 (95% CI 0.05–1.21; P = 0.090) for endovascular repair and 0.62 (95% CI 0.34–1.13; P = 0.116) for conservative treatment, comparing males to females (Supplementary Material, Appendix SV).
Figure 2:
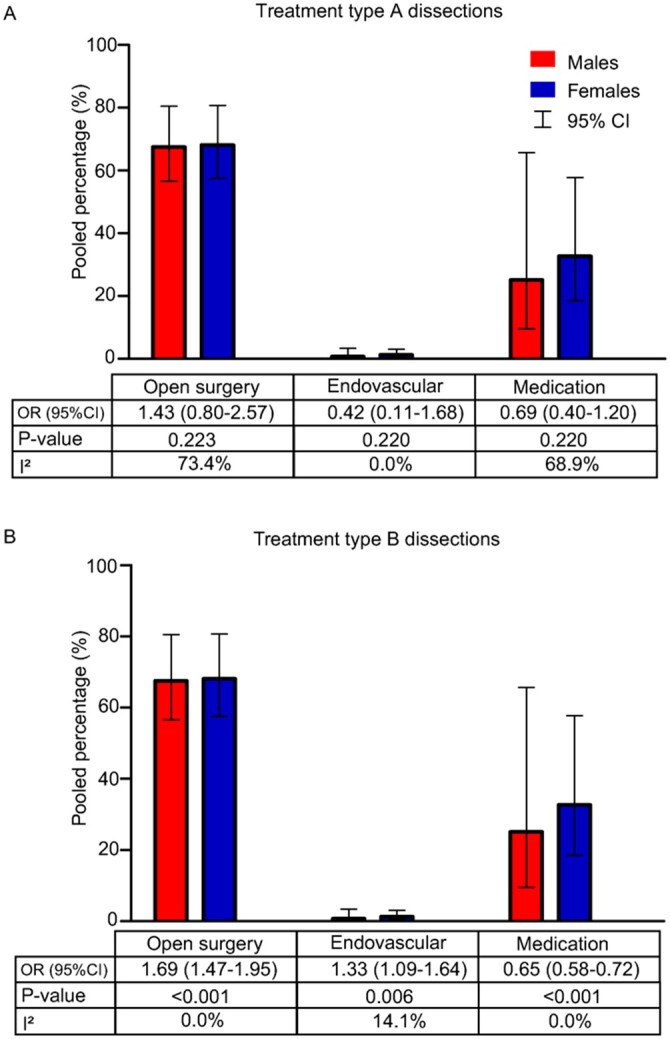
Meta-analysis of treatment in acute type A aortic dissection (A) and acute type B aortic dissection (B) patients. CI: confidence interval; OR: odds ratio.
Figure 3:
Meta-analysis of early mortality in male versus female surgically treated acute type A aortic dissection patients (A) and acute type B aortic dissection patients receiving any treatment (B). CI: confidence interval; RE Model: random-effects model; RR: risk ratio.
Operative characteristics
Of the 8 studies [6, 17–19, 21–24], the pooled operative characteristics of surgically treated AD-A patients are shown in Table 3. No operative characteristics were available on AD-B patients.
Table 3:
Operative characteristics of AD-A patients
| Males, pooled estimate (95% CI) | Females, pooled estimate (95% CI) | OR/SMD (95% CI) | P-value | Studies reported | I 2 (%) | |
|---|---|---|---|---|---|---|
| Isolated supracoronary ascending aortic replacement (%) | 43.3 (25.2–74.5) | 53.0 (34.3–81.9) | 0.70 (0.58–0.84)a | <0.001 | 3 | 36.1 |
| Bentall procedure (%) | 23.8 (19.8–28.7) | 17.3 (15.4–19.4) | 1.49 (1.19–1.86)a | <0.001 | 5 | 30.0 |
| David procedure (%) | 5.33 (3.18–8.93) | 3.19 (2.22–4.58) | 1.95 (1.31–2.91)a | 0.001 | 4 | 15.5 |
| Aortic valve repair or replacement (%) | 10.8 (4.18–28.1) | 9.50 (5.58–16.2) | 1.36 (0.87–2.11)a | 0.174 | 2 | 0.0 |
| Aortic valve replacement (%) | 11.0 (6.37–19.0) | 8.95 (4.34–18.5) | 1.54 (1.05–2.26)a | 0.028 | 4 | 0.0 |
| Total arch replacement (%) | 13.4 (7.26–24.9) | 8.91 (5.71–13.9) | 1.76 (1.09–2.84)a | 0.021 | 6 | 79.9 |
| Concomitant CABG (%) | 10.9 (7.94–14.9) | 9.95 (7.26–13.6) | 1.08 (0.65–1.81)a | 0.772 | 5 | 45.8 |
| Total operation time (min), mean | 321.6 (289.0–357.8) | 271.0 (226.9–323.7) | 0.29 (0.17–0.41)b | <0.001 | 6 | 69.5 |
| Cardiopulmonary bypass time (min), mean | 173.2 (148.2–202.4) | 161.3 (135.7–191.6) | 0.19 (0.13–0.24)b | <0.001 | 7 | 0.0 |
| Aortic cross-clamp time (min), mean | 104.0 (97.8–110.5) | 91.2 (84.3–98.6) | 0.25 (0.11–0.39)b | <0.001 | 6 | 73.1 |
| DHCA/circulatory arrest time (min), mean | 28.6 (25.1–32.6) | 28.4 (26.1–31.0) | 0.07 (−0.06 to 0.19)b | 0.293 | 6 | 62.3 |
a OR. b SMD.
AD-A: acute type A aortic dissection; CABG: coronary artery bypass graft; CI: confidence interval; DHCA: deep hypothermic circulatory arrest; OR: odds ratio; SMD: standardized mean difference.
Early and late outcomes
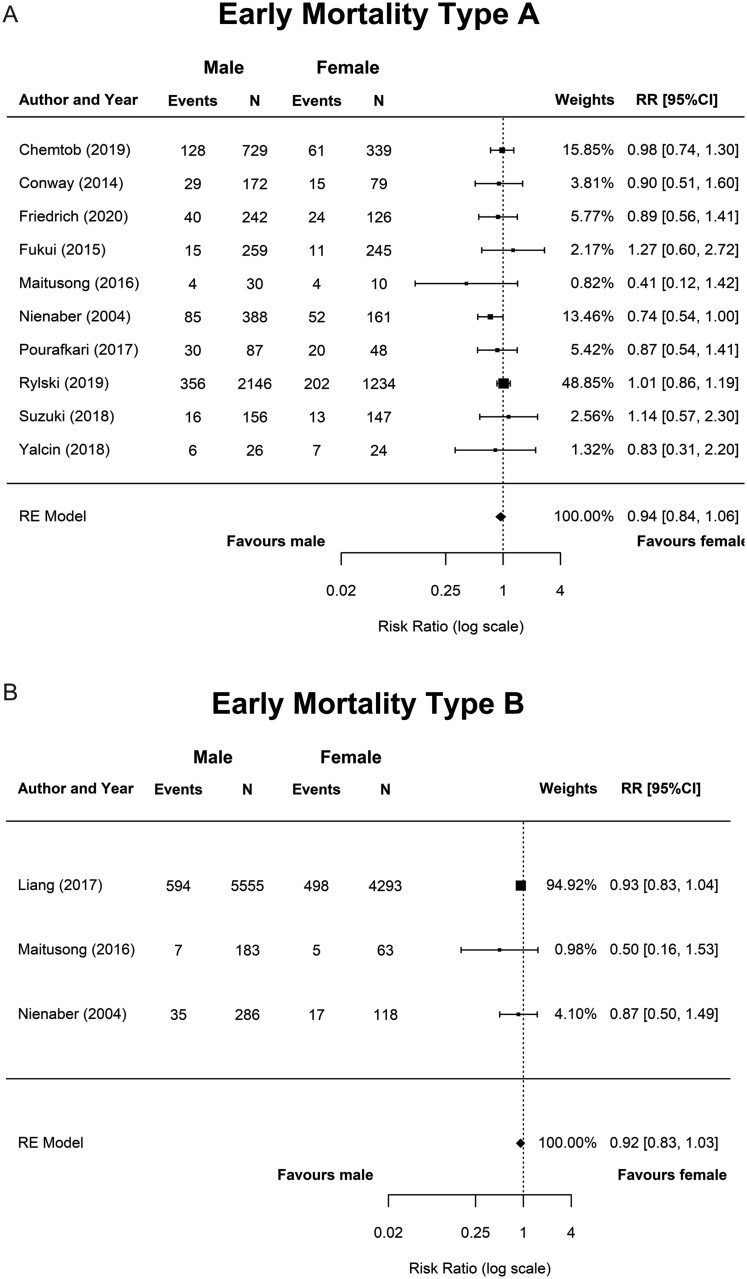
The forest plots of early mortality in male versus female patients for AD-A and AD-B are shown in Fig. 2. Results of meta-analyses for early mortality, morbidity and the sensitivity analyses are depicted in Table 4.
Table 4:
Meta-analyses for early mortality and morbidity in AD-A and AD-B
| Cohort | Outcome | Males (95% CI) | I 2 (%), Q-testa | Females (95% CI) | I 2, Q-testa | RR male vs female (95% CI) | P-value RR | I 2 (%), Q-testa | Studies |
|---|---|---|---|---|---|---|---|---|---|
| AD-A | Early mortality all diagnosed patients (%) | 36.9 (25.9–52.6) | 90.9, <0.001 | 52.6 (34.6–80.1) | 92.9, <0.001 | 0.78 (0.66–0.93) | 0.006 | 0.0, 0.723 | 3 |
| AD-A | Early mortality surgical patients (%) | 16.8 (13.7–20.6) | 83.2, <0.001 | 19.3 (14.1–26.4) | 89.5, <0.001 | 0.94 (0.84–1.06) | 0.308 | 0.0, 0.718 | 10 |
| AD-A | Early mortality surgical patients (%) (sensitivity 1)b | 19.8 (16.6–23.6) | 78.1, <0.001 | 23.4 (17.4–31.3) | 87.5, <0.001 | 0.94 (0.84–1.05) | 0.282 | 0.0, 0.731 | 7 |
| AD-A | Early mortality surgical patients (%) (sensitivity 2)c | 14.8 (12.1–18.0) | 73.8, <0.001 | 14.9 (11.2–19.7) | 79.8, <0.001 | 1.00 (0.88–1.13) | 0.965 | 0.0, 0.982 | 7 |
| AD-A | Early mortality surgical patients (%) (sensitivity 3)d | 14.6 (11.4–18.7) | 83.4, <0.001 | 14.8 (9.7–22.5) | 91.4, <0.001 | 0.95 (0.84–1.08) | 0.450 | 0.0, 0.515 | 6 |
| AD-A | Stroke (%) | 13.1 (10.2–16.8) | 54.1, 0.069 | 12.7 (9.16–17.8) | 59.7, 0.042 | 0.99 (0.79–1.23) | 0.911 | 0.0, 0.904 | 5 |
| AD-A | Acute renal failure (%) | 17.1 (12.2–24.0) | 82.3, <0.001 | 12.3 (8.5–17.9) | 68.0, 0.010 | 1.24 (0.93–1.66) | 0.135 | 19.5, 0.286 | 5 |
| AD-A | Re-exploration for bleeding (%) | 18.1 (12.9–25.3) | 81.0, <0.001 | 12.9 (8.7–19.2) | 65.8 <0.02 | 1.34 (1.08–1.67) | 0.010 | 0.0, 0.645 | 5 |
| AD-B | Early mortality all diagnosed patients (%) | 9.46 (6.56–13.6) | 76.2, 0.015 | 11.6 (10.7–12.6) | 0.0, 0.426 | 0.92 (0.83–1.03) | 0.143 | 0.0, 0.543 | 3 |
| AD-B | Early mortality surgicallye treated patients (%) | 6.21 (0.45–85.7) | 92.6, 0.001 | 11.4 (3.05–42.3) | 65.8, 0.088 | 0.77 (0.26–2.31) | 0.637 | 27.2, 0.241 | 2 |
The P-value of the Q-test is reported.
Exclusion of studies performed in Asia: Fukui et al. [6], Maitusong et al. [27] and Suzuki et al. [23].
Exclusion of studies which included all diagnosed patients: Nienaber et al. [5], Maitusong et al. [25], Pourafkari et al. [20], leaving studies which included only surgical patients.
Inclusion of studies with a good study quality.
Surgical treatment included open surgical repair and endovascular treatment.
AD-A: acute type A aortic dissection; AD-B: acute type B aortic dissection; CI: confidence interval; RR: risk ratio.
Forest plots of the meta-analyses for early morbidity in AD-A and an overview of all reported early outcomes in AD-A and AD-B are shown in Supplementary Material, Appendices SVI and SVII.
Overall mortality for AD-A was described in 6/12 studies [6, 18–20, 23, 26], of which none found a significant difference between males and females. For AD-B patients, 1/1 reporting study [26] found no significant male–female difference in late mortality. Late reoperation for AD-A was described in 2/12 studies [6, 23] and none for AD-B.
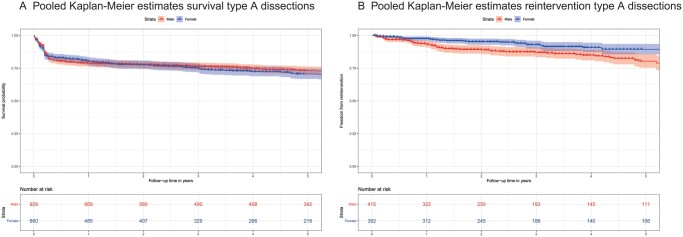
Pooled KM estimates for overall survival [6, 18–20, 23] and reoperation [6, 23] for AD-A patients are depicted in Fig. 4. Overall survival between male and female AD-A patients was not different, while more reoperations in male patients were observed compared to females.
Figure 4:
Pooled Kaplan–Meier estimates survival (A) and reinterventions (B) in acute type A aortic dissection patients.
Risk factor analyses
Six [6, 17, 19, 21, 23, 26] out of the 13 included studies performed risk factor analyses for early and/or late mortality, none of which found sex/gender to be an independent risk factor. Suzuki et al. [23] and Friedrich et al. [19] included male–female-specific risk factor analyses. Suzuki et al. [23] found different independent risk factors for late mortality: older age and preoperative cerebrovascular disease in males and tamponade, chronic obstructive pulmonary disease (COPD) and longer operation time in females. In Friedrich et al. [19], the independent preoperative and intraoperative risk factors for early mortality were cardiopulmonary resuscitation, longer cardiopulmonary bypass time and a higher amount of transfused red blood cells for males, and COPD, peripheral arterial disease, higher amount of transfused red blood cells and intubation prior to surgery for females. In a model including preoperative risk factors only, coronary heart disease and cardiopulmonary resuscitation were found as independent risk factors for both males and females, and additionally for females of older age, and hypolipoproteinaemia [19].
Risk of bias across studies
The publication bias could only be assessed for the meta-analysis of early mortality in surgical AD-A patients, as it contained more than 10 studies and was eligible for risk of bias analyses. The funnel plot and trim-and-fill are shown in Supplementary Material, Appendix SVIII. In the trim-and-fill analysis, we observed that 2 hypothetical studies were possibly missing that would have had higher mortality in male patients.
DISCUSSION
This systematic review shows that existing literature on potential differences between males and females in presentation, management and outcome of acute aortic dissection is scarce and reporting not uniform and incomplete. Clear male–female differences were observed in presentation and treatment; however, early or overall mortality was comparable.
Presentation
Our findings confirm the observations in the population-based Oxford Vascular study that the age of onset in AD is ∼10 years later in females compared to males [3]. A later age of onset is in line with the incidence of cardiovascular disease in general, such as acute coronary syndrome [28]. Literature suggests that the risk of cardiovascular disease is lower in premenopausal females than in postmenopausal females [29], due to the protective effect of oestrogen. Oestrogen decreases the proportion of collagen [30] and stimulates the formation of fibrillin [31] in the aortic wall, thereby decreasing the wall stiffness of the aorta and other arteries [30, 32]. It seems plausible that this protective effect of oestrogen also plays an important role in AD.
Active smoking was more common in males [20, 23, 26, 27]. Population-based studies on risk factors for aortic diseases [2, 33] found that smoking was a significant risk factor for the incidence of AD. However, Sidloff et al. [34] showed that smoking was not associated with age-standardized mortality in thoracic aortic disease. The association between smoking and abdominal aneurysms on the other hand has been well established [35]. This finding emphasizes that the aorta is a heterogeneous entity and different factors influence aneurysm formation in the thoracic and abdominal aorta [36].
Two AD-A studies [17, 20] found a higher prevalence of hypertension in females. Hypertension was found to be the most important modifiable risk factor in the development of AD in prospective population-based studies [3, 33]. An increased prevalence of hypertension in female AD patients can be explained by the older age at presentation since systolic blood pressure and prevalence of hypertension increase with age [37]. Hypertension is a well-known risk factor for AD and other cardiovascular diseases and might be more important in females compared to males, independently of age [28].
Three AD-A studies [6, 17, 18] showed that males presented with higher ‘creatinine levels’. As the absolute differences were small, we assume these differences to be physiological.
Female patients were diagnosed more frequently with more proximally located dissection, such as DeBakey type II [6, 17, 23]. One explanation for the proximally extended dissections could lie in geometric differences in the aorta. Rylski et al. [38] found that with increasing age, healthy females have a greater increase in the ascending aorta and aortic arch diameters than healthy males. Furthermore, the body surface area-adjusted diameters of the aforementioned aortic segments were greater in females than in males [38]. As observed in clinical trials on cardiovascular disease, female patients are often underrepresented [39]. Whether a referral delay of female patients with a high risk of AD plays a role in these studies remains to be elucidated. A second explanation could be that biomechanical differences between males and females in the aortic wall influence the extension of the dissections. Mean wall thickness in both the ascending and the descending aorta is higher in males [40] and the peak wave velocity is lower in healthy females [41]. In addition, the blood flow dynamics in the aorta are significantly different between males and females [42].
Management
This systematic review suggests that females receive conservative treatment more frequently than males in both AD-A and AD-B. The reasons for conservative treatment in AD-A were described in the original papers. First, refusal of emergency surgery was seen more often in females [5, 27]. Other reasons were advanced age, comorbidity, intramural haematoma, preoperative death [1, 5] or irreversible brain damage [20]. Apart from preoperative status and comorbidities, it is possible that literature reporting on poor outcomes for female patients after surgery [43] may influence the physician’s choice for a conservative approach.
Procedural times seemed longer in male surgically treated AD-A patients compared to females. This can be explained by more simplified procedures in females, such as the supracoronary artery replacement alone [17, 19, 21], while male patients more often underwent extensive repair with valve replacement [17–19, 27]. As stated earlier, male patients seemed to have more widespread dissections. Additionally, the increased age and comorbidities in females may have led to the decision of less complicated procedures, resulting in comparable surgical mortality.
Outcomes
The included AD-A studies showed no significant effect of sex/gender on early or overall mortality. Contrasting these findings are 3 population-based studies on AD [2, 4, 44]. McClure et al. [2] found a higher hospital mortality in females compared to males across a 12-year study period for AD-A (45.65 per 100 000 in males vs 64.21 in females) and Smedberg et al. [4] found an overall 30-day mortality in AD of 26% in males and 21% in females (P < 0.001). The Kaiser Permanente Registry of Aortic Dissections showed a significantly higher incidence of aorta-related morbidity and mortality for females versus males (50% vs 34%, P = 0.01) [44]. As our systematic review concerned hospital-based studies, all patients, who did not reach the hospital or were not operated on, were excluded by design. A possible explanation for the increased mortality in the population-based studies may be that females in worse conditions are more often denied surgical treatment and/or females die more frequently before reaching the hospital. The Oxford Vascular Study [3] confirms that females with acute aortic dissection die more frequently (61.1%) than males (38.9%) (P = 0.07) before reaching the hospital and in Smedberg et al. [4], the proportion of females was higher in patients who died out of the hospital than in admitted patients (42% vs 36%, P = 0.001).
Male patients had higher reoperation rates during follow-up in 2 AD-A studies [6, 23]. This can be explained by the different types of dissections in male and female patients: DeBakey type I dissections, which were more prevalent among males, require a late operation more frequently than DeBakey type II dissections [43].
Our meta-analysis revealed no significant difference between males and females with AD-B for early mortality. A study on male–female differences in thoracic endovascular aortic repair [45], not included in our systematic review, found non-significance on late mortality between male and female AD-B patients.
Furthermore, we found that male AD-B patients more often had complications that involved aortic branch vessels. Male patients more often had acute renal failure, paraplegia and limb ischaemia. Imaging characteristics on artery involvement were only available in Maitusong et al. [27], which show more involvement of the coeliac trunk and the superior mesenteric artery in male patients.
Male–female specific risk factors
Two AD-A studies performed male–female-specific risk factor analyses [19, 23] that showed different independent risk factors for late mortality such as COPD in females and cerebrovascular disease in males. Male–female risk factor differentiation would allow for the development of male–female-specific risk profiles to optimize treatment decision-making and outcomes. Therefore, we recommend male–female-specific sub-analyses in future risk factor studies for AD.
Sex versus gender
According to working definitions of the World Health Organization, sex refers to the biological characteristics [46] and gender to the socially constructed characteristics that define us being female or male [47]. Several biological factors may play a role in the incidence and prognosis of AD in males and females as previously described: hormones, vascular haemodynamics and cardiovascular risk factors. However, the sociological aspect of male–female differences also may not be underestimated. Jansen Klomp et al. [48] found that AD was less likely to be recognized in females by physicians. In addition, it seems that female AD patients die more often out of the hospital [3, 4]. These diagnostic delays might be due to a gender bias; females can be less likely to access medical care and/or to be recognized by physicians. Awareness of patients and physicians of the existing gender biases will hopefully decrease the gender gap.
Clinical relevance
Considering the 13 included studies, the in-hospital outcomes between male and female patients seem similar. However, as it is plausible that the diagnosis of AD is more frequently missed in females [2, 4, 48], awareness of less typical symptoms is required. Furthermore, close monitoring of patients with hypertension, especially females, is crucial in the prevention of AD. In case of presentation with AD, male patients with cerebrovascular disease and coronary heart disease are at high risk of mortality, whereas female patients with tamponade and COPD require attention. Physicians’ awareness of these differences will help them to actively ascertain relevant sex-specific risk factors, whilst preventing adverse outcomes.
Limitations
All included studies were retrospective hospital-based studies with inherent selection bias. The amount of out-of-hospital death and these patients’ clinical profiles remain unknown.
The quality of included studies was graded fair in some studies, mainly because clear definitions of variables and outcomes were not reported.
Significant heterogeneity was detected between the studies, which may have influenced the results of the meta-analyses. Between study differences may be due to disparities in management, operative techniques or health care systems in the participating countries, and differences in variable definitions.
Finally, there are limitations inherent of meta-analyses and combining data from retrospective studies, which should be acknowledged [49].
Recommendations
Prospective cohort studies studying male–female differences with a focus on late outcomes are recommended. The study quality should be improved by following international guidelines for study and outcome reporting, and by using standardized variable definitions. As AD is a rare disease, international collaboration and data sharing need to be stimulated to increase the power of studies. Furthermore, translational science can help elucidate underlying mechanisms for male–female disparities, such as hormonal changes or histological differences in the vascular wall. At the same time, gender-related factors that may affect diagnosis and referral patterns are important to consider moving forward. Most importantly, we recommend male–female-specific reporting of outcomes and risk factors in AD studies to fill the current knowledge gap.
CONCLUSION
This systematic review shows male–female differences in baseline and treatment characteristics in AD, however, comparable early and overall mortality. Published literature is scarce and heterogeneous and large prospective studies with more details and complete registration are clearly warranted. Improved knowledge of male–female differences can lead to the recognition of high-risk patients and help shape optimal individualized care for both males and females.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Wichor Bramer from the Erasmus University Medical Center Medical Library for conducting the literature search.
Funding
This study was supported by The Netherlands Organization for Health Research and Development ZonMW [849200014].
Conflict of interest: none declared.
Author contributions
Frederike Meccanici: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Visualization; Writing—original draft. Arjen L. Gökalp: Conceptualization; Data curation; Investigation; Methodology; Resources; Software; Validation; Writing—review & editing. Carlijn G.E. Thijssen: Conceptualization; Validation; Writing—review & editing. Mostafa M. Mokhles: Validation; Writing—review & editing. Jos A. Bekkers: Validation; Writing—review & editing. Roland van Kimmenade: Validation; Writing—review & editing. Hence J. Verhagen: Validation; Writing—review & editing. Jolien W. Roos-Hesselink: Conceptualization; Funding acquisition; Supervision; Validation; Writing—review & editing. Johanna J.M. Takkenberg: Conceptualization; Funding acquisition; Methodology; Supervision; Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Marco Moscarelli, Derrick Y. Tam and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Glossary
ABBREVIATIONS
- AD
Acute thoracic aortic dissection
- AD-A
Acute type A aortic dissection
- AD-B
Acute type B aortic dissection
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- KM
Kaplan–Meier
- OR
Odds ratio
REFERENCES
- 1. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL. et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 2. McClure RS, Brogly SB, Lajkosz K, Payne D, Hall SF, Johnson AP.. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: a population-based study. J Thorac Cardiovasc Surg 2018;155:2254–64.e4. [DOI] [PubMed] [Google Scholar]
- 3. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM.. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smedberg C, Steuer J, Leander K, Hultgren R.. Sex differences and temporal trends in aortic dissection: a population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur Heart J 2020;41:2430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M. et al. ; International Registry of Acute Aortic Dissection. Gender-related differences in acute aortic dissection. Circulation 2004;109:3014–21. [DOI] [PubMed] [Google Scholar]
- 6. Fukui T, Tabata M, Morita S, Takanashi S.. Gender differences in patients undergoing surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;150:581–7.e1. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- 8. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H. et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–926. [DOI] [PubMed] [Google Scholar]
- 9. Steuer J, Bjorck M, Mayer D, Wanhainen A, Pfammatter T, Lachat M.. Distinction between acute and chronic type B aortic dissection: is there a sub-acute phase? Eur J Vasc Endovasc Surg 2013;45:627–31. [DOI] [PubMed] [Google Scholar]
- 10.National Heart Lung aBI. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (14 October 2019, date last accessed).
- 11. Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 13. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010;36: 5–40. [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guyot P, Ades AE, Ouwens MJ, Welton NJ.. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell M, Muftakhidinov B, Winchen T. Engauge Digitizer Software. http://markummitchell.github.io/engauge-digitizer (1 March 2021, date last accessed).
- 17. Chemtob RA, Hjortdal V, Ahlsson A, Gunn J, Mennander A, Zindovic I. et al. Effects of sex on early outcome following repair of acute type A aortic dissection: results from the Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD). Aorta (Stamford) 2019;7:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conway BD, Stamou SC, Kouchoukos NT, Lobdell KW, Hagberg RC.. Effects of gender on outcomes and survival following repair of acute type A aortic dissection. Int J Angiol 2015;24:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedrich C, Salem MA, Puehler T, Hoffmann G, Lutter G, Cremer J. et al. Sex-specific risk factors for early mortality and survival after surgery of acute aortic dissection type a: a retrospective observational study. J Cardiothorac Surg 2020;15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pourafkari L, Ghaffari S, Tajlil A, Safaei N, Parizad R, Chavoshi M. et al. Gender-related differences in presentation and outcomes of acute type A aortic dissection. Int Cardiovasc Res J 2017;11:89–95. [Google Scholar]
- 21. Rylski B, Georgieva N, Beyersdorf F, Büsch C, Boening A, Haunschild J. et al. Gender-related differences in patients with acute aortic dissection type A. J Thorac Cardiovasc Surg 2019;162:528–35.e1. [DOI] [PubMed] [Google Scholar]
- 22. Sabashnikov A, Heinen S, Deppe AC, Zeriouh M, Weymann A, Slottosch I. et al. Impact of gender on long-term outcomes after surgical repair for acute Stanford A aortic dissection: a propensity score matched analysis. Interact CardioVasc Thorac Surg 2017;24:702–7. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki T, Asai T, Kinoshita T.. Clinical differences between men and women undergoing surgery for acute type A aortic dissection. Interact CardioVasc Thorac Surg 2018;26:944–50. [DOI] [PubMed] [Google Scholar]
- 24. Yalçin M, Gödekmerdan Katircioğlu E, Yazman S, Ürkmez M.. The differences between gender in intra- and postoperative results after surgical repair of acute type A aortic dissection. Turkiye Klinikleri J Cardiovasc Sci 2018;30:120–5. [Google Scholar]
- 25. Liang NL, Genovese EA, Al-Khoury GE, Hager ES, Makaroun MS, Singh MJ.. Effects of gender differences on short-term outcomes in patients with type B aortic dissection. Ann Vasc Surg 2017;38:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu YJ, Wang XZ, Wang Y, He RX, Yang L, Jing QM. et al. Correlation between Sex and prognosis of acute aortic dissection in the Chinese population. Chin Med J (Engl) 2018;131:1430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maitusong B, Sun HP, Xielifu D, Mahemuti M, Ma X, Liu F. et al. Sex-Related differences between patients with symptomatic acute aortic dissection. Medicine (Baltimore) 2016;95:e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leening MJ, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D. et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kannel WB, Hjortland MC, McNamara PM, Gordon T.. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 1976;85:447–52. [DOI] [PubMed] [Google Scholar]
- 30. Fischer GM, Swain ML.. Influence of contraceptive and other sex steroids on aortic collagen and elastin. Exp Mol Pathol 1980;33:15–24. [DOI] [PubMed] [Google Scholar]
- 31. Renard M, Muiño-Mosquera L, Manalo EC, Tufa S, Carlson EJ, Keene DR. et al. Sex, pregnancy and aortic disease in Marfan syndrome. PLoS One 2017;12:e0181166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajkumar C, Kingwell BA, Cameron JD, Waddell T, Mehra R, Christophidis N. et al. Hormonal therapy increases arterial compliance in postmenopausal women. J Am Coll Cardiol 1997;30:350–6. [DOI] [PubMed] [Google Scholar]
- 33. Landenhed M, Engstrom G, Gottsater A, Caulfield MP, Hedblad B, Newton-Cheh C. et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 2015;4:e001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sidloff D, Choke E, Stather P, Bown M, Thompson J, Sayers R.. Mortality from thoracic aortic diseases and associations with cardiovascular risk factors. Circulation 2014;130:2287–94. [DOI] [PubMed] [Google Scholar]
- 35. Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D. et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997;126:441–9. [DOI] [PubMed] [Google Scholar]
- 36. Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS.. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg 2008;136:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB. et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15. [DOI] [PubMed] [Google Scholar]
- 38. Rylski B, Desjardins B, Moser W, Bavaria JE, Milewski RK.. Gender-related changes in aortic geometry throughout life. Eur J Cardiothorac Surg 2014;45:805–11. [DOI] [PubMed] [Google Scholar]
- 39. Scott PE, Unger EF, Jenkins MR, Southworth MR, McDowell TY, Geller RJ. et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol 2018;71:1960–9. [DOI] [PubMed] [Google Scholar]
- 40. Lorbeer R, Schneider T, Quadrat A, Kühn JP, Dörr M, Völzke H. et al. Cardiovascular risk factors and thoracic aortic wall thickness in a general population. J Vasc Interv Radiol 2015;26:635–41. [DOI] [PubMed] [Google Scholar]
- 41. Harloff A, Mirzaee H, Lodemann T, Hagenlocher P, Wehrum T, Stuplich J. et al. Determination of aortic stiffness using 4D flow cardiovascular magnetic resonance—a population-based study. J Cardiovasc Magn Reson 2018;20:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garcia J, van der Palen RLF, Bollache E, Jarvis K, Rose MJ, Barker AJ. et al. Distribution of blood flow velocity in the normal aorta: effect of age and gender. J Magn Reson Imaging 2018;47:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung J, Stevens LM, Ouzounian M, El-Hamamsy I, Bouhout I, Dagenais F. et al. ; On behalf of the Canadian Thoracic Aortic Collaborative. Sex-related differences in patients undergoing thoracic aortic surgery. Circulation 2019;139:1177–84. [DOI] [PubMed] [Google Scholar]
- 44. Chalian A, Clare R, Thompson K, Shen A, Khan S, Jorgensen M. et al. Gender differences in presentation and long-term outcomes by type of aortic dissection in a large community based cohort. J Am Coll Cardiol 2014;63:A2058. [Google Scholar]
- 45. Czerny M, Hoebartner M, Sodeck G, Funovics M, Juraszek A, Dziodzio T. et al. The influence of gender on mortality in patients after thoracic endovascular aortic repair. Eur J Cardiothorac Surg 2011;40:e1–5. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Sexual and Reproductive Health. https://www.who.int/reproductivehealth/topics/gender_rights/sexual_health/en/ (08 December 2020, date last accessed).
- 47.World Health Organization. Gender and Health. https://www.who.int/health-topics/gender#tab=tab_1 (08 December 2020, date last accessed).
- 48. Jansen Klomp WW, Brandon Bravo Bruinsma GJ, Peelen LM, Nierich AP, Grandjean JG, van ’t Hof AWJ.. Clinical recognition of acute aortic dissections: insights from a large single-centre cohort study. Neth Heart J 2017;25:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ioannidis JP, Lau J.. Pooling research results: benefits and limitations of meta-analysis. Jt Comm J Qual Improv 1999;25:462–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.