Abstract
Background
Detailed prevalence estimates of BRAFV600 mutations and BRAF inhibitor (BRAFi) treatment responses in V600-mutant glioma will inform trial development.
Methods
Our systematic review analyzed overall prevalence of BRAFV600 mutations in glioma and BRAFi treatment response.
Results
Based on 13 682 patients in 182 publications, the prevalence of BRAFV600 in epithelioid glioblastoma (eGBM) was 69% [95% CI: 45–89%]; pleomorphic xanthoastrocytoma (PXA): 56% [48–64%] anaplastic pleomorphic xanthoastrocytoma (aPXA): 38% [23–54%], ganglioglioma (GG): 40% [33–46%], and anaplastic ganglioglioma (aGG): 46% [18–76%]. Prevalence in astroblastoma was 24% [8–43%], desmoplastic infantile astrocytoma (DIA): 16% [0–57%], subependymal giant cell astrocytoma (SEGA): 8% [0–37%], dysembryoplastic neuroepithelial tumor (DNET): 3% [0–11%], diffuse astrocytoma (DA): 3% [0–9%], and pilocytic astrocytoma (PA): 3% [2–5%]. We reviewed 394 V600-mutant gliomas treated with BRAFi from 130 publications. One hundred and twenty-nine pediatric low-grade gliomas showed 4 (3.1%) complete response (CR); 53 (41.1%) partial response (PR); 64 (49.6%) stable disease (SD) and 8 (6.2%) progressive disease (PD). 25 pediatric high-grade gliomas showed CR; PR; SD; PD in 4 (16.0%); 10 (40.0%), 4 (16.0%); and 7 (28.0%) respectively. Thirty-nine adult low-grade gliomas showed CR; PR; SD; PD of 4 (10.3%); 17 (43.6%); 16 (41.0%) and 2 (5.1%) respectively. Ninety-seven adult high-grade gliomas showed CR; PR; SD; PD of 6 (6.2%); 31 (32.0%); 27 (27.8%); and 33 (34.0%) respectively.
Conclusions
BRAFV600 prevalence is highest in eGBM, PXA, aPXA, GG, aGG, and lower in astroblastoma, DIA, SEGA, DNET, DA, and PA. Our data provide the rationale for adjuvant clinical trials of BRAFi in V600-mutant glioma.
Keywords: BRAF, BRAF inhibitors, glioma, prevalence, systematic review
Key Points.
Significant prevalence of BRAFV600 in five glioma entities.
Variable but promising response to BRAFi in V600-mutant glioma in a recurrent setting.
Provides rationale for adjuvant clinical trials of BRAFi in V600-mutant glioma.
Importance of the Study.
There is a need for accurate prevalence estimates of the biomarker BRAFV600 within glioma to inform decisions around testing and to consider the role of targeted therapeutic strategies. Our systematic review has identified glioma subtypes with high prevalence of BRAFV600 in which testing might be recommended. We have also identified subtypes with lower prevalence of BRAFV600 where testing might be considered. We describe the available evidence about the use of, and response to, BRAF inhibitors in V600-mutant glioma and conclude that adjuvant randomized trials of BRAF inhibitors in adult and pediatric low-grade and high-grade gliomas are needed.
Brain tumors are responsible for the most years of life lost by any cancer and there is a need for novel precision medicine approaches informed by detailed biomarker research.1
One such candidate is BRAF (v-raf Murine Sarcoma Viral Oncogene Homolog B1), a gene, responsible for production of the B-Raf protein, a member of the Raf serine/threonine kinase family.2BRAF is a key mediator in the mitogen activated protein kinase (MAPK) cell signaling pathway, which has a role in cell proliferation, survival, and differentiation.3 B-Raf activates MAPK extracellular signal-regulated kinase (MEK) proteins, which stimulate extracellular signal-regulated kinase (ERK) proteins which control cellular responses.3
BRAF is the most commonly mutated kinase in cancer; more than 75 unique BRAF mutations have been reported in melanoma, although many of these mutations yield no or unknown significance.4BRAF mutations are prevalent in melanoma (40–50%), thyroid cancers (10–70%), serous ovarian cancer (~30%), colorectal cancers (~10%), and non-small cell lung carcinoma (~3–5%).5–9
The most-reported oncogenic driver mutation in BRAF is V600E (~90%).10 There are others; for example, the E558K mutation promotes dimerization of the kinase domains which increases B-Raf activity.11 Additionally, KIAA1549-BRAF gene fusions are found in low-grade glioma, particularly pilocytic astrocytoma.12
BRAF V600E
V600E is caused by a point mutation (p.T1799A) in exon 15 of BRAF, which results in the substitution of valine with glutamic acid at codon 600. Valine can also be substituted with other amino acids such as lysine (V600K), aspartic acid (V600D), or arginine (V600R), but these are less common.6 V600 is found within the activation region of BRAF and results in constitutive activation of the MAPK signaling pathway, resulting in hyperactivation (~500x) of the signaling cascade and uncontrolled cell division, as well as insensitivity to negative feedback mechanisms, causing tumorigenesis.13,14
BRAF Inhibitors
BRAF inhibitors (BRAFi) are small kinase inhibitors that bind selectively to V600E mutated B-Raf proteins and stopping them from activating MEK, thus inhibiting the MAPK/ERK signaling cascade, preventing aberrant cell signaling.15 In melanoma models, constant BRAF signaling is needed to maintain tumor growth, and intervention with BRAFi has been observed to result in reversible tumor regression.16 BRAFi vemurafenib (Zelboraf), dabrafenib (Tafinlar), and Encorafenib (Braftovi) have approval from the FDA and EU Commission for the treatment of various cancers including non-resectable and metastatic melanoma.15,17,18
A phase II study of V600-positive melanoma found a 53% response rate and a median treatment duration of 6.7 months and overall survival of 15.9 months.19 The BRIM-3 study examined treatment with vemurafenib compared with dacarbazine in 675 V600E-positive melanoma patients. Interim results showed patients treated with BRAFi had a lower risk of death (relative risk reduction (RRR) = 63%) and lower risk of either tumor progression or death (RRR = 74%).20 Final results showed median overall survival was better for vemurafenib compared with dacarbazine (13.6 v 9.7 months).21
Therapy is generally well tolerated in patients, in monotherapy and in combination with MEK inhibitors (MEKi). Combinations of three different BRAFi/MEKi regimens were well tolerated with moderate, reversible adverse effects and a low discontinuation rate of 11.5% to 15.7% in metastatic melanoma patients.22
The use of small-molecule inhibitors is particularly advantageous in younger pediatric patients where intervention with conventional treatment regimens such as surgery, chemotherapy, or radiotherapy would prove to have more long-term and disruptive effects on the developing brain.
There are currently several clinical trials investigating the therapeutic use of BRAFi in glioma. The PNOC-002 study (NCT01748149) is a safety, phase 0 efficacy study of vemurafenib in children and young adults with recurrent/refractory BRAF V600E or BRAF T insertion mutant brain tumors.23
GlaxoSmithKline and Novartis Pharmaceuticals have recently finished recruiting for a phase 1/2 study to determine the safety, tolerability, and pharmacokinetics of oral dabrafenib in children and adolescent subjects with advanced BRAFV600 mutation-positive solid tumors.24 Other studies are investigating the use of MEKi in combination with BRAFi to treat BRAFV600 mutation-positive glioma. One study (NCT02034110) is observing the safety and clinical efficacy of dabrafenib and trametinib (MEKi) in BRAF V600E-mutated rare cancers, including glioma.25 Additionally, there are new emerging class 2 BRAF inhibitors such as TAK-580 (MLN2480) which are being experimented as a new therapy for low-grade gliomas.26
Objectives of Study
Objective 1: To determine the prevalence of BRAFV600 across diagnostic categories within the WHO classification.
Objective 2: To examine the use of BRAFi in pediatric and adult V600-mutant low-grade gliomas (LGG) and high-grade gliomas (HGG) and the response to treatment.
Materials and Methods
Protocol and Registration
The protocol for this systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42019127704 and CRD42019127824).27 This manuscript follows the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).28 Ethical approval was not required because all data was obtained from previously published papers.
Eligibility Criteria
To examine prevalence of BRAFV600 mutations, we sought studies that: (i) included patients or biopsied tumor material from patients with histopathologically diagnosed glioma or tumors with a glial component; (ii) included only primary brain tumors; (iii) evaluated the prevalence of BRAFV600; (iv) had 10 or more glioma patients tested for BRAFV600; and (v) were cross-sectional studies, cohort studies or randomized trials. To examine response to BRAFi, we sought studies that (i) included patients of any age and any stage of disease (primary or regressive) with glioma according to the WHO Revised Classification of Tumors of the CNS 29; (ii) included patients with BRAFV600 mutation-positive glioma; (iii) included patients who had been treated with a BRAFi, administered either alone or in combination with other therapies; and (iv) were randomized trials, other controlled or uncontrolled clinical trials, cohort studies, case series, or case reports. We imposed no language or data restrictions on eligibility of studies for the review.
Search Strategy
The principal database searches were of MEDLINE and Embase up to October 2020 (Supplementary Tables 1 to 4). For the BRAFi component we additionally searched the Cochrane Register of Controlled Trials and abstracts published by the American Society of Clinical Oncology (ASCO), or European Society for Medical Oncology (ESMO), using keywords “glioma”, “vemurafenib”, and “dabrafenib”. A further group of publications identified in the search criteria for BRAFV600 prevalence review were also considered. Additionally, we examined the reference lists of any accepted studies.
Data Extraction
Records identified from the searches on MEDLINE and Embase were transferred to an Endnote library and deduplicated.30 The remaining references were uploaded to Rayyan for title and abstract screening.31 Two independent reviewers assessed the eligibility of the publications, with conflicts being examined by a third reviewer. Full texts of the remaining articles were screened against the eligibility criteria by one reviewer, and this process was checked by a second reviewer.
Data extraction was performed by one reviewer into an Excel document, then checked and verified by a second reviewer. For all studies included in the review, data were extracted on publication information, country, age group (pediatric ≤18 years, adult >18 years), sex, glioma subtypes, and sample size. Where multiple publications related to the same study, all publications were used for data extraction, but classed as one study.
For studies of BRAFV600 mutation prevalence, details of the population studies, start and end dates, and information on prevalence were extracted. The method of mutational analysis was selected based on a hierarchical system, with the largest sample size taking preference over method of detection. If these conditions were the same a second hierarchical system came into place, with Sanger sequencing preferred to pyrosequencing, itself preferred to immunohistochemistry. To minimize risk of double reporting of patients, studies were grouped by institute/hospital and a single cohort selected from the same location within the same period. For this, we prioritized availability of patient samples for specific glioma subtypes over larger sample sizes (combined across subtypes).
For BRAFi studies, each patient was assigned a patient ID and patient characteristics were recorded. Where individual patient data were not available, aggregated data was collected such as median values of age, treatment duration, overall survival (OS), and progression free survival (PFS). Data on the primary tumor was collected including tumor type, WHO tumor grade, stage of glioma, tumor dissemination status and dissemination location. If WHO grade was not reported but tumor type was, then it was assigned the appropriate WHO grade, according to the 2016 WHO Classification of Tumors of the CNS. The following tumors were not reported in the above classification or the publication and were graded with the assistance of author KMK: optic pathway glioma (grade I), glioneuronal tumor (grade I), ganglioneurocytoma (grade I) fibrillary astrocytoma (grade II), pilomyxoid astrocytoma (grade II), anaplastic oligoastrocytoma (grade III), and anaplastic glioneuronal tumor (grade IV). Anaplastic astroblastoma are not graded according to WHO and were graded as not reported (NR). The type of BRAF mutation and any additional known mutations were recorded, as well as the mutation detection method. Any prior therapies were recorded. The type of BRAFi, dose, and therapy duration in months was recorded. We recorded whether therapy was continuous or intermittent; for patients who received multiple sessions of BRAFi therapy, we considered the therapy time cumulatively. We recorded MEKi type, dose, and duration (if applicable). Additionally, we also recorded any dose changes and associated adverse events that were reported due to therapy.
PFS was recorded as time from beginning of treatment to tumor progression or death and OS was recorded as time from beginning of treatment to death. We also recorded the time to best response and whether therapy was ongoing at the time of publication. Best radiological tumor response was recorded according to either RANO or RECIST criteria.32,33 Publications that did not disclose the best tumor response had the tumor evaluated based on tumor size changes according to RANO criteria. Any near-complete or significant responses were recorded as partial response.
Risk of Bias
Risk of bias was assessed in studies of BRAFV600 mutation prevalence using the tool by Hoy et al.34 We did not plan to perform risk-of-bias assessments on case reports, case series, or uncontrolled trials because they are descriptive studies from which estimates of treatment effect cannot be derived.
Statistical Analysis
Descriptive statistics were derived from categorical and continuous variables, such as WHO classification and sample size of cohort. Meta-analysis of prevalence estimates was carried out using the “metaprop” program in R, using the Freeman-Tukey double arcsine transformation and a random effects model.35 This was also used to create forest plots and to undertake subgroup analyses by glioma subtype according to WHO classifications, and by age group. Inconsistency between results of different studies was measured using the I2 statistic.
Analyses of BRAFi therapy focussed on individual patient data for PFS and OS and adopted a survival analysis approach. Patients who did not have PFS or OS recorded were assumed to be alive, without tumor progression, and thus data from these patients were censored. Where we had only aggregate values for groups of patients, the group values were applied to each patient in the group. Kaplan-Meier survival estimates were calculated, and survival curves were plotted. Outcomes were compared between age groups and WHO tumor grade groups.
Statistical analyses were performed using Microsoft Excel and RStudio (v1.3.1093).36,37
Results
Study Selection
Database searches of MEDLINE and Embase identified 5188 records for the BRAFV600 mutation prevalence search and 2220 for the BRAFi search (Supplementary Figures 1 and 2). After de-duplication and screening, 820 full text papers were examined for the BRAFV600 prevalence component, and 182 studies were included (Supplementary Figure 1). For the BRAFi component, 165 further records were identified from the search strategy for V600 prevalence; 222 full text papers were screened, and 93 studies were included, reported in 130 publications (Supplementary Figure 2).
BRAFV600 Prevalence
Study characteristics
The 182 included studies involved a total of 13 682 glioma patients (Supplementary Table 5 and 6). Patients ranged from 0 to 90 years of age. 2324 patients were included in studies of pediatric patients (≤18 years), 2109 in studies of adult patients (>18 years), 6983 in studies of both adult and pediatric patients, and 2266 in studies of unspecified age. Thirty-four studies (18.7%) included >100 glioma patients, while 61 studies (33.5%) had ≤20. Publication dates of studies ranged from 2004 to 2020. The method of detection of BRAFV600 mutation varied between included studies, most studies used multiple forms of detection (29.7%). Studies that only used NGS, PCR, and sequencing, IHC, and Sanger sequencing were 12.6%, 12.1%, 9.9%, and 9.9% respectively (Supplementary Table 7).
Sensitivity analyses
Seventy-three studies (40.1%) were low risk of bias, 81 studies (44.5%) were at moderate risk of bias and 28 studies (15.4%) were at high risk of bias (Supplementary Figure 3). All these studies were included within the meta-analysis as they were considered to provide valuable information for subgroup analysis. A further sensitivity analysis involved removing high risk of bias studies to see if there were alterations to overall prevalence; these changes were found to be minimal (Supplementary Table 8).
Overall prevalence of BRAFV600
The average prevalence of BRAFV600 mutation across all 182 included studies was 7%, with a 95% confidence interval (CI) of 5% to 8% (Supplementary Figure 4a). The I2 value was 83% indicating high heterogeneity between these studies, which is expected in studies of prevalence. Pediatric patients were found to have a higher prevalence of BRAFV600 mutation compared with adult patients. Pediatric patients had an overall average BRAFV600 prevalence of 7% (95% CI: 4 to 10%) (Supplementary Figure 4b), whereas adult patients had an average overall prevalence of 4% (95% CI: 1 to 8%) (Supplementary Figure 4c). There is variation between these estimates in both pediatric and adult, with I2 values of 63% and 75%, respectively, reflecting high heterogeneity.
Prevalence of glioma entities according to WHO
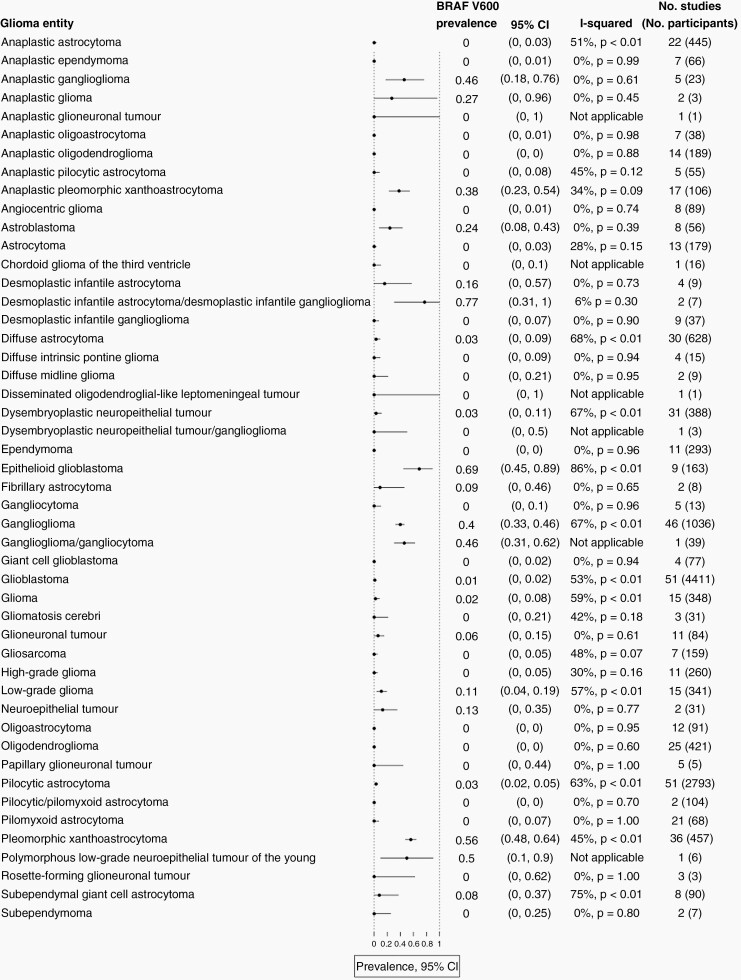
Glioma patients were classified diagnostically according to WHO guidelines. Summary estimates and I2 statistics from subgroup analyses by glioma entity are shown with numbers of contributing studies (Figure 1). Full study-level data for each of these meta-analyses are provided in Supplementary Figures 5a–5au. Among 48 glioma entities, BRAFV600 mutation was identified most commonly in epithelioid glioblastoma (eGBM) with an average prevalence of 69% (95% CI: 45 to 89%; I2 = 86%), followed by pleomorphic xanthoastrocytoma (PXA) 56% (95% CI: 48 to 64%; I2 = 45%), anaplastic pleomorphic xanthoastrocytoma (aPXA) 38% (95% CI: 23 to 54%; I2 = 34%), ganglioglioma (GG) 40% (95% CI: 33 to 46%; I2 = 67%), and anaplastic ganglioglioma (aGG) 46% (95% CI: 18 to 76%; I2 = 0%) (Figure 1; Supplementary Figure 5a–5e).
Fig. 1.
Summary forest plot displaying estimated BRAFV600 prevalence in glioma entities.
Other glioma entities were found to have a prevalence of BRAFV600 mutation greater than 1%, included astroblastoma 24% (95% CI: 8 to 43%), desmoplastic infantile astrocytoma (DIA) 16% (95% CI: 0 to 57%), LGG not otherwise specified (NOS) 11% (95% CI: 4 to 19%), subependymal giant cell astrocytoma (SEGA) 8%, (95% CI: 0 to 37%), glioneuronal tumor (GNT) 6%, (95% CI: 0 to 15%), dysembryoplastic neuroepithelial tumor (DNET) 3%, (95% CI: 0 to 11%), diffuse astrocytoma (DA) 3%, (95% CI: 0 to 9%), pilocytic astrocytoma (PA) 3%, (95% CI: 2 to 5%), glioma 2%, (95% CI: 0 to 8%), desmoplastic infantile astrocytoma/desmoplastic infantile ganglioglioma (DIA/DIG) 77%, (95% CI: 31 to 100%), neuroepithelial tumor 13%, (95% CI: 0 to 35%), anaplastic glioma (AG) 27%, (95% CI: 0 to 96%), ganglioglioma/gangliocytoma 46%, (95% CI: 31 to 62%), polymorphous low-grade neuroepithelial tumor of the young (PLNTY) 50%, (95% CI: 10 to 90%), and fibrillary astrocytoma (FA) 9%, (95% CI: 0 to 46%) (Figure 1; Supplementary Figure 5f–5s).
Use of BRAFi in BRAF V600-Mutant Glioma
Study characteristics: Study characteristics
Of the 93 studies, we found 24 publications relating to seven uncontrolled trials and 106 publications describing 86 case reports or case series (Supplementary Table 9).
Across these studies, we collected data for 394 patients: 241 pediatric patients, 144 adult patients, and 9 NR. In the pediatric group, the median age was 9.0 years (range; 0.1 to 17.0, n = 160), and of 142 pediatric patients, 69 (48.6%) were female. In the adult group, the median age was 34.0 years (range; 18.0 to 62.0 years, n = 143) and 60/118 (50.8%) were female (Supplementary Table 10).
Of the 241 pediatric patients, 158 (65.5%) had a V600E mutation. Three (1.2%) had a V600D mutation and 80 (33.2%) cases were V600 NOS. In adults, 120 (83.3%) tumors had V600E mutations and 24 (16.7%) V600 NOS (Supplementary Table 11).
In the pediatric cohort, there were 91 LGG; 66 (27.4%) grade I and 25 (10.4%) grade II. There were 27 HGG; 18 (7.5%) grade III and 9 (3.7%) grade IV. Seventeen (7.1%) gliomas were not graded, and 106 (44.0%) gliomas were not specified by grade or type. In the adult cohort, there were 41 LGG; 19 (13.2%) grade I and 22 (15.3%) grade II. There were 100 HGG; 46 (31.9%) grade III and 54 (37.5%) grade IV. Three (2.1%) gliomas were not graded (Supplementary Table 12).
In the pediatric patients, 52/241 (21.6%) glioma sites were specified. In adult patients, 55/144 (38.2%) glioma sites were specified. In both groups, the cerebrum was the region with the highest frequency of glioma; 22/52 (42.3%) in pediatrics and 46/55 (83.6%) in adults (Supplementary Table 13).
In the pediatric patients, the stage of glioma was reported in 42 patients. Nine (21.4%) had recurrent glioma and 33 (78.6%) had non-recurrent glioma (defined as progressive glioma or newly diagnosed glioma). In the adult patients, the stage of glioma was reported in 81 patients. 56 (69.1%) patients had recurrent glioma and 25 (30.9%) patients had non-recurrent glioma.
Prior Therapy Characteristics
Sixty out of 241 (24.9%) pediatric patients received surgical intervention, 68/241 (28.2%) patients received radiotherapy, and 93/241 (38.6%) patients received chemotherapy. One hundred and nine out of 144 (75.7%) adult patients had surgical intervention, 131/144 (91.0%) received radiotherapy, and 121/144 (84.0%) received chemotherapy. One hundred and thirty-five (56.0%) pediatric patients and 2 (1.4%) adult patients did not report any prior therapy, although it was not clear whether it was simply not reported.
Treatment Characteristics
Sixty-one (25.3%) pediatric patients were treated with vemurafenib monotherapy, 66 (27.4%) were treated with dabrafenib monotherapy and 47 (19.5%) were treated with dabrafenib with trametinib (a MEKi). BRAFi used to treat 67 patients (27.8%) were not specified. Median pediatric treatment time was 13.8 months (IQR; 17.2 months, range; 0.1 to 63 months), reported in 152/241 (63.1%) patients.
In adult patients, 43 patients were treated with vemurafenib monotherapy (29.9%), whilst 3 were treated in combination with trametinib (2.1%), and 4 with cobimetinib (2.8%). Thirteen out of 144 were treated with dabrafenib monotherapy (9.0%) whilst 81 were co-treated with trametinib (56.3%). Median adult treatment time was 8 months (IQR; 12.4 months; range 0.1 to 54.6 months), reported in 140/144 patients (97.2%) (Supplementary Table 14 and 15).
Nine HGG patients (1 pediatric, 8 adult) reported changing BRAFi during their treatment due to adverse events or lack of response to treatment. Six patients switched to dabrafenib from vemurafenib (n = 5) or PLX8394 (n = 1), and three changed to vemurafenib from dabrafenib.
Survival analysis: progression-free survival and overall survival
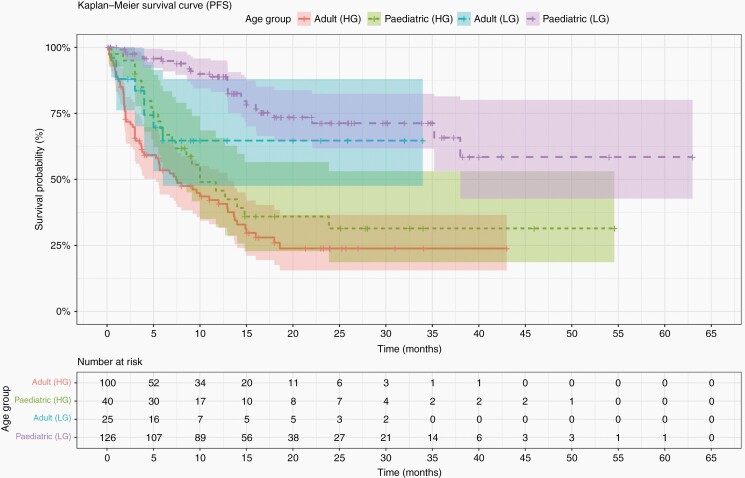
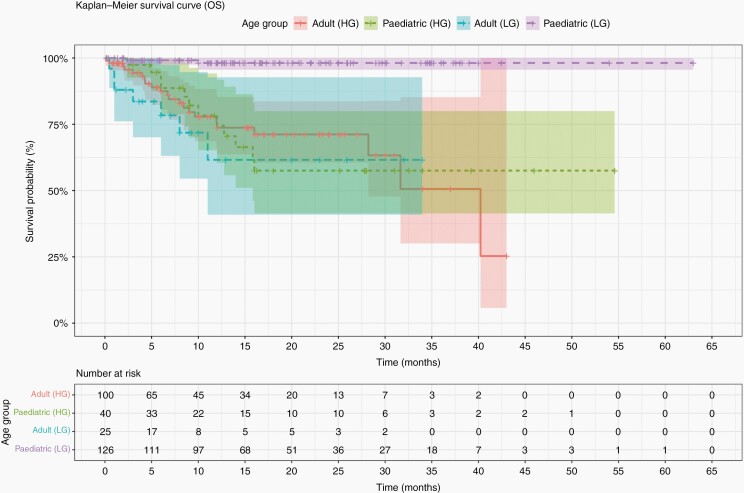
Median PFS for the pediatric cohort was 9 months (IQR; 10.7 months, range; 0.5 to 38 months) from data reported in 35/241 (14.5%) patients. In adult patients it was 3.8 months (IQR; 7.2 months, range; 0.1 to 40 months) from data reported in 90/144 (62.5%) patients. Median OS for the pediatric cohort was 4.5 months (IQR; 8.2, range; 0.5 to 22 months), from 10/11 (90.9%) of patients who died. The median OS for the adult cohort was 8.2 months (IQR; 7.5 months; range, 0.1 to 40 months), from 34/34 (100%) of patients who died (Table 1). A Mann-Whitney Wilcoxon (MWW) test was performed to determine if there was a significant difference between PFS and OS between age groups (P = .001 and P = .17, respectively). Furthermore, we performed a MWW test to determine significant differences in PFS and OS between LGG and HGG in each age group. MWW tests reported a P-value of P = .001 and P = .0002 for pediatrics, respectively, and P = .02 and P = .03 for adults, respectively.
Table 1.
Shows the Median PFS and OS, Split by Age and Tumor Grade, and the Number of Patients in Each Group
| Median (IQR) PFS (months) | No. of Patients | Median (IQR) OS (months) | No. of Patients | |
|---|---|---|---|---|
| Pediatric LGG | 13.0 (7.3) | 26 | 6.1 (3.9) | 2 |
| Adult LGG | 5.9 (6.1) | 24 | 9.5 (7.1) | 12 |
| Pediatric HGG | 3.5 (3.3) | 8 | 3.0 (6.0) | 7 |
| Adult HGG | 3.0 (5.8) | 65 | 6.8 (7.3) | 22 |
Additionally, we compared PFS and OS of treatment in both age groups, with and without dual therapy. Median PFS of the pediatric monotherapy group was 12.9 months (IQR; 10.7 months, range; 0.5 to 38 months) and dual therapy was 7.3 months (IQR; 8.4 months, range; 1.3 to 22.1 months). Median PFS of the adult monotherapy group was 5.5 months (IQR; 7.0 months, range; 0.1 to 18 months) and dual therapy was 3.0 months (IQR; 5.7 months, range; 0.2 to 23.9 months). MWW tests reported P-values of P = .63 and P = .06 for pediatrics and adults, respectively. Median OS of the pediatric monotherapy group was 3.0 months (IQR; 6 months, range; 0.5 to 10 months) and dual therapy was 11.0 months (IQR; 9.9 months, range; 2.2 to 22 months). Median OS of the adult monotherapy group was 9.6 months (IQR; 8 months, range; 0.1 to 40.2 months) and dual therapy was 5.0 months (IQR; 5.5 months, range; 2 to 9 months). MWW tests reported P-values of P = .17 and P = .06 for pediatrics and adults, respectively (Supplementary Table 16).
We compared the difference between LGG and HGG treated with dual therapy. One hundred and thirty-five out of 394 (34.2%) patients used MEKi; 62 LGG, 70 HGG, and 3 NR. However, only 65 (48.1%) reported progression-free survival data, and 11 (8.1%) reported overall survival data. Median PFS of the LGG dual therapy (n = 19) was 8.5 months (IQR; 7.9 months, range; 0.2 to 23.9 months) and HGG dual therapy (n = 46) was 2.9 months (IQR; 3.9 months, range; 0.3 to 18.6 months). Median OS of the LGG dual therapy (n = 3) was 8.5 months (IQR; 3.4 months, 2.2 to 9.0 months) and HGG dual therapy (n = 8) was 4.7 months (IQR; 5.5, range; 2 to 11 months). MWW tests reported P-values of P = .002 and P = .68 for PFS and OS, respectively.
OS and PFS data were not available for non-recurrent glioma patients and as such, survival data were derived from recurrent glioma patients and are the same as the results described above.
Kaplan-Meier survival curves were plotted for OS and PFS for all patients, split by age and grade (Figures 2 and 3, respectively).
Fig. 2.
Kaplan-Meier survival curves showing PFS of the cohort, split by age and tumor grade.
Fig. 3.
Kaplan-Meier survival curves showing OS of the cohort, split by age and tumor grade.
Response to BRAFi Therapy
One hundred and fifty-four pediatric patients had a recorded best tumor response. Eight (5.2%) pediatric patients showed complete response, 63 (40.9%) showed partial response, 68 (44.2%) showed stable disease and 15 (9.7%) showed progressive disease. One hundred and twenty-five (83.8%) were LGG (4 complete response, 53 partial, 64 stable, and 8 progressive), and 25 (16.2%) were HGG (4 complete, 10 partial, 4 stable, and 7 progressive).
One hundred and thirty-seven adult patients had a recorded best tumor response. Ten (7.3%) showed complete response, 48 (35.0%) showed partial response, 43 (31.4%) showed stable disease and 36 (26.3%) showed progressive disease. 39 (28.5%) were LGG (4 complete, 17 partial, 16 stable, 2 progressive) and 97 (70.8%) were HGG (6 complete, 31 partial, 27 stable, 33 progressive) (Supplementary Table 17).
Patient outcomes
Of the pediatric cohort, 94/241 (39.0%) were alive and continuing treatment with BRAFi at the end of the study. Thirty-eight out of 241 (15.8%) had stopped treatment but remained alive and 11/241 (4.6%) had died (the remaining 40.7% had unknown status). Fifty-four out of 144 (37.5%) adults were alive and receiving BRAFi therapy at the end of the study. Eighteen out of 144 (12.5%) had stopped treatment but remained alive and 34/144 (23.6%) had died (Supplementary Table 18).
Adverse reactions to BRAFi
One hundred and forty-two out of 241 (58.9%) pediatric patients and 109/144 (75.7%) adult patients had an adverse event associated with treatment with BRAFi. Adverse events were classified according to system organ class using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. In one pediatric patient and 49 adult patients, an adverse event was reported, but not specified. Some patients may have experienced an adverse event, but had not been reported (Supplementary Table 19).
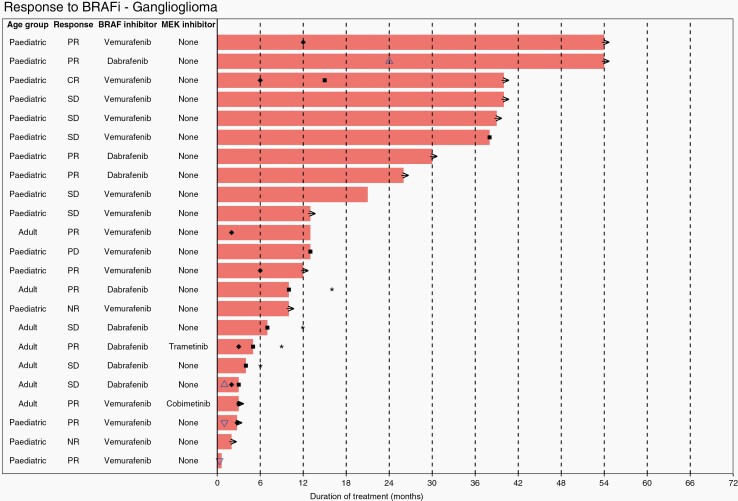
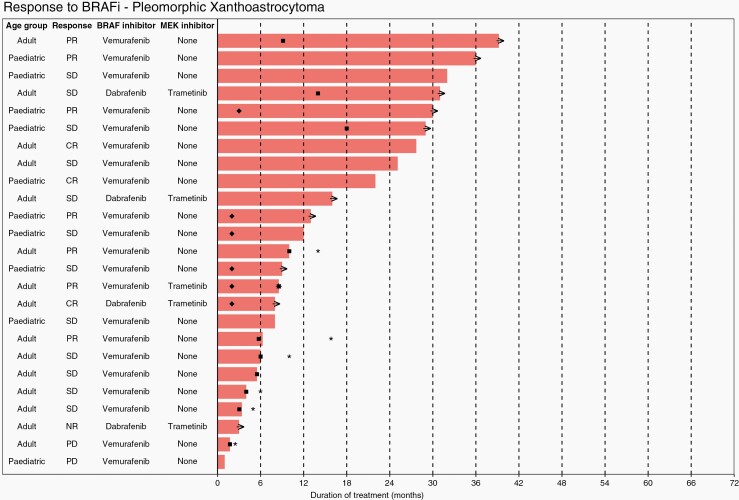
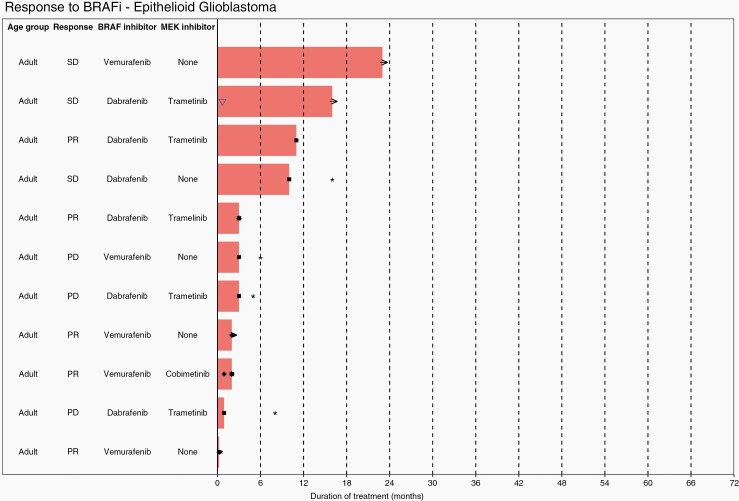
Waterfall plots show individual patient data for two subtypes of LGG; ganglioglioma (Figure 4) and PXA (Figure 5), and one subtype of HGG, epithelioid glioblastoma (Figure 6). Additional waterfall plots for other LGG and HGG subtypes can be found in supplementary material (Supplementary Figures 20a–20d and 21a–21g, respectively).
Fig. 4.
Waterfall plot showing individual patient data for treatment for ganglioglioma. KEY: → (ongoing treatment), ✱ (overall survival), ■ (progression free survival), ♦ (time to best response), △ (drug dosage increase), ▽ (drug dosage decrease).
Fig. 5.
Waterfall plot showing individual patient data for treatment of pleomorphic xanthoastrocytoma. KEY: → (ongoing treatment), ✱ (overall survival), ■ (progression free survival), ♦ (time to best response).
Fig. 6.
Waterfall plot showing individual patient data for treatment for epithelioid glioblastoma. KEY: → (ongoing treatment), ✱ (overall survival), ■ (progression free survival), ♦ (time to best response), ▽ (drug dosage decrease).
Discussion
This systematic review identified 182 studies of the prevalence of BRAFV600 mutations in glioma and 92 studies of the use of BRAFi in V600-mutant glioma. These studies provide evidence of variation in the prevalence of the mutation within different glioma subtypes, and an indication of when BRAFi might be considered for treatment.
Among studies of BRAFV600 mutation prevalence, 15.4% were in adults, 17.0% were in pediatric patients and the remainder were in mixed populations, or age was not reported. Pediatric patients were found to have a slightly higher prevalence of BRAFV600 mutation than adults, likely due to different subtypes of glioma in studies involving pediatric patients.
High average prevalence estimates of BRAFV600 mutation were observed in eGBM (69%), PXA (56%), aPXA (38%), GG (40%), and aGG (46%). The strongest evidence among these was for PXA (38 studies) and GG (47 studies). Other glioma entities were observed to have a prevalence of BRAFV600 above 10%, including astroblastoma (24%), DIA (16%), and LGG NOS (11%). For several entities there was strong evidence that the prevalence is much lower, including for DA, AA, PA, DIG, DNET, GBM, and oligodendroglioma.
The entities with high prevalence estimates may reflect similarities in their genetic landscapes. Although the relationship between PXA, aPXA, and eGBM is poorly understood, a study has observed eGBM to have clinical similarities to aPXA with epithelioid features, including leptomeningeal dissemination and recurrence within months of initial resection.38 Another study identified analogous MRI features between eGBM and PXA39; however, PXA was found to show degenerative changes, where eGBM showed less of these features. Case studies have identified eGBM arising from PXA.40
Our findings are broadly consistent with prevalence estimates presented in the WHO guidelines.41 WHO categorizes DIA and DIG subtypes together; we have found the prevalence of BRAFV600 mutation to be somewhat higher in DIA, compared with DIG. Our prevalence estimates were lower than WHO for SEGA and DNET. Our prevalence of 3% among PA, was as expected. An interesting finding was that 3 of 6 patients with PLNTY were found to have a BRAFV600 mutation. PLNTY is a rare entity not in current WHO guidelines which may warrant further investigation.
Over 99% of BRAFV600 mutations discovered in our cohort were the V600E mutation. Two V600K mutations were found in PA and ganglioglioma. A V600G mutation was discovered in a ganglioglioma patient. One study tested for BRAF V600E/E2/D mutations so the alterations in this cohort were undefined. Twenty-three papers (12.6%) used NGS alone. However, some of the studies included within our cohort (9.9%) used only IHC to identify the BRAFV600 mutation, but this method only looks at the V600E mutation and therefore will not identify the rarer forms of V600, whereas NGS can identify all mutations of BRAFV600.42
No comparative studies of the use (versus non-use) of BRAFi were identified. Across the noncomparative studies included, data was available on tumor outcomes after BRAFi for 154 pediatric patients and 137 adult patients. Complete or partial response was observed in 44% of pediatric and 54% of adult patients with low-grade glioma, and 56% of pediatric and 38% of adult patients with high-grade glioma. Progressive disease was observed for 6% of pediatric and 5% of adult patients with low-grade glioma, and 28% of pediatric and 34% of adult patients with high-grade glioma. Although response to BRAFi in the recurrent setting was variable, these results are promising and highlight the need for additional studies including the adjuvant setting in high prevalence glioma subtypes. Median PFS differed between pediatric and adult cohorts taking BRAFi therapy (9.0 versus 3.8 months). However, there was no clear difference in median OS. Both PFS and OS was, as would be expected, longer in low-grade glioma patients than in high-grade glioma patients (median PFS; 13.0 versus 3.5 months in pediatric patients, and 5.9 versus 3.0 months in adult patients, and median OS; 6.1 versus 3 months in pediatric patients, and 9.5 versus 6.8 months for adults). MWW tests showed these differences to be statistically significant (P < .05). We found no significant difference in the PFS or OS between pediatric or adult patients who had BRAFi monotherapy compared to dual therapy. However, median PFS differed significantly between LGG and HGG patients treated with dual therapy (8.5 versus 2.9 months), according to a MWW test (P < .05).
Although there is little difference in the number of LGG and HGG treated with dual therapy, there is a lower response rate in the LGG group due to the patients neither progressing nor dying, which we can assume is due to the less aggressive nature of LGG compared to HGG.
Whilst BRAFi therapy has proven to be effective in BRAF-mutated glioma, there is evidence that patients can develop resistance to these inhibitors, rendering them less effective and allowing tumor progression.43,44 However, several studies have found that the addition of MEKi to therapy tends to lengthen the time to tumor progression, improves PFS and outcomes, and delays the development of resistance to BRAFi in melanoma.45 Furthermore, some patients who develop resistance to monotherapy with BRAFi, still exhibit a response when treated in combination with MEKi.46
Strengths and Limitations
This is the largest systematic review and meta-analysis, to our knowledge, of BRAFV600 mutation prevalence in glioma and use of BRAFi in V600-mutant glioma.
Of the 182 included studies of prevalence, 110 were published since 2016, indicating that our findings are likely to be current. Most studies had a low or moderate risk of bias, although some high risk of bias studies were included in the meta-analyses. The evidence has limitations. Sample sizes of the included studies were often small because of the rarity of glioma. By separating the patients into different glioma entities, we were able to provide specific estimates for different entities, but this meant that confidence intervals were often wide. Despite our separation of glioma entities, large values of I² were observed for prevalence estimates, indicating variation between studies due to genuine differences; however high values of I2 are the norm in meta-analyses of prevalence. The differences between and within age groups are likely to be due to some extent by inclusion of different glioma entities. Techniques used to identify mutations varied between studies, with the most common methods being Sanger sequencing (regarded as the gold standard) and IHC (thought to have lower sensitivity and specificity).41 Some studies were seen to exclude tumors for reasons including insufficient material to investigate, tumors with an ambiguous diagnosis, and patients with loss of follow-up. Excluding patients such as these may influence the results of our studies as some tumor types could be selected against.
Evidence concerning outcomes following BRAFi therapy in V600-mutant glioma comprised only 394 individuals. Furthermore, our analysis is purely descriptive because no studies of efficacy were identified. There was variation in treatment protocols between studies with BRAFi administered at various time points across the included studies. Studies were mostly conducted in Western countries, China, and Japan, limiting generalizability: there were few patients from Western Asian or Southern Asian populations (Supplementary Table 8). One study mentioned that a patient from Pakistan had to travel to Canada to receive V600 mutation testing and BRAFi therapy, reflecting availability of screening for BRAFV600 mutations is not universal.
There were missing data for patients in many BRAFi studies (Supplementary Tables 9 to 18). Large numbers of patients were censored from analyses due to ongoing therapy at the time of publication. These patients may have progressed or died following the publication of the paper. In some larger studies, we could not obtain individual patient data which limited our ability to compare responses between groups; we could not examine different diagnoses of glioma and could group them only by grade. Whilst we acknowledge there is a small proportion of patients for whom we have long-term outcome data, which limits our findings to those who have likely the worst responses, therefore, invoking a high risk of bias, we believe that it is important to report the PFS and OS which may be useful for clinicians who are counseling patients about the use of these inhibitors. It is also important to note when interpreting our data that our PFS/OS findings relate to several published single-arm studies of BRAFi or dual therapy in glioma which lack a control arm for comparison.
Although WHO provides guidelines for grading CNS tumors, diagnosing different glioma subtypes is not straightforward, especially when there are discordant results between histology and genetic features.47 Thus, some of the glioma entities may have been misclassified, an issue that could affect the reliability of results.
Conclusion
In conclusion, targeted testing for BRAFV600 is indicated in eGBM, PXA, aPXA, GG, and aGG and may be considered in astroblastoma, DIA, SEGA, DNET, DA, and PA. Larger studies are required to better delineate BRAFV600 mutation prevalence in the rare entity PLNTY. Response to BRAFi in V600-mutant glioma in the recurrent setting were variable but promising, highlighting the need for new clinical trials of BRAFi therapy, alone or in combination with MEKi, undertaken in the adjuvant setting.
Supplementary Material
Contributor Information
Lily J Andrews, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Cancer Research Integrative Cancer Epidemiology Programme, University of Bristol, Bristol, UK.
Zak A Thornton, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Cancer Research Integrative Cancer Epidemiology Programme, University of Bristol, Bristol, UK.
Saanwalshah S Saincher, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Ian Y Yao, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Sarah Dawson, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Luke A McGuinness, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Hayley E Jones, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Sarah Jefferies, Department of Oncology, Addenbrooke’s Hospital, Cambridge, UK.
Susan C Short, Brain Cancer Research Group, Leeds Institute of Medical Research at St James’s, University of Leeds, Leeds, UK.
Hung-Yuan Cheng, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Alexandra McAleenan, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Cancer Research Integrative Cancer Epidemiology Programme, University of Bristol, Bristol, UK.
Julian P T Higgins, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Kathreena M Kurian, MRC Integrative Epidemiology Unit (IEU), Bristol Medical School, University of Bristol, Bristol, UK; Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Cancer Research Integrative Cancer Epidemiology Programme, University of Bristol, Bristol, UK; Brain Tumour Research Centre, Bristol Medical School, University of Bristol, Bristol, UK.
Funding
LJA is funded by Cancer Research UK (grant number C30758/A29791); ZAT is funded by Southmead Hospital Charitable Funds: Brain Tumour Bank and Research Fund 8036. This work was supported by Cancer Research UK (grant number C18281/A29019) and R101376-101). LAM is supported by an NIHR Doctoral Research Fellowship (DRF-2018-11-ST2-048). JPTH is a National Institute for Health Research (NIHR) Senior Investigator (NF-SI-0617-10145) and is supported by NIHR Bristol Biomedical Centre at University Hospitals Bristol Weston NHS Foundation Trust, University of Bristol. HEJ was supported by an MRC-NIHR New Investigator Research Grant (MR/T044594/1).
Conflict of interest statement. None of the authors have declared a conflict of interest.
Authorship statement. KMK conceived the study. AM and KMK designed the study. SD designed the search strategy. ZAT, LJA, IYY, SSS performed literature search, data extraction and initial interpretation. ZAT, LJA, and H-YC checked the data. ZAT LJA, H-YC, LAM, and HEJ performed statistical and meta-analysis. KMK, ZAT, and LJA drafted the manuscript. KMK, H-YC and JPTH supervised the project. KMK, H-YC, SCS, SJ and JPTH performed critical revision of the manuscript.
References
- 1. Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life lost (YLL) from cancer is an important measure of population burden–and should be considered when allocating research funds. Br J Cancer. 2005;92(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37(24):3183–3199. [DOI] [PubMed] [Google Scholar]
- 3. Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. [DOI] [PubMed] [Google Scholar]
- 4. Arkenau HT, Kefford R, Long GV. Targeting BRAF for patients with melanoma. Br J Cancer. 2011;104(3):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6(4):313–319. [DOI] [PubMed] [Google Scholar]
- 7. Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88(9):4393–4397. [DOI] [PubMed] [Google Scholar]
- 8. Jones JC, Renfro LA, Al-Shamsi HO, et al. (Non-V600) BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol. 2017;35(23):2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19(16):4532–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 11. Rajakulendran T, Sahmi M, Lefrançois M, Sicheri F, Therrien M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature. 2009;461(7263):542–545. [DOI] [PubMed] [Google Scholar]
- 12. Jones DT, Hutter B, Jäger N, et al. ; International Cancer Genome Consortium PedBrain Tumor Project . Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17(1):31–39. [DOI] [PubMed] [Google Scholar]
- 14. Pratilas CA, Taylor BS, Ye Q, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106(11):4519–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma A, Shah SR, Illum H, Dowell J. Vemurafenib: targeted inhibition of mutated BRAF for treatment of advanced melanoma and its potential in other malignancies. Drugs. 2012;72(17):2207–2222. [DOI] [PubMed] [Google Scholar]
- 16. Hoeflich KP, Gray DC, Eby MT, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66(2):999–1006. [DOI] [PubMed] [Google Scholar]
- 17. Ballantyne AD, Garnock-Jones KP. Dabrafenib: first global approval. Drugs. 2013;73(12):1367–1376. [DOI] [PubMed] [Google Scholar]
- 18. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–1327. [DOI] [PubMed] [Google Scholar]
- 19. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chapman PB, Hauschild A, Robert C, et al. ; BRIM-3 Study Group . Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chapman PB, Robert C, Larkin J, et al. Vemurafenib in patients with BRAF(V600) mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol. 2017;28(10):2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinzerling L, Eigentler TK, Fluck M, et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open. 2019;4(3):e000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicolaides T, Nazemi KJ, Crawford J, et al. Phase I study of vemurafenib in children with recurrent or progressive BRAFV600E mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget. 2020;11(21):1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geoerger B, Bouffet E, Whitlock JA, et al. Dabrafenib plus trametinib combination therapy in pediatric patients with BRAF V600-mutant low-grade glioma: Safety and efficacy results. J Clin Oncol. 2020;38(15):2. [Google Scholar]
- 25. Wen P, Stein A, Van Den Bent M, et al. Updated efficacy and safety of dabrafenib plus trametinib in patients with recurrent/ refractory BRAF V600E-mutated high-grade glioma (HGG) and low-grade glioma (LGG). Neuro-Oncology. 2019;21(Suppl 6):vi19–vi20. [Google Scholar]
- 26. Wright KD, Zimmerman MA, Fine E, Aspri T, Kieran MW, Chi S. Type II Braf inhibitor TAK-580 shows promise for upcoming Clinal trial as evidenced by single patient IND study. Neuro-Oncology. 2018;20:110–110. [Google Scholar]
- 27. Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 29. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 30. Hupe M. EndNote X9. J Electron Resource Med Lib. 2019;16(3-4):117–119. [Google Scholar]
- 31. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 34. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. [DOI] [PubMed] [Google Scholar]
- 35. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2021. Austria. https://www.R-project.org/ [Google Scholar]
- 37. RStudio Team. RStudio: Integrated Development for R. Boston, MA: RStudio, PBC; 2020. http://www.rstudio.com/. [Google Scholar]
- 38. Alexandrescu S, Korshunov A, Lai SH, et al. Epithelioid glioblastomas and anaplastic epithelioid pleomorphic Xanthoastrocytomas–Same entity or first Cousins? Brain Pathol. 2016;26(2):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furuta T, Miyoshi H, Komaki S, et al. Clinicopathological and genetic association between epithelioid glioblastoma and pleomorphic xanthoastrocytoma. Neuropathology. 2018;38(3):218–227. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe N, Ishikawa E, Kohzuki H, et al. Malignant transformation of pleomorphic xanthoastrocytoma and differential diagnosis: case report. BMC Neurol. 2020;20(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. IARC. WHO Classification of Tumours of the Central Nervous System. Geneva: World Health Organization; 2006. [Google Scholar]
- 42. Ihle MA, Fassunke J, König K, et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer. 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Yao Z, Jonsson P, et al. A secondary mutation in BRAF confers resistance to RAF inhibition in a BRAFV600E-mutant brain tumor. Cancer Discov. 2018;8(9):1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schreck KC, Morin A, Zhao G, et al. Deconvoluting mechanisms of acquired resistance of RAF inhibitors in BRAF V600E mutant human glioma. Clin Cancer Res. 2021. Aug 25:clincanres.CCR-21-2660-A.2021. https://clincancerres.aacrjournals.org/content/early/2021/09/28/1078-0432.CCR-21-2660.article-info [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 47. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.