Abstract
OBJECTIVES
Left atrial appendage occlusion (LAAO) at the time of implantation may reduce thromboembolic events (TEs) during continuous-flow left ventricular assist device support. The HeartMate 3 (HM3) reduces TEs overall, but the efficacy of LAAO in HM3 is unknown.
METHODS
Adults receiving first HM3 implantation from November 2014 through December 2019 at a single, large medical centre were retrospectively reviewed. TEs included device thrombosis and ischaemic stroke. Patients were classified by whether they received LAAO or not. Incidence of TEs was compared between groups using cumulative incidence curves with competing risks (death and heart transplant) and risk factors for TEs were assessed with Fine and Gray competing risk regression.
RESULTS
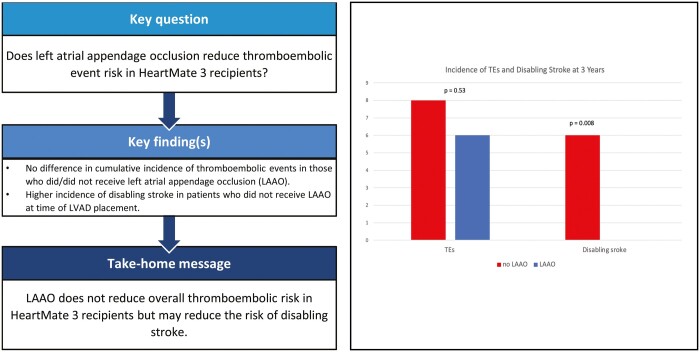
A total of 182 patients received HM3, of whom 99 (54%) received LAAO versus 83 (46%) who did not. There were 14 TEs, including 13 strokes (7%) and 1 pump thrombosis (0.5%). No significant difference in the incidence of TEs in each group was found (Gray’s test: P = 0.35). LAAO was not associated with TEs in multivariable Fine–Gray analysis (P = 0.10) and no significant risk factors for TEs were found. There were zero disabling strokes in those who received LAAO compared to 6 (7%) in those who did not receive LAAO (P = 0.008).
CONCLUSIONS
A low number of TEs was observed in HM3 recipients. LAAO did not further reduce the overall rate of TEs in this patient population, though its use may be beneficial in preventing disabling ischaemic strokes after HM3 implantation.
Keywords: Left atrial appendage occlusion, HeartMate 3, Mechanical circulatory support, Thromboembolism
Thromboembolic events (TEs) such as ischaemic stroke and device thrombosis have historically been significant complications associated with non-HeartMate 3 (HM3) continuous-flow left ventricular assist devices (LVADs) [1–4].
INTRODUCTION
Thromboembolic events (TEs) such as ischaemic stroke and device thrombosis have historically been significant complications associated with non-HeartMate 3 (HM3) continuous-flow left ventricular assist devices (LVADs) [1–4]. Reported risk factors for ischaemic stroke in LVAD patients include atrial fibrillation, female sex and hypertension [1, 2, 5]. Increased CHA2DS2–VASc scores have also been associated with TEs after LVAD implantation [6]. Left atrial appendage occlusion (LAAO) is associated with a decreased risk of stroke in patients with atrial fibrillation [7, 8], and concomitant LAAO at the time of LVAD placement increases TE-free survival relative to those who did not receive LAAO [9].
The HM3 is the newest generation of continuous-flow LVADs that utilizes a centrifugal pump system and fully magnetically levitated component parts [10]. The HM3 is associated with significantly improved TE-free survival at 2 years compared to previous generations of continuous-flow LVADs [10]. The HM3 is also associated with a dramatic reduction in pump thrombosis as well as reductions in both total and disabling ischaemic strokes at 2 years post-implantation [10]. The disabling ischaemic stroke rate between HM3 and previous continuous-flow LVADs is similar in the first 6 months after implantation, with the reduction in disabling ischaemic stroke risk primarily occurring after 6 months post-implantation [11]. Factors associated with survival free of haemocompatibility-related adverse events (TEs, neurological events, bleeding) after HM3 implantation include older age as well as international normalized ratio <1.5 and not being on aspirin at 30 days postoperatively [11]. LAAO was not associated with haemocompatibility-related adverse events following HM3 implantation. Prior studies on haemocompatibility-related adverse events in HM3 patients have included a low number of patients with LAAO in analyses and included all causes of stroke, including bleeding events, in the outcomes of interest. This study examines the effect of LAAO concomitant with HM3 implantation on TE risk specifically to determine if LAAO at the time of HM3 implantation reduces TEs.
PATIENTS AND METHODS
Ethical statement
This study was approved by the Columbia University Medical Center Institutional Review Board Protocol AAAE1866 with the waiver of informed consent.
Study design
Patients who received a first HM3 implant at a single, large medical centre from November 2014 through December 2019 were retrospectively reviewed. Primary and secondary end points of interest were the incidence of TEs and disabling stroke, respectively.
Data collection
All data were collected from the electronic medical record. All LVAD recipients included in this study were enrolled consecutively. Recorded data included baseline demographics such as past medical histories, INTERMACS score, preoperative laboratory data and use of an additional mechanical circulatory support tool prior to LVAD implant such as an extracorporeal membrane oxygenation, Impella or intra-aortic balloon pump. Haemodynamic data prior to implant including central venous pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure and Fick cardiac output were also collected. Operative characteristics including whether patients received LAAO or not and outcomes data including short- and long-term outcomes and TEs were collected. TEs included ischaemic stroke and device thrombosis. Consistent with the American Heart Association, in this study, stroke is defined as clinical symptoms attributable to ischaemia or transient ischaemic attack and excludes patients with the evidence of cerebral haemorrhage based on imaging [12]. Given this, and this study’s primary end point of TEs, patients with imaging indicative of haemorrhagic stroke were excluded from this study. Disabling stroke was defined as persistent clinical neurological deficit attributable to ischaemic stroke that required further therapy or resulted in coma or brain death. Patients were monitored for TEs via routine clinical follow-up. Short-term outcomes included in-hospital mortality and morbidity and postoperative atrial fibrillation. In-hospital morbidities included sepsis, urinary tract infection, takeback, use of renal replacement therapy and tracheostomy. Postoperative atrial fibrillation was defined as any atrial fibrillation occurring within 30 days of HM3 implantation regardless of the past history of atrial fibrillation.
Operative use of LAAO
During this study period, the standard approach for HM3 implantation was a full median sternotomy. Patients’ initial LVAD settings were determined intraoperatively by assessing haemodynamics, septal shift, degree of mitral regurgitation and size of aortic valve opening. LAAO was performed at the surgeon’s discretion but was generally indicated in all patients for TE prevention, regardless of atrial fibrillation history or prior stroke. All LAAOs were performed with the AtriClip LAA Exclusion System (AtriCure, Inc, Mason, OH, USA). The main obstacle to performing LAAO at the time of HM3 implantation was the difficulty in accessing the left atrial appendage from outside. Therefore, patients with repeat sternotomy were not generally candidates for concomitant LAAO. Complete closure and lack of residual communication between the left atrium and appendage was confirmed by external inspection and transoesophageal echocardiography.
Postoperative anticoagulation protocol
All patients were treated with antiplatelet therapy and warfarin with an international normalized ratio goal of 2–3. International normalized ratio goals were adjusted on an individual patient basis; a lower international normalized ratio was targeted for patients with bleeding events and patients with thrombotic events received therapeutic anticoagulation.
Follow-up
Patients were followed via routine clinical follow-up from the time of LVAD implantation to cardiac transplant, death or the end of the review period (31 January 2020). All patients were monitored via an outpatient LVAD clinic at this institution.
Statistical analysis
All analyses were conducted using R. P-values <0.05 were considered significant. Continuous variables were assessed for normality (Shapiro–Wilk test) and reported as median (interquartile range) or mean (standard deviation). Normally distributed data were compared using Student’s t-test and non-normally distributed data were compared using the Mann–Whitney U-test. Categorical variables were compared using Chi-square or Fisher’s exact test (any group having fewer than 10 observations). To compare the incidence of TEs between those who received LAAO and those who did not, cumulative incidence curves were created with death and heart transplant as competing risks. Predictors of TEs were investigated with Fine and Gray competing risk regression. Variables that were found to be significant in the univariable Fine and Gray regression (P < 0.05) were included in a multivariable analysis with LAAO. The incidence of disabling strokes between those who received LAAO and those who did not was compared using the chi-square test. Cumulative incidence curves and Fine and Gray regression could not be used to compare disabling strokes between groups as the LAAO group had zero disabling strokes.
Survival was compared between groups via Cox proportional hazards analysis in which patients were censored at the time of transplant or loss to follow-up. Variables found to be significantly associated with survival (P < 0.05) in univariable Cox models were included in a multivariable model with LAAO. Adjusted survival curves comparing survival in those who received LAAO and those who did not (adjusting for covariates significantly associated with mortality) were created. The effect of LAAO on in-hospital mortality was assessed via univariable logistic regression. A multi-variable regression could not be performed due to the low number of in-hospital mortalities. Variables with 5% or more data missing were excluded from all regression analyses, which included the history of peripheral vascular disease, discharge international normalized ratio, discharge haemoglobin and cardiopulmonary bypass time. Listwise deletion was utilized for variables with any missing data for regression analyses.
RESULTS
Patient characteristics
Of the 182 patients who received HM3 implants, 99 (54%) received concomitant LAAO. Those who received LAAO tended to be younger (median age 59 vs. 66 years, P < 0.001). The median CHA2DS2-VASc score was 3 in both groups (no LAAO IQR 3–4, LAAO IQR 2–4, P = 0.017). The LAAO group had a lower percentage of patients with a history of ischaemic cardiomyopathy (26% vs 63% in those without LAAO, P < 0.001). Four patients (4%) in the LAAO group had a previous sternotomy, compared to 43 (52%) in the no LAAO group (P < 0.001). Complete demographic comparisons between those who received LAAO and those who did not are shown in Table 1. Five patients had intracardiac thrombus discovered at the time of LVAD implant (2 in the no LAAO group vs 3 in the LAAO group, P = 1.00). Four intracardiac thrombi were discovered in the left ventricle intraoperatively and 1 in the left atrial appendage.
Table 1:
Baseline demographics and operative characteristics comparing patients who did and did not receive left atrial appendage occlusion
| Variable | No LAAO (n = 83) | LAAO (n = 99) | P-value |
|---|---|---|---|
| Baseline demographics | |||
| Age, years | 66 (56.03–70.37) | 59 (50.79–63.14) | <0.001 |
| Sex, male | 88 (73) | 80 (79) | 0.20 |
| HTN | 66 (55) | 60 (59) | 0.44 |
| Stroke/TIA | 16 (13) | 17 (16) | 0.97 |
| PVD | 11 (9) | 6 (5) | 0.26 |
| COPD | 10 (8) | 12 (12) | 0.77 |
| DM | 46 (38) | 35 (35) | 0.20 |
| Afib | 55 (45) | 48 (46) | 0.48 |
| ICM | 63 (52) | 26 (26) | <0.001 |
| Previous sternotomy | 52 (43) | 4 (4) | <0.001 |
| Previous tricuspid surgery | 1 (1) | 2 (2) | 1.00 |
| Previous mitral surgery | 8 (7) | 5 (5) | 0.55 |
| Prior CABG | 42 (35) | 0 (0) | <0.001 |
| BTT | 25 (21) | 14 (14) | 0.087 |
| CHA2DS2-VASc | 3 (3–4) | 3 (2–4) | 0.017 |
| INTERMACS | 0.71 | ||
| 1 | 14 (12) | 11 (11) | |
| 2 | 46 (38) | 54 (53) | |
| 3 | 33 (27) | 30 (30) | |
| 4 | 7 (6) | 5 (5) | |
| IABP | 31 (26) | 45 (44) | 0.076 |
| Impella | 6 (5) | 1 (1) | 0.094 |
| ECMO | 6 (5) | 8 (8) | 0.77 |
| mPAP, mmHg | 33.95 ± 10.62 | 35.52 ± 9.87 | 0.30 |
| PCWP, mmHg | 22.55 ± 9.15 | 23.85 ± 9.24 | 0.35 |
| CVP, mmHg | 10 (6–14) | 9 (6–15) | 0.96 |
| Fick cardiac output, l/min | 3.58 (2.94–4.46) | 3.59 (2.88–4.19) | 0.75 |
| Preoperative creatinine, mg/dl | 1.46 ± 0.45 | 1.4 ± 0.41 | 0.36 |
| Preoperative albumin, g/dl | 3.8 (3.2–4.15) | 3.8 (3.32–4.18) | 0.48 |
| Operative characteristics | |||
| CPB time, min | 103 (83–125) | 96.2 (72.8–132.5) | 0.52 |
| Concomitant surgery | |||
| Aortic valve surgery | 19 (16) | 15 (15) | 0.59 |
| Mitral valve surgery | 10 (8) | 19 (19) | 0.094 |
| Tricuspid valve surgery | 8 (7) | 7 (7) | 0.78 |
| Intraoperative intracardiac thrombus | 2 (2) | 3 (3) | 1.00 |
| Left ventricle | 2 | 2 | |
| Left atrium | 0 | 0 | |
| Left atrial appendage | 0 | 1 | |
Data are presented as % (n) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables.
Afib: atrial fibrillation; BTT: bridge to transplantation; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; DM: diabetes mellitus; ECMO: extracorporeal membrane oxygenation; HTN: hypertension; IABP: intra-aortic balloon pump; ICM: ischaemic cardiomyopathy; LAAO: left atrial appendage occlusion; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease.
Early and late outcomes
Data related to mortality and morbidity are shown in Table 2. The median time to event or last follow-up was similar between groups (422 days in the no LAAO group vs 461 in the LAAO group, P = 0.93). Those who received LAAO had similar incidences of postoperative morbidities compared to the no LAAO group, including similar incidences of postoperative atrial fibrillation (35% vs 41%, P = 0.46). In-hospital mortality was lower in the LAAO group (odds ratio: 0.92, 95% confidence interval [0.85–0.98], P = 0.013). Survival was not significantly different between the LAAO and no LAAO groups as assessed by multivariable Cox proportional hazards analysis (hazard ratio: 0.39, 95% confidence interval [0.12–1.23], P = 0.11; Fig. 1). Complete Cox proportional hazards data is shown in Table 3. There were no identified risk factors for mortality in multivariable analysis.
Table 2:
Outcomes Data comparing patients who did and did not receive left atrial appendage occlusion
| Variable | No LAAO (n = 83) | LAAO (n = 99) | P-value |
|---|---|---|---|
| Postoperative atrial fibrillation | 35 (29) | 41 (41) | 0.46 |
| Postoperative VT/VF | 20 (16) | 22 (22) | 0.77 |
| Postoperative sepsis | 16 (13) | 19 (19) | 0.72 |
| Postoperative UTI | 15 (12) | 21 (21) | 0.34 |
| Postoperative takeback | 12 (10) | 17 (17) | 0.47 |
| Postoperative RRT | 9 (7) | 4 (4) | 0.23 |
| Postoperative tracheostomy | 10 (8) | 9 (9) | 1.00 |
| Thromboembolic events | 10 (8) | 6 (6) | 0.53 |
| Ischaemic strokes | 10 (8) | 5 (5) | 0.26 |
| Time to ischaemic stroke, days | 7 (3.25–23.25) | 67 (12–123) | 0.093 |
| Disabling strokes | 7 (6) | 0 (0) | 0.008 |
| Cardiac transplants | 24 (20) | 18 (18) | 0.43 |
| Hospital stay, days | 32 (20.460–49) | 27.5 (21–36.25) | 0.25 |
| Overall mortality | 18 (15) | 5 (5) | 0.01 |
| In-hospital mortality | 11 (9) | 2 (2) | 0.030 |
Data are presented as % (n) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables.
LAAO: left atrial appendage occlusion; RRT: renal replacement therapy; UTI: urinary tract infection; VT/VT: ventricular tachycardiac/ventricular fibrillation.
Figure 1:
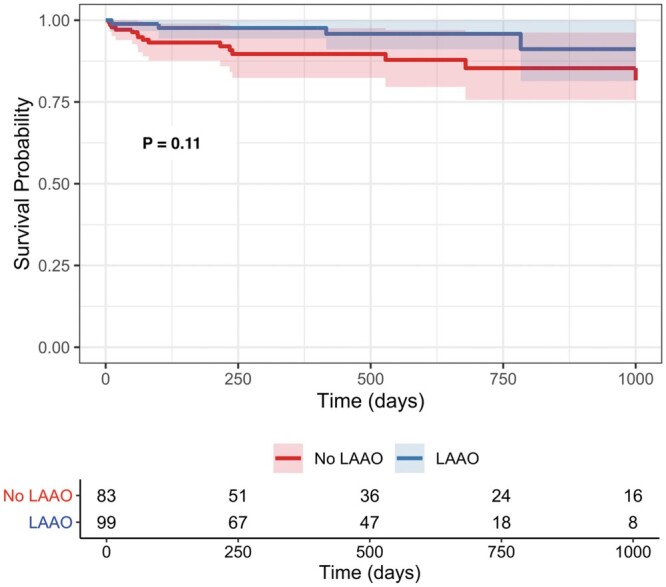
Adjusted survival curves comparing survival in patients who received left atrial appendage occlusion with HeartMate 3 implant and those who did not based on a multivariable Cox analysis including left atrial appendage occlusion, age and previous sternotomy. P-value is from left atrial appendage occlusion in the multivariable Cox model. LAAO: left atrial appendage occlusion.
Table 3:
Cox proportional hazards analysis
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% [CI] | P-value | HR | 95% [CI] | P-value | |
| LAAO | 0.29 | [0.10–0.80] | 0.017 | 0.39 | [0.12–1.23 | 0.11 |
| Previous sternotomy | 2.47 | [1.02–5.96] | 0.045 | 1.27 | [0.47–3.48] | 0.64 |
| Age, years | 1.05 | [1.01–1.10] | 0.027 | 1.04 | [0.99–1.09] | 0.088 |
| HTN | 1.78 | [0.65–4.91] | 0.26 | |||
| COPD | 1.44 | [0.42–4.91] | 0.56 | |||
| DM | 2.20 | [0.90–5.38] | 0.09 | |||
| INTERMACS score | ||||||
| 1 | 1.30 | [0.25–6.78] | 0.76 | |||
| 2 | 1.77 | [0.63–4.96] | 0.28 | |||
| 3 (reference) | ||||||
| 4a | ||||||
| Impella | 2.08 | [0.27–15.80] | 0.48 | |||
| IABP | 1.09 | [0.44–2.66] | 0.86 | |||
| ECMOb | 0.41 | |||||
| mPAP | 0.99 | [0.95–1.03] | 0.68 | |||
| PCWP | 0.99 | [0.94–1.04] | 0.65 | |||
CI: confidence interval; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; ECMO: extracorporeal membrane oxygenation; HR: hazard ratio; HTN: hypertension; IABP: intra-aortic balloon pump; LAAO: left atrial appendage occlusion; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure.
INTERMACS 4 had no mortalities that prevented our using it as a reference level and from putting it into the model as a covariate.
Mortality difference between patients bridged with ECMO and without determined with the Chi-square test due to there being zero cases of mortality in those bridged with ECMO.
Thromboembolic events and disabling strokes
There were 14 patients who developed TEs, including 13 strokes (7%) and 1 pump thrombosis (0.5%). All 13 strokes, which included patients with both disabling and non-disabling strokes, occurred within the first 6 months following implantation (8 in the no LAAO group vs 5 in the LAAO group, P = 0.26). A similar number of patients who did not develop TEs had a previous stroke or transient ischaemic attack as compared to those with TEs (17% vs 14%, P = 1.00). Those without TEs also had a similar number of patients with a past medical history of atrial fibrillation compared to those with TEs (50% vs 69%, P = 0.25). Complete demographic comparisons between those who developed TEs and those who did not are shown in Table 4. No significant difference in incidence of TEs in each group was found, with the rate of TE occurrence in each <10% at 3 years (Gray’s test: P = 0.35; Fig. 2). Regarding disabling strokes specifically, there were zero of such strokes in patients who received LAAO, compared to 6 (7%) in those who did not receive LAAO (P = 0.008). Age and previous sternotomy were associated with TEs in the univariable Fine–Gray analysis. LAAO was not associated with TEs in the multivariable Fine–Gray analysis (P = 0.10) and no significant risk factors for TEs were identified. Complete Fine–Gray analysis results are shown in Table 5. Two additional supplemental analyses were conducted. The first, in which patients with sternotomy were excluded from the comparison of baseline demographics (Supplementary Material, Table S1), outcomes data (Supplementary Material, Table S2) and cumulative incidence curves comparing TEs in the LAAO versus no LAAO groups (Supplementary Material, Fig. S1) were conducted to account for possible bias in the inclusion of patients with prior sternotomy. A second supplemental analysis comparing outcomes data between patients with non-disabling TEs and disabling TEs was completed assess for predisposing factors to disabling stroke (Supplementary Material, Table S3).
Table 4:
Baseline demographics and operative characteristics in those who did and did not develop thromboembolic events
| Variable | No TEs (n = 168) | TEs (n = 14) | P-value |
|---|---|---|---|
| Baseline demographics | |||
| Age | 61 (52–68) | 69.44 (56–72.37) | 0.053 |
| Sex, male | 83 (139) | 93 (13) | 0.54 |
| HTN | 61 (102) | 86 (12) | 0.12 |
| Stroke/TIA | 17 (27) | 14 (2) | 1.00 |
| PVD | 8 (12) | 14 (2) | 0.33 |
| COPD | 12 (20) | 0 (0) | 0.37 |
| DM | 40 (68) | 36 (5) | 0.78 |
| Afib | 50 (82) | 69 (9) | 0.25 |
| ICM | 42 (70) | 57 (8) | 0.28 |
| Previous sternotomy | 24 (40) | 50 (7) | 0.07 |
| Previous tricuspid surgery | 2 (3) | 0 (0) | 1.00 |
| Previous mitral surgery | 7 (11) | 7 (1) | 1.00 |
| Prior CABG | 17 (29) | 43 (6) | 0.031 |
| BTT | 20 (33) | 14 (2) | 1.00 |
| CHA2DS2-VASc | 3 (2–4) | 3.5 (3–4) | 0.10 |
| INTERMACS | 0.64 | ||
| 1 | 12 (20) | 21 (3) | |
| 2 | 51 (85) | 43 (6) | |
| 3 | 32 (53) | 29 (4) | |
| 4 | 6 (10) | 7 (1) | |
| IABP | 38 (64) | 46 (6) | 0.57 |
| Impella | 2 (4) | 14 (2) | 0.069 |
| ECMO | 7 (11) | 17 (2) | 0.21 |
| mPAP, mmHg | 34.91 ± 10.24 | 33.5 ± 10.35 | 0.65 |
| PCWP, mmHg | 23 (17–29.5) | 19 (15.5–25) | 0.60 |
| CVP, mmHg | 9 (6–14) | 12 (6.75–15.5) | 0.42 |
| Fick cardiac output, l/min | 3.55 (2.82–4.24) | 4.03 (3.27–4.68) | 0.10 |
| Preoperative creatinine, mg/dl | 1.36 (1.12–1.63) | 1.67 (1.43–1.89) | 0.10 |
| Preoperative albumin, g/dl | 3.8 (3.3–4.1) | 4.1 (3.62–4.27) | 0.14 |
| Operative characteristics | |||
| CPB time, min | 96 (73.5–124.5) | 115 (98.25–141.5) | 0.055 |
| Concomitant surgery | |||
| Aortic valve surgery | 16 (27) | 29 (4) | 0.26 |
| Mitral valve surgery | 16 (27) | 0 (0) | 0.23 |
| Tricuspid valve surgery | 8 (13) | 7 (1) | 1.00 |
| Intraoperative intracardiac thrombus | 3 (5) | 0 (0) | 1.00 |
| Left ventricle | 2 | 0 | |
| Left atrium | 0 | 0 | |
| Left atrial appendage | 1 | 0 | |
Data are presented as % (n) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables.
Afib: atrial fibrillation; BTT: bridge to transplantation; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; CVP: central venous pressure; DM: diabetes mellitus; ECMO: extracorporeal membrane oxygenation; HTN: hypertension; IABP: intra-aortic balloon pump; ICM: ischaemic cardiomyopathy; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease; TEs: thromboembolic events; TIA: transient ischemic attack.
Figure 2:
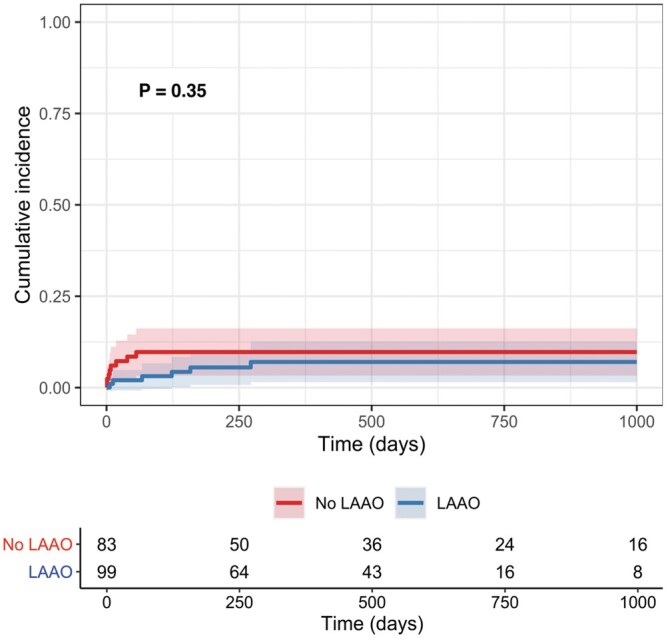
Cumulative incidence curves of thromboembolic events with death and heart transplant as competing events, comparing patients who received left atrial appendage occlusion with HeartMate 3 implant and those who did not. Differences in incidence were assessed via Gray’s test. LAAO: left atrial appendage occlusion.
Table 5:
Fine-Gray competing risk regression analysis for thromboembolic events
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| SHR | 95% [CI] | P-value | SHR | 95% [CI] | P-value | |
| LAAO | 0.61 | [0.21–1.74] | 0.35 | 0.41 | [0.14–1.20] | 0.10 |
| Previous sternotomy | 3.11 | [1.10–8.77] | 0.032 | 1.78 | [0.69–4.59] | 0.23 |
| Age, years | 1.05 | [1.00–1.10] | 0.062 | |||
| Sex | 2.60 | [0.33–20.4] | 0.36 | |||
| HTN | 3.61 | [0.80–16.20] | 0.094 | |||
| DM | 0.85 | [0.29–2.51] | 0.76 | |||
| PVD | 1.88 | [0.43–8.28] | 0.41 | |||
| Afib | 2.21 | [0.68–7.16] | 0.19 | |||
| Stroke/TIE | 0.80 | [0.19–3.41] | 0.76 | |||
| CHA2DS2-VASc | 1.34 | [0.93–1.94] | 0.12 | |||
| Impella | 6.15 | [1.35–28] | 0.019 | 1.67 | [0.21–13.50] | 0.63 |
| IABP | 1.35 | [0.46–4.01] | 0.58 | |||
| ECMO | 2.83 | [0.64–12.5] | 0.17 | |||
| mPAP | 0.99 | [0.94–1.04] | 0.59 | |||
| PCWP | 0.99 | [0.94–1.05] | 0.81 | |||
Afib: atrial fibrillation; CI: confidence interval; DM: diabetes mellitus; ECMO: extracorporeal membrane oxygenation; HTN: hypertension, IABP: intra-aortic balloon pump; LAAO: left atrial appendage occlusion; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease; SHR: subdistribution hazard ratio; TIE: transient ischaemic event.
DISCUSSION
This study investigated the effect of LAAO on the development of TEs after HM3 implantation. The rate of TEs was low, with only one pump thrombosis and 13 ischaemic strokes. TE rates did not differ significantly in LAAO versus non-LAAO recipients, and this finding remained true when patients with a previous sternotomy were removed (Supplementary Material, Table S1). However, it cannot be definitively concluded that there was no difference in the rate of TEs between these populations due to the small patient population and overall low number of TEs involved in this study. This is a departure from previous studies, which have demonstrated clear efficacy of LAAO in reducing TEs [9]. The absence of a confirmed difference in TEs between groups may be due to the lack of efficacy of LAAO in preventing TEs after HM3 implant. Were this to be true, a decrease in the efficacy of LAAO in decreasing TEs in HM3 recipients relative to prior generations of LVADs could be resultant from the improved haemocompatibility of the HM3, if the novel device design decreases overall TE risk to such a degree that LAAO provides no added benefit. The primary mechanism underlying pump thrombosis with previous continuous-flow LVADs was thought to be in situ thrombosis along the axial rotors. In the new HM3, the centrifugal design and magnetically levitated component parts are thought to reduce this in situ thrombosis that accounted for the majority of pump thromboses with previous continuous-flow LVADs.
Patients with LVADs are known to have the accumulation of thrombus in the left atrium and left atrial appendage despite adequate anticoagulation [13–15]. Thrombus from the left atrium can migrate to the left ventricle and enter the pump as ingested thrombus that can embolize systemically, causing stroke. While this study did not find an association between total ischaemic strokes and transient ischaemic attacks between the LAAO and no LAAO groups, it did show that there were zero disabling strokes in the LAAO group, compared to 6 (7%) in the no LAAO group. Disabling strokes after HM3 implant are more likely resultant from ingested thrombus from the left atrium that the larger gaps between component parts in the HM3 allow to embolize and cause a significant stroke. LAAO may reduce a common source of ingested thrombus that accounts for the lack of disabling strokes seen in patients who received LAAO. All of these disabling strokes occurred in the first 6 months postimplantation, which would be expected to be more related to the HM3 implantation procedure itself and less effected by the improved haemocompatibility of the HM3. In addition, patients’ medical histories affect stroke risk in the first 6 months following implantation. The no LAAO group had a higher-risk profile for perioperative stroke (older age, higher CHA2DS2-VASc, previous sternotomy). Of the 6 patients with disabling stroke, 3 had bacterial infections in the weeks prior to thrombotic events, indicating that they may have been in a state of hypercoagulability and thus more susceptible to the generation of LA thrombus. The comparison of postoperative outcomes between the non-disabling stroke and disabling stroke groups (Supplementary Material, Table S3) revealed no significant differences in additional postoperative outcome variables. Notably, no patients included in this study had mechanical mitral valves; thus, these were not a source of potential thrombus. LAAO may reduce disabling strokes in these high-risk patients, though due to the higher-risk profile for stroke in the no LAAO group, this study is unable to definitively determine the effect of LAAO on reducing postoperative disabling stroke risk and further study is needed to confirm this finding. In addition, LAAO requires more dissection and procedure time, which must be considered in balancing the possible benefit of LAAO with the increased operative risk associated with its use.
Patients with HM3 who develop bleeding following implant must be carefully monitored and receive anticoagulation based on their symptoms. However, there are little data on the safety of withholding anticoagulation in HM3 patients with regard to TE risk. While this study found no difference in the overall incidence of TEs between those receiving LAAO and those not receiving LAAO at the time of HM3 implantation, LAAO has been shown to decrease anticoagulation requirements and stroke risk in patients with atrial fibrillation [16–18]. LAAO may give clinicians more confidence in withholding anticoagulation for an HM3 patient who develops bleeding, though further studies are required to determine the safety of withholding anticoagulation in HM3 patients and the role of LAAO in reducing TE risk.
Limitations
Due to resternotomy patients not generally being candidates for LAAO, there is a source of bias in this study’s data. However, a large proportion of the cohort had a prior sternotomy and it was deemed most clinically useful to include these patients in analyses as many patients undergoing HM3 implant have had a prior sternotomy. This potential source of bias was accounted for by including previous sternotomy as a cofactor in the multivariable Fine and Gray analysis. For the interest of the reader, an analysis with previous sternotomy patients excluded has been provided, which includes a comparison of baseline demographics (Supplementary Material, Table S1), outcomes data (Supplementary Material, Table S2) and cumulative incidence curves comparing TEs in those who did and did not receive LAAO (Supplemental Material, Fig. S1). The incidence of TEs did not differ significantly between groups (Gray’s test: P = 0.39).
The retrospective, uncontrolled nature of this study creates additional limitations. First, the LAAO and no LAAO groups are not similar given differences in baseline characteristics that are representative of the widespread etiologies of heart failure (Table 1), and propensity score matching failed to adequately match patients because of these differences. This study is also limited in that LAAOs were done at the surgeon’s discretion and without a standardized protocol for determining specific patients for whom it is indicated, which may lead to bias in the study. Lastly, the retrospective design of this study prevents accounting for all possible confounding variables that prevented the determination of any causal relationships. Due to this being a single-centre study, outcomes may not be applicable to other centres and multicentre studies are needed to confirm these findings. With a very small sample size, this study may lack the statistical power to determine an association between LAAO and TE prevention after HM3 implantation, if present.
CONCLUSIONS
A low number of TEs was observed in HM3 recipients. No statistically significant difference in the rate of TEs between no LAAO and LAAO groups was found in this patient population. While it cannot be definitively concluded that LAAO did not further reduce the overall rate of TEs in this patient population, its use may be beneficial in preventing disabling ischaemic strokes after HM3 implantation. LAAO’s potential role in preventing disabling strokes presents an opportunity for the future study.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Funding
No funding was received for this study.
Conflict of interest: Paolo C. Colombo, Gabriel Sayer and Yoshifumi Naka have received consulting fees from Abbott. Nir Uriel has received grant support and consulting fees from Abbott and Medtronic. The remaining authors have no conflicts of interest to disclose.
Author Contributions
Andrew Melehy: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Gillian O'Connell: Data curation; Methodology; Writing—review & editing. Yuming Ning: Formal analysis; Methodology; Writing—review & editing. Paul Kurlansky: Formal analysis; Methodology; Writing—review & editing. Yuji Kaku: Conceptualization; Writing—review & editing. Veli Topkara: Conceptualization; Writing—review & editing. Melana Yuzefpolskaya: Conceptualization; Writing—review & editing. Paolo C. Colombo: Conceptualization; Writing—review & editing. Gabriel Sayer: Conceptualization; Writing—review & editing. Nir Uriel: Conceptualization; Writing—review & editing. Yoshifumi Naka: Conceptualization; Project administration. Koji Takeda: Conceptualization; Investigation; Project administration; Supervision; Writing—original draft; Writing—review & editing.
Reviewer Information
Interactive CardioVascular and Thoracic Surgery thanks Luca Bertoglio, Roberto Lorusso, Massimiliano Meineri and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
Glossary
- ABBREVIATIONS
- HM3
HeartMate 3
- LAAO
Left atrial appendage occlusion
- LVADs
Left ventricular assist devices
- TEs
Thromboembolic events
Presented at the Meeting of the International Society for Heart and Lung Transplantation, Virtual, 24–28 April 2021.
REFERENCES
- 1. Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier HO. et al. ; HeartMate II Clinical Investigators. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 2014;63:880–8. [DOI] [PubMed] [Google Scholar]
- 2. Kato TS, Schulze PC, Yang J, Chan E, Shahzad K, Takayama H. et al. Pre-operative and post-operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant 2012;31:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW. et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transpl 2014;33:12–22. [DOI] [PubMed] [Google Scholar]
- 4. Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA. et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33–40. [DOI] [PubMed] [Google Scholar]
- 5. Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM. et al. The spectrum of complications following left ventricular assist device placement. J Card Surg 2012;27:630–8. [DOI] [PubMed] [Google Scholar]
- 6. Koene RJ, Win S, Naksuk N, Adatya SN, Rosenbaum AN, John R. et al. HAS-BLED and CHA2DS2-VASc scores as predictors of bleeding and thrombotic risk after continuous-flow ventricular assist device implantation. Journal Card Fail 2014;20:800–7. [DOI] [PubMed] [Google Scholar]
- 7. Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK. et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P. et al. ; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988–98. [DOI] [PubMed] [Google Scholar]
- 9. Deshmukh A, Bhatia A, Sayer GT, Kim G, Raikhelkar J, Imamura T. et al. Left atrial appendage occlusion with left ventricular assist device decreases thromboembolic events. Ann Thorac Surg 2019;107:1181–6. [DOI] [PubMed] [Google Scholar]
- 10. Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT. et al. A fully magnetically levitated left ventricular assist device. N Engl J Med 2019;380:1618–27. [DOI] [PubMed] [Google Scholar]
- 11. Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ. et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation 2017;135:2003–12. [DOI] [PubMed] [Google Scholar]
- 12. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JB, Culebras A. et al. ; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fatkin D, Kelly RP, Feneley MP.. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol 1994;23:961–9. [DOI] [PubMed] [Google Scholar]
- 14. Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF.. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol 1994;24:755–62. [DOI] [PubMed] [Google Scholar]
- 15. Lewis RS, Wang L, Spinelli KJ, Ott GY, Abraham J.. Surgical occlusion of the left atrial appendage and thromboembolic complications in patients with left ventricular assist devices. J Heart Lung Transplant 2017;36:586–8. [DOI] [PubMed] [Google Scholar]
- 16. Kim R, Baumgartner N, Clements J.. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation-related cerebrovascular accident. J Thorac Cardiovasc Surg 2013;145:582–9. [DOI] [PubMed] [Google Scholar]
- 17. Johnson WD, Ganjoo A, Stone CD, Srivyas RC, Howard M.. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg 2000;17:718–22. [DOI] [PubMed] [Google Scholar]
- 18. García-Fernández M, Pérez-David E, Quiles J, Peralta J, Garcia-Rojas I, Bermajo J. et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003;42:1253–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.