Abstract
We have demonstrated that neuropeptide Y (NPY) can regulate pro-inflammatory signaling in the gut via cross-talk with the pro-inflammatory cytokine tumor necrosis factor (TNF). Here, we investigated if selective blocking of NPY receptors, NPY1R or NPY2R, using small molecule non-peptide antagonists (BIBP-3222 for NPY1R and BIIE-0246 for NPY2R) in the colon could attenuate intestinal inflammation by lowering TNF levels (BIBP - N-[(1R)]-4-[(Aminoiminomethyl)amino-1-[[[(4-hydroxyphenyl)methyl]amino]carbonyl]butyl-α-phenylbenzeneacetamide; BIIE - N-[(1S)-4-[(Aminoiminomethyl)amino]-1-[[[2-(3,5-dioxo-1,2-diphenyl-1,2,4-triazolidin-4-yl)ethyl]amino]carbonyl]butyl]-1-[2-[4-(6,11-dihydro-6-oxo-5H-dibenz[b,e]azepin-11-yl)-1-piperazinyl]-2-oxoethyl]-cyclopentaneacetamide). Colitis was induced using dextran sodium sulfate in drinking water for 7 days, or by adoptive T-cell transfer in RAG-/- mice. Colonic biopsies from healthy subjects (n = 10) and IBD patients (n = 34, UC = 20, CD = 14) were cultured ex vivo in presence or absence of NPY antagonists (100 µM, 20 h), and cytokine release into culture supernatants was measured by ELISA. Intracolonic administration of BIBP (but not BIIE) significantly reduced clinical, endoscopic, and histological scores, and serum TNF, interleukin (IL)-6, and IL-12p70 in DSS colitis; it also significantly attenuated histological damage and serum IL-6 in T-cell colitis (P < .05). Intracolonic administration of BIBP significantly reduced TNF and interferon (IFN)-γ release from UC biopsies, whereas BIIE downregulated only IFN-γ (P < .05). BIBP significantly reduced TNF and interferon (IFN)-γ release from UC biopsies, whereas BIIE downregulated only IFN-γ (P < .05). Our data suggest a promising therapeutic value for NPY1R inhibition in alleviating intestinal inflammation in UC, possibly as enemas to IBD patients.
Keywords: neuroimmune, ulcerative colitis, Crohn’s disease, TNF, NPY, mucosal explants
Introduction
The enteric nervous system (ENS) orchestrates the main function of gastrointestinal motility, and in addition, enteric neurotransmitters also play a key role in regulating inflammatory signaling in the gut.1–5 This role is effected due to a dynamic dialogue between the ENS and the enteric immune cells mediated by neuropeptide receptors expressed on immune cells and enteric ganglion cells.6,7 Inflammatory bowel disease (IBD) is a clinical condition characterized by intestinal inflammation in the gastrointestinal (GI) tract and is categorized into Crohn’s disease (CD- transmural inflammation occurring in any site between mouth and anus) and ulcerative colitis (UC, mucosal inflammation restricted to the colon). Though several studies have examined the role of enteric neuropeptides-immune cell interaction in IBD, a therapeutic approach based on enteric neuron-immune interactions has not yet been exploited to manage or cure intestinal inflammation in IBD.
Among the enteric neuropeptides, neuropeptide Y (NPY) is a potent modulator of enteric immune functions. Neuropeptide Y is a 36 amino acid peptide hormone that exerts pleiotropic effects on diverse functions including anxiety, appetite, fluid secretion, and vasodilation via 5 G-protein coupled receptors: Y1, Y2, Y4, Y5, and Y6.8 Among the NPY receptors, NPY1R and NPY2R have been implicated in regulating immune cell functions like antigen presentation, T-cell activation,2,9–11 macrophage nitric oxide production,12 as well as regulation of inflammatory pain.13 In addition, epithelial NPY1R and NPY2R receptors also regulate secretory functions and ion transport.14 Neuropeptide Y receptor antagonists to Y1R (BIBP-3222 or 3226) and Y2R (BIIE-0246) are small molecule nonpeptide antagonists and have been previously tested extensively in mouse models in the context of immune regulation, ion secretion, pain perception, and obesity (BIBP - N-[(1R)]-4-[(Aminoiminomethyl)amino-1-[[[(4-hydroxyphenyl)methyl]amino]carbonyl]butyl-α-phenylbenzeneacetamide; BIIE - N-[(1S)-4-[(Aminoiminomethyl)amino]-1-[[[2-(3,5-dioxo-1,2-diphenyl-1,2,4-triazolidin-4-yl)ethyl]amino]carbonyl]butyl]-1-[2-[4-(6,11-dihydro-6-oxo-5H-dibenz[b,e]azepin-11-yl)-1-piperazinyl]-2-oxoethyl]-cyclopentaneacetamide).2,13–16 In addition, BIBP, the NPY1R antagonist, shows high selective binding to the human Y1 receptor, sharing an overlapping binding site with NPY, and hence, has significant translational relevance.17,18
Previously, we demonstrated that NPY knockout (NPY KO) mice are resistant to chemical and infectious models of colitis due to enhanced antioxidant defenses and reduced oxidative stress in the germ-line null animals.4 We also demonstrated that enteric neurons from NPY KO mice produced less tumor necrosis factor (TNF), the main pro-inflammatory cytokine and therapeutic target in current IBD treatment strategies.5 We have also demonstrated the existence of a reciprocal regulation between NPY and TNF in the intestine via the regulation of NPY promoter by TNF via c-jun. We also demonstrated the role of NPY in increasing epithelial permeability via PI3-K-mediated regulation of pore-forming claudin-2.5 Further, we demonstrated the existence of TNF receptors (TNFR1 andTNFR2) on enteric neurons and that administration of TNF inhibitors can attenuate NPY expression during experimental colitis in mice.5 Thus, based on our published compelling findings, we now investigated if NPY inhibitors could be exploited to attenuate intestinal inflammation in IBD. Here, we hypothesized that NPY signaling plays a pro-inflammatory role in IBD, and hence, intracolonic administration of NPY1R and NPY2R receptor antagonists can reduce intestinal inflammation in mouse models of colitis and cytokine release from human IBD biopsies ex vivo.
Methods
Antibodies and Chemicals
The antibodies used were antineuropeptide Y (NPY, Santa Cruz Biotechnology, Dallas, TX) and anti-Tuj1 or anti-β-3 tubulin (Abcam, Cambridge, UK). Dextran sodium sulfate (DSS, molecular weight, 36,000-50,000 daltons) was obtained from MP Biomedicals. BIBP 3222 trifluoroacetate or BIBP-3226 (molecular weight 473.57, water soluble up to 10 mM)19,20 was used as the Y1 antagonist, and BIIE-0246 (molecular weight 932.5, soluble in DMSO)21–23 was used as Y2 antagonist; both were obtained from Tocris Bioscience (Bristol, UK).
Human Biopsy Collection
The sigmoid colon biopsies for the study were obtained by Dr. Tanvi Dhere, MD, and Dr. Heba Iskandar, MD, the GI physicians at Emory Clinic, Department of Medicine, Emory University, Atlanta, Georgia, USA. The protocol for the study was approved by the Emory Institutional Review Board (Protocol No. IRB00099851). The control group included both male and female subjects without inflammatory bowel disease who underwent colonoscopy for polyp/cancer screening (ages 34-71 years, n = 10) and who were without other disease conditions like diabetes or cancer. Clinical features of the IBD patients (ages 19-73 years, n = 34) are given in Table 1.
Table 1.
Clinical Characteristics of IBD Patients
| Disease Activity | ||||||
|---|---|---|---|---|---|---|
| Patient ID | Diagnosis | Gender | Age | Region Involved | Mayo Score (UC) or Simple Endoscopic Score (CD) | Medication |
| 1 | UC | F | 36 | Rectum | 2 | Mesalamine |
| 2 | UC | M | 40 | Ascending colon | 2 | Humira, prograf |
| 3 | UC | M | 25 | pan-UC | 3 | Humira, prograf |
| 4 | UC | M | 69 | pan-UC | 2 | Entyvio |
| 5 | CD | F | 35 | Ileum and Rectum | 12 | None |
| 6 | CD | M | 33 | Rectum | 3 | Entyvio |
| 7 | CD | F | 55 | Ileum | 4 | Mercaptopurine |
| 8 | CD | F | 43 | Colon | 0 | Azathioprine |
| 9 | CD | F | 66 | Small bowel/rectum | 3 | Humira, Prednisone |
| 10 | CD | F | 64 | Colon | 4 | Remicade |
| 11 | UC | F | 50 | Colon-pan | 3 | Mesalamine |
| 12 | UC | M | 35 | Colon-pan | 3 | Humira |
| 13 | UC | M | 28 | Small bowel, rectum | na | Remicade |
| 14 | UC | F | 42 | Colon-pan | 2 | Xeljanz |
| 15 | UC | F | 34 | Colon-pan | 0 | Sulfasalazine |
| 16 | CD | F | 27 | Left colon, rectum | 4 | Humira |
| 17 | UC | M | 52 | Pan-colon | 2 | Simponi |
| 18 | UC | F | 45 | Colon | na | None |
| 19 | CD | M | 73 | Colon | 20 | Entyvio |
| 20 | UC | F | 56 | Rectum | 2 | Mesalamine |
| 21 | CD | F | 60 | transverse/left colon | 4 | Mesalamine |
| 22 | CD | M | 71 | ileum | 6 | Vedolizumab |
| 23 | UC | F | 38 | pan-UC | 1 | Adalimumab |
| 24 | UC | F | 37 | pan-UC | 2 | Infliximab |
| 25 | CD | F | 60 | colitis | 18 | Adalimumab |
| 26 | UC | M | 40 | pan-uc | 1 | Vedolizumab |
| 27 | CD | M | 54 | ileum and colon | 11 | Ustekinumab |
| 28 | UC | F | 35 | extensive uc | 2 | Mesalamine/Enbrel |
| 29 | CD | F | 59 | left colon | 4 | Mesalamine |
| 30 | CD | M | 27 | ileum, rectum | 4 | Adalimumab |
| 31 | UC | M | 48 | pan uc | 0 | Mesalamine |
| 32 | UC | M | 37 | pan uc | 2 | Mesalamine |
| 33 | UC | M | 19 | pan uc | 2 | Mesalamine |
| 34 | UC | F | 40 | pan uc | 2 | Vedolizumab |
Biopsy Processing and Antagonist Treatment
Upon collection, control and IBD biopsies were immediately stored on ice and processed as recommended per published protocols.24–27 Briefly, within 2-3 hours of collection, biopsies were gently washed in sterile RPMI (Gibco), blotted, weighed, transferred to a 96-well plate in duplicate, and cultured overnight (20 h) in Roswell Park Memorial Institute Medium (RPMI) medium containing 10% heat-activated fetal bovine serum (FBS), L-glutamine (2 mM), and 100 U/mL of penicillin and 100 mg/mL of streptomycin at 37˚C in 5% CO2, in the presence of BIBP-3222 or BIIE-0426 (100 uM). The supernatants were collected the following day and stored at −80°C until multiplex ELISA analysis. The RPMI media incubated overnight in the same plate without biopsy served as negative control for the assay. The effects of antagonists on cytokine release from IBD biopsies were evaluated per published protocols.27
Antagonist-treated and untreated inflamed biopsies from the same patient were analyzed using a paired t test to assess the efficacy of antagonist-induced cytokine inhibition.
IBD Biopsy Cytokine Assay by Multiplex ELISA
Biopsy supernatants were assayed for pro-inflammatory cytokines by human V-plex pro-inflammatory panel 1 kit by multiplex ELISA (Meso Scale Diagnostics, MSD, Maryland. USA).28 The serum cytokine measurements that did not fall within the standard curve were not included for analysis. The patients on anti-TNF medications during the study (UC = 7 patients, CD = 5 patients) who showed normal TNF levels in ex vivo biopsy cultures were excluded from antagonist testing assays.
CLARITY Immunostaining of Biopsies
The human biopsies were fixed in 4% p-formaldehyde, embedded in hydrogel, and cleared using 8% sodium dodecyl sulfate (SDS) solution.29 The cleared tissue was incubated with anti-β-3-Tubulin or Tuj1 (1:800) or anti-NPY (1:500) antibodies over night at 4°C, followed by incubation with respective Alexa Fluor secondary antibodies AF 488 (NPY), AF 555 (Tuj1), and DAPI (nuclear stain, 1:10,000) at room temperature. The tissue was mounted in Vectashield and imaged using confocal microscope (Olympus FLUOVIEW FV1000). The z-stacks were captured, and a 3D image of the staining pattern was constructed to visualize the density of NPY staining within the biopsy.
Arbitrary Fluorescence Quantification
Staining intensity was assessed in a blinded fashion using image J software after the composite image was split into respective color channels (blue, DAPI; green, NPY; red, Tuj1, neuronal marker), and the intensity of green fluorescence from 3 different fields per biopsy was calculated and plotted as relative arbitrary intensity units (RFUs). The average intensity of NPY staining in control and IBD biopsies was compared.
Mice
Male C57BL6 mice between 8 and 10 weeks old (Jackson Laboratory, Stock No.000664) were used for modeling dextran sodium sulfate (DSS) model of acute colitis, and RAG knockout mice (B6.129S7-RAG1tm1Mom/J, Jackson Laboratory, Stock No. 002216) were used for modeling adoptive T-cell transfer colitis. After experimental procedures, mice were euthanized with CO2. All murine experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University and were performed according to the Emory guidelines for the ethical treatment of animals.
Induction of DSS Colitis and NPY Antagonist Administration
Mice were given 3% DSS in drinking water (wt/vol) for 1 week and then humanely killed on day 8. The mice received NPY1R (BIBP-3222) or NPY2R (BIIE-0246) antagonists (10 mg/kg body weight) via intracolonic injections on days 2, 4, and 6 (after isoflurane anesthesia) via a small polyethylene catheter (0.3 mm/0.07 mm, lubricated with Vaseline) inserted intrarectally at 4 cm from the anus.30,31 The control mice were administered saline intrarectally to serve as a negative control. Though BIBP19,20 and BIIE21–23 have been safely utilized in murine studies, we conducted pilot studies to ensure that intracolonic administration of both antagonists did not induce any adverse effects in mice by assessing for inflammation markers like body weight loss, occult blood, and histological architecture (Supplementary Figure 1). Mice were weighed daily for body weight changes, occult blood, diarrhea, and stool consistency, and clinical score was evaluated.5,32
T-Cell Transfer Colitis and NPY Antagonist Administration
RAG1 knockout mice were injected with 0.5 × 106 CD45RB+ high T cells intraperitoneally (day 1).33 The mice received the first dose of NPY1R (BIBP-3222) or NPY2R (BIIE-0246) antagonists via intracolonic injections (after isofluorane anesthesia) on week 2. Mice received NPY antagonists once a week up to week 8. Body weights were taken every week initially up to 3 weeks, and starting week 4, mice were monitored twice weekly for body weight. At the end of 8 weeks, mice were humanely killed and evaluated for inflammatory markers.
Assessment of Inflammation
The distal colon was processed for hematoxylin-eosin staining and histological score analysis based on crypt damage, ulcers, and neutrophil infiltration. The colon length of colitic mice (DSS/T cell transfer) receiving NPY antagonists were compared with that of respective control mice and with that of colitic mice (DSS/T cell transfer) without antagonists. Serum was used for analysis of cytokines by multiplex ELISA (mouse pro-inflammatory panel 1, V-plex plate, MSD).
Endoscopy in Mice and Assessment of Endoscopic Colitis Score
Coloview Veterinary Endoscope (Karl Stortz) which provides high-resolution was used as described previously.34 In brief, this miniature colonoscope system comprises a miniature rigid endoscope (1.9 mm outer diameter), a xenon light source, a triple-chip high-resolution charge-coupled device camera, and an operating sheath with instrument channels and an air/water injection bulb to regulate inflation of the mouse colon. The mice administered DSS for 1 week were subjected to endoscopy on the day of sacrifice. The endoscope was inserted into the mid-descending colon; the mucosa was surveyed up to the anorectal junction; and the colonic mucosal region was photographed to obtain high-resolution (1024 × 768 pixels) images. Two independent operators acquired the data in a blinded fashion, and the endoscopic colitis score was calculated using the decimal-weighted scoring system method.35
Serum Cytokine Assay by Multiplex ELISA
Serum was assayed for pro-inflammatory cytokines by using a mouse V-plex pro-inflammatory panel 1 kit by multiplex ELISA (Meso Scale Diagnostics, MSD, Maryland. USA) as published previously.28
Real Time PCR
Distal colon (control and colitic mice) and sigmoid biopsies (normal and IBD biopsies) were used to compare the expression of NPY, NPY1R, NPPY2, and TNF mRNA by real time polymerase chain reaction (qPCR). The RNA was extracted using RNAesy Kit (Qiagen). The cDNA was synthesized from RNA using the iscript supermix (Bio Rad), and SYBR green reaction mix (BioRad) was used for qPCR with primers for TNF, neuropeptide Y, NPY1R, and NPY2R using β-actin or GAPDH for normalization.
Colon Explant Culture
Mice were euthanized by CO2 asphyxiation, and the colons were dissected to remove fecal matter and washed in Phosphate Buffered Saline or PBS, pH 7.4 containing 1% penicillin/streptomycin (P/S, 10,000 U/mL; 1% P/S) for explant culture as previously published.5 The colon was divided into 0.5 to 1 cm segment pieces and cultured in triplicates in a 24-well plate in RPMI 1640 media supplemented with 10% FBS and 1% P/S and incubated at 37°C with 5% CO2 for 24 hours in the presence of RPMI only (untreated) or BIBP (100 µM) or BIIE (100 µM). The supernatants were removed and centrifuged at 12,000 g at 4°C for 15 minutes and stored at −80°C for cytokine analysis by multiplex ELISA. The cytokine concentration in the supernatant was normalized to the total protein estimated by Bradford assay in the respective colon segments and expressed as percentage of cytokine release relative to the untreated samples. An aliquot of the supernatant (50 µL) was also used to determine lactate dehydrogenase (LDH) release (Cyquant assay kit, Thermo Fischer Scientific) to ensure the integrity/viability of tissues after overnight culture.
Statistical Analysis
The data were analyzed using GraphPad Prism 9 (La Jolla, CA). One-way analysis of variance (ANOVA) was used to compare data in murine colitis experiments involving more than 2 treatment groups, followed by the Dunnett multiple comparison test. Paired t test was used to evaluate the efficacy of antagonists in inhibiting cytokine release from inflamed and inflamed + antagonist-treated biopsies. Differences were considered significant at P < .05. Data are represented as mean ± SEM in all experiments.
Results
NPY1R Receptor Antagonist (BIBP-3222) Is Protective in DSS Colitis
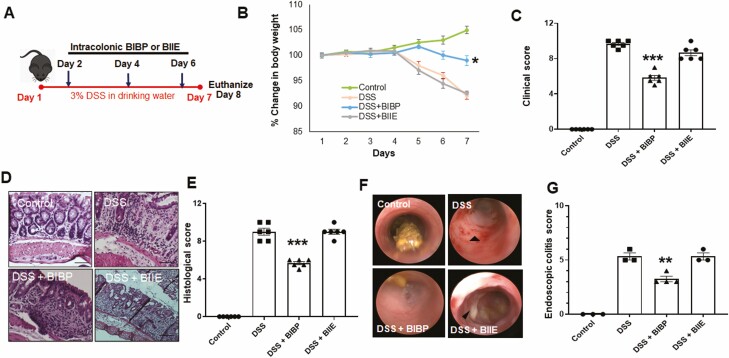
Our previous work has shown that NPY aggravates inflammation in mouse models of colitis.5,6 Hence, in the current study we assessed if intracolonic (rectal) administration of NPY1R receptor antagonist (BIBP-3222 at 10 mg/kg body weight) or NPY2R antagonist (BIIE-0246 at 10 mg/kg) in mice could reduce intestinal inflammation. The experimental outline is given in Figure 1A. We ensured that mice receiving the antagonists alone for 7 days showed no disease activity and healthy colon architecture (Supplemental Figure 1). In the acute colitis model with 7 days of 3% DSS, we observed that mice receiving BIBP, along with DSS, demonstrated significant protection from inflammation as depicted from reduced weight loss (Figure 1B) and reduced clinical score (Figure 1C) when compared with mice treated with DSS alone or DSS and BIIE. Also inflammation-induced colon shortening was significantly reduced in DSS and BIBP-treated mice (6.84 ± 0.1 cm, P < .05) compared with mice treated with DSS alone (5.4 ± 0.1 cm), or DSS and BIIE (6.16 ± 0.11 cm), whereas control mice presented with healthy colon lengths (8.25 ± 0.34 cm). We further validated the protective effects of BIBP-3222 by analyzing the histological architecture and indeed observed that mice treated with BIBP-3222 exhibited reduced ulceration, crypt damage, and neutrophil infiltration, as evident from hematoxylin-eosin staining of distal colon and histological score (Figure 1D and E). Further endoscopic colitis scores determined at day 8 also indicated that NPY1R inhibition offered protective effects (Figure 1F and G).
Figure 1.
NPY1R receptor antagonist (BIBP-3222) is protective in DSS colitis. A, Mice were administered dextran sodium sulfate (3%) in normal drinking water for 1 week. On days 2, 4, and 6, mice also received intracolonic injections of NPY1R antagonist (BIBP-3222) or NPY2R (BIIE-0246) at a dosage of 10 mg/kg body weight. Mice were monitored daily for body weight, occult blood, and diarrhea. Mice were killed humanely on day 8, and colon length was assessed. Distal colon was paraffin embedded and sections were stained with hematoxylin eosin (H&E) to assess histological damage. B, Change in body weight. C, Clinical score. D, Representative histological H&E sections of colon showing crypt damage, ulcers and neutrophil infiltration. E, Graphical representation of the intensity of histological damage by histological score. F, Endoscopy images on day 8. G, Endoscopic colitis score. Magnification 20X, Scale bar 50 µm. Values are mean ± SEM from 2 independent experiments, n = 3 per group per experiment, n = 3-4 for endoscopy assessment, *One-way ANOVA followed by Dunnett multiple comparison test, P < .05, **P < .01, ***P < .001.
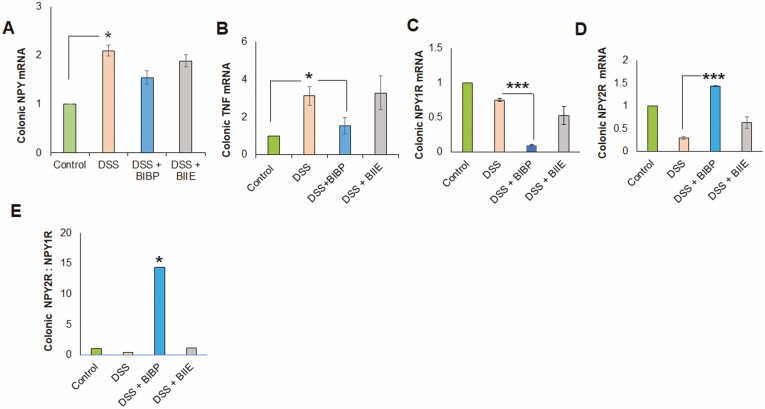
We also noted a significant inflammation-induced upregulation of colonic NPY mRNA (Figure 2A) upon DSS treatment, which was not altered upon administration of the NPY antagonists. Most importantly, we observed that NPY1R antagonist, BIBP, could significantly downregulate colonic TNF mRNA (Figure 2B). We achieved significant inhibition of the receptor expression by intracolonic administration of the drugs. Real time PCR analysis demonstrated that BIBP-3222 was effective in significantly downregulating localized NPY1R expression in the colon (Figure 2C). We also observed by qPCR that NPY2R mRNA was significantly downregulated by DSS, and administration of intracolonic BIIE during DSS treatment did not further downregulate NPY2R receptor expression (Figure 2D). Most importantly, we observed that ratio of Y2:Y1 was significantly downregulated in DSS colitis, which could be reversed by BIBP (Figure 2E). Taken together, these data suggested that NPY1R inhibition—but not NPY2R—is protective in the acute colitis model.
Figure 2.
NPY1R receptor antagonist (BIBP-3222) downregulates colonic TNF mRNA in DSS colitis. Mice were administered dextran sodium sulfate (3%) in normal drinking water for 1 week. On days 2, 4, and 6, mice also received intracolonic injections of NPY1R antagonist BIBP-3222 or BIIE-0246 (10 mg/kg body weight). Mice were killed humanely on day 8, and colons were flash frozen for RNA extraction. The colonic transcripts were assessed for changes in the expression of NPY, TNF, NPY1R, NPY2R by real time PCR (using primers given in Supplemental Table 1). Changes in (A) NPY, mRNA (B), TNF mRNA (C), NPY1R mRNA (D), NPY2R mRNA, and (E) NPY2R:NPY1R ratio. Values are mean ± SEM from 2 independent experiments, n = 6 (n = 3 per group per experiment). One-way ANOVA followed by Dunnett multiple comparison test, *P < .05, ***P < .001.
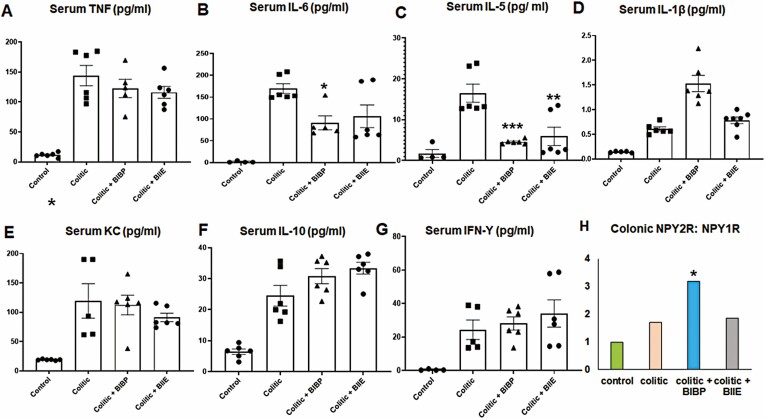
NPY1R Inhibition Was Effective in Reducing Serum TNF, IL-6, and IL-12p70 in DSS Colitis
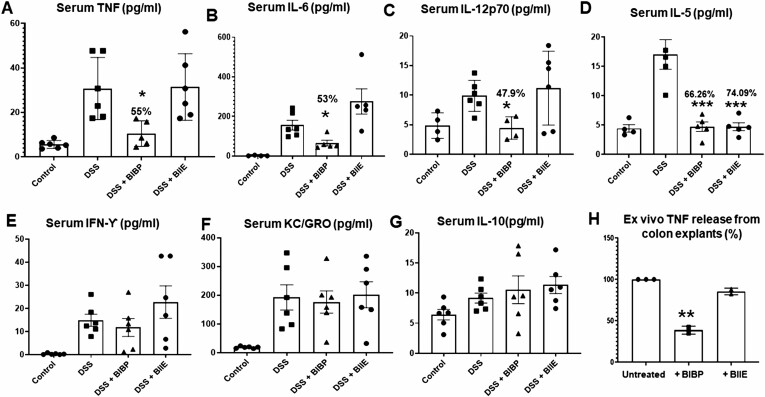
We observed that acute DSS treatment resulted in upregulation of serum TNF, IL-6, IL-12p70, IL-5, interferon-gamma (IFN-γ), chemokine (KC), IL-10, IL-1β, and IL-4 as determined using V-plex mouse pro-inflammatory panel by multiplex ELISA (Figure 3A). The NPY1R inhibition via intracolonic BIBP administration during DSS colitis resulted in significant inhibition of macrophage-driven T helper (Th)-1 cytokines like TNF (55.52% inhibition), IL-6 (53.8 % inhibition), and IL-12p70 (47.9 % inhibition; Figure 3A-C). We also noted that both BIBP (66.26%) and BIIE (74.09%) downregulated serum IL-5 (Figure 3D). However, BIBP did not alter serum IFN-γ, KC, and IL-10 (Figure 3E-G). The NPY2R inhibition via intracolonic BIIE-0246 failed to lower the serum levels of pro-inflammatory cytokines except IL-5, thus corroborating the lack of protective effects, as shown in Figure 1. In addition, we also observed that NPY1R inhibition was also effective in reducing TNF release from murine colon explant cultures ex vivo, whereas BIIE was ineffective (Figure 3H).
Figure 3.
Effects of NPY1R and NPY2R receptor antagonists on serum cytokines in DSS colitis. Mice were administered dextran sodium sulfate (3%) in normal drinking water for 1 week. On days 2, 4, and 6, mice also received intracolonic injections of NPY1R antagonist, BIBP-3222 or NPY2R antagonist, BIIE-0246 (10 mg/kg body weight). Mice were killed humanely on day 8, and serum was collected for cytokine analysis by V-plex mouse pro-inflammatory panel multiplex ELISA. Colonic explants from mice were cultured in RPMI media only (untreated) or in presence of NPY receptor antagonists BIBP (100 µM) or BIIE (100 µM) for 24 hours, and cytokine release into the supernatants was expressed as percentage. Changes in serum (A) TNF, (B) IL-6, (C) IL-12p70, (D) IL-5, (E) IFN-ϒ, (F) KC, and (G) IL-10. The ex vivo production of TNF from colon explant culture is given in (H). Values are mean ± SEM from 2 independent experiments, n = 6 (n = 3 per group per experiment), analyzed in duplicate. For ex vivo colon experiments, n = 3. One-way ANOVA followed by Dunnett multiple comparison test. Significant differences between the DSS group and the DSS+antagonist treated groups is expressed as *P < .05, **P < .01, ***P < .001.
NPY1R Antagonist Significantly Reduced Serum IL-6 Levels and Histological Damage in T-Cell Transfer Colitis
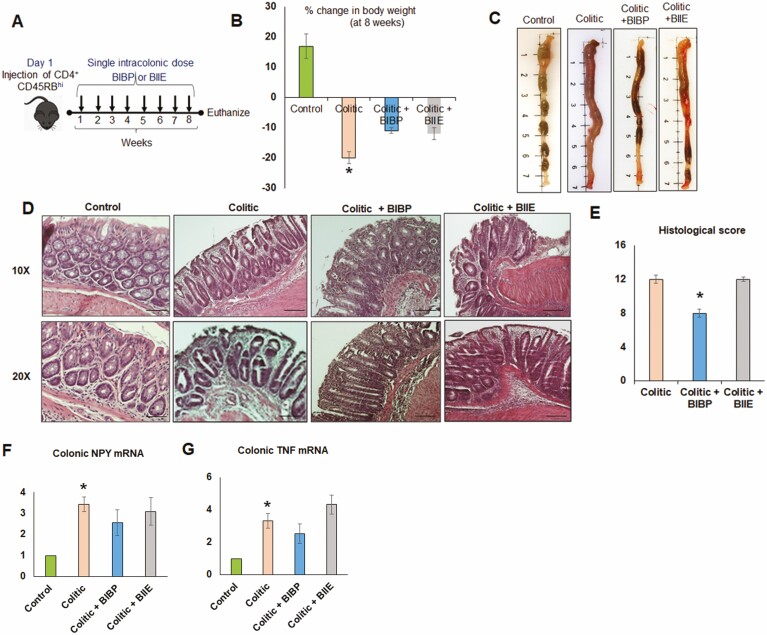
In the adoptive T-cell transfer model of colitis, BIBP or BIIE was administered once a week for 8 weeks via intracolonic route (Figure 4A). Both BIBP and BIIE administration resulted in comparable effects on body weight loss (Figure 4B) and colon morphology without significant changes in colon length (Figure 4C). Further histological analysis and comparison of histological scores indicated significant protection with BIBP compared with BIIE (Figure 4D and E). We found that upregulation of colonic NPY mRNA (Figure 4F) and colonic TNF mRNA (Figure 4G) were similar in both the NPY1R and NPY2R antagonist-treated groups, as well as the colitic group.
Figure 4.
NPY1R receptor antagonist (BIBP-3222) significantly reduced serum IL-6 and histological damage in T-cell transfer colitis model. A, RAG1 knockout mice were injected with 0.5 × 106 CD45RB+ high T cells intraperitoneally (day 1). On day 2, the mice received the first dose of NPY Y1 (BIBP-3222) or NPY2R (BIIE-0246) antagonists via intracolonic injections, after which the mice received NPY antagonists once a week up to week 8. Body weights were taken every week initially up to 3 weeks; and starting week 4, mice were monitored twice daily for body weight. At the end of 8 weeks, mice were killed humanely, and tissues were collected. B, Body weight loss at the end of 8 weeks. C, Colon morphology. D, H&E staining showing histological damage and (E) histological score. Real time PCR showing (F) colonic NPY mRNA and (G) colonic TNF mRNA. Values are mean ± SEM, n = 5-6. Magnification 20x, scale bar 50 µm. One-way ANOVA followed by Dunnett multiple comparison test, significant differences compared with the colitic group expressed as *P < .05.
Contrary to the acute DSS model, neither BIBP nor BIIE had any effects on serum TNF levels in the chronic T-cell colitis model (Figure 5A); BIBP induced significant inhibition of serum IL-6 (Figure 5B), and both antagonists downregulated IL-5 (Figure 5C). Other cytokines like IL-1β, KC, IL-10, and IFN-γ were significantly upregulated during colitis but were not affected by antagonists (Figure 5D-G). We also observed that colonic NPY2R:NPY1R ratio was significantly upregulated in the BIBP-treated group compared with colitic and BIIE-treated groups (Figure 5H).
Figure 5.
NPY1R Receptor antagonist (BIBP-3222) significantly reduced serum IL-6 in T-cell transfer colitis model. RAG1 knockout mice were injected with 0.5 × 106 CD45RB+ high T cells intraperitoneally (day 1). On day 2, the mice received the first dose of NPY Y1 (BIBP-3222) or NPY2R (BIIE-0246) antagonists via intracolonic injections (after isoflurane anesthesia), after which the mice received NPY antagonists once a week up to week 8. At the end of 8 weeks, mice were killed humanely, and serum was collected for evaluation of inflammatory markers. Serum levels of (A) TNF, (B) IL-6, (C) IL-5, (D) IL-1β, (E) KC, (F) IL-10, (G) IFN-γ, and (H) colonic NPY2R:NPY1R ratio. Values are mean ± SEM, n = 5-6. One-way ANOVA followed by Dunnett multiple comparison test. Significant differences compared with the colitic group expressed as *P < .05, **P < .01, ***P < .001.
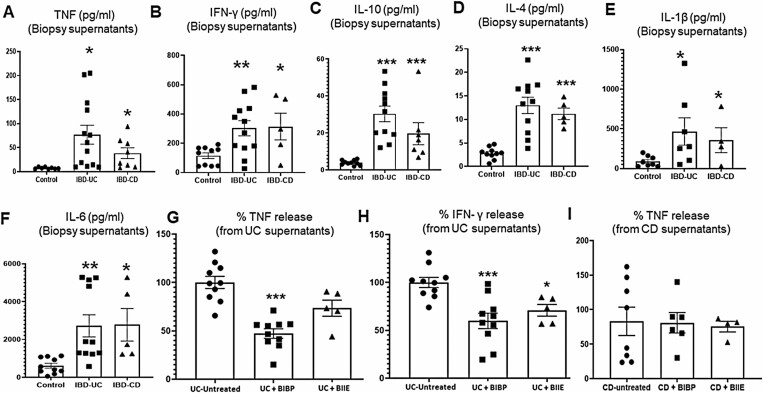
UC Patient Biopsies Incubated With NPY1R Antagonist Ex Vivo Depicted Significant Reduction in TNF and IFN-γ
We next investigated whether NPY receptor inhibition can attenuate the release of pro-inflammatory cytokines from IBD patient biopsies cultured ex vivo. Analysis of UC and CD biopsy supernatants by V-plex human pro-inflammatory multiplex ELISA assay demonstrated significantly higher levels of TNF, IFN-γ, IL-10, IL-4, IL-1β, and IL-6 compared with uninflamed control biopsies from healthy subjects (Figure 6A-F). We tested duplicate biopsies from the same patient and found that NPY1R antagonist (BIBP) treatment significantly reduced TNF release from UC biopsies (Figure 6G, P < .001) compared with untreated inflamed biopsies from the same patient. In addition, BIBP significantly downregulated IFN-γ from UC (Figure 6G, P < .001), whereas BIIE significantly downregulated IFN-γ release from UC biopsies (Figure 6H, P < .05). However, CD biopsies treated with BIBP and BIIE did not show significant changes in TNF release ex vivo (Figure 6I).
Figure 6.
Biopsies from IBD patients incubated with NPY1R antagonist BIBP-3222 ex vivo depicted significant reduction in TNF. Human IBD biopsies obtained from the sigmoid colon were incubated overnight in RPMI media with or without NPY1R (BIBP) or NPY2R antagonist (BIIE; 100uM) at 5% CO2, and supernatants were analyzed for TNF by multiplex ELISA using a human V-Plex pro-inflammatory MSD plate. UC and CD biopsy supernatants depicted enhanced release of (A) TNF, (B) IFN-γ, (C) IL-10, (D) IL-4, (E) IL-1β, and (F) IL-6 (n = 8-12 for control biopsies, n = 12-13 for UC, and n = 5-8 for CD). UC biopsies treated with BIBP showed significant downregulation of (G) TNF, whereas UC biopsies treated with BIBP and BIIE showed significant downregulation of IFN-γ (H). CD biopsies treated with BIBP or BIIE were ineffective in inhibiting TNF release (I); n = 10 for UC + BIBP, n = 5 for UC + BIIE, n = 6 for CD + BIBP, n = 4 for CD + BIIE. One-way ANOVA followed by Dunnett multiple comparison test was used to compare cytokine release in control, UC and CD biopsies. Paired t test was used to compare cytokine release between untreated and antagonist treated biopsies. Mean ± SEM, *P < .05, **P < .01, ***P < .001.
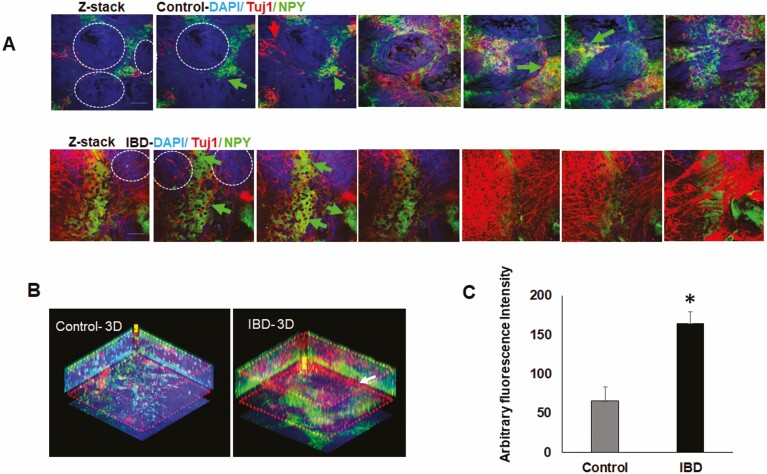
Confocal imaging of CLARITY-cleared patient biopsies revealed their rich innervation as seen from pan neuronal marker Tuj1 (β-3-tubulin) staining. Further, the control biopsies showed well-defined crypt architecture (crypt outlined by white dashed lines) compared with IBD biopsies (Supplemental Figure 2A). We observed enhanced NPY immunoreactivity in the z-stack images captured from inflamed colon biopsies compared with control biopsies (Figure 7A). The confocal z-stacks were used to reconstruct a 3D image (Figure 7B), and differences in NPY immunoreactivity were graphically expressed in arbitrary fluorescence units (Figure 7C) by Image J.
Figure 7.
CLARITY staining revealed significant increase in NPY immunoreactivity in inflamed biopsies. Human IBD biopsies obtained from the sigmoid colon were fixed in p-formaldehyde and processed for CLARITY as described in Methods. The cleared biopsies were stained with antibodies to NPY and neuronal marker Tuj1. Images were acquired with a confocal microscope and arbitrary fluorescence intensity assessed using Image J. A, Confocal z-stacks images of CLARITY-cleared biopsies stained for pan neuronal marker Tuj1 and NPY in control and IBD biopsies, white dashed circles show the colonic epithelial crypt in cross section, red arrows indicate Tuj1 positive neurons, and the green arrows show NPY positive neurons (B) 3D image constructed from z-stacks depicting higher intensity of NPY staining in inflamed tissue, (C) graphical representation of arbitrary NPY staining intensity using Image J. Magnification 40 ×, scale bar 50 µm. Values are mean ± S.E.M, n = 3 (control) and n = 4 (IBD). *P < .05.
Discussion
Neuronal regulation of immune functions at mucosal barriers via NPY receptors represent an important, evolutionarily, ancient pathway for host defense in species ranging from nematodes to mammals.36 Neuropeptide Y plays a direct and critical role in gastrointestinal immunity, as NPY-releasing neurons are found in proximity with enteric immune cells that harbor NPY1R (T cells and antigen-presenting cells [APC]) and NPY2R (T cells) receptors.15,37 Furthermore, NPY1R and NPY2R receptors are also present on enteric neurons, enabling a crucial enteric neuron-immune cross-talk in the gut.6
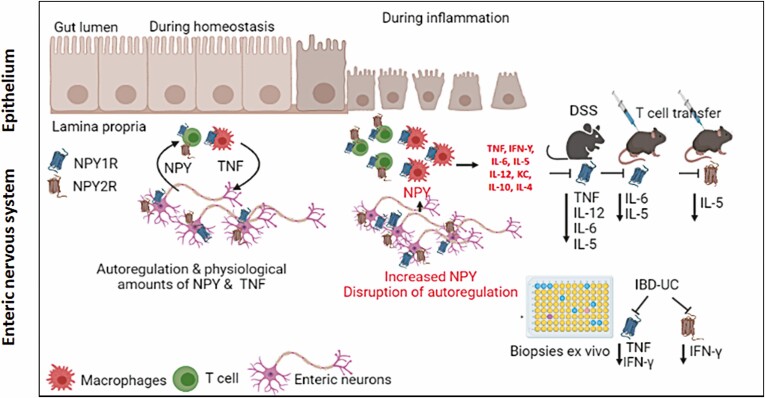
Under homeostasis, enteric neurons are the major producers of NPY, which, when produced in physiological amounts, stimulates enteric immune cells to produce TNF, which subsequently regulates enteric neuronal development, growth, and differentiation.5 However, during inflammation, extra neuronal NPY production induced from immune cells38 can fuel into the enteric neuron-immune feedback loop, resulting in imbalances in cytokine production, leading to intestinal inflammation (Figure 8).
Figure 8.
Graphical Abstract (illustrated using Biorender). At homeostasis, ENS is the major producer of NPY, and NPY is regulated via auto feedback regulation via Y1 and Y2 receptors in the ENS. NPY from ENS binds to Y1 receptors on enteric immune cells and induces TNF production. During inflammation, in addition to ENS, enteric immune cells also produce NPY, which binds to immune cells and induce more TNF via Y1. Hence, with intracolonic NPY1R inhibition using BIBP, we observed reduction of TNF release, and other cytokines like IL-12, IL-6, and IL-5 (DSS and adoptive T-cell transfer colitis), and TNF and IFN-ϒ (UC biopsies). However, Y2 receptor on ENS and T cells mediate feedback regulation of NPY production, and Y2 inhibition by BIIE caused reduction of IL-5 (adoptive T-cell transfer colitis) and IFN-ϒ (UC biopsies).
We and others have shown that NPY exerts a pro-inflammatory function in the mouse intestine.3,5,39,40 Our studies have also demonstrated an upregulation of NPY in human IBD.5 Several studies have tested NPY antagonists under systemic administration, yielding contrasting results in mouse models.41 This is because NPY has a bimodal role on immune cells and can differentially regulate immune responses based on the type of immune cells involved, such as antigen-presenting cells vs T cells39 and, due to unintended inhibition of NPY receptors, other various nonimmune cell types like epithelial and endothelial cells. Because NPY receptors are highly expressed in the colon, we investigated the potential of localized intraluminal administration of NPY1R (BIBP) and NPY2R (BIIE) antagonists via intracolonic injections in mouse models of DSS and T-cell colitis, and on human IBD biopsies ex vivo.
We observed significant protection with BIBP-mediated NPY1R inhibition in acute murine DSS colitis model, with a significant decrease in clinical, endoscopic, and histological scores, significant reduction in serum TNF, IL-12, and IL-6, suggestive of inhibition of macrophage-derived Th1 responses. However, NPY2R inhibition with BIIE did not offer protection in the DSS model. We also noted that NPY antagonists did not alter colonic NPY expression. Interestingly, we also observed that colonic explants from normal healthy mice treated ex vivo with BIBP also displayed significant reduction in TNF levels compared with BIIE.
Our study reveals a delicate balance between the NPY1R:NPY2R ratio, which is distorted during inflammation.9,42 Under homeostasis, the receptor ratio is coordinated so that NPY2R-mediated NPY production from enteric neurons (the main producers), immune cells (minimal production during homeostasis), and cytokine release from immune cells (via Y1) are in perfect balance via a regulatory feedback loop. But onset of inflammation triggers enhanced cytokine production and NPY upregulation from enteric neurons and immune cells,38 leading to distortion of the NPY1R:NPY2R ratio.
In acute DSS colitis, the main immune cell types involved are macrophages that harbor Y1 receptors.43 Y2 receptors regulate presynaptic NPY release from enteric neurons and T cells. We observed a decrease in Y2 expression with DSS, which could be an inherent compensatory mechanism of receptor desensitization in response to inflammation-induced NPY upregulation from enteric neurons4,5 or immune cells.38 Hence, we observed that NPY1R inhibition with BIBP was protective due to significant inhibition of macrophage-derived serum cytokines like TNF, IL-6, and IL-12. In addition, BIBP treatment prevented the DSS-induced decrease in NPY2R, suggesting that breach of epithelial barrier by DSS and activation of Y1 on mucosal macrophages may precede upregulation of NPY. Taken together, BIBP treatment induced NPY1R inhibition, attenuated intestinal inflammation by downregulating cytokines, and prevented DSS-induced Y2 downregulation, thus significantly reducing the Y1:Y2 ratio (or increase in NPY2R:NPY1R ratio). However, inhibiting NPY2R receptor with BIIE was not effective in downregulating cytokines, as inflammation-induced downregulation of Y2 served to upregulate the NPY1R:NPY2R ratio.
In the adoptive T-cell transfer colitis model, induced by transfer of CD4+ T cells into the immune deficient RAG1 knockout host, NPY1R inhibition offered significant protection compared with NPY2R inhibition. Although, both NPY1R and NPY2R inhibition had similar effects on body weight loss (10% weight loss) compared with 20% in untreated RAG knockout colitic mice; BIBP-treated mice showed significant histological protection, with significant reduction in serum IL-6 levels compared with untreated colitic mice. In contrast, BIIE treatment did not impart histological protection and failed to downregulate serum IL-6 levels. However, serum TNF levels showed similar upregulation in both antagonist-treated and untreated colitic groups. Similar to the DSS model, here we noted colonic NPY upregulation in colitic and antagonist-treated mice, which may be attributed to enhanced NPY production from immune cells in the context of chronic inflammation.38
The T-cell colitis is a highly complex systemic model and involves precise APC priming to activate T cells, implying the significance of sequential activation of Y1 on APC and T cells.39 Our data suggest that downregulating Y1 receptor using BIBP offered enhanced protection, as it helped downregulate a crucial cytokine like IL-6 in this model by more than 50%. Blocking Y2 receptor with BIIE was ineffective in downregulating IL-6, leading to histological damage similar to that in untreated colitic mice. Compared with the acute DSS colitis model, it was harder for us to fine tune and accomplish NPY receptor inhibition in the systemic T-cell transfer model with a chronic timeline of 8 weeks. We speculate that optimizing the time points at which Y1 and Y2 antagonists were administered during the 8-week timeline or co-administration of both antagonists might have resulted in enhanced protective effects.
Data from both the murine colitis models suggest that inhibiting Y1 offered more protection, as Y1-mediated events possibly precede Y2-mediated NPY upregulation, thus ultimately yielding a higher NPY2R:NPY1R ratio.
Most importantly, our study demonstrated that NPY1R antagonist significantly reduced TNF and IFN-γ release from UC biopsies ex vivo, whereas NPY2R antagonist downregulated IFN-γ release. Confocal 3D images from CLARITY-cleared biopsies revealed their rich innervation and significant increase in NPY immunoreactivity, suggestive of enhanced NPY secretion in the IBD colon. These data suggest that localized blocking of NPY1R signaling might be beneficial in UC, as it downregulates 2 crucial cytokines—TNF and IFN-γ.
Our data have significant translational value, as it has been demonstrated that BIBP has high selective binding affinity for the human Y1 receptor and shares an overlapping binding site with NPY.17,18 Our findings demonstrate that NPY1R inhibition might offer therapeutic value to improve the quality of life of UC patients, whereby a localized delivery of Y1 antagonist by enema could help alleviate colonic inflammation via downregulation of TNF and IFN-γ.
Our study reveals a dynamic dialogue between enteric NPY and systemic cytokines, the crucial role of NPY receptors, and the potential of NPY1R inhibition in managing and treating UC. A systematic study using biopsies from UC and CD patients investigating the relative distribution of Y1 and Y2 receptors on immune cells during the active phase and remission would be crucial in translating the findings to the clinic.
Supplementary Material
Author Contributions
B.C. conceived the research concept; B.C. and A.S.N. designed the research studies and provided the reagents; B.C., D.B., A.W., J.O., and B.S. conducted the experiments and acquired and analyzed the data. T.D. and H.I. performed colonoscopy and biopsy collection, B.C. and A.S.N. analyzed the data and drafted the manuscript; and all authors provided insight into the manuscript.
Funding
This research project was supported in part by the Emory University Integrated Cellular Imaging Core. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of Health.
Conflicts of Interest
None.
References
- 1. Bedoui S, von Hörsten S, Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides. 2007;28:373–376. [DOI] [PubMed] [Google Scholar]
- 2. Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides. 2007;28:453–458. [DOI] [PubMed] [Google Scholar]
- 3. Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem. 2007;7:1743–1752. [DOI] [PubMed] [Google Scholar]
- 4. Chandrasekharan B, Bala V, Kolachala VL, et al. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. Plos One. 2008;3:e3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandrasekharan B, Jeppsson S, Pienkowski S, et al. Tumor necrosis factor-neuropeptide Y cross-talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis. 2013;19:2535–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedoui S, Kawamura N, Straub RH, et al. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Di Giovangiulio M, Verheijden S, Bosmans G, et al. the neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front Immunol. 2015;6:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Salhy M, Hausken T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD). Neuropeptides. 2016;55:137–144. [DOI] [PubMed] [Google Scholar]
- 9. Taylor BK, Fu W, Kuphal KE, et al. Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons. Neuroscience. 2014;256:178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elitsur Y, Luk GD, Colberg M, et al. Neuropeptide Y (NPY) enhances proliferation of human colonic lamina propria lymphocytes. Neuropeptides. 1994;26:289–295. [DOI] [PubMed] [Google Scholar]
- 11. Dimitrijević M, Stanojević S, Vujić V, et al. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regul Pept. 2005;124:163–172. [DOI] [PubMed] [Google Scholar]
- 12. Dimitrijević M, Stanojević S, Mitić K, et al. The anti-inflammatory effect of neuropeptide Y (NPY) in rats is dependent on dipeptidyl peptidase 4 (DP4) activity and age. Peptides. 2008;29:2179–2187. [DOI] [PubMed] [Google Scholar]
- 13. Kostić S, Puljak L, Sapunar D. Attenuation of pain-related behaviour evoked by carrageenan injection through blockade of neuropeptide Y Y1 and Y2 receptors. Eur J Pain. 2013;17:493–504. [DOI] [PubMed] [Google Scholar]
- 14. Tough IR, Forbes S, Tolhurst R, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br J Pharmacol. 2011;164:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bedoui S, Kromer A, Gebhardt T, et al. Neuropeptide Y receptor-specifically modulates human neutrophil function. J Neuroimmunol. 2008;195:88–95. [DOI] [PubMed] [Google Scholar]
- 16. Bedoui S, Miyake S, Lin Y, et al. Neuropeptide Y (NPY) suppresses experimental autoimmune encephalomyelitis: NPY1 receptor-specific inhibition of autoreactive Th1 responses in vivo. J Immunol. 2003;171:3451–3458. [DOI] [PubMed] [Google Scholar]
- 17. Sautel M, Rudolf K, Wittneben H, et al. Neuropeptide Y and the nonpeptide antagonist BIBP 3226 share an overlapping binding site at the human Y1 receptor. Mol Pharmacol. 1996;50:285–292. [PubMed] [Google Scholar]
- 18. Rudolf K, Eberlein W, Engel W, et al. The first highly potent and selective nonpeptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol. 1994;271:R11–R13. [DOI] [PubMed] [Google Scholar]
- 19. Doods HN, Wienen W, Entzeroth M, et al. Pharmacological characterization of the selective nonpeptide neuropeptide Y Y1 receptor antagonist BIBP 3226. J Pharmacol Exp Ther. 1995;275:136–142. [PubMed] [Google Scholar]
- 20. Doods HN, Wieland HA, Engel W, et al. BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul Pept. 1996;65:71–77. [DOI] [PubMed] [Google Scholar]
- 21. Doods H, Gaida W, Wieland HA, et al. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–R5. [DOI] [PubMed] [Google Scholar]
- 22. Dumont Y, Cadieux A, Doods H, et al. BIIE0246, a potent and highly selective nonpeptide neuropeptide Y Y(2) receptor antagonist. Br J Pharmacol. 2000;129:1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ailanen L, Vähätalo LH, Salomäki-Myftari H, et al. Peripherally administered Y2-receptor antagonist BIIE0246 prevents diet-induced obesity in mice with excess neuropeptide Y, but enhances obesity in control mice. Front Pharmacol. 2018;9:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gustot T, Lemmers A, Louis E, et al. Profile of soluble cytokine receptors in Crohn’s disease. Gut. 2005;54:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vadstrup K, Galsgaard ED, Gerwien J, et al. Validation and optimization of an ex vivo assay of intestinal mucosal biopsies in Crohn’s disease: reflects inflammation and drug effects. Plos One. 2016;11:e0155335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loganes C, Valencic E, Pin A, et al. Ex vivo response to mucosal bacteria and muramyl dipeptide in inflammatory bowel disease. World J Gastroenterol. 2016;22:9734–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swanson KD, Theodorou E, Kokkotou E. Reproducing the human mucosal environment ex vivo: inflammatory bowel disease as a paradigm. Curr Opin Gastroenterol. 2018;34:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolfarth AA, Liu X, Darby TM, et al. Proline-rich acidic protein 1 (PRAP1) protects the gastrointestinal epithelium from irradiation-induced apoptosis. Cell Mol Gastroenterol Hepatol. 2020;10:713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandrasekharan B, Saeedi BJ, Alam A, et al. Interactions between commensal bacteria and enteric neurons, via FPR1 induction of ROS, increase gastrointestinal motility in mice. Gastroenterology. 2019;157:179–192.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cenac N, Garcia-Villar R, Ferrier L, et al. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. [DOI] [PubMed] [Google Scholar]
- 31. Lecci A, Carini F, Tramontana M, et al. Nepadutant pharmacokinetics and dose-effect relationships as tachykinin NK2 receptor antagonist are altered by intestinal inflammation in rodent models. J Pharmacol Exp Ther. 2001;299:247–254. [PubMed] [Google Scholar]
- 32. Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487–7491. [DOI] [PubMed] [Google Scholar]
- 34. Alam A, Leoni G, Wentworth CC, et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kodani T, Rodriguez-Palacios A, Corridoni D, et al. Flexible colonoscopy in mice to evaluate the severity of colitis and colorectal tumors using a validated endoscopic scoring system. J Vis Exp. 2013:e50843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wani KA, Goswamy D, Irazoqui JE. Nervous system control of intestinal host defense in C. elegans. Curr Opin Neurobiol. 2020;62:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dimitrijević M, Stanojević S. The intriguing mission of neuropeptide Y in the immune system. Amino Acids. 2013;45:41–53. [DOI] [PubMed] [Google Scholar]
- 38. Schwarz H, Villiger PM, von Kempis J, Lotz M. Neuropeptide Y is an inducible gene in the human immune system. J Neuroimmunol. 1994;51:53–61. [DOI] [PubMed] [Google Scholar]
- 39. Wheway J, Mackay CR, Newton RA, et al. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G550–G556. [DOI] [PubMed] [Google Scholar]
- 41. Ruiz HH, Becker S, Bai Y, et al. Pharmacological inhibition of NPY receptors illustrates dissociable features of experimental colitis in the mouse DSS model: implications for preclinical evaluation of efficacy in an inflammatory bowel disease model. Plos One. 2019;14:e0220156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin Q, Zou X, Ren Y, et al. Involvement of peripheral neuropeptide Y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience. 2004;123:337–347. [DOI] [PubMed] [Google Scholar]
- 43. Krieglstein CF, Cerwinka WH, Sprague AG, et al. Collagen-binding integrin alpha1beta1 regulates intestinal inflammation in experimental colitis. J Clin Invest. 2002;110:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.