Abstract
The mucilage surrounding hydrated Arabidopsis thaliana seeds is a specialized extracellular matrix composed mainly of the pectic polysaccharide rhamnogalacturonan I (RG-I). Although, several genes responsible for RG-I biosynthesis have been identified, the transcriptional regulatory mechanisms controlling RG-I production remain largely unknown. Here we report that the trihelix transcription factor DE1 BINDING FACTOR 1 (DF1) is a key regulator of mucilage RG-I biosynthesis. RG-I biosynthesis is significantly reduced in loss-of-function mutants of DF1. DF1 physically interacts with GLABRA2 (GL2) and both proteins transcriptionally regulate the expression of the RG-I biosynthesis genes MUCILAGE MODIFIED 4 (MUM4) and GALACTURONOSYLTRANSFERASE-LIKE5 (GATL5). Through chromatin immunoprecipitation-quantitative PCR and transcriptional activation assays, we uncover a cooperative mechanism of the DF1–GL2 module in activating MUM4 and GATL5 expression, in which DF1 binds to the promoters of MUM4 and GATL5 through interacting with GL2 and facilitates the transcriptional activity of GL2. The expression of DF1 and GL2 is directly regulated by TRANSPARENT TESTA GLABRA2 (TTG2) and, in turn, DF1 directly represses the expression of TTG2. Taken together, our data reveal that the transcriptional regulation of mucilage RG-I biosynthesis involves a regulatory module, comprising DF1, GL2, and TTG2.
Two transcription factors physically interact and cooperatively regulate Rhamnogalacturonan-I biosynthesis in Arabidopsis seed coat mucilage.
IN A NUTSHELL.
Background: Pectin is a complex cell wall polysaccharide that plays an important role in modulating cell adhesion, cell morphogenesis, and growth. The Arabidopsis seed coat mucilage is a specialized cell wall mainly composed of rhamnogalacturonan-I (RG-I; ∼90%), a type of pectic polysaccharide, making seed mucilage an ideal model system for studying the synthesis and regulation of pectin polysaccharides. Several genes involved in pectin synthesis have been identified, but the upstream transcription factors governing pectin synthesis and their regulatory mechanisms remain largely unknown.
Question: How is the biosynthesis of pectin transcriptionally regulated?
Findings: RG-I biosynthesis is significantly reduced in loss-of-function mutants of the trihelix transcription factor DE1 BINDING FACTOR 1 (DF1). DF1 physically interacts with GLABRA2 (GL2). The DF1–GL2 module cooperatively regulates the expression of the RG-I biosynthesis genes MUCILAGE MODIFIED 4 (MUM4) and GALACTURONOSYLTRANSFERASE-LIKE5 (GATL5). DF1 binds to the promoters of MUM4 and GATL5 through interacting with GL2 and facilitates the transcriptional activity of GL2. In addition, the expression of DF1 and GL2 is directly regulated by TRANSPARENT TESTA GLABRA2 (TTG2) and, in turn, DF1 directly represses the expression of TTG2. Altogether, our study uncovers the transcriptional regulation mechanism of mucilage RG-I biosynthesis dominated by the module comprising DF1, GL2, and TTG2.
Next steps: We are currently working to discover additional transcriptional regulators involved in pectic polysaccharides biosynthesis in the Arabidopsis seed coat mucilage. Further study will be focused on dissecting the transcriptional regulation networks and underlying mechanisms governing pectin biosynthesis.
Introduction
The mucilage surrounding hydrated Arabidopsis thaliana seeds is a specialized extracellular matrix synthesized by the outermost seed coat cells (mucilage secretory cells [MSCs]) (Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000). In mature dry seeds, the dehydrated mucilage is deposited between the radial cell wall and the secondary cell wall called columella. The imbibition of water triggers a rapid extrusion of mucilage, which then forms a gelatinous capsule around the seed. This capsule has a compact inner layer (adherent mucilage [AM]) that is attached to the seed surface and a diffuse outer layer (nonadherent mucilage [NM]) that is readily detached from the seed (Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000).
The pectic polysaccharide rhamnogalacturonan I (RG-I) accounts for ∼90% of the mucilage produced by Arabidopsis MSCs. Small amounts of homogalacturonan (HG), cellulose, and hemicelluloses are also present (Macquet et al., 2007; Voiniciuc et al., 2015). RG-I contains galacturonic acid (GalA) and rhamnose (Rha) glycosidically linked together in the repeating disaccharide unit [→4)-α-d-GalpA-(1→2)-α-l-Rhap-(1→]. HG is a linear polymer consisting of 1,4-linked α-d-GalA residues (Mohnen, 2008). Arabidopsis seed coat mucilage is now recognized as a model system for studying factors that regulate the biosynthesis and metabolism of pectin and other plant polysaccharides (Arsovski et al., 2010; North et al., 2014).
Numerous genes required for RG-I biosynthesis have been identified using the Arabidopsis seed coat mucilage system. For example, UDP-URONIC ACID TRANSPORTER1 (UUAT1) encodes a Golgi-localized protein that transports UDP-GlcA into the Golgi lumen. UDP-GlcA is itself a precursor for several other nucleotide sugars including UDP-GalA that are required for pectin biosynthesis (Saez-Aguayo et al., 2017). MUCILAGE-MODIFIED 4 (MUM4)/RHAMNOSE SYNTHESIS2 encodes a UDP-l-Rha synthase, which generates the UDP-l-Rha required for RG-I backbone biosynthesis (Usadel et al., 2004; Western et al., 2004; Oka et al., 2007). The glycosyltransferase that transfers the Rha residue from UDP-l-Rha to the RG-I backbone is encoded by RG-I:RHAMNOSYLTRANSFERASE1 (RRT1; Takenaka et al., 2018). Several other genes including GALACTURONOSYLTRANSFERASE11 (GAUT11) and MUCILAGE-RELATED 70 (MUCI70) are believed to be required for the biosynthesis of distinct domains of seed coat mucilage RG-I (Voiniciuc et al., 2018). GALACTURONOSYLTRANSFERASE-LIKE5 (GATL5) is also involved in the biosynthesis of RG-I and/or HG in Arabidopsis seed coat mucilage (Kong et al., 2013). Several of these genes have been shown to be transcriptionally regulated by upstream transcription factors (TFs). For example, MUM4 expression is regulated by APETALA2 (AP2), TRANSPARENT TESTA GLABRA1 (TTG1), and GL2, which are key regulators of the differentiation of seed coat cells (Jofuku et al., 1994; Western et al., 2001, 2004). GATL5 expression is also regulated by AP2, TTG1, GL2, and TTG2 (Kong et al., 2013). However, it has not been established whether these transcriptional regulations involve direct or indirect interactions.
A member of the trihelix family of TFs, DE1 BINDING FACTOR 1 (DF1), which is co-expressed with GL2, has been proposed to be involved in regulating mucilage production (Vasilevski et al., 2012; Voiniciuc et al., 2015). However, the functional mode of DF1 was not established. Here, we demonstrate that DF1 is a key regulator of RG-I biosynthesis in Arabidopsis seed coat mucilage. Our biochemical and genetic analyses show that DF1 physically interacts with GL2 to cooperatively promote RG-I biosynthesis. We also uncover the cooperative mechanism of the DF1–GL2 module in activating their direct target genes MUM4 and GATL5. In addition, we provide evidence for a negative feedback loop between DF1 and TTG2 at the transcriptional level. Our findings provide insight into the molecular mechanisms of the DF1–GL2 module in regulating seed coat mucilage RG-I biosynthesis and increase our understanding of the transcriptional regulation network involved in plant pectin biosynthesis.
Results
Expression of DF1 in the seed coat coincides with mucilage biosynthesis
The expression of DF1 in various tissues and organs was examined by reverse transcription quantitative PCR (RT-qPCR) analysis. The transcript abundance of DF1 was relatively higher in reproductive tissues (floral bud, flower, and silique) than in vegetative tissues (leaf, root, and stem) (Supplemental Figure S1A). To further investigate the tissue-specific expression pattern of DF1, we generated ProDF1:GUS transgenic Arabidopsis lines, in which the β-glucosidase (GUS) reporter gene was driven by the native DF1 promoter. GUS signals were detected in various tissues including leaves, roots, anther filaments, stigma, sepals, siliques, and developing seeds (Supplemental Figure S1, B–F). Most notably, strong GUS signals were detected in seed coat cells at 7–10 days post anthesis (DPA), a time coinciding with mucilage biosynthesis (Supplemental Figure S1G). We then performed in situ hybridization assays with sections from 4 DPA to 13 DPA seeds to obtain a spatiotemporal expression pattern for DF1. The antisense signals of the DF1 transcript were readily detected in MSCs at 4 and 7 DPA, whereas no signal was discernible at 10 and 13 DPA (Figure 1A). Thus, DF1 is expressed in MSCs largely during the early stages of mucilage biosynthesis.
Figure 1.
DF1 is required for mucilage biosynthesis. A, In situ hybridization detection of DF1 transcript in seed coat epidermal cells at 4, 7, 10, and 13 DPA. Scale bars = 50 μm. B, Release of mucilage in water containing 0.01% RR. Scale bars = 150 μm. C, Staining of the AM of WT and df1 seeds with 0.01% RR after shaking at 200 rpm for 1 h. Scale bars = 150 μm. D, Quantification of mucilage volumes after shaking at 200 rpm for 1 h. Data represent means ± standard deviation (sd) of 10 seeds. Means marked with different letters are significantly different according to the one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test (P < 0.05). E, Quantification of mucilage contents. Data represent means ± sd of three biological replicates. Means marked with different letters are significantly different based on the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05). F–M, Cross sections of the WT and df1-1 developing seeds at 4 DPA (F and G), 7 DPA (H and I), 10 DPA (J and K), and 13 DPA (L and M). Note the differences in the position of starch granules (J and K), the shape and size of mucilage pocket, and the shape of columella (L and M) between WT and df1-1. SG, starch granule; Mu, mucilage; Co, columella. Scale bars = 20 μm.
Mucilage biosynthesis is reduced in df1 seeds
Two homozygous loss-of-function mutants of DF1 (i.e. df1-1 and df1-2) were previously reported to have defects in mucilage extrusion or biosynthesis (Vasilevski et al., 2012; Voiniciuc et al., 2015). We re-examined the seeds of df1-1 and df1-2 using ruthenium red (RR) staining to clarify the effect of DF1 on mucilage production. Much less mucilage was released from the hydrated df1-1 and df1-2 seeds than from their wild-type (WT) counterparts (Figure 1B). The AM layer of df1 was significantly thinner than WT after shaking at 200 rpm for 1 h to remove the NM (Figure 1, C and D). Quantification of the mucilage revealed that both the NM and AM were decreased by ∼50% in the df1 mutants (Figure 1E). Since df1-1 and df1-2 have similar mucilage defect phenotypes, only df1-1 was used in our subsequent studies.
We next compared the morphology of MSCs in df1-1 and WT seeds during seed coat cell differentiation and mucilage production. df1-1 and WT MSCs had similar morphologies at the early stages (4–7 DPA) of seed coat differentiation. df1-1 and WT cells rapidly expanded in size and both formed and accumulated starch granules in a similar pattern (Figure 1, F–I). However, at the later developmental stages there were discernible differences between df1-1 and WT. At 10 DPA, WT MSCs deposited a substantial amount of mucilage and a narrow cytoplasmic column was formed in the center of the cells (Figure 1J). In contrast, the cytoplasm of df1-1 MSCs was located toward the bottom of the cells, rather than as a central columnar shape (Figure 1K). The mucilage pockets of WT MSCs at 13 DPA were plump and stained darkly with toluidine blue, and the volcano-shaped columellae were formed surrounding the cytoplasmic column (Figure 1L). In contrast, the mucilage pockets of df1-1 MSCs were relatively flattened in shape and smaller in size, and much less mucilage was deposited. In addition, the columella was wider and flattened compared to WT (Figure 1M).
Treating Arabidopsis seeds with ethylene diamine tetraacetic acid (EDTA) is known to enhance mucilage extrusion (Rautengarten et al., 2008; Arsovski et al., 2009; Saez-Aguayo et al., 2013; Voiniciuc et al., 2013). Thus, we treated df1 seeds with 50 mM EDTA for 1 h prior to RR staining to determine if WT amounts of mucilage were produced but not completely extruded after imbibition of water. No increase in the abundance of the extruded mucilage was observed on df1 seeds (Supplemental Figure S2). These results suggest that the mucilage defects in df1 seeds are due to reduced production of mucilage rather than compromised mucilage extrusion. Collectively, our observations confirm that disrupting DF1 leads to a significant reduction in mucilage biosynthesis and to substantial alterations in columella morphology in the MSCs.
RG-I and cellulose contents are decreased in the df1 mucilage
We used high-performance liquid chromatography to determine if the amounts and types of monosaccharides differ in the df1-1 and WT seed coat mucilage. Our data showed that the contents of Rha and GalA in the df1-1 mucilage decreased by 60% and 50%, respectively, compared with WT (Figure 2A). The contents of other monosaccharides in mucilage of df1-1 and WT were similar. Since RG-I is composed of Rha and GalA, these results suggest that the abundance of RG-I is decreased in the df1-1 mucilage. To confirm this, we performed whole mount immunolabeling assays of the df1-1 and WT mucilage with the CCRC-M36 antibody that specifically recognizes RG-I (Pattathil et al., 2010). CCRC-M36 labeling was substantially reduced in df1-1 relative to the WT (Figure 2B). The results of enzyme-linked immunosorbent assay (ELISA) of total mucilage using CCRC-M36 also revealed a significant reduction of RG-I in the df1-1 mucilage (Figure 2C).
Figure 2.
RG-I content is reduced in the df1 mucilage. A, Quantification of monosaccharide compositions in the WT and df1-1 total mucilage. Data represent means ± sd of three independent replicates. *P < 0.01; ns, not significant; Student’s t test. B, Whole mount immunolabeling of RG-I with the CCRC-M36 antibody in the WT and df1-1 mucilage. The cellulosic ray structure was counter stained with Calcofluor white, a fluorescent dye that binds to β-glycans. Scale bars = 200 μm. Three independent experiments (each with >20 seeds) were performed and similar results were obtained. C, ELISA of total mucilage from the WT and df1-1 seeds with the CCRC-M36 antibody. Data represent means ± sd of three biological replicates. **P < 0.01, Student’s t test. D, GalA:Rha molar ratio in the WT and df1-1 total mucilage. Data represent means ± sd of three biological replicates. Student’s t test.
To assess if the HG content of the df1-1 mucilage is altered, we compared the molar ratios of GalA and Rha in the df1-1 and WT mucilage. There was a small, but not statistically significant, increase in the GalA:Rha ratio in the df1-1 mucilage (Figure 2D), suggesting that the HG content of the df1-1 mucilage does not differ from WT. We then immunolabeled df1-1 and WT seeds with monoclonal antibodies that recognize moderately methylesterified HG (JIM5), highly methylesterified HG (JIM7), and sparsely methylesterified HG (CCRC-M38) (Willats et al., 2000; Pattathil et al., 2010). The fluorescence labeling areas of JIM5 and JIM7 were considerably reduced in df1-1 seeds, which is likely due to the reduced mucilage thickness of df1-1 (Supplemental Figure S3, A and B). However, the signal intensity of CCRC-M38, which labels the inner region of the AM, was increased in df1-1 (Supplemental Figure S3C). ELISA also showed that the signals of JIM5 and JIM7 were significantly decreased, while the CCRC-M38 signal was markedly increased in the df1-1 mucilage compared to the WT (Supplemental Figure S3D). These results suggest that the distribution of HGs with different degrees of methylesterification might be altered in the df1-1 mucilage.
We also observed reduced staining of the df1-1 mucilage when the seeds were counter stained with Calcofluor white or pontamine fast scarlet 4B (S4B) (Figure 2B;Supplemental Figure S4A), indicating that the amounts of cellulose are reduced. This was supported by a decrease in labeling of df1-1 relative to the WT with a family 3 carbohydrate-binding module (CBM3a) that recognizes crystalline cellulose (Supplemental Figure S4A). Examination of df1-1 seeds under polarized light showed a thin halo with a relatively weak signal, whereas WT seeds had a thick and bright halo with visibly discernible rays of crystalline cellulose (Supplemental Figure S4B). We also quantified the cellulose content and the results showed that the crystalline cellulose content was dramatically reduced in the df1-1 mucilage (∼2.0 mg g−1 seed) compared to that in the WT (∼4.5 mg g−1 seed) (Supplemental Figure S4C). These results demonstrate that cellulose content is reduced in the df1-1 mucilage.
DF1 physically interacts with GL2
GL2 is a key regulator governing MSC differentiation and mucilage biosynthesis (Western et al., 2001; Shi et al., 2012). Previous reports have shown that GL2 is expressed in the epidermal layer of seed coats during mucilage biosynthesis (Gonzalez et al., 2009) and that DF1 is co-expressed with GL2 (Vasilevski et al., 2012). We have provided evidence that DF1 expression in the seed coat coincides with mucilage biosynthesis (Figure 1A;Supplemental Figure S1G). This prompted us to determine if DF1 and GL2 interact to regulate mucilage biosynthesis. First, we examined the interaction using yeast two-hybrid (Y2H) system. Co-expression of DF1-fused GAL4 DNA binding domain (BD) and GL2-fused activation domain (AD) resulted in expression of the reporter genes HIS3, ADE2, and MEL1, suggesting that DF1 and GL2 physically interact in yeast (Figure 3A). We then performed bimolecular fluorescence complementation (BiFC) assays to examine this interaction in vivo. A strong fluorescence signal was detected in the cells co-transformed with N-terminus of YFP (nYFP)-DF1 and C-terminus of YFP (cYFP)-GL2 plasmids. A plasmid (35S:NLS-mCherry) containing a marker for the nucleus was co-transformed to indicate the position of the nuclei. The YFP signal resulting from the DF1–GL2 interaction overlapped with the fluorescence signal of 35S:NLS-mCherry, suggesting that DF1 and GL2 interact in the nucleus. No YFP signal was detected when the negative control BEL1-Like HOMEODOMAIN 2 (BLH2) was co-transformed with DF1 or GL2 (Figure 3B). A co-immunoprecipitation (Co-IP) experiment was then performed to confirm that DF1 and GL2 interact in vivo. MYC-tagged DF1 was co-expressed in Nicotiana benthamiana leaves with HA-tagged GL2 or with the HA tag alone. We then used an anti-HA antibody to precipitate the protein complex. MYC-DF1 was co-immunoprecipitated in the presence of GL2-HA but not with the control HA tag (Figure 3C). Taken together, these results provide evidence that DF1 physically interacts with GL2.
Figure 3.
DF1 physically interacts with GL2. A, Y2H assay showing that DF1 interacts with GL2. The combinations of BD-DF1 and empty AD plasmids, or AD-GL2 and empty BD plasmids were used as negative controls. B, The interaction between DF1 and GL2 detected by BiFC assay using Arabidopsis mesophyll protoplasts. The 35S:NLS-mCherry plasmid was cotransformed to indicate the nucleus. BLH2 was used as a negative control. Scale bars = 20 μm. For each combination, more than 10 protoplasts were observed with the same results. C, Co-IP assay of DF1 and GL2 interaction. MYC-DF1 was transiently coexpressed with GL2-HA or with the HA tag alone in N. benthamiana leaves. IP was performed using anti-HA antibody. After IP, MYC-DF1 was detected by immunoblot using anti-MYC antibody. Inputs show the total protein before IP. D, Y2H assay showed that the middle region of DF1 (DF1-M) was responsible for the interaction with GL2. E, Y2H assay showed that the N-terminus of GL2 (GL2-N) containing the homeodomain (HD) was required for the interaction with DF1.
Next, we mapped the domains required for the interaction between DF1 and GL2. Y2H assays showed that a region of DF1 between amino acid (aa) 151 and 399 was responsible for its interaction with GL2. The N-terminus (aa 1–150) and the C-terminus (aa 400–494) containing the GT1 DNA-BD have no discernible role in this interaction (Figure 3D). The N-terminus of GL2 (GL2-N, aa 1–250) containing the homeodomain (HD) is required for the interaction with DF1, whereas the region (GL2-M, aa 251–515) containing the START domain or the C-terminus (aa 516–776) do not interact with DF1 (Figure 3E). These results suggest that the interaction between DF1 and GL2 is mediated by specific regions of DF1 and GL2.
DF1 and GL2 have additive effects on mucilage biosynthesis
We generated a df1-1 gl2-8 double mutant and examined its mucilage phenotype to study the relationship between DF1 and GL2 in mucilage biosynthesis. Most of the gl2-8 seeds produced no mucilage upon imbibition, although small amounts of AM were discernible on the surface of a few seeds (Figure 4, C and E), which is consistent with previous studies (Shi et al., 2012). Little if any AM was discernible on the df1-1 gl2-8 double mutant (Figure 4, D and F). Quantification of total mucilage showed that df1-1 gl2-8 seeds produced significantly less mucilage than df1-1 and gl2-8 (Figure 4G).
Figure 4.
DF1 and GL2 have additive effects on mucilage biosynthesis. A–F, Mucilage phenotypes of WT (A), df1-1 (B), gl2-8 (C and E), and df1-1 gl2-8 (D and F) seeds. Red arrow heads in (E) indicate the small amount of AM on gl2-8 seeds. Scale bars = 150 μm. G, Quantification of total mucilage content in WT, df1-1, gl2-8, and df1-1 gl2-8 seeds. Data represent means ± sd of three biological replicates. Means marked with different letters are significantly different as determined by the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05). H–K, Scanning electron micrograph of the whole seeds of WT (H), df1-1 (I), gl2-8 (J), and df1-1 gl2-8 (K). Scale bars = 200 μm. For each mutant, more than 10 seeds were observed with identical phenotype. L–O, Magnifications of (H–K) showing the morphologies of MSCs and columella. Scale bars = 20 μm.
In addition, we used scanning electron microscopy to examine the columella morphology of seed coat cells. WT seeds had a bulged and dome-shaped columella (Figure 4, H and L), whereas df1-1 columella was broad and flat (Figure 4, I and M) and gl2-8 columella had a collapsed appearance (Figure 4, J and N). The seed coat cells of df1-1 gl2-8 were irregularly shaped and the columella was absent (Figure 4, K and O). These alterations were more severe in the double mutant than in either of the single mutants and indicate that DF1 and GL2 have additive effects on mucilage biosynthesis and columella morphology.
DF1 and GL2 regulate the expression of RG-I biosynthesis genes
MUM4, GATL5, RRT1, GAUT11, MUCI70, and UUAT1 are reported to be involved in the biosynthesis of seed coat mucilage RG-I (Usadel et al., 2004; Western et al., 2004; Kong et al., 2013; Saez-Aguayo et al., 2017; Takenaka et al., 2018; Voiniciuc et al., 2018). To gain insight into the regulatory mechanisms of DF1 and GL2 in mucilage biosynthesis, we used 7–10 DPA seeds to examine the expression of these genes in df1-1, gl2-8, and df1-1 gl2-8 by RT-qPCR. The expression of MUM4, GATL5, and RRT1 was significantly decreased in df1-1 and gl2-8. The reduction in MUM4 expression was somewhat more pronounced in df1-1 gl2-8 than in df1-1 or gl2-8 (Figure 5A). The expression of GAUT11, MUCI70, and UUAT1 was not significantly altered (Supplemental Figure S5A). This suggests that DF1 and GL2 act upstream of MUM4, GATL5, and RRT1 to promote RG-I biosynthesis and that DF1 and GL2 share common downstream genes in regulating this process.
Figure 5.
DF1 directly regulates MUM4 expression. A, Expression analysis of RG-I biosynthesis genes in WT, df1-1, gl2-8, and df1-1 gl2-8 developing seeds at 7–10 DPA. Data represent means ± sd of three biological replicates. Means marked with different letters are significantly different as determined by the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05). B, Schematic illustration of the MUM4 promoter and the positions of GT3 boxes. The positions of DNA probes (p1, p2) used in EMSA are indicated. I-III indicate the regions tested in the ChIP-qPCR assay. C, EMSA showing the binding of GST–DF1 fusion protein to the MUM4 promoter probes containing the GT3 box but not to the probes containing the mutated GT3 box. Unlabeled probes were used as the competitor. The arrow indicates the shifted bands. D, Association of DF1 with the MUM4 promoter examined by ChIP-qPCR assay. ACTIN8 (ACT8) was used as a negative control. Error bars indicate the sd of three biological replicates. Asterisks denote significant differences from the WT (*P < 0.05; **P < 0.01; Student’s t test). E, Mucilage phenotypes of mum4-2 and df1-1 mum4-2 seeds. Scale bars = 150 μm. F, Mucilage phenotypes of df1-1 and 35S:MUM4/df1-1 seeds. Scale bars = 150 μm. G, Quantification of the mucilage volumes of df1-1 and 35S:MUM4/df1-1 seeds. Data represent means ± sd of 10 seeds. Means marked with different letters are significantly different according to the one-way ANOVA followed by Tukey’s multiple comparison test (P < 0.05). H, Quantification of mucilage contents of df1-1 and 35S:MUM4/df1-1 seeds. Data represent means ± sd of three biological replicates. Means marked with different letters are significantly different based on the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05).
Since our data indicated that the cellulose content was decreased in the df1-1 mucilage, we examined the expression of genes involved in cellulose biosynthesis and assembly in seed coat mucilage in 7–10 DPA seeds. However, there was no significant difference in the expression levels of CELLULOSE SYNTHASE5 (CESA5; Sullivan et al., 2011), COBRA-LIKE 2 (COBL2; Ben-Tov et al., 2015), FEI2 (Harpaz-Saad et al., 2011), and HOMEODOMAIN GLABROUS2 (HDG2; Kong et al., 2021) in df1-1 and WT (Supplemental Figure S5B). Thus, DF1 is unlikely to be involved in regulating the expression of these genes.
DF1 directly activates MUM4 expression
DF1 has been shown to bind to the GT3 box (5′-GGTAAAT-3′) of target genes (Shibata et al., 2018). We found two GT3 boxes in the MUM4 promoter (Figure 5B), but no GT3 box in the GATL5 and RRT1 promoters. Therefore, we hypothesized that MUM4 is a direct target of DF1. To test this, we first performed electrophoretic mobility shift assays (EMSAs). The results showed that the Glutathione S transferase (GST)–DF1 fusion protein specifically bound to MUM4 probes containing the GT3 box. This binding was competed out by the unlabeled competitor probes. In contrast, the GST–DF1 fusion protein did not bind to the probe containing a mutated version of the GT3 box (Figure 5C).
We further examined the binding of DF1 to the MUM4 promoter by chromatin immunoprecipitation (ChIP)-qPCR assay. Chromatin was extracted from 7 to 10 DPA siliques of WT and DF1-overexpressing lines (35S:MYC-DF1) (Supplemental Figure S6, A and B). Immunoprecipitation was performed with an antibody that recognizes MYC. The MUM4 promoter fragments (II and III) containing the GT3 box were significantly enriched in the immunoprecipitated material from 35S:MYC-DF1 plants compared to the WT control. In contrast, no significant enrichment of the MUM4 promoter fragment (I) lacking the GT3 box was detected in 35S:MYC-DF1 (Figure 5D). These results indicate that DF1 specifically binds to the GT3 box of the MUM4 promoter.
Since DF1 acts upstream of MUM4, we suspected that the mucilage phenotype of the df1 mum4 double mutant would be similar to that of mum4. To test this, we generated df1-1 mum4-2 double mutant and examined the mucilage phenotype. As expected, no mucilage was extruded when df1-1 mum4-2 seeds were immersed in water (Figure 5E). The mucilage defect of df1-1 mum4-2 was indistinguishable from that of mum4-2, but was visibly more severe than that of df1-1 (Figure 5E), indicating that MUM4 is epistatic to DF1 in mucilage biosynthesis.
We next generated transgenic plants overexpressing MUM4 in the df1-1 background (35S:MUM4/df1-1). The expression of MUM4 increased by two- to four-fold relative to the WT in the 10 independent transgenic lines investigated (Supplemental Figure S6C). RR staining of seeds and quantification of mucilage showed that the mucilage defects of df1-1 are partially restored by overexpressing MUM4 (Figure 5, F–H). These analyses suggest that the role of DF1 in RG-I biosynthesis is largely dependent on MUM4 expression.
GL2 directly targets MUM4 and GATL5
GL2 is a typical HD-ZIP TF and thus can bind to the L1 box (5′-TAAATGT-3′) in its target genes (Tominaga-Wada et al., 2009). Promoter sequence analysis of MUM4 revealed the presence of an L1 box located between the two GT3 boxes (Figure 6A). Therefore, we assumed that MUM4 is also directly regulated by GL2. Yeast one-hybrid (Y1H) assay showed that GL2 binds to a MUM4 promoter fragment containing the L1 box (Figure 6B). EMSA further demonstrated that the GST-fused GL2 fragment (GST-nGL2, aa 1–250) containing the HD specifically bound to the L1 box, but not to a mutated version (Figure 6C).
Figure 6.
GL2 directly targets MUM4 and GATL5. A, Schematic illustration of the MUM4 promoter and positions of the GT3 and L1 boxes. The position of DNA probe used in EMSA is indicated. I–III indicate the regions tested in the ChIP-qPCR assay. B, Y1H assay showing that GL2 binds to the MUM4 promoter. The combinations of empty AD vector (EV) and ProMUM4-pHIS2.1 plasmids, or AD-GL2 and empty pHIS2.1 plasmids were used as negative controls. DDO and TDO represent SD-Trp-Leu and SD-Trp-Leu-His medium, respectively. C, EMSA showing the binding of GST–nGL2 fusion protein to the MUM4 promoter probe containing the L1 box but not to the probe containing the mutated L1 box. Unlabeled probes were used as competitors. The arrow indicates the shifted band. D, Association of GL2 with the MUM4 promoter examined by ChIP-qPCR assay. ACT8 served as a negative control. Error bars indicate the sd of three biological replicates. Asterisks denote significant differences from the WT (**P < 0.01, Student’s t test). E, Schematic illustration of the GATL5 promoter and the position of the L1 box. The position of DNA probe used in EMSA is indicated. I and II indicate the regions tested in the ChIP-qPCR assay. F, Association of GL2 and DF1 with the GATL5 promoter examined by ChIP-qPCR assay. ACT8 served as a negative control. Error bars indicate the sd of three biological replicates. Asterisks denote significant differences from the WT (**P < 0.01, Student’s t test). G, EMSA showing the binding of GST–nGL2 fusion protein to the GATL5 promoter probe containing the L1 box but not to the probe containing the mutated L1 box. Unlabeled probes were used as competitors. The arrow indicates the shifted band.
Next, we conducted ChIP-qPCR assays to assess the in vivo binding of GL2 to the MUM4 promoter. We generated transgenic plants harboring ProGL2:GL2-HA in the gl2-8 background (ProGL2:GL2-HA/gl2-8). The GL2–HA fusion protein was functionally active since the defects in mucilage production and trichome morphology were largely restored in the complemented lines (Supplemental Figure S7). ChIP assays were performed with a HA antibody using 7–10 DPA siliques of the WT and ProGL2:GL2-HA/gl2-8 plants. The presence of GL2 significantly enhanced the enrichment of MUM4 promoter fragments (II and III) that are adjacent to the L1 box (Figure 6D), indicating that GL2 binds to the L1 box in MUM4 promoter.
The GATL5 promoter contains an L1 box at 379-bp upstream of the ATG (Figure 6E). No L1 box is present in the RRT1 promoter. EMSA and ChIP-qPCR assays demonstrated that GL2 specifically binds to the GATL5 promoter fragment containing the L1 box (Figure 6, F and G), suggesting that GL2 also directly targets GATL5. As DF1 physically interacts with GL2, we examined the in vivo binding of DF1 to the GATL5 promoter through ChIP-qPCR assays. The results showed that the GATL5 promoter fragment containing the L1 box was significantly enriched by DF1 (Figure 6F), suggesting that DF1 associates with the GATL5 promoter through the interaction with GL2.
DF1 and GL2 cooperatively activate the expression of MUM4 and GATL5
To investigate the action mode of the DF1–GL2 module in activating MUM4 expression, we first performed dual-luciferase (LUC) reporter assays in Arabidopsis mesophyll protoplasts. As expected, when DF1 or GL2 was co-transformed with the reporter driven by the full-length MUM4 promoter, there was a significant increase in LUC activity compared with the control. Simultaneous expression of DF1 and GL2 generated a higher LUC activity relative to DF1 or GL2 alone (Figure 7A). However, neither DF1 nor GL2 had a significant effect on LUC activity when co-transformed with reporters driven by truncated versions of the MUM4 promoter lacking one or two of the GT3 boxes and the L1 box (Supplemental Figure S8A). These data suggest that the presence of the GT3 and L1 boxes is required for the activation of MUM4 by DF1 or GL2.
Figure 7.
DF1 and GL2 cooperatively activate the expression of MUM4 and GATL5. A, Dual-LUC reporter assays using ProMUM4:LUC and ProGATL5:LUC reporter plasmids. Means marked with different letters are significantly different according to the one-way ANOVA followed by Tukey’s multiple comparison test (P < 0.05). B, Schematic illustration of the MUM4 promoter constructs used in dual-LUC reporter assays. I–III indicate the regions tested in the ChIP-qPCR assay. C, Dual-LUC reporter assays using ProMUM4:LUC reporter plasmids containing mutated L1 box. Means marked with the same letter are not significantly different based on the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05). D, Dual-LUC reporter assays showing that DF1 failed to activate ProMUM4:LUC in gl2-8 background. ns, not significant. Student’s t test. E, ChIP-qPCR assays of DF1 binding to the MUM4 promoter in WT and gl2-8 protoplasts. Means marked with different letters are significantly different according to the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05). F, Dual-LUC reporter assays using ProMUM4:LUC reporter plasmids containing mutated GT3 box. Means marked with different letters are significantly different according to the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05). G, Dual-LUC reporter assays showing that GL2 failed to activate but significantly repressed ProMUM4:LUC in df1-1 background. **P < 0.01, Student’s t test. H, ChIP-qPCR assays of GL2 binding to the MUM4 promoter in WT and df1-1 protoplasts. Means marked with different letters are significantly different according to the one-way ANOVA and Tukey’s multiple comparison test (P < 0.05).
Next, we conducted transcriptional activation assays using reporter plasmids containing mutated forms of the L1 box or the GT3 box (Figure 7B). Mutation in the L1 box led to no activation of ProMUM4:LUC expression by either GL2 or DF1 (Figure 7C). This indicates that the L1 box that is occupied by GL2 may also be required for DF1 to activate MUM4. To verify this, we used gl2-8 leaf protoplasts to examine the effects of DF1 on ProMUM4:LUC expression. As expected, DF1 failed to activate ProMUM4:LUC expression in the gl2-8 background (Figure 7D). These results suggest that DF1-mediated activation of MUM4 is dependent on GL2 occupying the MUM4 promoter.
To explore whether the binding of DF1 to the MUM4 promoter is affected by the absence of GL2, we performed ChIP-qPCR assays using the WT and gl2-8 protoplasts transiently expressing 35S:MYC-DF1. The MUM4 promoter fragments (II and III) containing the GT3 box were significantly enriched by DF1 in the WT protoplasts (Figure 7E), consistent with the results obtained using transgenic plants (Figure 5D). However, the enrichment was abolished in the gl2-8 protoplasts (Figure 7E), suggesting that the binding of DF1 to the MUM4 promoter requires the participation of GL2.
GL2 failed to activate, but significantly repressed ProMUM4:LUC expression when the GT3 box was mutated or when DF1 was disrupted (Figure 7, F and G), suggesting that the presence of DF1 on the MUM4 promoter is required for GL2 to activate MUM4. Thus, we analyzed the binding of GL2 to the MUM4 promoter in the df1-1 background by ChIP-qPCR assays in protoplasts. The binding of GL2 to MUM4 promoter was not impaired in the df1-1 background (Figure 7H), suggesting that GL2 binds to the MUM4 promoter even when DF1 is absent. Such results indicate that DF1 is required for the activation of MUM4 by GL2 but is not required for GL2 to bind to the MUM4 promoter. These combined data unraveled a cooperative mechanism of the DF1–GL2 module in activating MUM4 expression. DF1 binding to the MUM4 promoter is dependent on GL2 occupancy and GL2 requires DF1 occupancy to activate MUM4.
Since DF1 and GL2 both directly target GATL5, and DF1 associates with GATL5 promoter through the interaction with GL2 (Figure 6F), we sought to investigate if the mechanism of DF1 and GL2 in activating GATL5 is similar to the mode of activating MUM4. To this end, we performed dual-LUC reporter assays using the ProGATL5:LUC reporter. GL2 was able to independently activate ProGATL5:LUC expression, whereas DF1 alone could not (Figure 7A), probably due to the lack of binding site of DF1 in the GATL5 promoter. Nevertheless, co-expression of GL2 with DF1 significantly increased the ProGATL5:LUC expression level compared to GL2 alone (Figure 7A), suggesting that DF1 enhances the transcriptional activation activity of GL2. These results suggest that DF1 and GL2 also act cooperatively to activate GATL5 expression.
We also investigated the interplay between DF1 and GL2 on their DNA-binding capability through EMSA. The binding affinity of DF1 to the GT3 box was not altered by adding nGL2 (Supplemental Figure S8B), nor was the binding affinity of GL2 to the L1 box affected by adding DF1 (Supplemental Figure S8C). Thus, DF1 and GL2 are not likely to affect each other’s DNA-binding activity in vitro.
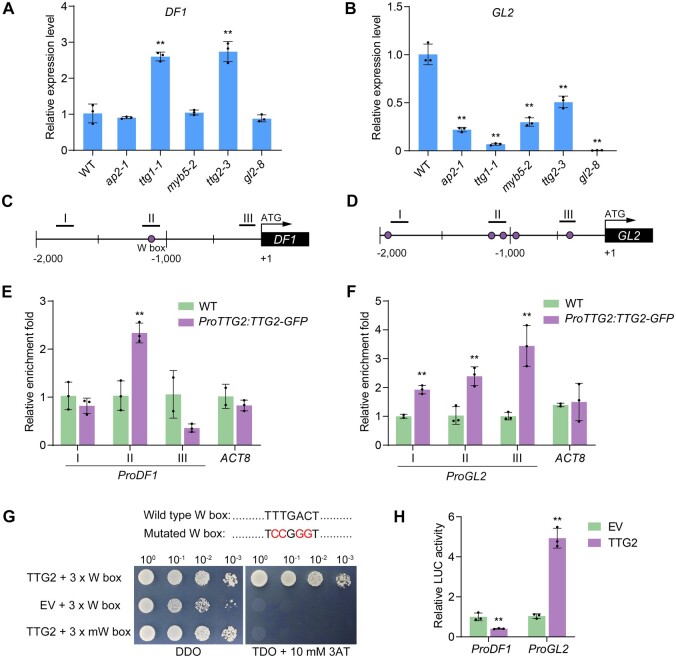
The expression of DF1 and GL2 are directly regulated by TTG2
A subset of TFs including AP2, TTG1, MYB5, TTG2, and GL2 have been reported to play key roles in MSC differentiation and mucilage production (Penfield et al., 2001; Johnson et al., 2002; Western et al., 2004; Li et al., 2009). Thus, we determined if any of these TFs regulate DF1 expression. To this end, we examined DF1 expression in 7–10 DPA seeds from the ap2-1, ttg1-1, myb5-2, ttg2-3, and gl2-8 mutants. The expression level of DF1 was significantly increased in the ttg1-1 and ttg2-3 mutants, but not significantly altered in the ap2-1, myb5-2, or gl2-8 mutants (Figure 8A). Since TTG2 is a downstream target of TTG1 (Zhao et al., 2008; Gonzalez et al., 2009), these results suggest that DF1 expression is negatively regulated by the TTG1–TTG2 cascade. In addition, GL2 expression was significantly decreased in all the mutants examined (Figure 8B), consistent with previous studies that its expression is regulated by AP2, the TTG1 complex, and TTG2 (Western et al., 2004; Ishida et al., 2007; Gonzalez et al., 2009).
Figure 8.
Expression of DF1 and GL2 are directly regulated by TTG2. A and B, Expression levels of DF1 (A) and GL2 (B) in the 7–10 DPA developing seeds of the ap2-1, ttg1-1, myb5-2, ttg2-3, and gl2-8 mutants. Data represent means ± sd of three biological replicates. Asterisks denote significant differences from the WT based on the one-way ANOVA and Dunnett's multiple comparison test (**P < 0.01). C and D, Schematic illustration of the promoters of DF1 (C) and GL2 (D) and the positions of the W boxes. I–III indicate the regions tested in the ChIP-qPCR assay. E and F, Binding of TTG2 to the DF1 promoter (E) and GL2 promoter (F) in vivo examined by ChIP-qPCR assay. ACT8 was used as a negative control. Error bars indicate the sd of three biological replicates. Asterisks denote significant differences from the WT (**P < 0.01, Student’s t test). G, TTG2 binding to the W box in vitro detected by Y1H assay. pHIS2.1-3×W box cotransformed with the EV and AD-TTG2 cotransformed with the mutated W box were used as negative controls. DDO and TDO represent SD-Trp-Leu and SD-Trp-Leu-His medium, respectively. H, Dual-LUC reporter assays showing that TTG2 directly suppresses DF1 expression and activates GL2 expression. Asterisks denote significant differences from the EV control (**P < 0.01, Student’s t test).
We then asked whether TTG2 directly regulates DF1 and GL2 expression. TTG2 belongs to the WRKY TF family, which binds to the W box (5′-C/TTGACT/C-3′) of target genes (Eulgem et al., 1999). Promoter sequence analysis showed that the DF1 promoter contains one W box (Figure 8C), and the GL2 promoter contains five W boxes (Figure 8D). We performed ChIP-qPCR assays to examine the association of TTG2 with the DF1 and GL2 promoters using complemented ttg2 plants (ProTTG2:TTG2-GFP/ttg2-3). The TTG2–GFP fusion protein was functionally active since the defects in mucilage production and trichome morphology were rescued in the complemented lines (Supplemental Figure S9). ChIP assays were performed with a GFP antibody using 7–10 DPA siliques of the WT and ProTTG2:TTG2-GFP/ttg2-3 plants. The presence of TTG2 significantly enhanced the enrichment of DF1 and GL2 promoter fragments containing the W box, but not the fragments without the W box (Figure 8, E and F). Such results indicate that TTG2 binds to the promoters of DF1 and GL2 via the W box.
In addition, Y1H assay also showed that TTG2 binds to the W box in vitro, but not to its mutated counterparts (Figure 8G). We further evaluated the transcriptional regulation of TTG2 on DF1 and GL2 expression through dual-LUC reporter assays in Arabidopsis mesophyll protoplasts. The 35S:TTG2 effector generated a more than two-fold decrease in the ProDF1:LUC expression and a more than four-fold increase in the ProGL2:LUC expression compared with the control (Figure 8H), demonstrating that TTG2 directly represses DF1 expression and activates GL2 expression. Taken together, the results of these analyses provide evidence that TTG2 binds to the promoters of DF1 and GL2 and directly regulates their expression.
To gain a better understanding of the transcriptional regulation network controlling RG-I biosynthesis, we also examined the expression levels of TTG2, MUM4, GATL5, and RRT1 in 7–10 DPA seeds of the ap2-1, ttg1-1, myb5-2, ttg2-3, and gl2-8 mutants. TTG2 expression was significantly decreased in ap2-1 and ttg1-1 mutants, consistent with the fact that TTG2 acts downstream of AP2 and TTG1 (Western et al., 2004; Gonzalez et al., 2009). However, TTG2 expression was not significantly altered in myb5-2 and gl2-8 (Supplemental Figure S10A), suggesting that TTG2 expression is independent of MYB5 or GL2. MUM4 and RRT1 expression was decreased in the ap2-1, ttg1-1, and gl2-8 mutants, but not in ttg2-3 (Supplemental Figure S10, B and C), suggesting that MUM4 and RRT1 expression is regulated by AP2, TTG1, and GL2, but independent of TTG2. In contrast, GATL5 expression was substantially reduced in all the mutants examined (Supplemental Figure S10D), suggesting that all these TFs regulate its expression. These data indicate that the RG-I biosynthesis genes in MSC are differentially regulated by specific upstream TFs, which constitute multiple transcriptional regulatory layers.
DF1 directly represses TTG2 expression
To further dissect the relationships between DF1 and the other TFs associated with mucilage production, we examined the expression of AP2, TTG1, TTG2, GL2, MYB5, TT2, TT8, EGL3, and MYB61 in the 7–10 DPA seeds of df1 mutants. Only the expression of TTG2 was significantly increased in df1 compared to WT (Figure 9A), suggesting that DF1 negatively affects TTG2 expression. Promoter sequence analysis showed that a GT3 box is present in the TTG2 promoter (Figure 9B), therefore we determined if DF1 directly represses TTG2 expression. EMSA and ChIP-qPCR assays revealed that DF1 binds to the TTG2 promoter via the GT3 box (Figure 9, C and D). Dual-LUC reporter assay further demonstrated that DF1 directly suppresses TTG2 expression (Figure 9E). Combined with the fact that TTG2 directly repressed DF1 expression, these findings revealed a negative feedback loop between DF1 and TTG2 at the transcriptional level.
Figure 9.
DF1 directly represses TTG2 expression. A, Expression of TF genes involved in mucilage production in WT and df1 mutants. Data represent mean ± sd of three biological replicates. Asterisks denote significant differences from the WT (**P < 0.01, Student’s t test). B, Schematic illustration of the TTG2 promoter and the position of the GT3 box. The position of DNA probe used in EMSA is indicated. I and II indicate the regions tested in the ChIP-qPCR assay. C, EMSA showing the binding of GST–DF1 fusion protein to the TTG2 promoter probe containing the GT3 box but not to the probe containing the mutated GT3 box. Unlabeled probes were used as competitors. The arrow indicates the shifted band. D, Binding of DF1 to the TTG2 promoter examined by ChIP-qPCR assay. ACT8 was used as a negative control. Error bars indicate the sd of three biological replicates. Asterisks denote significant differences from WT (**P < 0.01, Student’s t test). E, Dual-LUC reporter assays showing that DF1 represses TTG2 expression. Asterisks denote significant differences from the EV control (**P < 0.01, Student’s t test).
Discussion
DF1 plays a regulatory role in mucilage RG-I biosynthesis
Pectin comprises a family of GalA-containing polysaccharides that are abundant in plant primary cell walls and the mucilage surrounding hydrated Arabidopsis seeds (Cosgrove, 1997; Macquet et al., 2007). At least three major types of pectic polysaccharides are known to exist: HG, RG-I, and the substituted galacturonans, which include xylogalacturonan, apiogalacturonan, and RG-II (Mohnen, 2008). Due to this structural diversity and the fact that pectin structure may change during plant growth and development, the genetic factors that regulate pectin biosynthesis remain largely unknown.
Arabidopsis seeds are myxodiasporic since they produce a pectin-rich mucilage that is extruded upon imbibition of water. RG-I accounts for most of this pectin, thus mucilage formation is now recognized as a model system to study factors controlling pectin biosynthesis (Arsovski et al., 2010; North et al., 2014). A transcriptional hierarchy consisting of at least three tiers of TFs has been proposed to control Arabidopsis mucilage biosynthesis (North et al., 2014; Voiniciuc et al., 2015; Golz et al., 2018). The first tier of regulators includes AP2, NAC-REGULATED SEED MORPHOLOGY1 (NARS1), and NARS2, which control the differentiation of the outer ovule integument. AP2 and the TTG1–bHLH–MYB complex act in parallel to regulate the downstream expression of TTG2 and GL2 (Western et al., 2004; Gonzalez et al., 2009). Previous studies have reported that AP2, TTG1, and GL2 regulate the expression of MUM4 and GATL5 (Western et al., 2004; Kong et al., 2013), although the mechanisms involved were not determined. We have provided evidence that DF1 and GL2 physically interact and act in a cooperative module to directly activate MUM4 and GATL5 expression. Thus, DF1 is a key regulator of mucilage biosynthesis in the second tier (Figure 10A).
Figure 10.
The regulatory network of RG-I biosynthesis in Arabidopsis MSCs and the model depicting DF1–GL2 cooperation in activating MUM4 and GATL5 expression. A, The updated regulatory network of RG-I biosynthesis in Arabidopsis MSCs. DF1 acts in the second tier, it physically interacts with GL2 to cooperatively regulate the expression of MUM4 and GATL5, and forms a negative feedback loop with TTG2 at the transcriptional level. GL2 expression is also directly regulated by TTG2. Solid and dashed arrows indicate direct and putative indirect activation, respectively; blocked lines indicate direct repression. B, A proposed working model of the DF1–GL2 cooperation module in activating MUM4 expression. The binding of DF1 to GT3 box is dependent on the occupancy of GL2. Meanwhile, activation of MUM4 by GL2 requires the occupancy of DF1. C, A proposed working model of the DF1–GL2 cooperation module in activating GATL5 expression. DF1 associates with GATL5 promoter through the interaction with GL2, and cooperates with GL2 to activate GATL5 expression.
We have shown that DF1 expression is directly repressed by TTG2, whereas GL2 expression is directly activated by TTG2. Thus, TTG2 likely exerts opposite transcriptional regulation on DF1 and GL2 (Figure 10A). Moreover, we have also shown that DF1 represses TTG2 expression directly, and thus propose that at the transcriptional level a negative feedback loop exists between DF1 and TTG2 (Figure 10A).
It is notable that TTG2 does not affect the expression of MUM4 or RRT1 (Supplemental Figure S10). This may be a consequence of the opposing transcriptional regulation of DF1 and GL2 by TTG2. However, GATL5 expression was significantly decreased in the ttg2-3 mutant (Supplemental Figure S10). A possibility is that TTG2 regulates an as yet uncharacterized pathway(s) to modulate GATL5 expression.
Our updated transcriptional regulatory network for RG-I biosynthesis in seed coat mucilage (Figure 10A) is based on our new data and the cumulative results of previous studies. AP2 and the TTG1–bHLH–MYB complex act as the first-tier TFs controlling RG-I biosynthesis. They function in parallel pathways to activate the downstream expression of TTG2 and GL2, which encode the second-tier TFs. The expression of GL2 is also directly regulated by TTG2. DF1 also acts in the second tier, it physically interacts with GL2 to cooperatively activate the expression of MUM4 and GATL5, and forms a negative feedback loop with TTG2 at the transcriptional level. Our model provides insight into the complex regulatory steps required to control the biosynthesis of a single polysaccharide. Such a model also provides a foundation for identifying the factors that control the biosynthesis and assembly of polysaccharides present in the primary cell wall.
The cellulose content was reduced in the df1 mucilage but we found no discernible changes in the expression of genes (CESA5, COBL2, FEI2, and HDG2) reported to be involved in mucilage cellulose synthesis or assembly (Harpaz-Saad et al., 2011; Sullivan et al., 2011; Ben-Tov et al., 2015; Kong et al., 2021). Further studies are needed to elucidate the potential mechanism of the decrease of cellulose content in the df1 seed coat mucilage.
DF1 and GL2 cooperatively promote RG-I biosynthesis
Our data showed that DF1 and GL2 physically interact and both directly target MUM4 and GATL5. Through a series of ChIP-qPCR and dual-LUC reporter assays, we have demonstrated that cooperative binding of DF1 and GL2 to MUM4 promoter is required for the activation of MUM4. On the one hand, although GL2 could bind to MUM4 promoter independent of DF1, it requires DF1 occupancy on MUM4 promoter to activate MUM4. Surprisingly, GL2 significantly repressed ProMUM4:LUC expression when the occupation of DF1 in MUM4 promoter was disrupted (Figure 7, F and G). A plausible explanation is that the transcriptional activity of GL2 is affected by some yet unidentified co-factors (probably transcriptional repressors), in the absence of DF1 on MUM4 promoter. On the other hand, the binding of DF1 to the GT3 box in the MUM4 promoter requires the occupancy of GL2 on L1 box. Although EMSA showed that DF1 could independently bind to the GT3 box in vitro (Figure 5C), we propose that the in vivo binding is relatively weak, and thus requires the interaction with GL2 to be strengthened. Unexpectedly, EMSA showed that the DNA-binding capacity of DF1 could not be enhanced by GL2 (Supplemental Figure S8B). A plausible explanation for these seemingly contradictory results lies in the fact that the GT3 and L1 boxes could not be pooled in one DNA probe in EMSA due to the length restriction of DNA probes. Therefore, it could not resemble the circumstance they were actually arranged in chromosome in vivo. In other words, the cooperative binding of DF1 and GL2 to MUM4 promoter could not be accomplished in EMSA. Furthermore, the nGL2 fragment was used in EMSA because we could not obtain the full-length GL2 protein through prokaryotic expression. It is possible that the truncated nGL2 protein could not mimic the function of full-length GL2 regarding the cooperation with DF1.
Based on our data, we proposed a working model depicting a cooperative DF1–GL2 module that activates MUM4 expression. Binding of DF1 and GL2 to their respective GT3 and L1 boxes in the MUM4 promoter enables a cooperative interaction between DF1 and GL2, which is required for the activation of MUM4 (Figure 10B). We have also shown that DF1 and GL2 act cooperatively to activate GATL5 expression. Although no DF1-binding site is present in the GATL5 promoter, DF1 binds to the GATL5 promoter through the interaction with GL2, and cooperates with GL2 to activate GATL5 expression (Figure 10C). Taken together, the DF1-GL2 cooperation module is required for the normal expression of MUM4 and GATL5 in Arabidopsis MSCs. Disrupting DF1 or GL2 prevents this cooperation and results in impaired MUM4 and GATL5 expression, thereby reducing RG-I biosynthesis.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana mutant lines df1-1 (SALK_106258C), df1-2 (SALK_072465C), mum4-2 (CS3908), gl2-8 (SALK_130213), ttg2-3 (SALK_148838), ap2-1 (CS29), ttg1-1 (CS89), and myb5-2 (SALK_105723C) were obtained from the Arabidopsis Biological Resource Center (http://www.Arabidopsis.org).
To generate the 35S:MYC-DF1 transgenic lines, the coding sequence (CDS) of DF1 was amplified and fused with six copies of the MYC tag at the N-terminus under the control of CaMV35S promoter in the modified PBI221-MYC vector. The plasmid was introduced into Arabidopsis Columbia-0 (Col-0) plants by Agrobacterium tumefaciens mediated transformation (Clough and Bent, 1998). The CDS of MUM4 was also cloned into the modified PBI221-MYC vector and transformed into df1-1 plants, generating the 35S:MUM4/df1-1 transgenic lines. To generate the ProGL2:GL2-HA/gl2-8 complementary lines, the GL2 promoter (2,034-bp upstream of ATG) and the CDS of GL2 were cloned into a modified pDX2181-3HA vector, in which three copies of HA tags were fused with the C-terminus of GL2. The plasmid was introduced into gl2-8 plants by A. tumefaciens mediated transformation. To generate the ProTTG2:TTG2-GFP/ttg2-3 complemented lines, the 3,471-bp genomic sequence containing TTG2 promoter and CDS excluding the stop codon was amplified and cloned into a modified pCAMBIA2301-GFP vector, in which GFP was fused with the C-terminus of TTG2. The plasmid was introduced into ttg2-3 plants by A. tumefaciens mediated transformation. Primers used for these constructs are listed in Supplemental Data Set 1.
Seeds were surface sterilized and sown on half-strength Murashige and Skoog (1/2 MS) solid medium supplemented with 10 g L−1 sucrose. The 35S:MYC-DF1, 35S:MUM4/df1-1, and ProTTG2:TTG2-GFP/ttg2-3 transgenic lines were screened on 1/2 MS media containing 50 mg L−1 kanamycin. The ProGL2:GL2-HA/gl2-8 transgenic lines were screened on 1/2 MS media containing 50 mg L−1 hygromycin. Seeds were synchronized for 2 days at 4°C and then germinated at 22°C in a growth chamber under long-day conditions (14-h light/10-h dark) with a light intensity of 120 µmol m−2 s−1 (cool-white tubular LED lighting). After 7 days of growth the seedlings were transferred to soil and grown under the same conditions.
Gene expression analysis
Total RNA was isolated using the CTAB method (Jordon-Thaden et al., 2015) from developing seeds and using the TransZol reagent (TransGen, Beijing, China) from the other tissues following the manufacturer’s instructions. The first-strand cDNA was reverse transcribed using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen) according to the manufacturer’s instructions. RT-qPCR was performed using the TransStart Top Green qPCR SuperMix (TransGen) with the LightCycler 480 detection system (Roche, Basel, Switzerland). ACTIN2 was used as the internal control. Relative expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen, 2001). The gene-specific primers used are listed in Supplemental Data Set 1.
GUS staining assay
The DF1 promoter (2,600-bp upstream of ATG) was amplified from A. thaliana Col-0 genomic DNA using specific primers (Supplemental Data Set 1) and inserted into the pDX2181 binary vector containing the GUS reporter gene (Ye et al., 2012). The plasmid was introduced into Col-0 plants by A. tumefaciens mediated transformation. GUS histochemical assay was performed as described (Sieburth and Meyerowitz, 1997). The stained tissues were washed with 70% ethanol to remove plant pigments, and photographed with a stereomicroscope (MZ FLIII; Leica, Wetzlar, Germany).
Paraffin sections of GUS-stained developing seeds were prepared by fixing the seeds for 24 h at 4°C in formaldehyde:acetic acid:50% ethanol = 5:5:90 [v/v/v]. The fixed seeds were then dehydrated using a gradient series of 50%-100% ethanol. Ethanol was then gradually replaced with xylene. The seeds were embedded in paraffin wax and cut into 8–10 μm sections. Sections were de-waxed and photographed with a light microscope (BX51; Olympus, Tokyo, Japan).
In situ hybridization
Siliques at 4, 7, 10, and 13 DPA from Col-0 plants were fixed, embedded, and sectioned as described (Hu et al., 2016). For the preparation of digoxigenin-labeled RNA probes, a specific fragment of the coding region of DF1 was amplified (Supplemental Data Set 1) and inserted into the pGEM-T vector. Digoxigenin-labeled sense and antisense RNA probes were generated using the Digoxigenin RNA Labeling kit (Roche). Hybridization and immunological detection were performed as described (Mayer et al., 1998). Images were captured with a light microscope (BX51; Olympus).
RR staining
To observe mucilage release, mature dry seeds were directly imbibed for 30 min in water containing 0.01% (w/v) RR (Sigma-Aldrich, St Louis, MO, USA). To remove the NM layer, seeds suspended in water were subjected to shaking for 1 h at 200 rpm prior to staining with RR. The stained seeds were photographed using a BX51 light microscope (Olympus). To determine the mucilage volume, the length (2a) and width (2b) of each seed, as well as the length (2A) and width (2B) of the same seed plus the AM were measured using ImageJ software (version 1.8.1). The volume of the AM was calculated by subtracting the volume of the seed (V = 4/3 × π × a × b × b) from the volume of seed plus mucilage (V = 4/3 × π × A × B × B).
Resin sectioning
Siliques at 4, 7, 10, and 13 DPA were fixed overnight at 4°C with 2.5% (w/v) glutaraldehyde in phosphate-buffered saline (PBS, pH 7.0). Siliques were then washed with PBS and postfixed for 1 h in PBS containing 1% (v/v) osmium tetraoxide. The fixed siliques were washed, dehydrated, embedded in Spurr’s resin, and then cut into 1 µm sections. Sections were stained for 5 min with 0.1% (w/v) toluidine blue O in 0.1% (w/v) Na2CO3 and photographed using a BX51 light microscope (Olympus).
Mucilage extraction and monosaccharide quantification
Mature dry seeds (100 mg) were suspended in 4 mL of distilled water and shaken for 1 h at 200 rpm. The suspension was centrifuged for 5 min at 5000 rpm and the seeds were washed with 1 mL of distilled water. The supernatants were combined as the NM extract. The washed seeds were then re-suspended in 4 mL of distilled water and subjected to ultrasonic treatment (200 W, 2 min) as described (Zhao et al., 2017). After centrifugation for 5 min at 5000 rpm and a wash with 1 mL of distilled water, the supernatants were combined as the AM extract. The mucilage extracts were dialyzed and lyophilized. Quantification of the mucilage monosaccharide compositions was performed as described (Xu et al., 2020).
Immunolabeling assay and ELISA
Mature dry seeds were blocked for 1 h with PBS containing 3% (w/v) nonfat milk powder and then reacted for 2 h at room temperature with a 10-fold diluted primary monoclonal antibody (CCRC-M36/CCRC-M38/JIM5/JIM7/CBM3a) in the PBS-milk solution. After three gentle washes with PBS, the seeds were incubated for 1 h at room temperature in dark with 100-fold diluted Alexa Fluor488-tagged donkey anti-mouse immunoglobulin G (IgG) (for the CCRC series; Thermo Fisher Scientific, Waltham, MA, USA) or Alexa Fluor488-tagged donkey anti-rat IgG (for the JIM series) in PBS-milk solution. For CBM3a, a 1 h incubation with 100-fold-diluted anti-His monoclonal antibody (TransGen) in PBS-milk solution was performed prior to incubation with the AlexaFluor488-tagged donkey anti-mouse IgG. Seeds were gently washed in PBS and counterstained for 15 min with Calcofluor white or S4B (Sigma-Aldrich). Images were captured with a FluoView FV1000 confocal laser scanning microscope (Olympus). AlexaFluor488, Calcofluor white, and S4B were excited with a 488, 405, and 561 nm argon laser, respectively. Fluorescence emission was recorded at 500–560 nm for Alexa Fluor488, 420–480 nm for Calcofluor white, and 570–650 nm for S4B.
For ELISA, the total mucilage of 60 mg mature dry seeds was extracted by ultrasonic treatment in 6-mL distilled water. An aliquot of 200 μL of mucilage extracts was coated onto 96-well microtiter plates at 4°C overnight. The ELISA was performed as described (Xu et al., 2020).
Crystalline cellulose observation and content quantification
Dry seeds were hydrated with water and then mounted onto glass slides and examined with an Eclipse E600 POL microscope (Nikon) to observe the birefringence of crystalline cellulose under polarized light.
To determine the crystalline cellulose content in mucilage, 10 mg total mucilage was hydrolyzed for 2 h at 121°C with 2 mL of 2-M trifluoroacetic acid. The hydrolysate was centrifuged at 12,000 rpm for 10 min. The pellets were dissolved in 5 mL Updegraff reagent (acetic acid:nitric acid:water, 8:1:2 [v/v]) and heated at 100°C for 1 h (Updegraff, 1969). After centrifugation at 12,000 rpm for 10 min, the pellets were washed 3 times with acetone and vacuum-dried. The residue was hydrolyzed with 2 mL of 72% (w/v) H2SO4 for 30 min. The amounts of crystalline cellulose were quantified using the phenolsulfuric acid method with a dehydration factor of 0.9 (Ge et al., 2012).
Protein interaction assays
For the Y2H assay, the CDS of DF1 and GL2 were cloned into the pGBKT7-rec and pGADT7-rec vector (Clontech, Mountain View, CA, USA) respectively. The resulting BD-DF1 and AD–GL2 fusion protein constructs were co-transformed into yeast (strain Y2Hgold; Clontech). Transformants were examined for expression of the HIS3, ADE1, and MEL1 reporter genes on SD–Trp–Leu–His–Ade medium supplemented with 40 μg mL−1 5-Bromo-4-chloro-3-indoxyl-α-d-galactopyranoside (X-α-gal; Sigma Aldrich, St. Louis, MO, USA).
For BiFC assays, the CDS of DF1 and GL2 were cloned into the pVYNE(R) and pVYCE(R) vector (Waadt et al., 2008), respectively, with their N-terminus fused to the nYFP or the cYFP. The plasmids were co-transformed with the nucleus marker 35S:NLS-mCherry into Arabidopsis mesophyll protoplasts as described (Yoo et al., 2007). BLH2 cloned into the pVYNE(R) and pVYCE(R) vector were used as negative control plasmids. After overnight incubation, fluorescence was observed with a FluoView FV1000 confocal laser scanning microscope (Olympus). YFP and mCherry were excited with a 488 nm and 561 nm argon laser, respectively. Fluorescence emission was recorded at 500–540 nm for YFP and 570–620 nm for mCherry.
For Co-IP assays, the CDS of GL2 was cloned into a modified pCAMBIA1301-3HA vector, in which GL2 was fused with three copies of the HA tag under the control of CaMV35S promoter (35S:GL2-HA). The plasmid was transiently co-expressed with the 35S:MYC-DF1 in N. benthamiana leaves. After 40 h of infiltration, N. benthamiana leaves were harvested and total protein then extracted with the Co-IP buffer (50-mM Tris–HCl, pH 8.0, 150-mM NaCl, 1-mM EDTA, 0.5% Triton X-100, 1-mM DTT, 1-mM PMSF, and 1× protease inhibitor cocktail tablets [Roche]). IP was performed using an anti-HA antibody (TransGen) coupled to protein A + G agarose beads (CWBIO, Beijing, China). After IP, MYC-DF1 was detected by immunoblot using anti-MYC antibody (TransGen).
EMSA
The full-length CDS of DF1 and the CDS for aa 1–250 of GL2 (nGL2) containing the HD were cloned into the pGEX-4T-1 vector (GE Healthcare, Chicago, IL, USA) and expressed in Escherichia coli BL21 (DE3) cells (TransGen). The GST-DF1 and GST–nGL2 fusion proteins were purified using Glutathione Sepharose 4B (GE Healthcare). The EMSA reaction and detection was performed using the LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Y1H
The CDSs of GL2 and TTG2 were cloned into the pGADT7-rec (Clontech) vector. The MUM4 promoter fragment containing the L1 box (837-bp upstream of ATG) and three tandem repeats of the DF1 promoter fragment containing the W box (−1,306 to −1,257 upstream of ATG) were separately cloned into the pHIS2.1 vector (Clontech) to drive the HIS3 reporter gene. The AD-GL2 and AD-TTG2 constructs were co-transformed with the ProMUM4-pHIS2.1 and 3 × W-box-pHIS2.1 constructs into the yeast strain Y187 (Clontech), respectively. Transformants were selected on SD–Trp–Leu media and examined for the expression of HIS3 reporter gene on SD–Trp–Leu–His media supplemented with 3-amino-1, 2, 4-triazole (3-AT; Sigma Aldrich). 3-AT was supplemented at 15 mM for the detection of GL2 and ProMUM4 interaction, and at 10 mM for the detection of TTG2 and ProDF1 interaction.
ChIP assays
Siliques of WT and T2 generation of the 35S:MYC-DF1, ProGL2:GL2-HA/gl2-8, ProTTG2:TTG2-GFP/ttg2-3 transgenic plants were collected at 7–10 DPA. Chromatin extraction was performed as described (Bowler et al., 2004). For transient ChIP assays in protoplasts, the CDS of DF1 and GL2 were cloned into the pVYNE(R) and pVYCE(R) vector (Waadt et al., 2008), respectively, with their N-terminus fused with the MYC-tag or HA-tag, resulting in the 35S:MYC-DF1 and 35S:HA-GL2 constructs. About 500 µg plasmids were transformed into 2 × 107 protoplasts as described (Yoo et al., 2007), and the ChIP experiments were performed according to a previously described method (Lee et al., 2017). Immunoprecipitation was performed using the anti-MYC, anti-HA, or anti-GFP antibodies (TransGen) conjugated to Protein G Agarose (Millipore, Burlington, MA, USA) according to the manufacturer’s instructions. The precipitated DNA was analyzed by quantitative PCR using the specific primers listed in Supplemental Data Set 1.
Dual-LUC reporter assay
To create the effector constructs, the full-length CDS of DF1, GL2, and TTG2 were separately cloned into the pBI221 vector downstream of the CaMV35S promoter. To create the reporter constructs, the full length, truncated, and mutated forms of MUM4 promoter, the DF1 promoter (1,460-bp upstream of ATG), the GL2 promoter (2,066-bp upstream of ATG), and the TTG2 promoter (1,787-bp upstream of ATG) were separately inserted into the pGreenII 0800-LUC vector upstream of the firefly LUC (f-LUC) reporter gene. The renilla LUC (r-LUC) gene under the control of the CaMV35S promoter in the pGreenII 0800-LUC vector was used as an internal control for normalization. The isolation and transformation of Arabidopsis mesophyll protoplasts were performed as previously described (Yoo et al., 2007). LUC activities were measured using a Dual LUC Reporter Assay Kit (Vazyme, Nanjing, China) and the relative LUC activity (f-LUC/r-LUC) was calculated.
Statistical analysis
All statistical analyses were performed with Graphpad Prism version 8.0. Statistically significant differences are evaluated using Student’s t test or one-way ANOVA with Tukey’s or Dunnett’s multiple comparison test. Statistical tests and replicate numbers are as indicated in figure legends. Data from statistical analyses are provided in Supplemental Data Set 2.
Accession numbers
Sequence data used in this study can be found in the Arabidopsis Information Resource (https://www.arabidopsis.org) under the following accession numbers: DF1 (At1G76880), GL2 (At1G79840), TTG2 (At2G37260), AP2 (At4G36920), TTG1 (At5G24520), MYB5 (At3G13540), MUM4 (At1G53500), GATL5 (At1G02720), and RRT1 (At5G15740).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Tissue-specific expression profile of DF1.
Supplemental Figure S2. Mucilage phenotype of df1 and WT seeds after EDTA treatment.
Supplemental Figure S3. Immunolabeling of HG in the df1-1 and WT mucilage.
Supplemental Figure S4. Crystalline cellulose content is reduced in the df1-1 mucilage.
Supplemental Figure S5. Expression analysis of RG-I-related and cellulose-related genes.
Supplemental Figure S6. Generation of 35S:MYC-DF1 and 35S:MUM4/df1-1 transgenic plants.
Supplemental Figure S7. Generation and phenotypic analysis of ProGL2:GL2-HA/gl2-8 plants.
Supplemental Figure S8. Analysis of DF1 and GL2 interplay by dual-LUC reporter assays and EMSA.
Supplemental Figure S9. Generation and phenotypic analysis of the complemented ttg2 plants.
Supplemental Figure S10 . Transcriptional regulation among characterized TFs and RG-I biosynthesis genes during mucilage synthesis.
Supplemental Data Set 1 . Primers used in this study.
Supplemental Data Set 2 . Statistical analysis results.
Supplementary Material
Acknowledgments
We thank Michael G. Hahn (Complex Carbohydrate Research Center, University of Georgia) for providing the CCRC and JIM series antibodies.
Funding
This work was financially supported by the National Natural Science Foundation of China (31970322, 31770336, and 32070330), the National Key Scientific Research Project of China (2021YFD2200205), the Innovation Fund of Shandong Energy Institute (SEI I202119 and SEI I202130), the First Class Grass land Science Discipline Program of Shandong Province, the Taishan Scholar Program of Shandong (to G.Z.), and Grant DESC0008472 from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy (to M.O.).
Conflict of interest statement: The authors declare no conflict of interest.
R.H. and Y.K. designed the research. Y.X. performed most of the experiments and analyzed the data. Y.W., J.D., S.P., R.H., S.G., and D.W. assisted in experiments and discussed the results. Y.X. wrote the draft. R.H., Y.K., G.Z., S.L., and M.O. revised the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is Ruibo Hu (hurb@qibebt.ac.cn).
References
- Arsovski AA, Haughn GW, Western TL (2010) Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal Behav 5: 796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Popma TM, Haughn GW, Carpita NC, McCann MC, Western TL (2009) AtBXL1 encodes a bifunctional beta-D-xylosidase/alpha-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiol 150: 1219–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, De Rycke R, Viane R, Inze D (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113: 139–148 [Google Scholar]
- Ben-Tov D, Abraham Y, Stav S, Thompson K, Loraine A, Elbaum R, de Souza A, Pauly M, Kieber JJ, Harpaz-Saad S (2015) COBRA-LIKE2, a member of the glycosylphosphatidylinositol-anchored COBRA-LIKE family, plays a role in cellulose deposition in arabidopsis seed coat mucilage secretory cells. Plant Physiol 167: 711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Benvenuto G, Laflamme P, Molino D, Probst AV, Tariq M, Paszkowski J (2004) Chromatin techniques for plant cells. Plant J 39: 776–789 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13: 171–201 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18: 4689–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XM, Green VS, Zhang NN, Sivakumar G, Xu JF (2012) Eastern gamagrass as an alternative cellulosic feedstock for bioethanol production. Process Biochem 47: 335–339 [Google Scholar]
- Golz JF, Allen PJ, Li SF, Parish RW, Jayawardana NU, Bacic A, Doblin MS (2018) Layers of regulation - Insights into the role of transcription factors controlling mucilage production in the Arabidopsis seed coat. Plant Sci 272: 179–192 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Mendenhall J, Huo Y, Lloyd A (2009) TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev Biol 325: 412–421 [DOI] [PubMed] [Google Scholar]
- Harpaz-Saad S, McFarlane HE, Xu S, Divi UK, Forward B, Western TL, Kieber JJ (2011) Cellulose synthesis via the FEI2 RLK/SOS5 pathway and cellulose synthase 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J 68: 941–953 [DOI] [PubMed] [Google Scholar]
- Hu R, Li J, Wang X, Zhao X, Yang X, Tang Q, He G, Zhou G, Kong Y (2016) Xylan synthesized by Irregular Xylem 14 (IRX14) maintains the structure of seed coat mucilage in Arabidopsis. J Exp Bot 67: 1243–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Hattori S, Sano R, Inoue K, Shirano Y, Hayashi H, Shibata D, Sato S, Kato T, Tabata S, et al. (2007) Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. Plant Cell 19: 2531–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku KD, Denboer BGW, Vanmontagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene apetala2. Plant Cell 6: 1211–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Kolevski B, Smyth DR (2002) TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14: 1359–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordon-Thaden IE, Chanderbali AS, Gitzendanner MA, Soltis DE (2015) Modified CTAB and TRIzol protocols improve RNA extraction from chemically complex Embryophyta. Appl Plant Sci 3: apps.1400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Pei S, Wang Y, Xu Y, Wang X, Zhou G, Hu R (2021) HOMEODOMAIN GLABROUS2 regulates cellulose biosynthesis in seed coat mucilage by activating CELLULOSE SYNTHASE5. Plant Physiol 185: 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Zhou G, Abdeen AA, Schafhauser J, Richardson B, Atmodjo MA, Jung J, Wicker L, Mohnen D, Western T, et al. (2013) GALACTURONOSYLTRANSFERASE-LIKE5 is involved in the production of Arabidopsis seed coat mucilage. Plant Physiol 163: 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jin S, Kim SY, Kim W, Ahn JH (2017) A fast, efficient chromatin immunoprecipitation method for studying protein-DNA binding in Arabidopsis mesophyll protoplasts. Plant Methods 13: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SF, Milliken ON, Pham H, Seyit R, Napoli R, Preston J, Koltunow AM, Parish RW (2009) The Arabidopsis MYB5 transcription factor regulates mucilage synthesis, seed coat development, and trichome morphogenesis. Plant Cell 21: 72–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM (2007) In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 48: 984–999 [DOI] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11: 266–277 [DOI] [PubMed] [Google Scholar]
- North HM, Berger A, Saez-Aguayo S, Ralet MC (2014) Understanding polysaccharide production and properties using seed coat mutants: future perspectives for the exploitation of natural variants. Ann Bot 114: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Nemoto T, Jigami Y (2007) Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J Biol Chem 282: 5389–5403 [DOI] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW (2001) MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13: 2777–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Usadel B, Neumetzler L, Hartmann J, Bussis D, Altmann T (2008) A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J 54: 466–480 [DOI] [PubMed] [Google Scholar]
- Saez-Aguayo S, Ralet MC, Berger A, Botran L, Ropartz D, Marion-Poll A, North HM (2013) PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 25: 308–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Aguayo S, Rautengarten C, Temple H, Sanhueza D, Ejsmentewicz T, Sandoval-Ibanez O, Donas D, Parra-Rojas JP, Ebert B, Lehner A, et al. (2017) UUAT1 is a golgi-localized UDP-uronic acid transporter that modulates the polysaccharide composition of Arabidopsis seed mucilage. Plant Cell 29: 129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Katavic V, Yu Y, Kunst L, Haughn G (2012) Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J 69: 37–46 [DOI] [PubMed] [Google Scholar]
- Shibata M, Breuer C, Kawamura A, Clark NM, Rymen B, Braidwood L, Morohashi K, Busch W, Benfey PN, Sozzani R, et al. (2018) GTL1 and DF1 regulate root hair growth through transcriptional repression of ROOT HAIR DEFECTIVE 6-LIKE 4 in Arabidopsis. Development 145: dev159707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Meyerowitz EM (1997) Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9: 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Ralet MC, Berger A, Diatloff E, Bischoff V, Gonneau M, Marion-Poll A, North HM (2011) CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol 156: 1725–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka Y, Kato K, Ogawa-Ohnishi M, Tsuruhama K, Kajiura H, Yagyu K, Takeda A, Takeda Y, Kunieda T, Hara-Nishimura I, et al. (2018) Pectin RG-I rhamnosyltransferases represent a novel plant-specific glycosyltransferase family. Nat Plants 4: 669–676 [DOI] [PubMed] [Google Scholar]
- Tominaga-Wada R, Iwata M, Sugiyama J, Kotake T, Ishida T, Yokoyama R, Nishitani K, Okada K, Wada T (2009) The GLABRA2 homeodomain protein directly regulates CESA5 and XTH17 gene expression in Arabidopsis roots. Plant J 60: 564–574 [DOI] [PubMed] [Google Scholar]
- Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32: 420–424 [DOI] [PubMed] [Google Scholar]
- Usadel B, Kuschinsky AM, Rosso MG, Eckermann N, Pauly M (2004) RHM2 is involved in mucilage pectin synthesis and is required for the development of the seed coat in Arabidopsis. Plant Physiol 134: 286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevski A, Giorgi FM, Bertinetti L, Usadel B (2012) LASSO modeling of the Arabidopsis thaliana seed/seedling transcriptome: a model case for detection of novel mucilage and pectin metabolism genes. Mol Biosyst 8: 2566–2574 [DOI] [PubMed] [Google Scholar]
- Voiniciuc C, Dean GH, Griffiths JS, Kirchsteiger K, Hwang YT, Gillett A, Dow G, Western TL, Estelle M, Haughn GW (2013) Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell 25: 944–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Engle KA, Gunl M, Dieluweit S, Schmidt MH, Yang JY, Moremen KW, Mohnen D, Usadel B (2018) Identification of key enzymes for pectin synthesis in seed mucilage. Plant Physiol 178: 1045–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Yang B, Schmidt MH, Gunl M, Usadel B (2015) Starting to gel: how Arabidopsis seed coat epidermal cells produce specialized secondary cell walls. Int J Mol Sci 16: 3452–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56: 505–516 [DOI] [PubMed] [Google Scholar]
- Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, Haughn GW (2001) Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 127: 998–1011 [PMC free article] [PubMed] [Google Scholar]
- Western TL, Skinner DJ, Haughn GW (2000) Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 122: 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western TL, Young DS, Dean GH, Tan WL, Samuels AL, Haughn GW (2004) MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis seed coat. Plant Physiol 134: 296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TMIE, Visser J, Voragen A, Mikkelsen JD, Knox JP (2000) Analysis of pectin structure part 2 - Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohyd Res 327: 309–320 [DOI] [PubMed] [Google Scholar]
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AM (2000) Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J 22: 483–493 [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang Y, Wang X, Pei S, Kong Y, Hu R, Zhou G (2020) Transcription factors BLH2 and BLH4 regulate demethylesterification of homogalacturonan in seed mucilage. Plant Physiol 183: 96–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Zhou F, Lin Y (2012) Two novel positive cis-regulatory elements involved in green tissue-specific promoter activity in rice (Oryza sativa L ssp.). Plant Cell Rep 31: 1159–1172 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A (2008) The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development 135: 1991–1999 [DOI] [PubMed] [Google Scholar]
- Zhao X, Qiao L, Wu AM (2017) Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci Rep 7: 40672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.