Abstract
OBJECTIVES
Chemoradiotherapy (CRT) has been the backbone of guideline-recommended treatment for Stage IIIA non-small cell lung cancer (NSCLC). However, in selected operable patients with a resectable tumour, good results have been achieved with trimodality treatment (TT). The objective of this bi-institutional analysis of outcomes in patients treated for Stage IIIA NSCLC was to identify particular factors supporting the role of surgery after CRT.
METHODS
In a 2-centre retrospective cohort study, patients with Stage III NSCLC (seventh edition TNM) were identified and those patients with Stage IIIA who were treated with CRT or TT between January 2007 and December 2013 were selected. Patient characteristics as well as tumour parameters were evaluated in relation to outcome and whether or not these variables were predictive for the influence of treatment (TT or CRT) on outcome [overall survival (OS) or progression-free survival (PFS)]. Estimation of treatment effect on PFS and OS was performed using propensity-weighted cox regression analysis based on inverse probability weighting.
RESULTS
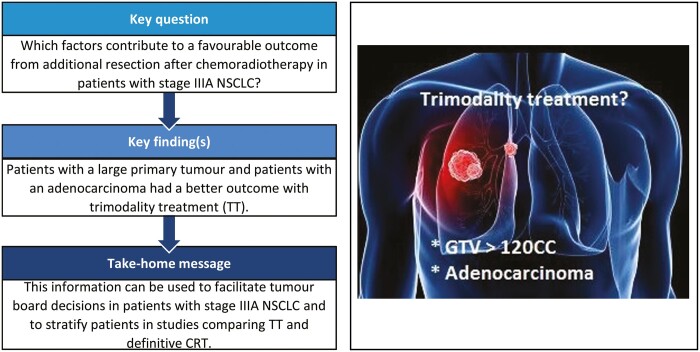
From a database of 725 Stage III NSCLC patients, 257 Stage IIIA NSCLC patients, treated with curative intent, were analysed; 186 (72%) with cIIIA-N2 and 71 (28%) with cT3N1/cT4N0 disease. One hundred and ninety-six (76.3%) patients were treated by CRT alone (high-dose radiation with daily low-dose cisplatin) and 61 (23.7%) by TT. The unweighted data showed that TT resulted in better PFS and OS. After weighting for factors predictive of treatment assignment, patients with a large gross tumour volume (>120 cc) had better PFS when treated with TT, and patients with an adenocarcinoma treated with TT had better OS, regardless of tumour volume.
CONCLUSIONS
Patients with Stage IIIA NSCLC and large tumour volume, as well as patients with adenocarcinoma, who were selected for TT, had favourable outcome compared to patients receiving CRT. This information can be used to assist multidisciplinary team decision-making and for stratifying patients in studies comparing TT and definitive CRT.
Keywords: Non-small cell lung cancer Stage IIIA, Chemoradiotherapy, Surgery, Tumour volume, Trimodality treatment
Approximately 30% of patients diagnosed with non-small cell lung cancer (NSCLC) present with Stage III disease [1].
INTRODUCTION
Approximately 30% of patients diagnosed with non-small cell lung cancer (NSCLC) present with Stage III disease [1]. For fit patients, concurrent chemoradiotherapy (CRT) or induction CRT followed by surgery is guideline-recommended radical treatment options, resulting in a 5-year survival of 15–32% and a median overall survival (OS) of 18–28 months [2–4]. Since the results of the PACIFIC study showed a significant improvement in survival with adjuvant durvalumab following CRT, this has changed the standard-of-care for patients with unresectable locally advanced NSCLC [5]. The optimal treatment strategy for patients with resectable locally advanced disease is an issue of ongoing debate during multidisciplinary tumour board discussions. This is fuelled by the fact that previous randomized trials have failed to show a survival benefit for patients receiving surgery after CRT for locally advanced NSCLC [4, 6, 7]. However, surgery is associated with favourable survival in patients with IIIA (e.g. T3N1/T4N0) disease, and in addition, other real-world data from several population-based studies suggest that selected patients with Stage IIIA-N2 locally advanced tumours might benefit from the addition of surgery to CRT (trimodality treatment, TT) [2, 6–9]. Awaiting data from phase III trials, investigating immunotherapy in the neoadjuvant setting, either alone, as dual therapy or in combination with chemotherapy and/or radiotherapy, refinement of treatment strategies for fit patients with potentially resectable, locally advanced NSCLC as to offering surgery following CRT would be helpful. To help multidisciplinary discussion and informed patient consent, the aim of this study was to try and identify those patients who might benefit the most from TT.
PATIENTS AND METHODS
Patients selection and data collection
After institutional review board (IRB) approval (Research Ethics Committee approval number IRBd20-277) and with an appropriate waiver of consent, patients with clinical Stage III NSCLC treated with definitive concurrent CRT or TT from January 2007 to December 2013 at the Netherlands Cancer Institute—Antoni van Leeuwenhoek (NKI-AVL) and at the Amsterdam University Medical Center (AUMC; location VU University Medical Center), were identified. Patients with Stage IIIB NSCLC were excluded because majority of these patients underwent definitive CRT, and therefore, we focused on patients with NSCLC Stage IIIA. Patients with a sulcus superior (Pancoast) tumour, a second primary lung cancer at initial presentation and patients operated on for a local recurrence after CRT, were excluded from this analysis. Only patients who completed treatment were included. OS was defined as the time between start of (induction) CRT and death of any cause. Progression-free survival (PFS), a secondary endpoint, was computed from the start of (induction) CRT to the date of progressive disease or death due to any cause.
Data concerning patient, tumour and treatment characteristics, as well as specific outcomes, were derived from patient records, date of death verified from the Dutch National Registry. All relevant data are within the manuscript and its supporting information files. Data on pre-treatment staging was collected, including computed tomography (CT) of the chest and upper abdomen and fluorodeoxyglucose positron emission tomography (FDG-PET) ± CT scan (PET-CT). If there was an FDG uptake in regional lymph nodes, involvement was preferably confirmed by e.g. endobronchial or endoscopic ultrasound techniques or by mediastinoscopy. When no pathological confirmation was obtained, the N-stage was based on PET-CT results. Pathological confirmation of the tumour was obtained by bronchoscopy or transthoracic biopsy or fine needle aspiration. A brain magnetic resonance imaging scan was performed to exclude brain metastases. All patients were staged using the seventh edition of the TNM staging system [10]. Because of the previously reported correlation between the gross tumour volume (GTV), recurrence and OS, the GTV of the primary tumour was extracted from the radiotherapy treatment planning system [11]. Shortly after completion of CRT, therapy response was evaluated by CT and/or PET-CT-scan [12] to select candidates for TT (with surgery typically being performed 4–8 weeks after CRT).
Treatment
After uniform staging according to the seventh edition of the Union for International Cancer Control (UICC) TNM classification, the treatment strategy for each individual patient was determined at a multidisciplinary tumour board meeting in the presence of dedicated specialists: a surgeon, pulmonary oncologist, radiation oncologist, radiologist, nuclear medicine physician, pathologist and specialized nurses. The recommendation for either CRT or TT was based on both patient factors (e.g. age, performance status, lung function, comorbidities) and tumour characteristics (e.g. resectability and size of the primary tumour and extent of mediastinal lymphadenopathy).
At institutional preference, different CRT treatment protocols were used: (i) at the NKI-AVL, CRT consisted of 24 administrations of daily low-dose cisplatin (6 mg/m2) concurrent with mildly hypo-fractionated radiotherapy: 24 fractions of 2.75 Gy to a total dose of 66 Gy [13]. (ii) At the AUMC, 3, occasionally 4 cycles of a full-dose cisplatin doublet were administered with radiotherapy typically starting at cycle 2 and consisting of 23–33 fractions of 2 Gy to total doses of 46 or 50 Gy if the tumour was deemed resectable at presentation, and higher doses of 60 or 66 Gy if there was doubt about resectability. A small number of patients were treated in Phase II and III clinical trials e.g. CRT with or without prophylactic cranial irradiation (NCT01282437) or CRT with concurrent olaparib or weekly cetuximab [14, 15].
Surgery consisted of resection of the primary tumour plus mediastinal lymph node dissection. Complete resection (R0) was defined as tumour-free bronchial, parenchyma, pleural and vascular margins. Pathologic complete response was reported if no vital tumour cells were present in the resection specimen or lymph nodes. Nodal down-staging was defined as pathological examination of the resection specimen revealing no tumour cells in initially (pre-treatment) positive (clinical or pathological) mediastinal lymph nodes.
Statistical analysis
PFS and OS were estimated using the Kaplan–Meier method. An estimation of treatment effect (i.e. TT versus CRT) on PFS and OS is complex because exposure to TT is likely to be confounded by selection based on patient and tumour characteristics. Therefore, a propensity-weighted analysis based on inverse probability weighting was performed. The objective of the propensity weighting is to compensate for imbalances stemming from the fact that the choice for either treatment was made by the patient and physician/multidisciplinary teams and not by randomization [16]. The statistical analysis was conducted in 2 steps which are described in the Supplementary Material, Text S1. All statistical analyses were performed in R version 4.0.3.
RESULTS
Patient and treatment characteristics
A total of 725 Stage III NSCLC patients, treated between 2007 and 2013, were identified. Two hundred and fifty-seven Stage IIIA patients were included for this analysis and 468 patients with Stage IIIB were excluded. One hundred and ninety-six (76.3%) patients received CRT alone (CRT group) and 61 (23.7%) patients were treated with induction CRT followed by surgery (TT group). Thirty-five out of 257 patients were selected in the AUMC—location VU University Medical Center of whom all were treated with TT. Before treatment, all patients had a CT of the chest and upper abdomen and FDG-PET CT scan. In 140 of the 186 patients in whom FDG uptake in regional lymph nodes was observed and endobronchial or endoscopic ultrasound techniques or mediastinoscopy were performed, pathologically proven N2-stage was confirmed. Adenocarcinoma (AC) was found in 76 (30%) patients [N0-1 n = 13 (17%), N2 n = 63 (83%)], squamous cell carcinoma in 90 (35%) patients [N0-1 n = 30 (33%), N2 n = 60 (67%)] and NSCLC not otherwise specified in 91 (35%) patients [N0-1 n = 28 (31%), N2 n = 63 (69%)]. To exclude brain metastases, all patients had pre-treatment cranial magnetic resonance imaging.
As is shown in Table 1, patients treated with TT were significantly younger, had significantly better pulmonary function, less comorbidity and presented with less advanced clinical N-status.
Table 1:
Patient and tumour characteristics of patients with clinical Stage IIIA NSCLC, treated with CRT or TT
| Characteristics | CRT | TT | All | P-value |
|---|---|---|---|---|
| (N = 196) | (N = 61) | (N = 257) | ||
| Age, years (median, IQR) | 65 (58–72) | 61 (55–67) | 64 (57–71) | 0.004 |
| Gender (no. of patients, %) | 1 | |||
| Male | 115 (59) | 36 (59) | 151 (59) | |
| Female | 81 (41) | 25 (41) | 106 (41) | |
| WHO performance (no. of patients, %) | 0.08 | |||
| 0 | 68 (35) | 14 (23) | 82 (32) | |
| 1 | 127 (65) | 12 (20) | 139 (54) | |
| Unknown | 1 (1) | 35 (57) | 36 (14) | |
| Charlson comorbidity index (no. of patients, %) | 0.02 | |||
| 2 | 7 (4) | 7 (11) | 14 (5) | |
| 3 | 13 (7) | 5 (8) | 18 (7) | |
| 4 | 3 (2) | 3 (5) | 6 (2) | |
| 5 | 1 (1) | 1 (2) | 2 (1) | |
| 6 | 70 (36) | 21 (34) | 91 (35) | |
| 7 | 62 (32) | 15 (25) | 77 (30) | |
| 8 | 19 (10) | 6 (10) | 25 (10) | |
| 9 | 17 (9) | 2 (3) | 19 (7) | |
| 10 | 4 (2) | 1 (2) | 5 (2) | |
| Preoperative FEV1 (% of predicted: median, IQR) | 76 (62–94) | 90 (76–102) | 80 (65–96) | 0.002 |
| GTV (cc, median, IQR) | 95 (53–172) | 74 (38–130) | 93 (49–165) | 0.07 |
| cT-stage (no. of patients, %) | 0.003 | |||
| T0/x | 4 (2) | 1 (2) | 5 (2) | |
| T1 | 39 (20) | 6 (10) | 45 (18) | |
| T2 | 79 (40) | 17 (28) | 96 (37) | |
| T3 | 39 (20) | 18 (30) | 57 (22) | |
| T4 | 35 (18) | 19 (31) | 54 (21) | |
| cN-stage (no. of patients, %) | 0.01 | |||
| N0 | 26 (13) | 16 (26) | 42 (16) | |
| N1 | 19 (10) | 10 (16) | 29 (11) | |
| N2 | 151 (77) | 35 (57) | 186 (72) | |
| Pathologically proven N2 stage (no. of patients, %) | 0.29 | |||
| Yes | 111 (56.6) | 29 (47.5) | 140 (54.5) | |
| No | 83 (42.3) | 32 (52.5) | 115 (44.7) | |
| Unknown | 2 (1.2) | 0 (0) | 2 (0.7) | |
| Pre-treatment histology (no. of patients, %) | 0.01 | |||
| AC | 54 (28) | 22 (36) | 76 (30) | |
| SCC | 78 (40) | 12 (20) | 90 (35) | |
| NSCLC NOS | 64 (33) | 27 (44) | 91 (35) | |
| Radiological response after CRT (no. of patients, %) | 0.06 | |||
| Complete response | 19 (10) | 11 (18) | 30 (12) | |
| Partial response | 108 (55) | 10 (16) | 118 (46) | |
| Stable disease or progression | 18 (9) | 5 (8) | 23 (9) | |
| Unknown | 51 (26) | 35 (57) | 86 (33) |
AC: adenocarcinoma; cN-stage: clinical nodal stage; cT-stage: clinical tumour stage; CRT: chemoradiotherapy; FEV1: forced expiratory volume in 1 second; GTV: gross tumour volume; IQR: interquartile; NSCLC NOS: non-small cell lung cancer not otherwise specified; SCC: squamous cell carcinoma; TT: trimodality treatment.
Low daily dose chemotherapy with concurrent 66 Gy/24 fractions radiotherapy was administered in 222 patients of whom 26 were treated with TT. Full-dose chemotherapy was administered in 35 patients, all treated with TT, of whom 19 underwent radiotherapy to a total dose of 46–50 Gy and higher doses of 56, 60 or 66 Gy were given in 16 patients. A small number of patients (N = 43) were treated in Phase II and III clinical trials. Lobectomy was performed in 52 of the TT patients (85.2%), pneumonectomy in 6 (9.8%) patients, a wedge resection in 2 (3.3%) patients and a mediastinal lymph node debulking in 1 (1.6%) patient. The complete pathological response was reported in 18% (n = 11) and an R0 resection in 83.6% (n = 51) of patients. Mediastinal lymph node down-staging was achieved in 79.3% (23/29) of pathologically confirmed N2 patients receiving surgery (Table 2).
Table 2:
Treatment and outcome characteristics of patients treated with TT
| TT (N = 61) | |
|---|---|
| Resection type (no. of patients, %) | |
| Lobectomy | 52 (85) |
| Pneumonectomy | 6 (9.8) |
| Wedge resection | 2 (3.3) |
| Mediastinal lymph node debulking | 1 (1.6) |
| Pathological complete response | |
| (tumour and lymph nodes, no. of patients, %) | |
| Yes | 18 (30) |
| No | 41 (67) |
| Unknown | 2 (3.2) |
| Resection margins (no. of patients, %) | |
| R0 | 51 (83) |
| R1 | 5 (8.2) |
| R2 | 0 (0) |
| Unknown | 5 (8.2) |
| ypT-stage (no. of patients, %) | |
| ypT0 | 19 (31) |
| ypT1 | 18 (30) |
| ypT2 | 14 (23) |
| ypT3 | 5 (8.2) |
| ypT4 | 1 (1.6) |
| yTis | 1 (1.6) |
| Unknown | 3 (4.9) |
| ypN-stage (no. of patients, %) | |
| ypN0 | 44 (72) |
| ypN1 | 6 (9.8) |
| ypN2 | 9 (15) |
| Unknown | 2 (3.3) |
| Mediastinal down-staging N2 (total cN2 = 35) | |
| (no. of patients, %) | |
| cN2 Path. proven → ypN1 or ypN0 | 23 (65.7) |
| cN2 Not path. proven → ypN1 or ypN0 | 4 (11.4) |
| cN2 Path. proven → ypN2 | 6 (17.1) |
| cN2 Not path. proven → ypN2 | 2 (5.7) |
| Post-resection 90-day mortality | |
| (no. of patients, %) | 3 (4.9) |
Follow-up and survival
The median follow-up of patients selected for TT and CRT was 33.5 [95% confidence interval (CI) 30.3–43.3] and 44.5 (95% CI 42.0–55.1) months, respectively. During the follow-up period, disease progression was observed less often in the TT group compared to the CRT group (41% vs 56%, respectively). There were 27 (44%) and 118 (60%) deaths in the TT and CRT groups, respectively.
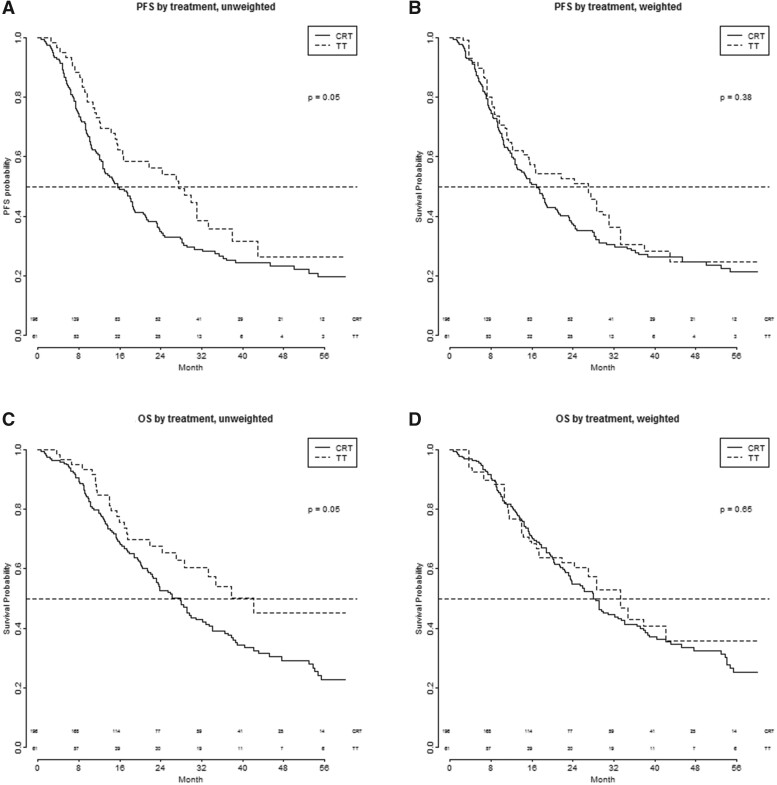
Figure 1 shows the PFS and OS after TT and CRT in the unadjusted and weighted analysis population (all patients included). In the unadjusted analysis, the median PFS was 27.6 months (95% CI: 16.6–43.0) for the TT group and 15.6 months (95% CI: 12.7–18.7) for the CRT group [hazard ratio (HR) 0.71 (95% CI: 0.5–1.01), P = 0.05 univariately and HR 0.75 (95% CI: 0.40–1.40), P = 0.37 multivariable]. The median OS was 42.3 months (95% CI 28.6—upper limit not attained) for patients receiving TT and 27.9 months (95% CI: 22.5–32.9) for patients receiving concurrent CRT [HR 0.66 (95% CI: 0:44–1.00), P = 0.05 univariately and HR 0.63 (95% CI: 0.29–1.34), P = 0.23 multivariable].
Figure 1:
(A–D) PFS and OS of patients with Stage IIIA NSCLC treated with CRT or TT. CRT: chemoradiotherapy; OS: overall survival; PFS: progression-free survival; TT: trimodality treatment.
After the inverse probability weighting was applied, the estimated median PFS in the TT group was 27.0 and 16.8 months in the CRT group [HR 0.82 (95% CI 0.54–1.27), P = 0.38 univariately and HR 0. 44 (95% CI: 0.39–2.28), P = 0.90 multivariable]. The estimated median OS in the TT group was 33.4 months, compared to 28.0 months in the CRT group [HR 0.87 (95% CI: 0.47–1.60), P = 0.64 univariately and HR 0.88 (95% CI: 0.24–3.23), P = 0.85 multivariable; Fig. 1].
Predictive and prognostic value of patient and tumour characteristics
Age, gender, WHO performance, tumour stage (N0-1 versus N2), Charlson Comorbidity Index (CCI), forced expiratory volume in 1 second and radiological response after CRT were not predictive for the effect of TT on PFS and OS. Tumour histology (AC vs squamous cell carcinoma + other) was predictive for OS (P for interaction 0.039) but not for PFS. GTV was predictive for PFS (P for interaction 0.043) but not for OS (P for interaction 0.061) when treated as a continuous variable. No prognostic effect of the characteristics on PFS was found. For OS, only age and gender were found to be prognostic after weighting (Supplementary Material, Tables S2 and S3).
Tumour nodal stage (N0-1 versus N2)
Figure 2 shows the PFS after TT and CRT in patients with nodal stage N0-1 versus nodal stage N2; for PFS, there was a slight benefit of TT in the N0-1 group. In the Supplementary Material, Text S2, the predictive effects of the nodal stage are described in more detail.
Figure 2:
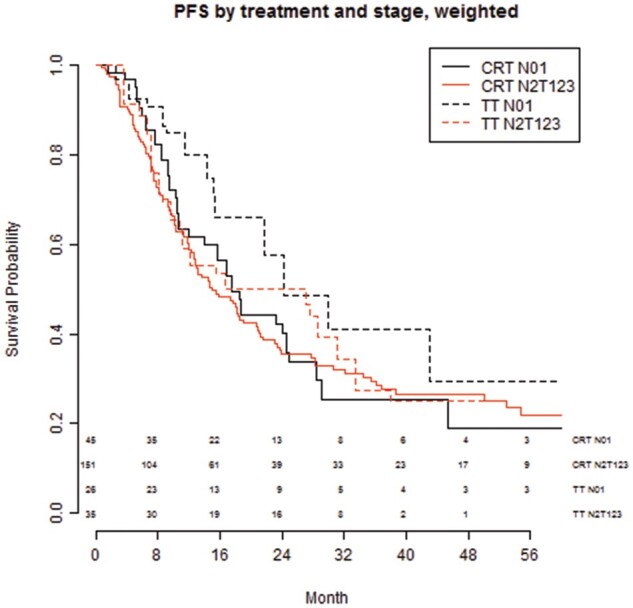
PFS by treatment and nodal stage (N0-1 vs N2) after CRT or TT. CRT: chemoradiotherapy; PFS: progression-free survival; TT: trimodality treatment.
Gross tumour volume
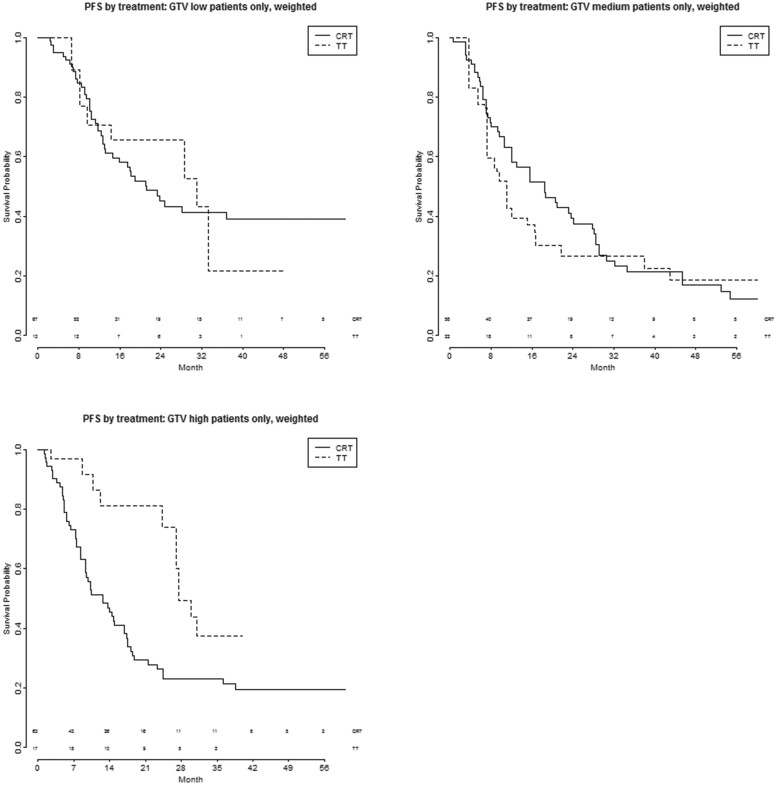
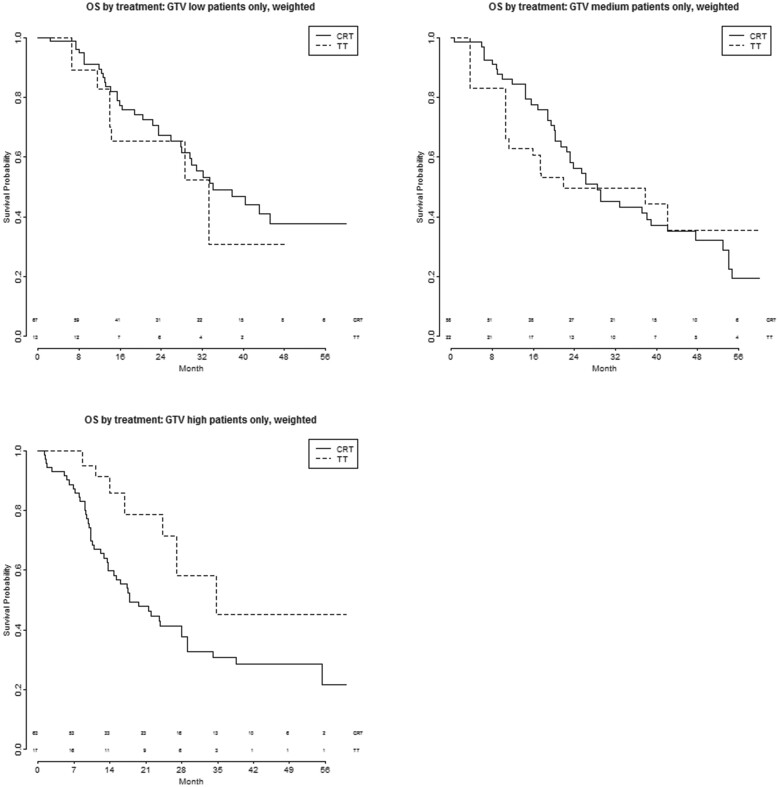
Figures 3A–C and 4A–C show the PFS and OS after TT and CRT, respectively, in patients with different volumes of the primary tumour, trichotomized into 3 categories with equal patient numbers (low GTV < 30 ml; medium GTV 30–92 ml; high GTV > 92 ml). Supplementary Material, Table S4 shows the tumour and nodal characteristics of these 3 groups—no significant differences were seen between them.
Figure 3:
(A–C) PFS by treatment and GTV trichotomized into 3 categories, after CRT or TT. CRT: chemoradiotherapy; GTV: gross tumour volume; PFS: progression-free survival; TT: trimodality treatment.
Figure 4:
(A–C) OS by treatment and GTV trichotomized into 3 categories, after CRT or TT. CRT: chemoradiotherapy; GTV: gross tumour volume; OS: overall survival; TT: trimodality treatment.
PFS is significantly better with TT (N0-1 n = 8, N2 n = 9) compared to CRT (N0-1 n = 25, N2 n = 38) in the group of patients with a high GTV: HR 0.39 (95% CI 0.19–0.83, P = 0.014) bivariate and 0.23 (95% CI 0.06–0.86, P = 0.029) multivariable. No significant differences were seen in the low (HR 0.96, 95% CI 0.44–2.1, P = 0.92 bivariate and HR 1.27, 95% CI 0.52–3.1, P = 0.61 multivariable) and medium (HR 1.2, 95% CI 0.58–2.3, P = 0. 67 bivariate and 1.7, 95% CI 0.78–3.6, P = 0.19 multivariable) GTV group (Supplementary Material, Table S5).
No significant differences were seen in OS in the 3 groups when comparing TT with CRT [GTV low: HR 1.3 (95% CI 0.56−2.95), P = 0.56 bivariate and 1.8 (95% CI 0.72−4.7), P = 0.20 multivariable; GTV medium: HR 1.05 (95% CI 0.45−2.5), P = 0.91 bivariate and 1.6 (95% CI 0.56–4.8), P = 0.37 multivariable; GTV high: HR 0.45 (95% CI 0.20−1.04), P = 0.06 bivariate and 0.33 (95% CI 0.083–1.3), P = 0.12 multivariable].
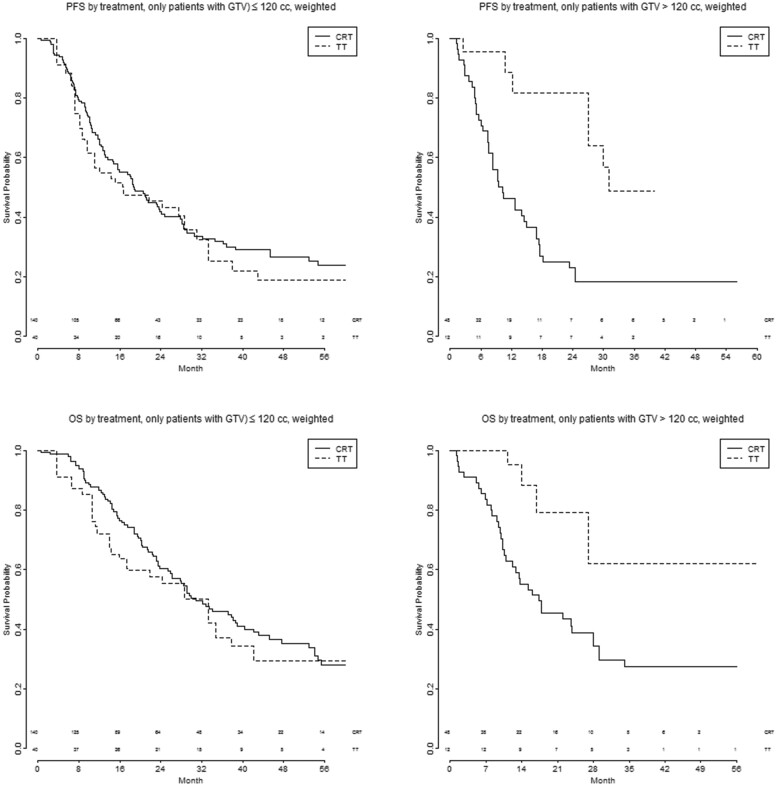
Figure 5 shows the PFS and OS after TT and CRT in patients with different GTV, dichotomized according to an optimized cut-off (small GTV ≤ 120 cc and large GTV > 120 cc). To optimize the TT-benefit in the ‘large’ tumour group, the cut-off was 120 cc, chosen as the ‘round’ number closest to the optimal cut-offs in our data (121 cc for PFS and 122 cc for OS). With this dichotomy, the effect of treatment seems to be different among the 2 GTV groups, not only for PFS but for OS as well (PFS: P for interaction 0.011 bivariate and 0.012 multivariable. OS: P for interaction 0.029 bivariate and 0.021 multivariable).
Figure 5:
(A–D) PFS and OS by treatment and GTV, dichotomized into 2 categories (small GTV ≤ 120 cc and large GTV > 120 cc), after CRT or TT. CRT: chemoradiotherapy; GTV: gross tumour volume; OS: overall survival; PFS: progression-free survival; TT: trimodality treatment.
PFS is significantly better with TT in patients with a large GTV: HR 0.27 (95% CI: 0.10–0.72, P = 0.008) bivariate and 0.20 (95% CI: 0.051–0.77, P = 0.03) multivariable. In the small GTV group, no difference is seen in PFS between CRT and TT: HR 1.105 (95% CI: 0.67–1.8, P = 0.70) bivariate and HR 1.74 (95% CI: 0.89–3.40, P = 0.11) multivariable.
For OS, significant differences were seen in the bivariate analysis in patients with a large GTV treated with TT HR 0.29 (95% CI: 0.093–0.90, P = 0.03), with a similar, but not statistically significant difference in the multivariable analysis: HR 0.28 (95% CI: 0.067–1.13, P = 0.073).
In the small GTV group, no significant OS-difference was found between CRT and TT: HR 1.18 (95% CI: 0.68–2.06, P = 0.44) bivariate and 1.86 (95% CI: 0.83–4.2, P = 0.13), multivariable.
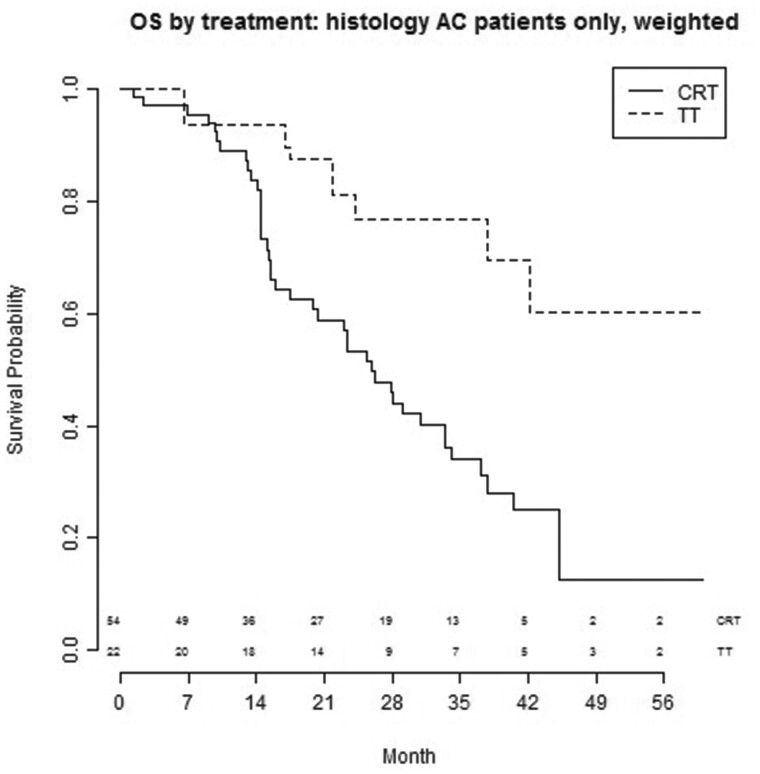
Histology
As presented in Fig. 6, patients with AC treated with TT (N0-1 n = 7, N2 n = 15) experienced a better OS compared to patients treated with CRT (N0-1 n = 6, N2 n = 48): HR 0.47 (95% CI: 0.21–1.09, P = 0.08), bivariate and HR 0.28 (95% CI: 0.078–0.99, P = 0.048), multivariable. In patients with squamous cell carcinoma, TT (N0-1 n = 5, N2 n = 7) yielded a comparable survival outcome to CRT (N0-1 n = 25, N2 n = 53): HR 1.4 (95% CI: 0.46–4.3, P = 0.55), bivariate and HR 0.78 (95% CI: 0.33–1.82, P = 0.47), multivariable (Supplementary Material, Table S5).
Figure 6:
OS by treatment of patients with AC after CRT or TT. AC: adenocarcinoma; CRT: chemoradiotherapy; OS: overall survival; TT: trimodality treatment.
DISCUSSION
Patients with Stage IIIA NSCLC had better PFS and OS when selected for TT compared with definitive CRT (in the unweighted analysis). Using propensity-weighted analysis, the effect of treatment on PFS and OS was most evident in patients with a large GTV and patients with locally advanced AC: those groups had improved PFS/OS and OS, respectively, when treated with TT. No differences were seen between the different treatment modalities in patients with nodal stage N0-1 and N2.
Prospective evidence for a survival advantage of adding surgery to CRT treatment for patients with locally advanced NSCLC is limited, with previous studies being compromised by including non-selected Stage IIIA patients, slow accrual, inadequate RT dose prescription, outdated radiotherapy techniques and allowing a pneumonectomy to be done, even when the outcome after pneumonectomy was considerably worse when compared to lobectomy [3, 4, 7, 17, 18]. A landmark Phase III study evaluated the potential benefit of surgical resection after CRT for Stage IIIA NSCLC which showed no significant benefit with modest evidence towards an increased 5-year survival after TT [4]. However, an unplanned exploratory analysis showed favourable OS for patients who underwent lobectomy versus a matched cohort with CRT alone, but not for those undergoing pneumonectomy [6]. Moreover, favourable outcome after induction therapy and surgery was found in patients with mediastinal down-staging after induction therapy, single level and ‘non-bulky’ N2 disease, a lobectomy (instead of a pneumonectomy) and a radical resection [6, 17, 19]. In previous studies, almost one-third of operated patients had a pneumonectomy, compared to 8.6% in our cohort. It is suggested that these high rates of pneumonectomy in literature might unfavourably affect the OS outcome [4, 6, 9]. Our incidence of pneumonectomy is relatively low, probably due to strict selection of patients in multidisciplinary tumour board meetings. Special care is needed when performing pneumonectomy after CRT due to the possibility of higher morbidity and mortality [18, 20]. However, in highly selected patients in whom an R0 resection is feasible, it may still be a potentially curative option with acceptable morbidity and mortality rates [6, 21, 22]. We have shown that surgical resection with dissection of the pulmonary artery branches and closure of the bronchus as required for lobectomy is safe in a highly irradiated field. When interpreting the data, it is notable that in the first months after treatment, survival was worse in the TT group, due, at least partly, to postoperative mortality [23].
From this analysis, we can add GTV as a predictive factor for improved PFS and potentially OS after TT. In addition, AC was a predictive factor for OS after TT. Previous reports have been published concerning the relationship between volume and survival and loco-regional failure for patients with Stage III NSCLC after CRT and/or surgery [14, 24, 25]. Although the tumour characteristics of these studies are heterogeneous, GTV is recognized as a prognostic factor for OS in Stage III patients treated with CRT [14, 24, 25]. However, the role of GTV in patients undergoing a resection has been unclear [24, 26]. Our findings may help treating physicians and multidisciplinary tumour board participants in choosing the most appropriate treatment for individual patients with locally advanced disease, as well as help in stratifying patients for future randomized trials comparing the 2 treatment modalities.
Limitations
We acknowledge some limitations of this work. The patients in the CRT group received high-dose, moderately hypo-fractionated radiotherapy with daily low-dose cisplatin [27, 28]. This schedule is used in a minority of institutions, conventional dose (e.g. 2 Gy)/fraction radiotherapy with e.g. a full-dose platinum doublet being the standard approach. The patients who were selected for TT also have intrinsically more favourable characteristics, the tumours are deemed resectable by the treating surgeon and the patients are fit enough to be medically operable. We compensated for this by introducing the propensity weighting and exploited multivariable models, but these methods can never fully replace randomization, so caution when interpreting the results is warranted. Moreover, since one of the participating centres included only patients who were selected for TT, we were unable to include ‘centre’ as a covariate in our models. No survival difference of TT versus definitive CRT was seen when patients were classified according to N0-1 or N2 status. However, in patients with suspected N2 disease on FDG-PET-CT, pathological confirmation was performed by mediastinal evaluation in only 75% of patients, so possible bias has to be taken into account.
Finally, we note that the role of surgery in the immunotherapy era needs to be clarified. Although there is improved systemic control and survival with adjuvant immunotherapy after CRT, surgery might still play an important role in maximizing local tumour control.
CONCLUSION
In conclusion, we found that for patients with Stage IIIA locally advanced NSCLC, large (>120 cc) tumour volume and AC had the most benefit from being treated in with TT, however, selection bias has to be taken into account. These results are relevant for decision-making in multidisciplinary teams and should be taken into consideration in any future trials comparing TT and CRT-based treatment approaches.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Author contributions
Pieter J.M. Joosten: Conceptualization; Data curation; Writing—original draft. Chris Dickhoff: Investigation; Methodology; Writing—review & editing. Vincent van der Noort: Formal analysis; Methodology; Resources; Software. Maarten Smeekens: Data curation; Writing—review & editing. Rachel C. Numan: Writing—review & editing. Houke M. Klomp: Writing—review & editing. Judi N.A. van Diessen: Data curation; Investigation; Writing—review & editing. Jose S.A. Belderbos: Writing—review & editing. Egbert F. Smit: Writing—review & editing. Kim Monkhorst: Writing—review & editing. Jan W.A. Oosterhuis: Writing—review & editing. Michel M. van den Heuvel: Conceptualization; Data curation; Writing—review & editing. Max Dahele: Writing—review & editing. Koen J. Hartemink: Conceptualization; Data curation; Supervision; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Pier Luigi Filosso, Francesco Petrella and the other anonymous reviewers for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CI
Confidence interval
- CRT
Chemoradiotherapy
- CT
Computed tomography
- FDG
Fluorodeoxyglucose
- GTV
Gross tumour volume
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PET
Positron emission tomography
- PFS
Progression-free survival
- TT
Trimodality treatment
REFERENCES
- 1. Morgensztern MD, Ng SH, Gao F, Govindan R.. Trends in stage distribution for patients with non-small cell lung cancer: a national cancer database survey. J Thorac Oncol 2010;5:29–33. [DOI] [PubMed] [Google Scholar]
- 2. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J. et al. ; ESMO Guidelines Committee. Early-stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol 2017;28:iv1–21. [DOI] [PubMed] [Google Scholar]
- 3. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE. et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non–small-cell lung cancer. J Clin Oncol 2020;38:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eberhardt WE, Pöttgen C, Gauler TC, Friedel G, Veit S, Heinrich V. et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194–201. [DOI] [PubMed] [Google Scholar]
- 5. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. ; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 6. Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C. et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pöttgen C, Eberhardt W, Stamatis G, Stuschke M.. Definitive radiochemotherapy versus surgery within multimodality treatment in stage III non-small cell lung cancer (NSCLC)—a cumulative meta-analysis of the randomized evidence. Oncotarget 2017;8:41670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickhoff C, Hartemink KJ, van de Ven PM, van Reij EJ, Senan S, Paul MA. et al. Trimodality therapy for stage IIIA non-small cell lung cancer: benchmarking multi-disciplinary team decision-making and function. Lung Cancer 2014;85:218–23. [DOI] [PubMed] [Google Scholar]
- 9. Xu XL, Dan L, Chen W, Zhu SM, Mao WM.. Neoadjuvant chemoradiotherapy or chemotherapy followed by surgery is superior to that followed by definitive chemoradiation or radiotherapy in stage IIIA (N2) nonsmall-cell lung cancer: a meta-analysis and system review. Onco Targets Ther 2016;9:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rami-Porta R, Crowley JJ, Goldstraw P.. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4–9. [PubMed] [Google Scholar]
- 11. van Diessen JNA, Chen C, van den Heuvel MM, Belderbos JS, Sonke JJ.. Differential analysis of local and regional failure in locally advanced non-small cell lung cancer patients treated with concurrent chemoradiotherapy. Radiother Oncol 2016;118:447–52. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 13. Uyterlinde W, Chen C, Nijkamp J, Obbink MG, Sonke JJ, Belderbos J. et al. Treatment adherence in concurrent chemoradiation in patients with locally advanced non-small cell lung carcinoma: results of daily intravenous prehydration. Radiother Oncol 2014;110:488–92. [DOI] [PubMed] [Google Scholar]
- 14. Van den Heuvel MM, Uyterlinde W, Vincent AD, de Jong J, Aerts J, Koppe F. et al. Additional weekly Cetuximab to concurrent chemoradiotherapy in locally advanced non-small cell lung carcinoma: efficacy and safety outcomes of a randomized, multi-center phase II study investigating. Radiother Oncol 2014;110:126–31. [DOI] [PubMed] [Google Scholar]
- 15. Walraven I, van den Heuvel M, van Diessen J, Schaake E, Uyterlinde W, Aerts J. et al. Long-term follow-up of patients with locally advanced non-small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab. Radiother Oncol 2016;118:442–6. [DOI] [PubMed] [Google Scholar]
- 16. Lunceford KJ, Davidian M.. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med 2004;23:2937–60. [DOI] [PubMed] [Google Scholar]
- 17. Bryan DS, Donington JS.. The role of surgery in management of locally advanced non-small cell lung cancer. Curr Treat Options Oncol 2019;20:27. [DOI] [PubMed] [Google Scholar]
- 18. Aggarwal C, Li L, Borghaei H, Somaiah N, Turaka A, Langer CJ. et al. Multidisciplinary therapy of stage IIIA non-small-cell lung cancer: long-term outcome of chemoradiation with or without surgery. Cancer Control 2014;21:57–62. [DOI] [PubMed] [Google Scholar]
- 19. Ziel E, Hermann G, Sen N, Bonomi P, Liptay MJ, Fidler MJ. et al. Survival benefit of surgery after chemoradiotherapy for stage III (N0-2) non-small-cell lung cancer is dependent on pathologic nodal response. J Thorac Oncol 2015;10:1475–80. [DOI] [PubMed] [Google Scholar]
- 20. Martin J, Ginsberg RJ, Abolhoda A, Bains MS, Downey RJ, Korst RJ. et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg 2001;72:1149–54. [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi M, Shimamatsu S, Edagawa M, Hirai F, Toyozawa R, Nosaki K. et al. Pneumonectomy after induction chemoradiotherapy for locally advanced non-small cell lung cancer: should curative intent pulmonary resection be avoided? Surg Today 2019;49:197–205. [DOI] [PubMed] [Google Scholar]
- 22. Steger V, Spengler W, Hetzel J, Veit S, Walker T, Mustafi M. et al. Pneumonectomy: calculable or non-tolerable risk factor in trimodal therapy for Stage III non-small-cell lung cancer? Eur J Cardiothorac Surg 2012;41:880–5. [DOI] [PubMed] [Google Scholar]
- 23. McMillan RR, Berger A, Sima CS, Lou F, Dycoco J, Rusch V. et al. Thirty-day mortality underestimates the risk of early death after major resections for thoracic malignancies. Ann Thorac Surg 2014;98:1769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Laar M, van Amsterdam WAC, van Lindert ASR, de Jong PA, Verhoeff JJC.. Prognostic factors for overall survival of stage III non-small cell lung cancer patients on computed tomography: a systematic review and meta-analysis. Radiother Oncol 2020;151:152–75. [DOI] [PubMed] [Google Scholar]
- 25. Ostheimer C, Mäurer M, Ebert N, Schmitt D, Krug D, Baumann R. et al. Prognostic impact of gross tumor volume during radical radiochemotherapy of locally advanced non-small cell lung cancer—results from the NCT03055715 multicenter cohort study of the Young DEGRO Trial Group. Strahlenther Onkol 2021;197:385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guberina M, Eberhardt W, Stuschke M, Gauler T, Aigner C, Schuler M. et al. Pretreatment metabolic tumour volume in stage IIIA/B non-small-cell lung cancer uncovers differences in effectiveness of definitive radiochemotherapy schedules: analysis of the ESPATUE randomized phase 3 trial. Eur J Nucl Med Mol Imaging 2019;46:1439–47. [DOI] [PubMed] [Google Scholar]
- 27. Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P. et al. ; EORTC LCG and RT Group. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur J Cancer 2007;43:114–21. [DOI] [PubMed] [Google Scholar]
- 28. Dieleman EMT, Uitterhoeve ALJ, van Hoek MW, van Os RM, Wiersma J, Koolen MGJ. et al. Concurrent daily cisplatin and high-dose radiation therapy in patients with stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2018;102:543–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.