Abstract
Introduction
Staphylococcus epidermidis (SE) is a common cause of bacterial keratitis in certain geographic areas. A high percentage of resistance to methicillin is shown, which gives it cross resistance to beta-lactams and sometimes resistance to other antibacterial groups. We analyzed clinical and microbiological variables in patients with infectious keratitis due to SE.
Methods
Medical records of 43 patients with suspected infectious keratitis and microbiological confirmation for SE, between October 2017 and October 2020, were retrospectively studied. Clinical characteristics (risk factors, size of lesions, treatment, evolution) and microbiological (susceptibility to antibiotics) were analyzed, and groups of patients with methicillin-resistant (MRSE) and methicillin-susceptible (MSSE) infection were compared.
Results
MRSE was present in 37.2% of infectious keratitis. All isolates were sensitive to vancomycin and linezolid. Rates of resistance to tetracyclines and ciprofloxacin were 50% and 56% in the MRSE group, and 11% and 7% in the MSSE group. The clinical characteristics, including size of lesion, visual axis involvement, inflammation of anterior chamber, presence of risk factors and follow-up time, did not show statistically significant differences between groups.
Conclusions
MRSE is a common cause of infectious keratitis caused by SE and shows a high rate of multidrug resistance. Clinically, it does not differ from MSSE keratitis. Additional work is needed to confirm these findings.
Keywords: keratitis, Staphylococcus epidermidis, methicillin-resistant
Abstract
Introducción
Staphylococcus epidermidis (SE) es una causa frecuente de queratitis bacteriana en ciertas áreas geográficas. Presenta un alto porcentaje de resistencia a meticilina, lo que confiere resistencia cruzada a beta-lactámicos y en algunas ocasiones también resistencia a otros grupos de anti-bacterianos. Analizamos variables clínicas y microbiológicas en pacientes con queratitis infecciosa por SE.
Métodos
Se analizaron retrospectivamente las historias clínicas de 43 pacientes con sospecha de queratitis infecciosa y confirmación microbiológica para SE, entre octubre de 2017 y octubre de 2020. Se analizaron las características clínicas (factores de riesgo, tamaño de las lesiones, tratamiento, evolución) y microbiológicas (susceptibilidad a antibióticos) y se compararon grupos de pacientes con infección resistente (MRSE) y sensible a meticilina (MSSE).
Resultados
El 37,2% de las queratitis fueron por MRSE. Todos los aislados fueron sensibles a vancomicina y linezolid. Las tasas de resistencia a tetraciclinas y ciprofloxacino fueron 50% y 56% en el grupo de MRSE, y 11% y 7% en el grupo de MSSE. Las características clínicas, incluido el tamaño de la lesión, la afectación del eje visual, la inflamación de la cámara anterior, la presencia de factores de riesgo y el tiempo de seguimiento, no mostraron diferencias estadísticamente significativas entre los grupos.
Conclusiones
MRSE es una causa frecuente de las queratitis infecciosas producidas por SE y presenta una alta tasa de resistencia a múltiples fármacos. Clínicamente, no muestra diferencias clínicas con la queratitis por MSSE. Se necesitan trabajos adicionales para confirmar estos hallazgos.
Keywords: queratitis, Staphylococcus epidermidis, resistente a meticilina
INTRODUCTION
Gram-positive bacteria are the most common culture-isolated microorganisms in bacterial keratitis according to several series [1-4]. Methicillin-resistant Staphylococcus aureus (MRSA) keratitis has been widely described [5-8]. However, literature related to methicillin-resistant Staphylococcus epidermidis (MRSE) is limited. Goodman et al. first reported two cases of MRSE keratitis in 1988 [9]. The prevalence has increased since then as showed in larger epidemiological studies, ranging from 34 to 79% [2,3,10].
S. epidermidis is opportunistic bacteria found in the normal skin microbiota and frequently acquires resistance to antibiotics [11-13]. MRSE is resistant to beta-lactams and frequently acquires resistance to other antibiotics for ophthalmological use such as quinolones or tetracyclines. At sites with poor immunosurveillance, such as foreign bodies, MRSE can cause infections and develop biofilms, making it difficult for antibiotics to interact with bacteria, increasing their resistance and making treatment more difficult [14]. The intact cornea and tear layer create a physical immune barrier that prevents opportunistic microorganisms, with limited pathogenicity, from causing infections, which is critical due to the almost complete absence of leukocytes in the tear fluid and corneal layer [15,16]. However, damage to this barrier facilitates microorganisms penetration and, consequently, infection.
We present the clinical characteristics, antibiotic resistance, and treatment results of patients with culture-confirmed MRSE keratitis compared to those with methicillin-sensitive S. epidermidis keratitis (MSSE) who attended the Ophthalmology Service in a period of three years.
METHODS
Corneal scrape samples were reviewed retrospectively at the Microbiology Service of the Hospital Clínico Universitario of Valencia during the period between October-2017 and October-2020.
All cases were diagnosed on the first visit as suspected infectious keratitis after slit lamp examination. The criteria considered were conventional: corneal lesion with stromal infiltration (with or without cells in the anterior chamber), pain, discomfort and redness, together with the patient’s anamnesis including previous ocular risk factors [1]. To avoid including colonization samples in our study, we included only symptomatic patients with inflammation signs in slit lamp examination.
Patients with monomicrobial culture for S. epidermidis and compatible clinical manifestations were included. The clinical history, microbiological results and available slit lamp photographs of all patients were reviewed. Clinical information collected included: patient’s age and sex, ocular risk factors, number of follow-up days required, size of the lesion at the time of diagnosis, visual axis involvement and clinical outcomes. All patients were managed in the outpatient clinic.
Patients were divided into severe and non-severe infectious keratitis, according to the following criteria: cells in the anterior chamber, size of the lesion>3 mm and/or involvement of the visual axis.
The size, maximum and minimum, of the infiltrate in millimeters was measured using the ruler adjusted to the slit lamp, as well as photographic control.
Sample collection and microbiological study. All samples were collected in the ophthalmology unit by corneal scraping procedure, direct inoculation onto appropriate culture media [3] and rapid shipment (within 2 hours) to the microbiology laboratory. Blood and chocolate agar plates were incubated in 10% carbon dioxide environments at 35°C for 5-7 days. To exclude accidental contaminants, the criterion to consider a positive culture was the monomicrobial growth of at least 10 colonies on a solid medium with similar morphology to the Gram stain. Cultures that isolated multiple organisms were excluded. Bacterial identification was carried out using MALDI-TOF technology (Bruker Daltonics). Antibiotic susceptibility studies were performed by broth microdilution (MicroScan, Beckman) and the minimum inhibitory concentrations (MIC) obtained were interpreted according to EUCAST guidelines. Antibiotics analyzed in all isolated bacterial strains were: cloxacillin, cotrimoxazole, erythromycin, clindamycin, ciprofloxacin, vancomycin, linezolid, daptomycin, tetracycline, rifampin, fusidic acid, mupirocin, chloramphenicol, and gentamicin.
Treatment and follow-up. After diagnosis, patients were empirically treated hourly, according to American Academy of Ophthalmology, with fortified eye drops of ceftazidime (50mg/ ml) and vancomycin (50mg/ml), or ceftazidime (50mg/ml) and tobramycin (50mg/ml), or with fluoroquinolones (3-5mg/ml), along with cyclopentolate 10 mg/ml every 8 hours; the final treatment decision was made according to physician’s discretion.
The first check-up was carried out 48 hours after the diagnosis in all cases, and at this time the evolution was classified as either good or suboptimal. The patients were followed-up and days until resolution of lesion (absence of fluorescein cor-neal lesion’s staining and absence of inflammation signs) were counted.
Statistical analysis. All the statistical analyses were performed using SPSS, Version 27, computer software (IBM, Armonk, NY, USA). Binary variables were analysed using chi-square or Fisher’s exact test (when expected value <5). Quantitative variables were analyzed with t-test when assuming normal distribution of the data and U-Mann-Whitney when it was not.
A p-value <0.05 was considered statistically significant. Antibiotic resistance was calculated in percentages.
RESULTS
During the study period, forty-three patients with clinical diagnosis of keratitis showed a monomicrobial culture for S. epidermidis, 16 of which were resistant to methicillin (37.2%).
Four patients were excluded due to probable contamination: three cases had clinically small marginal infiltrates associated with an epithelial defect and were mildly symptomatic, and the other case had a spongy margin ulcer and torpid evolution, in which subsequent cultures were positive for Aspergillus.
Patient characteristics. Patient’s mean age was 55.1 years, ranging from 11 to 89 years (MRSE group: 62.6 years, range 23-82 years; MSSE group: 50.7 years, range 11-89 years; p = 0.094). Twenty-two patients were women (51.2%), with no statistical differences between the two S. epidermidis groups.
Clinical characteristics. All cases presented with pain and redness of the affected eye, along with one or more epithelial defects and perilesional stromal infiltration.
The mean size of the lesion in maximum and minimum diameter was 1.68x1.05mm (MRSE group: 2.1x1.17mm; MSSE group 1.44x0.97mm; p=0.102 for maximum diameter and p=0.812 for minimum diameter).
Twenty-one patients (48,8%) presented with inflammation in the anterior chamber (MRSE group: n=10, 62.5%; MSSE group: n=11, 40.1%; p=0.083). Five patients had dense stromal edema, making the assessment of the anterior chamber impossible with slit lamp examination. Twenty-nine patients (67.4%) were classified after with criteria of severe keratitis (MRSE group: n=13, 81.3%; MSSE group: n=16, 59.3%; p=0.186). The lesion affected the visual axis in 15 cases (MRSE group: n=6, 37.5%; MSSE group: n=9, 33.3%; p=0.782).
All cases were unilateral, suggesting local risk factors rather than systemic conditions.
The mean follow-up time was 18,5 days until the cessation of the disease (MRSE group: 27.4 days, range 2-166 days; MSSE group: 13 days, range 2-59 days; p=0.129).
The medical records review did not show previous antibiotic treatment or hospital care in the last 4 weeks.
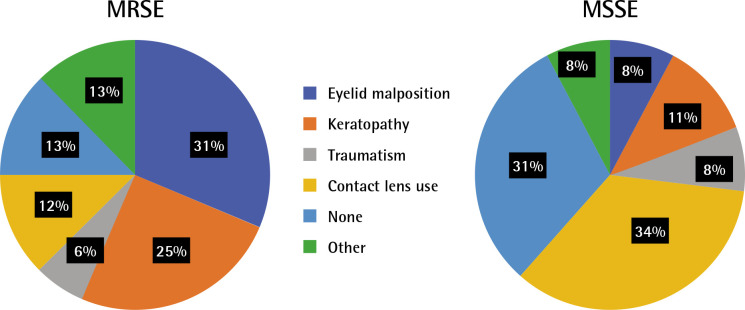
Risk factors and clinical outcomes. Ocular or systemic predisposing factors were present in 33 cases (76.7%). The risk factors include: eyelid malposition, keratopathy, traumatism, contact lens use, none and other (including antiglaucomatous topical medication, intellectual disability, systemic immunosuppression and chronic lacrimal obstruction). The MRSE keratitis are most commonly associated with eyelid malposition (31%) and previous keratopathy (25%) (Figure 1). The MSSE keratitis’ main risk factors are contact lens use (34%) and keratopathy (11%). Only 13% of cases in the MRSE group occurs without predisposing conditions, whereas 31% in the MSSE group.
Figure 1.
Risk factors of MRSE and MSSE keratitis cases.
MRSE: methicillin-resistant Staphylococcus epidermidis, MSSE: methicillin-susceptible Staphylococcus epidermidis.
MRSE group (n= 16). Fourteen cases of 16 (87.5%) occurred in eyes with previous risk factors or systemic predisposing conditions such as: lagophthalmos (3), contact lens use (2), neurotrophic keratopathy (2), distichiasis (2), traumatism (1), bullous keratopathy (1), penetrant keratoplasty (1), intellectual disability (1) and chronic use of antiglaucomatous topical medication (1). In 2 cases no prior risk factors were identified.
The outcomes were: corneal thinning (2), central leucoma affecting visual axis (2), penetrant keratoplasty (1) and total corneal opacity (1). The other 10 cases resolved without functional sequelae. The clinical and epidemiological characteristics are shown in Table 1.
Table 1.
Cases of keratitis in the MRSE group and clinical characteristics.
| Patient | Sex | Age (years) | Risk factor | Follow-up (days) | Size (max. x min. size) (mm) | Visual axis |
|---|---|---|---|---|---|---|
| 1 | Female | 82 | Lagophthalmos | 15 | 3.5x1.2 | No |
| 2 | Female | 75 | Bullous keratopathy | 8 | 4.5x4 | Yes |
| 3 | Female | 49 | Penetrant keratoplasty | 18 | 2x1 | No |
| 4 | Male | 82 | Lagophthalmos | 36 | 2x1 | Yes |
| 5 | Male | 60 | Traumatism | 2 | 0.4x0.2 | No |
| 6 | Male | 47 | Lagophthalmos | 166 | 0.9x0.8 | Yes |
| 7 | Female | 65 | Neurotrophic keratopathy | 59 | 1x0.8 | Yes |
| 8 | Female | 82 | Distichiasis | 26 | Not available | Yes |
| 9 | Female | 37 | Contact lens use | 9 | 0.2x0.2 | No |
| 10 | Female | 59 | None | 10 | 1.7x1 | No |
| 11 | Female | 66 | Neurotrophic keratopathy | 29 | 5.8x3.5 | Yes |
| 12 | Female | 41 | Intellectual disability | 8 | 1.7x0.2 | No |
| 13 | Male | 76 | None | 20 | 4x0.2 | No |
| 14 | Female | 80 | Antiglaucomatous topical medication | 13 | 1.3x1 | No |
| 15 | Female | 77 | Distichiasis | 8 | 1x1 | No |
| 16 | Female | 23 | Contact lens use | 11 | 1.5x1.5 | No |
MRSE: methicillin-resistant Staphylococcus epidermidis.
MSSE group (n= 27). Eight of the 27 cases (29,6%) did not present ocular risk factors or systemic conditions. The risk factors present in the other 19 patients were: contact lens use (10), traumatism (2), neurotrophic keratopathy (2), bullous keratopathy (1), distichiasis (1), systemic immunosuppression (1), chronic lacrimal obstruction (1) and floppy eyelid syndrome (1).
The outcomes were: mild leucoma without visual impairment in 23 cases, total corneal opacity (2), endophthalmitis (1) and corneal thinning (1).
A p=0.337 was calculated comparing the presence of any risk factor between MRSE and MSSE group.
Treatment and bacterial sensitivity to antibiotics. Of all cases with severe keratitis criteria (29), 20 (69%) were empirically treated with fortified eye drops of antibiotics administered hourly for 48 hours: 9 cases were treated with ceftazidime and tobramycin and 11 cases with ceftazidime and vancomycin. The other 9 (31%) cases were empirically treated with commercial antibiotics: 2 with topical moxifloxacin (5mg/ ml) and 7 with ciprofloxacin (3mg/ml). At the 48-hour checkup, if the clinical evolution was suboptimal, the treatment was adjusted according to the antibiogram.
The initial regime with commercial topical quinolones was maintained in all 9 (100%) cases due to good clinical evolution (less corneal infiltrate, less symptoms and/or decreased inflammation in the anterior chamber).
Of the 9 cases initially treated with ceftazidime and tobramycin, in 3 (33%) cases the same antibiotics were maintained due to good clinical evolution and rapid clinical resolution. The remaining 6 (66%) were changed after receiving antibiogram results: 2 to fortified vancomycin and one to amikacin, whereas the other 3 were shifted to commercial ciprofloxacin.
Out of the 11 cases treated initially with fortified ceftazi-dime and vancomycin, 2 (18%) patients were kept with the same treatment due to rapid resolution of the infection in one case and because of suboptimal response in the other case. The rest (9, 82%) were adjusted after antibiogram results. 1 was changed to fortified vancomycin after showing resistance to the other antibiotics commercially available, and the rest (8) were switched to commercial medication: one to gentamycin (3mg/ml), 3 to ciprofloxacin (3mg/ml), 3 to moxifloxacin (5mg/ ml) and 1 to tobramycin (3mg/ml).
Of the cases that did not meet criteria for severe keratitis (14), 10 (71%) were treated initially with topical ciprofloxacin hourly for 48 hours; this regime was maintained after the complete resolution of the infiltrate, with good clinical evolution and no side effects in all cases. Two (14%) cases were treated initially with ceftazidime and tobramycin (both with good clinical evolution), and 2 (14%) with ceftazidime and vancomycin (one of them with suboptimal evolution).
Comparative results of antibiotic susceptibility are shown in the Table 2. In the MRSE group, there were significant rates of resistance to ciprofloxacin (56%), tetracycline (50%), fusidic acid (45%) mupirocin (73%), and erythromycin (100%). Changes in MIC90 of the following antibiotics were detected: linezolid, vancomycin, ciprofloxacin, tetracycline, fusidic acid, mupirocin and aminoglycosides. In vitro, the best results were obtained with linezolid, vancomycin, daptomycin, chloramphenicol and rifampicin.
Table 2.
Percentages of antibiotics resistance in MRSE and MSSE groups.
| MRSE (N=16) | MSSE (N=27) | p | |||
|---|---|---|---|---|---|
| % Resistance | MIC90 | % Resistance | MIC90 | ||
| Cloxacillin | 100 | >2 | 0 | ≤0.25 | <0.001* |
| Cotrimoxazole | 13 | ≤2/38 | 7 | ≤2/38 | 0.578 |
| Erythromycin | 100 | >4 | 74 | >4 | 0.026* |
| Clindamycin | 75 | 0.5 | 33 | 0.5 | 0.063 |
| Ciprofloxacin | 56 | >2 | 7 | ≤1 | <0.001* |
| Vancomycin | 0 | 4 | 0 | 2 | |
| Linezolid | 0 | 4 | 0 | 2 | |
| Daptomycin | 0 | ≤1 | 0 | ≤1 | |
| Tetracycline | 50 | >8 | 11 | 2 | 0.005* |
| Rifampicin | 0 | ≤0.5 | 0 | ≤0.5 | |
| Fusidico | 45 | >2 | 8 | ≤2 | 0.013* |
| Mupirocin | 73 | >256 | 24 | <256 | 0.02* |
| Chloramphenicol | 0 | ≤8 | 0 | ≤8 | |
| Gentamicin | 38 | >8 | 18 | 4 | 0.168 |
| Tobramycin | 44 | >8 | 30 | 8 | 0.348 |
MRSE: methicillin-resistant Staphylococcus epidermidis, MSSE: methicillin-susceptible Staphylococcus epidermidis. MIC90 value was defined as the lowest concentration of the antibiotic at which 90% of the isolates were inhibited. *P<0.05
Treatment outcomes in MSSE and MRSE groups. Cases of severe and non-severe keratitis in MRSE and MSSE groups with good clinical response after empirical treatment are showed in Table 3. At 48-hour follow-ups, 20 (74%) cases of MSSE keratitis presented with good clinical evolution, and 13 (81%) cases in the MRSE group (p= 0,719).
Table 3.
Cases of severe and non-severe keratitis in MRSE and MSSE groups with good clinical response after 48 hours of empirical treatment.
| Severe (N=29) | Non-severe (N=14) | % total | |||
|---|---|---|---|---|---|
| MRSE (N=13) | MSSE (N=16) | MRSE (N=3) | MSSE (N=11) | ||
| Fluoroquinolones | 3 (100%) | 5 (83%) | 3 (100%) | 7 (100%) | 18 (95%) |
| Ceftazidime + vancomycin | 4 (80%) | 2 (33%) | 0 (0%) | 1 (50%) | 7 (54%) |
| Ceftazidime + tobramycin | 3 (60%) | 3 (75%) | 0 (0%) | 2 (100%) | 8 (73%) |
MRSE: methicillin-resistant Staphylococcus epidermidis, MSSE: methicillin-susceptible Staphylococcus epidermidis.
Of those with suboptimal clinical response (10/43; 23%), the initial treatment was: quinolones (1, 1%; in MSSE group); ceftazidime and tobramycin (3, 30%; 2 MRSE group and 1 MSSE group) and ceftazidime and vancomycin (6, 60%; 1 MRSE group and 5 MSSE group).
Of all the 19 cases of keratitis treated initially with commercial ciprofloxacin, 18 (95%) cases showed a good clinical evolution after 48 hours. The only patient with suboptimal response was classified as severe keratitis in the MSSE group (n=1; 5%).
In severe keratitis caused by MRSE, the ceftazidime and vancomycin regime showed an 80% efficacy (4 out of 5), and a 60% efficacy (3 out of 5) with ceftazidime and tobramycin combination treatment, p>0,99. In MSSE group, treatment with ceftazidime and tobramycin showed outcomes of 33% and 75% respectively in severe keratitis and 50% and 100% in non-severe, p>0,99
DISCUSSION
According to recommendations from the American Academy of Ophthalmology, bacterial keratitis are commonly treated with ciprofloxacin as empirical treatment [17]. However, MRSE is associated with in vitro resistance to this antibiotic, as well as to others commonly used in the ophthalmic clinical practice such as tobramycin [12,18].
Some (75%) of the infections presented in this study resolved after antibiotic treatment with fortified topical antibiotics. The rest were treated with commercial ciprofloxacin, also with good clinical response including severe cases. We found no differences among the different treatment regimes in both groups. Therefore, the evolution of the infection not only depends on treatment, but other aspects such as patient’s risk factors or initial presentation of the lesion must also be taken into account.
In case of progression to endophthalmitis, oral linezolid may be a valid choice, since it has shown excellent antibacterial sensitivity and has a good intraocular penetration that has been previously reported [19].
In the infectious pathology of the anterior pole of eye, ocular microbiota are the most frequently documented causing infection, showing that the border between commensal micro-biota and pathogenic microorganism is increasingly thin. Previous studies highlight the colonization rate of Staphylococcus species on the ocular surface of healthy eyes, with S. epidermidis being the most frequently isolated microorganism [11, 12, 20]. The patient’s medical history and a careful slit lamp ophthalmologic evaluation are important to rule out possible false positives from microbiological analysis.
These multidrug resistant bacteria are not rare findings in the clinical practice. In this study, a rate of 37.2% of MRSE is presented, which is similar to other publications [2,3]. Our study supports the hypothesis that the loss of ocular surface homeostasis can lead to corneal ulcers and stromal infiltration, since the majority of cases were associated with risk factors.
In our experience, MRSE infections have shown to be clinically similar to those caused by MSSE, as all the parameters analyzed showed no statistical significance (follow-up time, presence of risk factors, size of the lesion, anterior chamber inflammation or clinical response at 48h check-up). However, MRSE have shown to have higher in vitro resistances to common antibiotics.
Recent reports document multi-site infections with extremely resistant S. epidermidis to antibiotics, including linezolid, vancomycin, and teicoplanin [21,22]. This represents a major health problem in the near future, not only related to ophthalmological conditions but also to systemic infections that could lead to the death of a patient.
We consider the relevance of the microbiological analysis in all keratitis, not only in severe cases, in order to establish the etiology and to adequately treat patients with specific medication, so as not to contribute to increase antibiotic resistance in the future.
The main limitation of study is the small number of patients included, although to our knowledge there are no long comparative series of S. epidermidis keratitis. Other limitations are its retrospective design and the inherent differences in clinical and therapeutic actions during patient management. In order to minimize the risk of overdiagnosis and attribute the etiology to the local eye microbiota, samples from the healthy eye and the affected eye could have been analyzed in parallel, in order to confirm the findings of the cultures.
MRSE is a frequent cause of keratitis at our institution, especially in patients with ocular risk factors (eyelid abnormalities, previous keratopathy, traumatism or contact lens use). In our cohort, keratitis caused by MRSE and MSSE did not show differences in their clinical presentation, but MRSE showed multidrug resistance including resistance to fluoroquinolone and tetracycline antibiotics.
FUNDING
None to declare
CONFICTS OF INTEREST
All the authors state no conflicts of interest
References
- 1.Tena D, Rodríguez N, Toribio L, González-Praetorius A. Infectious keratitis: Microbiological review of 297 cases. Jpn J Infect Dis. 2019;72(2):121-23. doi: 10.7883/yoken.JJID.2018.269. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, Ramirez-Miranda A, Navas A, Pedro-Aguilar L, et al. Trends in microbiological and antibiotic sensitivity patterns in infectious keratitis: 10-year experience in Mexico City Cornea. 2015;34(7): 778-85. doi: 10.1097/ICO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 3.Mediero S, Boto de los Bueis A, Spiess K, Díaz-Almirón M, Del Hierro Zarzuelo A, Villalaín Rodes I, et al. Clinical and microbio-logical profile of infectious keratitis in an area of Madrid, Spain. Enferm Infecc Microbiol Clin. 2018;36(7):409-16. doi: 10.1016/j.eimc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Sand D, She R, Shulman IA, Chen DS, Schur M, Hugo YH, et al. Microbial keratitis in Los Angeles: The doheny eye institute and the los angeles county hospital experience. Ophthalmology. 2015;122(5):918-24. doi: 10.1016/j.ophtha.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Durrani AF, Atta S, Bhat AK, Mammen A, Dhaliwal D, Kowalski RP, et al. Methicillin-Resistant Staphylococcus aureus Keratitis: Initial Treatment, Risk Factors, Clinical Features, and Treatment Outcomes. Am J Ophthalmol. 2020; 214:119-26. doi: 10.1016/j.ajo.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Chang VS, Dhaliwal DK, Raju L, Kowalski PR. Antibiotic resistance in the treatment of Staphylococcus aureus Keratitis: A 20-year review. Cornea. 2015;34(6):698-703. doi: 10.1097/ICO.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong SJ, Huang YC, Tan HY, Ma DHK, Lin HC, Yeh LK, et al. Staphylococcus aureus keratitis: A review of hospital. PLoS One. 2013;8(11):6-11. doi: 10.1371/journal.pone.0080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson JC, Durkee H, Miller D, Maestre-Mesa J, Arboleda A, Aguilaret MC, et al. Molecular epidemiology and resistance profiles among healthcare-and community-associated Staphylococcus aureus keratitis isolates. Infect Drug Resist. 2019;12:831-43. doi: 10.2147/IDR.S190245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman DF, Gottsch JD. Methicillin-Resistant Staphylococcus epidermidis Keratitis Treated with Vancomycin. Arch Ophthalmol. 1988;106(11):1570-71. doi: 10.1001/archopht.1988.01060140738046. [DOI] [PubMed] [Google Scholar]
- 10.Tabbara KF, Antonios S, Alvarez H. Effects of fusidic acid on staphylococcal keratitis. Br J Ophthalmol. 1989;73(2):136-39. doi: 10.1136/bjo.73.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitazawa K, Sotozono C, Sakamoto M, Sasaki M, Hieda O, Yamasaki T, et al. Nasal and conjunctival screening prior to refractive surgery: An observational and cross-sectional study. BMJ Open. 2016;6(5):1-6. doi: 10.1136/bmjopen-2015-010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YH, Kang YC, Hou CH, Huang YC, Chen CJ, Shu JC, et al. Antibiotic susceptibility profiles of ocular and nasal flora in patients undergoing cataract surgery in Taiwan: An observational and cross-sectional study. BMJ Open. 2017;7(8):1-8. doi: 10.1136/bmjopen-2017-017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Cave R, Chen L, Yangkyi T, Liu Y, Liet K, et al. Journal of Global Antimicrobial Resistance Antibiotic resistance and molecular characteristics of methicillin-resistant Staphylococcus epidermidis re-covered from hospital personnel in China. 2020;22:195-201. doi: 10.1016/j.jgar.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Stewart PS. Mechanisms of antibiotic resistance in bacterial bio-films. Int J Med Microbiol. 2002;292(2):107-13. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 15.Eghrari AO, Riazuddin SA, Gottsch JD. Overview of the Cornea: Structure, Function, and Development. Vol 134. 1st ed. Elsevier Inc.; 2015. doi: 10.1016/bs.pmbts.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Hori J, Yamaguchi T, Keino H, Hamrah P, Maruyama K. Immune privilege in corneal transplantation. Prog Retin Eye Res. 2019;72(January):100758. doi: 10.1016/j.preteyeres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Lin A, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, et al. Bacterial Keratitis Preferred Practice Pattern. Ophthalmology. 2019;126(1):P1-P55. doi: 10.1016/j.ophtha.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Dave SB, Toma HS, Kim SJ. Ophthalmic antibiotic use and multidrug-resistant Staphylococcus epidermidis: A controlled, longitudinal study. Ophthalmology. 2011;118(10):2035-40. doi: 10.1016/j.ophtha.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 19.George JM, Fiscella R, Blair M, Rodvold K, Ulanski L, Stokes J, et al. Aqueous and vitreous penetration of linezolid and levofloxacin after oral administration. J Ocul Pharmacol Ther. 2010;26(6):579-86. doi: 10.1089/jop.2010.0022. [DOI] [PubMed] [Google Scholar]
- 20.Papa V, Blanco AR, Santocono M. Ocular flora and their antibiotic susceptibility in patients having cataract surgery in Italy. J Cataract Refract Surg. 2016;42(9):1312-17. doi: 10.1016/j.jcrs.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 21.Lee JYH, Monk IR, Gonçalves da Silva A, Seemann T, Chua KYL, Kearns A, et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat Microbiol. 2018;3(10):1175-85. doi: 10.1038/s41564-018-0230-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Chen C, Ye Y, Li X, Sun J, Xuet L, et al. The emergence of Staphylococcus epidermidis simultaneously nonsusceptible to linezolid and teicoplanin in China. Diagn Microbiol Infect Dis. 2020;96(2):114956. doi 10.1016/j.diagmicrobio.2019.114956 [DOI] [PubMed] [Google Scholar]