Abstract
Purpose
Remote patient monitoring (RPM) has contributed to improved patient-centered outcomes and prognosis in patients with end-stage renal disease on automated peritoneal dialysis (APD). However, evidence from prospective trials is lacking.
Methods
The participants (n = 15; median age: 65 years; males: 10; peritoneal dialysis vintage: 6.4 ± 3.5 years) randomly received APD therapy using the Kaguya® APD system either with or without the connective use of the cloud-based RPM software Sharesource® for 12 weeks. The primary outcome was patient satisfaction assessed using a modified nine-item Treatment Satisfaction Questionnaire for Medication (TSQM-9) questionnaire. The secondary outcomes were healthcare resource consumption, the health-related quality of life (HRQOL) subscales assessed with the Kidney Disease Quality of Life-Short Form questionnaire, and clinical laboratory parameters.
Results
Significant improvements were observed in the TSQM-9 subscales of Effectiveness (64.4 ± 18.8 vs. 57.8 ± 18.8; P = 0.006) and Convenience (76.3 ± 15.4 vs. 63.3 ± 17.3; P < 0.001) in patients on Sharesource®. Moreover, Sharesource® reduced the total amount of healthcare resource consumption (0.80 ± 1.32 vs. 1.87 ± 2.39 times/12 weeks; P = 0.02) and consultation time during regular monthly visits (813 ± 269 vs. 1024 ± 292 s; P < 0.001). A significant increase in ultrafiltration volume was found associated with more frequent modification of APD prescription in patients with Sharesource®. Sharesource® also improved the HRQOL subscale of General Health and Vitality.
Conclusion
Sharesource® can improve patient-centered outcomes in patients on APD while reducing the treatment burden for both patients and medical staff.
Trial registration: The study was registered in the Japan Registry of Clinical Trials (jRCT Number: jRCTs032190005).
Supplementary Information
The online version contains supplementary material available at 10.1007/s11255-022-03178-5.
Keywords: Kaguya, Patient satisfaction, Healthcare resource, Ultrafiltration, Quality of life
Introduction
To date, the number of dialysis patients in Japan has exceeded 340,000, and soaring medical costs and the burden of nursing care associated with hospital visits have become social problems [1]. In this context, peritoneal dialysis (PD) is performed as a home-based treatment, which requires only one or two visits to the hospital per month and allows the treatment to be tailored to the patient's lifestyle pattern, although it currently accounts for less than 3% of dialysis patients in Japan [2]. In particular, automated PD (APD) can be automatically performed using a cycler machine, while the patient is sleeping, leading to more time for work and family and social activities, which greatly contributes to improved health-related quality of life (HRQOL) in patients on PD [3, 4].
Remote patient monitoring (RPM) in patients undergoing APD, specifically the new cloud-based software Sharesource® (Baxter Healthcare Corporation, Deerfield, IL, USA), has proven to be beneficial in terms of reducing medical care costs [5], disease burden, and time associated with patients, physicians, and nurses [6], hospitalization rates and days [7], technique failure rate [8] and the improvement of treatment adherence with blood pressure (BP) control [9].
The Kaguya® (Baxter Healthcare Corporation) APD device and Sharesource® connectivity platform have been simultaneously available in Japan since 2018. The connective use of Sharesource® with Kaguya® is also expected to be highly useful in multiple ways to Japanese patients undergoing APD; however, the extent of its usefulness has not been established. Moreover, studies suggesting the usefulness of RPM in patients with APD were performed retrospectively or with a simulation model in which a lot of biases are unavoidable [5, 7, 8, 10, 11]. If not, previous studies used a historical control group for comparison, which was likely to overestimate the usefulness of RPM [6, 12]. Therefore, we designed an open-label, pilot, randomized controlled crossover trial involving patient-initiated APD using Kaguya® to clarify the impact of the additional use of Sharesource® on patient satisfaction, the use of healthcare resources, PD-related parameters, and HRQOL.
Methods
Study population
Outpatients undergoing PD at Keio University Hospital in Tokyo, Japan, were included in this randomized controlled trial (RCT), which was started in April 2019 and completed in March 2021. The criteria for trial participation included stable patients aged > 20 years who had undergone continuous ambulatory PD (CAPD) or APD using Yume®, an older APD model, and newly started APD with the use of Kaguya®. The patient exclusion criteria consisted of advanced malignancies, a life expectancy of less than 1 year, uncontrolled uremia (serum urea nitrogen > 100 mg/dL and/or potassium > 6 mEq/L and/or bicarbonate < 18 mmol/L), and severe cognitive impairment that made operating the APD device, understanding the study protocol, or answering the questionnaire consistently difficult.
Study design and randomization
The study protocol was reviewed and approved by the Certification of Clinical Trials Review Board of Keio University Hospital (Approval Number: N20180007). The study was conducted in adherence to the principles of the Declaration of Helsinki, the Consolidated Standards of Reporting Trials (CONSORT), and the Clinical Trials Act. Written informed consent was obtained from all participants. The study was registered in the Japan Registry of Clinical Trials (jRCT Number: jRCTs032190005). After the baseline assessment, an investigator not associated with this study performed simple randomization using computer-generated random numbers.
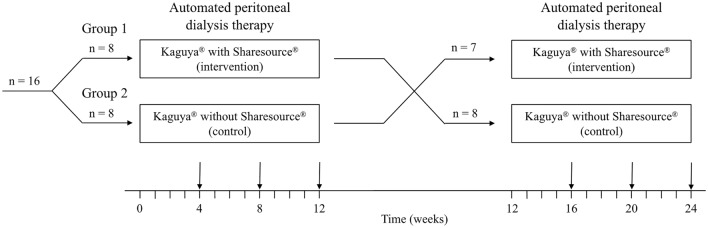
The participants received APD therapy using Kaguya® either with or without the connective use of Sharesource® for 12 weeks each in a counterbalanced fashion (i.e., participants received both therapies in random order) as per an open-label, crossover design (Fig. 1). The concept of medication half-life does not apply to treatment with Kaguya® and/or Sharesource®; therefore, only minimal evidence was available to determine the length of the washout period and we did not set a washout period between the trials. Anthropometric and blood test data were obtained from all patients at the first and last visits (at 0 and 12 weeks, respectively) of each 12-week trial. Additionally, patients were instructed to collect urine and PD fluid samples over a full 24-h period and were trained to complete the questionnaires on patient satisfaction and HRQOL at the last visit of each 12-week trial. Considering the innate character of RPM, blinding of the participants and the PD doctors was impossible.
Fig. 1.
Crossover flowchart of the trial process. Consultation time was measured in regular visits of 4, 8, 12 weeks of each trial and averaged, and all other variables were obtained within 12 weeks of each trial
Intervention
Available only in Japan, Kaguya® is an innovative APD system that automatically identifies the type of dialysate and connects it to Kaguya® through the PD fluid exchange set. The Sharesource® RPM system can perform two-way communication with Kaguya® (https://renalcare.baxter.com/products/kaguya). With Sharesource®, all home operation data of patients on APD, including performing status of APD session, details on the PD fluid (amount and time of the infusing, dwelling, and drainage), alarms detected, and entering information such as daily body weight (BW) and BP were accessible anytime from the nephrology clinic through the software. Moreover, the APD prescription could be performed and changed remotely. All these operations were performed by PD doctors, and the information obtained was shared with PD nurses. In this trial, PD doctors were required to check the information on Sharesource® at least once a week, including just before the patients’ visits to the clinic and on-demand (e.g., when the patients call doctors regarding their troubles with the APD treatment).
Outcome measures
The primary study outcome was patient satisfaction with the treatment (APD therapies using Kaguya® with and without Sharesource®). The secondary outcomes included HRQOL, the total cost of healthcare resources used during each trial, and PD-related anthropometric and biochemical parameters.
Patient satisfaction
Patient satisfaction with the treatment was assessed using the Japanese version of the nine-item Treatment Satisfaction Questionnaire for Medication (TSQM-9) questionnaire [13, 14] after requesting a TSQM license for this trial from IQVIA, Inc. (Danbury, CT, USA). The questionnaire was modified to measure the satisfaction of medical equipment. TSQM-9 assessed three key dimensions of treatment satisfaction, namely, Effectiveness, Convenience, and Overall satisfaction, which were calculated according to the user manual.
Healthcare resources
The consumption of healthcare resource for each 12-week trial period was quantified in five different categories as per previous simulation studies [10, 11]: unplanned hospital visits (including PD clinic and emergency department), calls from the clinic to patients, and from patients to the clinic, unplanned hemodialysis, and hospitalizations. Only the total amount of healthcare resources used was statistically compared between the trials. Additionally, the number and rate of patients who underwent modification of their APD prescriptions were recorded separately as a positive aspect. Moreover, consultation time during regular monthly visits to the PD clinic was recorded and averaged in each trial period.
HRQOL
HRQOL was assessed using the Kidney Disease Quality of Life-Short Form (KDQOL-SF) Japanese version 1.3, with the QOL subscales specific to kidney disease and dialysis (KDQOL) and the general HRQOL (SF-36) for 12 weeks of each trial [15]. The kidney disease component summary was calculated from the KDQOL scores. The physical, mental, and role/social component summaries were calculated from the SF-36 scores, as described previously [16, 17].
Anthropometric and biochemical data analyses
During regular visits to the PD clinic every 4 weeks, BP in the seated position and BW were measured using automated measuring devices, and blood samples were collected for routine clinical PD practice. Only data obtained at the 0 and 12th weeks of each 12-week trial were used for statistical comparison. Specifically, levels of urea nitrogen (mg/dL), creatinine (mg/dL), sodium (mEq/L), potassium (mEq/L), albumin (mg/dL), corrected calcium (mg/dL), phosphorus (mg/L), parathyroid hormone (PTH) (pg/mL), C-reactive protein (mg/dL), brain natriuretic peptide (BNP) (pg/mL), and hemoglobin (g/dL) were obtained as PD-related serum parameters. Body mass index (BMI, kg/m2) was calculated using height (cm) and BW (kg), and the geriatric nutritional risk index (GNRI) was calculated from the BMI and serum albumin level. Additionally, at the 12th week of each trial, patients collected their urine (if any) and PD fluid samples over a full 24-h period. From those samples, renal Kt/V, renal creatinine clearance (CCr, L/week/1.73 m2), ultrafiltration volume (mL/day), PD Kt/V, and PD CCr (l/week/1.73 m2) were calculated.
Statistical analysis
Continuous data were tested for normal distribution using the Shapiro–Wilk test. Normally distributed and skewed variables were expressed as mean ± standard deviation (SD) and median (interquartile range), respectively. The categorical data were presented as n (%). The paired Student’s t test or Wilcoxon signed-rank test was employed for comparisons of continuous parameters between the trials for both APD settings. The unpaired t test or Mann–Whitney U test was used for comparisons between both groups divided by the sequence of APD therapy. The percentage was compared between the trials using McNemar’s test. Linear mixed models were fit for analyzing carry-over and order effects in the primary outcome (patient satisfaction). The adjustment was performed for group, trial (first or second), and treatment as fixed effects, and for patient number as a random effect [18, 19]. The effects of group, trial, and treatment were regarded as carry-over effect, order effect, and treatment effect, respectively.
Although few studies have evaluated patient satisfaction with TSQM-9 in patients with chronic kidney disease, including dialysis patients, based on studies on other diseases that employed TSQM-9, we found that a total of 13–26 patients were required to detect an 11–13 increase in each subscale of TSQM-9 with SD = 16–18, β = 0.1–0.2 and α = 0.05 (two-tailed) with the paired Student’s t test [14, 20]. Furthermore, inflating this for an estimated attrition rate of approximately 20%, we calculated a maximum target sample size of 30. Nevertheless, a sample size of 15 was allowed, considering this was a pilot study that primarily examined patient-reported outcome measures. SPSS software for Mac (ver. 26; IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. P < 0.05 indicated statistical significance.
Results
Baseline characteristics
Among the 18 patients receiving therapies with CAPD or APD using Yume® who fulfilled the inclusion criteria, 16 gave consent to participate in the study. We randomized the 16 participants in a crossover design to first prescribe APD therapy using Kaguya® either with Sharesource® (group 1, n = 8) or without Sharesource® (group 2, n = 8), however, one patient assigned to group 2 developed cerebral infarction accompanied with long-term hospitalization and physical disabilities as a sequela in the first trial without Sharesource® and was thus excluded from the analysis. The overall cohort of 15 participants who completed the study consisted of 10 male and 5 female patients with a median age of 65 years (range 62–67 years) and PD vintage of 6.4 ± 3.5 years. The baseline characteristics did not differ significantly between the two groups. Furthermore, a paired t test or Wilcoxon signed-rank test for values from the first visit of each trial demonstrated that baseline anthropometric and biochemical data were nearly comparable between the trials (Table S1) [21].
Effect of Sharesource® on patient satisfaction
Among the subscales of TSQM-9, effectiveness and convenience were significantly higher during the APD trial with Sharesource® (64.4 ± 18.8 vs. 57.8 ± 18.8; P = 0.006; and 76.3 ± 15.4 vs. 63.3 ± 17.3; P < 0.001, respectively) (Table 1). However, the subscale of Overall satisfaction was not significantly different between the trials (66.7 ± 16.6 vs. 59.4 ± 24.0; P = 0.21). During the trial whether using Kaguya with or without Sharesource®, there were no statistically significant differences between the groups on most subscales of TSQM-9 (Tables S2 and S3). The carry-over effect was negligible in Effectiveness, Convenience, and Overall satisfaction (P = 0.33, 0.24, and 0.52, respectively). In addition, the order effect was absent in Effectiveness and Overall satisfaction (P = 0.84 and 0.55, respectively). Although the presence of order effect could not be statistically avoided in Convenience (P = 0.03), the treatment effect was independent and statistically significant (P < 0.001).
Table 1.
Effects of Sharesource® on treatment satisfaction and use of healthcare resource
| Variables | With sharesource® | Without sharesource® | P value |
|---|---|---|---|
| TSQM-9 | |||
| Effectiveness | 64.4 ± 18.8 | 57.8 ± 18.8 | 0.006 |
| Convenience | 76.3 ± 15.4 | 63.3 ± 17.3 | <0.001 |
| Overall satisfaction | 66.7 ± 16.6 | 59.4 ± 24.0 | 0.21 |
| Healthcare resource consumption (times/12 weeks) | 0.80 ± 1.32 | 1.87 ± 2.39 | 0.02 |
| Unplanned hospital visits | 0.27 ± 0.59 | 0.40 ± 1.12 | |
| Calls from the clinic to patients | 0.33 ± 0.72 | 0.60 ± 0.91 | |
| Calls from patients to the clinic | 0.20 ± 0.41 | 0.60 ± 0.74 | |
| Unplanned hemodialysis | 0 ± 0 | 0.13 ± 0.35 | |
| Hospitalizations | 0 ± 0 | 0.13 ± 0.35 | |
| Consultation time in regular visits (s)a | 813 ± 269 | 1024 ± 292 | <0.001 |
| Patients with modification of APD prescription (%) | 9 (60%) | 1 (7%) | 0.01 |
TSQM Treatment Satisfaction Questionnaire for Medication, APD automated peritoneal dialysis
aFor this variable, the mean values at 4, 8, and 12 weeks of each trial were calculated
Effect of Sharesource® on healthcare resource consumption
The total number of pre-specified healthcare resource consumption was significantly lower during the trial with Sharesource® compared with without Sharesource® (0.80 ± 1.32 times/12 weeks vs. 1.87 ± 2.39 times/12 weeks; P = 0.02) (Table 1). Two patients underwent hospitalization accompanied by unplanned hemodialysis during the trial without Sharesource® due to fluid retention and uremia, respectively. The percentage of patients who had modified APD prescriptions was significantly higher during the trial with Sharesource®, suggesting increased personalization of the APD treatment (60% vs. 7%; P = 0.01). The prescription modification included shortening or extending dwelling and drainage time, increasing estimated ultrafiltration volume, adjusting tidal volume, and increasing the total amount of dialysate used. Moreover, the mean consultation time of regular PD clinic visits was significantly shorter during the trial with Sharesource® (813 ± 269 s vs. 1024 ± 292 s; P = 0.01).
Effect of Sharesource® on HRQOL
No statistically significant differences were observed among the KDQOL subscales between the trials of APD therapies with or without Sharesource® (Table 2). On the other hand, the SF-36 subscales of General Health and Vitality were significantly higher in the trial with Sharesource® (49.0 ± 23.2 vs. 39.5 ± 22.5; P < 0.001; and 51.7 ± 21.3 vs. 45.0 ± 24.6; P < 0.05, respectively).
Table 2.
Effect of Sharesource® on health-related quality of life
| Variables | With sharesource® | Without sharesource® | P value |
|---|---|---|---|
| KDQOL | |||
| Symptoms/problems | 77.5 ± 14.6 | 75.2 ± 12.9 | 0.53 |
| Effects of kidney disease | 79.0 ± 17.2 | 74.6 ± 16.3 | 0.14 |
| Burden of kidney disease | 42.5 ± 23.0 | 37.9 ± 21.2 | 0.09 |
| Work status | 66.7 ± 40.8 | 66.7 ± 36.2 | 1 |
| Cognitive function | 92.0 ± 9.2 | 92.0 ± 8.8 | 1 |
| Quality of social interaction | 87.8 ± 9.7 | 89.8 ± 14.7 | 0.57 |
| Sleep | 55.4 ± 21.9 | 56.7 ± 16.3 | 0.74 |
| Social support | 76.1 ± 28.0 | 77.2 ± 26.4 | 0.79 |
| Satisfaction for care | 84.4 ± 13.3 | 84.4 ± 13.3 | 1 |
| Encouragement from staff | 81.7 ± 21.1 | 76.7 ± 24.9 | 0.32 |
| KDCS | 74.3 ± 9.7 | 73.1 ± 10.8 | 0.48 |
| SF-36 | |||
| Physical functioning | 70.3 ± 21.3 | 64.3 ± 26.2 | 0.05 |
| Physical role functioning | 69.2 ± 31.3 | 61.3 ± 28.7 | 0.25 |
| Body pain | 63.7 ± 26.4 | 57.1 ± 23.1 | 0.17 |
| General health | 49.0 ± 23.2 | 39.5 ± 22.5 | <0.001 |
| Vitality | 51.7 ± 21.3 | 45.0 ± 24.6 | <0.05 |
| Social functioning | 65.8 ± 32.9 | 53.3 ± 37.9 | 0.13 |
| Emotional role functioning | 76.1 ± 31.2 | 72.2 ± 33.3 | 0.59 |
| Mental health | 75.3 ± 18.8 | 72.7 ± 18.7 | 0.30 |
| PCS | 37.9 ± 15.2 | 34.1 ± 14.5 | 0.09 |
| MCS | 50.9 ± 10.2 | 48.5 ± 8.5 | 0.09 |
| RCS | 43.7 ± 15.7 | 41.3 ± 17.9 | 0.51 |
KDQOL Kidney Disease Quality of Life, KDCS kidney disease component summary, SF-36 Medical Outcomes Study 36-Item-Short Form Health Survey MOS, PCS physical component summary, MCS mental component summary, RCS role/social component summary
Effect of Sharesource® on PD-related parameters
Among PD-related parameters, ultrafiltration volume, PD Kt/V, and PD CCr were significantly increased during the trial with Sharesource® (1388.2 ± 426.1 mL/day vs. 1209.5 ± 395.9 mL/day; P = 0.003; 1.79 ± 0.55 vs. 1.62 ± 0.34; P = 0.04; and 43.2 ± 18.4 l/week/1.73 m2 vs. 37.4 ± 12.1 l/week/1.73 m2; P = 0.02, respectively) (Table 3). In addition, serum PTH was significantly lower during the trial with Sharesource® (215 [156–371] vs. 304 [163–489] pg/mL; P = 0.03). The other parameters were not significantly different between the trials with or without Sharesource®.
Table 3.
Effect of Sharesource® on peritoneal dialysis-related parameters
| Variables | With Sharesource® | Without Sharesource® | P-value |
|---|---|---|---|
| Systolic BP (mmHg) | 148.3 ± 8.8 | 143.8 ± 18.5 | 0.33 |
| Diastolic BP (mmHg) | 79.5 ± 12.9 | 80.7 ± 14.3 | 0.75 |
| BMI (kg/m2) | 24.0 ± 4.7 | 23.7 ± 4.9 | 0.15 |
| Urea nitrogen (mg/dL) | 59.6 (48.7–64.3) | 51.5 (44.1–59.7) | 0.17a |
| Creatinine (mg/dL) | 12.7 (11.0–13.8) | 12.5 (11.3–13.1) | 1a |
| Sodium (mEq/L) | 137.4 ± 3.0 | 137.7 ± 2.7 | 0.52 |
| Potassium (mEq/L) | 4.51 ± 0.69 | 4.39 ± 0.62 | 0.48 |
| Albumin (g/L) | 3.47 ± 0.34 | 3.54 ± 0.38 | 0.29 |
| GNRI | 97.2 ± 8.5 | 97.6 ± 9.1 | 0.66 |
| Calcium (mg/dL) | 9.44 ± 0.49 | 9.54 ± 0.51 | 0.36 |
| Phosphorus (mg/dL) | 5.70 ± 1.07 | 5.70 ± 1.05 | 1 |
| Parathyroid hormone (pg/mL) | 215 (156–371) | 304 (163–489) | 0.03a |
| CRP (mg/dL) | 0.12 (0.06–0.15) | 0.14 (0.06–0.22) | 0.71a |
| BNP (pg/mL) | 403.9 ± 554.6 | 238.4 ± 252.0 | 0.28 |
| Hemoglobin (g/dL) | 10.7 ± 0.9 | 11.4 ± 1.3 | 0.13 |
| Ultrafiltration (mL) | 1388.2 ± 426.1 | 1209.5 ± 395.9 | 0.003 |
| PD Kt/V | 1.79 ± 0.55 | 1.62 ± 0.34 | 0.04 |
| PD CCr | 43.2 ± 18.4 | 37.4 ± 12.1 | 0.02 |
BP blood pressure, BMI body mass index, GNRI geriatric nutritional risk index, CRP C-reactive protein, BNP brain natriuretic peptide, PD peritoneal dialysis, CCr creatinine clearance
aFor these variables, Wilcoxon signed-rank test was performed to test statistical significance
Discussion
Although previous retrospective and simulation studies suggested that RPM or Sharesource® possessed several multifaceted positive impacts on patients with APD [5–12, 22], the present study is the first pilot RCT to describe the effect of using Kaguya® with Sharesource® in patient-initiated APD therapy. Specifically, the system improved patient satisfaction for the treatment, reduced healthcare resource consumption, and improved HRQOL subscales and PD-related parameters.
The advantage of APD includes the ability to increase dialysis dose while patients can continue to engage in the daily activities of work, education, or family, leading to better HRQOL [23–25]. Moreover, APD was reported to improve patient/technique survival and peritonitis rate compared with CAPD [26], although another study reported conflicting results [27]. Kaguya®, a Japanese-specific APD system enabling automated connection with dialysates, may further reduce the incidence of peritonitis especially caused by touch contamination. However, no studies investigating the benefit of Kaguya® itself or Kaguya® with connective use of Sharesource® have been found. Several studies have previously reported a high level of satisfaction with RPM in both patients and medical staff [6, 28–31]. However, our study firmly demonstrated an improvement in patient satisfaction with the use of an RPM software using the TSQM, a well-validated scale widely used in clinical studies [13, 14]. The significant scores in the TSQM subscales of Effectiveness and Convenience could be due to the increased individualization of the APD treatment through frequent prescription changes and improved adherence [9, 12, 22, 32], as well as the noncompulsory reporting of treatment progress and initiation of treatment changes. Without Sharesource®, the PD staff could access only some of the patient information, such as daily ultrafiltration volume of APD, BW, and BP, by checking the APD recording notebook of the patient during their visits to the clinic. Additionally, patients must request for changes in their APD prescription with the help of PD doctors, nurses, or Baxter call centers. Although we were concerned about patient privacy with the use of RPM, this did not affect patients’ HRQOL. On the contrary, RPM improved the SF-36 subscales of General Health and Vitality, and all the patients actually elected to use Sharesource® after the clinical trial period. The use of the KDQOL-SF to assess HRQOL was also one of the strengths of this trial.
Ultrafiltration volume, PD Kt/V, PD CCr, and serum PTH were also improved with the use of Sharesource®. This could be also due to the optimization in PD prescription and increased treatment adherence. Nonetheless, caution is warranted in interpreting these results, given that only the parameters measured at a single point, i.e., week 12 of each trial, were used for comparisons, which might have resulted in increased variability in parameters that are affected by a variety of factors, including medication nonadherence, stress, and diet. Nonadherence, defined as the performance of less than 90% of prescribed PD therapy, was often observed in patients undergoing PD and was associated with negative clinical outcomes, including death, technique failure, and hospitalization [32, 33]. Adherence to APD treatment in the trial with Sharesource® was almost 100%.
In addition to clinical outcomes, the monetary costs of healthcare resources are an important aspect in the management of patients with end-stage renal disease (ESRD). Previous retrospective and simulation studies and the present trial showed that RPM-APD reduced the disease burden, consultation time, healthcare resource consumption, including the interaction of patients on APD with doctors or nurses and associated net costs, which in one study amounted to US $121,233 per year [5, 6, 10, 11]. Although we did not calculate the cost savings in this trial, reduced healthcare consumption with RPM-APD due to fewer clinic calls, unplanned clinic visits or hemodialysis, and hospitalizations, as well as reduced regular PD clinic consultation times, would have highly probably reduced net costs. Regarding the length of consultation, additional time is necessary for PD doctors to check the information on Sharesource®. On the other hand, PD doctors can check the status of patients on Sharesource® (e.g., during downtimes between consultations and laboratory experiments) without the constraints of scheduled consultations. Additionally, the use of Sharesource® involves only doctors, while consultation involves doctors, as well as patients and other medical staff (e.g., nurses and medical clerks). Moreover, an RPM-APD program proved beneficial during the COVID-19 pandemic by reducing on-site evaluation and more strictly controlling hypertension and serum phosphorus and PTH levels [34, 35]. However, this trial did not take into account the impact of the COVID-19 pandemic on the results.
The present trial has several limitations. First and most importantly, this study was a pilot study that included a small number of patients who were monitored for a short follow-up duration. The effects of Sharesource® with Kaguya® in improving patient satisfaction, reducing healthcare resource consumption, and improving HRQOL subscales and PD-related parameters were definitive. Future investigations should assess the effect of Sharesource® with Kaguya® on the long-term outcomes of patients on PD, including peritonitis, technique failure, and death. Second, owing to the nature of Sharesource® or RPM that made blinding of the participants and medical staff impossible, the effect of several biases, including the Hawthorne effect and observational bias, was undeniable, although PD-related parameters were relatively objective variables. Finally, the crossover design adopted in this trial could also potentially add several limitations, including the carry-over effect and the order effect, although both effects were nearly negligible statistically in the primary outcome and treatment effects remained after the adjustment of both effects. From these limitations, future RCTs with larger sample size, longer term follow-up, and parallel-group design are necessary for a more definitive demonstration of the positive impacts of Sharesource® on the outcomes of patients undergoing APD. However, the long-term assignment of a control group without the use of RPM is unethical, given the potentially high usefulness of RPM [33].
In conclusion, this RCT indicates the multidimensional beneficial effects of the Kaguya® with Sharesource® RPM-APD system in terms of patient-centered and clinical outcomes in ESRD patients undergoing APD. However, our results should be interpreted cautiously with due consideration of the several limitations of the study. Evidence from continuous research, especially RCTs and/or large observational studies is necessary to establish the value of existing and future RPM models among patients on APD and healthcare providers [36, 37].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the participants for their dedication to this research project. The authors wish to acknowledge the assistance of Dr. Shigeaki Ohtsuki for the valuable technical and statistical assistance.
Authors’ contributions
Research idea and study design: KU, KM, and NW; data acquisition: KU, KM, EK, TN, and TK; data analysis/interpretation: KU and KM; statistical analysis: KU; supervision or mentorship: NW, SW, and HI.
Funding
This study was conducted with the financial support of Baxter Ltd (Grant no. 04-080-5103). The funder did not influence the reporting of the data or results presented in this trial.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
We have read and understood International Urology and Nephrology’s policy on disclosing conflicts of interest and declare that KM and NW received salaries from an endowed chair at Baxter Ltd. and that and HI received research funding, also from Baxter Ltd.
Ethics approval
The study protocol was reviewed and approved by the Certification of Clinical Trials Review Board of Keio University Hospital (approval number: N20180007). The study was conducted with adherence to the principles of the Declaration of Helsinki, Consolidated Standards of Reporting Trials (CONSORT), and Clinical Trials Act.
Consent to participate
Written informed consent was obtained from all participants to participate in this study.
Consent for publication
Written informed consent was obtained from all participants for their anonymized information to be published in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nitta K, Masakane I, Hanafusa N, et al (2020) 2019 Annual Dialysis Data Report, JSDT Renal Data Registry. J Jpn Soc Dial Ther 53:579–632. (Japanese)
- 2.Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90–103. doi: 10.1038/nrneph.2016.181. [DOI] [PubMed] [Google Scholar]
- 3.Rabindranath KS, Adams J, Ali TZ, et al. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2007;22:2991–2998. doi: 10.1093/ndt/gfm515. [DOI] [PubMed] [Google Scholar]
- 4.De Wit GA, Merkus MP, Krediet RT, et al. A comparison of quality of life of patients on automated and continuous ambulatory peritoneal dialysis. Perit Dial Int. 2001;21:306–312. doi: 10.1177/089686080102100313. [DOI] [PubMed] [Google Scholar]
- 5.Ariza JG, Walton SM, Sanabria M, et al. Evaluating a remote patient monitoring program for automated peritoneal dialysis. Perit Dial Int. 2020;40:377–383. doi: 10.1177/0896860819896880. [DOI] [PubMed] [Google Scholar]
- 6.Milan Manani S, Rosner MH, Virzì GM, et al. Longitudinal experience with remote monitoring for automated peritoneal dialysis patients. Nephron. 2019;142:1–9. doi: 10.1159/000496182. [DOI] [PubMed] [Google Scholar]
- 7.Sanabria M, Buitrago G, Lindholm B, et al. Remote patient monitoring program in automated peritoneal dialysis: impact on hospitalizations. Perit Dial Int. 2019;39:472–478. doi: 10.3747/pdi.2018.00287. [DOI] [PubMed] [Google Scholar]
- 8.Corzo L, Wilkie M, Vesga JI, et al. Technique failure in remote patient monitoring program in patients undergoing automated peritoneal dialysis: A retrospective cohort study. Perit Dial Int. 2020 doi: 10.1177/0896860820982223. [DOI] [PubMed] [Google Scholar]
- 9.Yeter HH, Akcay OF, Ronco C, et al. Automated remote monitoring for peritoneal dialysis and its impact on blood pressure. Cardiorenal Med. 2020;10:198–208. doi: 10.1159/000506699. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama K, Washida N, Yube N, et al. The impact of a remote monitoring system of healthcare resource consumption in patients on automated peritoneal dialysis (APD): a simulation study. Clin Nephrol. 2018;90:334–340. doi: 10.5414/CN109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makhija D, Alscher MD, Becker S, et al. Remote monitoring of automated peritoneal dialysis patients: assessing clinical and economic value. Telemed J E Health. 2018;24:315–323. doi: 10.1089/tmj.2017.0046. [DOI] [PubMed] [Google Scholar]
- 12.Milan Manani S, Crepaldi C, Giuliani A, et al. Remote monitoring of automated peritoneal dialysis improves personalization of dialytic prescription and patient's independence. Blood Purif. 2018;46:111–117. doi: 10.1159/000487703. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi: 10.1186/1477-7525-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green J, Fukuhara S, Shinzato T, et al. Translation, cultural adaptation, and initial reliability and multitrait testing of the Kidney Disease Quality of Life instrument for use in Japan. Qual Life Res. 2001;10:93–100. doi: 10.1023/A:1016630825992. [DOI] [PubMed] [Google Scholar]
- 16.Suzukamo Y, Fukuhara S, Green J, et al. Validation testing of a three-component model of Short Form-36 scores. J Clin Epidemiol. 2011;64:301–308. doi: 10.1016/j.jclinepi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama K, Washida N, Morimoto K, et al. Home-based aerobic exercise and resistance training in peritoneal dialysis patients: a randomized controlled trial. Sci Rep. 2019;9:2632. doi: 10.1038/s41598-019-39074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grizzle JE. The two-period change-over design and its use in clinical trials. Biometrics. 1965;21:467–480. doi: 10.2307/2528104. [DOI] [PubMed] [Google Scholar]
- 19.Grieve AP. The two-period changeover design in clinical trials. Biometrics. 1982;38:517. [PubMed] [Google Scholar]
- 20.Contoli M, Rogliani P, Di Marco F, et al. Satisfaction with chronic obstructive pulmonary disease treatment: results from a multicenter, observational study. Ther Adv Respir Dis. 2019;13:1753466619888128. doi: 10.1177/1753466619888128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bovée DM, Visser WJ, Middel I, et al. A randomized trial of distal diuretics versus dietary sodium restriction for hypertension in chronic kidney disease. J Am Soc Nephrol. 2020;31:650–662. doi: 10.1681/ASN.2019090905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunch A, Vesga JI, Camargo DO, et al. Remote automated peritoneal dialysis management in Colombia. Kidney Int Rep. 2019;4:873–876. doi: 10.1016/j.ekir.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunkhorst R, Wrenger E, Krautzig S, et al. Clinical experience with home automated peritoneal dialysis. Kidney Int Suppl. 1994;48:S25–S30. [PubMed] [Google Scholar]
- 24.Schaefer F, Klaus G, Müller-Wiefel DE, et al. Current practice of peritoneal dialysis in children: results of a longitudinal survey. Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS) Perit Dial Int. 1999;19:S445–S449. doi: 10.1177/089686089901902S73. [DOI] [PubMed] [Google Scholar]
- 25.Bro S, Bjorner JB, Tofte-Jensen P, et al. A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int. 1999;19:526–533. doi: 10.1177/089686089901900606. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez AR, Madonia C, Rascon-Pacheco RA. Improved patient/technique survival and peritonitis rates in patients treated with automated peritoneal dialysis when compared to continuous ambulatory peritoneal dialysis in a Mexican PD center. Kidney Int Suppl. 2008;108:S76–S80. doi: 10.1038/sj.ki.5002606. [DOI] [PubMed] [Google Scholar]
- 27.Li PK, Szeto CC, Piraino B, et al. ISPD Peritonitis Recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez García MA, Fernández Rosales MS, López Domínguez E, et al. Telemonitoring system for patients with chronic kidney disease undergoing peritoneal dialysis: usability assessment based on a case study. PLoS One. 2018;13:e0206600. doi: 10.1371/journal.pone.0206600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnus M, Sikka N, Cherian T, et al. Satisfaction and improvements in peritoneal dialysis outcomes associated with telehealth. Appl Clin Inform. 2017;8:214–225. doi: 10.4338/ACI-2016-09-RA-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker RC, Tong A, Howard K, et al. Patients’ and caregivers’ expectations and experiences of remote monitoring for peritoneal dialysis: a qualitative interview study. Perit Dial Int. 2020;40:540–547. doi: 10.1177/0896860820927528. [DOI] [PubMed] [Google Scholar]
- 31.Walker RC, Tong A, Howard K, et al. Clinicians’ experiences with remote patient monitoring in peritoneal dialysis: a semi-structured interview study. Perit Dial Int. 2020;40:202–208. doi: 10.1177/0896860819887638. [DOI] [PubMed] [Google Scholar]
- 32.Drepper VJ, Martin PY, Chopard CS, et al. Remote patient management in automated peritoneal dialysis: a promising new tool. Perit Dial Int. 2018;38:76–78. doi: 10.3747/pdi.2017.00054. [DOI] [PubMed] [Google Scholar]
- 33.Bernardini J, Nagy M, Piraino B. Pattern of noncompliance with dialysis exchanges in peritoneal dialysis patients. Am J Kidney Dis. 2000;35:1104–1110. doi: 10.1016/S0272-6386(00)70047-3. [DOI] [PubMed] [Google Scholar]
- 34.Yeter HH, Gok Oguz E, Akcay OF, et al. The reliability and success of peritoneal dialysis during the COVID-19 pandemic. Semin Dial. 2020 doi: 10.1111/sdi.12940.10.1111/sdi.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunch A, Ardila F, Castaño R, et al. Through the storm: Automated peritoneal dialysis with remote patient monitoring during COVID-19 pandemic. Blood Purif. 2020 doi: 10.1159/000511407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giuliani A, Crepaldi C, Milan Manani S, et al. Evolution of automated peritoneal dialysis machines. Contrib Nephrol. 2019;197:9–16. doi: 10.1159/000496302. [DOI] [PubMed] [Google Scholar]
- 37.Wallace EL, Rosner MH, Alscher MD, et al. Remote patient management for home dialysis patients. Kidney Int Rep. 2017;2:1009–1017. doi: 10.1016/j.ekir.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.