Abstract
The aim of the present study was to investigate the influence of millimeter-wave electromagnetic (MW) irradiation on normal and pathological human sperm in vitro, and to evaluate a possible role of polyamines (PA) in this process. The stability of sperm membranes, the number of apoptotic gametes, and the content of seminal plasma PA in the ejaculates of fertile and subfertile men were compared before and after short-term MW electromagnetic exposure in vitro. The ejaculate samples were collected from healthy donors [n=25, age 22-38 years old (y.o.), average age 30.6±1.1 y.o. (mean ± SEM)] and from subfertile men (n=78, age 25-48 y.o., average age 34.1±0.8 y.o.) and exposed to MW radiation. The electromagnetic field had a wavelength of 7.1 mm, a frequency of 42.194 GHz and an exposure time of 20 min. The fragility of sperm membranes was evaluated by their resistance to sodium chloride solution (Milovanov test) and to acetic acid (Joel test). Acrosin activity was assayed spectrophotometrically. Apoptosis was determined by the externalization of phosphatidylserine on the outer side of the sperm membrane and propidium iodide staining. The PA levels were determined by agar gel electrophoretic fractionation. An increase in the resistance of sperm membranes, a decrease in acrosin activity, a decrease in the number of apoptotic gametes and a decrease in the seminal plasma PA concentrations were found after exposure of the native human sperm to low-intensity MW irradiation. Two types of reactions were revealed for the subfertile samples. The results revealed positive bio-effects of specific microwaves on the human semen and the participation of PA in the realization of these effects.
Keywords: millimeter-wave electromagnetic radiation, polyamines, sperm, seminal plasma, apoptosis
Introduction
Millimeter-wavelength electromagnetic waves (MWs) possess wavelengths of 1-10 mm. A healthy living cell is characterized by a spectrum of acoustoelectric oscillations (normal oscillations) in the plasma membrane that belong to the MW range, with the amplitude lowering and fading away when a cell is impaired resulting in loss of viability and death (1,2). The biological effects of electromagnetic irradiation depend on characteristics such as induction, frequency and duration of exposure. The short-wave range (30-300 GHz), corresponding to the cell's natural oscillations, is the most attractive range for investigations (3-5). It is considered that the MW action on the cells leads to the correction or restoration of their natural oscillations, which results in increased membrane stability and the maintenance or extension of the cell viability. Millimeter-wave irradiation is applied for complex therapy (Millimeter Wave Therapy; MW therapy) of various diseases, such as diabetes, cardiovascular and skin diseases, rheumatoid arthritis and disorders of the male reproductive system (6,7). For example, the complex treatment of chronic prostatitis includes MW therapy with wavelengths of 7.1 or 5.6 mm, and an exposure duration of 20 mins (5).
Despite the increased interest in bioelecromagnetic studies in the recent years, the mechanism of MW action remains incompletely understood. A possible explanation of its therapeutic effects includes its direct and indirect interaction with the skin structural components and further neurogenic activation of endogenous opioid systems, and is further discussed in a more specific review (2). However, there is very little information on the influence of MW on seminal liquid, male gametes, and their apoptosis in particular.
One of the most abundant components of the seminal plasma is polyamines (PAs), which are widely present in all tissues and biological fluids of the body (Fig. 1). They are essential for cell growth, cell proliferation and differentiation (8,9), and have the ability to coordinate the process of cell apoptosis (9-13). The contents of the PAs spermine (Spm) and spermidine (Spd) in the seminal plasma are significantly higher than in any other biological fluid, which highlights the importance of these low molecular weight organic polycations for human reproductive function, especially in men (10,14). However, there is a lack of studies aiming to understand the roles of the human seminal plasma PA. It has been established that higher PAs levels, such as that of Spd and Spm, are produced by the prostate gland from putrescin and decarboxylated SAM, participate in the regulation of the pH of seminal plasma, stabilize the structure of sperm DNA, activate the motility of gametes, and are considered to be a de-capacitating factor that participates in the regulation of the sperm membrane integrity during fertilization (8,14-16). The cell membranes are considered the main targets for MW at the molecular level (2,17). In this regard, the influence of short-term low-intensity MW exposure on the sperm cell membranes and the role of the seminal plasma components are of interest. It was hypothesized that if any biological effects of the MW irradiation on the sperm cells can be detected then the PA may be involved in the development of these effects. The current study aimed to reveal the biological effects of microwaves on male gametes and the role of PA in the realization of these effects.
Figure 1.
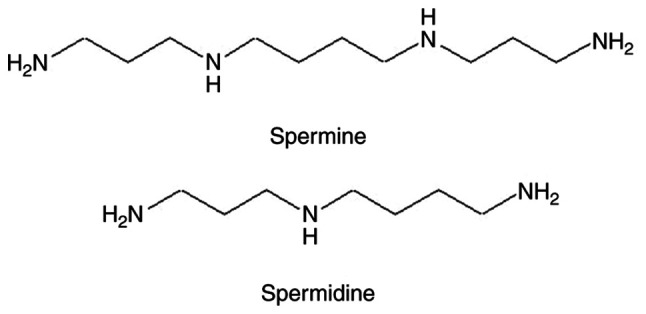
Polyamines of body tissues and biological liquids. The polyamines contain two or more amino groups with basic properties.
Materials and methods
Study design
The ejaculates of healthy fertile men (n=25) aged from 22-38 years old (y.o.) [(30.6±1.1 y.o. (mean ± SEM)], and of subfertile men (n=78) aged 25-48 y.o. (34.1±0.8 y.o.) were studied. The identification of normal and subfertile samples was based on the spermogram parameters described below. As the investigation was performed with participation of the Reproduction Center, the preliminary basis for the identification of samples as subfertile was also the inability for the donors to have children. The durations of ‘barren marriages’ ranged from 6 months to 15 years. No reproductive organ diseases were diagnosed in donors with abnormal spermograms, the donors did not abuse alcohol and were not smokers. The fertile samples were taken from the donors who had children in the previous 3 years.
First, we studied the influence of MW irradiation on spermatozoa from healthy donors. The whole ejaculate after complete liquefaction were examined for the standard spermogram parameters. Each sample was divided into three parts: Control F, Exp1 F and Exp2 F. The Control F was not exposed to the MW irradiation, Exp1 F was exposed to the MW irradiation as a whole ejaculate, and Exp2 F was centrifugated at 12,580 rcf for 15 min at room temperature, and divided into seminal plasma and sperm cells, after which the seminal plasma and spermatozoa were exposed to MW irradiation separately from each other. The Control F and Exp1 F group, after MW exposure, were then also centrifuged as above, and divided into seminal plasma and spermatozoa. The seminal plasma and spermatozoa from all three experiments were used for the specific assays. The spermogram parameters were also evaluated in the Exp1 F and Exp2 F groups after MW exposure. Next, the effect of MW treatment on spermatozoa with regard to metabolic processes was assessed. The ejaculates from donors with pathospermia (Control S, Exp1 S) were exposed to the MW treatment and processed similarly to those of healthy donors. Figs. 2 and S1 outlines the study design briefly.
Figure 2.
Study design and experiments. After complete liquefaction, the complete ejaculate samples were divided before or after millimeter-wavelength electromagnetic wave-exposure into two fractions, the seminal plasma and the spermatozoa, that were then further examined.
Ethical approval
The present study was performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) (18) and was approved by the Local Ethics Committee of the Astrakhan State Medical University of the Health Ministry of the Russian Federation (protocol no. 2; 21st May 2021). Informed consent for participation in the study and use of their samples was obtained from all participants.
MW field generation
The electromagnetic field was produced by a generator of monochromatic electromagnetic waves ‘Yav-1-7.1’ (Scientific Production Association, Istok). The MW parameters (λ=7.1 mm, υ=42.194 GHz) as well as the duration of the experiment have previously been determined to induce the intended bioeffects without a heating effect, and are recommended for clinical and experimental use (4,5,19). The absence of a thermal effect ensured the specificity of biological action (5,19,20).
Spermatozoa investigation
The assessment of the primary standard spermogram parameters was performed according to the generally accepted methods recommended by WHO and leading experts (21,22). Microscopic studies of sperm were performed on a transmission electron microscope; morphology and motility characteristics were explored (21,22). The motile sperm cells were counted using a Goryaev chamber. All these parameters were determinants for the verifying the samples as normal or subfertile ones at the zero point of the study. The biochemical investigations included membrane resistance tests, acrosin activity assay and apoptosis investigation by evaluation of PS externalization by AnnexinV-propidium iodide (PI) staining.
Membrane resistance assessment
The sperm membrane resistance to the 1% sodium chloride solution (Milovanov test) was explored (23). Briefly, the ejaculate was diluted gradually with 1% sodium chloride solution and incubated for 3 min at 37˚C with counting of motile sperm cells. The first dilution (1:500) was performed by adding 10 ml 1% sodium chloride solution to 0.02 ml ejaculate, next dilutions (1:1,000, 1:2,000, 1:4,000, 1:6,000, etc.) were obtained by adding 0.5 ml volumes of 1% NaCl solution to the corresponding volumes of the first diluted (1:500) solution. The number of motile sperms were counted in ejaculate samples before and 3 min after each dilution under a light microscope (magnification, x100), using a Goryaev chamber, and accordance with methods recommended by WHO and leading experts (21,22). The diluting was performed gradually starting from 1:500 up to the complete cessation of spermatozoa motility (23). The higher the dilution at which the motility remained, the greater the membrane resistance was considered to be. The membrane resistance was evaluated in equivalent units (EU) corresponding to the degree of dilution; for example, if the highest dilution maintaining sperm motility was 1:4,000, then the resistance was considered 4,000 EU. The mean ± SEM were then calculated.
The membrane resistance to acetic acid was evaluated in the Joel test (23). The ejaculate was diluted with 0.5% acetic acid solution (1:1) and incubated at 37˚C. At 0 h (before the dilution) and every 10 min after dilution, the number of motile spermatozoa was counted in the ejaculate samples, up to the complete cessation of motility. The duration of motility in these conditions was a marker of membrane resistance.
Acrosine activity assay
The spermatozoa were washed using PBS (pH 6.5) to remove the seminal plasma and extracted with 0.2 M acetate buffer (pH 2.4). The activity of free acrosine (EC 3.4.21.10) was determined spectrophotometrically as described by Schill (24). Briefly, benzoylarginine ethyl ester was hydrolyzed by acrosine producing ethanol, the latter was then assayed in an alcohol dehydrogenase reaction with formation of NADH(H+) detected at 366 nm (24) on the Novaspec III spectrophotometer (Amersham Biosciences). The total activity of acrosin was determined following rapid thawing (at 23˚C for 30 min) of the pre-frozen (at -196˚C liquid nitrogen) ejaculate. The pro-enzyme activity was calculated by subtracting the activity of free acrosine from the total acrosine activity. Acrosin activity was expressed in international units per 1 million spermatozoa (µU/106 cells) (24,25).
Apoptosis investigation
Sperm cells were washed to remove the seminal plasma and incubated in Menezo B-2 medium (BioMerieux) in a humidified incubator with 5% CO2 at 37˚C. The apoptotic process of gametes was assessed using fluorescein-labeled Annexin V (AnV), which binds to PS residues on the membrane surface of apoptotic cells, and PI, a fluorescent DNA dye that allows differentiation of cells with damaged membranes from those with intact membranes. The FITC Annexin V Apoptosis Detection Kit was purchased from BD Biosciences, Inc, and used according to the manufacturer's protocol. During the early stage of apoptosis, cells bind AnV, but like living cells, are impermeable to PI, therefore, they are AnV+/PI-. The percentage of such cells among the total number of cells was calculated (10,11). The microscopic studies were performed on a fluorescent microscope at a magnification of x100. The AnV+/PI-(green fluorescence without red fluorescence) cells were counted using a Goryaev chamber.
Assay of PA levels
PA was extracted from seminal plasma using n-butanol (1:1; pH=10) for 1 h at room temperature, and after evaporation of the butanol phase, the remaining solution was electrophoretically separated in 1.5% agar gel with 0.1 M citric acid buffer (pH=3.4-3.6) for 1 h at room temperature, with a voltage of 200 V and an amperage of 40 mA (Patent for invention RUS no. 2225981 dated 28.02.2002). Spm and Spd were visualized as pinkish-violet spots after staining with ninhydrin and identified using standard solutions (Fluka). The amount of PA in the sperm plasma of each man was determined using scanning electrophoregrams, transferring the image into a digital format on a PC using a specific computer program ‘PN 5108’ (certificate of registration of a computer program: RUS 2003612170 dated 21.07.2003). The concentrations of Spm and Spd were calculated using a classical calibration curve (plots reflecting the concentration-dependent peak areas for the standard PA solutions). The method was tested with natural semen samples (n=10) and showed the linearity within the working range of 0.05-40 µmol/ml, the detection limit for seminal plasma PA was 0.064 µmol/ml, variability interval 0.4 µmol/ml, maximum relative error ≤15%.
Statistical analysis
Statistical evaluation was performed after checking the distribution for normality (Shapiro-Wilk test) in Statistica version 7.0 (StatSoft Inc.). Comparisons between the studied values were assessed using an unpaired Student's t-test (P<0.05) or a one-way ANOVA followed by a post-hoc Tukey's test for multiple comparisons in Microsoft Excel 2016 (Microsoft Corporation) or SPSS version 24 (IBM Corp.), respectively. Data are presented as the mean ± SEM. Assessment of the correlation between parameters was performed using a Pearson correlation coefficient analysis (r). P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the spermatozoa of the fertile donors
The standard spermogram characteristics were obtained for the sperm cells from Exp1-2 F and Control F samples (Fig. S2A). An extended cytological study of the spermatozoa of fertile men did not reveal significant changes in the number of the head (amorphous, small, tapered, round, double-headed forms, etc.), neck (‘bent’ neck, thin midpiece, etc.) or tail defects (two-tailed forms, a short tail, etc.) in the experimental samples after exposure to low-intensity MW irradiation compared to the Control Samples (P>0.05, Table SI). Also, no alterations in the motility of the spermatozoa were observed after MW treatment of fertile men spermatozoa both in Exp1 F and Exp2 F (P>0.05, Table SII).
The evaluation of the functional state of sperm membranes by their resistance to 1% sodium chloride solution revealed that a short-term exposure to MW radiation resulted in a 26% increase in membrane resistance when applied to the whole ejaculate (Exp1 F), and in a 17% increase when the cells were separated from the plasma (Exp2 F). These findings are summarized in Table I.
Table I.
Changes in the resistance of sperm membranes and in apoptosis of fertile men after low-intensity millimeter-wavelength electromagnetic wave exposure.
| Parameter | Control F | Exp1 F | Exp2 F | p0-1 | p0-2 | p1-2 |
|---|---|---|---|---|---|---|
| Membrane resistance to sodium chloride, EUc | 4,250.32±320.63 | 5,325.41±291.80 | 4,963.01±278.91 | 0.033a | 0.214 | 0.666 |
| Percentage of AnV+/PI-spermatozoa,%c | 10.47±0.50 | 8.50±0.39 | 10.39±0.67 | 0.028a | 0.993 | 0.037a |
| Apoptotic indexc,d | NA | 0.81±0.02 | 0.99±0.01 | NA | NA | <0.01b |
aP<0.05,
bP<0.01.
cMean ± standard deviation.
dExp F/Control F. AnV, AnnexinV-FITC; PI, propidium iodide; EU, equivalent units; p0-1, comparison of Control F and Exp1 F, p0-2, comparison of Control F and Exp2 F, p1-2, comparison of Exp1 F and Exp2 F; P<0.05.
The Joel test showed a significant increase in spermatozoa resistance to acetic acid after the MW treatment. The differences in both the Control F and the Exp F were insignificant when incubated for <10 min, but after 20-30 min of incubation, the differences became statistically significant (P<0.05). Generally, the motility of sperm in samples after MW exposure was maintained for ~10 min longer than in Control F samples (Table SIII).
PS externalization in the spermatozoa of fertile donors
The next step of the present study was the determination of apoptotic markers in spermatozoa. The results are presented in Table I; Fig. S3A and B, E and F. The content of АnV+/PI-sperms in the Control F group was 10.47±0.50%. The percentage of these early apoptotic gametes was reliably reduced by almost 20% (P<0.01) in Exp1 F after MW exposure. The changes in the content of АnV+/PI-spermatozoa in the Exp2 F did not significantly differ rom the Control F (P>0.05). To make the results of the short-term MW impact assessment more demonstrative, the apoptosis index (AI) was calculated as follows: AI=Apoptotic percentage the in experimental group/apoptotic percentage in the Control F group.
This index shows the ratio of the AnV+/PI- gametes after exposure to the MW radiation (Exp1 F) to the number of such cells in the Control F group. AI values <1 (as in the Exp1 F group) were indicative of inhibition of apoptosis in gametes. The sodium chloride resistance of sperm membranes after MW exposure (Exp1 F) correlated inversely with the content of AnV+/PI- spermatozoa in ejaculates (r=-0.5; P<0.01) and with AI (r=-0.4; P<0.05).
Enzymatic activity of acrosine in spermatozoa of fertile donors
The determination of the enzymatic activity of acrosine is used as a diagnostic test for evaluating spermatozoa fertilization in vitro (24,25). Serine proteinase EC 3.4.21.10 acrosine is one of the key acrosomal enzymes and plays an important role in the process of oocyte fertilization (25). However, there is no data on the functioning of the acrosine system under the influence of MW-irradiation in the available literature. The activity of free acrosine was significantly lower in the experimental samples, particularly in Exp1 F, and was 87% of that observed in the Control F group (Table II). The total activity of acrosin did not change compared with the Control F group. The pro-enzyme activity of acrosin in ejaculate samples after MW-exposure compared to the control did not increase significantly, increasing by ~4% (Table II). The ratio of pro-acrosin/free acrosin was calculated, and its value increased on average by almost 20% in Exp1 F compared with the Control F (Table II).
Table II.
Acrosin activity in the spermatozoa of fertile donors before and after the low-intensity millimeter-wavelength electromagnetic wave exposure.
| Enzyme activity | Control F | Exp1 F |
|---|---|---|
| Free acrosin activity, µU/106 cellsb | 1.34±0.07 | 1.17±0.05a |
| Total acrosin activity, µU/106 cellsb | 5.16±0.15 | 5.15±0.13 |
| Pro-acrosin activity, µU/106 cellsb | 3.82±0.12 | 3.98±0.12a |
| Pro-acrosin/free acrosin ratiob | 2.85±0.11 | 3.40±0.12a |
aP<0.05 vs. Control F.
bMean ± standard deviation.
PA concentrations in seminal plasma of fertile donors
The next step of the study was to reveal the role of the seminal plasma PA on the effect of MW on spermatozoa. The assessment of the PA content was performed on Control F, Exp1 F and Exp2 F groups. The Control Samples showed that the concentration of Spm was 1.197±0.111 µmol/ml, and Spd was 1.265±0.150 µg/ml (Table III, Fig. 3). The MW treatment caused a significant decrease in the concentrations of both PAs in the Exp1 F group (P<0.05). The Spd concentration changed to a greater degree, so these alterations were not just quantitative, but also involved the profile of PA content. The change in the spectrum of PA levels in the seminal plasma after MW exposure is illustrated by the shift in the Spm/Spd ratio (0.95→1.22). The PA concentrations after MW treatment were not altered in the absence of spermatozoa (Exp2 F, P>0.05, Table III). However strong correlations (r=0.9, P<0.05) between the number of apoptotic cells and PA levels were observed for the Control F and Exp1 F samples.
Table III.
The concentration of polyamines in the seminal plasma of fertile donors after low-intensity millimeter-wavelength electromagnetic wave exposure in the presence (Exp1 F) and absence (Exp2 F) of the sperm cells.
| Parameter | Control F | Exp1 F | Exp2 F | p0-1 | p0-2 | p1-2 |
|---|---|---|---|---|---|---|
| Spm, µmol/mlc | 1.197±0.111 | 0.803±0.092 | 1.178±0.126 | 0.036a | 0.992 | 0.049a |
| Spd, µmol/mlc | 1.265±0.152 | 0.804±0.055 | 1.281±0.145 | 0.030a | 0.996 | 0.024a |
| Spm/Spdc | 0.95±0.03 | 1.22±0.03 | 0.93±0.05 | <0.001b | 0.944 | <0.001b |
aP<0.05,
bP<0.01.
cMean ± standard deviation. Spm, spermine; Spd, spermidine; p0-1, p comparison of Control F and Exp1 F, p0-2, comparison of Control F and Exp2 F; p1-2, comparison of Exp1 F and Exp2 F.
Figure 3.
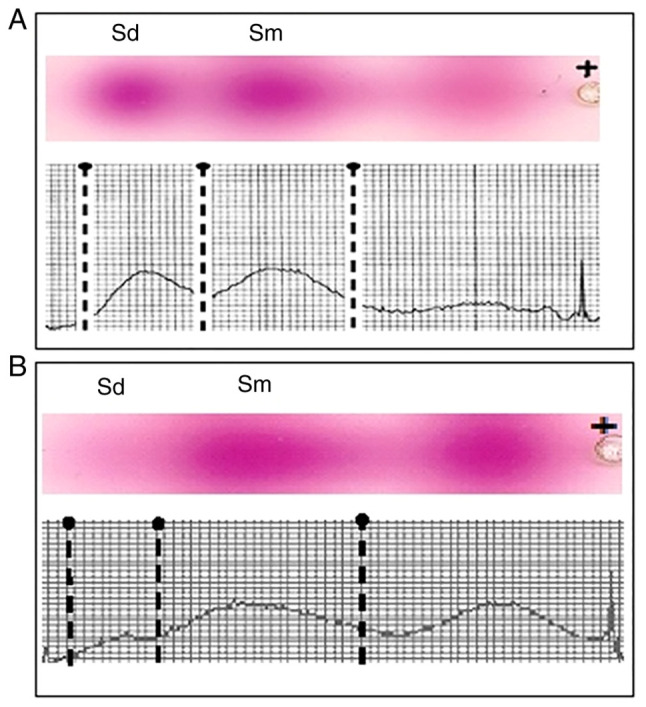
Electrophoregram and densitogram of the PA in the seminal plasma of a fertile man's (A) native seminal plasma, separated from the sperm cells, without MW exposure (Control F) and (B) in the seminal plasma, separated from the sperm cells, after exposure to low-intensity MW waves (Exp1 F). MW, millimeter-wavelength electromagnetic wave; PA, polyamine.
The PA electrophoregram and densitogram of seminal plasma of a fertile man before and after the exposure of the ejaculate to low-intensity MW are shown in Fig. 3. Two separate spots can be seen on the platelets corresponding to Spd and Spm visualized in the ninhydrin reaction. An additional spot of unknown substance appears in the electrophoregram after MW exposure. This substance is present not far from the PA, therefore it may be considered as a substance similar to PAs. At the same time its velocity of migration is lower than that of PA, suggesting it has a different molecular weight/charge ratio. Presumably this spot might belong to an acetylated Spd or Spm. Unfortunately, it was not possible to determine the identity of the molecule accounting for this spot due to a lack of standard components, and will thus form the focus of future investigations.
The results gained for the fertile samples provide evidence of the positive action of MW treatment, such as the increase in membrane stability and improved functionality of spermatozoa (lowered acrosin activity, inhibition of apoptosis). This effect was observed mainly for the complete ejaculates against the background of seminal plasma PA levels lowering, and was absent or less pronounced in the sperm cells exposed to MW in the absence of seminal plasma. The PAs did not ‘disappear’ from the spermatozoa-free plasma (Exp2 F) after MW treatment. Taken together these results reveal the involvement of the PA system on the effect of MW upon sperm cells.
Characteristics of the spermatozoa from the subfertile samples
The investigation of the influence of MW irradiation on spermatozoa of subfertile samples was performed in order to reveal any potential therapeutic effects (Fig. S2B; Fig. S3C and D). The complete ejaculates were tested only (Control S and Exp1 S).
Due to the large variability in the parameters of spermograms of the patients in the group of pathospermia, the changes in the morphology of the gametes, in the functional state of their membranes and in PS externalization as a marker of sperm apoptosis, were not statistically significant (all P>0.05). The reaction of subfertile spermatozoa to MW differed from that of the normozoospermia samples. In particular, the number of progressively motile gametes increased to 24% (Exp1 S) from 16% (Control S), while the number of non-progressively motile spermatozoa decreased from 20% in Control S to 12% in Exp1 S (P<0.05, Table IV). The quantity of immotile spermatozoa did not change significantly (P>0.05, Table IV).
Table IV.
Motility of spermatozoa from subfertile men before and after exposure to the millimeter-wavelength electromagnetic wave irradiation.
| Spermatozoa percentage, % | |||
|---|---|---|---|
| Spermatozoa motility characteristics | Control S | Exp1 S | P-value |
| Progressively motile, % | 16.9±2.3 | 24.0±1.3a | P<0.05 |
| Non-progressively motile, % | 20.3±2.4 | 12.1±1.7a | P<0.05 |
| Immotile, % | 62.8±1.3 | 63.9±1.5 | P<0.05 |
aP<0.01 vs. Control S.
PA concentrations in the seminal plasma of subfertile samples
First the levels of PA in the spermoplasm of subfertile men revealed that their levels drastically differed from those of fertile men, in particular the residual concentration of Spm and Spd was 58 and 15% from that of healthy donors, respectively. The study of the dynamics of the PA in the subfertile spermoplasm after MW treatment revealed two types of responses. In the first type, there was no significant change in the levels of PA, there was only a slight tendency to an increase in the levels of Spd, although this increase was not significant. In the second type of response, both Spm and Spd levels were significantly decreased, which resembled the response in normozoospermia and led to an increase in the Spm/Spd ratio.
Discussion
The parameters applied in the MW-electromagnetic field are in accordance with those that are already well-characterized and are known to induce a positive biological effect without heating. These frequencies and regimens are applied in therapeutic purposes, namely for the treatment of chronical prostatitis (3,5). The duration of treatment in the present study was within the therapeutically applied parameters. An alternative to the constant EM field is a pulsed-radiation that is used in telecommunication systems. The lack of the specific equipment has limited our investigation to the constant field only. Our preliminary experiments included the 20 and 40 min MW exposures, but the results within this range of time exposures did not differ (data not shown), therefore the experiments in the present study were limited to 20 min of exposure only.
There have been several theories suggested that may explain the effect of MW on cells. For example, changes in cell membranes, water molecule rotation induced by MW, and direct and indirect stimulation of nerve pathways are among possible mediators of the actions of MW (1,2,6). The aim of the present study was to determine any effect of MW on the complete ejaculate and the role of the seminal plasma PA on the effects of MW. The investigation of low-intensity MW radiation impact on sperm cells in their native seminal plasma environment and on isolated sperm cells (in cultural liquid) showed this treatment increased sperm membrane stability and lowered apoptosis of the sperm cells. The effect did not appear at the level of morphology or motility of the cells, which is an expected result, since it is unlikely that a short-term exposure can cause visible morphological changes in spermatozoa (2,6).
A documented positive effect of MW irradiation identified in the present study is the increased sperm membrane stability, and this is consistent with the previous literature reports (2-4). To assess the functional state of sperm membranes, two methods were used: i) A method using an external factor with increasing strength (sodium chloride solution, Milovanov test) and ii) a method using a constant external factor acting over an elongated time period (acetic acid, Joel test). Both methods showed an increase in the resistance of the membranes of sperm cells of fertile men after exposure to MW. A more pronounced stabilizing effect was observed for the cells in the presence of seminal plasma PA than in the cultural liquid. The inhibition of cell apoptosis was correlated with the degree of membrane stabilization and with the PA concentrations.
The function of the acrosomal enzyme acrosin is fundamental for ensuring the realization of the procreative function of spermatozoa, and is closely related with the state of the spermatozoa membranes (25). The results of the present study indicated that in normozoospermia, the MW treatment resulted in an increase in the pro-acrosin/free acrosin ratio; thus, the premature activation of acrosin was prevented, which is a favorable factor contributing to the effective realization of the fertilizing activity of spermatozoa. The increase in the pro-enzyme activity of acrosin may have been due to a decrease in the activity of free acrosin as a result of stabilization of the acrosomal membrane of the spermatozoa under the influence of MW. However, the direct influence of the MW field on the activity of acrosine by altering its conformation through modification of its solvate shell cannot be excluded, and it is likely the case that both mechanisms take place, and the extent of the effects of each mechanism should be determined.
Spm and Spd (Fig. 1) belong to the PA group, along with putrescine and cadaverine, which are often termed diamines (8). According to our previous results the content of Spm in human semen ranges from 138.4-21,749.9 µmol/l and Spd from 17.9-1,170.8 µmol/l (14), while the levels of PA in the blood serum (and blood plasma) do not exceed 1 µmol/l (26). Thus, the seminal plasma PA concentration greatly exceeds that of the other tissues, indicating the importance of PA for normal sperm function (27-29). The study design included the investigation of PA levels in separated sperm plasma and in the complete ejaculate. The separated sperm plasma (Exp2 F) did not show any changes in PA levels after MW exposure, indicating that the lowering of PA levels occurred in the presence of the sperm cells only (Exp1 F) and therefore may be attributed to the interaction of PAs with these cells. Moreover, the lack of direct change in PA concentration in Exp2 F showed the absence of an effect on PA from MW irradiation. The mechanism by which PA concentration was lowered in the Exp1 F group requires further study, but it is hypothesized that PAs may be taken up by the cells or absorbed by their membranes. Additional experiments are required to clarify the fate of the PAs within the sperm cells. The simultaneous effect of increased sperm membrane stability and lowered expression of apoptotic markers together with the lowered PA concentration in the environment (seminal plasma) may indicate interaction of PA with the membrane and its stabilization through this interaction. A previous study has reported on the ability of PA to behave as a membrane-bound component of sperm cells (30). Thus, the uptake of PA by the sperm cells or PA absorption into the membranes seems like a plausible possibility. The decrease in concentrations observed for Spd and Spm were comparable (64 and 67% vs. the Control F, respectively). The membrane stabilizing effect of the PAs may also result from the modulation of membrane fluidity under the action of PA (31), which prevents changes in the dynamics of the lipids of the cell membrane. Unfortunately, it was not possible to measure the PA levels inside the sperm cells, and further studies are required to clarify a possible mechanism by which PA is taken up by the cells.
Infertility is known to be associated with a change in PA concentrations in the ejaculate, which seems to influence gamete viability (14,32,33). Excessive production of PA results in a decreased number of mature spermatozoa (34), whereas lowered PA concentrations in the seminal liquid is correlated with increased sperm apoptosis (35). Our results correspond with previous studies, which reported a decrease in PA levels in subfertile men (27-29). This decrease may be due to their lowered synthesis or enhanced degradation of PAs, and should form the focus of future studies. A possible role in the regulation of sperm apoptosis may be attributed to these polycationic molecules (35,36). The antiapoptotic and cytoprotective effect of PA upon cells has been well documented (8,37-41). The MW irradiation of specific frequencies may induce apoptosis, and this effect is studied as a possible therapeutic tool for the management of cancer (42). The electromagnetic frequency used in the present study did not result in an upregulation of apoptotic cell markers compared with the intact Control F as shown in Table III. One of the methods of detecting apoptosis is the study of membrane phospholipid distribution based on Annexin V-PI staining. The predominant localization of exact classes of phospholipids within the inner or outer leaflets of the cytoplasm membrane is one of the prerequisites for maintaining cell viability. Phospholipid asymmetry in the membrane is disturbed and PS moves to the outer leaflet; when apoptosis is initiated and the cell loses its viability (10,12). The results of the present study demonstrated that PS translocation to the outer leaflet was reduced after MW treatment when compared with the Control F. The correlation between the decrease in PA concentration in the seminal plasma and the decrease in the number of apoptotic gametes in Exp1 F serves as an indirect proof that PA may serve as an inhibitory factor for apoptosis after MW treatment. Our previous in vitro studies on human peripheral blood lymphocytes showed the ability of PA to prevent apoptosis at physiological concentrations (11). Taken together, these results suggest that the mechanism of membrane stabilization and prevention of PS migration to the outer membrane leaflet under the action of MW radiation involves the seminal plasma PA system.
Seminal plasma is the natural environment and can be considered the primary source of PAs for the sperm cells, as the PA contents (32) and the activity of PA synthesizing enzymes is considerably higher in seminal plasma than inside the gametes (43). Thus, it is possible to conclude that the effects of MW on the fate of ejaculate seem to be mediated and potentiated by the PA system.
The comparison of results gained for normospermia and pathospermia samples reveals some interesting differences. In fertile samples, exposure to the MW field does not lead to a significant change in sperm motility, likely due to the motor potential of a normal spermatozoid being at its maximum under physiological conditions. However, in pathospermia samples, where the motility of spermatozoa is impaired and far from optimal, the effect of MW appears to slightly, but significantly improve motility, namely through an increase in the number of progressively motile spermatozoa.
However, the response of the PA system to MW exposure in pathospermia differed from that in normospermia and demonstrated two types. In the first type of response, there was no significant change in the levels of PA. In the second type of response, the levels of both Spm and Spd significantly decreased, but the Spm/Spd ratio increased, which resembled the response in normozoospermia. It is interesting to note that according to the medical history sheets data, the effectiveness of medical therapy in pathospermia patients with the second type of response was 30-40% higher than in patients with the first type of response (data not shown). Likely, the pathological changes in the membranes are more entrenched with regard to the actual cell pathophysiology in the patients who exhibit the first type of response than in the patients who exhibit the second type of response, thus, their cells do not respond as a physiological cell would be expected to, and this may explain the poorer curability of such patients.
In conclusion, the biological effects of microwaves on male gametes were shown. The protective and membrane-stabilizing effects of MW are described, and the role of PA in the biological effects of microwaves was determined. Differences in biological reactions to MW irradiation in normospermia (healthy) and pathospermia (men with impaired fertility) donors were also revealed. Electromagnetic irradiation at a frequency of 42.194 GHz and a wavelength of 7.1 nm, when used to treat human sperm for 20 min, resulted in certain specific biological effects without thermal side-effects: i) No changes in morphology and practically no effects on motility of spermatozoa were observed in healthy samples; ii) it prevented the premature activation of acrosin in samples from healthy donors, increasing the proacrosin/free acrosin ratio by ~20%; iii) it increased the stability of the membranes of sperm in the fertile samples, which was confirmed by the Milovanov and Joel tests; iv) it caused a decrease in the levels of the seminal plasma PA, and the decrease in the levels of Spd were more pronounced; v) it reduced the number of apoptotic (AnV+/PI-) gametes in the complete ejaculate from fertile men; vi) a decrease in the number of apoptotic (AnV+/PI-) gametes was correlated with a decrease in the levels of spermoplasm PA, which may indicate the participation of seminal plasma PA in the biological effects of microwaves; vii) it caused an increase in the motility of gametes in pathospermia samples from the men with impaired fertility; viii) in pathospermia, two types of reactions to the effects of MW exposure were noted: in the first type of reaction, there were no dynamics in the levels of spermoplasm PA; in the second type, the trend in PA changes were similar to the reaction of normospermia samples.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: This study has been supported by the RUDN University Strategic Academic Leadership Program.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MVP conceived and designed the study, performed the experiments and wrote the manuscript. DFZ, NRK and SVR recruited the patients and controls, and collected the samples. EVN analyzed the data, wrote and edited the manuscript and contributed to conceptualization of the study. MLB, SPS, KS and AH analyzed the data and edited the manuscript. MVP, DFZ, NRK and SVR confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Local Ethics Committee of the Astrakhan State Medical University of the Health Ministry of the Russian Federation (protocol no. 2; 21st May 2021). Informed consent for participation in the study and use of their samples was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Thackston KA, Deheyn DD, Sievenpiper DF. Limitations on electromagnetic communication by vibrational resonances in biological systems. Phys Rev E. 2020;101(062401) doi: 10.1103/PhysRevE.101.062401. [DOI] [PubMed] [Google Scholar]
- 2.Ziskin MC. Millimeter waves: acoustic and electromagnetic. Bioelectromagnetics. 2013;34:3–14. doi: 10.1002/bem.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbotina TI, Tereshkina OV, Khadartsev AA, Yashin AA. Effect of low-intensity extremely high frequency radiation on reproductive function in wistar rats. Bull Exp Biol Med. 2006;142:189–190. doi: 10.1007/s10517-006-0324-8. (In English, Russian) [DOI] [PubMed] [Google Scholar]
- 4.Nikoghosyan А, Heqimyan А, Ayrapetyan S. Non-thermal microwave radiation-induced brain tissue dehydration as a potential factor for brain functional impairment. Int J Basic Appl Sci. 2016;5:188–195. [Google Scholar]
- 5.Betskii OV, Devyatkov ND, Kislov VV. Low intensity millimeter waves in medicine and biology. Crit Rev Biomed Eng. 2000;28:247–268. doi: 10.1615/critrevbiomedeng.v28.i12.420. [DOI] [PubMed] [Google Scholar]
- 6.Tambiev AK, Kirikova NN. Novel concepts of the causes of EHF-radiation-induced stimulating effects. Crit Rev Biomed Eng. 2000;28:60–76. doi: 10.1615/critrevbiomedeng.v28.i56.90. [DOI] [PubMed] [Google Scholar]
- 7.Iryanov YM, Kiryanov NA. Reparative osteogenesis and angiogenesis in low intensity electromagnetic radiation of ultra-high frequency. Vestn Ross Akad Med Nauk. 2015;70:334–340. doi: 10.15690/vramn.v70i3.1330. (In Russian) [DOI] [PubMed] [Google Scholar]
- 8.Bae DH, Lane DJR, Jansson PJ, Richardson DR. The old and new biochemistry of polyamines. Biochim Biophys Acta Gen Subj. 2018;1862:2053–2068. doi: 10.1016/j.bbagen.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi K, Kashiwagi K. The functional role of polyamines in eukaryotic cells. Int J Biochem Cell Biol. 2019;107:104–115. doi: 10.1016/j.biocel.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Ploskonos MV, Zulbalaeva DF, Kurbangalieva NR, Ripp SV. Laboratory assessment of sperm apoptosis ability in men with different fertility. The problems of reproduction. 2020;26:77–84. [Google Scholar]
- 11.Hilal A, Ploskonos MV, Terentyev AA, Syatkin SP, Neborak EV, Blagonravov ML, Protasov A, Kaitova Z, Chibisov SM. Regulation of apoptosis of human immunocompetent cells under the effect of polyamines. FEBS Open Bio. 2018;8: (Suppl 1)(234) [Google Scholar]
- 12.Fratini E, Cervelli M, Mariottini P, Kanamori Y, Amendola R, Agostinelli E. Link between spermine oxidase and apoptosis antagonizing transcription factor: A new pathway in neuroblastoma. Int J Oncol. 2019;55:1149–1156. doi: 10.3892/ijo.2019.4878. [DOI] [PubMed] [Google Scholar]
- 13.Ploskonos MV, Syatkin SP, Neborak EV, Hilal A, Sungrapova KY, Sokuyev RI, Blagonravov ML, Korshunova AY, Terentyev AA. Polyamine analogues of propanediamine series inhibit prostate tumor cell growth and activate the polyamine catabolic pathway. Anticancer Research. 2020;40:1437–1441. doi: 10.21873/anticanres.14085. [DOI] [PubMed] [Google Scholar]
- 14.Ploskonos MV, Evdokimov VV. Polyamines of urogenital tract men as factors of apoptosis regulation spermatozoids. Urologiia. 2019;4:74–79. (In Russian) [PubMed] [Google Scholar]
- 15.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359(eaan2788) doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 16.Ozer Kaya S, Gur S, Erisir M, Kandemir FM, Benzer F, Kaya E, Turk G, Sonmez M. Influence of vitamin E and vitamin E-selenium combination on arginase activity, nitric oxide level and some spermatological properties in ram semen. Reprod Domest Anim. 2020;55:162–169. doi: 10.1111/rda.13601. [DOI] [PubMed] [Google Scholar]
- 17.Gapeev AB, Shved DM, Mikhaĭlik EN, Korystov IuN, Levitman MKh, Shaposhnikova VV, Sadovnikov VB, Alekhin AI, Goncharov NG, Chemeris NK. Antitumor effect of low-intensity extremely high-frequency electromagnetic radiation on a model of solid Ehrlich carcinoma. Biofizika. 2009;54:1128–1136. (In Russian) [PubMed] [Google Scholar]
- 18.World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. World Medical Association. [DOI] [PubMed] [Google Scholar]
- 19.Korolev YN, Bobrovnitskii IP, Geniatulina MS, Nikulina LA, Mikhailik LV. The ultrastructure of Sertoli cells and spermatogonia in the rats exposed to radiation under conditions of therapeutic and prophylactic application of low-intensity electromagnetic emission. Vopr Kurortol Fizioter Lech Fiz Kult. 2018;95:35–40. doi: 10.17116/kurort201895135-40. (In Russian) [DOI] [PubMed] [Google Scholar]
- 20.Korolev YN, Bragina EE, Nikulina LA, Mikhailik LV. Action features of the of low-intensity electromagnetic radiation at an early stage of the experimental metabolic syndrome development induced by a diet high in carbohydrates and fats. Vopr Kurortol Fizioter Lech Fiz Kult. 2021;98:47–52. doi: 10.17116/kurort20219801147. (In Russian) [DOI] [PubMed] [Google Scholar]
- 21.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization (WHO): WHO laboratory manual for the examination and processing of human semen. Vol 270. 5th edition. WHO, Geneva, 2010. [Google Scholar]
- 23.Dolgov VV, Lugovskaya SA, Fanchenko ND. Laboratory diagnostics of male infertility: issued by the Department of СLD, Moscow, p.145, 2006 (in Russian). [Google Scholar]
- 24.Schill WB. Acrosin activity in human spermatozoa: Methodological investigations. Arch Dermatol Forsch. 1973;248:257–273. doi: 10.1007/BF00594903. [DOI] [PubMed] [Google Scholar]
- 25.Zahn A, Furlong LI, Biancotti JC, Ghiringhelli PD, Marijn-Briggiler CI, Vazquez-Levin MH. Evaluation of the proacrosin/acrosin system and its mechanism of activation in human sperm extracts. J Reprod Immunol. 2002;54:43–63. doi: 10.1016/s0165-0378(01)00080-8. [DOI] [PubMed] [Google Scholar]
- 26.Song J, Shan Z, Mao J, Teng W. Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin Endocrinology. 2019;90:727–736. doi: 10.1111/cen.13946. [DOI] [PubMed] [Google Scholar]
- 27.Shohat B, Maayan R, Singer R, Sagiv M, Kaufman H, Zukerman Z. Immunosuppressive activity and polyamine levels of seminal plasma in azo-ospermic, oligospermic, and normospermic men. Arch Androl. 1990;24:41–50. doi: 10.3109/01485019008986857. [DOI] [PubMed] [Google Scholar]
- 28.Fair WR, Clark RB, Wehner N. A correlation of seminal polyamine levels and semen analysis in the human. Fertil Steril. 1972;23:38–42. doi: 10.1016/s0015-0282(16)38707-6. [DOI] [PubMed] [Google Scholar]
- 29.Shah GV, Sheth AR, Mugatwala PP, Rao SS. Effect of spermine on adenyl cyclase activity of spermatozoa. Experientia. 1975;31:631–632. doi: 10.1007/BF01944596. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein S, Breitbart H. Cellular localization of polyamines: Cytochemical and ultrastructural methods providing new clues to polyamine function in ram spermatozoa. Biol Cell. 1994;81:177–183. doi: 10.1016/s0248-4900(94)80008-1. [DOI] [PubMed] [Google Scholar]
- 31.Benavides MP, Groppa MD, Recalde L, Verstraeten SV. Effects of polyamines on cadmium- and copper-mediated alterations in wheat (Triticum aestivum L) and sunflower (Helianthus annuus L) seedling membrane fluidity. Arch Biochem Biophys. 2018;654:27–39. doi: 10.1016/j.abb.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Lefèvre PL, Palin MF, Murphy BD. Polyamines on the reproductive landscape. Endocr Rev. 2011;32:694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- 33.Lundy SD, Sangwan N, Parekh NV, Selvam MKP, Gupta S, McCaffrey P, Bessoff K, Vala A, Agarwal A, Sabanegh ES, et al. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur Urol. 2021;79:826–836. doi: 10.1016/j.eururo.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov IP, Rohrwasser A, Terreros DA, Gesteland RF, Atkins JF. Discovery of a spermatogenesis stage-specific ornithine decarboxylase antizyme: Antizyme 3. Proc Natl Acad Sci USA. 2000;97:4808–4813. doi: 10.1073/pnas.070055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korzun IA, Ploskonos MV, Syatkin SP, Blagonravov ML, Gushchina YuSh, Eremina IZ, Kaitova ZS, Navid MN, Aissa AA. Components of seminal plasma as factors regulating apoptosis of male gametes. FEBS Open Bio. 2019;9 (Suppl 1)(370) [Google Scholar]
- 36.Hilal A, Ploskonos MV, Syatkin SP, Neborak EV, Maksimova TV, Terentev AA. Apoptosis markers of spermatozoids and polyamines of human spermoplasm. FEBS Open Bio. 2019;9 (Suppl 1)(165) [Google Scholar]
- 37.Agostinelli E. Biochemical and pathophysiological properties of polyamines. Amino Acids. 2020;52:111–117. doi: 10.1007/s00726-020-02821-8. [DOI] [PubMed] [Google Scholar]
- 38.Bekebrede AF, Keijer J, Gerrits WJJ, Boer VCJ. The molecular and physiological effects of protein-derived polyamines in the intestine. Nutrients. 2020;12(197) doi: 10.3390/nu12010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain T, Tan B, Ren W, Rahu N, Dad R, Kalhoro DH, Yin Y. Polyamines: Therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids. 2017;49:1457–1468. doi: 10.1007/s00726-017-2447-9. [DOI] [PubMed] [Google Scholar]
- 40.Syatkin SP, Kirichuk AA, Soldatenkov AT, Kutyakov SV, Neborak EV, Shevkun NA, Kuznetsova OM, Skorik AS, Terent'ev AA. Screening of some dioxaboreninopyridine and aniline derivatives for carcinogenic properties using a model cell-free system of regenerating rat liver. Bull Exp Biol Med. 2017;162:801–807. doi: 10.1007/s10517-017-3717-y. [DOI] [PubMed] [Google Scholar]
- 41.Syatkin SP, Neborak EV, Khlebnikov AI, Komarova MV, Shevkun NA, Kravtsov EG, Blagonravov ML, Agostinelli E. The investigation of structure-activity relationship of polyamine-targeted synthetic compounds from different chemical groups. Amino Acids. 2020;52:199–211. doi: 10.1007/s00726-019-02778-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhao R, Liu Y, Liu S, Luo T, Zhong GY, Liu A, Zeng Q, Xin SX. Apoptosis-promoting effects on A375 human melanoma cells induced by exposure to 35.2-GHz millimeter wave. Technol Cancer Res Treat. 2020;19(1533033820934131) doi: 10.1177/1533033820934131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jänne J, Hölttä E, Haaranen P, Elfving K. Polyamines and polyamine-metabolizing enzyme activities in human semen. Clin Chim Acta. 1973;48:393–401. doi: 10.1016/0009-8981(73)90418-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.