Abstract
Comforts in modern society have generally been associated with longer survival rates, enabling individuals to reach advanced age as never before in history. With the increase in longevity, however, the incidence of neurodegenerative diseases, especially Alzheimer’s disease, has also doubled. Nevertheless, most of the observed variance, in terms of time of clinical diagnosis and progression, often remains striking. Only recently, differences in the social, educational and occupational background of the individual, as proxies of cognitive reserve (CR), have been hypothesized to play a role in accounting for such discrepancies. CR is a well-established concept in literature; lots of studies have been conducted in trying to better understand its underlying neural substrates and associated biomarkers, resulting in an incredible amount of data being produced. Here, we aimed to summarize recent relevant published work addressing the issue, gathering evidence for the existence of a common path across research efforts that might ease future investigations by providing a general perspective on the actual state of the arts. An innovative model is hereby proposed, addressing the role of CR across structural and functional evidences, as well as the potential implementation of non-invasive brain stimulation techniques in the causal validation of such theoretical frame.
Keywords: Aging, Alzheimer’s disease, cognitive reserve, diffusion tensor imaging, electroencephalography, functional magnetic resonance imaging, magnetic resonance imaging, positron emission tomography, transcranial magnetic stimulation
INTRODUCTION
Alzheimer’s disease (AD) is currently recognized as the most common type of neurodegenerative pathology, affecting nearly 5.5 million individuals in the United States alone, with a rate of one new diagnosis being made every 66 seconds [1]. AD is associated with a variety of cognitive symptoms, ranging from memory, language, and problem-solving impediments that ultimately affect the independence of the individual in everyday life, representing a massive socioeconomic and healthcare challenge [1]. Overall, structural and functional modifications are known to precede the cognitive impediments, with individuals displaying high degrees of variability in terms of the delay in symptomatology onset [2, 3]. Such inter-individual differences have most recently been approached by reserve-based models of resilience, which can be broadly divided into passive and active models of reserve. Within the former, the concept of reserve is seen more in terms of a physical threshold [4]—embracing the notion of a Brain Reserve (BR) [5, 6]—based on which the extent of the underlying neurological substrate may determine a favorable or unfavorable role depending on the number of neurons and brain size (e.g., the more solid the underlying physical substrate, the more resilient the brain will be in the face of damage). However, this earlier passive approach has been challenged by a more modern perspective—that of the Cognitive Reserve (CR) [7]—according to which, the involvement of the individual in various cognitive stimulating activities during life, plays a crucial role when it comes to determine the effectiveness in recruiting additional areas or in implementing compensatory strategies, behaving more flexibly and dynamically than a passive threshold. Both models, BR and CR, cannot be considered mutually exclusive and rather they have been suggested to operate at two different “levels of analysis”, in which the former looks at the quantity of available substrates (i.e., a more “static” perspective), whereas the latter focuses on their quality and at a more “dynamic” possibility for adaptation [8]. Furthermore, the neural implementation of CR can be broken down in terms of both neural reserve and neural compensation [9, 10], which operate by aiding better performances in everyday task when faced with increased task requirements or major brain insults, either by relaying on inter-individual differences in the strength of the underlying brain functional and structural networks or through a more efficient recruitment of alternative structures respectively [8]. Several different indexes have been used in the past as proxies of CR, especially in terms of education, leisure activities, active lifestyle, occupation, social engagement, and ultimately premorbid IQ [11, 12]. Within the aging population, the role of CR becomes even more important when considered in the clinical frame, given that several studies have reported CR as involved in exerting a protective role in the face of neurodegenerative pathologies [13–15], delaying their clinical worsening [3, 16], and in ensuring better cognitive performances [17]. However, once clinically reaching the time of AD diagnosis, individuals with higher CR have also been reported to progress faster within the disease [18–23] and to benefit less from interventional therapies [24, 25]. On the other hand, within the healthy population, CR has been generally associated with longer rates of survival [11] and an overall better quality of life [26]. Given the existence of such wide spectra on the role of CR in the aging process, most of the research interest has been directed toward analyzing the possible structural and functional differences that characterize both the normal and pathological processes of aging and how they may differentiate in individuals with high or low CR.
Here, we aim to summarize recent early literature addressing this issue, with a major focus on how the documented structural and functional changes in the brain associated with CR might come in useful terms when addressing biological markers of reserve. Moreover, given the identification of appropriate neural substrates, possible approaches for enhancing, promoting, and assessing CR will be described within the framework of non-invasive brain stimulation (NIBS). Over the last three decades, methods involving transcranial stimulation of cortical brain regions have been promoted as a safe and reliable tool for causal validation of theoretical models as well as for modulation of brain activity [27–29] with clinical [30] and cognitive enhancement applications [31, 32]. A few studies have explored the possibility of leveraging such technology to target regions and systems supposedly playing a role in normal and pathological aging [33–37], but with no attempt focused on CR so far. At last, we will review the literature on NIBS and CR, and discuss their potential future integration to better monitor disease progression and possibly increase individual resilience to neurodegeneration.
METHODS
Review of relevant published studies was carried out through online search in the CINAHL, MEDLINE, Google Scholar, and PsychINFO databases. Considered the abundance of material published focusing on the relationship between CR and AD, restriction criteria was set such as that mainly the abstracts of published work in the last 10 years were scrutinized, together with their associated relevant references. Papers ultimately considered for the review were searched based on the combination of pertinent keywords including: “Alzheimer Disease”, “aging”, “cognitive reserve”, “magnetic resonance imaging- MRI”, “functional magnetic resonance imaging- fMRI”, “positron emission tomography- PET”, “diffusion tensor imaging- DTI”, “electroencephalography- EEG”, “transcranial magnetic stimulation- TMS”. Only published work in English was considered. A graphical walkthrough of the literature search and of the model we hereby propose is presented in Fig. 1.
Fig. 1.
Schematic flowchart of the literature search and rationale behind the study.
THE ROLE OF COGNITIVE RESERVE ON STRUCTURAL CHANGES
Magnetic resonance imaging (MRI) studies of the aging brain have revealed the occurrence of structural modifications in the form of decreased brain weight and volume, such as that by the age of 90, the average loss of brain tissue reaches the 14% in the cerebral cortex and up to the 35% in the hippocampus, with similar patterns in apolipoprotein (APOE) ε4 allele carriers and non-carriers [38]. When the association between proxies of reserve and volumetric brain estimates was first analyzed, significant findings were reported in literature proving both global cortical involvement as well as region-specific associations. In the healthy population, increased CR was proven to boost the resilience of the individual from developing into mild cognitive impairment (MCI) or dementia by ensuring greater amount of white matter fibers among regions [39]; whereas, in a pathological sample, measures of cortical thickness were found to help predict progression from MCI into AD up to 24 months before former diagnosis [40]. Since that CR appears to act in ensuring cognitive functioning even in the face of significant thinning—thus masking the effect of the pathology by delaying its clinical manifestations, structural measures may be a more sensitive index of disease progression, capable of detecting changes that otherwise may not be observable on the basis of cognitive performances alone [40]. Indeed, there is evidence that individuals with higher reserve maintain relatively normal and stable cognitive functioning even in the face of significant reductions in their brain structural properties, mostly as a result of the compensatory increase in functional activation [41]. Congruently with the previously mentioned measures of cortical thickness, the existence of a negative correlation between CR and whole brain volume has been reported both in patients with AD and MCI [41], but not in healthy controls, for which the opposite pattern was observed—consistently with previous studies correlating measures of gray matter alone with IQ [42, 43]—and further proving the role of CR in ensuring a protective role by delaying clinical manifestations. Indeed, previous data in the literature have linked brain size as contributing to measures of general intelligence [44], such as that when premorbid IQ measures (e.g., tested through the administration of the American National Adult Reading Test) were utilized as proxies of CR, inter-individual variability in the clinical population could be independently related back to the association between premorbid IQ and both structural (in terms of increased atrophy) and functional (t-tau protein and amyloid-β(Aβ) depositions in the cerebrospinal fluid (CSF)) changes, with the former showing the strongest correlation [45]. Similar evidence of the role of CR as neuroprotective in healthy subjects and as neurocompensatory in the pathological population has been reported in studies correlating neuroimaging measures of cortical thickness and functional connectivity (diffusion tensor imaging, DTI) with indexes of cognitive functioning proving that for the former, greater cortical thickness in relevant regions, such as the frontal lobes, and decrease measures of fractional anisotropy (FA), possibly representing a more efficient reorganization of fibers [46], were associated with better neuropsychological performances, such as that the higher the CR (assessed in terms of years of education), the stronger the observed correlations [47, 48]. Using measures of cortical fractal structure, it has been further suggested that the relationship between CR and cortical thickness may relay on the complexity of the underlying layers rather than on their overall thickness [49], ensuring better cognitive abilities and less decline with increased age, thus providing a substrate of CR.
Similar studies investigating the role of education have reported slightly different regions showing the greatest variability in thickness among high and low CR individuals, focusing mainly over the frontal, parietal, and temporal regions [41, 50–54]. On this regard, evidence for a double dissociation comes from the notion that not only individuals with higher CR show greater resilience over those same regions, but also that certain cognitive abilities that are known to be implemented over those structures act by augmenting their resilience to aging. One of the greatest example is that of language; bilingual individuals have been reported to show increased grey and white matter over frontal and temporal regions compared to monolinguals [55].
Nevertheless, studies considering different proxies of reserve have revealed the involvement of different brain regions when comparing high versus low CR individuals. For instance, studies focusing on the association between cortical thickness and years of education have mostly reported greater regional thickness within the frontal lobes as a function of reserve (see [48] for example), which has been further restricted to the left anterior cingulate cortex and left dorsomedial prefrontal cortex when considering individuals with extremely low education (<4 years) [56]. On the other hand, when occupation, socioeconomic status, and engagement in physical activities are considered as proxies, measures of reserve tended to be skewed toward temporal regions [57–60]. It must be noted, however, that some of these studies specifically looked at the effects of CR in the hippocampal formation only—based on its known involvement in the neurodegenerative process—and it is therefore not possible to exclude that other regions might have shown changes as well, which were, however, not reported. Indeed, large cohort investigations have revealed less clear-cut differences in the association between proxies of reserve and regional cerebral changes, such as that education was observed to significantly correlate with both frontal and parieto-temporal regions, whereas no meaningful association was observed for either occupation or leisure activities [61]. Future investigations are needed to clarify whether if different proxies of CR entail equally different anatomical correlates.
All the aforementioned studies and previous investigations [62–65] have further contributed in consolidating the role of volumetric measures of both white and gray matter as fundamental in determining the existence of an underlying protective substrate as a function of proxies of CR. However, a small number of investigations have also reported no clear correlation between proxies like occupation and education with global volumetric measures (i.e., brain size) in predicting cognitive functions in later age [66]. Rather, these studies emphasized the association between general intelligence (factor ‘g’) and specific cognitive domains with an uneven profile of brain structures [67]. This same association was, however, no longer significant when the factor ‘g’ was removed from the specific cognitive scores [67]. In another study, factors such as age, gender, education, and white matter hyperintensities were assessed as possible predictors of cognitive functioning among the elders [68], proving that when cognitive performance was broadly divided into the components of memory, executive functions, and visuospatial skills, each of the aforementioned proxies contributed with different extents, with education having the higher predictive values for the latter two [68].
Despite structural measures being solid evidence in favor of more passive models of reserve, their contribution cannot be completely excluded from active models as well, especially if considering that the former may embrace a different level of analysis, yet being inevitably strongly interconnected with the latter [8]. Overall, structural findings in relation to the concept of reserve can be summarized as follow [51]: first of all, in the healthy population, measures of CR have been associated with greater amount of volume and cortical thickness, whereas the opposite (negative correlation) holds true in the case of pathological aging [51, 69]. In line with the proposed distinction in terms of neural reserve and neural compensation [10], the increased readiness of available connections and nodes within the system can be assumed as posing a protective factor in the face of major insults in terms of physical threshold [4], whereas the documented decrease in those same measures within the pathological population proves the compensatory role of CR in ensuring cognitive functioning even in the face of widespread atrophy (Fig. 2). Importantly, modern theories recognize a continuous transition in the role of CR from neuroprotective to neurocompensatory [70], such as that at the very first stages, CR may act in ensuring preserved functioning for longer in time. Once symptom severity reaches a threshold resulting in a formal diagnosis, CR may still act in modulating the link between pathology and cognition, for example by means of a more efficient recruitment of functional compensatory mechanisms (see next section).
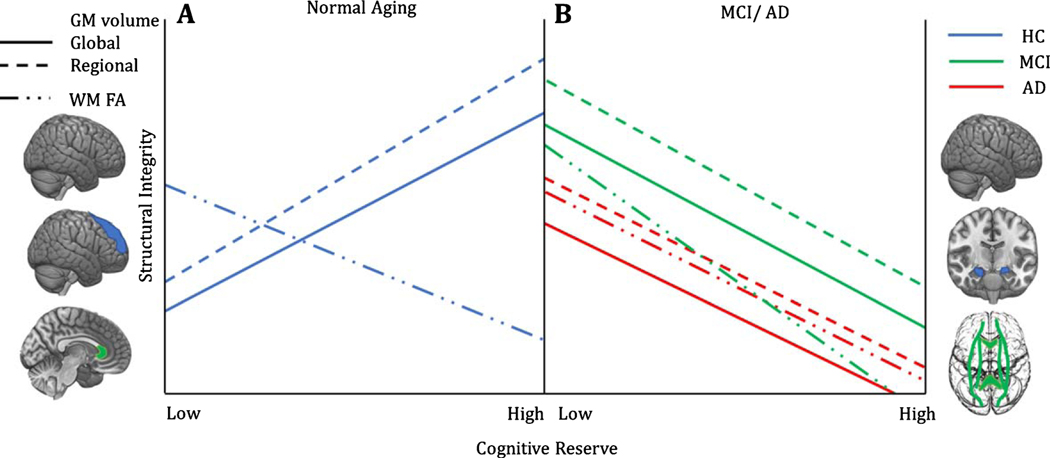
Fig. 2.
Associations between structural measures and CR in healthy and pathological brain aging. A) In healthy aging, a positive association has been reported between grey matter volume and CR, both at the whole brain level, as well as within specific regions, i.e., mainly the fronto-parietal sites [51]. In contrast, a negative correlation between CR and measures of FA in the genu of the corpus callosum—a region showing great physiological changes with age—has been reported [51]. According to the authors, the observed negative, rather than positive, relationship may reflect “a greater capacity to tolerate age-related structural damage without concomitant impact on cognitive performance,” In other words, individuals at preclinical/genetic risk conditions, who nonetheless have high CR, could lose white matter integrity without manifesting cognitive impairment, thus representing a transitional or intermediate brain status between healthy and pathological aging. Overall, grey and white matter changes support the continuous protective role of CR in sustaining preserved functioning for longer during time. B) In contrast with healthy cognitive aging, MCI and AD show negative associations between CR and grey matter volume at the global and regional level. In particular, the hippocampal formation represents one of the regions showing the greatest atrophy as a function of reserve, which suggests CR may enable patients to sustain more advanced stages of pathology compared to their low CR counterpart. A similar pattern has been observed in terms of FA reduction over pathologically relevant fibers’ tracts, including the corpus callosum, the cingulum, the inferior and superior longitudinal fasciculi and the fronto-occipital bundle [51], with a greater negative slope in MCI compared to AD patients [51].
The concept of CR has been theorized in terms of a prediction error [71]—the difference between the expected outcome of a patient based on the severity of the pathology and his observable real aftereffects. Through this decomposition approach, CR was again found to predict conversion rates from MCI to dementia, to influence the long-term decline and to overall act in protecting against atrophy, thus mirroring what was already known at the population level [71]. So far, only a single exception has been reported [40], wherein thinner cortices in relation to high CR were reported in both the healthy subjects and the pathological population alike.
The second major evidence within structural neuroimaging measures is based on the observation of region-specific changes associated to the degree of CR, such as those showing a limited effect of CR within the hippocampal areas but not elsewhere in the brain [72]. The reported evidence that patients with lower CR showed greater hippocampal volume has also been interpreted as evidence for the MCI individuals with low reserve being less resistant to the pathology [73], thus showing cognitive malfunctioning much earlier compared to the high CR, which can instead sustain greater hippocampal atrophy before showing any effect at the behavioral level [74]. Factors such as socioeconomic status in childhood have been proven to exert an effect over brain development, ultimately influencing hippocampal volumes, that may remain detectable many years into adulthood, thus perhaps providing a first starting point for lower CR [49]. Similarly, occupational complexity in the adult population has been linked with reduced hippocampal volume and an overall greater cerebral atrophy, even in the face of spared functioning among high CR individuals [75]. Within the healthy adult population, measures of CR were also found to be associated with decreased FA over the genu of the corpus callosum, a typical situ undergoing changes within normal aging [76]; whereas amnestic MCI patients showed an overall association with decreased white matter integrity and gray matter volumetric measures in regions belonging to the default mode network (DMN), counterbalanced by an increased functional activation [51]. Neural substrates of CR can therefore entail both global and regional specificity depending upon the population being examined [51]. For instance, the existence of an association between low CR and the entorhinal cortex volume has been reported in predicting development into MCI of a set of cognitively intact individuals [77]; similarly, in MCI and AD patients, those with lower CR showed both decreased volume in the hippocampal and entorhinal regions as well as decreased cognitive performance, whereas high CR were able to compensate despite having the same volumetric impediments [78]. Other studies focusing on the use of DTI have replicated similar results showing regional specificity dependent upon the population being examined, such as that the correlation between CR and white matter integrity in those areas most affected by the age-related decline was found to be even more negative in the healthy population compared to MCI or AD patients [79]. Of the latter, all of those who progressed into AD were found to belong to the high CR subgroup [79]. Taken together, this study validates three major concepts of interest reported so far:
In the healthy population, higher CR is associated with a better tolerance for age-related cortical changes and it acts in ensuring protection against pathological aging. Corroborating data come from postmortem brain analyses, reporting individuals with high amounts of both Aβ and neurofibrillary tangles who failed to show overt behavioral changes during life [80];
CR can be assumed to exert both global and regional-specific effects in terms of neural reserve and neural compensation. Those underlying substrates vary depending on the pathology and interact with both gray and white matter such as that, in the healthy controls, differences between individuals with high and low CR can be observed along those regions that are generally most affected with normal aging, whereas the same difference will be noticeable in the MCI/AD patients mostly along those regions involved in the pathological degeneration;
Despite high CR acting in ensuring better cognitive performances in the face of the same underlying degree of pathology, once diagnosed, those same patients tend to progress faster and to benefit less from interventional therapies (as evidenced by few studies comparing the cognitive benefits of AD patients with high or low CR following extensive cognitive and motor trainings [24, 25]).
THE ROLE OF COGNITIVE RESERVE ON FUNCTIONAL CHANGES
One major distinction between active and passive models of reserve comes in terms of the different mechanisms of action [7], such as that if passive models rely more on the underlying extent of available substrates, active ones work by assuming the existence of different recruitment capacities and activation patterns across individuals with different life backgrounds. So far, the reported evidence on structural changes has largely focused upon the role of volumetric properties of different brain regions during both normal and pathological aging, which, however, poses more favorably toward BR theories. Despite so, a considerably amount of investigations has also been carried out highlighting not only the mere quantitative aspects, but also their internal complexity [49] and the degree of connections among relevant structures [46]. Studies have looked at the relationship between functional architecture of the brain, its intrinsic resilience toward lesions [81], and cognitive profile, providing early quantitative markers of the brain’s resilience. Intriguingly, results show a positive correlation between the brain’s resilience toward insults and higher IQ, suggesting that individuals with higher intelligence levels are able to afford more functional lesions (i.e., loss of network nodes) before showing a significant decrease in their brain efficiency levels [82]. More recent work has also found trace of a link between coping style and specific networks’ activity, again suggesting that even psychological attitude in response to stressors might have a functional fingerprint, possibly representing one additional factor entailing a protective role from the development of mental distress, ensuring a better quality of life and ultimately act as a secondary proxy of reserve [83]. Taken together, those findings establish the importance of considering structural and functional approaches altogether, as presumably they reflect two concurrent implementations of a rather similar measure.
In the past years, one large cohort study [84] has been conducted assessing brain connectivity changes as a function of the interaction between age and IQ which, despite not directly assessed by the authors as a proxy of reserve, provides one of the few longitudinal evidences for the protective role of the latter. Indeed, starting from an equal IQ level at the age of 11 years, members of the Aberdeen 1936 Cohort study were divided into “maintainers” or “decliners” as a matter of their obtained cognitive scores at the age of 60–70 years and subsequently evaluated in their high-order cognitive abilities and patterns of functional activity [84]. In line with the CR hypothesis, individuals entailing more successful aging were observed to show functional MRI (fMRI) activation patterns closer to the ones of healthy younger individuals, whereas reduced patterns were observed for the subgroup of decliners [84]. Of importance, IQ levels at age 11 were similar but not identical across the volunteering subjects (SD = 0.55), but further distinguished apart as a function of age (SD = 2.25 when 60–70 years old) [84] and perhaps life-experiences. Overall, the possibility to examine changes in the effect of CR as a function of age, from an equal starting point in the earliest years of life, further sustains the notion that the building up of CR is a continuous process occurring as a function of time, rather than a fixed measure [8, 85].
Other studies focusing on the effect of age differences in relation to CR and brain activity have made use of positron emission tomography (PET) scanning, revealing a subset of regions—right inferior temporal gyrus, right postcentral gyrus, cingulate, and left cuneus—where significant differences could be observed representing aging-related compensatory mechanisms in highly functional individuals, such as that a stronger positive correlation was observed in the young group over all regions except for the cuneus, where instead a stronger positive slope was observed only among the eldery [86]. Age-related differences in terms of networks recruitment have also been reported during a memory task [10], for which healthy young individuals with higher measures of reserve showed an increased in the activity over right hippocampus, posterior insula, thalamus, and the operculum bilaterally with a concomitant decrease over the lingual gyrus, association cortex and inferior parietal lobe, the cingulate and calcarine cortex relative to the increase in the task’s difficulty. By comparison, the response of high CR elders consisted in a decrease in the recruitment over those same regions proportional to their CR estimate, interpreted as an expression of functional reorganization in trying to overcome natural decline in cognitive performances [10]. In particular, frontal areas may be seen as the substrates that are more greatly involved in aiding the compensatory mechanisms that underpin CR in successful aging [87], such as that a greater recruitment of those regions could be observed during a memory task compared to healthy young individuals, who showed the opposite pattern instead. Nevertheless, frontal areas are not the only ones showing a direct involvement with compensatory mechanisms and rather a much broader network has been reported, especially over regions were A has been accumulated. As a function of education, MCI patients, but not AD, showed greater florbetapir-PET and fluorodeoxiglucose (FDG)-PET uptake, meaning that the same regions of the brain were characterized by both greater Aβ depositions and increased glucose metabolism, respectively [88]. Such findings have been interpreted as evidence of a compensatory mechanisms counteracting disease pathology since the earliest stages (i.e., MCI) [88].
Overall, CR has therefore been observed to display opposite patterns in the healthy population versus the pathological group [51, 89]: within the former, greater reserve translates in a better and more efficient recruitment of cerebral regions resulting into a negative correlation being observed among measures of reserve and brain activity, as opposed to the positive association (greater reserve equals increased activity) indicative of a compensatory mechanism that can be appreciated within the clinical population. Few studies have reported the presence of those inverted associations in different brain regions that overall appear to broadly overlap with frontal, temporal, and parietal locations, over which structural changes have also been reported to occur in the previous section, including: the inferior frontal cortex [90], the superior temporal gyrus and superior parietal lobe [41], left frontal areas, anterior and posterior cingulate cortex, angular gyrus, and overall in regions belonging to the DMN [89, 91]. Indeed, dysfunctions within the DMN are present early in the stages of pathological aging [92], for which CR has been reported to exert a modulatory role over the deactivation of this network such as that in MCI/AD subjects, higher CR is associated with reduced deactivation, independently from volumetric indexes of brain atrophy, opposite to what was observed in the healthy individuals [89].
A positive correlation has also been observed between high education and the functional connectivity over the posterior cingulate cortex (PCC) within the DMN at the population level, but not in the healthy controls, reinforcing the assumption that the observed effect mirrors the presence of compensatory mechanisms visible even during resting state fMRI [93]. Further, PCC disconnection from the temporal lobe has been suggested as a crucial step in the conversion from MCI into AD [93]. Interestingly, an inverted correlation has also been observed between the PCC and precuneus metabolism with measures of reserve in AD candidates carrying the apolipoprotein (APOE) ε4 allele, suggesting that CR may act as protective mechanism even in the face of genetic predispositions [94].
Further functional evidence has also been collected with the use of PET scanning, which can be used not only to assess changes in the cerebral uptake of glucose but also of Aβ levels as a function of both brain pathology and CR, such as that better performances over cognitive measures have been noticed in individuals with higher degree of education in the face of greater 11- labeled Pittsburgh Compound B (PiB) uptake [14]. The modulatory role of CR over two specific regions in the brain of AD patients, the medial and inferior temporal gyri, has been reported [95], such that a negative interaction was observed between high levels of education, A markers in the CSF, and the extent of glucose uptake (assessed by means of FDG-PET), whereas a positive association was present over the same regions in individuals with less than 5 years of education, here used as a proxy of CR. Similarly, CR was found to be significantly correlated with the extent of tau deposition, but not of Aβ, over the inferior temporal lobe, meaning that better cognitive performances were observed in high CR individuals, over low CR ones, at similar levels of pathology in both the healthy controls as well as in the MCI and AD populations [96]. When looking at the detectable biomarkers of AD within the CSF, a significant decrease over Aβ and tau alterations could be observed as a function of education, resulting in a significant attenuation of the association between age and AD biomarkers, proving a protective mechanism [97].
Furthermore, CR was also found to be modulatory of the known association between increased amyloid deposition within the brain and the resulting poorer cognitive outcome, such as that, instead, increased brain pathology was associated with less dramatically reduced performances [98]. The existence of a meaningful correlation between proxies of CR and brain regions part of the memory system, which are known to be early affected in the AD course, has also been remarked [99], such as that a positive association was present between education and acetylcholinesterase (AChE), the hydrolyzing enzyme of the neurotransmitter acetylcholine, important in memory and learning functions [100], activity in the bilateral hippocampi and between occupation and AChE in the right posterior cingulate gyrus, respectively. The notion that different proxies of CR may interact with different regions of the brain does not come surprising, as it does reflect what observed regarding the structural changes over the fronto-parietal and temporal regions when considering education or socioeconomic status as indexes of CR [48]. However, differently from what was previously discussed, few studies [99] have further claimed that, based on the proved association between CR and changes occurring at the molecular level, it may seem reasonable to suggest that the preserved or stimulated cholinergic activity may be one of the main factors sustaining reserve.
Frontal connections have been proposed as the most likely underlying compensatory mechanisms on the behalf of CR [87]. Hence, global connectivity measures of the left frontal cortex to the rest of the brain have also been investigated, showing that for higher measures of reserve, a stronger connectivity was present [101]. Within the same study, greater hypometabolism over the precuneus in individuals with higher CR was also observed, congruently with the studies mentioned above, but the association between reduced metabolism and deterioration of memory performances was further modulated by the increased connectivity over frontal areas, which is assumed to be the underlying substrate of CR [101]. Connections from the basal forebrain and frontal regions may show an association with CR at the very first stages of the pathology [102], i.e., within the MCI population, such that greater metabolism was present over those regions in MCI compared to the healthy or AD groups. Since that an inverted U shape correlation could be observed between cognitive performances and brain metabolism over those same regions in individuals with high CR, the authors suggested that early initial increase in brain activity may prove the presence of compensatory mechanisms mediated by CR in the MCI population [102]. The notion that CR may exert its biggest effect early within the disease, possibly delaying clinical diagnosis, is well supported by the evidence that at one point, no compensatory mechanism may be sufficient to overcome the neuropathology, especially at the more advanced stages [103].
Large cohort studies of clinical and healthy populations have identified other variables that may still play a role when considering CR. One such factor is gender; glucose metabolic uptake over the temporal lobe has been observed to correlate with better verbal memory in females with MCI, but not in the healthy control or in the AD sample, compared to men with a similar degree of pathology, suggesting that the observed advantage in women may indeed be a reflection of different life impact factors that ultimately shape CR [104]. However, the same advantage was previously found to be inverted in a meta-analytic study [105], in which males with AD, rather than females, were suggested as having better performances at both verbal and visuo-spatial tasks. The authors interpreted this relationship as evidence not only of gender-related difference within the pathology itself, but also a reflection of different degrees of reserve. Because of the contrasting results, further studies may be needed to clarify the differences in reserve due to gender, which perhaps may reflect a continuous and ever-changing path modeled upon society’s structure.
Overall, the functional changes associated with CR have been nicely summarized in few exemplificative studies [13, 106] reporting all three of the main observations made so far:
A decrease in the metabolism over the posterior regions of the brain, mainly temporal and occipital regions of interests, has been observed in individuals with higher degree of reserve compared to individuals with lower CR. Such decrease is, however, compensated by an augmented activity over the frontal areas and more in general over regions where accumulation of Aβ plaques occurs.
A greater activity over frontal areas appears to be proportional to the magnitude of the reserve itself, such as that an increased functional connectivity is present between frontal regions and the rest of the brain, confirming the proposed role of being involved in recruiting additional compensatory network elsewhere in the brain that may aid the individual in maintaining an efficient level of functionality, even in the face of major pathology. Such reorganizational pattern appears to be present not only during task execution, but it’s rather readily visible during resting state as well (Fig. 3).
Similarly to what has been reported regarding structural modifications, an inverted pattern among the healthy and the pathological group can also be appreciated [51, 89]. In particular, healthy individuals who benefit of greater underlying resources have been noticed to possess more efficient recruitment and functional organization within their networks, such as that smaller activation is required for them to outperform compared to the low healthy CR individuals (Fig. 3).
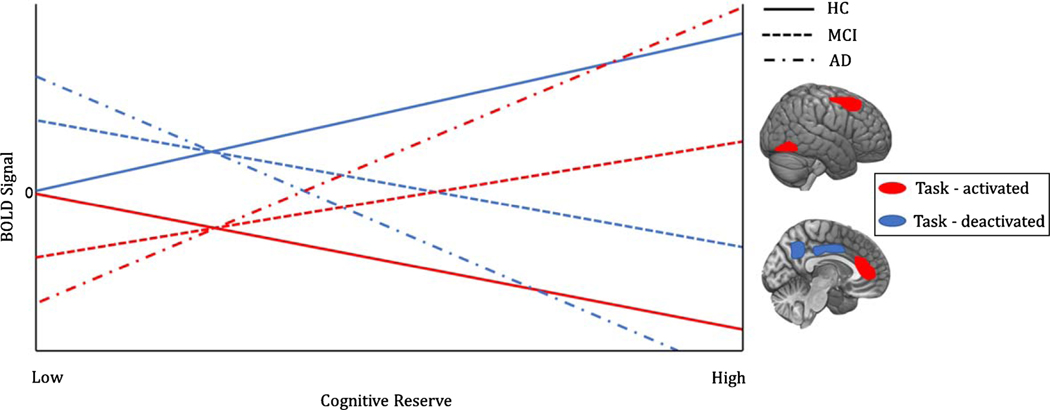
Fig. 3.
Task-associated functional activation/deactivation and CR in healthy and pathological cognitive aging. Task-induced activation and deactivation in regions showing greatest difference in the regression slope between healthy subjects and the pathological sample [89]. As a function of CR, healthy elderlies show a decrease in the recruitment of regions active during the task at hand (in this case, language related areas- middle/superior frontal gyrus and anterior cingulate cortex mainly), and reduced deactivation in some of the regions belonging to the DMN (precuneus, posterior cingulate cortex). Such finding suggests higher CR is associated with a more efficient allocation of metabolic resources and information processing. On the other hand, AD patients need greater activation to carry out the same task, thus proving better compensatory capacities as a function of CR. Greater reduction is observed in regions typically showing task-induced deactivations, i.e., DMN regions. Amnestic-MCI patients show less dramatic effects; a tendency mirroring that of the AD sample can still be appreciated.
ELECTROENCEPHALOGRAPHY EVIDENCE OF COGNITIVE RESERVE
Electroencephalography (EEG) is a widely assessed approach capable of providing direct evidence of the ongoing activity of the brain specifically for each frequency band [107]. Despite its prevalence, so far very few studies have been carried out investigating the possible association between this technique and the underlying occurring cortical changes as a function of CR. A study [108] investigated the modulatory role of education over the event related potentials (ERPs) changes, which have been observed to occur with aging and that are considered to reflect the progressive worsening in the retrieval process of episodic memories. In the young group, the authors observed that both high and low educated individuals showed no difference in the recruitment of frontal areas during a recollection tasks, suggesting that processes like familiarity and monitoring may not be sensible to proxies of CR. On the other hand, the involvement of parietal regions during recollection was observed to be limited to the left hemisphere in the low educated group and to be instead bilaterally distributed in the higher group, showing earlier, longer, and greater amplitude ERPs [108]. Within the elder population, the effect of education over ERPs changes was such that, in the high functioning group, the frontal involvement appeared to be equal to that of the younger counterpart; whereas a decrease in the effect amplitude over the parietal sites was observed, with a strong bilateral involvement for the older group compared to the younger one. Intact familiarity processes but impaired recollection, reflected by modulation of the P300 component [108], suggests the presence of a possible restriction in the attention allocation process [109] that might as a consequence negatively affect the encoding phase. Therefore, a progressive frontal shift as a function of age in the high functioning group was hypothesized to explain the preserved memory performances among the elders, whereas impairment in both familiarity and recognition processes was observed alike in the old low educated group, failing to show any effect over the frontal sites and with a significant slowing of parietal components, limited only to the right hemisphere and completely absent over the left side of the brain instead [108].
Overall, those findings can be considered as possible evidence of the protective role of certain proxies of CR, such as education, against the documented cognitive slowing naturally occurring with aging, ensuring better performances [109, 110]. Most importantly, the reported findings appear to be in line with two primary neuroscientific models of aging: the Hemispheric Asymmetry Reduction in Older Adults (HAROLD) and the Posterior Anterior Shift in Aging (PASA) models, which specifically aim to deal with the observed neural shifts in the elderly, upon which CR can supposedly exert its modulatory influence. In particular, according to the HAROLD model, high performing elders tend to show greater bilateral hemispheric engagement during most cognitive tasks compared to younger individuals or lower functioning elders [111–115]. According to the PASA model, compensatory mechanisms may be implemented by the elderlies in terms of a progressive greater recruitment of anterior areas to overcome deficits over more posterior regions, resulting in a progressive shift in functions [114, 116]. Congruently with the PASA model, the presence of an additional frontal involvement has been observed in a group of high CR elders during a source memory task not present in their younger counterpart [117], suggesting that the further frontal involvement could indeed underlie the adaptive recruitment of additional areas to help overcoming normally age-related deficits.
It should be noted that assumptions that CR exacerbates the PASA and HAROLD model may be overly simplistic and the above discussion is more of an attempt to fit CR theory within the more classical models of aging. A recent study [118] has proposed the existence of a “task-invariant CR network” comprehensive of a variety of regions ranging from basic motor and cognitive functions up to higher-order control processes, which overall show patterns of increased or decreased activity as a function of CR across a broad range of tasks. Future research should attempt to address whether fMRI-derived evidences of the existence of a unified CR network might help in addressing EEG rearrangements as well, which may underlie easily-addressable profiles of greater reserve.
During the past few years, the NEIL Research Unit Study [119] has also been initiated, recruiting over 1,000 healthy elders over 50 years of age which will undergo follow-up sessions every two years to monitor any change occurring within basic physical, neuropsychological, psychological, and resting-state EEG measures in respect to the monitored lifestyles factors as proxies of CR. When data from the population under investigation will become available to be analyzed, useful introspective considerations will possibly be suggested on the association between EEG and CR.
With regard to AD pathology, changes in the underlying neural activity have been widely documented as a function of pathology progression [120–125], which translate into a significant slowing of the EEG activity due to the progressive decrease in the expression of the high frequencies power bands (mainly alpha and beta) in favor of the lower ones (delta and theta) [120, 126–131]. The documented rearrangement of the power spectra in this category of patients becomes interesting when considering few steps: first of all, the progressive decrease in cognitive functioning has been documented through a variety of different structural measures, among which those assessing the deterioration of myelinated fibers, such as diffusion tensor MRI. A decrease in the white matter FA over frontal regions and the corpus callosum has indeed been observed [132]. The authors argue this reduction possibly mirrors fronto-temporal disconnections in the AD population, especially since it was observed to be both lower than healthy controls and significantly correlated with Mini-Mental State Examination (MMSE) scores [133, 134]. From a functional perspective, the same frontal regions were observed to show a significant decrease in the expressed beta power in favor of lower rhythmic activity [132], where the beta power has long been known to reflect cognitive and attentional processing [135]. It is therefore not surprising that a significant correlation was also documented between lower beta power and worse cognitive functioning of the individual, as assessed through MMSE total score [132]. At the same time, the low frequency spectra (delta and theta bands) was also found to correlate with the severity of the damage in the white matter fibers over the frontal regions [132]. Overall, those findings were interpreted by the authors as reflecting the presence of a fronto-temporal disconnection due to the progressive loss of basal cholinergic neurons projections to the frontal areas that as a consequence would “pathologically disclose the cortico-fugal EEG rhythms triggering thalamus-cortical delta rhythm” [132].
Despite not yet being investigated, CR in the high functioning elders can be hypothesized to have a role over three different components at equally different sets of time:
During the early stages of aging, CR might play a neuroprotective role in delaying the progressive loss of power over the posterior regions and in ensuring preservation of hemispheric asymmetries, leading to the maintenance of activity patterns that are closer to the ones observed in healthy younger individuals.
When CR is no longer able to delay the neural shift toward a possible pathological manifestation, it may still act by ensuring a more efficient recruitment of frontal areas to maintain cognitive functions in the face of cortical degeneration. The hypothesized switch in the role of CR from protective to compensatory in delaying overt behavioral manifestations would be in line with the already reported association between CR and the structural and functional changes observed over the frontal areas and here represented by the progressive rearrangement of the power spectra.
At the time of diagnosis, occurring later in individuals with greater reserve, the pathology will have reached such a degree of severity to result in a marked loss of cholinergic neurons underlying fronto-temporal connections and the associated behavioral symptoms. Given that high-reserve individuals will reach the time of the diagnosis at a more severe stage of pathology, thanks to its initial protective power, we can hypothesize the existence, at this stage, of a positive correlation among measures of reserve and the loss of high frequency components, in favor of the slower ones.
NIBS AND ITS POTENTIAL CONTRIBUTION TO CR THEORY
Given the neurodegenerative nature of AD, one of the most prominent underlying pathological changes affecting the brain consists in the progressive loss of neurons and the consequent decrease in the amount of grey matter and associated degree of neural plasticity, which starts in the entorhinal cortex and hippocampal areas and progressively involves the entire brain [136]. A better understanding of the underlying changes in neuroplasticity have been investigated through the implementation of transcranial magnetic stimulation (TMS) [137], leading to the development of ad-hoc interventional approaches targeting spared plasticity mechanisms and consequently potentiate cognition [138, 139]. As stated in Faraday’s Law of electromagnetic induction, TMS can be operated to modulate cortical excitability by means of a magnetic pulse delivered by the coil to an underlying population of neurons, directly causing a discharge of action potentials [140, 141].
One of the most well shared notions regarding the AD pathology and TMS is that of the documented decrease in the resting motor threshold (RMT) compared to healthy controls, whereas MCI individuals collocate themselves halfway between those two groups [142]. At least early in the disease, these findings have been mainly considered to reflect a functional change within the underlying networks to compensate for the progressive loss of cortical neurons [34] and to slowly move toward representing structural changes due to the gradual thinning of the whole cerebral cortex, such as that increase in the RMT may no longer represent cortical excitability per se, but rather simply reflect the augmented scalp-to-cortex distance [143]. To demonstrate that any change in the RMT is not merely due to the loss of cortical neurons alone but it may rather reflect the presence of an underlying hyperexcitability of the brain, measures of cortical thickness and scalp-to-brain distance have been correlated with those of cortical excitability as reflected by the electrical field (EF) intensity induced in the cortex by the TMS pulse [143]. This approach has enabled the researchers to obtain a measure of excitability that was independent from the degree of brain atrophy (i.e., the distance between the brain and the skull), leading to the observation that the EF over the motor cortex correlated negatively with the cortical thickness in relevant regions of the brain (sensorimotor cortex, cuneus, and precuneus). This translates, accordingly to the authors, into the notion that the thinner is the cortex, the greater the intensity of the stimulation needed to induce a MEP [143]. Because of that, one could expect that AD patients would have required a greater stimulation for MEPs to be elicited. However, this was not observed and instead lower intensities were needed. Again, this finding is congruent with the notion that AD brains are characterized by a compensatory hyperactivation that has been hypothesized to reflect alterations in the cholinergic activity as well as a dysfunction in the glutamatergic receptors. On the other hand, changes in excitability may also be related to GABA-ergic dysfunctions [144], with AD patients showing increased contralateral silent period (CSP) and transcallosal inhibition (TI) as well, both of which have been linked to GABA inhibitory circuits. Furthermore, both the RMT and the CSP were found to increase as a function of disease severity, congruently with what was already reported in a previous study [145], which presumably reflects the widespread cortical atrophy that characterizes the most advanced stages of the disease. Based on those notions, it might be possible to plot the change in the RMT as a U-shaped line as a function of AD severity such as that, assuming a starting point as healthy, the RMT will progressively decrease with the AD diagnosis and will keep decreasing as the disease progresses until when it becomes so severe (due to the spreading of the atrophy) that the RMT will start to increase again because the brain will eventually no longer be hyperexcitable, up to the point when higher degrees of stimulation will be needed to induce a MEP to counterbalance the cortical thinning [143, 145] (Fig. 4). Because of the slow decreasing of the curve, it may be that no change will be observed in the RMT between healthy subjects and MCI or very mild AD patients, whereas those differences will become prominent at the more advanced stages. Furthermore, it seems plausible to assume that interindividual differences could play a role in determining the shift of the curve on the x-axis, which could become an interesting facet of the role of reserve, such as that the higher the CR, the greater the shift over the right, thus the more delayed the changes in the RMT and therefore the changes in plasticity that one could aspect to observe (Fig. 4).
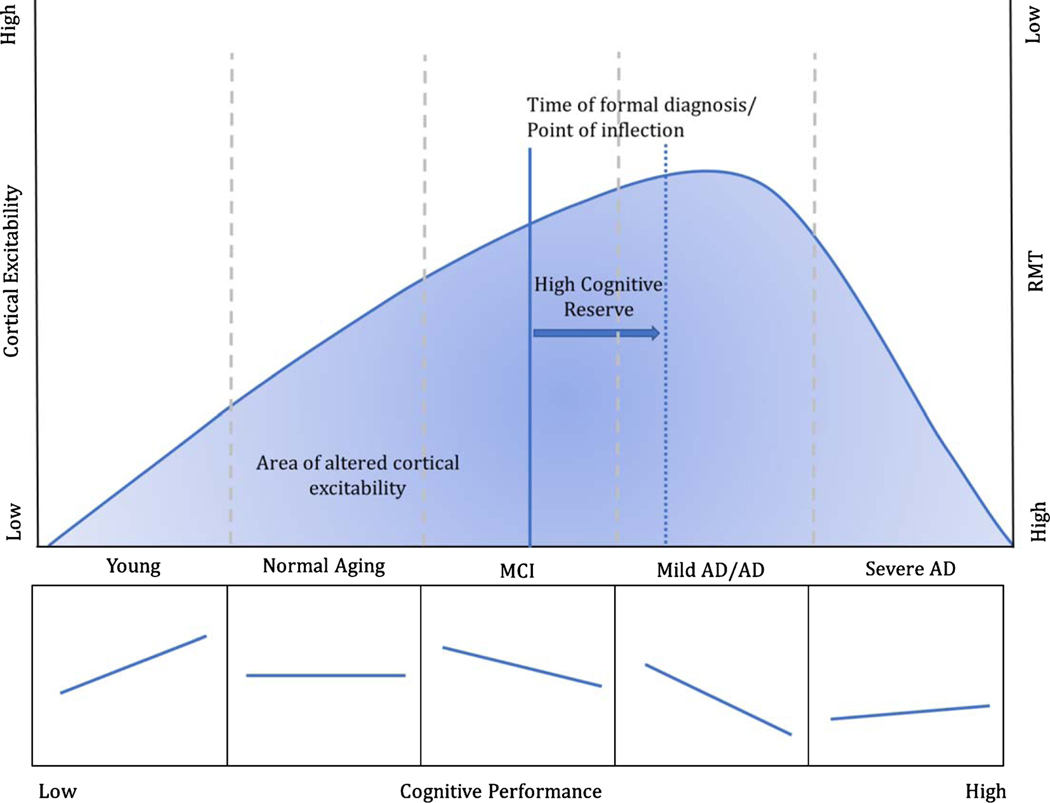
Fig. 4.
Proposed model of cortical excitability as a function of disease progression and cognitive performance. Lower RMT (i.e., higher cortical excitability) has been observed in pathological cognitive aging compared to healthy cohorts, reflecting a possible compensatory mechanism for the progressive loss of cortical neurons. We speculate that the decrease in the RMT would be observed until the most advanced stages of the disease when a critical point in cortical thinning is reached. In line with this notion, higher cortical excitability is associated with better cognitive performance in young subjects and to progressively shift toward a negative association at the earliest stages of AD. The progressive change in slope between cortical excitability and cognitive performance is thought to reflect the point of inflection where hyperexcitability no longer acts as a compensatory mechanism, but rather starts to become detrimental for the subject, possibly reflecting a greater impediment in cognitive resource allocation. CR is hereby proposed to ensure preserved functioning for longer during time, delaying the time when the inflection point is reached by the individual.
However, when the obtained measures of cortical excitability were correlated with the mean scores obtained with the MMSE [144], what was observed was that lower RMTs corresponded to lower MMSE scores, whereas a negative correlation was present between MMSE scores and CSP duration (reported as greater for the left hemisphere compared to the right). A significant correlation was also observed between the MMSE and TI for the right hemisphere solely. In this particular study, no meaningful correlation was observed between MEP amplitude and MMSE. Accordingly to the authors, the most plausible explanation may be that of a relative preserved integrity of the cortico-spinal tracts in the face of a reduced threshold in the rest of the brain, such as that when the delivered stimulation is increased up to the 130% of the RMT to elicit MEPs, “it recruits the same extra proportion of output as in healthy subjects” [144]. From this early explanation, it comes that the RMT may be a more sensible measure of change in the cortical excitability of the brain that might, as a consequence, translate into changes in cognitive functioning (e.g., as expressed by the MMSE). The decrease in the intracellular glutamate levels appears to play a role in altering the homeostasis and in inducing a decrease in membrane resting potential that consequently may facilitate depolarization [144]. For what concerns the cholinergic dysfunctions, the progressive degeneration of the nucleus basalis of Meynert— from which most of the cholinergic projections originate [146]—has been found to start early in the disease and to mirror the changes in cognitive functions, especially those relying on the attentional processes [147]. Given the role that acetylcholine is known to play in regard of both motor and higher order cognitive functions (e.g., memory and attention among others), it may be interesting to better evaluate the possible existent correlation between activity in the motor cortex and its association with cognition, assuming the presence of an underlying shared mechanism. Indeed, the existence of an association between the RMT and cognitive functions has been observed even in healthy subjects performing a 2-n back task [148], but not in the control condition (0back) or in a more difficult setting (3-n back). The authors reported the presence of a negative correlation between RMT and working memory such as that lower thresholds were found to be indicative of a better memory load which has been further interpreted as a proof of the RMT to be not only a measure of corticospinal excitability, but to rather represent broader cortical activity [148]. Given that those findings were reported independently of whether the TMS pulse was delivered before or after the cognitive task, it was possible to assume that, within the present study, the RMT was indeed representative of underlying cortical activity rather than mirroring modulation of the cognitive task over the measured RMT or the effect of TMS over M1 [149].
The presented body of evidence seems to suggest the existence of a correlation between cortical excitability (i.e., RMT) and cognitive performance with two inverted patterns among healthy subjects and patients, such as that for the former, lower RMT correlates with better performance whereas the opposite seems to be true in the pathological population (higher RMT equals a better performance) (Fig. 4).
Therefore, it may be tempting to conclude that hyperexcitability plays a favorable role in a healthy brain because it may lead to a more prone responsiveness of the subject to a specific task, whereas the same hyperexcitability is detrimental in AD patients, even if it’s assumed to reflect a compensatory mechanism. One possible explanation may come in terms of the fact that given a limited amount of resources that the brain has, the hyperexcitability of a region may result in a sudden decrease in the activity in the rest of the brain to counterbalance, including those regions that presumably contribute to the ongoing task. Given the widespread of the pathology in AD, the overall cognitive resources of the AD populations will be significantly reduced compared to the healthy group, thus making them more sensible to sudden shifts in their allocations to compensate for a given task. Despite acting as a compensatory mechanism, individuals with higher RMT may be the ones who either find themselves on the left side of the curve (i.e., not severe enough to show a significant change in the RMT compared to healthy) or on the extreme right side (so severe to show greater RMT because of the widespread cortical thinning). Only the former, however, will benefit of higher RMT as it is perhaps indicative of lower severity, thus making them performing almost as good as the healthy, but significantly different from the moderate/severe AD.
An alternative explanation to the hyperexcitability hypothesis is that of a progressive reduced capacity of the inhibitory circuits to exert their effects over the cortex, thus resulting into greater disinhibition [149]. However, measures of cholinergic inhibition (as assessed by short latency afferent inhibition) and measures of GABAergic inhibition (as assessed by short latency intracortical inhibition) were not found to be significantly correlated with the RMT, even though the former was found to be significantly decreased in AD patients [150]. This was interpreted by the authors as evidence of the fact that the documented hyperexcitability in these patients is less likely to be related to a deficiency with the inhibitory system (either cholinergic or GABAergic), but rather to reflect a dysfunction in the cortical excitatory system related to an increased glutamatergic response [150]. Despite so, no significant modifications have been observed in terms of intracortical facilitation in AD patients compared to healthy controls [142]. Because intracortical facilitation is considered to rely upon NMDA receptor mediated activity, the glutamatergic dysfunction cannot be explained in terms of NMDAr dysfunction, as it appears to be preserved in AD patients, but rather to an imbalance between NMDA and non-NMDA receptors, with the altered functioning of the latter [151]. Indeed, administration of ketamine (a drug that is known to enhance non-MNDA receptor activity by at the same time blocking the activity of NMDA receptors) in AD patients has been proven to increase cortical excitability to TMS by “increasing high-frequency glutamatergic neurotransmission” mediated by non-NMDA receptors [151].
In conclusion, future approaches could also embrace relatively novel techniques, as those relative to the field of NIBS, to assess changes in cortical excitability and plasticity across the lifespan, that might modulate the interaction with CR estimates, affecting both the individual starting point as well as their critical change in slope [152], but for which few relevant works appear to have been published so far. Moreover, such techniques could be used to induce targeted modulation of cortical excitability, which if performed in a systematic and repeated way might slow down pathological decrease. Various NIBS approaches have already proven their feasibility in inducing overt enhancement of cognitive functions. Protocols capable of directly affecting mechanisms of cortical plasticity, promoting synaptic receptivity (i.e., through mechanisms of long term potentiation) as observed in theta-burst TMS, or in aiding neural synchronization of widespread neural networks, as within transcranial alternating current stimulation approaches [32], come of great interest as possible interventions to augment individuals’ resilience to degenerative pathologies.
AN INTEGRATED MODEL OF COGNITIVE RESERVE
Within the wide evidence collected in literature, the role of CR appears crucial in explaining inter-individual differences among subjects that might otherwise display similar neuropathological conditions. Congruent with the initial description [8], CR may exert both a protective role, thus delaying clinical manifestations and ensuring the maintenance of adequate cognitive functioning, as well as a compensatory role, through the recruitment of adjacent neural networks, when facing a major pathology.
From a neuroimaging perspective, the mechanisms through which CR has been observed to operate can be hypothesized to vary depending on the different stages at which the individual is found to belong to. Indeed, if we consider a time-frame that starts from the progressive thinning of the cerebral cortex observed during normal healthy aging, toward the progressive pathological side of it; the weight of CR will be different and gradually less impactful, until when a certain degree of clinical severity is reached for which CR can no longer play a meaningful role. Therefore, at the very beginning, CR will act as a protective agent, such as that individuals who have previously engaged in an active and cognitively stimulating life will benefit, as a consequence, of enriched neural networks both in terms of volumetric measures as well as greater and stronger white matter interconnections across regions.
Consequently, the strengthened cortical networks will be more resistant to the pathology, similarly to what was hypothesized in the past by passive models of reserve—like the Brain Reserve theory [6]—with the only difference that neural substrates will not reflect innate properties of the brain, but rather be the result of previous active engagement. Both global and region-specific effects can be hypothesized [51], with the latter being centered in protecting those regions of the brain that are most commonly affected by the pathology. The role of CR in protecting and delaying the clinical diagnosis of a neurodegenerative condition, such as that of AD, may account for the reported evidence of postmortem brain autopsies showing clear presence of widespread amyloid pathology in individuals that never displayed behavioral symptoms in life [80]. On the other hand, as the underlying pathology progresses, a gradual shift can be hypothesized from structural changes to functional ones and thus from a protective role of CR toward more of a compensatory function [70].
Again, different patterns can be appreciated between healthy individuals and AD patients such as that, for the former, higher measures of CR have been found to be associated with finer patterns and decrease network activation during task execution whereas, in the clinical population, the opposite patterns holds true, with high reserve being associated with greater network activation, proving its compensatory role through the recruitment of adjacent cortical regions [51, 89]. This enlarged network activation mirrors the posterior to anterior shift in cognitive functioning observed during aging—perhaps exacerbated by the role of CR—congruently with the already proposed models in literature explaining networks’ rearrangement, gradually losing specificity (the HAROLD model) and progressively moving toward frontal regions involvement (the PASA model). Such cortical reorganization in favor of more anterior regions is supported by the empirical data showing progressive reduced metabolism posteriorly and increased activity in frontal areas as a function of reserve [13, 106], as well by the gradual shift in the power spectra activity that favors low frequency frontal activity (i.e., theta) in face of posterior faster cortical synchronization (i.e., mainly alpha) [127].
One hypothesis that could aid in explaining the association between CR and frontal regions might rely in the proposed role of frontal cholinergic projections as underpinning reserve [99], consistently with the known role of acetylcholine in sustaining memory [153], one of the first functions undergoing degeneration within the AD pathology and which often brings the individual under clinical attention. Furthermore, fronto-temporal disconnections due to the progressive loss of basal cholinergic neurons projections to the frontal areas could results in a “pathologically disclosure of the cortico-fugal EEG rhythms triggering thalamus-cortical delta rhythm” [132], thus providing a neural explanation for the reported changes in the individual’s power spectra. In addition, recent evidence has also been collected proving the existence of a negative correlation between frontal theta power and activity in the regions belonging to the DMN in healthy subjects [154], which increased activity has in turn been reported to be positively associated with high CR measures [89]. More recently, evidence of the increased amount of left frontal connectivity in individuals with higher levels of education, a proxy of CR, has been interpreted as further evidence sustaining frontal projection as the more likely neural substrate of cognitive reserve [101].
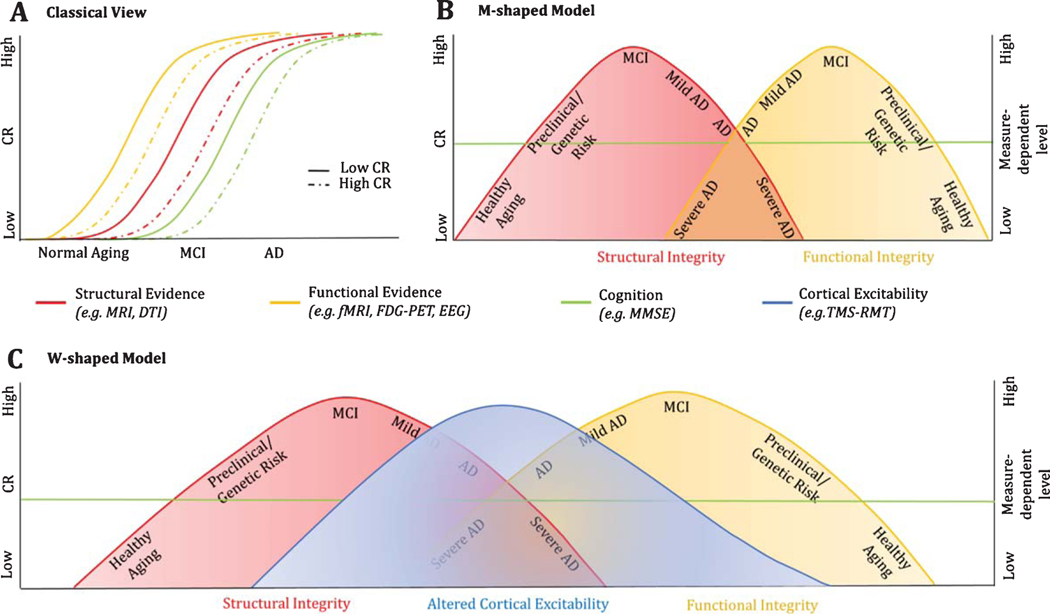
By taking in consideration the overall collected evidence, we hereby propose a M-shaped model summarizing the structural and functional modifications from normal aging to the pathological side of it as a function of cognitive reserve (Fig. 5B). Differently from the traditional view on CR, which simply looks at the translational shift in time, reflecting the delay in symptomatology (Fig. 5A), this model considers the step-by-step role of CR in guaranteeing the maintenance of a functional cognitive profile along both structural and functional pattern’s modification. Furthermore, the potential modification of cortical excitability as assessed through TMS protocols is proposed as the best easily-assessable quantitative estimate of the individual collocation along the aging process, from the healthy to the pathological side of it (Fig. 5C).
Fig. 5.
Contrasting models of the impact of CR on the manifestation and progression of pathological brain states. The traditional view on the role of CR (A) hypothesizes that CR simply acts in protecting the individual, delaying the time of symptomatology onset and of clinical diagnosis. On the other hand, a M-shaped model (B) reflexes a much more complex reality in which not only healthy subjects and pathological individuals show gradual opposite changes in slope as a function of CR, but also structural and functional modifications show mirror-like behavior. In the proposed model, the term structural integrity refers to the collected evidence of global and regional changes in the cerebral volume, such as that, in healthy aging, higher reserve correlates with more robust neural substrates, i.e., greater grey matter volumes and higher measures of FA in the connecting fibers. On the other hand, a progressive shift in slope is observed in the pathological sample (from MCI to AD), where higher CR becomes associated with thinner cortices, providing its compensatory role in sustaining greater degree of severity for longer in time. Similarly, functional integrity refers to the reported changes in the functional recruitment of regions that ensure the individual to maintain an appropriate level of cognitive functioning. In respect to CR, healthy elderlies show less functional activation over task-relevant regions and reduced deactivation of the DMN (not shown in the model; see Fig. 3), proving more finely tuned patterns of activity and effortless processing. On the other hand, in the AD pathology, CR acts in ensuring greater functional activation and recruitment of adjacent regions (mainly frontal areas), to maintain an efficient cognitive profile despite disease progression. The reported slope change in functional activity represent therefore task-driven, more than resting state, accommodations as a function of CR, with similar patterns reported in both fMRI and FDG-PET studies [156]. Notably, preclinical stages of disease or genetic conditions present intermediate brain statuses as a function of CR between healthy and pathological aging. C) represents a W-shaped model, where the altered cortical excitability is seen as the most easily and non-invasive assessable measure summarizing both structural and functional evidences, further proving the potential implementation of NIBS in clinical practice in the evaluation of the individual collocation along the healthy-pathological axis.
One note worth mentioning regards the inconsistency in the approach of measuring CR across different studies, for which years of education, working activity, or social engagement have most often been utilized as estimates of reserve, based on arbitrary cut-off points. Due to this variability in the proxies used to estimate CR, it becomes particularly challenging to compare across different studies, for which the need of using a unified measure of reserve becomes mandatory. In this regard, few questionnaires have come into use, such as the Lifetime of Experiences Questionnaire [155] and the Cognitive Reserve Index questionnaire [85], both of which provide a quantitative continuous estimate of reserve that takes into account a complex set of activities considered cognitively stimulating, into which the individual might have engaged in his or her life, both in terms of duration and frequency. The importance of framing CR as a composite score reflects its original theoretical framework [7] and for this reason should strongly be preferred over the use of a single proxy measure.
CONCLUSIONS
Individuals with AD show wide inter-individual differences at the time of clinical diagnosis, only recently linked to reserve-based model of resilience, especially that of the CR theory. Within the present review, we have therefore gathered the current state of the art evidence for the role of CR in both healthy aging and AD, starting from the reserve’s role in counterbalancing cortical neural loss toward ensuring greater compensatory mechanisms. In conclusion, we have proposed a model looking at the stage-based role of CR in relation to both structural and functional modifications and we propose NIBS techniques, especially TMS and its ability to monitor alterations in cortical excitability, as a possible easily assessable biomarker of the individual collocation along the aging process, from healthy to possibly pathological.
ACKNOWLEDGMENTS
ES and APL were supported by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014-13121700007. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the ODNI, IARPA, or the U.S. Government. ES and APL are supported by the BROAD Institute at Harvard-MIT (Boston, MA, USA) via 2016P000351. ES and APL are supported by Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. ES is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) grant 2017. APL is further supported by the Berenson-Allen Foundation, the Sidney R. Baer Jr. Foundation, grants from the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, the Sidney R. Baer Jr. Foundation. The contributions of PJF were supported in part by the NIH (R21 AG051846).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0549r1).
REFERENCES
- [1].Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 13, 325–373. [Google Scholar]
- [2].Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB (2006) Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly.AnnNeurol 60, 688–695. [DOI] [PubMed] [Google Scholar]
- [3].Snowdon DA (2003) Healthy aging and dementia: Findings from the Nun Study. Ann Intern Med 139, 450–454. [DOI] [PubMed] [Google Scholar]
- [4].Satz P (1993) Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology 7, 273–295. [Google Scholar]
- [5].Katzman R (1993) Education and the prevalence of dementia and Alzheimer’s disease. Neurology 43, 13–20. [DOI] [PubMed] [Google Scholar]
- [6].Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A (1988) Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23, 138–144. [DOI] [PubMed] [Google Scholar]
- [7].Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. JIntNeuropsychol Soc 8, 448–460. [PubMed] [Google Scholar]
- [8].Stern Y (2009) Cognitive reserve. Neuropsychologia 47, 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Steffener J, Reuben A, Rakitin BC, Stern Y (2011) Supporting performance in the face of age-related neural changes: Testing mechanistic roles of cognitive reserve. Brain Imaging Behav 5, 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R (2005)Brain networks associated with cognitive reservein healthy young and old adults. Cereb Cortex 15, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deary I (2008) Why do intelligent people live longer? Nature 456, 175–176. [DOI] [PubMed] [Google Scholar]
- [12].Harrison SL, Sajjad A, Bramer WM, Ikram MA, Tiemeier H, Stephan BCM (2015) Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J Clin Exp Neuropsychol 37, 253–264. [DOI] [PubMed] [Google Scholar]
- [13].Kemppainen NM, Aalto S,Karrasch M, Någren K,Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO (2008) Cognitive reserve hypothesis: Pittsburgh Compound Band fluorodeoxy glucose positron emission tomography inrelation to education in mild Alzheimer’s disease. Ann Neurol 63, 112–118. [DOI] [PubMed] [Google Scholar]
- [14].Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC (2008) Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11labeled Pittsburgh Compound B uptake. Arch Neurol 65, 1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 10041010. [PubMed] [Google Scholar]
- [16].Stern Y (2006) Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 20, S69–S74. [DOI] [PubMed] [Google Scholar]
- [17].Hindle JV, Martyr A, Clare L (2014) Cognitive reserve in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 20, 1–7. [DOI] [PubMed] [Google Scholar]
- [18].Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M (2006) The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. J Int Neuropsychol Soc 12, 147–152. [DOI] [PubMed] [Google Scholar]
- [19].Bruandet A, Richard F, Bombois S, Maurage CA, Masse I, Amouyel P, Pasquier F (2008) Cognitive decline and survival in Alzheimer’s disease according to education level. Dement Geriatr Cogn Disord 25, 74–80. [DOI] [PubMed] [Google Scholar]
- [20].Helzner EP, Scarmeas N, Cosentino S, Portet F, Stern Y (2007) Leisure activity and cognitive decline in incident Alzheimer disease. Arch Neurol 64, 1749–1754. [DOI] [PubMed] [Google Scholar]
- [21].Stern Y, Tang MX, Denaro J, Mayeux R (1995) Increased risk of mortality in alzheimer’s disease patients with more advanced educational and occupational attainment. Ann Neurol 37, 590–595. [DOI] [PubMed] [Google Scholar]
- [22].Teri L, McCurry SM, Edland SD, Kukull WA, Larson EB (1995) Cognitive decline in Alzheimer’s disease: A longitudinal investigation of risk factors for accelerated decline. J Gerontol Ser A 50A, M49–M55. [DOI] [PubMed] [Google Scholar]
- [23].Wilson RS, Bennett DA, Gilley DW, Beckett LA, Barnes LL, Evans DA (2000) Premorbid reading activity and patterns of cognitive decline in Alzheimer disease. Arch Neurol 57, 1718–1723. [DOI] [PubMed] [Google Scholar]
- [24].Mondini S, Madella I, Zangrossi A, Bigolin A, Tomasi C, Michieletto M, Villani D, Di Giovanni G, Mapelli D (2016) Cognitive reserve in dementia: Implications for cognitive training. Front Aging Neurosci 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Olazaran J, Muniz R, Reisberg B, Pena-Casanova J, del Ser T, Cruz-Jentoft AJ, Serrano P, Navarro E, Garcia de la Rocha ML, Frank A, Galiano M, Fernandez-Bullido Y, Serra JA, Gonzalez-Salvador MT, Sevilla C (2004) Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology 63, 2348–2353. [DOI] [PubMed] [Google Scholar]
- [26].Lara E, Koyanagi A, Caballero F, Domènech-Abella J,` Miret M, Olaya B, Rico-Uribe L, Ayuso-Mateos J, Haro J (2017) Cognitive reserve is associated with quality of life: A population-based study. Exp Gerontol 87, 67–73. [DOI] [PubMed] [Google Scholar]
- [27].Pascual-Leone A (1999) Transcranial magnetic stimulation: Studying the brain–behaviour relationship by induction of ‘virtual lesions.’ Philos Trans R Soc B Biol Sci 354, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120, 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rossini PM, Rossi S (2007) Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology 68, 484–488. [DOI] [PubMed] [Google Scholar]
- [30].Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2, 145–156. [DOI] [PubMed] [Google Scholar]
- [31].Luber B, Lisanby SH (2014) Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 85, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Santarnecchi E, Brem AK, Levenbaum E, Thompson T, Kadosh Cohen R, Pascual-Leone A (2015) Enhancing cognition using transcranial electrical stimulation. Curr Opin Behav Sci 4, 171–178. [Google Scholar]
- [33].Alagona G,Bella R,Ferri R,Carnemolla A,Pappalardo A, Costanzo E, Pennisi G (2001) Transcranial magnetic stimulationin Alzheimer disease: Motor cortex excitabilityand cognitive severity. Neurosci Lett 314, 57–60. [DOI] [PubMed] [Google Scholar]
- [34].Ferreri F, Pauri F, Pasqualetti P, Fini R, Forno GD, Rossini PM (2003) Motor cortex excitability in Alzheimer’s disease: A transcranial magnetic stimulation study. Ann Neurol 53, 102–108. [DOI] [PubMed] [Google Scholar]
- [35].Oliviero A, Profice P,Tonali P,Pilato F, Saturno E, Dileone M, Lazzaro V (2006) Effects of aging on motor cortex excitability. Neurosci Res 55, 74–77. [DOI] [PubMed] [Google Scholar]
- [36].Reuter-Lorenz PA, Cappell KA (2008) Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci 17, 177–182. [Google Scholar]
- [37].Rossini P, Rossi S, Babiloni C, Polich J (2007) Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Prog Neurobiol 83, 375–400. [DOI] [PubMed] [Google Scholar]
- [38].Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001) Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22, 581–594. [DOI] [PubMed] [Google Scholar]
- [39].Mortamais M, Portet F, Brickman AM, Provenzano FA, Muraskin J, Akbaraly TN, Berr C, Touchon J, Bonafe A,´ le Bars E, Menjot de Champfleur N, Maller JJ, Meslin C, Sabatier R, Ritchie K, Artero S (2014) Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry 22, 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Querbes O, Aubry F, Pariente J, Lotterie J-A, Demonet J-´F, Duret V, Puel M, Berry I, Fort J-C, Celsis P (2009) Early diagnosis of Alzheimer’s disease using cortical thickness: Impact of cognitive reserve. Brain 132, 2036–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sole-Padulĺ es C, Bartrès-Faz D, Junqu e C, Vendrell P,´ Rami L, Clemente IC, Bosch B, Villar A, Bargallo N,´ Jurado MA, Barrios M, Molinuevo JL (2009) Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 30, 1114–1124. [DOI] [PubMed] [Google Scholar]
- [42].Colom R, Jung R,Haier R(2006) Distributed brain sites for the g-factor of intelligence. NeuroImage 31, 1359–1365. [DOI] [PubMed] [Google Scholar]
- [43].Haier R, Jung R, Yeo R, Head K, Alkire M (2004) Structural brain variation and general intelligence. Neuroimage 23, 425–433. [DOI] [PubMed] [Google Scholar]
- [44].McDaniel M (2005) Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence 33, 337–346. [Google Scholar]
- [45].Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, Shaw LM, Decarli CS, Carmichael O, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR (2011) Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain 134, 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Johansen-Berg H, Baptista C, Thomas A (2012) Human structural plasticity at record speed. Neuron 73, 10581060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rapp SR, Espeland MA, Manson JE, Resnick SM, Bryan NR, Smoller S, Coker LH, Phillips LS, Stefanick ML, Sarto GE, Women’s Health Initiative Memory Study (2013) Educational attainment, MRI changes, and cognitive function in older postmenopausal women from the Women’s Health Initiative Memory Study. Int J Psychiatry Med 46, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vaque-Alcázar L, Sala-Llonch R, Valls-Pedret C, Vidal-´ Pineirõ D, Fernandez-Cabello S, Bargallo N, Ros E, Bartres-Faz D (2017) Differential age-related graý and white matter impact mediates educational influence on elders’ cognition. Brain Imaging Behav 11, 318–332. [DOI] [PubMed] [Google Scholar]
- [49].Whalley LJ, Staff RT, Fox HC, Murray AD (2016) Cerebral correlates of cognitive reserve. Psychiatry Res Neuroimaging 247, 65–70. [DOI] [PubMed] [Google Scholar]
- [50].Arenaza-Urquijo EM, Lanuzza B, La Joie R, Mevel K, Mezenge F, Perrotin A, Desgranges B, Bartrès-Faz D,´ Eustache F, Chetelat G (2013) Relationships betweeń years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 83, 450–457. [DOI] [PubMed] [Google Scholar]
- [51].Bartrés-Faz D, Arenaza-Urquijo EM (2011) Structuraĺ and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr 24, 340–357. [DOI] [PubMed] [Google Scholar]
- [52].Kim JP, Seo SW, Shin HY, Ye BS, Yang J-J, Kim C, Kang M, Jeon S, Kim HJ, Cho H, Kim J-H, Lee J-M, Kim ST, Na DL, Guallar E (2015) Effects of education on aging-related cortical thinning among cognitively normal individuals. Neurology 85, 806–812. [DOI] [PubMed] [Google Scholar]
- [53].Liu Y, Julkunen V, Paajanen T, Westman E, Wahlund L-O, Aitken A, Sobow T, Mecocci P, Tsolaki M, Vellas B, Muehlboeck S, Spenger C, Lovestone S, Simmons A, Soininen H, Consortium A (2012) Education increases reserve against Alzheimer’s disease—evidence From structural MRI analysis. Neuroradiology 54, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Serra L, Cercignani M, Petrosini L, Basile B, Perri R, Fadda L, Spano B, Marra C, Giubilei F, Carlesimo GA, Caltagirone C, Bozzali M (2011) Neuroanatomical correlates of cognitive reserve in Alzheimer disease. Rejuvenation Res 14, 143–151. [DOI] [PubMed] [Google Scholar]
- [55].Olsen RK, Pangelinan MM, Bogulski C, Chakravarty MM, Luk G, Grady CL, Bialystok E (2015) The effect of lifelong bilingualism on regional grey and white matter volume. Brain Res 1612, 128–139. [DOI] [PubMed] [Google Scholar]
- [56].Rzezak P, Squarzoni P, Duran FL, Alves T de TF, Tamashiro-Duran J, Bottino CM, Ribeiz S, Lotufo PA, Menezes PR, Scazufca M, Busatto GF (2015) Relationship between brain age-related reduction in gray matter and educational attainment. PLoS One 10, e0140945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, Sager MA (2014) Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 83, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Daugherty AM, Raz N (2016) Regional brain shrinkage and change in cognitive performance over two years: The bidirectional influences of the brain and cognitive reserve factors. Neuroimage 126, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ (2012) Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann Neurol 71, 653–660. [DOI] [PubMed] [Google Scholar]
- [60].Suo C, Leon I, Brodaty H, Trollor J, Wen W, Sachdev P, Valenzuela MJ (2012) Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. NeuroImage 63, 1542–1551. [DOI] [PubMed] [Google Scholar]
- [61].Foubert-Samier A, Catheline G, Amieva H, Dilharreguy B, Helmer C, Allard M, Dartigues J-F (2012) Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol Aging 33, 423.e15–423.e25. [DOI] [PubMed] [Google Scholar]
- [62].Teipel SJ, Meindl T, Wagner M, Kohl T, Burger K, Reiser MF, Herpertz S, Moller H-J, Hampel H (2009) White matter microstructure in relation to education in aging and Alzheimer’s disease. J Alzheimers Dis 17, 571–583. [DOI] [PubMed] [Google Scholar]
- [63].Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung L-K, Brown TR, DeCarli C, Stern Y (2011) White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging 32, 15881598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Draganski B, Lutti A, Kherif F (2013) Impact of brain aging and neurodegeneration on cognition: Evidence from MRI. Curr Opin Neurol 26, 640–645. [DOI] [PubMed] [Google Scholar]
- [65].Perneczky R, Wagenpfeil S, Lunetta KL, Cupples LA, Green RC, DeCarli C, Farrer LA, Kurz A (2010) Head circumference, atrophy, and cognition: Implications for brain reserve in Alzheimer disease. Neurology 75, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Staff RT, Murray AD, Deary IJ, Whalley LJ (2004) What provides cerebral reserve? Brain 127, 1191–1199. [DOI] [PubMed] [Google Scholar]
- [67].Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ (2006) Generality and specificity in cognitive aging: A volumetric brain analysis. Neuroimage 30, 14331440. [DOI] [PubMed] [Google Scholar]
- [68].Kaplan RF,Cohen RA,Moscufo N,Guttmann C,hasman J, Buttaro M, Hall CH, Wolfson L (2009) Demographicand biological influences on cognitive reserve. J Clin Exp Neuropsychol 31, 868–876. [DOI] [PubMed] [Google Scholar]
- [69].Porat S, Goukasian N, Hwang KS, Zanto T, Do T, Pierce J, Joshi S, Woo E, Apostolova LG (2016) Dance experience and associations with cortical gray matter thickness in the aging population. Dement Geriatr Cogn Disord Extra 6, 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Arenaza-Urquijo EM, Wirth M, Chetelat G (2015) Cog-´ nitive reserve and lifestyle: Moving towards preclinical Alzheimer’s disease. Front Aging Neurosci 7, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Hinton L, DeCarli C (2010) Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133, 2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Valenzuela M, Brodaty H, Wen W, Chen X, Sachdev P (2009) Lifespan mental activity predicts diminished rate of hippocampal atrophy. Alzheimers Dement 5, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mondragon JD, Celada-Borja C, Barinagarrementeria-´ Aldatz F, Burgos-Jaramillo M, Barragan-Campos HM´ (2016) Hippocampal volumetry as a biomarker for dementia in people with low education. Dement Geriatr Cogn Disord Extra 6, 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schroder J, Pantel J (2016) Neuroimaging of hippocampal¨ atrophy in early recognition of Alzheimeŕs disease - a critical appraisal after two decades of research. Psychiatry Res Neuroimaging 247, 71–78. [DOI] [PubMed] [Google Scholar]
- [75].Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Sager MA, Hermann BP, Johnson SC, Okonkwo OC (2015) Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin Neuropsychol 30,634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Medina DA, Gaviria M (2008) Diffusion tensor imaging investigations in Alzheimer’s disease: The resurgence of white matter compromise in the cortical dysfunction of the aging brain. Neuropsychiatr Dis Treat 4, 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Soldan A, Pettigrew C, Lu Y, Wang M-C, Selnes O, Albert M, Brown T, Ratnanather JT, Younes L, Miller MI (2015) Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum Brain Mapp 36, 2826–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Osone A, Arai R, Hakamada R, Shimoda K (2015) Impact of cognitive reserve on the progression of mild cognitive impairment to Alzheimer’s disease in Japan. Geriatr Gerontol Int 15, 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Arenaza-Urquijo EM, Bosch B, Sala-Llonch R, Sole-´ Padulles C, Junqú e C, Ferń andez-Espejo D, Bargalĺ o N, Rami L, Molinuevo JL, Bartres-Faz D (2011) Spe-´ cific anatomic associations between white matter integrity and cognitive reserve in normal and cognitively impaired elders. Am J Geriatr Psychiatry 19, 33–42. [DOI] [PubMed] [Google Scholar]
- [80].Roe CM, Xiong C, Miller JP, Morris JC (2007) Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology 68, 223–228. [DOI] [PubMed] [Google Scholar]
- [81].Albert R, Jeong H, Barabasi A-L (2000) Error and attack´ tolerance of complex networks. Nature 406, 378. [DOI] [PubMed] [Google Scholar]
- [82].Santarnecchi E, Rossi S, Rossi A (2015) The smarter, the stronger: Intelligence level correlates with brain resilience to systematic insults. Cortex 64, 293–309. [DOI] [PubMed] [Google Scholar]
- [83].Santarnecchi E, Sprugnoli G, Tatti E, Mencarelli L, Neri F, Momi D, Di Lorenzo G, Pascual-Leone A, Rossi S,Rossi A (2018) Brain functional connectivity correlates of coping styles. Cogn Affect Behav Neurosci 18, 495–508. [DOI] [PubMed] [Google Scholar]
- [84].Waiter GD, Fox HC, Murray AD, Starr JM, Staff RT, Bourne VJ, Whalley LJJ, Deary IJ (2008) Is retaining the youthful functional anatomy underlying speed of information processing a signature of successful cognitive ageing? An event-related fMRI study of inspection time performance. Neuroimage 41, 581–595. [DOI] [PubMed] [Google Scholar]
- [85].Nucci M, Mapelli D, Mondini S (2012) Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin Exp Res 24, 218–226. [DOI] [PubMed] [Google Scholar]
- [86].Scarmeas N, Zarahn E, Anderson KE, Hilton J, Flynn J, van Heertum R, Sackeim HA, Stern Y (2003) Cognitive reserve modulates functional brain responses during memory tasks: A PET study in healthy young and elderly subjects. Neuroimage 19, 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Springer MV, McIntosh AR, Winocur G,Grady CL (2005) The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology 19, 181–192. [DOI] [PubMed] [Google Scholar]
- [88].Arenaza-Urquijo EM, Bejanin A, Gonneaud J, Wirth M, La Joie R, Mutlu J, Gaubert M, Landeau B, de la Sayette V, Eustache F, Chetelat G (2017) Association betweeń educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: Neuroimaging evidence for protection and compensation. Neurobiol Aging 59, 72–79. [DOI] [PubMed] [Google Scholar]
- [89].Bosch B, Bartres-Faz D, Rami L, Arenaza-Urquijo EM,´ Fernandez-Espejo D, Junqú e C,Soĺ e-Padulĺ es C,Ś anchez-´ Valle R, Bargallo N, Falć on C, Molinuevo JL (2010)´ Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex 46, 451–461. [DOI] [PubMed] [Google Scholar]
- [90].Bartres-Faz D, Soĺ e-Padulĺ es C, Junqú e C, Rami L, Bosch B, Bargallo N, Molinuevo JL (2009) Interactions of cogni-´ tive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol Psychol 80, 256–259. [DOI] [PubMed] [Google Scholar]
- [91].Ewers M, Insel PS, Stern Y, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2013) Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology 80, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL (2003) Functional deactivations: Change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A 100, 1450414509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bozzali M, Dowling C, Serra L, Spano B, Torso M,` Marra C, Castelli D, Dowell NG, Koch G, Caltagirone C, Cercignani M (2015) The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. J Alzheimers Dis 44, 243–250. [DOI] [PubMed] [Google Scholar]
- [94].Garibotto V, Borroni B, Sorbi S, Cappa SF, Padovani A, Perani D (2012) Education and occupation provide reserve in both ApoE 4 carrier and noncarrier patients with probable Alzheimer’s disease. Neurol Sci 33, 1037–1042. [DOI] [PubMed] [Google Scholar]
- [95].Carapelle E, Serra L, Modoni S, Falcone M, Caltagirone C, Bozzali M, Specchio LM, Avolio C (2017) How the cognitive reserve interacts with -amyloid deposition in mitigating FDG metabolism: An observational study. Medicine (Baltimore) 96, e5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rentz DM, Mormino EC, Papp KV, Betensky RA,Sperling RA, Johnson KA (2017) Cognitive resilience in clinical and preclinical Alzheimer’s disease: The Association of Amyloid and Tau Burden on cognitive performance. Brain Imaging Behav 11, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Almeida RP, Schultz SA, Austin BP, Boots EA, Dowling NM, Gleason CE, Bendlin BB, Sager MA, Hermann BP, Zetterberg H, Carlsson CM, Johnson SC, Asthana S, Okonkwo OC (2015) Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol 72, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA (2010) Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 67, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Garibotto V, Trattamanti M, Marcone A, Florea I, Panzacchi A, Moresco R, Virta JR, Rinne JO, Cappa SF, Perani D (2013) Cholinergic activity correlates with reserve proxies in Alzheimer’s disease. Neurobiol Aging 34, 2694.e13–2694.e18. [DOI] [PubMed] [Google Scholar]
- [100].Francis PT (2005) The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr 10, 6–9. [DOI] [PubMed] [Google Scholar]
- [101].Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M, Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2017) Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology 88, 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kim M-J, Lee K-M, Son Y-D, Jeon H-A, Kim Y-B, Cho Z-H (2012) Increased basal forebrain metabolism in mild cognitive impairment: An evidence for brain reserve in incipient dementia. J Alzheimers Dis 32, 927–938. [DOI] [PubMed] [Google Scholar]
- [103].Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA (2008) Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology 70, 1732–1739. [DOI] [PubMed] [Google Scholar]
- [104].Sundermann EE, Maki PM, Rubin LH, Lipton RB, Landau S, Biegon A, Alzheimer’s Disease Neuroimaging Initiative (2016) Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 87, 1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Irvine K, Laws KR, Gale TM, Kondel TK (2012) Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J Clin Exp Neuropsychol 34, 989–998. [DOI] [PubMed] [Google Scholar]
- [106].Morbelli S, Perneczky R, Drzezga A, Frisoni GB, Caroli A, van Berckel BNM, Ossenkoppele R, Guedj E, Didic M, Brugnolo A, Naseri M, Sambuceti G, Pagani M, Nobili F (2013) Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: A European Alzheimer Disease Consortium Project. J Nucl Med 54, 894–902. [DOI] [PubMed] [Google Scholar]
- [107].Basar E, Basar-Eroglu C, Karakas S,Schurmann M (2001) Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39, 241–248. [DOI] [PubMed] [Google Scholar]
- [108].Angel L, Fay S, Bouazzaoui B, Baudouin A, Isingrini M (2010) Protective role of educational level on episodic memory aging: An event-related potential study. Brain Cogn 74, 312–323. [DOI] [PubMed] [Google Scholar]
- [109].Friedman D (2003) Cognition and aging: A highly selective overview of event-related potential (ERP) data. J Clin Exp Neuropsychol 25, 702–720. [DOI] [PubMed] [Google Scholar]
- [110].Salthouse TA (1996) The processing-speed theory of adult age differences in cognition. Psychol Rev 103, 403–428. [DOI] [PubMed] [Google Scholar]
- [111].Cabeza R (2002) Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17, 85–100. [DOI] [PubMed] [Google Scholar]
- [112].Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002) Aging gracefully: Compensatory brain activity in high-performingolderadults.Neuroimage 17,1394–1402. [DOI] [PubMed] [Google Scholar]
- [113].Daselaar S, Veltman D, Rombouts S, Raaijmakers J, Jonker C (2003) Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 126, 43–56. [DOI] [PubMed] [Google Scholar]
- [114].Dennis NA, Cabeza R (2008) Neuroimaging of Healthy Cognitive Aging, Taylor & Francis Group. [Google Scholar]
- [115].Rosen AC, Prull MW, O’hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD (2002) Variable effects of aging on frontal lobe contributions to memory. Neuroreport 13, 2425–2428. [DOI] [PubMed] [Google Scholar]
- [116].Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R (2008) Que PASA? The Posterior-Anterior Shift in Aging.´ Cereb Cortex 18, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Czernochowski D, Fabiani M, Friedman D (2008) Use it or lose it? SES mitigates age-related decline in a recency/recognition task. Neurobiol Aging 29, 945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Stern Y, Gazes Y, Razlighi Q, Steffener J, Habeck C(2018) A task-invariant cognitive reserve network. Neuroimage 178, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hannigan C,Coen RF, Lawlor BA, Roberts on IH, Brennan S (2015) The NEIL Memory Research Unit: Psychosocial, biological, physiological and lifestyle factors associated with healthy ageing: Study protocol. BMC Psychol 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual-Marqui RD, Rodriguez G, Romani GL, Salinari S, Tecchio F, Vitali P, Zanetti O, Zappasodi F, Rossini PM (2004) Mapping distributed sources of cortical rhythms in mild Alzheimer’s disease. A multicentric EEG study. Neuroimage 22, 57–67. [DOI] [PubMed] [Google Scholar]
- [121].Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, Cordone S, Gesualdo L, Del Percio C (2016) Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int J Psychophysiol 103, 88–102. [DOI] [PubMed] [Google Scholar]
- [122].Dierks T, Frolich L, Ihl R, Maurer K (1995) Correlation¨ between cognitive brain function and electrical brain activity in dementia of Alzheimer type. J Neural Transm Gen Sect 99, 55–62. [DOI] [PubMed] [Google Scholar]
- [123].Fraga FJ, Falk TH, Kanda PAM, Anghinah R (2013) Characterizing Alzheimer’s disease severity via resting-awake EEG amplitude modulation analysis.PLoSOne 8,e72240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Jeong J (2004)EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 115, 1490–1505. [DOI] [PubMed] [Google Scholar]
- [125].Neto E, Biessmann F, Aurlien H, Nordby H, Eichele T (2016) Regularized linear discriminant analysis of EEG featuresindementiapatients.FrontAgingNeurosci 8,273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Anghinah R, Kanda PAM, Lopes HF, Basile LFH, Machado S, Ribeiro P, Velasques B, Sameshima K, Takahashi DY, Pinto LF, Caramelli P, Nitrini R (2011) Alzheimer’s disease qEEG: Spectral analysis versus coherence. which is the best measurement? Arq Neuropsiquiatr 69, 871–874. [DOI] [PubMed] [Google Scholar]
- [127].Babiloni C, Binetti G, Cassetta E, Forno GD, Percio CD, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM (2006) Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: A multicenter study. Clin Neurophysiol 117, 252–268. [DOI] [PubMed] [Google Scholar]
- [128].Bennys K, Rondouin G, Vergnes C, Touchon J (2001) Diagnostic value of quantitative EEG in Alzheimer’s disease. Neurophysiol Clin Neurophysiol 31, 153–160. [DOI] [PubMed] [Google Scholar]
- [129].Brenner RP, Ulrich RF,Spiker DG, Sclabassi RJ, Reynolds CF, Marin RS, Boller F (1986) Computerized EEG spectral analysis in elderly normal, demented and depressed subjects. Electroencephalogr Clin Neurophysiol 64, 483492. [DOI] [PubMed] [Google Scholar]
- [130].Coben LA, Danziger WL, Berg L (1983) Frequency analysis of the resting awake EEG in mild senile dementia of Alzheimer type. Clin Neurophysiol 55, 372–380. [DOI] [PubMed] [Google Scholar]
- [131].Moretti DV, Babiloni C, Binetti G, Cassetta E, Forno GD, Ferreric F, Ferri R, Lanuzza B, Miniussi C, Nobili F, Rodriguez G, Salinari S, Rossini PM (2004) Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin Neurophysiol 115, 299–308. [DOI] [PubMed] [Google Scholar]
- [132].Scrascia F, Curcio G, Ursini F, Trotta L, Quintiliani L, Migliore S, Altamura C, Pitocco F, Altavilla R, Melgari JM, Quattrocchi CC,Vernieri F (2014) Relationship among diffusion tensor imaging, EEG activity, and cognitive status in mild cognitive impairment and Alzheimer’s disease patients. J Alzheimers Dis 38, 939–950. [DOI] [PubMed] [Google Scholar]
- [133].Chen T-F, Chen Y-F, Cheng T-W, Hua M-S, Liu H-M, Chiu M-J (2009) Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp 30, 3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX (2006) Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology 66, 1845–1849. [DOI] [PubMed] [Google Scholar]
- [135].Pijnenburg Ya L, vd Made Y, van C van Walsum AM, Knol DL, Scheltens P, Stam CJ (2004) EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol 115, 1332–1339. [DOI] [PubMed] [Google Scholar]
- [136].Cantone M, Di Pino G, Capone F, Piombo M, Chiarello D, Cheeran B, Pennisi G, Di Lazzaro V (2014) The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol 125, 1509–1532. [DOI] [PubMed] [Google Scholar]
- [137].Koch G, Di Lorenzo F, Bonní S, Ponzo V, Caltagirone C, Martorana A (2012) Impaired LTP- but not LTDLike cortical plasticity in Alzheimer’s disease patients. J Alzheimers Dis 31, 593–599. [DOI] [PubMed] [Google Scholar]
- [138].Cespon J, Miniussi C, Pellicciari MC (2018) Interven-` tional programmes to improve cognition during healthy and pathological ageing: Cortical modulations and evidence for brain plasticity. Ageing Res Rev 43, 81–98. [DOI] [PubMed] [Google Scholar]
- [139].Tatti E, Rossi S, Innocenti I, Rossi A, Santarnecchi E (2016) Non-invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Res Rev 29, 66–89. [DOI] [PubMed] [Google Scholar]
- [140].Barker AT, Jalinous R, Freeston IL (1985) Non-Invasive magnetic stimulation of human motor cortex. Lancet 325, 1106–1107. [DOI] [PubMed] [Google Scholar]
- [141].Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117, 847858. [DOI] [PubMed] [Google Scholar]
- [142].Nardone R, Tezzon F, Holler Y, Golaszewski S, Trinka E, Brigo F (2014) Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand 129, 351–366. [DOI] [PubMed] [Google Scholar]
- [143].Niskanen E, Kon¨ onen M, M¨ aätt¨ a S, Hallikainen M,¨ Kivipelto M, Casarotto S, Massimini M, Vanninen R, Mervaala E, Karhu J, Soininen H (2011) New insights into Alzheimer’s disease progression: A combined TMS and structural MRI study. PLoS One 6, e26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Khedr EM, Ahmed MA, Darwish ES, Ali AM (2011) The relationship between motor cortex excitability and severity of Alzheimer’s disease: A transcranial magnetic stimulation study. Neurophysiol Clin Neurophysiol 41, 107–113. [DOI] [PubMed] [Google Scholar]
- [145].Perretti A, Grossi D, Fragassi N, Lanzillo B, Nolano M, Pisacreta AI, Caruso G, Santoro L (1996) Evaluation of the motor cortex by magnetic stimulation in patients with Alzheimer disease. J Neurol Sci 135, 31–37. [DOI] [PubMed] [Google Scholar]
- [146].Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR (1982) Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 215, 1237–1239. [DOI] [PubMed] [Google Scholar]
- [147].Winkler J, Thal LJ, Gage FH, Fisher LJ (1998) Cholinergic strategies for Alzheimer’s disease. JMolMed 76,555–567. [DOI] [PubMed] [Google Scholar]
- [148].Schicktanz N, Schwegler K, Fastenrath M, Spalek K, Milnik A, Papassotiropoulos A, Nyffeler T, de Quervain DJ-F (2014) Motor threshold predicts working memory performance in healthy humans. Ann Clin Transl Neurol 1, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chandra SR, Issac TG, Nagaraju BC, Philip M (2016) A study of cortical excitability, central motor conduction, and cortical inhibition using single pulse transcranial magnetic stimulation in patients with early frontotemporal and Alzheimer’s dementia. Indian J Psychol Med 38, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA (2004) Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Tonali PA (2003) Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease: Evidence of impaired glutamatergic neurotransmission? Ann Neurol 53, 824–824. [DOI] [PubMed] [Google Scholar]
- [152].Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A (2011) Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr 24, 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hasselmo ME, Bower JM (1993)Acetylcholine and memory. Trends Neurosci 16, 218–222. [DOI] [PubMed] [Google Scholar]
- [154].Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P (2008) Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol 67, 242–251. [DOI] [PubMed] [Google Scholar]
- [155].Valenzuela MJ, Sachdev P (2007) Assessment of complex mental activity across the lifespan: Development of the Life time of Experiences Questionnaire(LEQ).Psychol Med 37, 1015–1024. [DOI] [PubMed] [Google Scholar]
- [156].Buckner RL (2004) Memory and executive functions in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208. [DOI] [PubMed] [Google Scholar]