Abstract
Background and aim:
To assess the incidence of Type 1 Diabetes Mellitus (T1DM) during the period 2012-2017, the frequency and severity of ketoacidosis (DKA) at diabetes onset, and the factors associated with DKA in children and adolescents younger than 18 years old in the Abruzzo region, Italy.
Methods:
All incident cases of T1DM (0-17 years old) diagnosed between January 2012 and December 2017 were included. Data about the patients were obtained from two independent sources; insulin prescriptions and medical records. Clinical data at diabetes onset, as well as demographic and non-demographic data, including center of first hospitalization, distance to regional reference center and number of pediatricians (per 1000 residents younger than 18 years) were collected and evaluated.
Results:
During 2012-2017 period, 177 patients were diagnosed with T1DM. In 2012, T1DM incidence was 15.6 per 100,000/year; in 2013, 16.4 per 100,000/year; in 2014, 11.6 per 100,000/year; in 2015, 14.2 per 100,000/year; in 2016, 16.2 per 100,000/year and in 2017, 12.2 per 100,000/year. DKA was present in 29.3% of patients, 6.9% with severe DKA. The DKA presence was correlated to age (p<0.02), ethnicity (p<0.04), being transferred to a specialist center instead of being directly admitted to one (p<0.002) and the number of pediatricians in the population (p<0.01). The DKA severity was associated with the delay of transfer (p<0.04).
Conclusions:
Being admitted directly to a specialist center is very important and it could be expression of high alertness of pediatricians. Availability of well-trained pediatricians is necessary for the prevention of DKA. (www.actabiomedica.it)
Keywords: Diabetic Ketoacidosis, Diabetes Mellitus, Type 1, Incidence, Child, Delivery of Health Care
Introduction
Type 1 Diabetes Mellitus (T1DM) is one of the major chronic diseases of childhood. Approximately, there are 80,000 new cases (under 15 years of age) worldwide every year (1). The incidence of T1DM varies greatly between different countries, but also within them. The highest incidence rates are observed in Finland, Northern Europe, and Canada (2). The overall pattern is one of an approximately 3% per annum increase, though in some high-incidence countries such as Finland (3) and Norway (4) the incidence rate of T1DM seems to show a temporary deceleration (5).
It is well established that unrecognized and untreated T1DM leads to ketoacidosis (DKA), with an increased risk of cerebral edema, cognitive deficits, or even death. Overall mortality in children with DKA varies from 0.15% to 0.35% in developed countries (6). Even subtle cerebral injury may occur, with deficits in attention, spatial memory, executive function and other cognitive function (7-9). Furthermore, the costs of DKA-related hospital admission are high.
The frequency of DKA at T1DM onset varies from 12.8% to 80% worldwide (10). The lowest rates are found in Canada and the Scandinavian countries, the highest in Saudi Arabia (11). The frequency of DKA is lower in countries where the background incidence of T1DM is higher (10, 12-13). There is evidence regarding the association of DKA with the increased awareness of the disease and having a first-degree relative with T1DM (14-15). Younger age, lower socioeconomic status, lower parental education (16) and living in smaller cities (17) confer an increased risk of DKA at onset of disease. Higher rates of DKA are more common in countries with lower development index (11).
Having a regular healthcare provider is associated with a reduced risk of DKA at diabetes onset (17). Other factors related with a decreased risk of DKA are the development of specialist pediatric diabetes services and the widespread availability of glucose meters (12). However, the beneficial effect of additional factors, including direct admission to a specialist center (18-19), prevention campaigns and raising public awareness (20-23) has not been always confirmed (20, 24).
Italy is one of the countries with the greatest variability in T1DM incidence. The highest rate is observed in Sardinia (25). In the 1990-2003 period, the incidence rate in peninsular Italy (0-14 years) was 12.26/100,000 with an increasing temporal trend of 2.94% per year. In the Veneto region, an incidence rate of 16.5 per 100,000 person-years was reported (26). DKA frequency at onset of T1DM in Italy is increasing (38.5% to 47.6%) (24).
Abruzzo is a region of central-eastern Italy; it is divided in 4 provinces (L’Aquila; Chieti; Pescara; Teramo). During 1990-1995 period, the overall age-adjusted incidence rate (0-14 years) in the Abruzzo region was 9.34 (95% CI 7.76-10.95) (27).
In Abruzzo region, the model of territorial and hospital healthcare delivery consists of 4 autonomous areas. During the study period, T1DM management in the region was mostly managed by a regional reference center for T1DM in childhood and a small peripheral healthcare unit, necessary for the orographic characteristics of the region.
The aim of this study was to assess the incidence of T1DM during the period 2012-2017 as well as the frequency and severity of DKA at diabetes onset in children and adolescents younger than 18 years in the Italian region of Abruzzo. We wanted to investigate the possible factors associated with DKA. We also evaluated the factors affecting DKA severity. By identifying modifiable risk factors, it would be possible to reduce the rate and severity of DKA through targeted interventions.
Methods
Study population
Children and adolescents aged <18 years old at diagnosis of T1DM were included in the study. All incident cases between January 2012 and December 2017 were considered. T1DM diagnosis was established based on clinical and biological parameters. Clinical diagnoses of T1DM were validated through the presence of at least one diabetes autoantibody (Islet Cell cytoplasmic Autoantibodies - ICA, Insulinoma-Associated-2 Autoantibodies - IA2, Antibodies to Glutamic Acid Decarboxylase - Anti GAD); the clinical judgment of a specialist in pediatric diabetology was also used (2). All children with Maturity Onset Diabetes of the Young, Type 2 Diabetes Mellitus, Cystic Fibrosis-related Diabetes, Neonatal Diabetes or other forms were excluded.
DKA presence at onset and its severity were defined according to the International Society for Pediatric and Adolescent Diabetes Guidelines (28) (mild: pH <7.3, bicarbonate <15 mmol/l; moderate: pH <7.2, bicarbonate <10mmol/l; severe: pH<7.1, bicarbonate <5 mmol/l). Ketone levels were not consistently reported.
Data about the patients were obtained from two independent sources. The primary source was territorial pharmaceutical insulin prescriptions (2012-2017). As a secondary source, medical records of patients were consulted. Completeness of registration was assessed by capture–recapture method (29). Population data from the Italian National Institute for Statistics (http://dati.istat.it/) were used for the calculation of the incidence.
Data collected from patients’ files included: gender, date of diagnosis, age at onset, place of residence, laboratory data [blood glucose concentration, pH, bicarbonate, c-peptide, hemoglobin A1c (HbA1c), autoimmunity for diabetes (ICA, IA2, Anti GAD)], presence of first-degree relative with T1DM, personal medical history (including duration of symptoms and/or recent infections), ethnicity, first hospitalization in specialist center or transfer to it. The study population was divided into four age groups: 0-4 years, 5-9 years, 10-14 years and 15-17 years old group.
The distance from patient’s residence to regional reference specialist center was measured by online distance calculator (https://maps.google.it/). The number of inhabitants (<10000, 10-50000, 50-250000 inhabitants) and degree of urbanization (densely populated, intermediate density, rural) of each municipality of residence was determined according to Eurostat classification (https://www.istat.it/it/archivio/156224).
Moreover, the number of pediatricians (per 1000 residents younger than 18 years) was calculated for each province using data from the Italian National Institute for Statistics.
Data analysis
Statistical analyses were performed using SPSS 16.0 for windows software (SPSS Inc, Chicago, IL, USA). Variables were assessed for normal distribution. Descriptive statistics were described as means ± standard deviations for continuous variables and as frequencies for categorical variables. The incidence of Type 1 diabetes was evaluated as punctual and 95% confidence interval (95% CI). Student’s t test and the χ2 test or fisher exact test were used to evaluate the differences between patients with and without DKA at T1DM diagnosis. Stepwise logistic regression was applied to determine independent risk factors for DKA occurrence at onset. Comparison between continuous variables to assess the factors contributing to DKA severity was performed using one-way analysis of variance (ANOVA). A p value< 0.05 was considered statistically significant.
Ethical approval
This study was carried out in accordance with the Declaration of Helsinki and approved by the local Ethics Committee, University of Chieti-Pescara, Italy.
Results
Incidence of T1DM
The total number of children aged 0-17 years-old diagnosed with T1DM in the Abruzzo region during 2012-2017 was 177 (male: 56.3%; mean age at diagnosis: 9.77 ± 4.38 years); three patients were excluded from the analysis for incomplete data.
Thirty-two new cases of T1DM were identified in 2012 (incidence: 15.58 per 100,000/year; 95% CI 11.02-22.04), thirty-four new cases were identified in 2013 (incidence: 16.39 per 100,000/year; 95% CI 11.72-22.95) and twenty-four new cases were identified in 2014 (incidence: 11.64 per 100,000/year; 95% CI 7.81-17.38). Twenty-nine, thirty-three and twenty-five new cases were identified in 2015 (incidence: 14.20 per 100,000/year; 95% CI 9.80-20.40), 2016 (incidence: 16.16 per 100,000/year; 95% CI 11.49-22.73) and 2017 (incidence: 12.24 per 100,000/year; 95% CI 8.27-18.12), respectively.
The majority of patients (88.5%) were of Italian origin. The distribution of the residence at disease onset among the provinces was as follows: 19.5% in L’Aquila province, 32.2% in Chieti province, 27.6% in Pescara province, 20.7% in Teramo province. The highest number of patients was distributed along the coast of the region.
According to age, the T1DM incident cases were distributed as follows: 13.2% in 0-4 years old group, 27.6% in 5-9 years old group, 40.2% in 10-14 years old group, 19% in 15-17 years old group.
Of the total patients, 23% were diagnosed in spring, 21.3% in summer, 29.3% in autumn, and 26.4% in winter, so more patients were diagnosed during the autumn, but the difference was not significant (p=0.442).
Diabetes onset-Presence and severity of DKA and associated factors
The mean blood glucose concentration at the onset was 431 ± 157.32 mg/dl. The mean HbA1c level was 11.80 ± 2.33% (105 ± 25.6 mmol/mol), the mean c-peptide level was 0.47 ±0.44 ng/ml. Patients with first-degree relative with T1DM accounted for 8.6% of cases. The presence of infection before diagnosis was reported in 20.07% of cases. The mean duration between the first symptoms and the diagnosis was 16.20 ± 18.74 days. In 67.2% of cases, patients were admitted first in a specialist center for the management of Type 1 diabetes. Of those who were transferred (57 patients), 70.71% were transferred on the same day.
DKA was present in 51 patients (29.3%) (male: 63%; mean age: 8.86 ± 4.73 years). The frequency of mild and moderate DKA was 11.5% and 10.9%, respectively. The frequency of severe DKA was 6.9% (male: 50%; mean age: 9.28 ± 4.24 years). Cerebral edema following DKA treatment was not observed. One patient experienced a hyperglycemic hyperosmolar state at onset of T1DM (for the purposes of the study, this patient was considered without DKA). The characteristics of patients according to the presentation at onset are shown in Table 1.
Table 1.
Characteristics of patients according to the presentation at the onset of diabetes.
| Characteristic | With DKA | Without DKA | p Value |
|---|---|---|---|
|
Year of diagnosis (n, %)
2012 2013 2014 2015 2016 2017 |
7 (21.88%) 11 (32.35%) 6 (25%) 8 (27.59%) 11 (33.33%) 8 (36.36%) |
25 (78.12%) 23 (67.65%) 18 (75%) 21 (72.41%) 22 (66.67%) 14 (63.64%) |
0.842 |
|
Season of diagnosis (n, %)
Spring Summer Autumn Winter |
9 (22.5%) 11 (29.75%) 18 (25.25%) 13 (28.26%) |
25 (78.12%) 23 (67.65%) 33 (64.75%) 33 (71.74%) |
0.614 |
| Age at onset (years) (mean ± SD) | 8.86 ± 4.73 | 10.15 ± 4.18 | 0.077 |
|
Age group (n, %)
0-4 years old 5-9 years old 10-14 years old 15-17 years old |
13 (56.52%) 9 (18.75%) 21 (30%) 8 (24.24%) |
10 (43.48%) 39 (81.25%) 49 (70%) 25 (75.76%) |
0.01 |
| Glucose concentration (mg/dL) (mean ± SD) | 491.18 ± 152.97 | 406.82 ± 152.98 | 0.001 |
| HbA 1 c % (mmol/mol) (mean ± SD) | 12.55 ± 1.93 (114 ± 21.2) |
11.48 ± 2.40 (102 ± 26.5) |
0.005 |
| c-peptide (ng/ml) (mean ± SD) | 0.29 ± 0.25 | 0.54 ± 0.48 | 0.001 |
|
First-degree relative with T1DM (n, %)
No Yes |
49 (30.82%) 2 (13.34%) |
110 (69.18%) 13 (86.66%) |
0.115 |
|
Presence of infection (before diagnosis) (n, %)
No Yes |
36 (26.09%) 15 (41.67%) |
102 (73.91%) 21 (58.33) |
0.067 |
|
Caucasian Italians (n. %)
No Yes |
10 (50%) 41 (26.62%) |
10 (50%) 113 (73.38%) |
0.031 |
|
First hospitalization in specialist center at the onset (n, %)
No Yes |
27 (47.37%) 24 (20.51%) |
30 (52.63%) 93 (79.49%) |
0.000 |
| Specific symptoms duration (days) (mean ± SD) | 15.73 ± 16.60 | 16.39 ± 19.62 | 0.832 |
|
Province of residence (n, %)
Aquila Chieti Pescara Teramo |
15 (44.12%) 12 (21.43%) 11 (22.92%) 13 (36.12%) |
19 (55.88%) 44 (78.57%) 37 (77.08%) 23 (63.88%) |
0.071 |
| Distance from the patient’s residence to the regional reference center (Km) (mean ± SD) | 63.53 ± 33.99 | 49.19 ± 32.88 | 0.010 |
|
Number of inhabitants (n, %)
<10000 10-50000 50-250000 |
23 (35.94%) 19 (32.02%) 9 (17.65%) |
41 (46.06%) 40 (67.98%) 42 (82.35%) |
0.084 |
|
Degree of urbanization (n, %)
Densely populated Intermediate density Rural |
1 (5.88%) 28 (31.46%) 22 (32.35%) |
16 (94.12%) 61 (68.54%) 46 (67.56%) |
0.082 |
| Pediatricians/1000 residents/province (mean ± SD) | 0.85 ± 0.68 | 0.88 ± 0.66 | 0.009 |
The characteristics according to the DKA severity are shown in Table 2.
Table 2.
Characteristics of patients presenting DKA at the onset according to severity.
| Characteristic | Mild DKA | Moderate DKA | Severe DKA | p Value |
|---|---|---|---|---|
|
Year of diagnosis (n)
2012 2013 2014 2015 2016 2017 |
1 5 3 4 4 3 |
3 4 1 2 6 3 |
3 2 2 2 1 2 |
0.808 |
|
Season of diagnosis (n)
Spring Summer Autumn Winter |
5 3 7 5 |
3 4 8 4 |
1 4 3 4 |
0.749 |
| Age at onset (years) (mean ± SD) | 9.21 ± 5.06 | 8.22 ± 4.84 | 9.28 ± 4.24 | 0.766 |
|
Age group (n)
0-4 years old 5-9 years old 10-14 years old 15-17 years old |
3 4 4 9 |
5 2 8 4 |
1 2 5 4 |
0.484 |
| Glucose concentration (mg/dL) (mean ± SD) | 498.40 ± 173.22 | 493 ± 140.34 | 475 ± 148.05 | 0.917 |
| HbA 1 c % (mmol/mol) (mean ± SD) | 12.59 ± 1.88 (114 ± 20.7) |
12.51 ± 1.81 (114 ± 20.5) |
12.57 ± 2.27 (114 ± 25) |
0.992 |
| c-peptide (ng/ml) (mean ± SD) | 0.32 ± 0.25 | 0.28 ± 0.26 | 0.24 ± 0.22 | 0.637 |
|
First-degree relative with T1DM (n)
No Yes |
19 1 |
19 0 |
11 1 |
0.483 |
|
Presence of infection (before diagnosis) (n)
No Yes |
15 5 |
14 5 |
7 5 |
0.565 |
|
Caucasian Italians (n)
No Yes |
1 19 |
6 19 |
3 9 |
0.09 |
|
First hospitalization in specialist center at the onset (n)
No Yes |
8 12 |
10 9 |
9 3 |
0.158 |
| Delay of transfer (days) (mean ± SD) | 0.45 ± 0.60 | 0.68 ± 0.74 | 1.33 ± 1.43 | 0.035 |
| Specific symptoms duration (days) (mean ± SD) | 15.5 ± 13.74 | 14.16 ± 10.17 | 18.85 ± 27.29 | 0.775 |
|
Province of residence (n)
Aquila Chieti Pescara Teramo |
7 5 5 3 |
7 5 3 4 |
1 2 3 6 |
0.32 |
| Distance from the patient’s residence to the regional reference center (Km) (mean ± SD) | 61.42 ± 40.33 | 66.95 ± 28.48 | 61.65 ± 32.89 | 0.863 |
|
Number of inhabitants (n)
<10000 10-50000 50-250000 |
9 7 4 |
7 9 3 |
7 3 2 |
0.756 |
|
Degree of urbanization (n)
Densely populated Intermediate density Rural |
0 12 8 |
0 12 7 |
1 4 7 |
0.243 |
| Pediatricians/1000 residents/province (mean ± SD) | 0.85 ± 0.71 | 0.84 ± 0.69 | 0.85 ± 0.067 | 0.864 |
There were no major changes in the frequency and the severity of DKA over the years (p=0.842; p=0.808) and there was no seasonality (p=0.614; p=0.749). Patients presenting with DKA were younger than patients without DKA at diagnosis (8.86 ± 4.73 years vs 10.15 ± 4.18 years) (p=0.077). DKA presence was different within various age groups (56% of children in the 0-4 years old group, 19% in the 5-9 years old group, 30% in the 10-14 years old group, and 24% in the 15-17 years old group; p<0.020). The severity of DKA was not correlated to age (p=0.484).
The mean blood glucose concentration at the onset of disease was lower in children without DKA (406.82 ± 152.98 vs 491.18 ± 152.97 mg/dl) (p<0.002). Mean HbA1c level for patients presenting in DKA was 12.55 ± 1.93% (114 ± 21.2 mmol/mol) and higher than that for patients not affected by DKA (11.48 ± 2.40%, 102 ± 26.5 mmol/mol) (p<0.010). Mean c-peptide was lower in patients with DKA at the onset of disease (p<0.002).
Having a first-degree relative with T1DM and the presence of infection were not significantly associated with DKA (p=0.115 and p=0.067, respectively). On the contrary, ethnicity other than Caucasian Italians was associated with DKA at the onset of the disease (p=0.031), but not with its severity (p=0.090). DKA was less frequent in patients admitted to specialist center directly than those transferred later (p<0.002). Delay of transfer was associated with the severity of DKA (p<0.040).
The duration of diabetes-specific symptoms (polyuria, polydipsia, weight loss) prior to the admission to the health care unit was not correlated with the DKA presence (p=0.832).
No associations were found between the province of residence at disease onset and the presence of DKA (p=0.071) or its severity (p=0.320). However, six patients of twelve with severe DKA at onset of T1DM resided in Teramo province. Moreover, the mean distance from the patient’s residence to the regional reference center was greater for patients who developed DKA (p<0.02). The distribution of residences of patients with DKA is shown in Figure 1.
Figure 1.
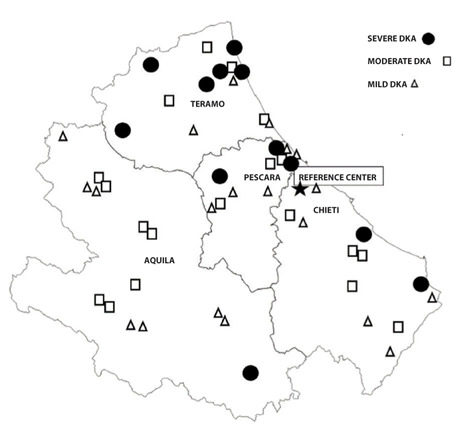
Distribution of the residences of the patients presenting with ketoacidosis (DKA) at the onset of diabetes according to DKA severity. The map also shows the regional reference center for pediatric Type 1 diabetes in Abruzzo, Italy.
The number of inhabitants and the degree of urbanization of each municipality of residence were not associated with DKA at onset of diabetes (p=0.084 and p=0.082, respectively).
The mean of number of pediatricians (calculated per 1000 residents younger than 18 years in each province) was lower in the group of patients affected by DKA (p<0.01).
Discussion
Published data show that the incidence rate of T1DM increases across Europe by an average of approximately 3% per annum with periods of less and more rapid rise and, in some centers, showing a cyclical pattern (5, 26, 30). It has been pointed out that T1DM incidence is very heterogeneous even in neighboring countries within a well-defined geographical region (31). Italy is one of the countries with the greatest variability (25, 26). For this reason, knowing local T1DM and DKA epidemiological data is of paramount importance for resource allocation and healthcare services provision. With this study, we can classify Abruzzo as a region with intermediate-high risk for T1DM. This is in contrast with previous studies reporting that the lowest T1DM rates in Italy were observed in the southern regions, including Abruzzo (27, 32), but in accordance with the high incidence rate of T1DM in Apulia (annual incidence of 25.2 per 100,000), another region in South Italy (33).
T1DM incidence is lower in the 0-4 years old group, increases with age, and meets the peak in children aged from 9 to 14 years, almost comparable to what was found in other countries (33-34). In the large SWEET database, seasonality at T1DM clinical onset is documented with higher percentage of incident cases in autumn and winter and lower in spring and summer (35). We have not detected a significant seasonality, with the highest percentage of incident cases seen in autumn (29.3%) and the lowest in summer (21.3%).
In our study, the frequency of DKA at onset of T1DM is 29.3%, which is high (in Denmark 14.7%, in Sweden 18%, in Canada 18.6%) (10-11). Even so, it is lower than what is reported in other studies from Italy (38.5 - 47.6 - 56%) (24, 36-37). The frequency of severe DKA is also lower (6.9% versus 10.3% (36) and 15%) (24, 37). The frequency of DKA in Abruzzo region remains stable over the study period. Similarly, stable prevalence in DKA has also been noted in other countries (20, 38).
Although the association between mean age at onset and DKA is not significant, when divided in age groups, younger patients (0-4 years) are more likely to present with DKA (of any severity) in our region. Younger children may have more aggressive β-cell destruction (39) and less well-developed compensatory mechanisms, which could result in faster development of acidosis and dehydration (17, 40-41). Difficulties in diagnosing T1DM, as well as a wrong preliminary diagnosis (42), are a significant cause of DKA development; mostly in younger children (43).
Pediatricians have a key role in the diagnostic and therapeutic assistance of children with diabetes. They should be able to promptly identify alterations of clinical parameters (respiratory rate, state of dehydration, water balance) and T1DM symptoms, which are often nonspecific and/or masked by the presence of concomitant diseases (44, 45). Pediatricians should have a high medical index of suspicion (15) and a low threshold to check blood or urine sugar and ketone (18). They are responsible for early diagnosis and direct admission to the specialist center.
Similarly, to previous findings (15), in our study, children diagnosed with DKA have higher HbA1c and lower c-peptide, which may indicate longer duration of the preclinical disease state. Surprisingly, having a first-degree relative with T1DM and the presence of infection are not associated with DKA. Ethnicity is associated with DKA perhaps because of lack of awareness, language and cultural barriers, and practical difficulties in accessing healthcare (14). The need for appropriate provision of healthcare services for the increasing population of immigrants is key.
The mean distance from the patient’s residence to the regional reference specialist center is associated with the presence of DKA at disease onset. Our analysis shows also that DKA at diabetes onset is less frequent in patients admitted to a specialist center directly than those transferred later. Moreover, delay of transfer is associated with the severity of DKA. Malachowska et al. have shown in their region that being admitted directly to the specialist center is a protective factor against DKA regardless of the patient’s place of residence and its distance from the specialist center (19). A multicenter analysis conducted in Germany and Austria shows that patients with DKA at onset of diabetes are admitted to the nearest hospital, independent of center size (46). Hospitalization in a non-specialist department, such as an emergency room, and the delay of transfer could be justified by the more serious clinical conditions due to the diagnostic delay, probably because of a lower degree of alertness of pediatricians further away from the regional reference center. During the study period, 75% of severe DKA arrived in the most accessible emergency room, to then be transferred to the specialist center. Correct diagnostic and therapeutic management, in collaboration with specialist centers, and rapid transfer to the specialist center play a crucial role in the therapy of DKA and in the prevention of complication.
In our study, the duration of specific symptoms (polyuria, polydipsia, weight loss) before the admission to the health care unit is not correlated with the DKA presence. However, relevant data were collected retrospectively, based on patients’ reports. Province of residence, number of inhabitants, and degree of urbanization of each municipality are not associated with DKA in our analysis. The number of pediatricians (calculated per 1000 residents younger than 18 years old) is lower in Teramo and L’Aquila provinces, which are also among the areas furthest from the regional reference center. The mean number of pediatricians (per 1000 residents younger than 18 years in each province) is lower in the group of children with DKA.
In conclusion, smaller density of pediatricians and their lower accessibility to the regional reference center, as well as deferred referral of patients to a specialist center, likely expression of lower alertness levels of attending pediatricians, could account for the high incidence of DKA at T1DM diagnosis. Ethnicity could also contribute. Epidemiological registry provides information that is useful not only for clinical assessments, but also for determining health care policy. Specific resources could be earmarked for immigrant children. Multidisciplinary specialist center should be able to provide all aspects of diabetes care according to chronic care model (47), even continuing education of pediatricians and educational sessions for medical doctors (21-22). In our region, the regional reference center periodically delivers continuing medical education courses about DKA and diabetes to pediatricians. We believe it is of great importance to equip the whole region with sufficient number of well-trained pediatricians, capable of early diagnosis of diabetes and promptly admitting all children to a specialist center.
Conflict of Interest:
ST has participated in advisory boards for Novo Nordisk, Sanofi Aventis, Eli Lilly and Lifescan. He has also received speaker honoraria from Roche Diagnostics, Novo Nordisk, Sanofi Aventis and Harmonium Pharma. The remaining authors have no disclosure.
References
- Atkilt HS, Turago MG, Tegegne BS. Clinical Characteristics of Diabetic Ketoacidosis in Children with Newly Diagnosed Type 1 Diabetes in Addis Ababa, Ethiopia: A Cross-Sectional Study. PLoS One. 2017;12(1):e0169666. doi: 10.1371/journal.pone.0169666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Suppl. 27):7–19. doi: 10.1111/pedi.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of Type 1 Diabetes in Finland. JAMA. 2013;310(4):427–428. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G Group NCDS. Incidence of type 1 diabetes in Norway among children aged 0–14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia. 2014;57(1):57–62. doi: 10.1007/s00125-013-3090-y. [DOI] [PubMed] [Google Scholar]
- Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–17. doi: 10.1007/s00125-018-4763-3. [DOI] [PubMed] [Google Scholar]
- Poovazhagi V. Risk factors for mortality in children with diabetic keto acidosis from developing countries. World J Diabetes. 2014;5(6):932–8. doi: 10.4239/wjd.v5.i6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Lee JK, Sims CE, Demaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156(1):109–14. doi: 10.1016/j.jpeds.2009.07.054. [DOI] [PubMed] [Google Scholar]
- Aye T, Mazaika PK, Mauras N, et al. Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care. 2019;42(3):443–9. doi: 10.2337/dc18-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol. 2017;10(4):409–28. doi: 10.1080/17512433.2017.1293521. [DOI] [PubMed] [Google Scholar]
- Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878–94. doi: 10.1007/s00125-012-2690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of Diabetic Ketoacidosis of New-Onset Type 1 Diabetes in Children and Adolescents in Different Countries Correlates with Human Development Index (HDI): An Updated Systematic Review, Meta-Analysis, and Meta-Regression. Horm Metab Res. 2018;50(03):209–22. doi: 10.1055/s-0044-102090. [DOI] [PubMed] [Google Scholar]
- Jefferies C, Cutfield SW, Derraik JGB, et al. 15-year incidence of diabetic ketoacidosis at onset of type 1 diabetes in children from a regional setting (Auckland, New Zealand) Sci Rep. 2015;5:10358. doi: 10.1038/srep10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014 May;133(4):e938–45. doi: 10.1542/peds.2013-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092. doi: 10.1136/bmj.d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn ER, Albert BB, Hofman PL, Cutfield WS, Gunn AJ, Jefferies CA. Pathways to reduce diabetic ketoacidosis with new onset type 1 diabetes: Evidence from a regional pediatric diabetes center: Auckland, New Zealand, 2010 to 2014. Pediatr Diabetes. 2017;18(7):553–8. doi: 10.1111/pedi.12456. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Yu HW, Jung HW, et al. Factors Associated with the Presence and Severity of Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes in Korean Children and Adolescents. @BULLET J Korean Med Sci. 2017;32(2):303–9. doi: 10.3346/jkms.2017.32.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhla M, Rahme E, Simard M, Larocque I, Legault L, Li P. Risk of ketoacidosis in children at the time of diabetes mellitus diagnosis by primary caregiver status: A population-based retrospective cohort study. Cmaj. 2018;190(14):E416–21. doi: 10.1503/cmaj.170676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli L, Flitter B, Pyle L, et al. A survey of youth with new onset type 1 diabetes: Opportunities to reduce diabetic ketoacidosis. Pediatr Diabetes. 2017;18(7):547–52. doi: 10.1111/pedi.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małachowska B, Małachowska K, Pietrzyk J, Fendler W, Rzeznik D, Mlynarski W. Accessibility of the reference center as a protective factor against ketoacidosis at the onset of diabetes in children. J Pediatr Endocrinol Metab. 2014;27(11–12):1137–43. doi: 10.1515/jpem-2014-0067. [DOI] [PubMed] [Google Scholar]
- Fritsch M, Schober E, Rami-Merhar B, Hofer S, Fröhlich-Reiterer E, Waldhoer T. Diabetic ketoacidosis at diagnosis in Austrian children: a population-based analysis, 1989-2011. J Pediatr. 2013;163(5):1484–8.e1. doi: 10.1016/j.jpeds.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care. 1999;22(1):7–9. doi: 10.2337/diacare.22.1.7. [DOI] [PubMed] [Google Scholar]
- Vanelli M, Chiari G, Lacava S, Iovane B. Campaign for diabetic ketoacidosis prevention still effective 8 years later. Diabetes Care. 2007;30(4):e12. doi: 10.2337/dc07-0059. [DOI] [PubMed] [Google Scholar]
- Choleau C, Maitre J, Elie C, et al. Effet à un an de la campagne nationale de prévention de l’acidocétose au moment du diagnostic de diabète de type 1 chez l’enfant et l’adolescent. Arch Pédiatrie. 2014:1–9. doi: 10.1016/j.arcped.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Rabbone I, Maltoni G, Tinti D, et al. Diabetic ketoacidosis at the onset of disease during a national awareness campaign: A 2-year observational study in children aged 0-18 years. Arch Dis Child. 2020;105(4):363–6. doi: 10.1136/archdischild-2019-316903. [DOI] [PubMed] [Google Scholar]
- Bruno G, Maule M, Merletti F, et al. Age-period-cohort analysis of 1990-2003 incidence time trends of childhood diabetes in Italy: the RIDI study. Diabetes. 2010;59(9):2281–7. doi: 10.2337/db10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigliano M, Tadiotto E, Morandi A, et al. Epidemiology of Type 1 Diabetes Mellitus in the pediatric population in Veneto Region, Italy. Diabetes Res Clin Pract. 2015;107(3):e19–21. doi: 10.1016/j.diabres.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Altobelli E, Chiarelli F, Valenti M, Verrotti A, Tumini S, Di Orio F. Incidence of insulin-dependent diabetes mellitus (0-14 years) in the Abruzzo Region, Italy, 1990-1995: results from a population-based register. J Pediatr Endocrinol Metab. 1998;11(4):555–62. doi: 10.1515/jpem.1998.11.4.555. [DOI] [PubMed] [Google Scholar]
- Wolfsdorf J, Craig ME, Daneman D, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10:118–33. doi: 10.1111/j.1399-5448.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- Laporte RE, Mccarty D, Bruno G, Tajima N, Baba S. Counting diabetes in the next millennium: Application of capture-recapture technology. Diabetes Care. 1993;16(2):528–34. doi: 10.2337/diacare.16.2.528. [DOI] [PubMed] [Google Scholar]
- Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–7. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- Vukovic R, Jesic MD, Vorgucin I, et al. First report on the nationwide incidence of type 1 diabetes and ketoacidosis at onset in children in Serbia: a multicenter study. Eur J Pediatr. 2018;177(8):1155–62. doi: 10.1007/s00431-018-3172-4. [DOI] [PubMed] [Google Scholar]
- Carle F, Gesuita R, Bruno G, et al. Diabetes Incidence in 0-to 14-Year Age-Group in Italy A 10-year prospective study FOR THE RIDI STUDY GROUP*. Diabetes Care. 2004;27(12):2790–6. doi: 10.2337/diacare.27.12.2790. [DOI] [PubMed] [Google Scholar]
- Fortunato F, Cappelli MG, Vece MM, et al. Incidence of Type 1 Diabetes among Children and Adolescents in Italy between 2009 and 2013: The Role of a Regional Childhood Diabetes Registry. J Diabetes Res. 2016;2016:1–7. doi: 10.1155/2016/7239692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DA, Islam N, Sutherland J, Reimer K, Amed S. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018;19:501–505. doi: 10.1111/pedi.12566. [DOI] [PubMed] [Google Scholar]
- Gerasimidi Vazeou A, Kordonouri O, Witsch M, et al. Seasonality at the clinical onset of type 1 diabetes-Lessons from the SWEET database. Pediatr Diabetes. 2016;17:32–7. doi: 10.1111/pedi.12433. [DOI] [PubMed] [Google Scholar]
- Zucchini S, Scaramuzza AE, Bonfanti R, et al. A Multicenter Retrospective Survey regarding Diabetic Ketoacidosis Management in Italian Children with Type 1 Diabetes. J Diabetes Res. 2016;2016:1–6. doi: 10.1155/2016/5719470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salardi S, Porta M, Maltoni G, et al. Ketoacidosis at diagnosis in childhood-onset diabetes and the risk of retinopathy 20 years later. J Diabetes Complications. 2016;30(1):55–60. doi: 10.1016/j.jdiacomp.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Szypowska A, Ramotowska A, Grzechnik-Gryziak M, Szypowski W, Pasierb A, Piechowiak K. High frequency of diabetic ketoacidosis in children with newly diagnosed type 1 diabetes. J Diabetes Res. 2016;2016:1–5. doi: 10.1155/2016/9582793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szypowska A, Skórka A. The risk factors of ketoacidosis in children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes. 2011;12(14):302–6. doi: 10.1111/j.1399-5448.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- Eyal O, Oren A, Almasi-Wolker D, Tenenbaum-Rakover Y, Rachmiel M, Weintrob N. Ketoacidosis in newly diagnosed type 1 diabetes in children and adolescents in israel: Prevalence and risk factors. Isr Med Assoc J. 2018;20(2):100–3. [PubMed] [Google Scholar]
- Mallare JT, Cordice CC, Ryan BA, Carey DE, Kreitzer PM, Frank GR. Identifying Risk Factors for the Development of Diabetic Ketoacidosis in New Onset Type 1 Diabetes Mellitus. Clin Pediatr (Phila) 2003;42(7):591–7. doi: 10.1177/000992280304200704. [DOI] [PubMed] [Google Scholar]
- PawÅowicz M, Birkholz D, NiedÅwiecki M, Balcerska A. Difficulties or mistakes in diagnosing type 1 diabetes in children? - demographic factors influencing delayed diagnosis. Pediatr Diabetes. 2009;10(8):542–9. doi: 10.1111/j.1399-5448.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Bui H, To T, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156(3):472–7. doi: 10.1016/j.jpeds.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lokulo-Sodipe K, Moon RJ, Edge JA, Davies JH. Identifying targets to reduce the incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in the UK. Arch Dis Child. 2014;99(5):438–42. doi: 10.1136/archdischild-2013-304818. [DOI] [PubMed] [Google Scholar]
- Townson J, Cannings-John R, Francis N, Thayer D, Gregory JW. Presentation to primary care during the prodrome of type 1 diabetes in childhood: A case-control study using record data linkage. Pediatr Diabetes. 2019;20(3):330–8. doi: 10.1111/pedi.12829. [DOI] [PubMed] [Google Scholar]
- Nagl K, Rosenbauer J, Neu A, et al. Children with onset-ketoacidosis are admitted to the nearest hospital available, regardless of center size. J Pediatr Endocrinol Metab. 2020;33(6):751–759. doi: 10.1515/jpem-2020-0038. [DOI] [PubMed] [Google Scholar]
- Williamson S. The best model of care for children and young people with diabetes. J R Coll Physicians Edinb. 2010;40(Suppl 17):25–32. [Google Scholar]


