Abstract
Background:
Anxiety and depression may affect asthma control. Previously, it has been reported that the hospital anxiety depression scale (HADS) questionnaire was fruitful in the management of adolescents with asthma. This study compared the scores of two different questionnaires, namely the Childhood Anxiety Sensitivity Index (CASI) and Children’s Depression Inventory (CDI), with asthma control level and lung function in asthmatic adolescents, evaluated in a real-life setting.
Methods:
A group of adolescents with asthma was consecutively enrolled. Asthma was diagnosed according to the GINA document, and consistently the symptom control grade was assessed. The adolescents completed the CASI, CDI, and Asthma Control Test (ACT) questionnaires. Visual Analogue Scale (VAS) for asthma symptoms perception and doctor’s asthma control evaluation were considered. Lung function and clinical characteristics were also assessed.
Results:
Totally, 87 asthmatic adolescents (60 males, 27 females, median age 14.2 years) were evaluated. 16.1% of asthmatic adolescents had anxious symptoms detected by CASI, and 11.5% depressive symptoms revealed by CDI. High scores of both CASI and CDI were significantly associated with uncontrolled asthma (p= 0.013 and 0.043, respectively).
Conclusions:
This study showed that anxiety and depression affected asthma control. Thus, in clinical practice, the psychological assessment could be included in asthmatic adolescents’ asthma work-up. (www.actabiomedica.it)
Keywords: asthma, adolescents, anxiety, depression, asthma control, lung function, treatment
Introduction
Anxiety and depression may affect adolescents who have asthma (1). Also, their parents frequently report anxious and depressive symptoms (2). It has been evidenced that asthmatics caregivers are more anxious and depressed than caregivers of healthy subjects (3). As a result, asthma course could be significantly affected by adolescents’ emotional problems and their parents. In this regard, the adolescent experiences new behaviors, including the definition of his/her identity and personality. So, the adolescent maturates personal experience with plenty of new emotions. The illness may per se significantly and negatively influence the psychological evolutive process. Consistently, asthma severity, asthma control, and asthma medications are closely linked with anxiety and depression in adolescents (3). Adolescence asthma could represent a particular asthma phenotype that deserves adequate identification and management (4). Asthmatic adolescents may have problems concerning the acceptance of being asthmatic, the perception of symptoms, the compliance and the adherence to the prescribed treatment, the self-management of asthma, and mainly the decision to take reliever drugs (5).
On the other hand, asthmatic patients should attain and maintain control of asthma (6). Few asthmatics have, unfortunately, well-controlled asthma (7). Unsatisfactory asthma control may recognize different causes, including emotional disorders. A study conducted on asthmatic adults revealed that uncontrolled asthma was frequently associated with anxiety and depression, assessed by the hospital depression and anxiety scale (HADS) questionnaire (8). A further study explored this issue in adolescents with asthma using HADS (9). The outcomes confirmed that controlled asthma was associated with low HADS scores for both anxiety and depression.
However, many other questionnaires are available to assess emotional problems, including the Childhood Anxiety Sensitivity Index (CASI) for anxiety (10) and Children’s Depression Inventory (CDI) for depression (11). Therefore, the current study aimed to compare emotional aspects, using these questionnaires, with asthma control and lung function in asthmatic adolescents.
Methods
Study Design
This real-life cross-sectional study consecutively enrolled adolescents who were newly diagnosed with asthma at the third-level pediatric clinic of the Policlinico San Matteo of Pavia (Italy).
Inclusion criteria were: age between 12 and 18 years, both genders, and asthma diagnosis. Exclusion criteria were: current respiratory infections, severe chronic disorders (e.g., metabolic disorders, autoimmunity, neuropsychiatric diseases, cancer), and use of medications, including immune-suppressants, psychiatric drugs, chemotherapy, able to interfere with the interpretation of the results.
The internal ethics review Committee obtained approval for the study (code number: 22253/2017; in the Italian Project “ControL’Asma” promoted by the Italian Society of Paediatric Allergy and Immunology). Both the parents signed informed consent.
Asthma diagnosis was confirmed according to the 2018 GINA document (6). The asthma symptom control was measured according to the GINA guidelines (6).
Allergy was defined by a positive skin prick test and consistent history.
Anxiety and depression were evaluated in adolescents using specific questionnaires (CASI and CDI) below described.
Inhaled corticosteroid (ICS) dosage was assessed according to with GINA document.
Lung function assessment
Spirometry was performed using a computer-assisted spirometer (Pulmolab 435-spiro 235, Morgan, England – predictive values ECCS 1993), with an optoelectronic whirl flow meter. According to guidelines, this spirometer fulfills the ATS/ERS standards, and it was performed as stated by the European Respiratory Society (12).
Asthma control level
Asthma control level was assessed according to the GINA criteria: patients were classified as having: well-controlled, partly controlled, or uncontrolled asthma (6). Briefly, the assessment of symptom control considered four questions concerning the past four weeks: daytime symptoms more than twice/week, any night waking due to asthma, reliever needed more than twice/week, and any activity limitation due to asthma (6).
Asthma Control Test
ACT questionnaire consisted of 5 questions with five possible responses, exploring the patient’s perception of his/her asthma control (13). The result could range between 0 and 25, where 25 is the optimal asthma control. The adolescents were stratified according with the ACT-based control: 25 = well control; 20–24 = partly controlled; <20 uncontrolled.
Allergic Rhinitis
Rhinitis was considered if there was a history of typical nasal symptoms, such as itching, sneezing, watery rhinorrhea, and nasal obstruction apart from common cold, consistent with sensitization.
Allergy
If the symptom occurrence was consistent with the exposure to sensitizing allergen, allergic rhinitis was diagnosed. The tested allergens include house dust mites, cat, dog, Alternaria, Aspergillus, Cladosporium, Phleum pratense, Parietaria Judaica, and birch hazelnut tree, olive tree, cypress, and ragweed.
Visual analog scale (VAS)
The VAS consisted of one ruler asking for asthma symptoms perception (14). VAS was performed both by adolescents and doctors. Adolescents assessed their perception of symptom severity; doctors evaluated their perception of asthma control.
The VAS was a 10-cm vertical line on which 0 implied the most severe respiratory symptoms or worst asthma control, while 10 corresponded to no respiratory symptom or optimal control for the patients or doctors, respectively. With a movable marker, the subject could mark any point on the 10-cm segment, which best described his/her perception. No interval marker was visible on the line. A value >6 was considered normal (14).
Questionnaires
CASI: there are 18 items exploring anxiety [10]. The CASI-3 sub-scales are scored as follows: physical concerns = sum of items 3, 4, 7, 8,12, 15; cognitive concerns = sum of items 2, 5, 10, 14, 16, 18; social concerns = sum of items 1, 6, 9, 11, 13, 17. Each CASI item is presented with a five-phrase answer format varying from “Very Little” to “Very Much.” The respondent chooses the one phrase that best represents the extent to which he/she agrees with the item. Each item is scored on a 0 to 4 -point scale: very little (scored 0), a little (1), some (2), much (3), and very much (4). The total score is the sum of all 18 items. The individual’s ASI score is the sum of the points for all sixteen items. The lowest possible ASI score is 0, and the highest is 64. The total ASI score is used for comparison with norms. A cut-off value > 35 identifies the most anxious subjects.
CDI: there are 27 items quantifying symptoms such as depressed mood, hedonic capacity, vegetative functions, self-evaluation, and interpersonal behaviors (11). Each item consists of three statements graded in order of increasing severity from 0 to 2; adolescents selected the one that characterized their symptoms best during the past two weeks. The item scores are combined into a total depression score, which ranges from 0 to 54. A higher CDI score means a higher depressive state. A cut-off value > 20 identifies more depressed subjects.
Both questionnaires were administered during the visit.
Statistical Analysis
Descriptive statistics of the study patients were firstly calculated; qualitative data were reported in terms of absolute frequencies and percentages; quantitative data were reported in terms of medians, first and third quartiles (1st – 3rd q). The normality of distributions was evaluated using the Shapiro-Wilk test. A comparison of frequencies was performed by the Chi-square test or Fisher’s Exact test (in case of expected frequencies less than 5). Quantitative data of three groups were analyzed using the non-parametric Analysis of Variance (Kruskal-Wallis test). Correlations were calculated using the Spearman test. All statistical tests were 2-sided, and a P value less than 0.05 was considered statistically significant. All the analyses were performed using GraphPad Prism version 8.4.0 for Mac OS X, GraphPad Software, San Diego, California USA, www.graphpad.com.
Results
This real-life cross-sectional study included 87 consecutive adolescents (60 males, 27 females, median age 14.2 years), as reported in Table 1. Only three subjects were excluded as suffering from diabetes mellitus Type I, cancer, and epilepsy. All adolescents completed the questionnaires.
Table 1.
Description of the study patients (n=87).
| N. (%) | |
|---|---|
| Gender: Male | 60/87 (69 %) |
| Female | 27/87 (31 %) |
| Age (years) | 14.2 [13.4 – 17.6] |
| VAS for asthma symptoms (patient) | 8 [8 – 9] |
| VAS for asthma control (physician) | 9 [8 – 9.5] |
| FVC (% pred.) | 101 [91.5 – 108.5] |
| FEV1 (% pred.) | 98 [91 – 108] |
| FEV1/FVC | 84.4 [80 – 89.7] |
| FEF 25–75 (% pred.) | 92 [77.5 – 106.5] |
| Bronchial obstruction (FEV1-< 80% pred.), N/n (%) | 8/87 (9.1 %) |
| Allergic Rhinitis, n/N (%) | 64/87 (73.5 %) |
| Allergy, n/N (%) | 83/87 (95.4 %) |
| GINA-based Asthma Control level, N/n (%): | |
| Well controlled | 48/87 (55.2 %) |
| Partly controlled | 30/87 (34.5 %) |
| Uncontrolled | 9/87 (10.3 %) |
| Asthma Control Test (ACT) score | 23 [22 – 24] |
| ACT-based asthma control level N/n (%): | |
| Well controlled (ACT = 25) | 15/87 (17.2 %) |
| Partly controlled (ACT 20–24) | 61/87 (70.1 %) |
| Uncontrolled (ACT < 20) | 11/87 (12.7 %) |
| High dose ICS | 5/87 (5.7 %) |
| CDI score | 7 [4 – 11] |
| CDI (>20), N/n (%) | 10/87 (11.5 %) |
| CASI score | 29 [25 – 33] |
| CASI (≥35), N/n (%) | 14/87 (16.1 %) |
Figures represent median values (unless otherwise specified) and figures in squared parentheses represent 1st and 3rd quartiles; figures in round parentheses represent percentages. N = total number of patients, n = percentage of patients.
The median VAS score was 8 in adolescents, and 9 in doctors. Lung function parameters are reported in Table 1; bronchial obstruction, such as FEV1 <80% of predicted, was observed in 8 (9.1%) subjects. Allergic rhinitis was diagnosed in 64 (73.5%) adolescents, allergy in 83 (95.4%).
The GINA-based asthma control grade was well-controlled in 48 (55.2%) subjects, partly controlled in 30 (34.5%), and uncontrolled in 9 (10.3%). The median ACT score was 23; the ACT-based asthma control grade was well-controlled in 15 (17.2%) subjects, partly controlled in 61 (70.1%), and uncontrolled in 11 (12.7%). High dose ICS was assumed by 5 (5.7%) subjects.
The median CDI score was 7; 10 (11.5%) had a value ≥ 20.
The median CASI score was 29; 14 (16.1%) had a value ≥ 35.
The adolescents were then stratified considering the GINA-based asthma control level in 3 sub-groups (Table 2). There were significant differences among sub-groups concerning: allergic rhinitis comorbidity (p=0.0045), adolescents’ and doctors’ VAS (p=0.002 and 0.01, respectively), presence of bronchial obstruction (p=0.0005), FEF25-75 (p=0.034), ACT score and ACT-based asthma control level (p<0.0001, 0.0006, <0.0001, and <0.0001, respectively), CDI score (p=0.013), and CASI ≥ 35 (p=0.043).
Table 2.
Comparison among the three groups of patients: well-controlled, partially controlled, and uncontrolled according to GINA guidelines.
| Asthma control (GINA) | ||||
|---|---|---|---|---|
| Well controlled [N = 48] | Partly controlled [N = 30] | Uncontrolled [N = 9] | ##P | |
| Gender: Male, n/N (%) | 33/48 (68.7 %) | 22/30 (73.3 %) | 5/9 (55.5 %) | 0.59 |
| Age (years) | 14.8 [13 – 17] | 14.5 [13.5 – 17.3] | 14 [12.7 – 17.1] | 0.85 |
| Allergic Rhinitis, n/N (%) | 37/48 (77 %) | 19/30 (63.3 %) | 8/9 (88.9 %) | 0.0045 # |
| Allergy, n/N (%) | 46/48 (95.8 %) | 28/30 (93.3 %) | 9/9 (100 %) | 0.68 # |
| VAS for asthma symptoms (patient) | 9 [8 – 10] | 8 [8 – 8] | 7 [7 – 8] | 0.002 |
| VAS for asthma control (physician) | 9 [8.7 – 10] | 9 [8 – 9] | 8 [7 – 9] | 0.01 |
| FVC - at baseline (% pred.) | 101.5 [93.7 – 109.5] | 101.5 [91.2 – 108.7] | 95 [82 – 96] | 0.13 |
| FEV1- at baseline (% pred.) | 97.5 [91.7 – 107.2] | 101.5 [93.5 – 111.7] | 90 [72 – 100] | 0.05 |
| Bronchial obstruction (FEV1-< 80 %pred.), n/N (%) |
3/48 (6.2 %) | 1/30 (3.3 %) | 4/9 (44.4 %) | 0.0005 # |
| FEV1/FVC - at baseline | 83.4 [79.9 – 87.8] | 87.6 [84.1 – 90.9] | 83.7 [70 – 90.8] | 0.10 |
| FEF 25–75 - at baseline (% pred.) | 89 [75.2 – 99.2] | 99 [84.4 – 113.7] | 87 [42 – 97] | 0.034 |
| Asthma Control Test (ACT) score | 24 [23 – 25] | 22 [21 – 22] | 16 [15 – 17] | < 0.0001 |
| ACT-based asthma control level n/N (%): | ||||
| Well controlled (ACT = 25) | 15/48 (31.2 %) | 0/30 (0 %) | 0/9 (0 %) | 0.0006 # |
| Partly controlled (ACT 20–24) | 33/48 (68.8 %) | 27/30 (90%) | 1/9 (11.1 %) | < 0.0001 # |
| Uncontrolled (ACT < 20) | 0/48 (0 %) | 3/30 (10 %) | 8/9 (88.9 %) | < 0.0001 # |
| High dose ICS | 2/48 (4.1 %) | 2/30 (6.6 %) | 1/9 (11.1 %) | 0.68 # |
| CDI score | 5 [4 – 9] | 7 [4 – 9] | 10 [7.5 – 14] * | 0.013 |
| CDI ≥20, n/N (%) | 6/48 (12.5 %) | 3/30 (10 %) | 1/9 (11.2 %) | 0.9 # |
| CASI score | 29 [25 – 32.2] | 30 [25.2 – 32.7] | 29 [27 – 37] | 0.59 |
| CASI (≥35), n/N (%) | 7/48 (14.6 %) | 3/30 (10 %) | 4/9 (44.4 %) | 0.043 # |
Figures represent median values (unless otherwise specified) and figures in squared parentheses represent 1st and 3rd quartiles; figures in round parentheses represent column percentages; # P: Chi-square test; ##P: Kruskal-Wallis test. VAS: the higher the score less severe is the symptom. Post-hoc analysis: * well-controlled vs uncontrolled (p<0.01); partly-controlled vs uncontrolled (p<0.01).
There was mild correlation between CASI and CDI (r=0.32); CASI and ACT (r= -0.24); CDI and ACT (r= -0.27), as reported in Figure 1. Adolescents with CASI ≥ 35 showed mild correlations between CASI and VAS (r= 0.24); CASI and ACT (r= 0.32); CASI and FEF25-75 (r= -0.20), as reported in Figure 2.
Figure 1.
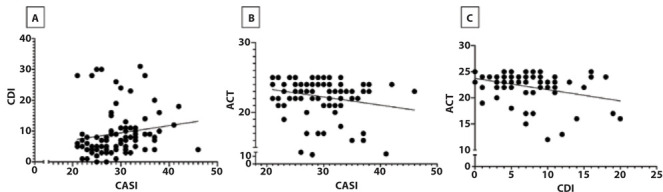
A = Relationship between CASI and CDI; B = relationship between CASI and ACT; C = relationship between CDI and ACT.
Figure 2.
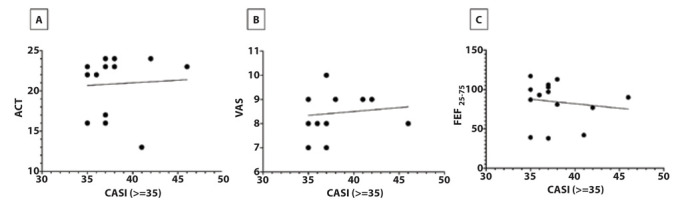
A = Relationship between CASI and ACT; B = relationship between CASI and VAS; C = relationship between CASI and FEF25–75. In adolescents with CASI level ≥ 35.
Discussion
The current study investigated the clinical relevance of emotional problems on asthma control in a group of asthmatic adolescents visited at a third-level hospital.
There was a clear predominance of males, consistently with other studies (9,15). Uncontrolled asthma affected more than 10% of the visited adolescents, and also partly controlled asthma was observed in about 1/3 of patients. Consistently, an overt bronchial obstruction was documented in approximately 10% of subjects. This finding underlines the concept that asthma control is a demanding challenge in clinical practice. On the other hand, the perception of asthma symptoms was optimistic in the vast majority of patients and the perception of good asthma control by the doctors. This discrepancy is usual as the subjective perception conveys the influence of many influencing factors, including emotional aspects, illness past, sociocultural issues.
This study evidenced that emotional impairment, mainly anxiety, was present in some adolescents with asthma, and there was an association with asthma symptom control. In detail, anxiety, evaluated by the CASI questionnaire, amounted to 16% and depression, considered by the CDI questionnaire, to 11.5%. However, these numbers seem to be underestimated if compared with our previous study that reported an anxiety prevalence of 43% and depression prevalence of 18% in asthmatic adolescents (9). This difference, particularly remarkable for anxiety, could depend on the different abilities provided by the different questionnaires in intercepting symptomatic patients. HADS seems to be more reliable than CASI and CDI in detecting emotional problems in asthmatic adolescents. Also, HADS allows us to consider both anxiety and depression contextually.
Moreover, the present study confirmed the relevance of allergic rhinitis comorbidity as a potential risk factor associated with uncontrolled asthma. The nose-bronchi axis is widely recognized, and upper airways diseases impact asthma history significantly. In this regard, nasal obstruction is the key symptom involved in asthma worsening as it entails the impaired nasal function, such as conditioning the inspired air (16). Also, FEF25-75 was significantly different in the asthma control sub-groups to the detriment of other spirometry parameters. FEF25-75 is an early marker of bronchial impairment in patients with rhinitis and asthma worsening in symptomless asthmatics (17).
The current study confirmed the association between emotional disorders and asthma control. Adolescents with anxiety and/or depression had more frequently uncontrolled asthma.
This outcome is recognized in many documents, but very few studies addressed this issue. Sundom and colleagues reported that young asthmatics reporting anxiety and depression had poor asthma control (18). Howell demonstrated that suboptimal quality of life, mainly concerning emotional aspects, depended on poor asthma control in children and adolescents with asthma (19).
The Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric asthma study showed that reported outcomes, including anxiety and depression, were correlated with worsened social aspects in children with asthma (20). Moreover, Inner-city Black adolescents with poorly controlled asthma had a high prevalence of anxiety (21). Another study demonstrated that adolescents with asthma-related anxiety, measured by a specific questionnaire, had poor asthma control assessed by the intensity of asthma care (22). In Asian adolescents with asthma, poorly controlled asthma was associated with psychiatric comorbidity, including anxiety, depression, neuroticism, and perceived stress (23). Consistently, children and adolescents with well-controlled asthma had a low risk of anxiety, depression, and poor self-esteem (24). Therefore, the current findings confirmed the outcomes reported in the literature. There is convincing evidence that emotional disorders exert essential effects on asthmatic adolescents. The current study provided corroborating findings consistent with the concept that anxiety and depression are associated with poor-controlled asthma in adolescents.
However, the present study has some limitations, including the cross-sectional design, the lack of a control group, and psychological questionnaires alone. In particular, the study’s cross-sectional nature did not allow to establish the direction of causality between clinical outcomes and psychological variables. Moreover, some confounding factors, including parental education, parental beliefs about medication necessity, environmental triggers, adherence to treatment, and the family’s socioeconomic status, which could affect the current results, were not adequately considered in the present study. In this regard, a study investigating most of these variables is ongoing.
However, the current outcomes could be interpreted from another perspective, such as poor-controlled asthma per se could induce anxiety and depression. It is well known that chronic and severe disorders cause emotional distress or worsen pre-existing anxiety and depression. In other words, a vicious circle.
In conclusion, the current study showed that anxiety and depression were common in adolescents who have asthma. Emotional disorders significantly affected asthma control and could induce airflow impairment. Thus, in clinical practice, the psychological assessment could be included in asthmatic adolescents’ asthma work-up.
Conflicts of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Vila G, Nollet-Clemencon C, de Blic J, Falissard B, Mouren-Simeoni MC, Scheinmann P. Assessment of anxiety disorders in asthmatic children. Psychosomatics. 1999;40(5):404–413. doi: 10.1016/S0033-3182(99)71205-3. doi: 10.1016/S0033-3182(99)71205-3. [DOI] [PubMed] [Google Scholar]
- Brew BK, Lundholm C, Gong T, Larsson H, Almqvist C. The familial aggregation of atopic diseases and depression or anxiety in children. Clin Exp Allergy. 2018;48(6):703–711. doi: 10.1111/cea.13127. doi: 10.1111/cea.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter G, Sharpe L, Hunt CJ. Systematic review and meta-analysis of anxious and depressive symptoms in caregivers of children with asthma. J Ped Psychol. 2015;40(7):623–632. doi: 10.1093/jpepsy/jsv012. doi: 10.1093/jpepsy/jsv012. [DOI] [PubMed] [Google Scholar]
- Holley S, Walker D, Knibb R, et al. Barriers and facilitators to self-management of asthma in adolescents: an interview study to inform development of a novel intervention. Clin Exp Allergy. 2018;48(8):944–956. doi: 10.1111/cea.13141. doi: 10.1111/cea.13141. [DOI] [PubMed] [Google Scholar]
- Sundbom F, Malinovschi A, Lindberg E, Alving K, Janson C. Effects of poor asthma control, insomnia, anxiety, and depression on quality of life in young asthmatics. J Asthma. 2016;53(4):398–403. doi: 10.3109/02770903.2015.1126846. doi: 10.3109/02770903.2015.1126846. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma. GINA guidelines. Global Strategy for Asthma Management and Prevention. 2019 Available at: htpp://www.ginasthma.org/ . Last accessed on September 2020. [Google Scholar]
- Licari A, Marseglia GL, Tosca MA, Ciprandi G. Asthma control in children and adolescents: a study in clinical practice. J Asthma. 2020;57(6):645–647. doi: 10.1080/02770903.2019.1594889. doi: 10.1080/02770903.2019.1594889. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Schiavetti I, Riciardolo F. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol. 2015;115(5):408–414. doi: 10.1016/j.anai.2015.08.007. doi: 10.1016/j.anai.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Licari A, Ciprandi R, Marseglia G, Ciprandi G. Anxiety and depression in adolescents with asthma and their parents: a study in clinical practice. Monaldi Archives for Chest Diseases. 2019;89(3) doi: 10.4081/monaldi.2019.1063. doi: 10.4081/monaldi.2019.1063. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Fleisig W, Rabian B, Peterson RA. Childhood anxiety sensitivity index. J Clin Child Psychol. 1991;20:162–168. [Google Scholar]
- Bang YR, Park JH, Kim SH. Cut-off scores of the children’s depression inventory for screening and rating severity in Korean adolescents. Psychiatry Inv. 2015;12(1):23–8. doi: 10.4306/pi.2015.12.1.23. doi: 10.4306/pi.2015.12.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey form assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Tosca MA, Silvestri M, Olcese R, Pistorio A, Rossi GA, Ciprandi G. Breathlessness perception assessed by the visual analog scale and lung function in children with asthma: a real-life study. Ped Allergy Immunol. 2012;23(6):537–542. doi: 10.1111/j.1399-3038.2012.01316.x. doi: 10.1111/j.1399-3038.2012.01316.x. [DOI] [PubMed] [Google Scholar]
- Delmas MC, Guignon N, Chee CC, Fuhrman C, Herbet JB, Gonzalez L. Asthma and major depressive episode in adolescents in France. J Asthma. 2011;48(6):640–646. doi: 10.3109/02770903.2011.585410. doi: 10.3109/02770903.2011.585410. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Cirillo I, Klersy C, Marseglia GL, Caimmi D, Vizzaccaro A. Nasal obstruction is the key symptom in hay fever patients. Otolaryngol Head Neck Surg. 2005;133(3):429–435. doi: 10.1016/j.otohns.2005.05.049. doi: 10.1016/j.otohns.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Cirillo I, Klersy C, et al. Role of FEF25-75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am J Rhinol. 2006;20(6):641–647. doi: 10.2500/ajr.2006.20.2914. doi: 10.2500/ajr.2006.20.2914. [DOI] [PubMed] [Google Scholar]
- Sundbom F, Malinovschi A, Lindberg E, Alving K, Janson C. Effects of poor asthma control, insomnia, anxiety, and depression on quality of life in young asthmatics. J Asthma. 2016;53(4):398–403. doi: 10.3109/02770903.2015.1126846. doi: 10.3109/02770903.2015.1126846. [DOI] [PubMed] [Google Scholar]
- Howell CR, Lindsay AT, Gross HE, Reeve BB, Huang SW, DeWalt DA, et al. Association of consistently suboptimal quality of life with consistently poor asthma control in children with asthma. Ann Allergy Asthma Immunol. 2017;119(6):562–564.e1. doi: 10.1016/j.anai.2017.09.053. doi: 10.1016/j.anai.2017.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CMJ, DeWalt DA, Huang IC. Impaired patient-reported outcomes predict poor school functioning and daytime sleepiness: the PROMIS Pediatric Asthma Study. Acta Pediatr. 2017;17(8):850–854. doi: 10.1016/j.acap.2017.07.010. doi: 10.1016/j.acap.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams MR, Bruce AC, Fitzpatrick AM. Anxiety contributes to poorer asthma outcomes in inner-city Black adolescents. J Allergy Clin Immunol Pract. 2018;6(1):227–235. doi: 10.1016/j.jaip.2017.06.034. doi: 10.1016/j.jaip.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzese JM, Reigada LC, Lamm A, et al. The association of youth and caregiver anxiety and asthma care among urban young adolescents. Acad Pediatr. 2016;16(8):792–798. doi: 10.1016/j.acap.2016.03.009. doi: 10.1016/j.acap.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ho R, Li TK, et al. Psychiatric comorbidities in Asian adolescents asthma patients and the contributions of neuroticism and perceived stress. J Adolesc Health. 2014;55(2):267–275. doi: 10.1016/j.jadohealth.2014.01.007. doi: 10.1016/j.jadohealth.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Letitre SL, de Groot EP, Draaisma E, Brand PLP. Anxiety, depression, and self-esteem in children with well-controlled asthma: case-control study. Arch Dis Child. 2014;99(8):744–8. doi: 10.1136/archdischild-2013-305396. doi: 10.1136/archdischild-2013-305396. [DOI] [PubMed] [Google Scholar]


