Abstract
Objective:
We performed a systematic review and meta-analysis for exploring clinical benefits and safety of tocilizumab in addition to standard of care (SOC) in treating patients with coronavirus disease 2019 (COVID-19).
Methods:
An electronic search was carried out in PubMed, EMBASE, Cochrane Library, and Science Direct, as well as in medRxiv preprint server, to identify eligible studies. Only randomized Controlled Trials (RCTs) that compared mortality events and/or adverse events between a tocilizumab + SOC group and a SOC-only control group were included. The primary outcome was 28-day mortality. Secondary outcomes include progression to severe disease, defined as need for mechanical ventilation (MV) or intensive care unit (ICU) admission, and adverse events (AE).
Results:
A total of nine studies (6,490 participants) could be included in this meta-analysis, with 3,358 participants in the tocilizumab + SOC group and 3,132 participants in the SOC-only group. The overall mortality rate was lower in the tocilizumab group compared to the SOC-only group, though the difference was not statistically significant (odds ratio [OR], 0.87; 95% CI, 0.73-1.04; I2, 15%). This finding was unaffected by subgroup analyses based on initial use of steroids or mechanical ventilation at baseline. Patients receiving tocilizumab were 26% less likely to progress to MV, and this difference was statistically significant (OR, 0.74; 95% CI, 0.64-0.86; I2, 0%). Among patients who were not in ICU at randomization, the tocilizumab group had 34 % lower rate of ICU admission compared to the SOC-only group (OR, 0.66; 95% CI, 0.40-2.14; I2, 29%). The occurrence of serious infections was lower in the tocilizumab group (OR, 0.57; 95% CI, 0.36-0.89; I2, 21%).
Conclusion:
Tocilizumab is generally well-tolerated in COVID-19. Although this drug does not appear to have a significant benefits on survival, it may have a role in preventing progression to intensive care and MV. (www.actabiomedica.it)
Keywords: Covid-19, SARS-COV-2, Tocilizumab, IL-6 inhibitor, Mortality
Introduction
Presentations of coronavirus disease 2019 (COVID-19) range from asymptomatic to severe pneumonia with respiratory failure (1). While the majority of people with COVID-19 experience a mild and uncomplicated course, approximately 10-15% develop moderate or severe disease necessitating hospitalization and oxygen supplementation, and 3-5% require intensive care unit (ICU) admission (2).
Severe COVID-19 is primarily characterized by Acute Respiratory Distress Syndrome (ARDS), but can then evolve into a systemic disease, with considerably high risk of developing multiple organ failure (MOF). Although the pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is complex and only partly understood, it is thought to involve a dysregulated host immune response resulting in a hyperinflammatory state and immune-mediated thrombosis and organ damage. Several immunosuppressive drugs have been investigated for use in severe COVID-19, but many have been unsuccessful.
Interleukin 6 (IL-6) is a pleiotropic, pro-inflammatory cytokine. Elevated IL-6 levels have been observed in patients with severe COVID-19, thus characterizing the so-called cytokine storm that develop in a large number of patients with severe/critical illness (3). Tocilizumab is a monoclonal antibody direct against the IL-6 receptor, thus effective to block downstream IL-6 signaling. This drug, which has been widely used for rheumatoid arthritis and giant cell arteritis, has been approved by the Food and Drug Administration (FDA) for use in severe COVID-19. However, despite the results of several trials and meta-analyses have been published, no definite conclusion has hitherto been reached as to the efficacy and tolerability of tocilizumab (4–6). Furthermore, new randomized controlled trials (RCTs) have been published after the earlier meta-analyses. Here we present an updated meta-analysis of RCTs on tocilizumab for COVID-19, specifically its effects on preventing mortality and the need for mechanical ventilation (MV), as well as its adverse effect profile. In addition, the current analysis offers meta-regression and subgroup analysis that did not feature in previous published meta-analyses.
Methods
Study protocol and registration
The present meta-analysis was conducted in strict conformity with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (7). The protocol for this study has been reported in the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42021266563).
Definitions
Severe COVID-19 defined as patients with respiratory rate ≥30 breaths/min, or peripheral capillary oxygen saturation (SpO2) ≤93% or PaO2/FiO2 ≤ 300 mmHg, or combination of these findings. Critical COVID-19 described as patients with confirmed COVID-19 who ICU management due to mechanical ventilation.
Literature search strategy
A systematic search of literature up to May 2021 was conducted on the electronic databases PubMed, EMBASE, Cochrane library, Preprint server of medRxiv and Science Direct to identify studies eligible for inclusion. The search terms used included, but were not limited to, “Tocilizumab and covid-19”, “Anti-interleukin 6 antibody and covid-19”, “Tocilizumab and coronavirus 2019”, “Tocilizumab and SARS-CoV-2”, and “Anti-interleukin 6 antibody and coronavirus 2019.” No language restriction or date limit was set. Using references from included articles, a manual search was also performed to identify additional eligible studies.
Eligibility Criteria
Studies were screened and assessed for eligibility by two independent reviewers (VM, I.C). The search results were screened by title and abstract and those of potential relevance evaluated by full text. Studies were considered eligible for inclusion when fulfilling the following criteria: (1) reported clear extractable data on study outcome of mortality; (2) compared tocilizumab plus standard of care (SOC) to SOC (control); (3) RCT study designs, either blinded or open-label; (4) the subjects were patients with molecularly confirmed SARS-CoV-2 infection.
Studies were excluded if: (1) there was any intervention beyond tocilizumab that was given only to one of either groups, or (2) they reported the effects of tocilizumab on mortality with unclear data collection time points. Any disagreements between investigators arising during the eligibility assessment were settled through a consensus.
Data Extraction and Quality Assessment
Data extraction was conducted by two independent reviewers (V.M, I.C). The following information was extracted for each study: the surname of the first author and the year of publication, the geographical area where the study was conducted, baseline demographic characteristics, sample size, and number of events (mortality, MV, ICU admission and adverse events) in the tocilizumab and SOC-only groups respectively.
Quality assessment and analysis of risk of bias for all selected full-text articles was performed using the Cochrane risk-of-bias tool. The risk of bias evaluation evaluations for the included studies is presented as Figure 2.
Figure 2.
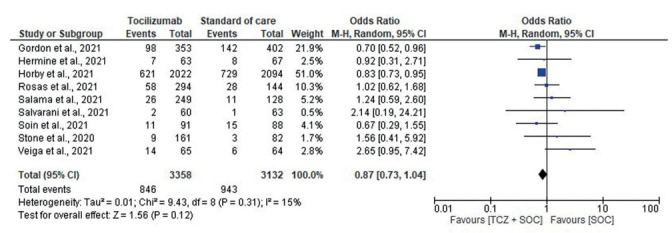
Forest plot for mortality between Tocilizumab and SOC group.
Outcomes of Interest
The primary outcome were mortality events in the tocilizumab + SOC group versus the SOC only group. Secondary outcomes were the progression to MV within 28 days, ICU admissions and the number of adverse events. To account for all of these aspects separately, four different meta-analyses were performed.
Statistical Analysis
Data was meta-analyzed using a random-effects model using the DerSimonian and Laird method (8) with RevMan version 5.3 (Copenhegen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We assessed heterogeneity between studies by estimation of the I2 statistic, where an I2 greater than 60% was considered to indicate substantial heterogeneity (9). A random effects meta-regression using log ORs was performed to evaluate the impact of covariates (baseline characteristics and other treatments) on the association between treatment and mortality. Univariate meta-regression and leave-one-out meta-analysis was performed with Open Meta-Analyst statistical software (Providence, RI: CESH, Brown University).
Results
Study Identification
The initial search produced 890 articles. Following removal of duplicates and primary screening, 18 articles were assessed by full-text for eligibility in the meta-analysis. Of these, 9 were excluded because they failed to match the primary and secondary outcome of this review. Thus, a final number of 9 articles could be included in this systematic review and meta-analysis (Fig. 1)
Figure 1.
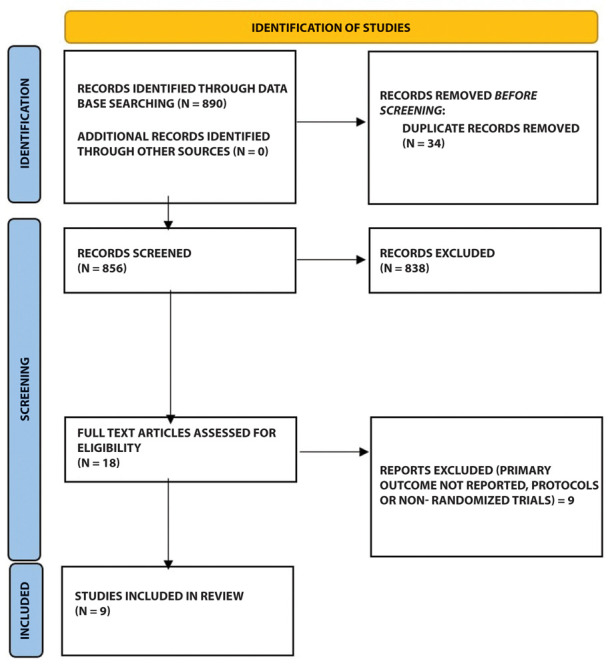
Flow of studies through the meta-analysis.
Characteristics of included studies
All RCTs included in the analysis involved hospitalized patients with COVID-19 and were multicenter in design. The geographical distribution of the studies was wide, with 6 exclusively conducted in one country, while 3 were multinational investigations. Six trials had open-label design and three were double-blinded. A total of 9 studies were included, totaling 6490 participants. Of these, 3358 (51.74%) were assigned to the tocilizumab group and 3132 (48.26%) were assigned to the SOC-only. A total of 810 (24.1%) deaths out of 3358 participants were reported in the tocilizumab group compared to 926/3132 (29.6%) in the SOC-only group. Only one RCT used a single dose of tocilizumab, whilst the other 8 RCTs allowed a second dose if needed. Baseline characteristics were generally similar across the tocilizumab group and SOC-only group. The characteristics of the study populations varied and are summarized in Table 1 and 2.
Table 1.
Study design characteristics and inclusion criteria for randomized controlled studies included in the meta-analysis
| Author | Country | Study design | SOC treatment | Tcz regimen | Recruitment | Inclusion criteria |
|---|---|---|---|---|---|---|
| (Salvarani et al., 2021) | Italy | Open-label | H,A,S,L/R or D/C or D/R, Heparin | Two doses. 2nd dose 12 hrs later | NS | Covid-19 positive, fever, PaO2/FiO2 between 200 and 300, and/or CRP levels of >10mg/dL and/or CRP level increases to at least twice the admission value |
| (Salama et al., 2021) | USA, Africa | Double-blind | R*, S | Single dose with possibility of 2nd dose 8-24 hrs later | Within 48 hrs of hospital admission | Confirmed Covid-19 with radiologic features SpO2 <94% on room air |
| (Hermine et al., 2021) | France | Open-label | H,A,S,L/R OR R*, anticoagulants | Single dose with possibility of 2nd dose 48 hrs later | Within 72 hrs of COVID-19 diagnosis | Covid-19 positive and/or CT chest findings Moderate, severe or clinical pneumonia O2 >3L/min WHO-CPS score ≥ 5 |
| (Rosas et al., 2021) | Europe & North America | Double-blind | S, antivirals, convalescent plasma | Single dose with possibility of 2nd dose 8-24 hrs after 1st dose | NS | Covid-19 positive CXR or CT findings PaO2/FiO2 <300 and/or SpO2 <93% on room air |
| (Soin et al., 2021) | India | Open-label | S, R* | Single dose with possibility of 2nd dose 12-168 hrs later | NS | Confirmed Covid-19 Respiratory rate >24/min and SpO2 <95% on room air, or septic shock or ARDS |
| (Stone et al., 2020) | USA | Double-blind | H,S, antivirals | Single dose | Upon hospital admission | Covid-19 positive, fever Pulmonary infiltrates or need for supplemental oxygen. At least one of the following labs: CRP >50 mg/L, ferritin >500 ng/ml, D-dimer >1000 ng/ml, or LDH >250U/L |
| (Veiga et al., 2021) | Brazil | Open-label | H,A,S, heparin, antibiotics | Single dose | NS | Confirmed severe or critical Covid-19. Receiving supplemental O2 or receiving MV for <24 h before analysis. At least two of following: D-dimer >1000ng/mL, CRP >50 mg/L, ferritin >300 μg/L |
| (Gordon et al., 2021) | UK, USA, France | Open-label | S,H,R* | Single doze with possibility of 2nd 12 to 24 hrs later | Within 24hrs of ICU admission | Confirmed Covid-19, ICU admission or on respiratory or cardiovascular support |
| (Horby et al., 2021) | UK | Open-label | H,A,S,L/R | Single doze with possibility of 2nd doze 12-24 hrs later | Within 21 days of primary randomization | Confirmed SARS-CoV-2 infection, SpO2 <92% on room air or receiving oxygen therapy, CRP ≥ 75mg/L |
H, Hydoxychloroquine; L/R, Lopinavir/Ritonavir;D/C, Darunavir/Covicostat; D/R, Darunavir/Ritonavir; R*, Remdesivir; F, Favipiravir, S, Steroid; A, Azithromycin; IFN, Interferon; TCZ, Tocilizumab; SOC, Standard of care; NS, Not specified.
Table 2.
Baseline patient characteristics and outcome of randomized controlled studies included in the meta-analysis
| Author | Total subjects | Tocilizumab | SOC | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Male n (%) | Age (median (range) or mean ± SD | Mortality n (%) | n | Male n (%) | Age (median (range) or mean ± SD | Mortality n (%) | |||
| (Salvarani et al., 2021) | 126 | 60 | 40 (66.7%) | 61.5 (51.5-73.5) | 2 (3.3%) | 66 | 37 (56%) | 60 (54-69) | 1 (1.5%) | Mortality, ICU admission, progression to MV |
| (Salama et al., 2021) | 377 | 249 | 150 (60%) | 56 ± 14.3 | 26 (10.4%) | 128 | 73 (57%) | 55.6 ± 14.9 | 11 (8.6%) | Mortality, composite outcome (MV or death by day 28) |
| (Hermine et al., 2021) | 130 | 63 | 44 (69.8%) | 64 (57.1-74.3) | 7 (11.1%) | 67 | 44 (65.7%) | 63.3 (57.1-72.3) | 8 (11.9%) | Mortality, proportion needing noninvasive or MV, composite outcome |
| (Rosas et al., 2021) | 438 | 294 | 205 (69.7%) | 60.9 ± 14.6 | 58 (19.7%) | 144 | 101 (70%) | 60.6 ± 13.7 | 28 (19.4%) | Clinical failure (defined as death, initiation of MV or ICU admission) |
| (Soin et al., 2021) | 179 | 91 | 76 (83.5%) | 56 (47-63) | 11 (12.1%) | 88 | 76 (86%) | 54 (43-63) | 15 (17%) | Mortality, incidence of MV up to day 21 |
| (Stone et al., 2020) | 243 | 161 | 96 (59.6%) | 61.1 (46.4-69.7) | 9 (5.6%) | 82 | 45 (54.8) | 56.5 (44.7-67.8) | 3 (3.7%) | Mortality, ICU admission, composite outcome |
| (Veiga et al., 2021) | 129 | 65 | 44 (67.7%) | 57.4 ± 15.7 | 14 (21.5%) | 64 | 44 (69%) | 57.5 ± 13.5 | 6 (9.4%) | Mortality, progression to MV, composite outcome |
| (Gordon et al., 2021) | 755 | 353 | 261 (73.9%) | 61.5 ± 12.5 | 87 (24.6%) | 402 | 283 (70%) | 61.1 ± 12.8 | 160 (39.8%) | Mortality, composite outcome |
| (Horby et al., 2021) | 4116 | 2,022 | 1,335 (66%) | 63.3 ± 13.7 | 596 (29.5) | 2,094 | 1437 (68.6%) | 63.9 ± 13.6 | 694 (33.1%) | Mortality by day 28, composite outcome |
Quality assessment
There was a risk of performance and detection bias due to the open labelled design in six studies. Risk of bias for the included studies is illustrated in Supplementary fig. 1.
Figure 1:
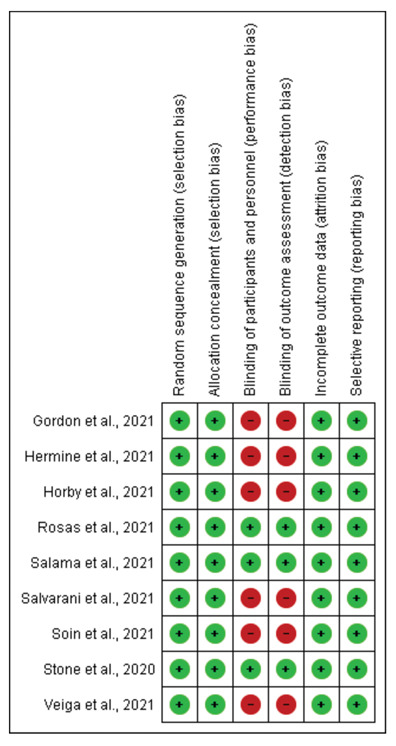
Risk of bias summary
Meta-regression analysis
The results of the meta-regression are summarized as Table 3. The meta-regression showed an effect of azithromycin (coefficient −2.41; p = 0.029) on mortality. Based on the B-coefficient, azithromycin was associated with enhanced risk of death. The analysis showed no significant effect of other covariates on mortality. The mean or median age ranged from 54 to 64 years and men comprised 67.03% (tocilizumab group) and 68.33 % (SOC-only group). Diabetes, hypertension, chronic lung disease and obesity were the common comorbidities reported, none of which seemed to significantly influence mortality. In addition, four studies reported IL-6 baseline levels, which also did not influence overall mortality.
Table 3.
Univariate Meta-regression analysis of covariates (baseline characteristics & treatments) on Mortality rate
| Covariate | No. of Studies | Beta coefficient (95% CI) | SE | R2 | p-value |
|---|---|---|---|---|---|
| Age | 9 | -2.16 (-4.63-0.32) | 1.26 | -0.56 | 0.088 |
| Sex(Male) | 9 | -0.246 (-0.584-0.092) | 0.172 | -0.79 | 0.153 |
| CRP | 8 | 0.20 (-0.16-0.56) | 0.18 | -1.74 | 0.273 |
| D-dimers | 6 | -0.19 (-0.46-0.07) | 0.14 | 0.00 | 0.155 |
| Ferritin | 9 | -0.03 (-0.39-0.34) | 0.19 | -0.33 | 0.890 |
| IL-6 | 4 | 0.04 (-0.33-0.41) | 0.19 | 0.00 | 0.835 |
| LDH | 3 | -0.18 (-10.01- 9.65) | 5.02 | NA | 0.971 |
| Lymphocytes count | 4 | -0.66 (-2.67-1.35) | 1.02 | -1.22 | 0.520 |
| Platelet count | 3 | -1.45 (-4.94-2.04) | 1.78 | NA | 0.415 |
| Patients on other treatments at recruitment: | |||||
| Invasive ventilation | 6 | 0.13 (-0.27-0.52) | 0.20 | -0.18 | 0.534 |
| Steroids | 8 | -0.06 (-0.29-0.16) | 0.12 | -0.04 | 0.584 |
| Antivirals | 8 | -0.06 (-0.41-0.53) | 0.24 | -0.58 | 0.804 |
| Anticoagulants | 3 | 6.458 (-6.14-19.05) | 6.43 | NA | 0.315 |
| Azithromycin | 4 | -2.41 (-4.57 - -0.25) | 1.10 | -2.58 | 0.029* |
| Hydroxychloroquine | 4 | -0.76 (-2.01-0.49) | 0.64 | -0.88 | 0.235 |
| Comorbidities | |||||
| Diabetes | 9 | -0.19 (-0.67-0.30) | 0.25 | -0.12 | 0.447 |
| Hypertension | 6 | -0.30 (-1.24-0.63) | 0.476 | 1.00 | 0.527 |
| Obesity | 5 | 0.26 (-0.03-0.55) | 0.15 | 0.00 | 0.076 |
| Chronic lung disease | 8 | -0.087 (-0.36-0.19) | 0.14 | -0.05 | 0.541 |
Asterisk (*) shows significant P value
Outcomes
Overall Mortality
The overall mortality rate was lower in the tocilizumab group compared to the SOC-only group, but the difference did not reach statistical significance (OR, 0.87; 95% CI, 0.73-1.04; I2, 15%) (Fig. 2). A leave-one-out analysis showed association between tocilizumab and mortality was not influenced by any single study (Supplementary fig. 2). Subgroup analysis based on disease severity, initial use of steroids or MV at randomization/baseline did not show any statistically significant findings.
Figure 2:
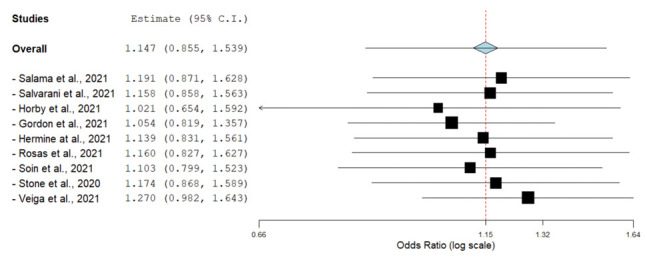
Leave-one-out forest plot
Four studies (10–13) reported mortality in patients with severe or critical COVID-19, four (14–17) in patient with mild to moderate disease, and one (18) in both moderate and severe patients. A total of 799 deaths out of 2784 participants were reported in the tocilizumab group compared to 919/2745 participants in the SOC-only group (OR, 0.84; 95% CI, 0.63-1.12; I2, 57%). In patients with mild or moderate disease, 47 deaths out of 574 participants were reported in the tocilizumab group compared to 24/387 in the SOC-only group (OR, 1.30; 95% CI, 0.77-2.20; I2, 0%) (Supplementary fig. 3).
Figure 3:
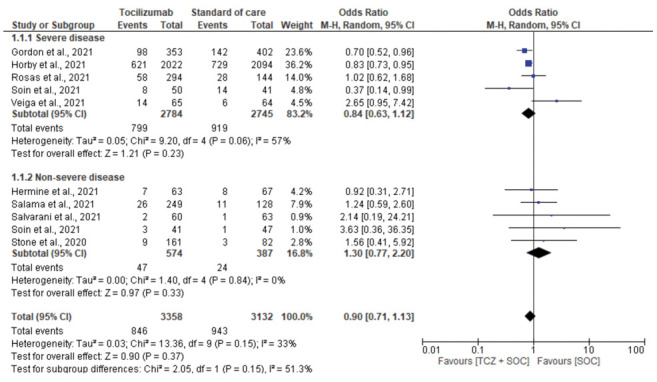
Subgroup analysis for mortality based on severity of disease
A pooled analysis of 5 studies in which over 50% of patients were using steroids at baseline(recruitment) evidenced mortality rates of 27.7% and 32.5% in tocilizumab and SOC-only groups, respectively (OR, 0.87; 95% CI, 0.66-1.13; I2, 46%) (10,11,13,15,18). The pooled analysis of 4 studies in which less than 50% patients were using steroids at baseline evidenced mortality rates of 13.1% and 11.2% in the tocilizumab and SOC-only cohorts, respectively (OR, 1.07; 95% CI, 0.95-1.64; I2, 0%) (Supplementary fig. 4, supplementary Table 1).
Figure 4:
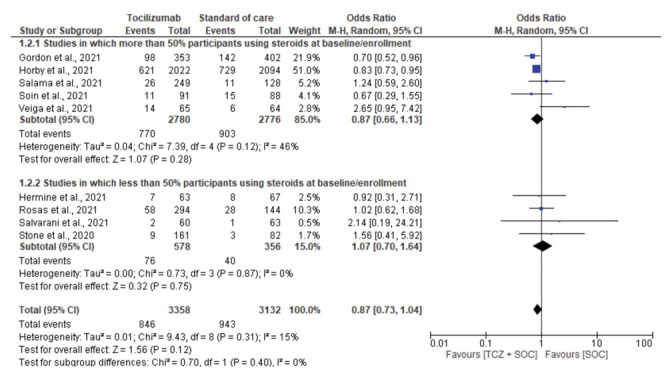
Subgroup analysis for mortality based on percentage of participants on steroid use at enrollment
Table 1.
Summary on use of steroids and mechanical ventilation
| Study | Use of corticosteroids, n (%) | Use of Mechanical ventilation, n (%) | ||
|---|---|---|---|---|
| TCZ | SOC | TCZ | SOC | |
| (Salvarani et al., 2021) | NA | NA | 0 | 0 |
| (Salama et al., 2021) | 200 (80.3) | 112 (87.5) | 0 | 0 |
| (Hermine et al., 2021) | 21 (33) | 41 (61) | 0 | 0 |
| (Rosas et al., 2021) | 57 (19.4) | 41 (28.5) | 111 (37.8) | 54 (37.5) |
| (Soin et al., 2021) | (85) | (89) | 5 (10) | 5 (10) |
| (Stone et al., 2020) | 18 (11) | 5 (6) | 0 | 1 (1) |
| (Veiga et al., 2021) | 45 (69) | 47 (73) | 11 (17) | 10 (16) |
| (Gordon et al., 2021) | 50 (14.2) | 52 (12.9) | 104 (29) | 121 (30) |
| (Horby et al., 2021) | 1664 (82) | 1721 (82) | 268 (13) | 294 (14) |
A pooled analysis of 4 studies which did not include patients undergoing MV at enrollment showed mortality rates of 8.3% and 6.8% in patients in tocilizumab and SOC-only groups, respectively (OR, 1.23; 95% CI, 0.71-2.11; I2, 0%) (14–17). The pooled analysis of 5 studies which included MV patients showed mortality rate of 28.4% and 32.9% among in the patients in the tocilizumab and SOC-only group, respectively (OR, 0.85; 95 CI, 0.67-1.08; I2, 42%) (10–13,18) (Supplementary fig. 5, supplementary Table 1).
Figure 5:
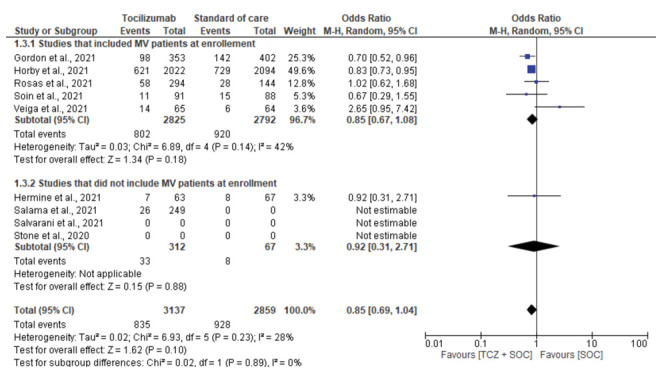
Subgroup analysis for mortality based on use of Mechanical ventilation (MV) at enrollment.
Mechanical Ventilation
Seven studies assessed the need for MV. Patients receiving tocilizumab were at lower risk of progressing to MV, with difference between tocilizumab and SOC-only cohorts achieving statistical significant (OR, 0.74; 95% CI, 0.64-0.86; I2, 0%) [Fig. 3]. Six studies reported the composite outcome of MV or death within 28 days. A 22% lower rate of this composite outcome was observed in the tocilizumab group compared to the SOC-only group (31.4% vs 39.4%; OR, 0.78; 95% CI, 0.63-0.96; I2, 25%) [Supplementary Fig. 6].
Figure 3.
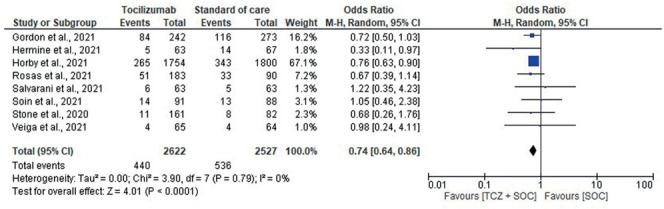
Forest plot for need/progression to Mechanical ventilation.
Figure 6:
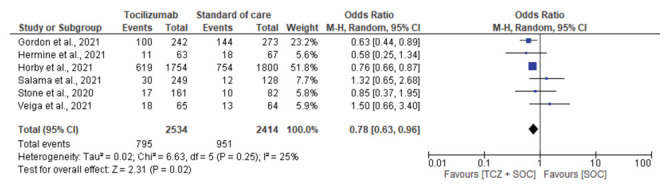
Forest plot for progression to mechanical ventilation or death
ICU admission
Four RCTs reported ICU admissions as secondary outcome. Among patients who were not in ICU at randomization, those in the tocilizumab group had a lower rate of ICU admission compared to the SOC-only cohort (OR, 0.66, 95% CI, 0.40-1.08, I2, 29%) (Fig. 4).
Figure 4.
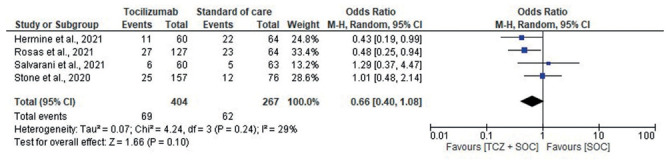
Forest plot for ICU admission among patients who were not already in ICU at randomization.
Rate of adverse events (AEs)
The comparison of the risk of AEs between the tocilizumab and SOC group is shown in Fig. 5. Five studies assessed events of serious infections which were significantly lower in the tocilizumab group (OR, 0.57; 95% CI, 0.36-0.89; I2, 21%). There was no significant difference between the two groups for the number of patients with at least 1 AE (OR, 1.38; 95% CI, 0.87-2.19; I2, 70%), the occurrence of serious AEs (OR, 0.90; 95% CI, 0.70-1.14; I2, 0%) and infection (OR, 0.89, 95% CI, 0.65-1.23). Notably, an over 43% lower risk of severe infection (OR, 0.57; 95% CI, 0.36-0.89; I2, 21%).
Figure 5.
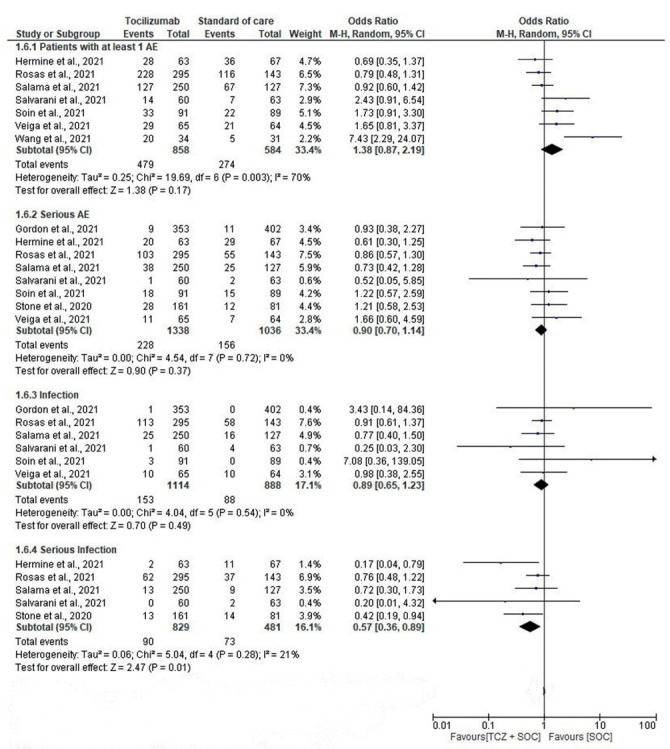
Forest plot for the risk of adverse event(s) between the tocilizumab and control group.
Discussion
The role of IL-6 in severe COVID-19 remains incompletely understood. Our meta-analysis of RCTs that compare tocilizumab with SOC did not find evidence for a statistically significant benefit on mortality. Severity of illness did not seem to play an important role in determining the benefit of tocilizumab. Our findings on mortality were consistent with previous meta-analyses which included 5,6 & 7 RCTs, respectively (19,20,6).
Although the pooled analysis of the nine studies showed no significant benefit of tocilizumab on survival, we found that use of this drug was associated with a significantly lower rate of progression to MV. This finding was also, in keeping with previous studies (6,21,22). Two previous meta-analyses, which included 7 and 8 studies respectively, reported that ICU admissions and progression to MV were significantly lower in the tocilizumab group compared to SOC-only group. Our results, support the evidence of lower rate of ICU admission with tocilizumab treatment, though such difference was not statistically significant. This finding might be very beneficial to countries facing a challenging shortage of ICU beds and ventilators amidst the present pandemic. In resource-constrained situations, tocilizumab may reduce the load on limited resources such as ventilators and, potentially, ICU beds.
Baseline data on use of azithromycin was not balanced in the Tocibras (13) study. This could have contributed to the significant association noted between this drug and mortality in our meta-regression. That said, studies have shown azithromycin to be almost ineffective for patients admitted to hospital with COVID-19 (23–25).
In the studies we examined, tocilizumab was generally well-tolerated. Our meta-analysis found no association with higher risk of AEs, serious AEs or infection compared with the SOC-only group. Rather, a lower rate of serious infections was found in the tocilizumab group compared to the SOC-only group. Although this may even seem paradoxical, given the immunosuppressive effects of tocilizumab, in the setting of COVID-19-associated ARDS, tocilizumab could act not as indiscriminate immunosuppressant but rather as immunoregulator, thus reducing inappropriate inflammation and bolstering the immune system’s overall effectiveness. Another, although speculative, explanation might be that some severe infections might have been unnoticed among the fatalities in the tocilizumab group, as tocilizumab suppresses the acute phase response, leading to falsely low levels of CRP during bacterial infections. In general, our finding indicate tocilizumab has an acceptable tolerability for treating COVID-19. These findings are consistent with results reported by three previous meta-analyses of observational studies and one meta-analysis of RCTs (5,6,22)
Tocilizumab’s beneficial effect in COVID-19 is thought to result from attenuating the hyperinflammatory state (26). Impaired viral clearance and hyperinflammation might contribute to the pathophysiology of different stages of severe COVID-19. The timing of an immunomodulatory intervention is likely to be crucial: immunostimulants could improve the antiviral response, but worsen hyperinflammation; immunosuppressants can attenuate hyperinflammation but further impair viral clearance. Thus, immunomodulation must be tailored to the underlying immunopathology. Lack of immunobiological stratification of patients included in clinical trials might potentially explain why most clinical trials have failed to show significant benefits of tocilizumab on mortality in a general COVID 19 population. It is also possible that, in critically ill patients it may be too late for tocilizumab to blunt immune response and thus prevent mortality, but seems to prevent progression to MV. Also, maybe tocilizumab may blunt ARDS but not multi-organ injury (cardiac, renal, liver), which is an important driver of mortality. We recommend further study on tocilizumab, specifically in patient subpopulations who are exhibiting evidence of hyperinflammation.
Recent evidence also shows that SARS-CoV-2 hyperinflammation is accompanied by significant anti-inflammatory cytokine response that is associated with disease progression and multi-organ injury, and characterized by a high IL-10 and IL-10/lymphocyte count ratio, in adult patients with laboratory confirmed SARS-CoV-2 infection (27). Thus, in some patients, a more prominent hypoinflammatory response may occur, even in the setting of hyperinflammation. Indeed, Henry at al. (27) reported that IL-10 value had a more significant impact on COVID-19 disease progression than IL-6. Finally, although COVID-19 was originally classified as a primary respiratory disease, recent evidence has shown that the inflammation associated with COVID-19 causes dysregulation of multiple biological pathways leading to profound hemostasis disturbance, in the form of localized and systemic coagulopathies and thrombotic events (28). In addition to ARDS, this COVID-19 associated coagulopathy is an important hallmark and predictor of outcome in SARS-CoV-2 infection, and tocilizumab may have a limited impact in reducing the formation of microthrombi which may drive pulmonary and multi-organ injury.
Our meta-analysis has several limitations. Four studies had a relatively small sample size (fewer than 100 patients enrolled in each arm of the trial), which might have reduced the power to adequately to reflect a statistical difference. The number of patients in the RECOVERY and REMAP-Cap trials were much higher compared to other RCTs (study weights 51.0% and 21.9%, respectively) (10,11). Six studies had an open-label design, which may have led to a higher risk of selection and performance bias. There were differences in terms of enrollment criteria, the time at which tocilizumab therapy was initiated, doses and frequency of tocilizumab administered, lack of a uniform-SOC across studies and presence of other treatments. With regard to the latter, a univariate analysis of other treatments was performed and found no significant effects on mortality (Table 2). Lastly, information on neutropenia, thrombocytopenia and GI perforation was not provided in these RCTs. All are well known AEs of tocilizumab in the rheumatoid arthritis population. Especially thrombocytopenia is of key interest, given the association with severe covid-19.
In conclusion, tocilizumab is a generally a well-tolerated treatment, and possibly effective to preventing progression to MV, but does not appear to have a statistically significant survival benefit in a generalized COVID-19 population. In order to further evaluate its efficacy on mortality and identify subsets of patients most likely to benefit from the inclusion of tocilizumab within the standard of care, larger RCTs with significant statistical power and well-defined inclusion criteria are required, including subgroup analyses for patients with evidence of hyperinflammation.
Author Contributions:
Conceptualization- VM; Methodology- VM, BMH, CVC, IC; Data curation- VM, CVC, IC; Software & formal Analysis- VM, CVC, IC; Investigation- All authors; Writing - Original article- VM, BMH, CVC, JV, GL; Writing - Review & Editing- All authors
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020;2(8):113–22. [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Plebani M. Cytokine “storm”, cytokine “breeze”, or both in COVID-19? Clin Chem Lab Med CCLM. 2021;59(4):637–9. doi: 10.1515/cclm-2020-1761. [DOI] [PubMed] [Google Scholar]
- Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–30. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- Lan S-H, Lai C-C, Huang H-T, Chang S-P, Lu L-C, Hsueh P-R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W-T, Hung S-H, Lai C-C, Wang C-Y, Chen C-H. The effect of tocilizumab on COVID-19 patient mortality: A systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. 2021:107602. doi: 10.1016/j.intimp.2021.107602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Medrxiv. 2021 [Google Scholar]
- Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–16. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga VC, Prats JA, Farias DL, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. Bmj. 2021:372. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soin AS, Kumar K, Choudhary NS, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tleyjeh IM, Kashour Z, Damlaj M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow CS, Hasan SS. The effect of tocilizumab on mortality in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2021:1–6. doi: 10.1007/s00228-021-03087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Khatri M, Malik M, et al. Use of tocilizumab in COVID-19: a systematic review and meta-analysis of current evidence. Cureus. 2020;12(10) doi: 10.7759/cureus.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Lu J, Tang Y, Dai Y, Zhou J, Wu Y. Tocilizumab for treating COVID-19: a systemic review and meta-analysis of retrospective studies. Eur J Clin Pharmacol. 2020:1–9. doi: 10.1007/s00228-020-03017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–52. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado RH, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. The Lancet. 2020;396(10256):959–67. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby PW, Roddick A, Spata E, et al. Azithromycin in hospitalised patients with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2020 [Google Scholar]
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BM, Benoit SW, Vikse J, et al. The anti-inflammatory cytokine response characterized by elevated interleukin-10 is a stronger predictor of severe disease and poor outcomes than the pro-inflammatory cytokine response in coronavirus disease 2019 (COVID-19) Clin Chem Lab Med CCLM. 2021;59(3):599–607. doi: 10.1515/cclm-2020-1284. [DOI] [PubMed] [Google Scholar]
- Lippi G, Sanchis-Gomar F, Favaloro EJ, Lavie CJ, Henry BM. Coronavirus disease 2019–associated coagulopathy. In: Mayo Clinic Proceedings. Elsevier; 2021. p. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


