Abstract
Background
Based on the results of several recent randomized trials, European and American guidelines on valvular heart disease management have substantially expanded the indications for transcatheter aortic valve implantation (TAVI). We present an all-comer data on peri-operative risk profile and in-hospital outcomes from Germany for patients treated by TAVI or isolated surgical aortic valve replacement (iSAVR) in 2020, providing an opportunity to compare study data with data from daily clinical practice.
Methods
Data concerning all isolated aortic valve procedures performed in Germany in 2020 were retrieved from the mandatory nationwide quality control program. Expected mortality was calculated with the annually revised German Aortic valve score (AKL-score) based on the data of either catheter-based (AKL-CATH) or isolated surgical (AKL-CHIR) aortic valve replacement in Germany from the previous year (2019).
Results
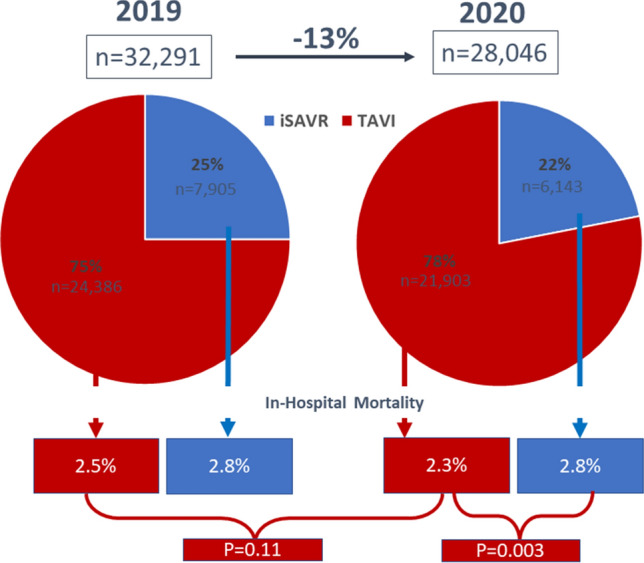
In 2020 21,903 TAVI procedures (20,810 transvascular (TV; vs. 2019: 22.973; − 9.4%), 1093 transapical (TA; vs. 2019: 1413; − 22.6%)) and 6144 (vs. 2019 7905; − 22.5%) iSAVR were performed in Germany. Patients who received TAVI showed a significantly higher perioperative risk profile than patients undergoing iSAVR based on older age and more severe co-morbidities. While in-hospital mortality after TAVI (2.3%) was numerically lower than in 2019 (2.5%), this difference was not significant (p = 0.11). In-hospital mortality after iSAVR was identical in 2020 and 2019 (2.8%) and thus higher than after TAVI (p = 0.003), resulting in an observed expected mortality ratio of 1.02 after TAVI and 1.05 after iSAVR. After exclusion of the emergency procedures, in-hospital mortality did not differ significantly between the groups (TAVI 2.2% vs. iSAVR 1.9%, p = 0.26).
Conclusion
Total numbers of both iSAVR and TAVI in Germany were lower in 2020 than in 2019, most likely due to the COVID-19 pandemic. However, the relative number of patients treated by TAVI as compared to iSAVR continues to increase. Despite older age and more severe comorbidities compared to patients undergoing iSAVR the in-hospital mortality after TAVI continued to decrease in 2020 and remains significantly lower than after iSAVR.
Graphical Abstract
Keywords: TAVI, TAVR, Aortic valve replacement, Real world, Registry
Introduction
The PARTNER 3 and Evolute low-risk studies both showed that trans-catheter aortic valve implantation (TAVI) is also a therapeutic option for patients with low surgical risk, with the equal outcome to surgical aortic valve replacement. This includes patients at a relatively young age [1–3]. In fact, the PARTNER 3 study demonstrated the superiority of TAVI using balloon-expandable prosthesis as compared to surgical valve replacement (iSAVR) regarding the occurrence of the combined primary endpoint of death, stroke or rehospitalization both after 1 year (8.5% vs. 15.1%, p = 0.001) and after 2-years of follow-up (11.5% vs. 17.4%, p = 0.007) [1, 2]. Large registries also provide promising results regarding the comparison of TAVI with iSAVR regarding patients outcome [4, 5].
Accordingly, the recently revised guidelines of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) on valvular heart disease recommend TAVI over conventional surgical aortic valve replacement in patients as soon as they reach the age of 75 years—provided that a transfemoral approach is possible and that there are no adverse anatomic features that increase the risk for the TAVI procedure [6]. The guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA) even recommend evaluating the possibility of TAVI in patients as young as 65 years of age, with both TAVI and surgical aortic valve replacement carrying a “class I” recommendation for patients between 65 and 80 years of age [7]. Still, prosthetic valve durability of the TAVI prostheses is a major aspect regarding the recommendation for TAVI in patients with a high life expectancy. In the PARTNER 2 study population, the incidence of paravalvular leaks after TAVI remained higher than after iSAVR, however, transvalvular gradients and thus effective valve opening areas remained stable and not significantly different in both groups over the period of 5 years [8].
In clinical practice, however, conditions are usually different from those in randomized trials. We present an analysis of all TAVI as well as iSAVR performed in patients with general health insurance in Germany to explore real-world indications and outcomes of the two treatment methods, in particular their relative changes over time.
Methods
In Germany, the recording of pre-procedural, peri-procedural and post-procedural data is mandatory for all patients with statutory health insurance who receive a heart valve replacement, whether surgical or catheter-based. The results presented in this study are based on the annual central analysis of these data. The data are primarily anonymized and analyzed by the Institute for Quality Assurance and Transparency in Healthcare (IQTIG), a governmental organization independent of healthcare insurers and providers. Data collection, control mechanisms and statistical analysis methods have been previously reported [9–12]. Data set were complete in at least 98% of the patients. Data was given for patients undergoing either isolated aortic valve replacement or TAVI—independent of the access route. Patients undergoing conversion to open-heart surgery after TAVI were included in the TAVI cohort.
Definitions
Procedural complications include device-malpositioning, coronary obstruction, aortic dissection, anulus rupture, pericardial tamponade, left ventricular (lv) decompensation, low cardiac output, cerebral emboli, aortic regurgitation (AR) > = II°, device embolization, rupture of a heart chamber, conversion, vascular complications, severe bleeding complications, rhythm disorders and others.
Access-related complications were further subdivided into infections, vessel rupture, vessel dissection, relevant bleeding/hematoma, ischemia, AV-fistula, aneurysm, sternum instability and others.
Risk stratification
Patients were stratified into risk groups based on the procedure—surgical or catheter-based. This stratification was performed according to either the German aortic valve (AKL) score for patients treated with iSAVR (AKL-CHIR) or for the AKL score for patients treated with TAVI (AKL-CATH). The special feature of these scores is their annual readjustment by recalibrating the weight of all individual parameters based on the results of the previous year [13].
The AKL-CATH Score includes the following risk factors: sex, age, height, weight, NYHA classification, presence of cardiogenic shock as well as the use of inotropes or mechanical circulatory support prior to valve replacement, decompensation or resuscitation within 48 h prior to procedures, pulmonary hypertension, heart rhythm at baseline, left ventricular function, peripheral artery disease, lung disease, preoperative renal function. Compared to AKL-CATH Score, the AKL-CHIR score 2020 additionally considers the presence of diabetes mellitus as well as the performance of surgery as reoperation or in the presence of endocarditis. Pulmonary hypertension and the patient's BMI are not taken into account.
The patients were divided into perioperative risk groups based on the AKL-Score: very high (> = 10%), high (6–< 10%), intermediate (3–< 6%) and low (< 3%9). The calculation of the observed vs. expected (O/E) mortality ratio is a valuable measure of treatment quality and was performed for the overall population of each therapy option as well as for each risk group. However, statistic comparison of the expected mortality and, therefore, the ratios is only of limited value given the distinct calculation models.
The urgency of the procedure and thus the classification as an emergency procedure was left to the assessment of the surgeon or interventionalist.
Statistical analysis
Categorical variables were analyzed by the Pearson´s chi2 test using a series of 2 × 2 tables. The alpha level to assume statistical significance was 0.05. For the total number of procedures, intraprocedural complications, the number of patients with endocarditis, and -hospital mortality, the data were compared to data from 2019 [12]. For comparison of the operative risk profile of patients who received TAVI, corresponding available clinical factors contributing to the logistic EuroScore were compared from the 2019 and 2018 reports with the 2020 results.
Results
Procedures
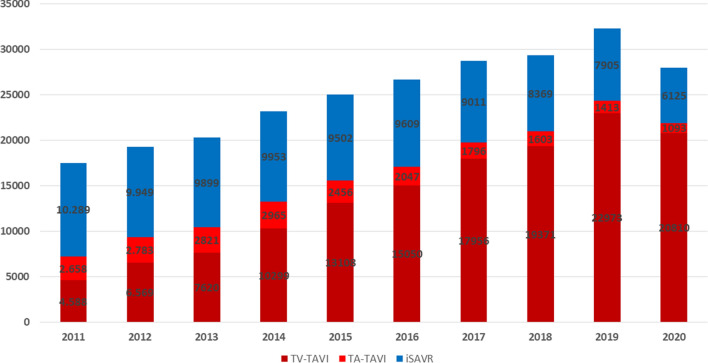
In 2020, 21,903 TAVI procedures (20,810 transvascular (TV), 1093 transapical (TA)) were performed. Compared to previous years, the number of TV-TAVI declined for the first time since 2011 (2019 22.973; − 2163, − 9.4%). The number of TA-TAVI showed a substantially larger reduction (2019: 1413; 2020: 1093; − 320; − 22.6%), and a continued trend to decline since 2013.The total number of iSAVR also declined substantially (2020: 6144, 2019: 7905; − 1780; − 22.5%; Fig. 1). Accordingly, in 2020 overall isolated aortic valve procedures decreased by 13% (2019:32,291; 2020: 28,028). 78.1% of isolated aortic valve procedures were TAVI procedures (74.2% TV-TAVI, 3.9% TA-TAVI, whereas this was 75.0% (71.1% TV-TAVI, 3.9% TA-TAVI; Fig. 1) in 2019.
Fig. 1.
Number of TV-, TA-TAVI and iSAVR procedures 2011–2020,
Baseline characteristics
Data on clinical details were complete in over 99% of the datasets.
Compared to patients undergoing iSAVR, the majority of patients who underwent TAVI in 2020 were over 75 years of age and were also more likely to have more severe symptoms including prior cardiogenic shock. In addition, these patients were significantly more likely to have additional coronary artery disease (CAD) with a high number of percutaneous coronary interventions (PCI) performed 6 months prior to TAVI as well as a higher number of patients with 3-vessel CAD and involvement of the left main (LM). Additionally, these patients were more likely to have poor LV function and to have noncardiac relevant conditions (Table1). Thus, these patients showed a significantly increased operative risk according to the ASA classification (Table 1) compared to patients undergoing iSAVR. Patients undergoing iSAVR were more often male, more often overweight and more often showed endocarditis. The percentage of patients with endocarditis stayed constant compared to 2019 (2020: 10.3%; 2019: 9.2% p = 0.06; Table 1). Compared to previous years patients undergoing TAVI in 2020 showed more often a BMI > = 30m2/kg, extracardiac arterial disease and cardiogenic shock within 48 h prior TAVI, while the number of patients with COPD, LV-EF < 30%, NYHA IV and inotropes used prior to the procedure has decreased. Other factors contributing to surgical risk, such as age, sex, or even previous cardiac surgery, remained the same or undulated between years. Regarding ASA classification, there was an increase in patients in ASA classifications 1 and 2, but a decrease in classes 3 and 4 over the years. The proportion of patients with ASA class 5 was always low over the years and showed no change (Table 2).
Table 1.
Baseline characteristics of patients undergoing iSAVR and TAVI 2020
| iSAVR 2020 | TAVI 2020 | p-value | |
|---|---|---|---|
| N = 6125 | N = 21,517 | ||
| Age < 65 years | 40.8% (2497) | 2.1% (453) | < 0.001 |
| 65– < 75 years | 38.5% (2359) | 11.3% (2436) | < 0.001 |
| > = 75 years | 20.7% (1269) | 86.6% (18,628) | < 0.001 |
| Gender (male) | 65.3% (2124) | 50.5% (10,622) | < 0.001 |
| BMI < 18.5 | 0.9% (54) | 1.4% (301) | 0.003 |
| > = 30 | 35.5% (2147) | 26.2% (5638) | < 0.001 |
| NYHA IV | 5.6% (345) | 8.3% (1784) | < 0.001 |
| History of cardiogenic shock | 11.8% (725) | 23.2% (4935) | < 0.001 |
| Atrial fibrillation | 16.0% (979) | 38.6% (8294) | < 0.001 |
| PM/ICD prior surgery | 5.0% (303) | 12.0% (2571) | < 0.001 |
| LV-EF < = 30% | 4.3% (260) | 6.7% (1439) | < 0.001 |
| CAD (> = 1vessel) | 25.1% (1539) | 57.3% (12,322) | < 0.001 |
| 3-Vessel CAD | 6.4% (390) | 22.0% (4723) | < 0.001 |
| LM CAD | 3.6% (222) | 4.8% (1021) | 0.0002 |
| PCI within 6 months prior procedure | 6.5% (398) | 21.7% (4658) | < 0.001 |
| Prior heart/aortic surgery | 13.4% (820) | 15.3% (3299) | 0.0002 |
| Endocarditis | 10.3% (631) | 0.03% (6) | < 0.001 |
| Diabetes | 24.3% (1486) | 30.5% (6564) | < 0.001 |
| Extracardiac arterial disease | 18.0% (1104) | 29.3% (6299) | < 0.001 |
| COPD | 9.6% (585) | 12.5% (2686) | < 0.001 |
| Neurological disorders | 10.9% (669) | 13.8% (2969) | < 0.001 |
| Chronic dialysis | 2.3% (143) | 5.3% (1140) | < 0.001 |
| ASA > = III | 89.5% (5482) | 91.8% (19,751) | < 0.001 |
Data presented in percent. BMI: body-mass index; NYHA: New York Heart Association class of heart failure; PM: pacemaker; ICD: implantable cardioverter defibrillator; CAD: coronary artery disease; LM: left main; PCI: percutaneous coronary intervention; PAOD: peripheral arterial occlusive disease; COPD: chronic obstructive pulmonary disease; ASA: classification of the American Society of Anaesthesiologists for perioperative risk
Table 2.
Risk-characteristics of patients undergoing TAVI over the years 2018–2020
| TAVI 2020 (n = 21,544) |
TAVI 2019 (n = 22,922) |
p-value 2020 vs. 2019 |
TAVI 2018 (n = 19,392) |
p-value 2020 vs. 2018 |
|
|---|---|---|---|---|---|
| Patient Characteristics influencing logES and AKL | |||||
| Age Median (IQR) | 81 (78–85) | 81 (78–85) | – | 81 (78–85) | – |
| Female gender | 49.6% | 48.6% | 0.34 | 49.4% | 0.26 |
| BMI < 18.5 kg/m2 | 1.4% | 1.5% | 0.44 | 1.4% | 0.78 |
| BMI > = 30 kg/m2 | 26.2% | 24.9% | < 0.001 | 24.8% | < 0.001 |
| Extracardiac arterial disease | 29.3% | 26.5% | < 0.001 | 26.2% | < 0.001 |
| COPD | 12.5% | 13.1% | 0.65 | 14.6% | < 0.001 |
| Endocarditis | 0.03% | 0.04% | 0.87 | 0.03% | 0.64 |
| Previous cardiac/aroticsurgery | 15.3% | 16.9% | 0.004 | 15.4% | 0.65 |
| Shock (< 48 h) | 3.4% | 2.4% | < 0.001 | 3.0% | 0.02 |
| Using of inotropes | 0.9% | 1.3% | < 0.001 | 1.6% | < 0.001 |
| Sinus rhythm at beginning of procedure | 66.8% | 65.4% | 0.002 | 65.1% | < 0.001 |
| LF-EF < 30% | 6.8% | 6.2% | 0.04 | 8.4% | < 0.001 |
| NYHA IV | 8.3% | 8.6% | 0.86 | 10.2% | < 0.001 |
| Recent MI (< 48 h) | 0.6% | 0.5% | 0.16 | 0.6% | 0.94 |
| CCS IV | 1.1% | 0.9% | 0.04 | 1.4% | 0.03 |
| Pulmonary hypertension | * | 46.8% | – | 46.5% | – |
| Emergency | 0.6% | 0.6% | 0.86 | 0.7% | 0.66 |
| sCR (mean; mg/dl)** | 1.18 | 1.17 | – | 1.19 | – |
| ASA-classification | < 0.001 | < 0.001 | |||
| 1 | 1.2% | 0.5% | < 0.001 | 0.3% | < 0.001 |
| 2 | 7.0% | 4.9% | < 0.001 | 5.1% | < 0.001 |
| 3 | 64.2% | 67.7% | < 0.001 | 69.3% | < 0.001 |
| 4 | 26.9% | 26.4% | 0.03 | 24.5% | < 0.001 |
| 5 | 0.7% | 0.6% | 0.15 | 0.9% | 0.05 |
*Information not available in 2020 (only mean PA pressure given)
**Standard deviation not available
IQR: interquartile range; COPD: chronic obstructive pulmonary disease; LV-EF: systolic left ventricular ejection fraction; NYHA: New York Heart Association; MI: myocardial infarction; CCS: Canadian Cardiovascular society; sCR: serum Creatinine; ASA: American Society of Anesthesiologists
Complications
Procedural complications remain higher in patients undergoing TAVI (6.4%) than in patients undergoing iSAVR (3.2%, p < 0.001). Compared to previous years the distribution of the complications remained nearly stable in patients undergoing TAVI, with a slow decline of AR > = 2 to 0.25% in 2020 (vs 2019 0.3%, p = 0.44; vs. 2018 0.5%, p = 0.0003) and a slow increase of intraprocedural vascular complications to 2.8% (vs. 2019: 2.7%, p = 0.50; vs. 2018: 2.3%, p = 0.0009; Table 3). Overall access-related complications were present in 6.5% after TAVI; most common were bleeding/hematoma (69.2%), aneurysm (25.6%), vessel rupture (7.7%), dissection (5.1%).
Table 3.
Intraprocedural complications of patients undergoing iSAVR in 2020 and TAVI in 2020, 2019 and 2018
| iSAVR 2020 | TAVI 2020 | TAVI2019 | TAVI 2018 | |
|---|---|---|---|---|
| 6143 | 21,544 | 24,386 | 21,056 | |
| Intraprocedural complications | 3.24% | 6.42% | 5.90% | 5.95% |
| Device malposition | 0.13% | 0.42% | 0.53% | 0.53% |
| Coronary occlusion | 0.08% | 0.19% | 0.16% | 0.18% |
| Aortic dissection | 0.02% | 0.09% | 0.10% | 0.12% |
| Aortic regurgitation > = II | 0.11% | 0.25% | 0.28% | 0.45% |
| Annulus rupture | 0.08% | 0.11% | 0.15% | 0.13% |
| Tamponade | 0.10% | 0.45% | 0.57% | 0.64% |
| LV decompensation | 0.21% | 0.39% | 0.37% | 0.51% |
| Cerebral embolism | 0.03% | 0.10% | 0.07% | 0.08% |
| Rhythm disorders | 0.39% | 1.71% | 1.47% | 1.68% |
| Device embolisation | 0.02% | 0.25% | 0.21% | 0.25% |
| Vascular complications | 0.31% | 2.83% | 2.72% | 2.32% |
The proportion of patients who required a new pacemaker or defibrillator after the procedure was significantly higher after TAVI than after iSAVR (10.0% vs. 4.3%, p < 0.001). The proportion of these patients increased compared with recent years (vs 2019: 8.4% (p < 0.001); vs 2018 7.8% (p < 0.001)).
In-hospital mortality
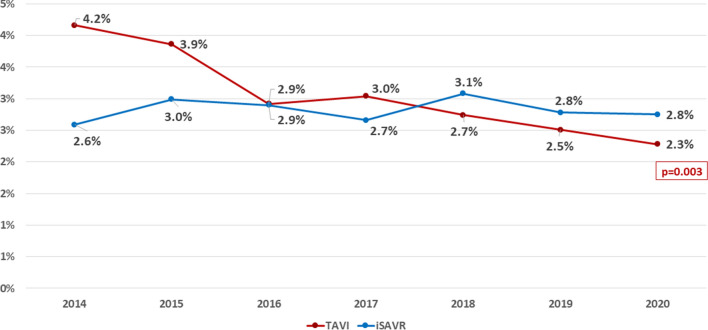
Overall in-hospital mortality after TAVI including TV-TAVI and TA-TAVI keeps declining over the years and was 2.3% in 2020, while in-hospital mortality after iSAVR remained constant over the years (2.8%), resulting in a significantly lower in-hospital mortality after TAVI as compared to iSAVR in 2020 (p = 0.033). Compared to the overall mortality after TAVI in 2019 (2.5%), in-hospital mortality after TAVI in 2020 (2.3%) was only numerically lower (p = 0.11; Fig. 2).
Fig. 2.
All-cause in-hospital mortality 2014–2020 after TAVI and iSAVR
According to the calculation by the AKL-CATH or the AKL-CHIR score respectively, the majority of patients undergoing both TAVI and iSAVR were at low-risk (AKL-score < 3%). TAVI patients showed an observed to expected mortality ratio (O/E) of 1.02. The O/E mortality ratio was 0.97 in low-risk patients, 1.08 in intermediate-risk patients, 1.16 in high risk and 0.99 in very high-risk patients. Patients undergoing iSAVR showed an O/E mortality ratio of 1.05: 1.04 in low risk, 1.07 in intermediate risk, 1.02 in high and 1.07 in very high-risk patients (Table 4).
Table 4.
In-hospital mortality rates 2020 stratified by either AKL-CATH (TAVI) or AKL-CHIR (iSAVR) Score
| TAVI 2020 | iSAVR 2020 | |
|---|---|---|
| All patients | ||
| Observed | 2.28% | 2.75% |
| Expected | 2.24% | 2.61% |
| O/E | 1.02 | 1.05 |
| Distribution by AKL-score | ||
| < 3% | 83.48% | 84.05% |
| ≥ 3% and < 6% | 12.28% | 7.43% |
| ≥ 6% and < 10% | 2.83% | 3.76% |
| ≥ 10% | 1.41% | 1.18% |
| Low-risk patients (AKL 0–< 3%) | ||
| Observed 0–< 3% | 1.45% | 1.13% |
| Expected 0–< 3% | 1.49% | 1.09% |
| O/E 0–< 3% | 0.97 | 1.04 |
| Intermediate-risk patients (AKL 3–< 6%) | ||
| Observed 3–< 6% | 4.36% | 4.41% |
| Expected 3–< 6% | 4.02% | 4.12% |
| O/E 3–< 6% | 1.08 | 1.07 |
| High-risk patients (AKL 6–< 10%) | ||
| Observed 6–< 10% | 8.70% | 7.83% |
| Expected 6–< 10% | 7.53% | 7.69% |
| O/E 6–< 10% | 1.16 | 1.02 |
| Very high-risk patients (AKL > = 10%) | ||
| Observed ≥ 10% | 20.39% | 24.74% |
| Expected ≥ 10% | 20.58% | 23.14% |
| O/E > 10% | 0.99 | 1.07 |
Of all iSAVR procedures, 4.0% (n = 247) were rated as emergency procedures. These procedures were associated with significantly increased in-hospital mortality (22.3%; 55/247). Thus, the in-hospital mortality for non-emergency procedures was 1.9% (113/5864).
Only 0.6% (n = 130) of TAVI procedures were rated to be emergency cases. These procedures also showed high in-hospital mortality (27/130; 20.8%). The in-hospital mortality rate of patients who underwent non-emergency TAVI was 2.2% (463/21,373) (Table 3). After exclusion of emergency procedures, in-hospital mortality rates after TAVI and iSAVR show no significant difference (p = 0.26).
Discharge
Most patients after both TAVI (45.2%) and iSAVR (56.4%; p < 0.001) stayed 8–14 days in the hospital that performed the procedure (= index-hospital). However, while 31.4% of patients who received TAVI could be discharged after less than 7 days, only 7.9% did so after iSAVR (p < 0.001). More patients after iSAVR stayed more than 14 days (35.8% vs. 23.5%, p < 0.001). While most (75.5%) patients who received TAVI could be discharged directly home, this was the case in only 52% of patients who received iSAVR (p < 0.001).
Discussion
The data presented here for all isolated surgical or transcatheter aortic valve interventions performed in Germany in 2020 in patients with statutory health insurance show the following important facts:
All procedures—iSAVR, endovascular, and transapical TAVI were performed less frequently in 2020 than in 2019.
While in-hospital mortality after iSAVR has remained constant over the past several years, in-hospital morality after TAVI continues to decline steadily -albeit only numerically when compared to the previous year.
Nearly 1 out of 4 patients undergoing TAVI had significant CAD with PCI within the preceding 6 months.
Patients after TAVI have shorter index-hospital stays than patients after iSAVR. In addition, patients after TAVI are more often discharged directly home.
In Germany, as in USA, the number of TAVI procedures overall as well as the share of TAVI procedures of all isolated aortic valve replacements has been rising steadily and exponentially since 2011 [9–12, 14]. On the one hand, more and more patients with high-grade aortic valve stenosis are being treated [9–12], on the other hand TAVI has become the therapeutic gold-standard in many patients [6, 7]. Whereas the further decrease in iSAVR and TA-TAVI procedures could be anticipated due to the data from previous years, the first-time decline in TV-TAVI procedures is surprising. Still, given the overall decline in all procedures—the percentage of TV-TAVI within all isolated aortic valve replacements grew.
In medicine, and thus also in interventional cardiology, the year 2020 was highly influenced by the impacts of the COVID-19 pandemic causing a temporary suspension of non-emergency procedures [15]. Thus, the decrease of TAVI might be linked to the postponement of just these very procedures. The known poor outcome of untreated patients with symptomatic severe aortic valve stenosis [16] suggests a possible link between the decrease of the procedures and the recently described increased cardiovascular mortality during the pandemic [15]. A small monocentric observational study showed that patients scheduled for TAVI who were postponed due to the pandemic had frequent cardiovascular events during the waiting period (10%), and some of these patients (3%) even died during this period [17]. This in combination with the decrease in procedures are, therefore, alarming results for the care of patients with aortic valve disease in Germany during the pandemic.
While in-hospital mortality after iSAVR remained almost unchanged over the years, in-hospital mortality after TAVI continued to decrease also in 2020– albeit not statistically significant [9–12]. This is even more remarkable, as for the first time data for TAVI are now pooled from all patients treated with either a TV or a TA access. In a theoretical subtraction of TA-TAVI patients with their inherent higher in-hospital mortality risk (approximately 6% in the years 2014–2019 [9–12]) from the complete cohort, one can assume an extrapolated mortality after endovascular TAVI of only around 2.1% in 2020. These data were also consistent with data from the U.S. SVT registry, demonstrating the transferability of German data to other western countries [14]. In addition to the greater experience of the physicians and the constantly improving techniques and devices, the decrease of in-hospital mortality can also be attributed to a slow but steady change of the patients’ risk profile. The comparison of the ASA classifications of the patients undergoing TAVI 2018–2020 in particular shows that there is an overall trend towards treating less ill patients with TAVI. The individual clinical factors partly contradict this trend, however, it might be necessary to collect more long-term data than only a period of the last 3 years.
It is important to note that the in-hospital mortality rates reported here cannot be immediately compared between patients treated with TAVI or iSAVR. The cohorts reflect real-world patients and are not randomized or matched. Patients undergoing iSAVR are younger but include more emergency procedures and endocarditis cases, which carry a higher risk. On the other side, patients undergoing TAVI are more than a decade older and suffer from significantly more severe comorbidities. However, since the AKL score takes these factors into account, especially the presence of endocarditis, an almost identical O/E mortality ratio is a clear indicator that in an all-comer collective the outcome of TAVI and iSAVR is the same. Additionally, it should be noted that after exclusion of the emergency procedures, the intra-hospital mortality of TAVI and iSAVR patients remained the same, despite the above-mentioned differences due to comorbidities [17]. This fact supports the data of all PARTNER and Evolute trials in recent years, which have consistently shown either non-inferiority or superiority of TAVI over iSAVR in patients undergoing elective isolated aortic replacement [1–3, 18–20].
An interesting and very important aspect of the data presented here is the frequency of PCI within 6 months before the aortic valve procedure. Nearly 1 in 4 patients receiving TAVI received a PCI within this time period. This corresponds to the proportion of patients in some of the randomized trials in whom revascularization was performed addition to aortic valve replacement [1, 18]. This fact indicates that patients undergoing TAVI in clinical practice are not only patients with isolated aortic valve stenosis but rather patients with aortic valve stenosis and concomitant relevant coronary artery disease requiring treatment. According to the quality data from the year 2019 in Germany, in-hospital mortality of patients undergoing combined aortic valve and coronary artery bypass surgery is much higher at 4.7% [21]. In most of the large randomized trial, this fact was taken into account and these patients were then partly excluded either completely [22] or the need for revascularization was considered in the randomization [3, 23]. For the interpretation of our data, this means that the lower mortality after TAVI is even more remarkable, as it should actually be compared to the overall mortality after both, iSAVR and after combined aortic valve and bypass surgery. However, a valid comparison would only be possible with the exact knowledge of the individual severity of the CAD.
In addition to the steadily decreasing mortality rate and other data indicating a long and good durability of TAVI prostheses, which have led to a significant expansion of the indication, economic considerations also play an important role in the treatment of patients today. Therefore, it should be emphasized that despite their older age and higher number of comorbidities one third of the patients after TAVI stayed less than 7 days in the hospital performing the procedure, whereas after iSAVR one third stayed more than 14 days. The majority of the patients after TAVI could be discharged directly home, whereas this was true for only half of the patients after iSAVR. These data confirm data from randomized trials [1]. Importantly, these results mean that performing a TAVI not only saves costs [24], but also enables rapid reintegration into the home environment and more efficient use of the low resources—an important point regarding the shortage of hospital beds during a pandemic.
Limitations
This year, the IQTIG analyzed data for procedural data and in-hospital mortality for patients who received a TV and TA TAVI in aggregate. Unfortunately, it is not possible to perform subgroup analyses with regard to the access route. Due to the significantly higher morbidity of TA-TAVI patients on the one hand, but also the known higher in-hospital mortality, on the other hand, this aggregated analysis changes the interpretation of the data considerably.
Furthermore, the different calculation of the risk score models depending on the procedure does not allow a direct comparison of the risk subgroups between iSAVR and TAVI.
Additionally, the data given are only procedure-specific: thus, patients with endocarditis and patients with isolated aortic regurgitation are all included in the analysis of the patients undergoing iSAVR. This fact also disables to clearly assign complications or re-procedures to a given patient and makes any subgroup analyses impossible. Moreover, there is no detailed data available on bioprosthetic valve dysfunction and failure.
In addition, the lack of a clear definition of emergency procedures, might even cause a bias in the comparison of only non-emergency procedures.
The definition of intraprocedural complications—although defined identically for both procedures—primarily reflects the typical complications after TAVI. Therefore, a comparison between the procedures does not seem meaningful and was not discussed. Finally, the quality control program is not mandatory for patients with private medical insurance. Therefore, these patients may not fully be included in the present analysis.
Conclusion
The overall numbers of both TAVI and iSAVR in 2020 are most likely to have decreased due to the COVID-19 pandemic. However, the overall percentage of TAVI procedures continues to grow steadily.
Despite a still significantly higher operative risk profile with regard to pre-existing conditions and age as well as the inclusion of patients who underwent TA-TAVI, in-hospital mortality in 2020 is again lower after TAVI than after iSAVR. Of note is the high proportion of surgeries due to endocarditis in patients who received iSAVR and, conversely, the high proportion of patients with PCI (including left main PCI) performed shortly before TAVI. These factors, which have increased significantly in the individual groups in recent years, make it increasingly difficult to directly compare the two groups with each other.
Remarkable and independent of these factors is a significantly shorter length of stay and a significantly more frequent discharge of patients after TAVI directly home. This shows an important socioeconomic advantage of TAVI compared to iSAVR in an all-comer cohort.
Abbreviations
- TAVI
Transcatheter aortic valve implantation
- iSAVR
Isolated surgical aortic valve replacement
- ESC
European Society of Cardiology
- EACTS
European Association for Cardio-Thoracic Surgery
- ACC
American College of Cardiology (ACC)
- AHA
American Heart Association (AHA)
- AKL Score
German Aortic Valve Score
- AR
Aortic regurgitation
- LV-EF
Systolic left ventricular ejection fraction
- NYHA
New York Heart Association classification
- TV
Transvascular
- TA
Transapical
Declarations
Conflict of interest
Luise Gaede, Johannes Blumenstein and Helge Möllmann receive either lecture, advisory board and/or proctor fees from Edwards Lifesciences, Boston Scientific and Abbott Vascular. All other authors have no conflict of interest to report.
References
- 1.Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/nejmoa1814052. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Mack MJ, Hahn RT, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–1161. doi: 10.1016/j.jacc.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 3.Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/nejmoa1816885. [DOI] [PubMed] [Google Scholar]
- 4.Bekeredjian R, Szabo G, Balaban Ü, et al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: In-hospital data and 1-year results from the German Aortic Valve Registry (GARY) Eur Heart J. 2019;40:1323–1330. doi: 10.1093/eurheartj/ehy699. [DOI] [PubMed] [Google Scholar]
- 5.Hamm CW, Möllmann H, Holzhey D, et al. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J. 2014;35:1588–1598. doi: 10.1093/eurheartj/eht381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Nishimura RA, Bonow RO, et al (2021) 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. [DOI] [PubMed]
- 8.Makkar RR, Thourani VH, Mack MJ, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382:799–809. doi: 10.1056/nejmoa1910555. [DOI] [PubMed] [Google Scholar]
- 9.Gaede L, Blumenstein J, Liebetrau C, et al. Outcome after transvascular transcatheter aortic valve implantation in 2016. Eur Heart J. 2018 doi: 10.1093/eurheartj/ehx688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaede L, Blumenstein J, Liebetrau C, et al. Transvascular transcatheter aortic valve implantation in 2017. Clin Res Cardiol. 2020 doi: 10.1007/s00392-019-01509-8. [DOI] [PubMed] [Google Scholar]
- 11.Möllmann H, Husser O, Blumenstein J, et al. Lower mortality in an all-comers aortic stenosis population treated with TAVI in comparison to SAVR. Clin Res Cardiol. 2020 doi: 10.1007/s00392-019-01548-1. [DOI] [PubMed] [Google Scholar]
- 12.Gaede L, Blumenstein J, Husser O, et al. Aortic valve replacement in Germany in 2019. Clin Res Cardiol. 2021;110:460–465. doi: 10.1007/s00392-020-01788-6. [DOI] [PubMed] [Google Scholar]
- 13.Kötting J, Schiller W, Beckmann A, et al. German aortic valve score: a new scoring system for prediction of mortality related to aortic valve procedures in adults. Eur J Cardio-thoracic Surg. 2013;43:971–977. doi: 10.1093/ejcts/ezt114. [DOI] [PubMed] [Google Scholar]
- 14.Mack M, Vemulapalli S, Herrmann H, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 15.Nef HM, Elsässer A, Möllmann H, et al. Impact of the COVID-19 pandemic on cardiovascular mortality and catherization activity during the lockdown in central Germany: an observational study. Clin Res Cardiol. 2021;110:292–301. doi: 10.1007/s00392-020-01780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38:V61. doi: 10.1161/01.CIR.38.1S5.V-61. [DOI] [PubMed] [Google Scholar]
- 17.Ro R, Khera S, Tang GHL, et al. Characteristics and outcomes of patients deferred for transcatheter aortic valve replacement because of COVID-19. JAMA Netw Open. 2020;3:e2019801. doi: 10.1001/jamanetworkopen.2020.19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 19.Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 20.Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 21.Aortenklappenchirurgie B zum E 2019: KK (2019) Bundesauswertung zum Erfassungsjahr 2019: Kombinierte Koronar- und Aortenklappenchirurgie. www.iqtig.org
- 22.Smith C, Leon M, Mack M, Miller D, Moses J, Svnesson L, Tuzcu E, Webb J, Fontana G, Makkar R, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani V, Corso P, Pichard A, Bavaria J, Herrmann H, Akin J, Anderson W, Wang DPSD. Transcatheter versus surgical aotic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 23.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 24.Shah KK, Elder D, Nguyen MTH, et al. Transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement for aortic stenosis (SAVR): a cost-comparison study. Hear Lung Circ. 2021;30:1918–1928. doi: 10.1016/j.hlc.2021.05.088. [DOI] [PubMed] [Google Scholar]