Abstract
Objective
CXCR4 is a chemokine with multiple effects on the immune system. In murine lupus models, we demonstrated that monocytes, neutrophils, and B cells over-expressed CXCR4, and its ligand, CXCL12, was up-regulated in diseased kidneys. We sought to determine whether CXCR4 and CXCL12 expressions were increased in human systemic lupus erythematosus (SLE) peripheral blood leukocytes (PBLs) and kidneys.
Methods
PBLs from 31 SLE patients, eight normal human controls, and nine rheumatoid arthritis (RA) patients were prospectively analyzed by flow cytometry for CXCR4 expression. Human lupus nephritis (LN) biopsies (n=14) were immunostained with anti-CXCL12 antibody.
Results
CD19+ B cells and CD4+ T cells from SLE patients displayed an over two-fold increase (p=0.0001) and three-fold increase (p<0.0001), respectively, in median CXCR4 expression compared to controls (n=8). Moreover, CXCR4 expression on B cells was 1.61-fold higher (p=0.0008) in patients with SLEDAI > 10 (n=8) versus SLEDAI ≤ 10 (n=16), 1.71-fold higher in patients with class IV LN (n=5) versus other classes of LN (n=7) (p=0.02), and 1.40-fold higher in active (n=6) versus inactive (n=18) neuropsychiatric SLE (NPSLE) patients (p=0.01). CXCL12 was significantly up-regulated in the tubules and glomeruli of LN kidneys (n=14), with the percentage of positive cells positively correlating with severity of LN.
Conclusions
CXCR4 appears to be up-regulated in multiple leukocyte subsets in SLE patients. The heightened expression of CXCR4 on B cells in active NPSLE, and CXCL12 in nephritic kidneys suggests that the CXCR4/CXCL12 axis might be a potential therapeutic target for SLE patients with kidney and/or CNS involvement.
Keywords: lupus, chemokine, lupus nephritis
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease associated with the production of pathogenic anti-nuclear autoantibodies (ANAs) that affects roughly 1 in 2500 people in the United States; 90% are women between the ages of 15 and 45 (1-3). SLE presents with a diverse array of clinical symptoms, which often reflect the consequences of injury to multiple organ systems, such as the kidney, brain, and skin.
The renal manifestations of SLE, termed lupus nephritis (LN), is the leading cause of morbidity and mortality (4). LN is thought to be initiated by deposition of preformed circulating ANA-containing immune complexes (ICs) in the glomerular capillary wall (5). After IC deposition, it is thought that the complement cascade and FcR-mediated mechanisms play important roles in mediating leukocyte recruitment (6).
Multiple etiologies, such as vascular occlusion, cytokine production, and nerve injury, can promote formation of neuropsychiatric SLE (NPSLE). Similar to LN, autoantibodies may be largely responsible for NPSLE, in part because of their abilities to form ICs that clog vessels, and induce nerve damage (7). Two autoantibodies, one that cross-reacts with the NMDA glutamate receptor, NR2 (8), and another against ribosomal P (9), have been associated with NPSLE, and shown to promote apoptosis in neurons (10-11).
One molecule recently documented to be crucial to the pathogenesis of murine lupus is CXCR4 (12). A seven-membrane G-protein coupled receptor located on multiple cell types such as CD34+ hematopoietic progenitor cells (13), CD4+ T cells (14), and B cells (15), CXCR4 binds CXCL12, or stromal cell derived factor-1 (SDF-1) (16). CXCL12 has been documented to be expressed by cells in several different tissues, including endothelial cells in the bone marrow (17), distal tubular cells of the kidney (18), and dendritic cells in the skin (19). The interaction of CXCL12 and CXCR4 results in migration, integrin activation, and chemotaxis of lymphocytes, monocytes, neutrophils (14), CD34+ hematopoietic progenitor cells (13), and leukemic cells (20), which may play key roles in their recruitment to affected peripheral tissues in lupus, such as the kidney and skin. In addition, this binding enhances survival and proliferation, and transcription in the CXCR4-expressing cells (21). Mice deficient in CXCL12 or CXCR4 have demonstrated defects in vascularization (22), bone marrow myelopoiesis (23), and limb innervation (24).
Intricately involved in B cell lymphopoiesis (23), and chemotaxis of B cells (25) and T cells, CXCR4 may play a vital role in the development of SLE, which involves cross-talk between these two subsets of lymphocytes. Moreover, it is well established that T cells from SLE patients demonstrate a higher chemotactic response to CXCL12 than those from normal and rheumatoid arthritis patients (26-27). We also recently demonstrated in the mouse the importance of the CXCR4/CXCL12 axis in lupus pathogenesis (12). We showed clearly that CXCR4 was hyper-expressed in several murine lupus models, suggesting that CXCR4 hyper-expression was a generalized feature of lupus and was independent of the underlying genetic basis. In addition, CXCL12 was significantly enhanced in the nephritic kidneys of lupus mice. To assess the contribution of CXCR4/CXCL12 up-regulation on lupus pathogenesis, mice were treated with a peptide antagonist of CXCR4, which reduced renal infiltration by inflammatory myeloid cells and prolonged survival (12). However, while it is unknown whether CXCR4/CXCL12 participates in the development of NPSLE, CXCR4/CXCL12 could potentially play a pivotal role in NPSLE pathogenesis by recruiting B and T cells and promoting autoantibody formation.
In this study, we tested whether SLE patients, and, in particular, those hospitalized for severe active SLE sequellae such as LN and NPSLE, demonstrated expression abnormalities in the CXCR4/CXCL12 axis. We evaluated the expression of CXCR4 on peripheral blood leukocyte subsets of SLE inpatients, compared these levels to various clinical and laboratory parameters, and investigated CXCL12 expression within LN biopsies.
Patients and Methods
Patients and controls
A total of 45 lupus patients were recruited from Cochin Hospital (Université de Paris V, Paris, France) (n=3), and University of Texas Southwestern (UTSW) Medical Center (Dallas, TX) (n=14). Lupus patients were classified as having SLE, if they met at least four of the American College of Rheumatology (ACR) diagnostic criteria for SLE. All patients classified as having “lupus nephritis” (Cochin Hospital (n=13), UTSW Medical Center (n=14)) had evidence of nephritis by histopathological analyses of renal biopsy. All patients classified as having “neuropsychiatric SLE” (Cochin Hospital (n=8)) were diagnosed with at least one of the 19 NPSLE syndromes established by the ACR that was not attributed to another etiology (28). Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores were calculated for each lupus patient, based on clinical and laboratory findings at the time of study enrollment (29). In addition, 8 normal adult controls and 9 rheumatoid arthritis (RA) patients from Cochin Hospital were included in the study.
Patient blood samples and corresponding historical, clinical, and laboratory information were prospectively collected and analyzed in a blind fashion. Renal biopsies (n=14) used for staining for CXCL12 were collected at UTSW Medical Center. Lupus nephritis classifications were determined by a renal pathologist (XJZ) using the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 Classification of Lupus Nephritis (30-31) guidelines. Concurrent blood samples were not available from these patients who had renal biopsies. The design and execution of the studies were authorized by the Internal Review Boards of each hospital, and conformed to the standards and guidelines of the ethics board. All patients consented by written agreement to inclusion in this study, which was approved by each medical center's Institutional Review Board.
Flow Cytometry and Antibodies
Antibodies to the following human antigens were used for flow cytometry analyses: CD4-PE, CD4-PECy5, CD8-PECy7, CD14-FITC, CD15-PE, CD19-FITC, CD19-PECy5, CD38-APC, CXCR4-APC, CXCR4-PE, IgD-FITC (BD Biosciences, Franklin Lakes, NJ), CD3-AlexaFluor® 700, CCR7-APC, and CD45RA-FITC (eBioscience, San Diego, CA). Whole blood was labeled with antibodies. Red blood cells were lysed after staining using the BD Fix and Lyse solution. Flow cytometer voltage parameters were normalized using BD Spectral Beads (BD Biosciences, Franklin Lakes, NJ). At least 5 × 104 cells were acquired in the live gate, as defined by size and granularity. Samples were acquired on BD Canto and BD FACSAria flow cytometers (BD Biosciences, Franklin Lakes, NJ), and analyzed using Flow Jo software (Tree Star, Inc., Ashland, OR). MFIs represent median fluorescent intensities. Subsets of CD19+ B cells (n=9) and CD4+ T cells (n=4) were analyzed using the BD FACSAria flow cytometer to increase the relative sensitivity of the assay.
Immunohistochemistry
Paraffin sections of human LN biopsies (n=14) and minimal change disease biopsies (n=3) (5 μm) were boiled in 10 mM citrate, quenched with 0.01% NaBH4, blocked with human IgG, and stained with mouse monoclonal anti-human SDF-1/CXCL12 IgG antibody (R & D Systems, Minneapolis, MN) at 4°C overnight. Sections were then washed and incubated with Cy3-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) for 1 hr. at room temperature. Sections were washed and then mounted with Vectashield (Vector Laboratories, Burlingame, CA) with DAPI, visualized and photographed with a Zeiss Axioplan 2 and digital camera (Carl Zeiss International, Germany), and analyzed with Axiovision software (Carl Zeiss International, Germany). The average percentage of positive cells over total number of cells per high power field in ten different fields for each LN section was calculated by three independent observers (YD, LT, MP).
Statistical analyses
Data were analyzed using GraphPad 4 (GraphPad, San Diego, CA). For comparisons of CXCR4 MFIs between two groups, p-values were calculated using unpaired t-test, with Welch's correction for groups with unequal variances. In correlation analyses, p-values were determined by Pearson's method. R2 values were generated using Gauss-Markov linear regression analyses. For comparisons of percentage of positive cells amongst at least three groups, p-values were calculated using one-way ANOVA values.
Results
Patient Characteristics
From January 1995 to June 2008, 31 hospitalized SLE patients (26 females, 5 males; median age = 42±14 years) were prospectively sampled at Cochin Hospital in Paris, France. Relevant patient attributes are presented in Table 1. Five ethnicities, Caucasian (n=17), French Caribbean (n=5), African (n=4), Asian (n=2), and Maghreb (n=3), were represented in this population. At the time of sampling, five had active nephritis (class III (n=1), class IV (n=3), class V (n=1)), and eight had inactive renal involvement (histopathologically documented as class II (n=1), class IV (n=3), class V (n=2), class III and class V (n=1), and class IV and class V (n=1)). Clinically, active LN was defined by the presence of hematuria (defined as ≥3 red blood cells per high-power-field), proteinuria (≥500 mg/24 hours), or increase in blood creatinine by ≥20%. Histologically, active and chronic LN was based on the ISN/RPS 2003 Classification of LN guidelines (30-31). In addition, six exhibited neuropsychiatric symptoms attributed to active SLE, and two patients in remission at the time of sampling had NPSLE in their past medical history. Most patients were on treatments of less than 10 mg/day of corticosteroids with or without 400 mg/day of hydroxychloroquine, while three patients were on 50 mg/day of azathioprine, two patients were on 0.6 mg/m2/day intravenous cyclophosphamide, and two patients were on mycophenylate mofetil (<3 g/day) at the time of sampling. All nine RA patients were female and Caucasian; the median age was 59.9±7.5 years. The eight normal human controls consisted of five females and three males, with an average age of 32.8±8.6 years.
TABLE 1. Patient Characteristics.
| PATIENT | SEX | AGE | RACE | Dx | SLEDAI | GN | Extra-renal | Rx |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 27 | Asian | SLE | 34 | V | D, V, N, M, S | CS(1mkd), CYC |
| 2 | F | 15 | African | SLE | 4 | IV | A, D | HC, CS(5), CYC |
| 3 | F | 53 | Asian | SLE | 19 | V | N, H | CS5, AZ |
| 4 | F | 45 | African | SLE | 20 | IV | N, H | CS(5), AZ |
| 5 | M | 32 | FC | SLE | 10 | IV | D, A | CS(7.5), Col |
| 6 | F | 44 | Cauc | SLE | 0 | - | D, A, H | CS(5), HC |
| 7 | F | 24 | African | SLE | 22 | - | C, S, P, N | - |
| 8 | M | 46 | FC | SLE | 2 | V | D, S, APS | CS(5) |
| 9 | F | 72 | Cauc | SLE | 2 | - | APS, A, D, SS | CS(10), MMF(2) |
| 10 | F | 60 | Cauc | SLE | 2 | - | N, SS, H, O | CS (5) |
| 11 | F | 50 | Maghreb | SLE | 2 | III/V | D, A, S, APL, V | MMF(3),CS( 8) |
| 12 | F | 58 | African | SLE | 4 | - | D, H | - |
| 13 | F | 52 | Cauc | SLE | 0 | - | D, A | - |
| 14 | F | 39 | Maghreb | SLE | 10 | III | D, A | CS( 5), HC |
| 15 | F | 45 | FC | SLE | 26 | IV | D, A, N, S, MAS | CS(5), HC |
| 16 | F | 46 | Cauc | SLE | 0 | V/IV | D, A | CS(5), HC |
| 17 | F | 39 | Cauc | SLE | 0 | - | D, A, S, APS | CS(5) |
| 18 | F | 60 | Cauc | SLE | 12 | - | D, A, N | - |
| 19 | F | 45 | Cauc | SLE | 10 | - | A | CS(10) |
| 20 | F | 39 | Cauc | SLE | 7 | - | A, S, C | CS(10), HC |
| 21 | M | 30 | FC | SLE | 15 | - | D, A | CS(10) |
| 22 | F | 20 | FC | SLE | 12 | IV | S, C, A, D | CS(0.7mkd) |
| 23 | F | 60 | Cauc | SLE | 2 | II | S, D, A, N, MAS | CS(5) |
| 24 | F | 52 | Cauc | SLE | 4 | - | A, V | CS(5), HC |
| 25 | F | 47 | Cauc | SLE | 9 | - | A, H, APS | HC |
| 26 | M | 40 | Cauc | SLE | 19 | IV | S, APS, D, A | - |
| 27 | F | 41 | Cauc | SLE | 1 | - | H, A, APS | CS(1 mkd) |
| 28 | F | 26 | Maghreb | SLE | 9 | - | D, A, H, S | CS(30) |
| 29 | M | 24 | Cauc | SLE | 4 | - | D, H | - |
| 30 | F | 20 | Cauc | SLE | 8 | - | D, A, S | - |
| 31 | F | - | Cauc | SLE | - | - | D, A, H, S | - |
NOTE: All patients were recruited from Cochin Hospital in Paris, France.
Abbreviations: French Carribean (FC), Caucasian (Cauc), Dermatologic (D), Vascular (V), Neurological (N), Muscular (M), Serositis (S), Arthralgia (A), Hematological (H), Anti-phospholipid syndrome (APS), Macrophage activation syndrome (MAS), Cardiac (C), Ophthalmologic (O), Sjogren's syndrome (SS); Corticosteroids (CS) [mg/d indicated in (), otherwise mg/kg/d (mkd)], azathioprine (AZ), cyclosporine (CYC), colchicine (Col), hydroxychloroquine (HC), mycophenylate mofetil (MMF)
CXCR4 expression on CD19+ B cells and CD4+ T cells in SLE patients
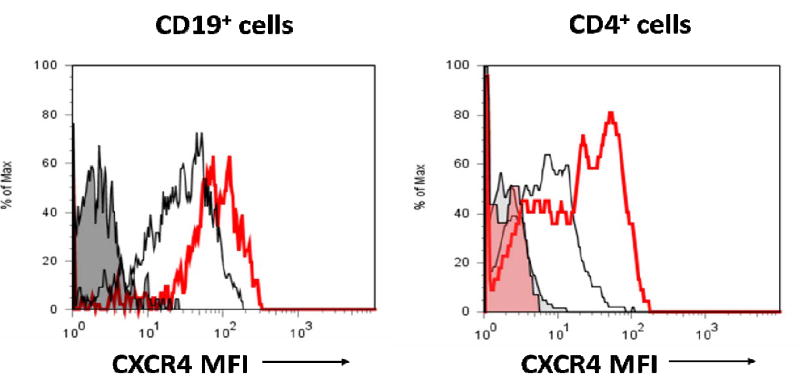
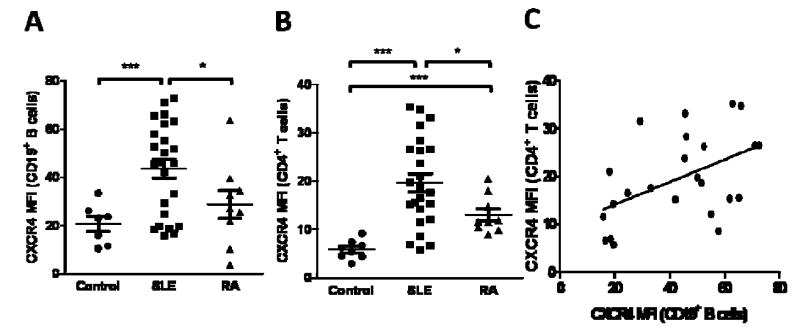
Peripheral blood B cells, T cells, monocytes, and neutrophils from SLE patients were analyzed for CXCR4 expression. Representative plots of CD19+ B cell and CD4+ T cell expression of CXCR4 are shown in Figure 1. SLE patients demonstrated a 2.12-fold and 1.52-fold increase in median CXCR4 expression on CD19+ B cells compared to the healthy controls (n=7, p=0.0001), and RA patients (n=9, p=0.05) (Figure 2A). CD4+ T cells from SLE patient PBLs displayed a 3.36-fold and 1.51-fold increase in median CXCR4 expression compared to the healthy controls (n=8, p<0.0001) and RA patients (n=9, p=0.007), respectively (Figure 2B). There were no significant differences in CXCR4 surface expression on CD8+ T cells, neutrophils, or monocytes between SLE and control samples (data not shown).
Figure 1.
CXCR4 expression on peripheral blood CD19+ B and CD4+ T cells in a systemic lupus erythematous (SLE) patient and a normal control. Displayed are two representative flow cytometry plots of the median fluorescent intensity (MFI) of CXCR4 on CD19+ B and CD4+ T cells in a SLE patient (red) and a normal control (black). The shaded area represents the isotype control used for the SLE patient.
Figure 2.
CXCR4 expression on peripheral blood CD19+ B and CD4+ T cells in SLE patients at Cochin Hospital and correlation of CXCR4 levels on each cell population. A and B, CD19+ B cells (A) and CD4+ T cells (B) from SLE and rheumatoid arthritis (RA) patients and healthy controls were analyzed for CXCR4 expression by flow cytometry. Plotted for each patient group are the MFIs for CXCR4. p-values comparing two groups were calculated using unpaired t-test with Welch's correction for groups with unequal variances. Error bars represented standard error measurements. C, Correlation scatter plots of CXCR4 MFIs of CD19+ B and CD4+ T cells was displayed. p-values were generated using unpaired t-test. R2 values were calculated using Gauss-Markov linear regression analyses. Abbreviations: *, p ≤ 0.05; **, p ≤ 0.005; ***, p ≤ 0.0005.
Further analysis for CXCR4 expression was also performed in subsets of CD19+ B cells (n=9) and CD4+ T cells (n=4). There was a 24.1-fold increase in CXCR4 median fluorescent intensity (MFI) in CD19+ B cells (MFI=3046) versus isotype controls (MFI=127), and a 6.6-fold increase in CD4+ T cells (MFI = 668) compared to isotype controls (MFI = 102). There was a global up-regulation of CXCR4 MFI in the vast majority of the CD19+ B cell and CD4+ T cell subpopulations that were studied compared to isotype controls. Of all the CD19+ B cell subsets, naïve B cells (CD38+ IgD+) expressed the highest average CXCR4 MFI (3915) and second-highest fold increase (30.8) over the isotype control MFI. Transitional B cells (CD38++ IgD+) had the second-highest average MFI of 2732, and the highest fold increase (32.5) over the isotype control MFI. Other CD19+ B cell subsets including a subpopulation of naïve Bm1 cells and memory B cells (32) (CD38- IgD+, avg. MFI = 2255, 15.3-fold increase), memory B cells (CD38- IgD-, avg. MFI = 1178, 8.1-fold increase), early memory B cells (CD38+ IgD-, avg. MFI = 1065, 7.4-fold increase), and plasmablasts (CD38++ IgD-, avg. MFI = 416, 3.5-fold increase) were examined. Three CD4+ T cell subsets were evaluated for CXCR4 expression; naïve T cells (CD45RA+ CCR7+) had the highest average MFI (1108) and fold increase (10.1) over isotype controls, followed by effector memory T cells (CD45RA- CCR7-, avg. MFI = 904, 2.4-fold increase). Central memory T cells (CD45RA- CCR7+) had expressed an average CXCR4 MFI of 58, which was 0.6 fold lower than the isotype control MFI.
There was a statistically significant positive correlation between CXCR4 expression on CD19+ and CD4+ cells (p=0.01, r2=0.24) (Figure 2C). Moreover, the MFI of CXCR4 on CD19+ B cells was also found to positively correlate with systemic lupus erythematosus disease activity index (SLEDAI) scores (r2=0.15), but this relationship did not reach significance (p=0.06) (data not shown). CD4+ T cell CXCR4 surface expression did not correspond with SLEDAI scores (p=0.98, r2=0.00003) (data not shown). Correlations between CXCR4 surface expression on either CD19+ B or CD4+ T cells, and various laboratory parameters including anti-dsDNA titer, 24-hour proteinuria, BUN/creatinine, CH50, C3, or C4 were not found to be significant (data not shown).
CXCR4 expression on CD19+ B cells and CD4+ T cells in subsets of SLE patients
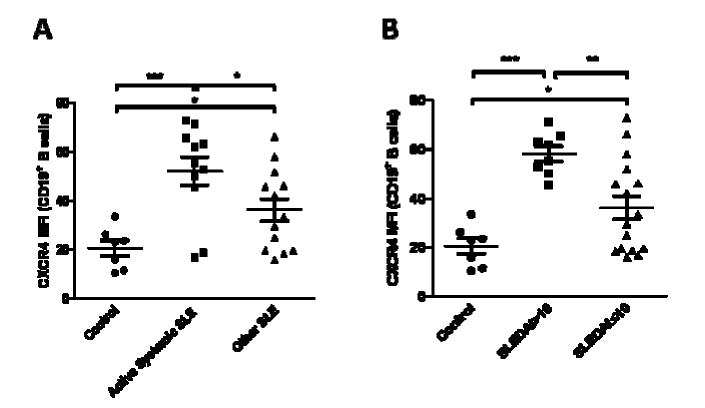
As we noted a positive trend between CXCR4 expression on CD19+ B cells and SLEDAI scores in the SLE patients, we further divided the patient population into two groups including those with active systemic involvement (i.e. hematological, cardiac, renal and/or neuropsychiatric involvement) (n=11), and those with cutaneous and/or rheumatological manifestations or remitted SLE (n=13). Patients with active systemic SLE yielded 1.44-fold (p=0.04) elevation in CXCR4 B cell MFIs compared to their SLE counterparts (Figure 3A) but not in CD4+ T cell MFIs (0.99-fold and p=0.95) (data not shown). Thus, we chose to compare the patients with the highest SLEDAI scores and those with the lowest, and we found that SLE patients with SLEDAI >10 (n=8) yielded 1.61-fold (p=0.0008) elevation in CXCR4 B cell MFIs compared to those with SLEDAI ≤10 (n=16) (Figure 3B). CD4+ T cells only showed 1.18-fold higher difference in the SLEDAI score >10 group compared to the SLEDAI ≤10 group (p=0.38) (data not shown).
Figure 3.
CXCR4 expression on peripheral blood B cells in other subsets of SLE patients. A, CXCR4 MFIs were measured on CD19+ B cells from SLE patients with active systemic involvement (i.e. hematological, cardiac, renal and/or neuropsychiatric involvement) and with cutaneous and/or rheumatological manifestations or remitted disease activity (denoted as other SLE), and normal controls. B, CXCR4 MFIs were measured on CD19+ B cells from SLE patients with SLEDAI scores > 10 or ≤ 10, and normal controls. p-values comparing two groups were calculated using unpaired t-test. Error bars represented standard error measurements. Abbreviations: *, p ≤ 0.05; **, p ≤ 0.005; ***, p ≤ 0.0005.
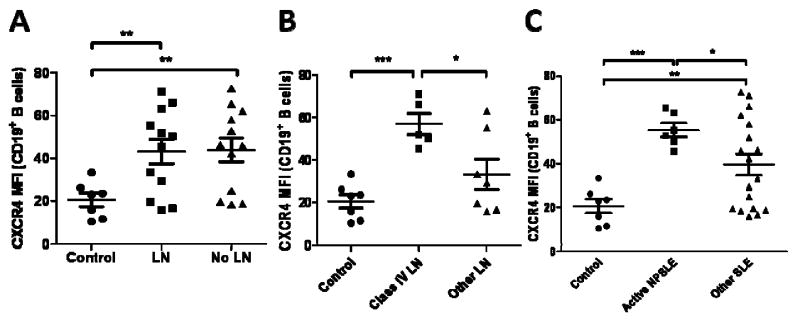
Next, we sub-categorized patients according to their different end-organ involvement, including those with LN and NPSLE. We did not discern any significant changes in CD19+ B cell (0.98-fold difference in LN versus non-LN patients, p=0.93) and CD4+ T cell (1.07-fold difference, p=0.73) expression of CXCR4 in SLE patients with (n=12) and without nephritis (n=12), even though both populations expressed CXCR4 significantly higher than the controls (Figure 4A). Even sub-dividing patients into those with active LN (n=4) and those without active LN (n=20), which included those with inactive LN (n=8) and without LN (n=12), yielded no significant differences on CD19+ B cells (1.10-fold difference in active LN versus non-LN patients, p=0.75) and CD4+ T cells (0.86 fold difference, p=0.61) (data not shown). However, patients with active or inactive class IV LN (n=5), which have previously shown worse renal function and outcome than the other subclasss (33-35), demonstrated a 1.71-fold increase in CXCR4 expression on their B cells (p=0.02) (Figure 4B) and 1.44-fold increase on CD4+ T cells (p=0.19) (data not shown) compared to those with other classes of LN (n=7).
Figure 4.
CXCR4 expression on peripheral blood B cells in subsets of SLE patients at Cochin Hospital. A, CXCR4 MFIs were measured on CD19+ B cells from SLE patients with lupus nephritis (LN) or without lupus nephritis (LN), and normal controls. B, CXCR4 MFIs were measured on CD19+ B cells from SLE patients with class IV LN or other LN, and normal controls. C, CXCR4 MFIs were measured on CD19+ B cells from active NPSLE and other SLE patients, including those with a previous history of NPSLE and no active neuropsychiatric involvement and those with no prior history of NPSLE, and normal controls. p-values comparing two groups were calculated using unpaired t-test with Welch's correction for groups with unequal variances. Error bars represented standard error measurements. Abbreviations: *, p ≤ 0.05; **, p ≤ 0.005; ***, p ≤ 0.0005.
Next, we examined patients with NPSLE. Interestingly, patients with active NPSLE (n=6), as defined by the SLEDAI scoring index (29) (i.e. seizure, psychosis, visual disturbance), demonstrated a 1.40-fold increase (p=0.01) in CXCR4 expression on CD19+ B cells compared to those who did not have active NPSLE (n=18), which included those with a prior history of active NPSLE but no active neurological involvement (n=2) and those without a history of NPSLE (n=16) (Figure 4C). Once again, no differences were noted in CD4+ T cell CXCR4 expression in these different patient groups (p=0.43).
CXCL12 expression in renal biopsies of lupus patients
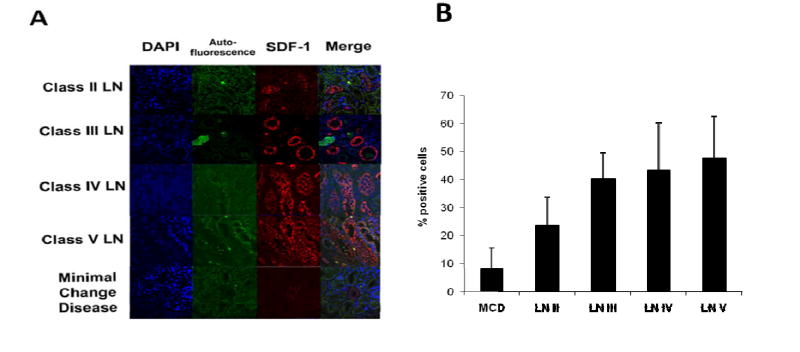
Besides CXCR4, differential expression of its ligand, CXCL12, could constitute an additional factor dictating end-organ disease, drawing from the lessons learned in murine lupus (12). Analyses of renal biopsies obtained from 14 different LN patients (n=3 each for class II, III, and V LN, n=5 for class IV LN) recruited at UTSW Medical Center revealed that CXCL12 expression correlated with renal pathology. We first observed CXCL12 in both glomeruli and tubules of these LN kidneys (Figure 5A). As renal pathology worsened, CXCL12 expression increased in terms of the percentage of positive cells (p<0.05) (Figure 5B). Specifically, classes III, IV, and V LN displayed over 40% of total cells expressing CXCL12. Indeed, in patients with class III, IV, and V LN, CXCL12 was detected at high levels in virtually all renal segments, and resembled the staining patterns seen in the kidneys from 8-10 month old lupus mice (12). As a non-inflammatory control, biopsies from patients with minimal change disease were also analyzed. CXCL12 expression in these control kidneys was minimal, and was localized to substructures in the glomerular tufts (Figure 5A).
Figure 5.
Increased CXCL12 expression in renal biopsies of SLE patients at UTSW Medical Center. A, Paraffin-fixed sections of renal biopsies obtained from SLE patients were stained for CXCL12 in a blinded fashion. Displayed are representative 40× images of 14 independent patient kidneys with various WHO classes of GN. CXCL12 staining was found to be positive in tubular cells in LN samples. Samples obtained from patients with minimal change disease were used as a non-inflammatory control. B, The percent of cells staining positive for CXCL12 was calculated for each subclass of lupus nephritis biopsy and minimal change disease.
Discussion
We have investigated CXCR4 expression on different subsets of PBLs from a total of 31 lupus inpatients. We determined that the SLE inpatient cohort demonstrated significant increases in CD19+ B and CD4+ T cell expression of CXCR4, with a bias towards the naïve cell subsets. We also documented a positive trend in CXCR4 levels on CD19+ B cells of SLE patients with SLEDAI scores, implying a possible role of CXCR4 in driving active disease in SLE. In particular, compared to those with SLEDAI ≤ 10, SLE patients with SLEDAI > 10 exhibited uniformly high CXCR4 levels on their B cells. Obtaining a larger group of SLE patients with severe activity could potentially be helpful in determining whether B cell CXCR4 could become a reliable marker of disease severity or activity in SLE.
The direct correlation in CXCR4 expression in CD19+ B cells of SLE patients with increasing disease severity may be due to the systemic activation of cytokines. We previously showed that up-regulation of CXCR4 can be dependent on several different cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6, and toll-like receptor (TLR) ligation. However, type I interferons, which have widespread influences on SLE pathogenesis (36-37), failed to up-regulate CXCR4 (12).
We recently demonstrated hyper-expression of CXCR4 on several PBL subsets in multiple murine lupus models with nephritis, resulting in prolonged B cell lifespan and augmented B cell chemotaxis (12). The nephritic kidneys from these mice subsequently revealed enhanced levels of CXCL12. A CXCR4 antagonist ameliorated several symptoms in these lupus-prone mice including splenomegaly and nephritis, and lowered mortality rates (12). The similar findings in humans and mice underscore the potential importance of the CXCR4/CXCL12 axis in SLE pathogenesis.
When analyzing the histories of patients with elevated SLEDAI scores, we noted that one half of these patients had a history of NPSLE. While a comparison of NPSLE versus non-NPSLE patients yielded increases in B cell CXCR4 expression, singling out patients with active NPSLE further widened the gap in B cell levels of CXCR4 compared to non-active NPSLE patients (Figure 4C). Furthermore, we measured the levels of CXCL12 in the cerebrospinal fluid (CSF) of an active NPSLE patient, and discovered up-regulated levels of CXCL12 compared to controls (data not shown).
While a larger number of CSF samples from active NPSLE patients needs to be analyzed to confirm an up-regulation of CXCL12, this trend of higher CXCR4 expression in active NPSLE suggests that CXCR4+ cells could potentially target these peripheral tissues to induce pathology. Present on the surface of glial and neuronal cells (38), CXCR4 has functional properties endemic to the central nervous system (CNS), including neuron migration during cerebellar development (39) and modulation of neuronal signaling (40). Neither CXCR4 nor CXCL12 have been postulated before to have a role in the pathogenesis of NPSLE, although both have been shown to influence the recruitment of lymphocytes across the blood-brain barrier (41). Interestingly, strong CXCR4 expression has been documented on lymphocytes isolated from the CSF of patients with acute lymphoblastic leukemia with extramedullary involvement (42) and brains of patients with primary CNS lymphoma (43). Of relevance to autoimmunity, elevated CXCL12 levels have been reported in the CSF of multiple sclerosis patients (44). Clearly, further exploration of the lymphocytes in the CSF and brains of NPSLE patients is warranted to establish the level of involvement of the CXCR4/CXCL12 axis in NPSLE.
Another end-organ where CXCR4/CXCL12 could potentially be playing a pathogenic role is the kidney. While a comparison of patients with active LN and those without LN displayed similar CXCR4 levels, patients with class IV LN, which portend a worse prognosis (33-35), exhibited a 1.71-fold increase in CXCR4 levels on B cells compared to patients who developed other classes of LN (p=0.02, Figure 4B). The absence of increased CXCR4+ cells in the peripheral blood of SLE patients with active LN may be due to the homing of these cells to the diseased kidneys or to other sites expressing elevated CXCL12, thereby diminishing their numbers in circulation. Nevertheless, the augmented CXCR4 expression on circulating B cells in class IV LN patients suggests that the trafficking of these cells to the kidneys may be important in mediating renal damage. In view of these interesting leads, a larger number of LN patients need to be studied to confirm the prognostic value of monitoring CXCR4 expression in this disease.
Since the target of these circulating CXCR4+ cells is the chemokine CXCL12, we examined the expression of CXCL12 in human LN kidneys using immunohistochemistry. The present report constitutes the first demonstration of increased CXCL12 expression within the end-organs in human LN. The widespread expression of CXCL12 within LN kidneys implies that CXCR4+ cells can potentially traffic to multiple sites within these kidneys to inflict end-organ damage. In addition, we have demonstrated an increasing trend in CXCL12 positive cells with worsening renal pathology, particularly in class III, IV, and V LN. One can envisage the increased cellularity in the class III and IV kidneys being driven by the enhanced expression of CXCL12 within these kidneys, compared to the kidneys with WHO class II glomerulonephritis and minimal change disease. Indeed, in murine lupus models with renal involvement, podocytes from renal glomeruli produced elevated amounts of CXCL12 (45), and large aggregates of CXCR4+ cells have been observed in these nephritic kidneys (12). By significantly diminishing tubulo-interstitial disease, glomerulonephritis, and crescent formation in these mice, the CXCR4 peptide antagonist CTCE-9908 prolonged their lifespan, confirming the crucial roles of the CXCR4/CXCL12 axis in mediating LN (12).
While we could document the up-regulation of CXCR4 on CD4+ T cells in the peripheral blood of SLE patients, no significant increases in CD4+ T cell expression of CXCR4 were noted in specific sub-classes of SLE patients analyzed, including those with neuropsychiatric and renal involvement, or those with severe disease. It is possible that besides CXCR4, other chemokine receptors could be playing critical roles in mediating T cell trafficking to these end-organs in SLE. In addition, it would be important to examine CXCR4 expression in other end-organ manifestations in lupus that are also known to be driven by T cells. Indeed, a small preliminary study of CD3+ T cells from the peripheral blood of patients with cutaneous lupus at UTSW Medical Center displayed higher CXCR4 levels than their SLE counterparts and controls by over three-fold and two-fold respectively (data not shown).
Our examination of CXCR4 expression in CD19+ B cell and CD4+ T cell subsets implies that both naïve cell populations contained relatively higher amounts of CXCR4 compared to memory cells. Diminished CXCR4 expression in memory cells is likely due to the homing of these cells to peripheral tissue, since they are more likely than naïve cells to position themselves in these areas. As we have demonstrated enhanced levels of CXCL12 in tubules and glomeruli of lupus nephritis kidneys, these memory cells are likely migrating to areas enriched with CXCL12. However, further confirmation of their enhanced presence in peripheral tissue is warranted.
Furthermore, previous to this study, CXCR4 expression has only been examined in a small number of subsets of PBLs of SLE patients. Fritsch et al examined the expression of CXCR4 in three subtypes of memory CD4+ T cells, including CCR7+/CD27+, CCR7-/CD27+, and CCR7-/CD27- (46). Differentiation of these cells starts from naïve cells, progressing to CCR7+/CD27+, then to CCR7-/CD27+, and finally CCR7-/CD27- (47). The percentage of CXCR4 levels in all three subclasses of memory CD4+ T cells were lower in four active SLE patients, and eight total SLE patients compared to controls (46). Henneken et al investigated CXCR4 levels in subsets of B cells in 4 SLE patients, and showed that fewer naïve, memory, and total B cells displayed CXCR4 expression compared to healthy controls (48). Both of these studies were limited in that they sampled a small number of patients and did not relate any clinical data to CXCR4 levels. Amoura et al sampled a larger SLE population (n=44) and did not detect a significant difference in CXCR4 levels on CD4+ and CD8+ T cells in SLE patients vs. healthy controls (n=22) (49). However, because the clinical characteristics of the SLE patient population were not well delineated in these studies, the apparent discrepancies in the reported CXCR4 expression profiles amongst these studies may relate to differences in disease severity and/or end-organ (notably neuropsychiatric) involvement.
In conclusion, CXCR4 levels are elevated in CD19+ B cells and CD4+ T cells of SLE patients, with naive cells expressing higher amounts than their memory counterparts. B cell expression of CXCR4 correlates positively with disease severity. Patients with peripheral organ involvement, particularly the kidneys or the brain, showed evidence of dysregulation of CXCR4 and CXCL12. Noteworthy were the increased B cell CXCR4 levels in the peripheral blood of active NPSLE and class IV LN patients and the elevated CXCL12 in LN kidney biopsies. Though the mechanistic basis for this up-regulation remains to be defined, these findings suggest that CXCR4/CXCL12 hyperexpression may play a vital role in the pathogenesis of SLE, particularly in patients with central nervous system or kidney involvement. Given that CXCR4/CXCL12 interactions are highly involved in leukocyte recruitment to areas of inflammation, resulting in end-organ damage, blockade of this axis represents a potential therapeutic avenue for preserving peripheral organ function in SLE.
Acknowledgments
We wish to thank Lin-chiang Tseng and Manali Patel for providing cell counts. This project was funded by NIH Grants #P01 AI 039824 and #P50 CORT AR 055503.
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
References
- 1.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Advances in immunology. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Pascual V. Type I Interferon in Systemic Lupus Erythematosus and Other Autoimmune Diseases. Immunity. 2006;25(3):383–92. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85(3):303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 4.Davis JC, Tassiulas IO, Boumpas DT. Lupus nephritis. Curr Opin Rheumatol. 1996;8(5):415–23. doi: 10.1097/00002281-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh US, Bagavant H, Fu SM. Role of anti-DNA antibodies in the pathogenesis of lupus nephritis. Autoimmunity Reviews. 2006;5(6):414–8. doi: 10.1016/j.autrev.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT. Complement Activation Contributes to Both Glomerular and Tubulointerstitial Damage in Adriamycin Nephropathy in Mice. J Immunol. 2006;177(6):4094–102. doi: 10.4049/jimmunol.177.6.4094. [DOI] [PubMed] [Google Scholar]
- 7.Rhiannon JJ. Systemic lupus erythematosus involving the nervous system: presentation, pathogenesis, and management. Clin Rev Allergy Immunol. 2008;34(3):356–60. doi: 10.1007/s12016-007-8052-z. [DOI] [PubMed] [Google Scholar]
- 8.Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):675–8. doi: 10.1002/art.21547. [DOI] [PubMed] [Google Scholar]
- 9.Yoshio T, Hirata D, Onda K, Nara H, Minota S. Antiribosomal P protein antibodies in cerebrospinal fluid are associated with neuropsychiatric systemic lupus erythematosus. J Rheumatol. 2005;32(1):34–9. [PubMed] [Google Scholar]
- 10.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 11.Matus S, Burgos PV, Bravo-Zehnder M, Kraft R, Porras OH, Farias P, et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J Exp Med. 2007;204(13):3221–34. doi: 10.1084/jem.20071285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F, et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol. 2009;182(7):4448–58. doi: 10.4049/jimmunol.0801920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–96. [PubMed] [Google Scholar]
- 14.Nanki T, Lipsky PE. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol. 2000;164(10):5010–4. doi: 10.4049/jimmunol.164.10.5010. [DOI] [PubMed] [Google Scholar]
- 15.Honczarenko M, Douglas RS, Mathias C, Lee B, Ratajczak MZ, Silberstein LE. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood. 1999;94(9):2990–8. [PubMed] [Google Scholar]
- 16.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382(6594):829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 17.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106(11):1331–9. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67(5):1772–84. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 19.Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155(5):1577–86. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91(12):4523–30. [PubMed] [Google Scholar]
- 21.Wong D, Korz W. Translating an Antagonist of Chemokine Receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14(24):7975–80. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 23.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 24.Odemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, Gierschik P, et al. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30(4):494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Vicente-Manzanares M, Montoya MC, Mellado M, Frade JM, del Pozo MA, Nieto M, et al. The chemokine SDF-1alpha triggers a chemotactic response and induces cell polarization in human B lymphocytes. Eur J Immunol. 1998;28(7):2197–207. doi: 10.1002/(SICI)1521-4141(199807)28:07<2197::AID-IMMU2197>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178(3):1938–47. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 27.Harada T, Kyttaris V, Li Y, Juang YT, Wang Y, Tsokos GC. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity. 2007;40(1):1–8. doi: 10.1080/08916930601095148. [DOI] [PubMed] [Google Scholar]
- 28.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 30.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 31.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 32.Bohnhorst JO, Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J Immunol. 2001;167(7):3610–8. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 33.Hiramatsu N, Kuroiwa T, Ikeuchi H, Maeshima A, Kaneko Y, Hiromura K, et al. Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology (Oxford) 2008;47(5):702–7. doi: 10.1093/rheumatology/ken019. [DOI] [PubMed] [Google Scholar]
- 34.Derksen RH, Hene RJ, Kater L. The long-term clinical outcome of 56 patients with biopsy-proven lupus nephritis followed at a single center. Lupus. 1992;1(2):97–103. doi: 10.1177/096120339200100207. [DOI] [PubMed] [Google Scholar]
- 35.Ferluga D, Jerse M, Vizjak A, Hvala A, Rozman B, Kos-Golja M, et al. Correlation among WHO classes, histomorphologic patterns of glomerulonephritis and glomerular immune deposits in SLE. Wien Klin Wochenschr. 2000;112(15-16):692–701. [PubMed] [Google Scholar]
- 36.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, et al. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 1999;73(6):2348–57. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- 39.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 40.Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 2007;38(3):365–76. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- 41.Wu DT, Woodman SE, Weiss JM, McManus CM, D'Aversa TG, Hesselgesser J, et al. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6(1):S82–5. [PubMed] [Google Scholar]
- 42.Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, et al. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol. 2001;115(3):545–53. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- 43.Simon C, Juarez J, Nicolson GL, Boyd D. Effect of PD 098059, a specific inhibitor of mitogen-activated protein kinase kinase, on urokinase expression and in vitro invasion. Cancer Res. 1996;56(23):5369–74. [PubMed] [Google Scholar]
- 44.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(Pt 1):200–11. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 45.Balabanian K, Couderc J, Bouchet-Delbos L, Amara A, Berrebi D, Foussat A, et al. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol. 2003;170(6):3392–400. doi: 10.4049/jimmunol.170.6.3392. [DOI] [PubMed] [Google Scholar]
- 46.Fritsch RD, Shen X, Illei GG, Yarboro CH, Prussin C, Hathcock KS, et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006;54(7):2184–97. doi: 10.1002/art.21943. [DOI] [PubMed] [Google Scholar]
- 47.Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175(10):6489–97. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 48.Henneken M, Dorner T, Burmester GR, Berek C. Differential expression of chemokine receptors on peripheral blood B cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2005;7(5):R1001–13. doi: 10.1186/ar1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amoura Z, Combadiere C, Faure S, Parizot C, Miyara M, Raphael D, et al. Roles of CCR2 and CXCR3 in the T cell-mediated response occurring during lupus flares. Arthritis Rheum. 2003;48(12):3487–96. doi: 10.1002/art.11350. [DOI] [PubMed] [Google Scholar]