Abstract
Background:
Sunitinib has a narrow therapeutic window, with considerable differences between patients. Dosing based on pharmacokinetics (PK) may help overcome some of those issues. This study aims to evaluate and compare the cost-effectiveness of PK-guided individualized treatment of sunitinib with its standard dose in patients with metastatic renal cell carcinoma (mRCC).
Methods:
A comprehensive literature search was performed, and relevant values were used to provide information for the decision analysis model. Utility data were derived from published studies, and costs were obtained from the perspective of payers in China and the United States. A Markov model was established to evaluate the associated costs and health outcomes for patients. The primary outputs of the model included lifetime costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICER). One-way and probability sensitivity analyses were conducted to evaluate the potential uncertainties of parameters.
Results:
Cost-effective analysis showed that the QALY of the PK-guided group increased by 0.83 compared with that in the standard dose group. From the perspective of both countries’ health systems, the cost of PK-guided dose was lower than that of standard dose. Hence, PK-guided treatment was the dominant strategy. One-way and probability sensitivity analyses confirmed the reliability of these results.
Conclusion:
On the basis of currently available data, PK-guided sunitinib treatment may be a safe, effective, and economical intervention for patients with mRCC.
Keywords: cost-effectiveness, metastatic renal cell carcinoma, pharmacokinetic guidance, standard dose, sunitinib
Introduction
Sunitinib is an oral multi-target tyrosine kinase inhibitor acting on signaling cascades involved in proliferation and tumor progression. This drug has been approved by the Food and Drug Administration as a first-line treatment for advanced and/or metastatic renal cell carcinoma (mRCC) 1 and a second-line treatment in locally advanced inoperable and metastatic gastrointestinal stromal tumors. 2 The recommended starting dose and schedule for sunitinib is 50 mg/day for 28 days, followed by a 14-day break. At this daily dose of sunitinib, the target total trough concentration (TTL, sum of trough concentrations of sunitinib and its metabolites) that the patient should achieve is 50–100 ng/ml.3,4 Owing to the large individual patient variability, drug levels may exceed this range, with severe toxicity such as thrombocytopenia, anorexia, fatigue, hand–foot syndrome, and bleeding events. Sunitinib could also induce rare but potentially life-threatening events such as intestinal perforation and interstitial lung disease.1,5 Given that these toxicities are difficult to treat and predict, doctors must closely monitor all patients who have started sunitinib treatment. Individual differences in patients will lead to high systemic exposure to sunitinib and its active metabolite concentrations for some patients, resulting in differences in toxicity. In this context, predictive indicators must be identified for the prevention of severe toxicity caused by sunitinib. High sunitinib blood levels are associated with longer progression-free survival (PFS), overall survival (OS), and other curative effects. 6 However, the therapeutic index of this drug is narrow, and its systemic exposure varies greatly among patients. Pharmacokinetics (PK)-guided dosing has been proposed as a strategy to regulate sunitinib medication 7 and obtain optimal dose adjustment, thereby improving drug efficacy and avoiding adverse side effects.
Although therapeutic drug monitoring (TDM) has long been implemented for aminoglycosides, vancomycin, and antiepileptics and has been proven as cost-effective, 8 this strategy is not widely applied for sunitinib. To date, only five studies have reported the clinical efficacy and safety of PK-guided sunitinib dosing,9–13 and no cost-effectiveness analysis has been conducted. On one hand, PK guidance can improve dose management, reduce costs, and enhanced treatment outcomes. On the other hand, this strategy also increases the cost of patient care, and its economic remains unclear. Here, an inductive analysis of existing clinical research and a cost-effectiveness analysis on PK-guided sunitinib treatment were conducted to provide patients with economical and effective treatment strategies.
Methods
Systematic review
Search strategy
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplementary Tables S3), a comprehensive search was conducted on PubMed, Embase and Cochrane Library, CNKI, Wanfang Database, and VIP Database to identify eligible papers published up to January 2021. The search terms were as follows: sunitinib, pharmacokinetics, PK, TDM, therapeutic drug monitoring, individualized dose adjustment, patient specificity, and individualized. Subject and free terms were used. The search strategy was detailed in Supplementary Tables S1. The reference lists of retrieved articles and related reviews were also examined manually for additional studies. Inclusion criteria were as follows: (1) human subject research; (2) randomized controlled trials (RCTs) or cohort studies; (3) sunitinib dosage was adjusted via PK tools (PK equations or software) in the intervention group; (4) sunitinib was taken as standard dosing without the aid of PK tools in the control group; and (5) in the case of pooled articles based on similar patients, only the high-quality or the most recent study was selected. Exclusion criteria were as follows: (1) outcome indicators did not include PFS, OS, or toxicity; (2) study subjects were not patients with advanced/mRCC; (3) insufficient clinical data – for example, data were reported as a conference abstract, or the detailed dosing method was not provided; and (4) publications were not written in Chinese or English.
Quality assessment and data abstraction
Quality assessment and data extraction were independently performed by two of the authors, and disagreements were resolved through discussion or consultation with the third author. Cochrane risk of bias criteria was used to assess the potential risk of bias in RCTs. 14 For cohort studies, quality was assessed using the Newcastle–Ottawa Scale (NOS): a study can be rated up to nine stars, and a final score of six stars or more is considered as high quality. 15 Collected information included author name, publication year, country of origin, study design, disease type, tumor response and adverse events (AEs) evaluation criteria, treatment group, number of people, treatment regimens, and outcome indicators (including OS, PFS, and toxicity).
Cost-effectiveness analysis
Model design
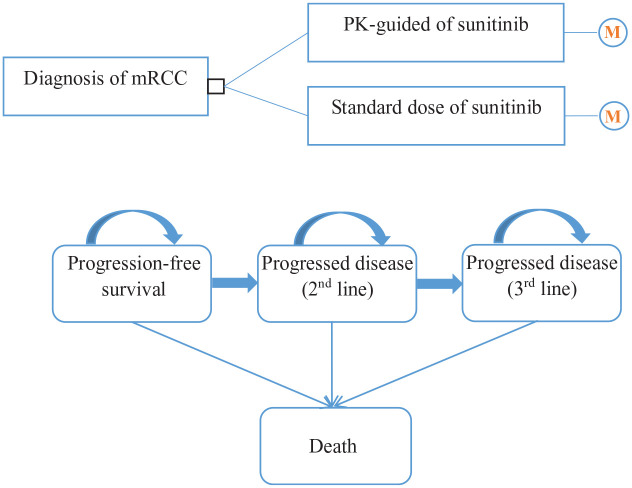
A Markov model was constructed to evaluate the costs and health outcomes of mRCC treatment with sunitinib standard dose or PK-guided dose. The model included three health states reflecting different characteristics of the disease: PFS, progressed disease (PD), and death (Figure 1). PD status was treated by second-line treatment and third-line treatment depending on treatment. Patients in PFS could transit to second-line treatment or death after the initial treatment, those in the second-line treatment phase might continue to receive third-line treatment or entered a state of death, and those in the third-line treatment might only deteriorate to death. Another possibility is to remain in the same condition after a cycle. Irrespective of salvage therapy effectiveness, patients could not return to the former state once the disease has progressed. According to the actual data on clinical sunitinib treatment, patients generally receive continuous treatment for 4 weeks, and medication is stopped for 2 weeks. Hence, the cycle length was set to 6 weeks in this work. The time horizon was 10 years because the survival rate of kidney cancer patients after 5 years was lower than 10%. 16
Figure 1.
Markov model.
TreeAge (TreeAge Software Inc, Williamstown, MA, USA) was used to program and analyze the model. The primary outputs of the model included total cost, QALY, and ICER. All costs and health output were discounted at a rate of 5%. Willingness to pay (WTP) threshold was set as US$100,000 in the United States and US$31,500 in China (3× the per capita gross domestic product of China in 2020). 17 This study was performed in accordance with the recommendations of the Consolidated Health Economic Evaluation Reporting Standards checklist; details are presented in Supplementary Table S4.
Transition probabilities
The probabilities of transition from PFS to PD and from PD to death in the PK-guided group were obtained by fitting the PFS and OS curves in the phase II clinical trial. 9 The probability of the standard dose group was derived from another phase II clinical trial of patients with similar eligibility criteria by using the curve data for the standard dose. 18 PFS and OS Kaplan–Meier curves were digitized using GetData (version 2.26). An algorithm was applied to generate pseudo-individual patient data, and five survival distributions (Weibull, Log-logistic, Log-normal, Gompertz, and Exponential) were used to parameterize the model using the R software (Version 4.1.1). On the basis of Akaike information criterion (AIC), Bayesian information criterion (BIC), and clinical plausibility, Log-normal was chosen as the optimal fit for OS and PFS curve. The specific OS and PFS parameters are shown in Table 1. Survival probability was calculated at time (λ and γ are the scale parameters and shape parameters). Grade 3/4 AEs, such as fatigue, diarrhea, hypertension, thrombocytopenia, hand–foot syndrome, were included. Ratio parameters were derived from clinical trial data.9,18
Table 1.
Key clinical data in the model.
| Variables | Baseline value (range) | |
|---|---|---|
| PK-guided | Standard dose | |
| PFS | Scale = 2.5998; Shape = 0.9564; r2 = 0.98989 | Scale = 2.1714; Shape = 0.8085; r2 = 0.990818 |
| OS | Scale = 3.5995; Shape = 0.6925; r2 = 0.98599 | Scale = 3.1127; Shape = 0.8291; r2 = 0.990518 |
| Probability of third-line treatment | 0.1880 (0.1504–0.2256) 9 | |
| Probability of total AEs (grades 1 and 2) | 0.1917 (0.15336–0.23004) 9 | 0.3453 (0.27624–0.41436) 18 |
| AEs (grade ⩾ 3) incidence | ||
| Fatigue | 0.0926 (0.07408–0.11112) 9 | 0.1795 (0.1436–0.2154) 18 |
| Diarrhea | 0.0277 (0.02216–0.03324) 9 | 0.0855 (0.0684–0.1026) 18 |
| Hypertension | 0.2820 (0.2256–0.3384) 9 | 0.2740 (0.2192–0.3288) 18 |
| Thrombocytopenia | 0.0370 (0.0296–0.0444) 9 | 0.1453 (0.11624–0.17436) 18 |
| Hand foot syndrome | 0.0513 (0.04104–0.06156) 9 | 0.1111 (0.08888–0.13332) 18 |
| Probability of total AEs (grade ⩾ 3) | 0.0981 (0.07848–0.11772) a | 0.1591 (0.12728–0.19092) a |
AEs, adverse events; OS, overall survival; PFS, progression free survival; PK, pharmacokinetics.
Probability of total AEs is a weighted average of the five adverse events.
Cost and utility estimates
Cost estimation from the perspective of China and US health systems only considered direct medical costs, including the costs of sunitinib’s drug treatment, TDM, second-line treatment, third-line treatment, management of treatment-related grade 3/4 AEs, best supportive care (BSC), and terminal care19–25 as shown in Table 2. According to clinical trial data, the standard prescribed dose of sunitinib is 50 mg/day for 4 weeks, followed by 2 weeks of discontinuation. The PK-guided individual dose will either be increased or decreased depending on the TTL of sunitinib and its metabolites (SU012662). Given the lack of data on the actual dose intensity of PK-guided sunitinib, the drug cost of PK-guided group was assumed to be basically the same as that of standard group. Drug prices were acquired from Yaozhi.com in China and Red Book Wholesale Acquisition Cost in the United States. On the basis of data on actual clinical drug regimen and relevant literature, the patients’ choice of second-line treatment included axitinib, pazopanib, nivolumab, and everolimus. Sorafenib was assumed as the third-line treatment. The proportion of each second- and third-line treatment was allocated according to the clinical data from RCTs. 9 Patients received BSC after the failure of third-line therapy and treated with terminal care prior to death. The cost of AEs was based on the incidence of grade ⩾ 3 AEs in clinical trials and the treatment cost of each AE and calculated as a weighted average. All costs of China health system were converted at the average exchange rate of US dollars in 2020 (1 USD = 6.901 RMB) and discounted until 2020.
Table 2.
Cost estimates value and health preference data.
| Parameters | Value (range) | Distribution | |
|---|---|---|---|
| Cost ($) | China | United States | |
| Cost of sunitinib/cycle | 2515.58 (1257.79–5031.16) 19 | 19,701.85 (9850.93–39,403.70) 22 | Gamma |
| Cost of TDM | 60 (48–72) a | 80 (64–96) 23 | Gamma |
| Cost of second-line treatment/cycle | 3794.6 (1897.3–7589.2) 19 | 19,437.7 (9718.85–38,875.4) 22 | Gamma |
| Cost of third-line treatment/cycle | 555 (277–832.5) 19 | 11,190.48 (5595.24–16,785.72) 22 | Gamma |
| Cost of BSC/cycle | 323 (258.4–387.6) 20 | 1404.20 (1123.36–1685.04) 24 | Gamma |
| Cost of terminal care/patient | 1940 (1552–2328) 20 | 12,401.64 (9921.31–14,881.97) 24 | Gamma |
| Cost of managing AEs (grade ⩾ 3) per event | |||
| Fatigue | 107.66 (86.13–129.19) 21 | 160.91 (128.73–193.09) 25 | |
| Diarrhea | 38.55 (64.32–96.48) 21 | 60.18 (48.14–72.22) 25 | |
| Hypertension | 80.4 (9.88–14.82) 21 | 233.72 (186.98–280.46) 25 | |
| Thrombocytopenia | 3313.89 (2651.11–3976.67) 21 | 4646.71 (3717.37–5576.05) 25 | |
| Hand–foot syndrome | 14.85 (11.88–17.82) 21 | 137.53 (110.02–165.04) 25 | |
| Total AEs cost of PK (grade ⩾ 3) | 157.09 (125.67–188.51) b | 261.46 (209.17–313.75) b | Gamma |
| Total AEs cost of standard (grade ⩾ 3) | 527.81 (422.25–633.37) b | 788.51 (630.81–946.21) b | Gamma |
| Utility | |||
| Utility of PFS | 0.73 (0.58–0.88) 26 | Beta | |
| Utility of second-line treatment | 0.66 (0.53–0.79) 26 | Beta | |
| Utility of third-line treatment | 0.55 (0.44–0.66) 27 | Beta | |
| Disutility due to AEs (grades 1 and 2) | 0.014 (0.008–0.02) 24 | Beta | |
| Disutility due to AEs (grade ⩾ 3) | 0.157 (0.11–0.204) 24 | Beta | |
| Other | |||
| Discount rate | 5% (0–8%) | Fixed | |
AE, adverse event; BSC, best supportive care; PFS, progression-free survival; PK, pharmacokinetics; TDM, therapeutic drug monitoring.
The costs come from local charge.
The total cost is a weighted average of the cost per adverse event and the incidence per adverse event.
Life years were adjusted to health-related QALYs by using the utility value in the range of 0–1 (1 means complete health, 0 means death, QALY = health status utility value × life years). Health utility values for PFS, second-line treatment, and third-line treatment were derived from literature,26,27 and disutility due to AEs was included in the model to account for the effect of AEs on the quality of life. 24
Sensitivity analysis
One-way sensitivity analysis was performed to examine the individual uncertainty of each parameter range, and the results were presented using a tornado diagram. The ranges of the parameters used in the one-way sensitivity analyses were obtained from literature; ±20% of the base-case value was used when data are not available. An assumed 50% discount of the price of sunitinib and related drugs for second-line therapy was used for one-way sensitivity analyses. For probabilistic sensitivity analysis, the Monte Carlo method was applied to simulate all variables with uncertainties within 95% CI for 1000 times, and the results were presented as a scatter plot of incremental cost effects. Probability parameters and utility values with values between 0 and 1 were set to beta distribution, and cost parameters with values greater than 0 and positively biased were set to gamma distribution. A cost-effectiveness acceptability curve was constructed to summarize the uncertainty of the evaluation under different WTP thresholds.
Results
Study characteristics
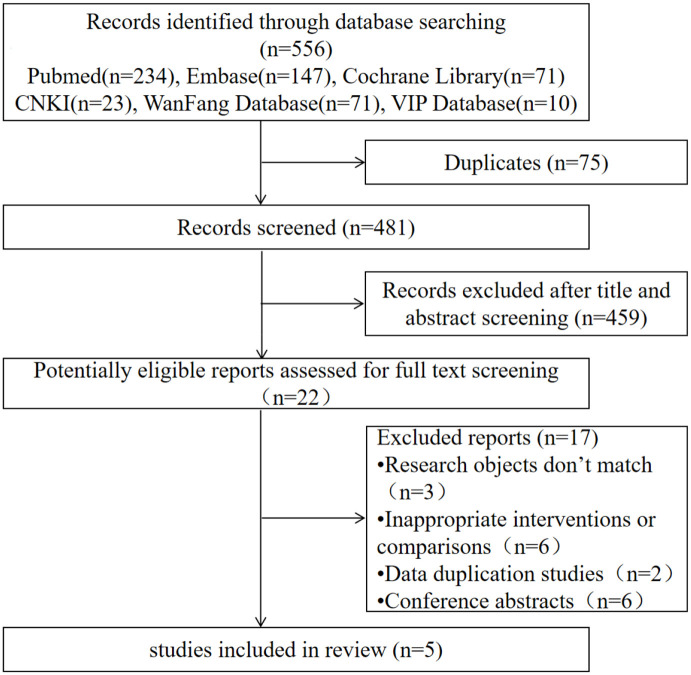
Among the 556 possibly relevant reports, 22 were proven to be eligible after duplicate removal and abstract screening. Subsequently, 17 studies were excluded (6 were conference abstracts, 6 were inappropriate interventions or comparisons, 2 were data duplication, and 3 had inconsistent research objects). Upon full-text screening, five studies9–13 were ultimately included in the systematic review, of which one was a phase II clinical trial 9 and four were cohort studies.10–13 A total of 519 patients were included, 306 of whom were in the PK-guided arm and 213 were in the standard dose arm. The flowchart of study selection is shown in Figure 2, and the characteristics of these trials are summarized in Table 3.
Figure 2.
Flowchart of study selection.
Table 3.
Summary of basic characteristics of studies.
| Author(s) (Year) |
Country | Study design | Disease type | 1. Tumor response evaluation 2. Adverse events evaluation |
Regimens | No. of Patients | Sunitinib concentration measurement | Dose adjustment | Outcome indicators |
|---|---|---|---|---|---|---|---|---|---|
| Bjarnason et al. 9 | Canada | Phase 2 clinical trial | mRCC | 1.RECIST v.1.1 2.NCI CTCAE v.3.0 |
Standard dose | 147 | 50 mg/day, 4/2 | PFS, ORR, OS, toxicity | |
| PK-guided dose | 117 | HPLC | Based on sunitinib and SU012662 concentrations | ||||||
| Noda et al. 10 | Japan | Retrospective | mRCC | 1.RECIST v.1.1 2.NCI CTCAE v.4.0 |
Standard dose | 8 | 50 mg/day, 4/2 | PFS, OS | |
| PK-guided dose | 13 | HPLC | The total concentration of sunitinib (sunitinib + SU12662)100 ng/ml is the target concentration | ||||||
| Lankheet et al. 11 | Netherlands | Prospective | aRCC | 1.RECIST v.1.1 2.NCI CTCAE v.4.0 |
Standard dose | 13 | 37.5 mg/day, once daily continuously | Toxicity | |
| PK-guided dose | 29 | LC-MS/MS | TTL < 50 ng/ml is the target concentration, Sunitinib dose levels allowed were 12.5, 25, 37.5, 50, and 62.5 mg QD | ||||||
| Bjarnason et al. 12 | Canada | Retrospective | mRCC | 1.RECIST v.1.1 2.NCI CTCAE v.4.0 |
Standard dose | 38 | 50 mg/day, 4/2 | PFS, OS | |
| PK-guided dose | 134 | HPLC | Based on sunitinib concentration to adjust dose; Dose adjustment methods are divided into the following four schemes: (1) 50 mg/day, 2/1; (2) 50 mg/day-7;(3) 37.5 mg/day-7;(4) 25 mg/day-7 |
||||||
| Takasaki et al. 13 | Japan | Prospective | mRCC | 1.RECIST v.1.1 2.NCI CTCAE v.3.0 |
Standard dose | 7 | 50 mg/day, 4/2 | PFS, TTF, toxicity | |
| PK-guided dose | 13 | LC-MS | the effective range of total sunitinib (sunitinib + N-desethyl sunitinib) concentration is 50–100 ng/ml |
aRCC, advanced renal cell cancer; HPLC, high-performance liquid chromatography; LC-MS, liquid chromatography–mass spectrometry; mRCC, metastatic renal cell cancer; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events version; ORR, objective response rate; OS, overall survival; PFS, progression free survival; PK, pharmacokinetics; QD, once daily; RECIST, Response Evaluation Criteria in Solid Tumours; TTF, time to treatment failure; TTL, total trough level.
Quality of included studies
The results of the quality assessment based on the Cochrane risk of bias tool for the included RCT are shown in Supplementary Table S2a. A major source of bias was its non-blind study design (open-label nature). The source of two other biases was unclear because of lack of data. The results of the quality assessment using NOS for the included nonrandomized studies are shown in Supplementary Table S2b. Only three studies received a score of 6 points or more (indicating high quality), and one cohort study received a score of 5 points due to insufficient article information (indicating low quality). For comparability, all studies were scored 0 because of the lack of report on whether the intervention and control groups were matched according to specific factors. Given that the studies with a score of 6 or more were retrospective or prospective cohort, the number of patients included was relatively small, and complete outcome indicators were lacking. After comprehensive examination, the cost-effectiveness analysis was conducted using phase II clinical trial data.
Cost-effectiveness analysis
Base-case analysis
Model validation results showed that the OS and PFS generated by the model were close to those obtained in the clinical trials (Supplementary Figures S1–S4), indicating the consistency between the data from the fitting curve and the original data.
Table 4 shows the costs and health outcomes of two strategies obtained after running the model. The QALY of the patients in the PK-guided group increased by 0.83 compared with that in the standard dose group. The ICER for PK-guided dose versus standard dose was −21,594.83 US$/QALY in China and −120,192.60 US$/QALY in the United States. Therefore, PK-guided individualized dosing is cost-effective because of its low price and additional health outcomes.
Table 4.
Base-case results.
| Strategy | Costs (US$) | ∆Costs (US$) | QALY | ∆QALY | ICER (US$/QALY) | Dominance |
|---|---|---|---|---|---|---|
| China | ||||||
| PK-guided | 133,547.95 | — | 2.77 | — | — | |
| Standard dose | 151,376.73 | 17,828.78 | 1.94 | −0.83 | −21,594.83 | Dominated |
| United States | ||||||
| PK-guided | 884,982.65 | 99,231.52 | 2.77 | — | — | |
| Standard dose | 984,214.16 | — | 1.94 | −0.83 | −120,192.60 | Dominated |
ICER, incremental cost-effectiveness ratio; PK, pharmacokinetics; QALY, quality-adjusted life years.
Sensitivity analysis
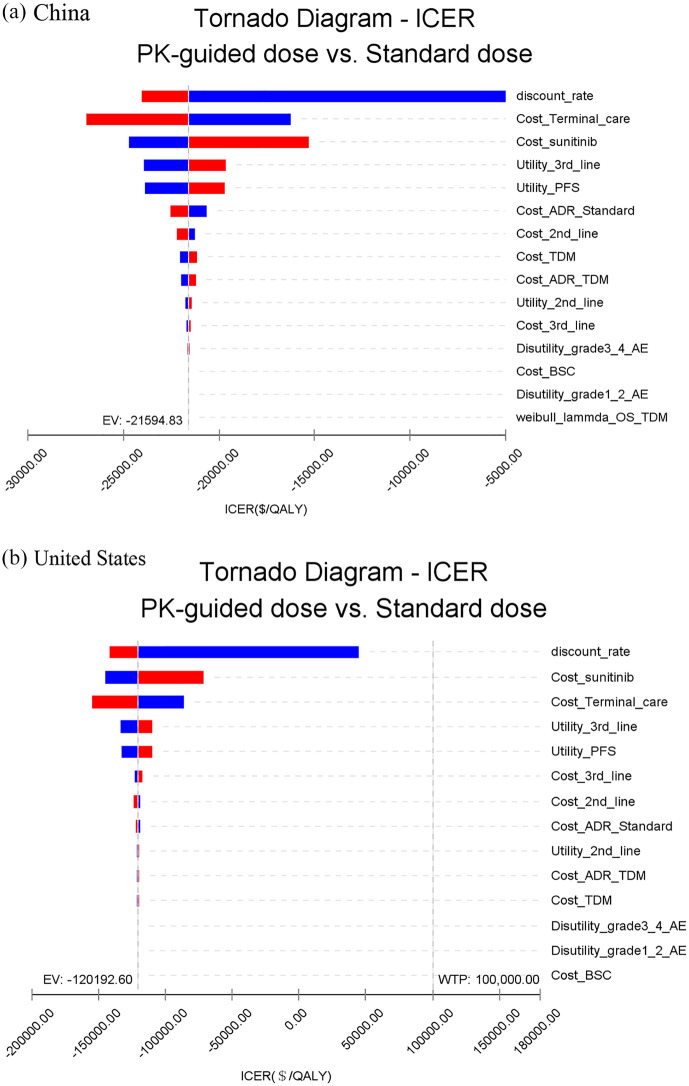
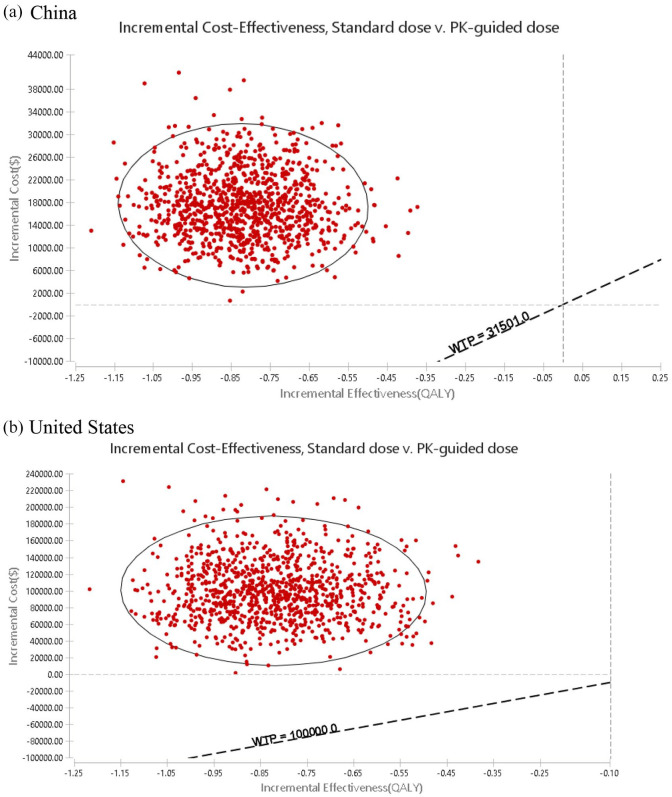
One-way sensitivity analysis showed that the results were robust to parameter changes. Within the variation range of each parameter, the results had the highest sensitivity to the discount rate, followed by the cost of terminal care. However, the variation did not exceed the threshold, that is, the result cannot be reversed. For the United States, the results were also most sensitive to the discount rate, followed by the cost of sunitinib. Similarly, these parameters cannot reverse the results (Figure 3). For incremental cost-effectiveness, probabilistic sensitivity analysis showed that 100% of the scatter points were above the threshold line in China and the United States. This finding indicated that the economical results of the standard dose group were inferior to those of PK-guided group, and this result was reliable (Figure 4).
Figure 3.
Tornado diagram: results of one-way deterministic sensitivity analysis.
Figure 4.
Incremental cost-effective scatter plot of standard group versus PK-guided group.
Discussion
Owing to the small number of included studies and the inconsistent quality of the articles, the intervention and control groups were not matched by propensity, and the resulting large heterogeneity prevented any formal meta-analysis. Therefore, the results used in the decision analysis model were based on the best relevant data of existing research without statistical synthesis. This study is the first to evaluate the cost-effectiveness of PK-guided sunitinib administration in untreated patients with mRCC. The PK-guided dose had lower costs and produced more QALYs than the standard dose, suggesting the cost-effectiveness of the former. Sensitivity analysis was conducted on the major influencing factors to verify the stability of the model and the reliability of the advantage strategy. Given the variation in drug prices across different regions due to local affordability and market scheme, diverse health settings were considered in the economic evaluation for easy transferability among different regions. Given that the clinical data and utility values were obtained from foreign countries, the economics of the two strategies was analyzed from the perspective of Chinese and American health systems to reflect realistic results.
Although sunitinib has evidence-based clinical efficacy, its severe toxicity to certain patients has become an important issue in clinical decision-making for appropriate treatment. Considerable drug exposure may be one of the reasons for the serious toxicity of this medication. 13 For patients of different body weights, genders, or ages, the recommended dose is 50 mg/day (dose reduction is only considered when serious AEs have occurred); hence, the PK variability has remarkably increased among individuals. 28 With the subsequent emergence of treatment-related AEs, maintaining the standard dosing regimen of sunitinib might be difficult. Therefore, other schedules have been proposed to improve the safety and increase the dose intensity of sunitinib. One alternative is sunitinib 50 mg/day with 2 weeks on treatment and 1 week off (schedule 2/1) and 37.5 mg/day on the continuous daily dosing (CDD) schedule. Based on the available evidence, the 2/1 schedule is relatively more effective and safer than the 4/2 schedule, and is feasible way to maintain drug level.29,30 Comparison of intermittent versus continuous dosing regimens revealed that CDD does not have any advantage over 4/2 schedule in terms of the incidence or severity of AEs or patient-reported outcomes. 18
Clinically, because the AEs risk from low-dose sunitinib is lower than that from high-dose sunitinib, many clinicians will choose to directly give low-dose sunitinib to patients with mRCC to avoid the risk. This practice is actually not recommended. Clinicians are required to balance the clinical efficacy and the risk of toxicity and choose the appropriate treatment regimen for the patient, rather than reducing the risk of AEs only by reducing the dose. Admittedly, 2/1 schedule is safer and more effective than 4/2 schedule and more convenient than TDM, but unfortunately it may not be suitable for all patients. Regardless of the schedule, good treatment results and few adverse reactions are not guaranteed for all patients. Therefore, it is important to adjust the drug concentration appropriately according to the actual situation of the patient, rather than a unified schedule. In addition, TDM could also be helpful in unique populations, such as in age or weight extremes (children, elderly, patients with limb amputation, or obese patients), or rare ethnic/genetic groups of patients.
This study has limitations. First, the phase II clinical trial data used in the cost-effectiveness analysis was a single-arm study. The control group was based on patients with similar qualification standards who received standard dose, resulting in differences in patients’ baseline characteristics and follow-up personnel. This phenomenon would have a certain impact on the reliability of current results. Therefore, a prospective randomized clinical trial is needed to finally determine the value of PK-guided dose. Second, the clinical trial data used in the model were obtained from a foreign phase II clinical trial, and the utility value was derived from the EQ-5D (EuroQol five-dimension questionnaire) scale score for Dutch patients with mRCC. Therefore, the current findings may not reflect Chinese data. However, sensitivity analysis showed that these parameters have minimal impact on the cost-effectiveness of treatment strategies. Third, the actual dose intensity of drugs after adopting PK guidance was not provided in the evaluation of drug cost, leading to the possibility of dose downward or upward adjustment. Hence, the drug cost of PK group was not accurately estimated. Although the current hypothesis was consistent with the standard dose with minimal deviations, the results cannot be reversed. Fourth, various phase 3 studies showed that the actual median dose of sunitinib ranges 30–46 mg/day for patients using a fixed dose of 50 mg/day. Given that this median dose is difficult to accurately quantify, the 50 mg dose was still used in the model. However, setting the cost of sunitinib at ±50 to reduce the difference caused by hypothesis would consequently decrease its impact on the actual results. Fifth, the cost of grade 1/2 AEs was excluded because of lack of evidence indicating the notable differences between PK-guided and standard doses. In addition, cost data sources were currently unavailable. To date, many large hospitals in China have not yet launched the TDM of sunitinib. Despite these limitations, the results reflect the general clinical conditions of Chinese patients with mRCC and provide important reference for Chinese decision-makers.
Conclusion
From the perspective of China and US health systems, the PK-guided treatment of sunitinib may be a safe, effective, and economical intervention for patients with mRCC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221085212 for Systematic review and cost-effectiveness of pharmacokinetically guided sunitinib individualized treatment for patients with metastatic renal cell carcinoma by Tingting Chen, Jiahe Chen, Chaoxin Chen, Jianming Guo, Xin He, Song Zheng, Maobai Liu and Bin Zheng in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Tingting Chen: Data curation; Formal analysis; Methodology; Software; Writing – original draft.
Jiahe Chen: Data curation; Methodology; Software; Writing – review & editing.
Chaoxin Chen: Formal analysis; Methodology; Software; Writing – review & editing.
Jianming Guo: Conceptualization; Methodology; Software; Validation.
Xin He: Investigation; Methodology; Validation.
Song Zheng: Investigation; Methodology; Validation.
Maobai Liu: Funding acquisition; Investigation; Project administration.
Bin Zheng: Conceptualization; Funding acquisition; Investigation; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Science and Technology Department of Fujian Province (grant number: 2020R0052) and Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2018Y9037).
Ethics statement: This study was based on a literature review and modeling techniques; this study did not require approval by an institutional research ethics board.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tingting Chen, College of Pharmacy, Fujian Medical University, Fuzhou, ChinaDepartment of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China.
Jiahe Chen, Department of Pharmaceutical and Health Economics, School of Pharmacy, University of Southern California, Los Angeles, CA, USALeonard D. Schaeffer Center for Health Policy & Economics, University of Southern California, Los Angeles, CA, USA.
Chaoxin Chen, College of Pharmacy, Fujian Medical University, Fuzhou, ChinaDepartment of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China.
Jianming Guo, College of Pharmacy, Fujian Medical University, Fuzhou, ChinaDepartment of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China.
Xin He, College of Pharmacy, Fujian Medical University, Fuzhou, ChinaDepartment of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China.
Song Zheng, Department of Urology, Fujian Medical University Union Hospital, Fuzhou, China.
Maobai Liu, Fujian Medical University Union Hospital, 29,Xinquan Road, Gulou District, Fuzhou City 350001, Fujian Province, China.
Bin Zheng, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian, ChinaCollege of Pharmacy, Fujian Medical University, Fuzhou, China.
References
- 1. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124. [DOI] [PubMed] [Google Scholar]
- 2. Casali PG, Abecassis N, Aro HT, et al. ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29(Suppl. 4): iv68–iv78. [DOI] [PubMed] [Google Scholar]
- 3. Demlová R, Turjap M, Peš O, et al. Therapeutic drug monitoring of sunitinib in gastrointestinal stromal tumors and metastatic renal cell carcinoma in adults – a review. Ther Drug Monit 2020; 42: 20–32. [DOI] [PubMed] [Google Scholar]
- 4. Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 2006; 24: 25–35. [DOI] [PubMed] [Google Scholar]
- 5. Flaig TW, Kim FJ, La Rosa FG, et al. Colonic pneumatosis and intestinal perforations with sunitinib treatment for renal cell carcinoma. Invest New Drugs 2009; 27: 83–87. [DOI] [PubMed] [Google Scholar]
- 6. Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010; 66: 357–371. [DOI] [PubMed] [Google Scholar]
- 7. de Jonge ME, Huitema AD, Schellens JH, et al. Individualised cancer chemotherapy: strategies and performance of prospective studies on therapeutic drug monitoring with dose adaptation: a review. Clin Pharmacokinet 2005; 44: 147–173. [DOI] [PubMed] [Google Scholar]
- 8. Touw DJ, Neef C, Thomson AH, et al. Cost-effectiveness of therapeutic drug monitoring: a systematic review. Ther Drug Monit 2005; 27: 10–17. [DOI] [PubMed] [Google Scholar]
- 9. Bjarnason GA, Knox JJ, Kollmannsberger CK, et al. The efficacy and safety of sunitinib given on an individualised schedule as first-line therapy for metastatic renal cell carcinoma: a phase 2 clinical trial. Eur J Cancer 2019; 108: 69–77. [DOI] [PubMed] [Google Scholar]
- 10. Noda S, Otsuji T, Baba M, et al. Assessment of sunitinib-induced toxicities and clinical outcomes based on therapeutic drug monitoring of sunitinib for patients with renal cell carcinoma. Clin Genitourin Cancer 2015; 13: 350–358. [DOI] [PubMed] [Google Scholar]
- 11. Lankheet NA, Kloth JS, Gadellaa-van Hooijdonk CG, et al. Pharmacokinetically guided sunitinib dosing: a feasibility study in patients with advanced solid tumours. Br J Cancer 2014; 110: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bjarnason GA, Khalil B, Hudson JM, et al. Reprint of: outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol 2015; 33: 171–178. [DOI] [PubMed] [Google Scholar]
- 13. Takasaki S, Kawasaki Y, Kikuchi M, et al. Relationships between sunitinib plasma concentration and clinical outcomes in Japanese patients with metastatic renal cell carcinoma. Int J Clin Oncol 2018; 23: 936–943. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chichester: Cochrane Collaboration, 2011, pp. 1–8. [Google Scholar]
- 15. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 10 June 2019).
- 16. Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in metastatic renal cell carcinoma. Future Oncol 2017; 13: 1967–1980. [DOI] [PubMed] [Google Scholar]
- 17. Eichler HG, Kong SX, Gerth WC, et al. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge. Value Health 2004; 7: 518–528. [DOI] [PubMed] [Google Scholar]
- 18. Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol 2012; 30: 1371–1377. [DOI] [PubMed] [Google Scholar]
- 19. Yaozhi Network, https://www.yaozh.com/ (accessed 15 August 2021).
- 20. Chen J, Hu G, Chen Z, et al. Cost-effectiveness analysis of pembrolizumab plus axitinib versus sunitinib in first-line advanced renal cell carcinoma in China. Clin Drug Investig 2019; 39: 931–938. [DOI] [PubMed] [Google Scholar]
- 21. Wu B, Dong B, Xu Y, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS ONE 2012; 7: e32530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RED BOOK Online, http://www.micromedexsolutions.com/ (2020, accessed 20 August 2021).
- 23. Physician fee schedule, https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/
- 24. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer 2018; 206: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perrin A, Sherman S, Pal S, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ 2015; 18: 200–209. [DOI] [PubMed] [Google Scholar]
- 26. de Groot S, Redekop WK, Versteegh MM, et al. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res 2018; 27: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Remák E, Charbonneau C, Négrier S, et al. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol 2008; 26: 3995–4000. [DOI] [PubMed] [Google Scholar]
- 28. Gao B, Yeap S, Clements A, et al. Evidence for therapeutic drug monitoring of targeted anticancer therapies. J Clin Oncol 2012; 30: 4017–4025. [DOI] [PubMed] [Google Scholar]
- 29. Chung DY, Kang DH, Kim JW, et al. Does an alternative sunitinib dosing schedule really improve survival outcomes over a conventional dosing schedule in patients with metastatic renal cell carcinoma? An updated systematic review and meta-analysis. Cancers 2019; 11: 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng H, Li M, Wu Q, et al. A 2/1 sunitinib dosing schedule provides superior antitumor effectiveness and less toxicity than a 4/2 schedule for metastatic renal cell carcinoma: a systematic review and meta-analysis. Front Oncol 2020; 10: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221085212 for Systematic review and cost-effectiveness of pharmacokinetically guided sunitinib individualized treatment for patients with metastatic renal cell carcinoma by Tingting Chen, Jiahe Chen, Chaoxin Chen, Jianming Guo, Xin He, Song Zheng, Maobai Liu and Bin Zheng in Therapeutic Advances in Medical Oncology