Abstract
Introduction:
We carried out systematic review and network meta-analysis to investigate the role of stem cell therapy (SCT) in the management of erectile dysfunction (ED) secondary to cavernous nerve injury in rats and post-radical prostatectomy (RP) in humans.
Patients and Methods:
The protocol was registered with PROSPERO database. We searched studies analyzing the efficacy of SCT for ED due to bilateral cavernous nerve injury (BCNI) in rats using Healthcare Databases Advanced Search (HDAS) Export software (MEDLINE, EMBASE, Scopus) from inception to September 2020. The outcome measurements, for 29 animal studies, were intracavernosal pressure (ICP), ICP/MAP (mean arterial pressure) ratio, and histological/molecular changes. All three available human trials evaluating SCT in post-RP ED were assessed for International Index for Erectile Function (IIEF) Score and Erection Hardness Score (EHS).
Results:
For ICP measurement, animal studies were divided into adipose-derived stem cells (ADSCs) subgroup and bone marrow–derived stem cells (BMSCs) subgroup. Pooled analysis of these studies showed a beneficial effect of SCT in improving erectile function in rats with BCNI using network meta-analysis (95% confidence interval, CI; p < 0.001). There was an increase in ICP/MAP ratio in stem cell groups (including co-intervention) compared with control BCNI group. Histological and molecular evaluation of penile tissue revealed an increase in neuronal nitric oxide synthase (nNOS), smooth muscle content, and anti-apoptotic activity. Human trials revealed improved IIEF (70–150% from baseline at 6 months) and EHS (80–200% from baseline).
Conclusion:
Our results confirm that SCT does improve the erectile function in rats having cavernous nerve injury. Similarly, early human results have shown promising results.
PROSPERO registration ID:
CRD42020201343.
Keywords: Radical Prostatectomy, Erectile dysfunction, Stem cells
Introduction
Prostate cancer (PCa) is the second most frequent cancer diagnosis made in men and the fifth leading cause of death worldwide. 1 Radical prostatectomy (RP) remains the most commonly employed procedure for localized PCa in patients with a life expectancy of at least 10 years, which represents 25% of patients with PCa.1,2 Erectile dysfunction (ED) is a common complication after RP. 3 It is estimated that 86% patients experience ED after RP.4,5 Several factors determine the development of ED after RP. The important ones include patient age, preoperative potency status (baseline Erectile function), comorbidities, performance of nerve-sparing RP (unilateral versus bilateral), type of surgery (intra- versus inter- versus extra-fascial), surgical technique (open versus laparoscopic versus robot-assisted RP), and level of surgical experience.4,6 It was thought that robotic techniques will reduce ED in RP, but a recent meta-analysis has not been able to establish the same. 7
Post-RP ED substantially decreases quality of life (QoL) of the afflicted men and their sexual partners. 8 The main stay of treatment is phosphodiesterase type-5 inhibitors (PDE5Is) with other strategies being intraurethral alprostadil, intracorporal injection therapy, vacuum erection devices, and surgical procedures like penile revascularization and penile prosthesis implantation. 9 It is important to mention that majority of these strategies treat ED symptomatically and do not address the underlying cause of ED. Furthermore, limitations to their use exist, such as intolerance to side effects, cost limitations, and unsatisfactory outcomes. 10 Newer modalities [such as stem cell therapy (SCT) and low intensity extracorporeal shock wave therapy (LI-SWT)] are being investigated to develop a curative treatment for post-RP ED with the aim to restore cavernous nerves and rehabilitate penile erectile tissue.10,11 Stem cells are hypothesized to address both of these goals and as such being looked into their potential role in curative management of post-RP ED.
SCTs have been used in various clinical conditions due to immunoregulatory, immunosuppressive, and regenerative properties. In recent years, it has been established that within penile tissue, stem cells can differentiate into endothelial, neuronal, or smooth muscle cells and are capable of restoring possible structural damage in the penile tissue. Based on these very properties, several animal and human trials have been performed to evaluate the role of mesenchymal stem cells (MSCs) in the treatment of post-RP ED. In ED research, three types of stem cells are commonly used, including adipose-derived stem cells (ADSCs), bone marrow–derived stem cells (BMSCs), and muscle-derived stem cells (MDSCs). 12
In this meta-analysis, an attempt has been made to summarize and analyze animal studies having SCT for ED due to cavernous nerve injury with outcome measurements being intracavernosal pressure (ICP), ICP/MAP (mean arterial pressure) ratio, and histological as well as molecular changes in penile tissue. All three available human trials evaluating SCT in post-RP ED were assessed for International Index for Erectile Function (IIEF) and Erection Hardness Score (EHS).
Methods
The study protocol was registered with PROSPERO (CRD42020201343).
Evidence acquisition
Criteria for considering studies for this review
The inclusion criteria for animal studies included the following:
Animals/population: Male rats, ED secondary to cavernous nerve injury
Intervention/exposure: SCT
Comparator/control: Sham versus bilateral cavernous nerve injury (BCNI)/vehicle/stem cell/co-intervention/combined
The inclusion criteria for human studies included the following:
Animals/population: Male patients, ED secondary to RP
Intervention/exposure: SCT
The exclusion criteria for animal studies included the following:
Animals/population: Use of stem cells in male rats in which ED was due to other causes like metabolic/neurological.
Intervention/exposure: If the interventions are ill defined or dose/frequency is not mentioned or/ structural methods are inadequate.
Comparator/control: Studies which lacked proper grouping into control, Sham, and intervention.
Study design: Studies which did not fulfill above criterion and lacked any defined outcomes.
The exclusion criteria for human trials included studies using stem cells in male patients having ED due to non-prostatectomy causes.
Outcome measures
Animal studies
Primary outcomes:
ICP measurements
ICP/MAP ratio
Secondary outcomes: (histological/molecular)
Increase in nitric oxide synthase (NOS) fibers
Increase in smooth muscle content
Miscellaneous immune response–related changes
Human studies
IIEF score
EHS
Change in penile length
Adverse effects
Search method for the identification of studies
Electronic searches
The systematic review was done in accordance with Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). The search was limited to the English language. Healthcare Databases Advanced Search (HDAS) Export software was used for searching MEDLINE, EMBASE, and also searched other databases (inception to September 2020). Search terms included ‘Prostatectomy’, ‘Erectile dysfunction’, ‘Stem cells’, ‘Rats’, ‘Animal experiments’, and ‘Human trials’. Boolean operators (‘And’/‘Or’) were used.
Data extraction
Two reviewers (M.M.W., S.M.) independently screened all abstracts and full-text articles for eligibility according to the criteria for considering studies for this review. Any disagreements were resolved by mutual consensus. Two authors (M.M.W., S.M.) extracted data from individual studies.
For animal studies, the data were grouped into data sheets having columns for type/source of stem cells, any co-intervention, total rats, randomization of rats, mode of cavernosal nerve injury (crush/electric/cryo), route (intracavernosal, intravenous, local) and dose of stem cells (most common dose used is 1.0 × 106 cells), and assessment interval (variable, most studies have performed at 4 weeks post-intervention) (Table 1).
Table 1.
Animal study: data extraction results.
| Reference | Stem cell | Type/source | Co-intervention | Total rats | Experiment randomization | Type of injury | Route (average time of treatment 2–4 h after injury) | Dose | Assessment interval | ED function assessment | Histological/Molecular assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Zheng et al.
13
China |
ADSC | Allogenic SD rat Groin |
ICA II | 42 SD | Divided into six groups a. Sham group = 7 b. BCNI only = 7 c. BCNI + PBS = 7 d. BCNI + ADSC = 7 e. BCNI + ICI II = 7 f. BCNI + ICI II + ADSC = 7 |
BCNI (transection injury) | ICI, ICA II intragastric | ADSCs (5 × 105) ICA (4.5 mg/kg/day) |
1 week | ICP value and ICP/MAP ratio | Western blotting CCK 8 assay RNA immunoprecipitation NOS content |
| 2. Yang et al.
14
China |
ADSC | Allogenic SD rat inguinal fat pad |
rAd expressing hBDNF | 40 SD | Divided into four groups a. Sham group = 10 b. BCNI only = 10 c. ADSCs infected with lenti-GFP = 10 d. ADSCs infected with lenti-GFP = 10 |
BCNI (crush) | ICI | ADSCs infected with lenti-GFP (1 × 106 in 20 µl) and lenti-rBDNF (1 × 106 in 20 µl) | 4 weeks | Maximal ICP/MAP ratio | Western blot Masson trichrome staining NOS content |
| 3. Yang et al.
15
China |
ADSC | Allogenic Rat epididymis |
Nil | 36 SD | Divided into six groups a. Sham group = 6 b. PBS = 6 c. ADSC = 6 d. VEGF (ADSC-V) = 6 e. GDNF (ADSC-G) = 6 f. VEGF and GDNF (ADSC-G&V) = 6 |
BCNI (crush injury) | MPG | Cell-fibrin scaffolds (1.5 × 106 cells, 100 µl per rat) were prepared | 2 weeks | Mean ICP | Western blotting |
| 4. Chen et al.
16
China |
ADSC Pluripotent stem cells |
Human adipose tissue | iMSC and human adMSC | Not available | Divided into four groups a. Sham operation (sham group) b. adMSC group (adipose-derived MSC group) c. Induced pluripotent stem cell-derived MSC (iMSC group). d. PBS Group (PBS only group) |
BCNI (crush injury) | Intracavernosal (ICI) | 1 × 106 adMSC cells 1 × 106 iMSC cells Both in 20 µl of PBS |
4 weeks | ICP/MAP ratio | Western blot Masson trichrome staining Immunoreaction detection |
| 5. Ying et al.
17
China |
ADSC NAS |
Allogenic Rat inguinal fat |
Nil | 45 SD rats | Divided into three groups a. Sham group = 15 b. BCNI with PBS = 15 c. BCNI with NAS = 15 |
BCNI (crush injury) | ICI | One million NAS | 4 weeks | Maximal ICP/MAP ratio | Masson s trichrome staining Toluidine staining NOS content |
| 6. Wu et al.
18
Taiwan |
BM-MSC | Allogenic Rat femur |
SPC | 24 SD rats | Divided into three groups a. Sham group = 8 b. Vehicle group = 8 c. SPC group = 8 |
BCNI (crush injury) | ICI | NA | 4 weeks | Maximal ICP/MAP ratio | Histology NOS content Immunoflorescence |
| 7. Matsuda et al.
19
Japan |
BM-MSCs | Allogenic Rat bone marrow |
Nil | 18 SD rats | Divided into three groups a. Sham group = 6 b. Vehicle derived MSC = 6 c. BM-MSC = 6 |
BCNI (electric coagulation) | Intravenous, right jugular vein | 1.0 × 106 cells | 4 weeks | ICP/MAP ratio, with ICP-AUC analysis | Masson s trichrome staining Fluorogold study Green fluorescence |
| 8. Ouyang et al.
20
China |
MSCs and MSC exosomes | Allogenic Rat bone marrow |
Nil | 32 SD rats | Divided into four groups a. Sham = 8 b. PBS = 8 c. MSC = 8 d. MSC exosomes = 8 |
BCNI (crush injury) | ICI | MSC cells: 1.5 × 106
MSC exosomes: 100 µg |
4 weeks | Maximal ICP increase | Western blotting TUNEL staining |
| 9. Li et al.
21
China |
ADSCs and BMSCs | NA | Exosomes derived from ADSC (ADSC-Exo) and BMSC (BMSC-Exo) | 48 SD rats | Divided into four groups a. Sham group = 12 b. BCNI + vehicle MSC = 12 c. BCNI + exosomes derived from ADSCs (ADSC-Exo group) = 12 d. BCNI + BMSC-derived exosomes (BMSC-Exo) = 12 |
BCNI (crush injury) | ICI | 100 µg proteins of exosomes derived from ADSC/BMSC | 3 weeks | Maximal ICP/MAP ratio | Masson s trichrome staining Imaging fluorescence |
| 10. Zheng et al.
22
China |
ADSCs | Allogenic SD rats |
ICA II promoted the differentiation of ADSCs to SCs | 36 SD rats | Divided into six groups a. Sham (n = 6) b. BCNI (n = 6) c. PBS (n = 6) d. ADSCs (n = 6) e. ICA II (n = 6) f. ICA II + ADSCs (n = 6) |
BCNI (crush injury) | ICI and intragastric for ICA II | ADSC, 1 × 106 cells, ICA II (4.5 mg/kg/day) | 4 weeks | ICP and MAP | Western blotting qRT-PCR |
| 11. Wu et al. 23 China | ADSCs | Allogenic SD rats |
Nanotechnology-assisted (Nano-ADSC)-injected group with an external magnetic field | 40 SD rats | Divided into four groups: a. Sham (n = 10) b. BCNI (n = 10) c. ADSCs (n = 10) d. Nano-ADSC (n = 10) |
BCNI (crush injury) | ICI | 2 × 105 cells | 4 weeks | ICP/MAP ratio | Western blotting Immunohistochemistry In Vivo ADSC tracing |
| 12. Chen et al.
24
China |
ADSCs | Allogenic SD rats |
Nil | 40 SD rats | Divided into four groups a. Sham = 32 b. BCNI = 32 c. PBS = 8 d. ADSC = 8 (four rats died in Sham and BCNI groups) |
BCNI (crush injury) | Intracavernosal (ICI) | 2 × 105 cells | 4 weeks | ICP/MAP ratio | Masson s trichrome staining Fluorescence Staining |
| 13. Fang et al.
25
China |
BM-MSC (r-MSC) | Allogenic Rat bone marrow |
Neural d-MSCs Using trans-retinoic acid for differentiation |
50 SD rats | Divided into five groups (10 in each) a. sham operation group = 10 b. BCNI + ICI of r-BM-MSCs (IC group) = 10 c. PPI of r-BM-MSCs (IP group) = 10 d. PPI of d-MSCs (IP-d group) = 10 e. PPI of PBS (PBS group) = 10 |
BCNI (crush injury) | Intracavernosal (ICI) and PPI | 1 × 106 cells | 2 weeks | ICP, MAP, and AUC | Western blotting Immunofluorescence staining Immunohistochemical staining |
| 14. Jeon et al.
26
Korea |
ADSC | Human fat | Low energy SWT | 50 SD rats | Divided into five groups a. Sham group = 10 b. BCNI group = 10 c. BCNI + ADSC group = 10 d. BCNI + SWT group = 10 e. BCNI + SWT + ADSC group = 10 |
BCNI (crush injury) | Around injured cavernous injury | 1 × 106 cells | 4 weeks | ICP, ICP/MAP | Immunostaining, Western blotting, and a cyclic guanosine monophosphate assay |
| 15. Song et al.
27
China |
Umbilical cord-MSCs | hUCB mesenchymal stem cells (hUCB-MSCs) | BDNF | 42 SD rats | Divided into four groups Group A: sham operation sham + PBS, n = 6 Group B: BCNI + PBS, 12 Group C: BCNI + hUCB-MSCs, n = 12 Group D: BCNI + BDNF-hUCB-MSCs n = 12 |
BCNI (electric coagulation) | Intracavernosal (ICI) | 2 × 106 cells | 4 weeks | ICP/MAP | Western blotting |
| 16. Yang et al.
28
China |
ADSC | Allogenic rats | Nil | 39 SD rats | Divided into three groups a. Sham group = 13 b. Cryo group = 13 c. ADSC group = 13 |
BCNI (cryoinjury) | ICI | 1 × 106 cells | 4 weeks | ICP/MAP | Western blotting Immunofluorescence staining Immunohistochemical staining |
| 17. Kim et al.
29
South Korea |
BM-MSCs | Human (iliac crest) | SPION-MSC | 30 SD rats | Divided into three groups a. Sham group = 10 b. BCNI group = 10 c. SPION-MSC group = 10 |
BCNI (crush injury) | ICI | 1 × 106 cells | 2 and 4 weeks | ICP/MAP | MRI Histological evaluation |
| 18. Lee et al.
30
South Korea |
ADSCs | Human | BDNF | 75 SD rats | Divided into five groups a. Normal (N) group = 15 b. Saline application = 15 c. Fibroblast growth factor-hydrogel injection (bFGF) = 15 d. ADSC application covered with BDNF – membrane after BCNI (ADSC/BDNF) = 15 e. The co-administration of bFGF-hydrogel injection and BDNF-membrane with ADSC after BDNF (bFGF + ADSC/BDNF) = 15 |
BCNI (crush injury) | ICI | 1 × 106 cells | 4 weeks | ICP/MAP | Masson s trichrome staining Western blotting NOS expression |
| 19. Kim et al.
31
South Korea |
ADSCs | Human | NGF-hydrogel | 25 SD rats | Divided into five groups a. Sham = 5 b. BCNI + Saline application = 5 c. BCNI + ADSCs = 5 d. BCNI + NGF = 5 e. BCNI + ADSC + NGF = 5 |
BCNI (crush injury) | MPG | 1 × 106 cells | 4 weeks | ICP/MAP ratio | Immunoflorescence staining Western Blot for eNOS and nNOS |
| 20. You et al.
32
South Korea |
BM-MSC | Human (hBMSC) | Nil | 40 SD rats | Divided into four groups a. Sham = 10 b. ICI of PBS + PPI of fibrin sealent = 10 c. hBMSCs (ICI group) = 10 d. hBMSCs (PPI + ICI group) = 10 |
BCNI (dissection) | MPG and ICI | 1 × 106 cells | 4 weeks | ICP/MAP ratio | Western blotting Immunohistochemical analysis |
| 21. You et al.
33
South Korea |
ADSCs | Human | Fibrin scaffolds for periprostatic injection of ADSCs | 60 SD rats | Divided into six groups a. Sham = 10 b. BCNI = 10 c. ICI of PBS + PPI of fibrin sealent = 10 d. PPI of ADSC-seeded fibrin sealant = 10 e. ICI of ADSC = 10 f. PPI of ADSC-seeded fibrin sealant and ICI of ADSCs = 10 |
BCNI (dissection) | MPG and ICI | 1 × 106 cells | 4 weeks | ICP/MAP ratio with AUC | Western blotting Immunohistochemical analysis |
| 22. Jeong et al.
34
South Korea |
ADSC | Human | BDNF, oral Udenafil in desired group | 30 SD rats | Divided into five groups a. Sham group = 6 b. BCNI = 6 c. BCNI + Oral Udenafil = 6 d. BCNI + ADSC + BDNF = 6 e. BCNI + ADSC + BDNF + Oral Udenafil = 6 |
BCNI (crush injury) | Local application to injured nerve | 1 × 106 cells | 4 weeks | ICP/MAP ratio | Immunohistochemical analysis Masson s trichrome cGMP eNOS |
| 23. Qiu et al.
35
USA |
ADSC | Autologous rat | Adipose-derived SVF (immediate group received SVF after BCNI, delayed group received SVF after 4 weeks) | 89 SD rats | Divided into four groups a. Sham group = 26 b. BCNI + Saline = 23 c. BCNI + Immediate SVF = 17 d. BCNI + Delayed SVF = 23 |
BCNI (crush injury) | ICI | 2 × 106 cells | 12 weeks | ICP | Masson s trichrome |
| 24. Kim et al.
36
South Korea |
BM-MSCs | Allogenic rats (femur/tibia) | Matrixen used (it is a collagen-based biocompatible polymer and it has been used widely as scaffolds in medical field) | 50 SD rats | Divided into five groups a. Sham group = 5 b. BCNI = 10 c. BCNI + MSCs = 10 d. BCNI + Matrixen = 10 e. BCNI + MSC + Matrixen = 10 |
BCNI (crush injury) | MPG | 1 × 106 cells Matrixen 20 µl |
4 weeks | ICP/MAP ratio | PKH-26/NGF co-staining into cavernous nerve, histological and immunohistochemical analysis |
| 25. Kim et al.
37
South Korea |
BM-MSCs | Allogenic rats (femur/tibia) |
BDNF (rAd/hBDNF represents MSCs infected with rAd expressing human BDNF) | 40 SD rats | Divided into four groups a. Sham group = 10 b. BCNI = 10 c. BCNI + MSC = 10 d. BCNI + MSC infected with rAd/hBDNF = 10 |
BCNI (crush injury) | MPG | 1 × 106 cells | 4 weeks | ICP/MAP ratio | Masson s trichrome staining eNOS and nNOS protein expression by Western blotting |
| 26. Woo et al.
38
South Korea |
Muscle derived-MSC (MD-MSC) |
Allogenic white rats (derived from femoral muscles) | MD-MSC was labeled with PKH-26 fluorescent cell linker | 15 White rats | Divided rats into three groups a. Sham group = 5 b. BCNI = 5 c. BCNI + MD-MSC = 5 |
BCNI (transection injury) | ICI | 1 × 106 cells | 4 weeks | ICP | Histology Measurement of cGMP |
| 27. Albersen et al.
39
USA |
ADSC | Autologous (paratesticular pad of adipose tissue) | Nil | 32 SD rats | Divided rats into four groups a. Sham group + PBS = 8 b. BCNI + PBS = 8 c. BCNI + ADSC Lysate = 8 d. BCNI + ADSC = 8 |
BCNI (crush injury) | ICI | 1 × 106 cells | 4 weeks | ICP/MAP | Histology TUNEL staining |
| 28. Kim et al.
40
USA |
MD-MSC and HBSS used (Hanks’ Balanced Salt Solution) | Allogenic (MD-MSC from gastrocnemius muscle of female SD rat) | Rat MDC were transduced with retrovirus carrying the β-galactosidase reporter gene | 30 SD rats | Divided rats into five groups a. Sham = 6 b. BCNI + MDSC + HBSS = 6 (2 weeks) c. BCNI + MDSC + HBSS = 6 (4 weeks) d. BCNI + HBSS = 6 (2 weeks) e. BCNI + HBSS = 6 (4 weeks) |
BCNI (transection injury) | ICI | 1 × 106 cells | 2/4 weeks | ICP | Histology |
| 29. Bochinski et al.
41
USA |
Embryonic stem cells Embryonal |
Allogenic rats (from blastocyst) | NES (embryonic stem cells were induced to differentiate into neural cells by transfecting them with BDNF) | 26 SD rats | Divided into four groups a. Sham group = 5 b. BCNI + culture medium into CC = 8 c. BCNI + NES to MPG = 4 d. BCNI + NES to CC = 9 |
BCNI (crush injury) | ICI and MPG | 500 µl (10,000 cells/ml) | 3 months | ICP | Histochemical analysis for NOS-containing fibers, tyrosine hydroxylase, and neurofilament staining |
adMSC, adipose MSC; ADSC, adipose-derived stem cell; AUC, Area under curve; BCNI, bilateral cavernous nerve injury; BDNF, brain-derived neurotrophic factor; BM-MSCs, bone marrow–derived stem cells; CC, corpus cavernosum; CCK8,Cell Counting Kit-8; cGMP, cyclic guanosine monophosphate; d-MSCs, differentiated MSCs; ED, erectile dysfunction; bFGF, Basic fibroblast growth factor; eNOS, endothelial nitric oxide synthase; GDNF, glial cell-derived nerve growth factor; GFP, green fluorescent protein; hBDNF, human brain–derived neurotrophic factor; hBMSC,human bone marrow stem cells;HBSS, Hanks Balanced Salt solution; hUCB, human umbilical cord blood; ICA II, Icariside II; ICI, intracavernosal injection; ICP, intracavernosal pressure; iMSC, induced pluripotent stem cell–derived MSC; MAP, mean arterial pressure; MD-MSC, Muscle derived stem cells; MPG, major pelvic ganglion; MRI, magnetic resonance imaging; MSC, mesenchymal stem cell; NA, not available; NAS, neural-like cells from adipose-derived stem cells; NES, neural stem cells; NGF-hydrogel, nerve growth factor–incorporated hyaluronic acid–based hydrogel; nNOS, neuronal nitric oxide synthase; NOS, nitric oxide synthase; PBS, phosphate-buffered saline; PKH-26, red-fluorescent dye; PPI, periprostatic implantation; qRT-PCR, quantitative reverse transcription polymerase chain reaction; rAd, recombinant adenoviruses; SCs, Schwann cells; SD, Sprague-Dawley rats; SPC, smooth muscle progenitor; SPION-MSC, super-paramagnetic iron oxide nanoparticle labeling was performed for MSC; SVF, stromal vascular fraction; SWT, shock wave therapy; TUNEL, terminal deoxynucleotidyltransferase-mediated nick-end labeling; VEGF, vascular endothelial growth factor.
For three human trials using SCT for post-RP, the data were categorized into total patients, age in years, duration since procedure, cell type used (adipose versus bone marrow), dose (most common dose being 1 × 109 cells), mode of delivery (intracavernosal, intravenous, local), side effects, mode of assessment (using standard tools like IIEF), and follow-up (Table 2).
Table 2.
Human study: data extraction results (post-radical prostatectomy).
| Reference | Patient No. | Age (years) | Duration since procedure | Cell type used | Dose | Mode of delivery | Side effects | Mode of assessment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1. Yiou et al.
42
(NCT01089387) |
12 | 63.6 (45–70) |
22.9 months (6 months-3 years) |
BM-MNCs | Four different doses 2 × 107 2 × 108 1 × 109 2 × 109 |
Intracavernous | Mild postoperative pain at the BM aspiration No evidence of prostate cancer reactivation |
IIEF-15 EHS Penile length, Color Doppler |
1 year initially, extended to 61 months in phase II for 9 patients |
| 2. Yiou et al.
43
(NCT01089387) |
6 | 59.9 | 26.3 months | BM-MNCs | 1 × 109 | Intracavernous | No serious adverse events were reported. | IIEF-15 EHS Penile length, Color Doppler |
6 months |
| 3. Haahr et al.
44
(NCT02240823) |
21 Continent = 14 Incontinent = 7 |
60.2 (46–69) |
10.7 months (6–15) | ADRC | 8.4–37.2 million ADRCs | Intracavernous | No serious adverse events were reported; eight men experienced transient redness and swelling at the injection sites, three reported reaction in the penile area; five men stated minor abdominal hematomas; one man had an abdominal hematoma which led to scrotal and penile hematomas; this patient had taken large doses of non-steroidal anti-inflammatory drugs for back pain in the days before the treatment; all reported hematomas resolved within 14 days without any sequel; finally, eight patients reported light abdominal discomfort after liposuction | IIEF-5 EHS |
I year |
ADRC, adipose-derived regenerating cell; BM-MNCs, bone marrow mononuclear cells; EHS, Erection Hardness Score; IIEF, International Index for Erectile Function.
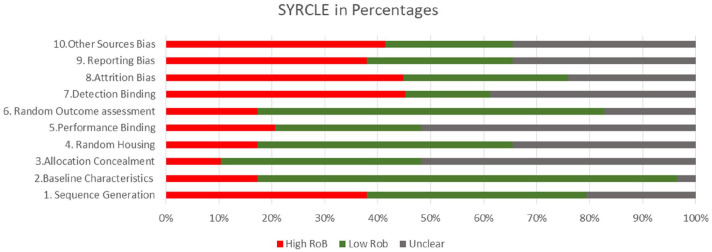
Quality assessment. SYRCLE’s RoB tool (Systematic Review Centre for Laboratory Animal Experimentation) which is an adapted version of the Cochrane RoB tool was used for animal studies (Figure 1). 45 All three human trials were evaluated in this study; no quality assessment was performed for them
Figure 1.
SYRCLE for animal studies.
Statistical analysis
Traditional meta-analysis was performed where possible. As all studies were unlikely to evaluate all treatments, a second set of analyses used a network meta-analysis approach to make indirect, as well as direct, comparisons between studies. A frequentist approach was utilized. 46 Specifically, the general approach used a model for treatment contrasts (the ‘contrast-based’), which considers treatment effects as fixed effects, and heterogeneity between studies as random effects. 47 All analyses were performed using the DerSimonian-Laird random-effects method, regardless of the amount of heterogeneity between studies. Statistical heterogeneity was assessed using the I2 statistics. Substantial statistical heterogeneity was assumed if the I2 value was above 50%.
An inverse variance method was used for continuous data and expressed as the mean difference with 95% confidence interval (CI), and for dichotomous data, a Mantel-Haenszel method was used. A p value of <0.05 was considered significant. The preferred method of variation was the standard deviation. Where this was not available, the value was imputed. The standard deviation was assumed to be a quarter of the data range.
The studies were divided into one of three subgroups depending on the source of adult stem cells: ADSC, BMSC, and Mixed. Meta-analyses were performed for the ADSC and BMSC subtypes, but due to a small number of studies (only two), no analysis of the mixed subgroup of studies was performed.
Results
Description of studies
Literature search
Animal studies
In total, 189 articles matched initial search. After removing duplicates, 71 were screened, of which further 37 were excluded (conference abstracts, language other than English, abstract-only studies). Furthermore, SQR3 (Survey, Question, Read, Recite, and Review) technique was used and 34 articles were found relevant; however, 5 articles were excluded (nerve injury by radiation, studies involving oral therapy or use of cells other than stem cells, studies evaluating role in apoptosis); 29 studies were included for qualitative and 22 studies in quantitative analysis (Table 1). PRISMA flowchart is attached.
Human studies
Five articles matched initial search. After screening, two articles were excluded and three were included in qualitative research. PRISMA flowchart is attached.
Included studies
Animal studies
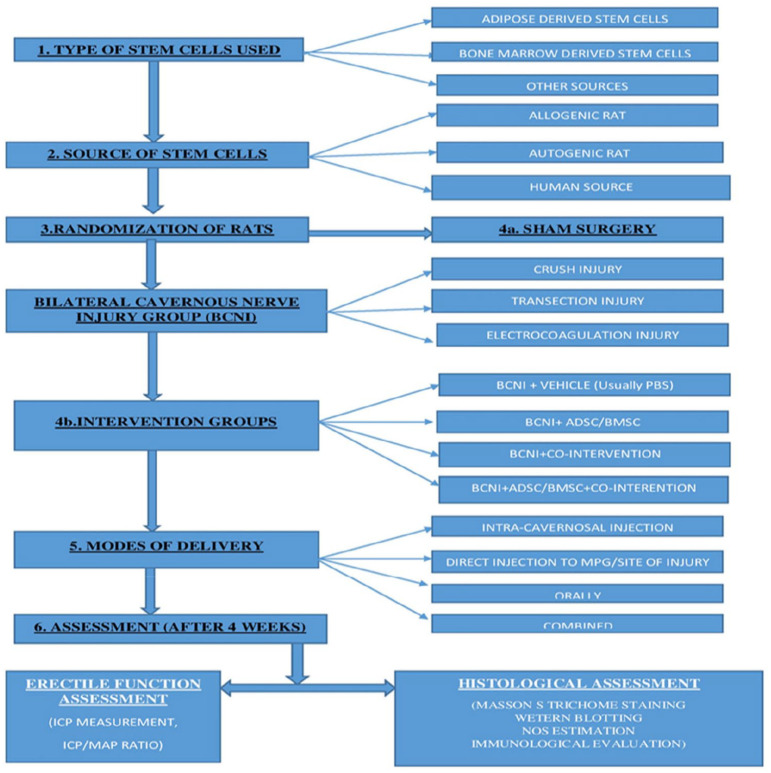
In 29 animal studies, a similar experimental protocol was followed. It involved dividing rats randomly in different groups – Sham, BCNI, Vehicle (in some), stem cell group, co-intervention group, and combined group (Stem cell and Co-intervention). Flowchart depicts stepwise approach carried out in animal experiments (Figure 2). Stem cells used included – ADSC (16 studies), BMSC (8 studies), and others (5 studies). Most studies used Sprague-Dawley (SD) rats, total of about 1,017, while only one study used white rats (15). Details are provided in Table 1.
Figure 2.
Flowchart depicting method of research in animal experiments.
Cavernous injury technique
Bilateral crush injury was most common form of cavernous nerve injury and used in 21 studies.14–18,20–26,30,31,34–39,41 Usually, this involved using a non-serrated hemostat away from major pelvic ganglion (MPG). In five studies, cavernous nerve injury was caused by transection injury,13,32,33,38,40 in two studies electric coagulation,19,27 and in one study Cryo-injury 28 was used.
Co-Interventions and modifications
Some studies used certain co-interventions or modifications to enhance the effect of stem cell intervention. Co-interventions included brain-derived neurotrophic factor (BDNF) in six studies;14,27,30,34,37,41 oral Icariside II (ICA II) in two studies;13,22 and low shock wave therapy and oral Udenafil were used in one study each.26,34 Modifications included use of fibrin scaffolds, Matrixen (biocompatible polymer), exosomes, nanotechnology, and super-paramagnetic iron oxide nanoparticles.
Route of administration
Most common route of administration of stem cells was intracavernosal route. It was used in 17 studies.13,14,16–18,20,21,23,24,27–30,35,38–40 The usual technique for IC injections (ICIs), included rolling up the prepuce to expose the penis and injecting to the lateral aspect of the penis. The needle was inserted around 3–4 mm. Before injection, drainage via the dorsal vein was halted by circumferential compression of the base of the penis using external compression (like an elastic band). The compression was released in around 1 min after injection of cells.
In four studies, stem cells were injected directly to MPG or cavernous nerves (periprostatic implantation, PPI).15,31,36,37 In five studies, ICI was used in combination with either MPG or PPI.22,25,32,33,41 For PPI of stem cells, different scaffolds like Fibrin or Matrixen were used. In most studies, mixtures were instilled into fissures between vesico-prostatic junction and seminal vesicles. In two studies, stem cell infusion was done around injured nerve.26,34 In one study, stem cells were administered through intravenous route (right jugular vein). 19 The most common dose of stem cells used is 1.0 × 106 cells.
ICP measurement
Initially, the number of studies providing data in a suitable format for analysis was evaluated, along with the number of animals. A total of 22 studies provided data suitable for meta-analysis. This consisted of 13 studies in the ADSC subgroup, 7 in the BMSC subgroup, and 2 in the Mixed subgroup. Data from a total of 909 animals were collected. Information on the number of studies on which data in each treatment group were collected, along with the pattern of the treatment combinations, was analyzed using a network map.
ADSCs
The first set of analyses considered the ADSC studies only. Both direct comparisons only and a network meta-analysis were performed. The results of both sets of analyses are shown in Table 3. Both analyses show the mean difference between each pair of treatments, in addition to corresponding CIs and the significance of the group differences. On comparing, stem cell group performed significantly better than BCNI group as traditional meta-analysis revealed difference of 27 (95% CI, P value <0.001) and network meta-analysis revealed difference of 28 (95% CI, P value <0.001). Overall, the traditional meta-analysis and network meta-analysis gave fairly similar results. Typically, the same pairs of groups varied from each other (or not) in both sets of analyses. As expected, all groups were found to have significantly lower values than the sham group.
Table 3.
Summary of meta-analysis results.
| Group 1 | Group 2 | Direct comparisons | Network meta-analysis | ||||
|---|---|---|---|---|---|---|---|
| Difference (95% CI) a | P value | I 2 | Difference (95% CI)a | P value | |||
| ADSC studies | Sham | BCNI | –71 (–84, –58) | <0.001 | 99% | –73 (–86, –59) | <0.001 |
| Vehicle | –68 (–85, –52) | <0.001 | 99% | –69 (–85, –53) | <0.001 | ||
| Stem cell | –44 (–54, –34) | <0.001 | 99% | –44 (–57, –32) | <0.001 | ||
| Co-intervention | –42 (–55, –28) | <0.001 | 99% | –42 (–57, 28) | <0.001 | ||
| Combined | –21 (–33, –9) | 0.001 | 99% | –23 (–39, –7) | 0.006 | ||
| BCNI | Vehicle | 17 (–4, 38) | 0.12 | 99% | 3 (–15, 21) | 0.73 | |
| Stem cell | 27 (15, 39) | <0.001 | 99% | 28 (15, 42) | <0.001 | ||
| Co-intervention | 31 (20, 50) | <0.001 | 99% | 30 (15, 45) | <0.001 | ||
| Combined | 48 (32, 65) | <0.001 | 99% | 49 (33, 66) | <0.001 | ||
| Vehicle | Stem cell | 30 (13, 48) | 0.001 | 99% | 24 (7, 40) | 0.005 | |
| Co-intervention | 10 (–5, 24) | 0.21 | 97% | 25 (6, 44) | 0.008 | ||
| Combined | 15 (2, 27) | 0.03 | 97% | 44 (25, 65) | <0.001 | ||
| Stem cell | Co-intervention | 5 (–4, 13) | 0.28 | 98% | 2 (–13, 16) | 0.83 | |
| Combined | 22 (7, 36) | 0.003 | 99% | 21 (5, 37) | 0.01 | ||
| Co-intervention | Combined | 25 (11, 39) | <0.001 | 99% | 20 (3, 37) | 0.02 | |
| BMSC studies | Sham | BCNI | –42 (–45, –39) | <0.001 | (+) | –48 (–67, –31) | <0.001 |
| Vehicle | –72 (–92, –51) | <0.001 | 99% | –71 (–82, –60) | <0.001 | ||
| Stem cell | –27 (–39, –16) | <0.001 | 98% | –27 (–38, –16) | <0.001 | ||
| Co-intervention | –32 (–37, –27) | <0.001 | 83% | –36 (–48, –24) | <0.001 | ||
| Combined | –7 (–16, 1) | 0.10 | 96% | –16 (–32, 0) | 0.05 | ||
| BCNI | Vehicle | (#) | – | – | –22 (–41, –4) | 0.02 | |
| Stem cell | 23 (11, 36) | <0.001 | 98% | 21 (5, 38) | 0.01 | ||
| Co-intervention | 8 (6, 10) | <0.001 | (+) | 13 (–5, 31) | 0.17 | ||
| Combined | 30 (28, 32) | <0.001 | (+) | 32 (12, 53) | 0.002 | ||
| Vehicle | Stem cell | 43 (33, 52) | <0.001 | 96% | 44 (32, 55) | <0.001 | |
| Co-intervention | 30 (12, 48) | 0.001 | 98% | 35 (22, 48) | <0.001 | ||
| Combined | 37 (35, 39) | <0.001 | (+) | 54 (38, 71) | <0.001 | ||
| Stem cell | Co-intervention | –7 (–18, 4) | 0.23 | 98% | –9 (–21, 4) | 0.17 | |
| Combined | 10 (4, 16) | 0.001 | 94% | 10 (–5, 26) | 0.18 | ||
| Co-intervention | Combined | 22 (20, 24) | <0.001 | 0% | 19 (3, 36) | 0.02 | |
ADSC, adipose-derived stem cell; BCNI, bilateral cavernous nerve injury; BMSC, bone marrow–derived stem cell; CI, confidence interval.
Differences reported as Group 2 minus Group 1.
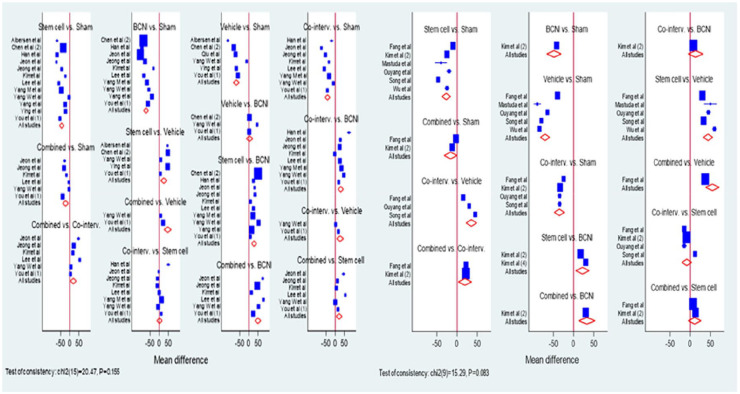
The BCNI group had significantly lower ICP values than all other groups, with the exception of the vehicle group, where no difference was observed. The traditional meta-analyses suggested a very high degree of heterogeneity between the study results for all comparisons. A graphical illustration of these direct comparisons between groups is shown in a Forest plot in Figure 3.
Figure 3.
Forest plots for direct comparisons (ADSC studies – left side, BMSC studies – right side).
BMSCs
A similar set of analyses was performed for the BMSC studies. The meta-analysis results, using both approaches, are summarized in Table 3. The results suggested that again the sham group tended to have the highest values. On comparing, again stem cell group performed significantly better than BCNI group as traditional meta-analysis revealed difference of 23 (95% CI, P value <0.001) and network meta-analysis revealed difference of 21 (95% CI, P value <0.001). For the traditional meta-analyses, the I2 values indicated heterogeneity between studies. For these studies, the vehicle group performed worst, with the network meta-analyses suggested significantly lower ICP values than all other groups. A graphical illustration of the results for the direct comparisons is shown in a Forest plot in Figure 3.
ICP/MAP ratio
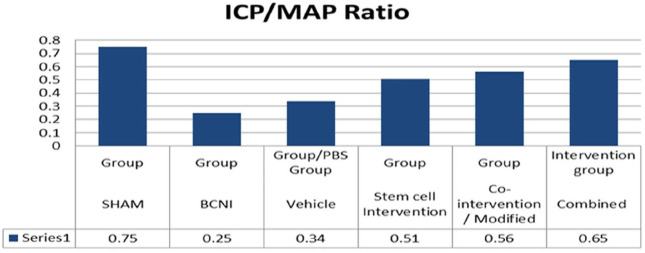
ICP and MAP ratio was evaluated from 26 studies;13–37,39 3 studies did not provide ICP/MAP ratio values.38,40,41 We found mean ICP/MAP ratio was higher in stem cell group (0.51) compared with BCNI group (0.25). Further for combined intervention group, ICP/MAP ratio was observed to be even higher than stem cell group at 0.65 suggesting that the co-interventions further enhanced the benefit (Figure 4).
Figure 4.
Mean ICP/MAP ratios.
Histological/molecular evaluation
All 29 studies evaluated penile tissue as well as MPG (studies which involved periprostatic intervention); 21 studies reported an increase in level of neuronal nitric oxide synthase (nNOS), and 26 studies reported either increase in smooth muscle content or increase in ratio of smooth muscle to collagen ratio. Other changes reported included anti-apoptotic role played by stem cells. The summarized results are given in Table 4.
Table 4.
Results from histological/molecular evaluation.
| Summarized results of tissue analysis | ||||
|---|---|---|---|---|
| Reference | NOS | Smooth muscle/collagen ratio | Smooth muscle content | Other finding |
| 1. Zheng et al. 13 | Increased nNOS fibers | Suppression of miR-33 expression | ||
| 2. Yang et al. 14 | Increased nNOS fibers | Increased | ||
| 3. Yang et al. 15 | Increased nNOS fibers | Increased | ||
| 4. Chen et al. 16 | Increased nNOS expression | Increased | Anti-apoptotic Increased endothelial content |
|
| 5. Ying et al. 17 | Increased nNOS fibers | Increased | Increased myelinated axons | |
| 6. Wu et al. 18 | No significant improvement | Increased | Anti-apoptotic | |
| 7. Matsuda et al. 19 | Increased | |||
| 8. Ouyang et al. 20 | Increased nNOS expression | Increased | Increased | Anti-apoptotic |
| 9. Li et al. 21 | Increased nNOS expression | |||
| 10. Zheng et al. 22 | Suppression of miR-33 expression, promoting diffusion of ADSC to SC | |||
| 11. Wu et al. 23 | Increased expression of α-SMA | Increased expression of β III tubulin and CD31 | ||
| 12. Chen et al. 24 | Increased nNOS fibers | Increased | Increased | |
| 13. Fang et al. 25 | Increased nNOS fibers | Increased | Increased | |
| 14. Jeon et al. 26 | Increased | Anti-apoptotic Increased VEGF expression |
||
| 15. Song et al. 27 | Increased nNOS fibers | Increased | Increased expression of α-SMA | |
| 16. Yang et al. 28 | Increased nNOS fibers | Elevation of neurotrophic factors (VEGF, NGF, and Neurturin) | ||
| 17. Kim et al. 29 | Increased | Increased | ||
| 18. Lee et al. 30 | Increased nNOS fibers | Increased expression of α-SMA | ||
| 19. Kim et al. 31 | Increased nNOS fibers | Decrease in smooth muscle atrophy | ||
| 20. You et al. 32 | Increased nNOS fibers | Increased | ||
| 21. You et al. 33 | Increased nNOS expression | Increased | ||
| 22. Jeong et al. 34 | Increased nNOS expression | Increased | Increase in cGMP level | |
| 23. Qiu et al. 35 | Increased nNOS fibers | Increased | Increased | |
| 24. Kim et al. 36 | Increase in expressions of the eNOS and nNOS | Increase in neuronal cells | ||
| 25. Kim et al. 37 | Increase in eNOS and nNOS | Increased | ||
| 26. Woo et al. 38 | Increase in cGMP level | |||
| 27. Albersen et al. 39 | Increased nNOS fibers | Increased | Reduced apoptosis | |
| 28. Kim et al. 40 | Increased | Nerve fiber regeneration | ||
| 29. Bochinski et al. 41 | Differentiation of embryonic stem cells into neural cells leading to improved ED | |||
ADSC, adipose-derived stem cells; cGMP, cyclic guanosine monophosphate; ED, erectile dysfunction; eNOS, endothelial nitric oxide synthase; miR-33, micro-RNA; NGF, nerve growth factor; nNOS, neuronal nitric oxide synthase; NOS, nitric oxide synthase; SC, Schwann cells; SMA, smooth muscle actin; VEGF, vascular endothelial growth factor.
Human studies
The three phase I/II trials included in this study included two open label clinical trials and one open label extension study.42–44 The trials included patients who had ED post-RP and failed to recover using conventional therapy. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. Since all three trials included patients who had failed to recover on conventional therapy, these three trials were non-comparative and looked at long-term effect of stem cells on post-RP ED. One of the trials was open label extension. 43 A total of 35 patients were included in three trials. The results are summarized in Table 5.
Table 5.
Outcomes of SCT in human trials.
| Baseline | 1 month | 3 months | 6 months | 1 year | |
|---|---|---|---|---|---|
| 1. Yiou et al. 42 | |||||
| IIEF-15 score (Max 75) | 25.3 | 28.3 | 39.7 | 43.5 | 44.4 |
| EHS | 1.3 | 1.7 | 2.3 | 2.6 | 3.0 |
| Penile length (cm) | 12.4 | 13.5 | 13.3 | 12.9 | NA |
| 2. Yiou et al. 43 | |||||
| IIEF-15 score (Max 75) | 18.7 | 33.2 | 47.4 | 46.6 | |
| EHS | 1.8 | 2.2 | 2.7 | 3.3 | |
| 3. Haahr et al. 44 | |||||
| IIEF -5 (SHIM score) | |||||
| Continent group (11 pat) | 6 | 6 | 11 | 16 | |
| Incontinent group (6 pat) | 5 | 5 | 5 | 5 | |
| EHS | |||||
| Continent group (11 pat) | 1 | 2 | 2 | 3 | |
| Incontinent group (6 pat) | 1 | 1 | 1 | 1 | |
EHS, Erection Hardness Score; IIEF, International Index for Erectile Function; NA, not available; SCT, stem cell therapy; SHIM, Sexual Health Inventory for men.
IIEF score (International Index For Erectile Dysfunction): Two trials used IIFF-15 score and found there was an increase in score; one study revealed an increase from baseline of 25.3 to 39.7 at 6 months, 42 and the other study indicated an increase from 18.7 to 46.6 for same duration. 43 One trial used IEFF-5 scoring and found that in continent group of patients, it increased from 6 to 16 in a 6-month period; however, there was no improvement in incontinent group.
EHS: One trial reported an elevation in EHS from baseline 1.3 to 2.6 in 6 months, 42 the second reported increase from 1.8 to 3.3 for same duration, 43 and third trial indicated an increase from 1 to 3 at 6 months in the continent group. 44 However, there was no change in incontinent group.
Penile length: In one trial, penile length was also considered. It increased from 12.4 to 13.3 cm by end of 3 months. However, by end of 6 months, it was around 12.9 cm. 42
Adverse outcomes: One trial reported mild postoperative pain at the bone marrow (BM) aspiration with no evidence of prostate cancer reactivation. 42 Second trial reported 8 men developed transient redness and swelling at injection site. 5 men in this series also developed minor abdominal wall hematomas (post-liposuction). 44 The third trial has not reported any adverse outcome 43 (Table 2).
Discussion
Cavernous injury is widely accepted to be responsible for post-RP ED due to the damage caused by incision, heat, and mechanical stress. 48 Although, nerve-sparing RP was introduced 30 years ago, ED still remains a challenge with RP surgery.49,50 A recent prospective series has shown that up to 75% of men reported ED, 1 year after RP with minimal difference between robotic and open surgery groups. 51 Therefore, it is now commonly believed that, although leaving the cavernosal nerves (CNs) intact, nerve-sparing RP still causes subtle changes that are not obvious to the surgeons. 52 These changes cause CNs to undergo Wallerian degeneration and eventually lose their connection to the corpora cavernosa. 53 Alternatively, the surgery-incurred insults may temporarily prevent the CNs from releasing nitric oxide (NO) into the corpus cavernosum (CC), and without NO-induced engorgement, the penile tissue becomes hypoxic and its cavernous musculature is replaced by collagens and fibrous scar tissue.52,53
SCT is among different novel approaches being investigated to manage post-RP ED. Stem cells are undifferentiated or partially differentiated cells and are classified as totipotent (e.g. zygote), pluripotent [e.g. embryonic stem cells (ESCs)], multipotent (e.g. hematopoietic and MSCs), and unipotent according to the number of cell lines in which they could be differentiated. Stem cells have been used for the treatment of cardiovascular, 54 neurological, 55 autoimmune, 56 and hematologic diseases. 57 In recent years, SCT has been proposed for the treatment of ED as stem cells can differentiate to endothelial, neuronal, or smooth muscle cells, and therefore restore possible structural damage in the penile tissue. 58 Most commonly used stem cells used in the treatment of ED are MSCs. MSCs are able to demonstrate therapeutic effects by their ability to produce an array of bioactive molecules including growth factors capable of inducing increased cell proliferation and immunomodulatory effects. 59 They lead to stimulation of angiogenesis and revascularization, modulation of immune and inflammatory responses, inhibition of apoptosis, and trophic effects such as stimulation of mitosis, proliferation, and differentiation of intrinsic stem/progenitor cells. 60 This is termed as paracrine action of stem cells, because few stem cells can be detected after transplantation, and almost no direct evidence supports the theory that transplanted stem cells have differentiated into vascular endothelial cells, smooth muscle cells, or nerves. 61
The current meta-analysis examined 29 preclinical studies of SCT in the treatment of ED secondary to BCNI in rats and also evaluated three human clinical trials. The animal studies were analyzed for ICP measurement, ICP/MAP ratio, and histological/molecular results. Our study focused mainly on ICP measurements as it is considered a reliable method for direct measure of erectile function. It allows for the acquisition of data on basal ICP, peak ICP, plateau ICP, time to erection and detumescence time, duration of response. 62
Meta-analysis of ICP values (post-SCT) including ADSC as well as BMSC stem cell groups revealed statistically significant improvement in ED in SCT intervention group as compared with BCNI group. There is minimal literature in the form of a systemic review or a meta-analysis available regarding ICP measurements in ED in BCNI rats to compare. We also evaluated ICP/MAP ratio for animal studies. We found that there is an increase in ICP/MAP ratio in stem cell intervention group as compared with BCNI group. A previous meta-analysis based on ICP/MAP ratio had also revealed that there is a significant difference of erectile functions between stem cell transplantation group and control group. 63 We noticed that for both ICP (meta-analysis) and ICP/MAP ratio, the efficacy of SCT was enhanced by the addition of co-interventions. This has been reported previously as well. 63
In case of animal studies, histology of penile tissue post-SCT was also analyzed. It revealed an increase in NOS, ratio of smooth muscle to collagen, and anti-apoptotic activity in intervention groups. In a previous systematic review, it has been established that SCT does lead to structural changes in CC resulting in increased endothelial and smooth muscle cell markers, increase in neural cell markers, decrease in apoptosis, as well as a decrease in collagen content. 48
The three human trials in post-RP patients did reflect improvement in SHIM and EHS scores after SCT. The trials have revealed that SCT in humans has a potential efficacy suggested by a significant improvement in IIEF scores, erectile function with minimal adverse effects. However, in one trial, the SCT therapy results could not produce desired results in men having urinary incontinence. 44 This has been already established that urinary continence seems to be a prerequisite for having sexual intercourse post-RP. 64
Adult stem cells in regenerative medicine do have issues and concerns. The most important concern being development of recurrences as well as other tumors in patients receiving SCT due to conflicting evidence that adult stem cells can promote tumor genesis. 65 Several hypotheses have proposed that the events in either stem and/or differentiated cells, such as genomic instability, inflammatory microenvironment, cell fusion, and lateral gene transfer, should be considered as the possible origin of cancer stem cells (CSCs), are responsible for the sustained and uncontrolled growth of malignant tumors, and are proposed to play significant roles in metastasis and recurrence 66 but also the chemotaxis and subsequent migration of patient stem cells to the hypoxic tumor mass promoting angiogenesis; however, the specific response of the stem cell to the malignant mass may well be due to the malignancy type. 59 In the majority of animal trials, animal was sacrificed at around 4 weeks, so this aspect has not been addressed. The issues with human trials included in this study include very limited number of patients, SCT treatment started after a gap of few months to years, sometimes overlapping both SCT with other therapies, no long-term follow-up details, and also pre-screening for risk factors, and the presence of secondary malignancy may well be required to reduce risk factors.
In order to understand SCT in post-RP patients, a double-blind randomized controlled study is need of hour; however, it will not be an easy task, as a number of questions need to be addressed – When to start? Which type of stem cell to use? What quality control of stem cells to be used? What source? What is ideal dose? Which route? How to do follow-up?
Conclusion
Our results confirm that SCT does improve the erectile function in rats having cavernous nerve injury. Furthermore, co-interventions and specific modifications do improve efficacy of SCT. Similarly, early human results have shown promising results. Thus, regenerative medicine approach to the treatment of ED appears to hold much promise.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872221086999 for Is there a role for stem cell therapy in erectile dysfunction secondary to cavernous nerve injury? Network meta-analysis from animal studies and human trials by Mudassir M. Wani, Bhavan P. Rai, William Richard Webb and Sanjeev Madaan in Therapeutic Advances in Urology
Footnotes
Author contributions: Mudassir M. Wani: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Visualization; Writing – original draft; Writing – review & editing.
Bhavan P. Rai: Supervision; Validation; Writing – review & editing.
William Richard Webb: Project administration; Validation; Visualization.
Sanjeev Madaan: Conceptualization; Data curation; Formal analysis; Project administration; Resources; Software; Supervision; Validation; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Authors recieved no financial support for this research.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: William Richard Webb  https://orcid.org/0000-0002-2701-626X
https://orcid.org/0000-0002-2701-626X
Sanjeev Madaan  https://orcid.org/0000-0003-4220-5613
https://orcid.org/0000-0003-4220-5613
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mudassir M. Wani, Royal Glamorgan Hospital, Cardiff, UK Institute of Medical Sciences, Faculty of Medicine, Health and Social Sciences, Canterbury Christ Church University, Kent, UK.
Bhavan P. Rai, Freeman Hospital, Newcastle, UK
William Richard Webb, SCRABEL, Institute of Medical Sciences, Faculty of Medicine, Health and Social Sciences, Canterbury Christ Church University, Kent, UK.
Sanjeev Madaan, Darent Valley Hospital, Darenth Wood Road, Dartford DA2 8DA, UK. Canterbury Christ Church University, Kent, UK.
References
- 1. Rawla P. Epidemiology of prostate cancer. World J Oncol 2019; 10: 63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011; 59: 61–71. [DOI] [PubMed] [Google Scholar]
- 3. Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med 2010; 7: 3572–3588. [DOI] [PubMed] [Google Scholar]
- 4. Salonia A, Burnett AL, Graefen M, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol 2012; 62: 261–272. [DOI] [PubMed] [Google Scholar]
- 5. Weyne E, Albersen M. Post-RP erectile dysfunction – therapies for the next decade. Nat Rev Urol 2014; 11: 616–618. [DOI] [PubMed] [Google Scholar]
- 6. Rabbani F, Stapleton AM, Kattan MW, et al. Factors predicting recovery of erections after radical prostatectomy. J Urol 2000; 164: 1929–1934. [PubMed] [Google Scholar]
- 7. Fode M, Ohl DA, Ralph D, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int 2013; 112: 998–1008. [DOI] [PubMed] [Google Scholar]
- 8. Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med 1998; 13: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lue Tom F. Erectile dysfunction. N Engl J Med 2000; 342: 1802–1813. [DOI] [PubMed] [Google Scholar]
- 10. Alwaal A, Hussein AA, Lin CS, et al. Prospects of stem cell treatment in benign urological diseases. Korean J Urol 2015; 56: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vardi Y, Appel B, Kilchevsky A, et al. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 2012; 187: 1769–1775. [DOI] [PubMed] [Google Scholar]
- 12. Yiou R. Stem-cell therapy for erectile dysfunction. Biomed Mater Eng 2017; 28(s1): S81–S85. [DOI] [PubMed] [Google Scholar]
- 13. Zheng T, Zhang T, Zhang W, et al. Icariside II facilitates the differentiation of ADSCs to Schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed Pharmacother 2020; 125: 109888. [DOI] [PubMed] [Google Scholar]
- 14. Yang M, Sun JY, Ying CC, et al. Adipose-derived stem cells modified by BDNF gene rescue erectile dysfunction after cavernous nerve injury. Neural Regen Res 2020; 15: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang W, Chen Z, Ma X, et al. Co-overexpression of VEGF and GDNF in adipose-derived stem cells optimizes therapeutic effect in neurogenic erectile dysfunction model. Cell Prolif 2020; 53: e12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Z, Han X, Ouyang X, et al. Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics 2019; 9: 6354–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ying CC, Yang M, Wang Y, et al. Neural-like cells from adipose-derived stem cells for cavernous nerve injury in rats. Neural Regen Res 2019; 14: 1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu YN, Chen KC, Liao CH, et al. Smooth muscle progenitor cells preserve the erectile function by reducing corporal smooth muscle cell apoptosis after bilateral cavernous nerve crush injury in rats. Biomed Res Int 2019; 2019: 8520523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuda Y, Sasaki M, Kataoka-Sasaki Y, et al. Intravenous infusion of bone marrow-derived mesenchymal stem cells reduces erectile dysfunction following cavernous nerve injury in rats. Sex Med 2018; 6: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ouyang X, Han X, Chen Z, et al. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther 2018; 9: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Lei H, Xu Y, et al. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology 2018; 6: 927–935. [DOI] [PubMed] [Google Scholar]
- 22. Zheng T, Zhang TB, Wang CL, et al. Icariside II promotes the differentiation of adipose tissue-derived stem cells to Schwann cells to preserve erectile function after cavernous nerve injury. Mol Cells 2018; 41: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu H, Tang WH, Zhao LM, et al. Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury: in vivo cell tracking, optimized injection dosage, and functional evaluation. Asian J Androl 2018; 20: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Yang Q, Zheng T, et al. Neurotrophic effect of adipose tissue-derived stem cells on erectile function recovery by pigment epithelium-derived factor secretion in a rat model of cavernous nerve injury. Stem Cells Int 2016; 2016: 5161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang JF, Jia CC, Zheng ZH, et al. Periprostatic implantation of neural differentiated mesenchymal stem cells restores cavernous nerve injury-mediated erectile dysfunction. Am J Transl Res 2016; 8: 2549–2561. [PMC free article] [PubMed] [Google Scholar]
- 26. Jeon SH, Shrestha KR, Kim RY, et al. Combination therapy using human adipose-derived stem cells on the cavernous nerve and low-energy shockwaves on the corpus cavernosum in a rat model of post-prostatectomy erectile dysfunction. Urology 2016; 88: 226.e1–226.e9. [DOI] [PubMed] [Google Scholar]
- 27. Song L, Zhu J, Zhang X, et al. BDNF-hypersecreting human umbilical cord blood mesenchymal stem cells promote erectile function in a rat model of cavernous nerve electrocautery injury. Int Urol Nephrol 2016; 48: 37–45. [DOI] [PubMed] [Google Scholar]
- 28. Yang R, Fang F, Wang J, et al. Adipose-derived stem cells ameliorate erectile dysfunction after cavernous nerve cryoinjury. Andrology 2015; 3: 694–701. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH, Lee HJ, Doo SH, et al. Use of nanoparticles to monitor human mesenchymal stem cells transplanted into penile cavernosum of rats with erectile dysfunction. Korean J Urol 2015; 56: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SH, Kim IG, Jung AR, et al. Combined effects of brain-derived neurotrophic factor immobilized poly-lactic-co-glycolic acid membrane with human adipose-derived stem cells and basic fibroblast growth factor hydrogel on recovery of erectile dysfunction [published correction appears in Tissue Eng Part A 2016; 22: 194]. Tissue Eng Part A 2014; 20: 2446–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim IG, Piao S, Lee JY, et al. Effect of an adipose-derived stem cell and nerve growth factor-incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng Part A 2013; 19: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. You D, Jang MJ, Lee J, et al. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology 2013; 81: 104–110. [DOI] [PubMed] [Google Scholar]
- 33. You D, Jang MJ, Lee J, et al. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate 2013; 73: 278–286. [DOI] [PubMed] [Google Scholar]
- 34. Jeong HH, Piao S, Ha JN, et al. Combined therapeutic effect of udenafil and adipose-derived stem cell (ADSC)/brain-derived neurotrophic factor (BDNF)-membrane system in a rat model of cavernous nerve injury. Urology 2013; 81: 1108.e7–1108.e14. [DOI] [PubMed] [Google Scholar]
- 35. Qiu X, Fandel TM, Ferretti L, et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol 2012; 62: 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim SJ, Park SH, Sung YC, et al. Effect of mesenchymal stem cells associated to matrixen on the erectile function in the rat model with bilateral cavernous nerve crushing injury. Int Braz J Urol 2012; 38: 833–841. [DOI] [PubMed] [Google Scholar]
- 37. Kim SJ, Choi SW, Hur KJ, et al. Synergistic effect of mesenchymal stem cells infected with recombinant adenovirus expressing human BDNF on erectile function in a rat model of cavernous nerve injury. Korean J Urol 2012; 53: 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woo JC, Bae WJ, Kim SJ, et al. Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury. Korean J Urol 2011; 52: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Albersen M, Fandel TM, Lin G, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 2010; 7: 3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim Y, de Miguel F, Usiene I, et al. Injection of skeletal muscle-derived cells into the penis improves erectile function. Int J Impot Res 2006; 18: 329–334. [DOI] [PubMed] [Google Scholar]
- 41. Bochinski D, Lin GT, Nunes L, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int 2004; 94: 904–909. [DOI] [PubMed] [Google Scholar]
- 42. Yiou R, Hamidou L, Birebent B, et al. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study. Eur Urol 2016; 69: 988–991. [DOI] [PubMed] [Google Scholar]
- 43. Yiou R, Hamidou L, Birebent B, et al. Intracavernous injections of bone marrow mononucleated cells for postradical prostatectomy erectile dysfunction: final results of the INSTIN clinical trial. Eur Urol Focus 2017; 3: 643–645. [DOI] [PubMed] [Google Scholar]
- 44. Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine 2016; 5: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salanti G, Higgins JPT, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008; 17: 279–301. [DOI] [PubMed] [Google Scholar]
- 47. White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate metaregression. Res Synth Methods 2012; 3: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mangır N, Türkeri L. Stem cell therapies in post-prostatectomy erectile dysfunction: a critical review. Can J Urol 2017; 24: 8609–8619. [PubMed] [Google Scholar]
- 49. Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012; 62: 418–430. [DOI] [PubMed] [Google Scholar]
- 50. Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment [published correction appears in J Clin Oncol 2011; 29: 779]. J Clin Oncol 2010; 28: 4687–4696. [DOI] [PubMed] [Google Scholar]
- 51. Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol 2015; 68: 216–225. [DOI] [PubMed] [Google Scholar]
- 52. Fode M, Ohl DA, Ralph D, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int 2013; 112: 998–1008. [DOI] [PubMed] [Google Scholar]
- 53. Albersen M, Kendirci M, Van der Aa F, et al. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J Sex Med 2012; 9: 385–403. [DOI] [PubMed] [Google Scholar]
- 54. Chen S, Liu Z, Tian N, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol 2006; 18: 552–556. [PubMed] [Google Scholar]
- 55. Mazzini L, Mareschi K, Ferrero I, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy 2012; 14: 56–60. [DOI] [PubMed] [Google Scholar]
- 56. Műzes G, Sipos F. Issues and opportunities of stem cell therapy in autoimmune diseases. World J Stem Cells 2019; 11: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ball LM, Bernardo ME, Roelofs H, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol 2013; 163: 501–509. [DOI] [PubMed] [Google Scholar]
- 58. Protogerou V, Chrysikos D, Karampelias V, et al. Erectile dysfunction treatment using stem cells: a review. Medicines (Basel) 2021; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 2014; 163: 399–408. [DOI] [PubMed] [Google Scholar]
- 60. Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 2014; 23: 1045–1059. [DOI] [PubMed] [Google Scholar]
- 61. Lin CS, Xin Z, Dai J, et al. Stem-cell therapy for erectile dysfunction. Expert Opin Biol Ther 2013; 13: 1585–1597. [DOI] [PubMed] [Google Scholar]
- 62. Pan F, Zhang J, Liu Y, et al. Intracavernosal pressure recording to evaluate erectile function in rodents. J Vis Exp 2018; 136: 56798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shan H, Chen F, Zhang T, et al. Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: a systematic review and meta-analysis. PLoS ONE 2015; 10: e0121428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koehler N, Holze S, Gansera L, et al. Erectile dysfunction after radical prostatectomy: the impact of nerve-sparing status and surgical approach. Int J Impot Res 2012; 24: 155–160. [DOI] [PubMed] [Google Scholar]
- 65. Chu DT, Nguyen TT, Tien NLB, et al. Recent progress of stem cell therapy in cancer treatment: molecular mechanisms and potential applications. Cells 2020; 9: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Afify SM, Seno M. Conversion of stem cells to cancer stem cells: undercurrent of cancer initiation. Cancers (Basel) 2019; 11: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872221086999 for Is there a role for stem cell therapy in erectile dysfunction secondary to cavernous nerve injury? Network meta-analysis from animal studies and human trials by Mudassir M. Wani, Bhavan P. Rai, William Richard Webb and Sanjeev Madaan in Therapeutic Advances in Urology