Abstract
Acute myeloid leukemia (AML) is the common type of acute leukemia in adults. Definitive prognostic significance of variants of unknown significance lacks for many commonly mutated genes, including the isocitrate dehydrogenase 1 (IDH1) synonymous single nucleotide polymorphism (SNP) variant c.315C>T. In this retrospective cohort study of 248 AML patients at the University of Maryland Greenebaum Comprehensive Cancer Center, we show that the IDH1 c.315C>T SNP, previously reported to be associated with poor prognosis by other studies with conflicting data, does not confer worse prognosis, with a median overall survival (OS) of 17.1 months compared to 15.1 months for patients without this SNP (P=0.57). The lack of negative effect on prognosis by IDH1 SNP c.315C>T is consistent with the absence of amino acid alteration (p.Gly105Gly).
Keywords: AML, IDH1 c.315C>T SNP, prognosis, survival, Myeloid mutations
Introduction
It is estimated that 20,050 new cases of acute myeloid leukemia (AML) will be diagnosed in the United States in 2022 (1). AML-related mortality remains high, with an estimated 11,540 deaths expected in 2022 (1). Over the last decade, there have been significant advances in understanding the genetic landscape and pathophysiology of AML, leading to the approval of nine new medications for AML treatment (2). Targeted therapies are now available against the Fms-like tyrosine kinase 3 gene (FLT3) and isocitrate dehydrogenase (IDH) 1 and 2 gene mutations in AML (3).
The detection of genetic variants has become essential in determining risk stratification of AML and may guide treatment. However, definitive information on prognostic significance of various well-characterized mutations is still lacking. Although FLT3-ITD, Nucleophosmin-1 gene (NPM1) and CCAT/enhancer binding protein a gene (CEBPA) mutations have become established as prognostic markers in cytogenetically normal AML (CN-AML), there is a large group of patients without these mutations (4). Thus, there is a need for additional markers that may predict the differential outcomes of these patients. One potential source for expanding prognostication of AML is variants of unknown significance (VUS), such as common single nucleotide polymorphisms (SNPs) found in the population. A VUS becomes classified as pathogenic or benign once its impact is better understood. Therefore, studying VUS in commonly mutated genes may improve risk stratification and prognostication for AML patients.
Pathogenic variants within IDH1 or IDH2 occur in approximately 20% of AML (5). These mutations include R132 (in IDH1) and R140/R172 (in IDH2), which lead to the production of the oncometabolite 2-hydroxyglutarate and are targeted with selective oral inhibitors (6, 7). Currently, the VUS SNP in codon 105 in exon 4 of the IDH1 gene (8), (c.315C>T (p.Gly105=), rs11554137), which occurs in approximately 5-10% of AML cases, is poorly understood. In two studies, the IDH1 c.315C>T SNP was associated with an inferior outcome in cytogenetically normal AML (9, 10). In a third study, outcomes were also inferior, but this was attributable to association with FLT3-ITD (11). These studies proposed that the “silent” SNP may affect gene function by way of decreasing mRNA stability and thereby changing rates of protein translation, folding, and ultimately, function. However, these proposed ideas have never been demonstrated in vitro or in clinic. To date, the IDH1 c.315C>T SNP is not commonly screened for in myeloid mutation panels. In this study, we hypothesized that presence of IDH1 c.315C>T SNP does not impact clinical outcome of AML patients because of the lack of amino acid (glycine, Gly) change in position 105.
Methods
Study Design
We conducted a single-site retrospective cohort study to compare overall survival (OS), event-free survival (EFS) and complete remission (CR) and complete remission with incomplete hematologic recovery (CRi) rates in adults with AML with and without the IDH1 c.315C>T (p.Gly105=) SNP from 2013 through 2020. OS was defined as the time from diagnosis to death from any cause. EFS was defined as the time from treatment initiation to induction failure, relapse, or death from any cause. Treatment response was evaluated according to the 2017 European LeukemiaNet (ELN) criteria (12). Composite CR rate included CR+CRi. The study was approved by the University of Maryland Baltimore Institutional Review Board (IRB).
Data Source
We reviewed the medical records of patients diagnosed with AML at the University of Maryland Greenebaum Comprehensive Cancer Center (UMGCCC) between 2013 and 2020. UMGCCC uses the Epic electronic medical record (EMR) system. We used Epic and its features such as Care Everywhere and CRISP to extract relevant chart data from our site as well as all other available clinical sites within University of Maryland Medical System. The Care Everywhere feature allows access to a health network connecting all hospitals that utilize Epic. CRISP is a state-designed Health Information Exchange for a Maryland online database to extract relevant data from other clinical sites (13). We included all patients whose blood or bone marrow aspirate were examined for IDH mutation with Sanger sequencing which started at UMGCCC in 2013; no exclusion was performed. Data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Maryland (14, 15).
Variables and Comparison Groups
Data extracted included age, gender, ethnicity, Eastern Cooperative Oncology Group (ECOG) performance status, baseline comorbidities, AML categories (de novo AML, myelodysplasia-related AML, myeloproliferative-related AML, therapy-related AML), cytogenetics, myeloid mutations including IDH1/IDH2/FLT3/Tumor Protein 53 (TP53), treatments received, and outcomes. Data were checked multiple times by independent data collectors. We compared patients with and without the IDH1 c.315C>T (p.Gly105=) SNP.
Molecular Testing
Analysis of IDH1 and IDH2 Gene Alterations
IDH1 and IDH2 gene alterations were investigated using Sanger DNA sequencing. IDH1 Codon 132, IDH2 codons 140 and 172, and the surrounding sequences within exon 4 were analyzed on whole blood or bone marrow aspirate by Sanger DNA sequencing on an Applied Biosystems 3730XL genetic analyzer, using Sequencher™ DNA Sequence Analysis Software (version 5). The c.315C>T SNP in IDH1 codon 105 is in the same exon as the R132 mutation. These sequences were compared to NCBI reference sequences for the IDH1 (NM_005896.3 and NP_005887.2) and IDH2 (NM_002168.3 and NP_002159.2) genes. The lower limit of detection for this assay is approximately 20% allele proportion.
Propensity Score Estimation
This study obtained the Average Treatment Effect on the Treated (ATT) (16). We included the following variables in the propensity score model: age at diagnosis, gender, ethnicity, comorbidities, ECOG performance status, type of AML, cytogenetics at diagnosis, FLT3, IDH1, IDH2 and TP53 mutational status and first-line treatment. Different methods for matching were attempted, including 1:1 nearest neighbor, 1:2 nearest neighbor, full matching, inverse probability weighting, and weighting by the odds. Full matching was chosen as the matching method because it achieved the lowest standardized biases differences, smallest coefficients of variations and smallest weights. Weights obtained from full matching were used to adjust outcomes. No patients were dropped in the matching process. The choice of estimand (ATT) was based on achieving standardized bias scores less than 0.25 (17). We used balance tables and Love plots to assess for covariate balance before and after matching. As a sensitivity check, we repeated the analysis using inverse probability weighting and obtained average treatment effect as an estimand. Generalized boosted model was used to calculate weights. The results of inverse probability weighting are provided as supplementary data. Cluster-robust standard errors were used to account for subclass membership in the matching process. The R statistical package “MatchIt” and “WeightIt” were used for propensity score weighting (18).
Statistical Analysis
Descriptive statistics were used to compare baseline characteristics of patients with and without IDH1 c.315C>T. Categorical variables were presented as absolute numbers and percentages. Continuous variables were presented as means with standard deviations or medians with interquartile ranges (IQR). Baseline characteristics were compared using Pearson chi-square or Fisher’s exact test when categorical or t-test when continuous. OS and EFS were compared using log-rank and Gehan Breslow-Wilcoxon rank tests. Multivariable and univariable Cox proportional hazards models were used to assess relative mortality. Regression diagnostics were used to evaluate model assumptions. All statistical tests were two-sided, and P-values <0.05 were considered statistically significant. R-statistical software (version 4.1.1) was used for statistical analyses.
Results
Cohort Characteristics
We identified a total of 444 AML patients treated at UMGCCC during the study period that we had Sanger sequencing data on patients (2013–2020). All patients tested for IDH1 mutations using the Sanger technique were included (2015-2020). Patients not tested for IDH1 mutations were excluded; ultimately, 248 patients were included. There was no other exclusion criteria. The median age was 65 years [IQR 54-75] and 42% were female. Median follow-up was 27.33 months [IQR 17.6-46.9]. Median OS for the whole population was 17.1 months (CI 13.8-21.8). The IDH1 c.315C>T SNP was found in 23 patients (9%). Table 1 shows propensity score-adjusted baseline characteristics in patients with and without the IDH1 c.315C>T SNP. After matching, there were no statistically significant differences in baseline characteristics between the two groups. Covariate balance before and after propensity score weighting is shown in Supplemental Figure 1 . Unadjusted baseline characteristics are shown in Supplementary Table 1 . In the unadjusted cohort, patients with the IDH1 c.315C>T SNP compared to patients without received the following treatments: intensive chemotherapy (30% vs. 34%), hypomethylating agent with or without others treatments (30% vs. 21%), hypomethylating agents with venetoclax (22% vs. 18%), clinical trial (9% vs. 19%), other treatments (0 vs. 4%) and none (9% vs. 4%).
Table 1.
Adjusted baseline characteristics of patients with IDH1 c.315C>T mutated vs. IDH1 wild-type AML.
| IDH1 c.315C>T Mutated | Percentage/SD/IQR | IDH1 wild-type AML | Percentage/SD/IQR | P-value | |
|---|---|---|---|---|---|
| Number | 23 | – | 51β | ||
| Female | 8 | 0.35 | 19 | 0.37 | 0.85 |
| Ethnicity | 0 | 0 | 0.85 | ||
| Caucasian | 13 | 0.57 | 30 | 0.59 | |
| Other | 10 | 0.43 | 21 | 0.41 | |
| Unknown | 0 | 0 | 0 | 0 | |
| Comorbidities | |||||
| Cardiovascular disease | 6 | 0.26 | 11 | 0.21 | 0.65 |
| Diabetes mellitus | 7 | 0.3 | 18 | 0.36 | 0.53 |
| Hypertension | 11 | 0.48 | 21 | 0.41 | 0.66 |
| CKD stage III-V/ESRD | 1 | 0.04 | 2 | 0.04 | 0.95 |
| Asthma/COPD | 4 | 0.17 | 8 | 0.15 | 0.77 |
| Active Cancer | 2 | 0.09 | 3 | 0.06 | 0.54 |
| AML type | 0.96 | ||||
| AML, de novo | 15 | 0.65 | 33 | 0.65 | |
| AML with MDS/CMML changes | 4 | 0.17 | 9 | 0.17 | |
| AML with prior MPN | 2 | 0.09 | 6 | 0.11 | |
| Therapy-Related AML | 2 | 0.09 | 4 | 0.07 | |
| Cytogenetic Category | 0.98 | ||||
| Favorable Risk | 1 | 0.04 | 2 | 0.04 | |
| Intermediate Risk | 29 | 0.82 | 43 | 0.83 | |
| Unfavorable Risk | 2 | 0.09 | 4 | 0.07 | |
| Not performed or Inadequate | 1 | 0.04 | 3 | 0.06 | |
| IDH1 mutated | 2 | 0.09 | 7 | 0.14 | 0.65 |
| IDH2 mutated | 3 | 0.13 | 6 | 0.12 | 0.93 |
| FLT3-ITD status | 0.99 | ||||
| FLT3-ITD mutated 1-49% | 1 | 0.04 | 2 | 0.04 | |
| FLT3-ITD mutated 50-100% | 1 | 0.04 | 2 | 0.039 | |
| FLT3 WT | 19 | 0.83 | 42 | 0.83 | |
| Not tested | 2 | 0.09 | 5 | 0.09 | |
| FLT3-TKD status | 0.95 | ||||
| FLT3-TKD mutated | 4 | 0.17 | 8 | 0.15 | |
| FLT3 WT | 17 | 0.74 | 39 | 0.76 | |
| Not tested | 2 | 0.09 | 5 | 0.09 | |
| P53 status | 0.98 | ||||
| P53 mutated | 3 | 0.13 | 7 | 0.14 | |
| P53 WT | 8 | 0.35 | 17 | 0.33 | |
| Not tested | 12 | 0.52 | 27 | 0.53 | |
| ECOG status III/IV | 1 | 0.04 | 4 | 0.08 | 0.6 |
| First treatment received | 0.95 | ||||
| Anthracycline-based regimen | 8 | 0.35 | 16 | 0.32 | |
| Other* | 13 | 0.57 | 31 | 0.6 | |
| None | 2 | 0.09 | 4 | 0.08 | |
| Age (Average ± SD) | 65.6 | 15.9 | 66.5 | 15.2 | 0.81 |
| Age (Median, IQR) | 68.5 | 59.6-77.7 | 69.1 | 58.1-77.9 | 0.8 |
βEstimated sample size, the unweighted control number is 225 patients. *Other therapies include but are not limited to Venetoclax, decitabine, and cytarabine regimens. N.B. In this propensity-score model, no patients were excluded from the analysis. FLT3, fms-like tyrosine kinase 3; ITD, internal tandem duplication; TKD, tyrosine kinase domain; WT, wild type.
Outcomes
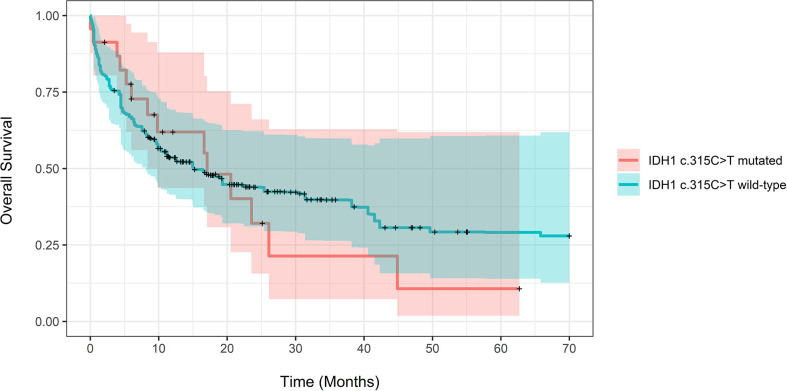
Excluding patients who did not have a bone marrow biopsy, the adjusted composite CR rate for patients with and without the IDH1 c.315C>T SNP was 77.10% compared to 65.30%; this finding was not statistically significant (P=0.53). The death at the end of observation in the IDH mutated group was 14 patients (39.10%) vs. 81 in the wildtype group (36%); P=0.944. The adjusted median OS for patients with compared to without the IDH1 c.315C>T SNP was 17.1 months (CI 8.37-Not calculable (NC)) compared to 15.1 months (CI 8.1-77.3, P=0.57). The unadjusted median OS for patients with and without the IDH1 c.315C>T SNP was 17.1 months (CI 9.8-NC) and 17 months (CI 13-22.2) (P=0.9). Adjusted OS difference at years 1-3 for patients with and without the IDH1 c.315C>T SNP showed no statistically significant difference ( Supplementary Table 2 ). On weighted-univariable Cox proportional hazards regression of the total cohort, there was no statistically significant difference in relative mortality (HR 1.08, CI 0.62-1.93, P=0.79). Figure 1 demonstrates propensity score-adjusted OS for patients with and without the IDH1 c.315C>T SNP.
Figure 1.
Propensity score-adjusted Overall Survival for patients with IDH1 c.315C>T mutated vs. IDH1 wild-type AML. Log-Rank adjusted P-Value was non-significant (P = 0.57).
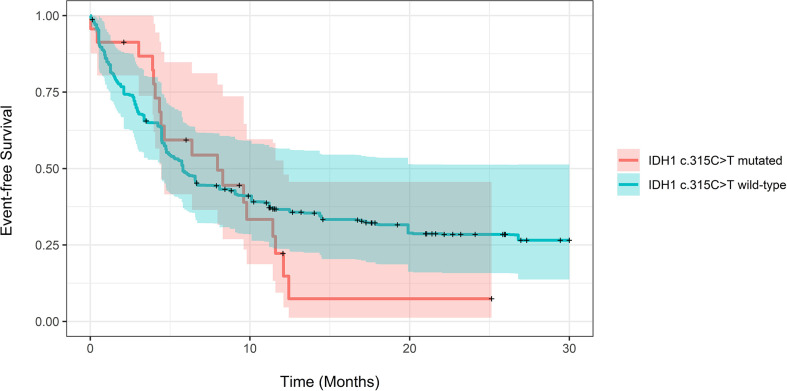
The adjusted median EFS for patients with and without the IDH1 c.315C>T SNP was 5.8 months (CI 4.47-74) compared to 7.97 (CI: 4.43-12.1, P=0.73). Adjusted EFS at years 1-3 for patients with and without the SNP also showed no statistical significance ( Supplementary Table 3 ). The relative mortality and progression were not different in patients with and without the SNP (HR 1.18, CI 0.71-1.98, P=0.5) using Cox proportional hazards regression. Figure 2 demonstrates propensity score-adjusted EFS for patients with vs. without the IDH1 c.315C>TSNP. As a sensitivity check, we repeated analysis using inverse probability weighting. There was no statistically significant difference in adjusted median OS or median EFS between the two groups. The results are provided in supplementary file ( Supplementary Tables 4–6 and Supplementary Figures 2–4 ).
Figure 2.
Propensity score-adjusted Event-free Survival for patients with IDH1 c.315C>T mutated vs. IDH1 wild-type AML. Log-Rank adjusted P-Value was non-significant (P = 0.73).
Discussion
Prognostic models for AML largely rely on cytogenetic aberrations and somatic mutations such as FLT3-ITD and NPM1 and CEBPA mutations in patients with a normal karyotype (4). However, many intermediate-risk patients have poorly understood AML genetic profiles, which may hinder accurate prognostication and clinical decision-making. We, therefore, aimed to study the IDH1 c.315C>T SNP, a poorly understood VUS seen in ~5-10% of AML cases (8, 9).
Previous data on the prognostic significance of this variant have been mixed; while it was shown to have inferior outcomes for cytogenetically normal AML in two studies (9, 10), another study showed inferior outcomes that were attributable to association with FLT3-ITD (11). Of the two studies that showed the adverse prognostic significance of the IDH1 c.315C>T SNP, one (10) (N=51, 8 with variant) reported that the SNP confers an inferior prognosis in NPM1/CEBPA wild-type Egyptian patients with AML. The other study (9) showed that the SNP had a negative effect on outcomes in univariate, but not multivariate, analysis, with the greatest impact in NPM1/FLT3 high-risk patients (either NPM1 wild-type or FLT3-ITD). These studies proposed that the synonymous SNP may induce genetic alteration at the mRNA level, such as alterations mRNA stability, folding, or splicing; however, all these studies were vulnerable to inadequate design (10). In order to evaluate such potential mechanisms, future RNA-seq studies to analyze transcriptome profile and to characterize changes in ribosome-associated mRNA (i.e. translatome) are warranted.
We hypothesized that due to the synonymous nature of IDH1 c.315C>T mutation resulting in uninterrupted presence of glycine in position 105 of the protein, the biochemical function of IDH1 enzyme remains intact; hence has no impact on the clinical outcome of patient. In this report, we confirm our hypothesis. Compared to prior studies, our study had greater power, with a large sample size (N=248), more patients with the variant (23 patients, 9%), and adjustment for more extensive disease profile data, allowing for many variables to be controlled. In addition, our study used propensity score weighting to adjust for baseline confounding, which showed no statistical prognostic difference between cohorts while controlling for baseline characteristics, including FLT3 mutations. This is consistent with the previous multivariate analysis (9). Our study adds to the current data, revealing that with greater power and tight statistical control of an extensive number of confounding variables, there was no negative prognostic value for the IDH1 c.315C>T SNP, with statistically insignificant differences in clinical outcome.
The major limitation of this study is that it was a retrospective single-site model. To control for observable confounding variables, we adjusted outcomes using propensity score analysis. After matching, the standardized mean difference was less than 0.2 in all variables. As a sensitivity analysis, we conducted weighted-multivariable Cox proportional hazards regression to adjust for possible remaining confounding, and there was no qualitative difference in outcomes.
Conclusion
Our retrospective cohort study showed that, unlike in previous studies and concordant with our mechanism-based hypothesis, the presence of IDH1 c.315C>T SNP was not associated with inferior OS, PFS or CR+CRi rates compared with its absence. Due to the rarity of this SNP, further collaborative study with multiple institutions is warranted to understand the impact of this SNP fully.
Data Availability Statement
The data that support the findings of this study are available from MMA, Moaath.mustafaali@umm.edu, upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Maryland Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
The authors confirm their contribution to the paper as follows: Study conception and design: EC, MMA, and AE. Data Collection: EC, MMA, HA, KK, and DS. Analysis and Interpretation: EC, MMA, HA, KK, DS, JL, SL, SN, VD, MB, and AE. Draft manuscript preparation: EC, MMA, and AE. Statistical analysis: MMA. Critical Review of Manuscript: HA, KK, DS, JL, SL, SN, VD, MB, and AE. Administrative and technical support: MMA. Supervision: MMA and AE. All authors reviewed the results and approved the final version of the manuscript.
Funding
This research was supported by funds through the National Cancer Institute - Cancer Center Support Grant (CCSG) - P30CA134274 and through the Maryland Department of Health’s Cigarette Restitution Fund Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.804961/full#supplementary-material
References
- 1. American Cancer Society . American Cancer Society: Cancer Facts and Figures 2022. Atlanta, Ga: American Cancer Society; (2022). [Google Scholar]
- 2. Estey E, Karp JE, Emadi A, Othus M, Gale RP. Recent Drug Approvals for Newly Diagnosed Acute Myeloid Leukemia: Gifts or a Trojan Horse? Leukemia (2020) 34(3):671–81. doi: 10.1038/s41375-019-0704-5 [DOI] [PubMed] [Google Scholar]
- 3. Lai C, Doucette K, Norsworthy K. Recent Drug Approvals for Acute Myeloid Leukemia. J Hematol Oncol (2019) 12(1):1–20. doi: 10.1186/s13045-019-0774-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and Management of Acute Myeloid Leukemia in Adults: Recommendations From an International Expert Panel, on Behalf of the European LeukemiaNet. Blood (2010) 115(3):453–74. doi: 10.1182/blood-2009-07-235358 [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N Engl J Med (2013) 369(1):98. doi: 10.1056/nejmx130028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ward PS, Lu C, Cross JR, Abdel-Wahab O, Levine RL, Schwartz GK, et al. The Potential for Isocitrate Dehydrogenase Mutations to Produce 2-Hydroxyglutarate Depends on Allele Specificity and Subcellular Compartmentalization. J Biol Chem (2013) 288(6):3804–15. doi: 10.1074/jbc.M112.435495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fathi AT, Wander SA, Faramand R, Emadi A. Biochemical, Epigenetic, and Metabolic Approaches to Target IDH Mutations in Acute Myeloid Leukemia. Semin Hematol (2015) 52(3):165–71. doi: 10.1053/j.seminhematol.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 8. COSMIC - Overview Cosm1741220. Available at: https://cancer.sanger.ac.uk/cosmic/mutation/overview?id=122230596 (Accessed October 3, 2021).
- 9. Wagner K, Damm F, Göhring G, Görlich K, Heuser M, Schäfer I, et al. Impact of IDH1 R132 Mutations and an IDH1 Single Nucleotide Polymorphism in Cytogenetically Normal Acute Myeloid Leukemia: SNP Rs11554137 Is an Adverse Prognostic Factor. J Clin Oncol (2010) 28(14):2356–64. doi: 10.1200/JCO.2009.27.6899 [DOI] [PubMed] [Google Scholar]
- 10. Ali MAM, Ahmed EK, Assem MMA, Helwa R. The Synonymous Isocitrate Dehydrogenase 1 315c>T SNP Confers an Adverse Prognosis in Egyptian Adult Patients With NPM1-/CEBPA-Negative Acute Myeloid Leukemia. Indian J Hematol Blood Transfusion (2018) 34(2):240–52. doi: 10.1007/s12288-017-0852-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ho PA, Kopecky KJ, Alonzo TA, Gerbing RB, Miller KL, Kuhn J, et al. Prognostic Implications of the IDH1 Synonymous SNP Rs11554137 in Pediatric and Adult AML: A Report From the Children’s Oncology Group and SWOG. Blood (2011) 118(17):4561–6. doi: 10.1182/blood-2011-04-348888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations From an International Expert Panel. Blood (2017) 129(4):424–47. doi: 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CRISP . (2021). Available at: https://www.crisphealth.org (Accessed October 4, 2021).
- 14. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J Biomed Inf (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)-A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inf (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harder VS, Stuart EA, Anthony JC. Propensity Score Techniques and the Assessment of Measured Covariate Balance to Test Causal Associations in Psychological Research. Psychol Methods (2010) 15(2):79. doi: 10.1037/a0019623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Software (2011) 42. doi: 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 18. Greifer N. WeightIt: Weighting for Covariate Balance in Observational Studies. R package version 0.9. 0. (2021). Available at: https://cran.r-project.org/web/packages/WeightIt/index.html (Accessed 1 Nov 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from MMA, Moaath.mustafaali@umm.edu, upon reasonable request.