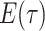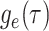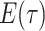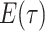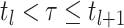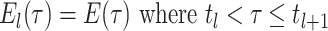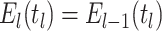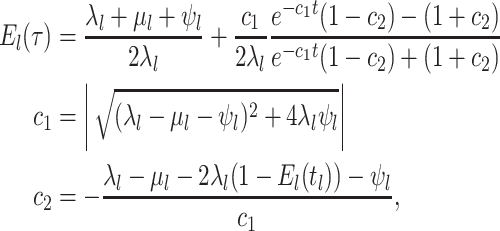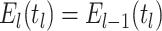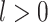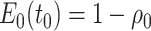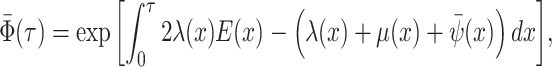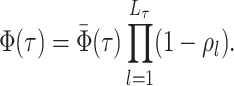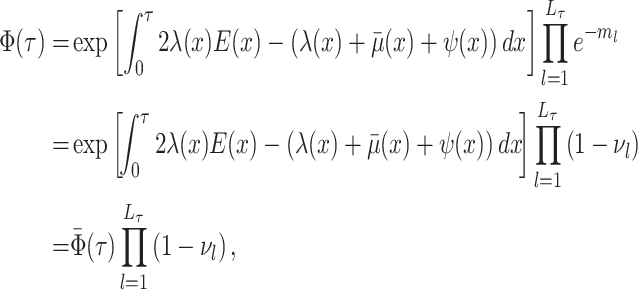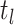Abstract
Birth–death stochastic processes are the foundations of many phylogenetic models and are widely used to make inferences about epidemiological and macroevolutionary dynamics. There are a large number of birth–death model variants that have been developed; these impose different assumptions about the temporal dynamics of the parameters and about the sampling process. As each of these variants was individually derived, it has been difficult to understand the relationships between them as well as their precise biological and mathematical assumptions. Without a common mathematical foundation, deriving new models is nontrivial. Here, we unify these models into a single framework, prove that many previously developed epidemiological and macroevolutionary models are all special cases of a more general model, and illustrate the connections between these variants. This unification includes both models where the process is the same for all lineages and those in which it varies across types. We also outline a straightforward procedure for deriving likelihood functions for arbitrarily complex birth–death(-sampling) models that will hopefully allow researchers to explore a wider array of scenarios than was previously possible. By rederiving existing single-type birth–death sampling models, we clarify and synthesize the range of explicit and implicit assumptions made by these models. [Birth–death processes; epidemiology; macroevolution; phylogenetics; statistical inference.]
Evolutionary, demographic, and epidemiological pro- cesses leave a footprint in the branch length distribution and topology of reconstructed phylogenetic trees. This insight has inspired a huge effort to extract information about these processes by fitting stochastic models. For example, in molecular epidemiology, researchers have leveraged the fact that for many viral pathogens, such as HIV and SARS-CoV-2, accumulate genetic diversity on the timescale of transmission (Drummond et al. 2003; Duffy et al. 2008). This genetic diversity can be used to reconstruct the evolutionary relationships between viral variants sampled from different hosts, which in turn can help elucidate the epidemiological dynamics of a pathogen over time (Grenfell et al. 2004; Volz 2012). Similarly, phylogenetic trees can provide unique insights into the temporal variation in speciation and extinction rates (Morlon 2014).
Phylogenetic branching models can be broadly grouped into two classes. The first, based on Kingman’s coalescent process (Kingman 1982), has been widely used to examine changes in the historical population size of pathogens (Pybus et al. 2000; Strimmer and Pybus 2001; Drummond et al. 2005; Volz et al. 2009). These coalescent methods have also been applied to reconstruct macroevolutionary dynamics (Morlon et al. 2010). Coalescent models are well suited for estimating deterministic population dynamics; however, fitting highly stochastic processes, such as the dynamics of an emerging pathogen, is computationally intensive and in some cases the assumptions of the coalescent may not be appropriate (Stadler et al. 2015; Boskova et al. 2014; Volz and Frost 2014). The second class of models, which are collectively referred to as birth–death-sampling (BDS) models (Kendall 1948; Maddison et al. 2007; Stadler 2009; Stadler 2010), is well suited for stochastic scenarios, and are thus becoming an increasingly favorable and popular alternative to coalescent models in epidemiology (Stadler et al. 2012) and have long been the foundation of most macroevolutionary studies—both for inferring speciation and extinction dynamics (Raup 1985; Nee et al. 1994) and for estimating divergence times (Gernhard 2008; Heath et al. 2014). As the name implies, the BDS process includes three types of events: birth (pathogen transmission between hosts, or speciation in a macroevolutionary context), death (host death or recovery, or extinction in macroevolution), and sampling (including fossil collection in macroevolution).
In the context of epidemiology, BDS models have the additional property that the model
parameters, which can be estimated from viral sequence data, explicitly correspond to
parameters in classic structured epidemiological models that are often fit to case
surveillance data. If we reparameterize these models, we can describe the dynamics of the
basic and effective reproductive ratios (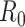 and
and
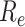 , respectively) over time (Stadler et al. 2012; Stadler et al. 2013) (see Box 1). A common research aim is to describe how the
frequency of birth, death, and sampling events, and other derived variables such as
, respectively) over time (Stadler et al. 2012; Stadler et al. 2013) (see Box 1). A common research aim is to describe how the
frequency of birth, death, and sampling events, and other derived variables such as
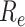 , change throughout the course of an epidemic.
There has been less work in macroevolution linking the parameters of a BDS model to those of
an underlying more mechanistic model (but see Ezard et al.
2016) but this seems like a promising avenue for future development. As we detail
below and in the Supplementary material
available at Dryad at https://doi.org/10.5281/zenodo.5028470, there has been an astounding rise in the
variety and complexity of BDS model variants. A key assumption in the specification of BDS
submodels is whether all lineages alive at some time point are exchangeable (Stadler 2013) (such models are hereafter “single-type”
models), meaning they diversify according to the same process, or if rather the
diversification process is variable (“multitype” models; e.g., Maddison et al. 2007; FitzJohn 2012; Stadler and Bonhoeffer 2013; Rasmussen and Stadler 2019; Barido-Sottani
et al. 2018), with lineages belonging to one of multiple possible states each
characterized by a unique process. Each of these diversification processes can then be
characterized by different dynamical assumptions. In the epidemiological case, these
assumptions specify, for example, the nature of viral transmission and the sampling procedure
(Stadler et al. 2013; Kühnert et al. 2014; Gavryushkina et al.
2014). While typically not explicitly tied to mechanistic evolutionary processes, there
are a similar abundance of dynamical assumptions employed in the macroevolutionary context
specifying the nature of biodiversity change through time (Nee
2006; Gernhard 2008; Morlon et al. 2011; Stadler 2011;
Morlon 2014; Heath et
al. 2014; Louca 2020).
, change throughout the course of an epidemic.
There has been less work in macroevolution linking the parameters of a BDS model to those of
an underlying more mechanistic model (but see Ezard et al.
2016) but this seems like a promising avenue for future development. As we detail
below and in the Supplementary material
available at Dryad at https://doi.org/10.5281/zenodo.5028470, there has been an astounding rise in the
variety and complexity of BDS model variants. A key assumption in the specification of BDS
submodels is whether all lineages alive at some time point are exchangeable (Stadler 2013) (such models are hereafter “single-type”
models), meaning they diversify according to the same process, or if rather the
diversification process is variable (“multitype” models; e.g., Maddison et al. 2007; FitzJohn 2012; Stadler and Bonhoeffer 2013; Rasmussen and Stadler 2019; Barido-Sottani
et al. 2018), with lineages belonging to one of multiple possible states each
characterized by a unique process. Each of these diversification processes can then be
characterized by different dynamical assumptions. In the epidemiological case, these
assumptions specify, for example, the nature of viral transmission and the sampling procedure
(Stadler et al. 2013; Kühnert et al. 2014; Gavryushkina et al.
2014). While typically not explicitly tied to mechanistic evolutionary processes, there
are a similar abundance of dynamical assumptions employed in the macroevolutionary context
specifying the nature of biodiversity change through time (Nee
2006; Gernhard 2008; Morlon et al. 2011; Stadler 2011;
Morlon 2014; Heath et
al. 2014; Louca 2020).
Box 1: The Connection between BDS and SIR Models
The single-type BDS model is intimately related to the SIR compartmental model used in classic theoretical epidemiology. This connection illustrates the explicit and implicit assumptions of the general BDS model and its sub models. Here, we define the SIR epidemiological model, discuss how it can inform and be informed by these diversification models, and examine the shared assumptions of the two frameworks.
The SIR Model
The SIR model partitions the host population via infection status into susceptible (S),
infected (I), and recovered (R) hosts. Infection of susceptible hosts occurs at a
per-capita rate 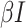 . Infected hosts may recover (at
rate
. Infected hosts may recover (at
rate 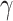 ), die of virulent cases (at rate
), die of virulent cases (at rate
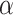 ), or be sampled (at rate
), or be sampled (at rate
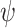 ). The cumulative number of sampled
hosts is represented in the SIR model (Figure B.1
top) by
). The cumulative number of sampled
hosts is represented in the SIR model (Figure B.1
top) by 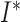 . Upon sampling, infected hosts may
be treated and hence effectively recover with probability
. Upon sampling, infected hosts may
be treated and hence effectively recover with probability 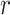 .
Hosts that have recovered from infection exhibit temporary immunity to future infection
which wanes at rate
.
Hosts that have recovered from infection exhibit temporary immunity to future infection
which wanes at rate 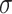 . The special case of the SIR
model with no immunity (the SIS model) is obtained in the limit as
. The special case of the SIR
model with no immunity (the SIS model) is obtained in the limit as
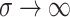 . In addition to these
epidemiological processes, the SIR model includes demographic processes, such as host
birth (rate
. In addition to these
epidemiological processes, the SIR model includes demographic processes, such as host
birth (rate 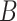 ) and death from natural causes (rate
) and death from natural causes (rate
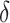 ). While not shown explicitly in
the figure, these epidemiological and demographic rates may change over time as a result
of host behavioral change, pharmaceutical and nonpharmaceutical interventions, or
host/pathogen evolution.
). While not shown explicitly in
the figure, these epidemiological and demographic rates may change over time as a result
of host behavioral change, pharmaceutical and nonpharmaceutical interventions, or
host/pathogen evolution.
The BDS Model
The BDS model follows the number of sampled and unsampled viral lineages over time,
analogous to the 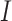 and
and 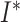 classes of the SIR model. A key element of general BDS model is that birth and death rates
may vary over time. This time dependence may be either continuous (Morlon et al. 2011; Rabosky and Lovette
2008b) or discrete (Stadler 2011; Stadler and Bonhoeffer 2013; Gavryushkina et al. 2014; Kühnert et
al. 2014) Although arbitrarily time-dependent, the birth, death, and sampling
rates in the general BDS model are assumed to be diversity-independent, analogous to the
assumption of density-dependent transmission (pseudo mass action) in the SIR model (Keeling and Rohani 2008). Incorporating such diversity
dependence into macroevolutionary models has been shown to increase the accuracy of
extinction rate estimates and are necessary to accurately capture the saturation of
diversity (Etienne et al. 2012). While some forms
of diversity-dependence in diversification rates may be incorporated implicitly capturing
deterministic diversity dependence as time dependence (Rabosky and Lovette 2008a), stochastic diversity-dependence (Etienne and Rosindell 2012) goes beyond the scope of
the BDS models considered here.
classes of the SIR model. A key element of general BDS model is that birth and death rates
may vary over time. This time dependence may be either continuous (Morlon et al. 2011; Rabosky and Lovette
2008b) or discrete (Stadler 2011; Stadler and Bonhoeffer 2013; Gavryushkina et al. 2014; Kühnert et
al. 2014) Although arbitrarily time-dependent, the birth, death, and sampling
rates in the general BDS model are assumed to be diversity-independent, analogous to the
assumption of density-dependent transmission (pseudo mass action) in the SIR model (Keeling and Rohani 2008). Incorporating such diversity
dependence into macroevolutionary models has been shown to increase the accuracy of
extinction rate estimates and are necessary to accurately capture the saturation of
diversity (Etienne et al. 2012). While some forms
of diversity-dependence in diversification rates may be incorporated implicitly capturing
deterministic diversity dependence as time dependence (Rabosky and Lovette 2008a), stochastic diversity-dependence (Etienne and Rosindell 2012) goes beyond the scope of
the BDS models considered here.
The single-type BDS model assumes all viral lineages are exchangeable—this has several implications. First, all viral lineages are epidemiologically identical hence all mutations between them are neutral. Incorporating nonneutral genetic variation requires a multitype approach as in Equation (16). Second, transmission is independent of lineage age. In the macroevolutionary case, such age-dependence has been suggested to reflect niche differentiation in novel species (Hagen et al. 2015) and in the epidemiological case may reflect adaptation towards increased transmissibility following a host species-jumping event. Third, lineage exchangeability is reflected in the absence of an exposed (E) class in the SIR model in which hosts can, for example, transmit infections but not be sampled or vice versa. Finally, the single-type BDS model assumes all lineages are sampled at random and does not include submodels with nonrandom representation of lineages (Stadler et al. 2012).
Model Connections
Given their shared model assumptions, the single-type BDS model can be constrained
explicitly to reflect an underlying SIR epidemic by setting the viral birth rate equal to
the per-capita transmission rate of the infectious class 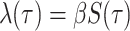 and the
viral death rate to the infectious recovery or removal rate
and the
viral death rate to the infectious recovery or removal rate 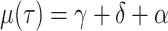 , whereas
the sampling rate
, whereas
the sampling rate 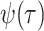 is identical across models
(Figure B1a). While constraining the birth, death, and sampling rates in this manner can
be used to parameterize compartmental models (Kühnert et
al. 2014) doing so is an approximation assuming independence between the exact
timing of transmission, recovery or removal from the population, and sampling events in
the SIR model and birth, death, and sampling events in the diversification model. The
resulting tree likelihood in terms of the compartmental model is given by:
is identical across models
(Figure B1a). While constraining the birth, death, and sampling rates in this manner can
be used to parameterize compartmental models (Kühnert et
al. 2014) doing so is an approximation assuming independence between the exact
timing of transmission, recovery or removal from the population, and sampling events in
the SIR model and birth, death, and sampling events in the diversification model. The
resulting tree likelihood in terms of the compartmental model is given by:
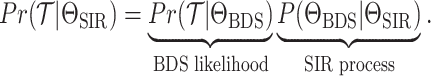 |
(B1) |
While they are not submodels of the general BDS process given by equation 13, likelihood
models have been developed that capture the full nonindependence of viral diversification
and epidemiological dynamics for the SIR model specifically (Leventhal et al. 2012) and in compartmental models in general (Vaughan et al. 2019). The connection between the BDS
process and SIR epidemiological models can also be used after the diversification rates
are inferred to estimate the basic and effective reproductive rates (Stadler et al. 2012; Stadler and
Bonhoeffer 2013). Specifically, the effective reproductive rate at time
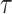 before the present day is given by
before the present day is given by
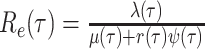 .
Although the SIR model is a useful epidemiological model for is simplicity, realistically
modeling epidemic dynamics requires far more complex compartmental models. As reflected by
their shared structure, the application of the single-type BDS model is restricted,
however, to the assumptions of the SIR model alone and further methodological advances in
multitype modeling are necessary for direct inference for the larger class of
epidemiological models.
.
Although the SIR model is a useful epidemiological model for is simplicity, realistically
modeling epidemic dynamics requires far more complex compartmental models. As reflected by
their shared structure, the application of the single-type BDS model is restricted,
however, to the assumptions of the SIR model alone and further methodological advances in
multitype modeling are necessary for direct inference for the larger class of
epidemiological models.
This flourishing of methods and models has facilitated critical insights into epidemics (du Plessis and Stadler 2015; Joy et al. 2016) and the origins of contemporary biodiversity (Morlon 2014; Schluter and Pennell 2017). However, this diversity of models has made it difficult to trace the connections between variants and to understand the precise epidemiological, evolutionary, and sampling processes that differ between them. Furthermore, despite their apparent similarities, these models have been derived on a case-by-case basis using different notation and techniques; this creates a substantial barrier for researchers working to develop novel models for new situations. And critically, it is imperative that we understand the general properties of BDS phylogenetic models and the limits of inferences from them (Louca and Pennell 2020a; Louca et al. 2021), and this is difficult to do without considering the full breadth of possible scenarios. Here, we address all of these challenges by unifying the whole class of phylogenetic BDS models. We do so by first deriving a likelihood for general single- and multitype BDS models; in the general case, we do not assume anything about the functional forms (i.e., temporal dynamics) of the various parameters including the sampling rate through time, the possibility of sampling ancestors (or not), or how the process was conditioned. While such general models may be useful for studying the mathematical properties of BDS models as a whole (Lambert and Stadler 2013; Louca and Pennell 2020a; Louca et al. 2021), statistical inference from these models requires researchers to make further constraints on the process. We prove that existing BDS model variants are indeed submodels of the more general case—and thereby clarify the specific assumptions made by different models—and provide a standardized notation and technique for deriving these and other submodels that have not previously been considered in the literature.
The Single-type Birth–Death-Sampling Model
Model specification
The BDS stochastic process begins with a single lineage at time
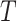 before the present day. We note
that this may be considerably older than the age of the most recent common ancestor of
an observed sample which is given by
before the present day. We note
that this may be considerably older than the age of the most recent common ancestor of
an observed sample which is given by 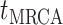 . While
we focus primarily on applications to epidemiology, our approach is agnostic to whether
the rates are interpreted as describing pathogen transmission or macroevolutionary
diversification.
. While
we focus primarily on applications to epidemiology, our approach is agnostic to whether
the rates are interpreted as describing pathogen transmission or macroevolutionary
diversification.
In the model, transmission/speciation results in the birth of a lineage and occurs at
rate 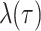 , where
, where
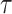 (
(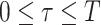 ) is measured in units
of time before the present day (
) is measured in units
of time before the present day (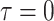 ), such that
), such that
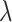 can be time-dependent. We
make the common assumption that lineages in the viral phylogeny coalesce exactly at
transmission events, thus ignoring the within-host coalescent processes in the donor
(Romero-Severson et al. 2016). Throughout, we
will use
can be time-dependent. We
make the common assumption that lineages in the viral phylogeny coalesce exactly at
transmission events, thus ignoring the within-host coalescent processes in the donor
(Romero-Severson et al. 2016). Throughout, we
will use 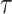 as a a general time variable and
as a a general time variable and
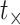 to denote the time at
which a specific event
to denote the time at
which a specific event 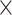 occurs as measured in units of
time before the present day (see Supplementary
Table S1). Lineage extinction, resulting from host recovery or death in the
epidemiological case or the death of all individuals in a population in the
macroevolutionary case, occurs at time-dependent rate
occurs as measured in units of
time before the present day (see Supplementary
Table S1). Lineage extinction, resulting from host recovery or death in the
epidemiological case or the death of all individuals in a population in the
macroevolutionary case, occurs at time-dependent rate 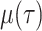 . We allow for two distinct
types of sampling: lineages are either sampled according to a Poisson process through
time
. We allow for two distinct
types of sampling: lineages are either sampled according to a Poisson process through
time 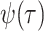 or binomially at very
short intervals, which we term “concerted sampling attempts” (CSAs), where lineages at
some specified time
or binomially at very
short intervals, which we term “concerted sampling attempts” (CSAs), where lineages at
some specified time 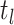 are sampled with probability
are sampled with probability
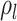 (
(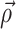 denotes a vector of
concerted sampling events at different time points). In macroevolutionary studies based
only on extant lineages, there is no Poissonian sampling, but a CSA at the present
(
denotes a vector of
concerted sampling events at different time points). In macroevolutionary studies based
only on extant lineages, there is no Poissonian sampling, but a CSA at the present
(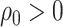 ). In epidemiology, CSAs
correspond to large-scale testing efforts (relative to the background rate of testing)
in a short amount of time (relative to the rates of viral sequence divergence); for full
explanation, see Appendix. We call these attempts rather than events because if
). In epidemiology, CSAs
correspond to large-scale testing efforts (relative to the background rate of testing)
in a short amount of time (relative to the rates of viral sequence divergence); for full
explanation, see Appendix. We call these attempts rather than events because if
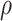 is small or the infection is
rare in the population, few or no samples may be obtained. CSAs can also be incorporated
into the model by including infinitesimally short spikes in the sampling rate
is small or the infection is
rare in the population, few or no samples may be obtained. CSAs can also be incorporated
into the model by including infinitesimally short spikes in the sampling rate
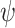 (more precisely, appropriately
scaled Dirac distributions). Hence, for simplicity, in the main text we focus on the
seemingly simpler case of pure Poissonian sampling through time except at the
present-day, where we allow for a CSA to facilitate comparisons with macroevolutionary
models; the resulting formulas can then be used to derive a likelihood formula for the
case where past CSAs are included (see Appendix).
(more precisely, appropriately
scaled Dirac distributions). Hence, for simplicity, in the main text we focus on the
seemingly simpler case of pure Poissonian sampling through time except at the
present-day, where we allow for a CSA to facilitate comparisons with macroevolutionary
models; the resulting formulas can then be used to derive a likelihood formula for the
case where past CSAs are included (see Appendix).
In the epidemiological case, sampling may be concurrent (or not) with host treatment or
behavioral changes resulting in the effective extinction of the viral lineage. Hence, we
assume that sampling results in the immediate extinction of the lineage with probability
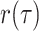 . As with the CSAs, this
arbitrary time dependence allows for the incorporation of Dirac spikes in any of these
variables, for example, with mass extinctions (
. As with the CSAs, this
arbitrary time dependence allows for the incorporation of Dirac spikes in any of these
variables, for example, with mass extinctions (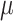 ) and lagerstätten in the fossil
record (
) and lagerstätten in the fossil
record (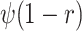 ) (Magee and Höhna 2021). Similarly, in the case of past CSAs, we must
include the probability,
) (Magee and Höhna 2021). Similarly, in the case of past CSAs, we must
include the probability, 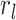 , that sampled hosts are removed
from the infectious pool during the CSA at time
, that sampled hosts are removed
from the infectious pool during the CSA at time 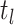 . Poissonian sampling without the
removal of lineages (
. Poissonian sampling without the
removal of lineages (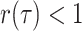 ) can be employed in the
macroevolutionary case to explicitly model the collection of samples from the fossil
record (such as the fossilized-birth–death process; Heath et al. 2014).
) can be employed in the
macroevolutionary case to explicitly model the collection of samples from the fossil
record (such as the fossilized-birth–death process; Heath et al. 2014).
For our derivation, we make no assumption about the temporal dynamics of
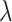 ,
, 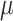 ,
,
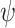 , or
, or 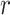 ;
each may be constant, or vary according to any arbitrary function of time given that it
is biologically valid (nonnegative and between 0 and 1 in the case of
;
each may be constant, or vary according to any arbitrary function of time given that it
is biologically valid (nonnegative and between 0 and 1 in the case of
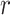 ). Specifically, the time-varying
functions may be any piecewise-continuous functions of time with at most finite number
of discontinuities (see Rate Assumption section in the Appendix). Note that these
functions need not be differentiable. We make the standard assumption that at any given
time any given lineage experiences a birth, death or sampling event independently of
(and with the same probabilities as) all other lineages. We revisit this assumption in
Box 1 where we discuss how the implicit assumptions of the single-type BDS process are
well summarized by the diversification model’s relationship to the SIR epidemiological
model. Our resulting general time-variable BDS process can be fully defined by the
parameter set
). Specifically, the time-varying
functions may be any piecewise-continuous functions of time with at most finite number
of discontinuities (see Rate Assumption section in the Appendix). Note that these
functions need not be differentiable. We make the standard assumption that at any given
time any given lineage experiences a birth, death or sampling event independently of
(and with the same probabilities as) all other lineages. We revisit this assumption in
Box 1 where we discuss how the implicit assumptions of the single-type BDS process are
well summarized by the diversification model’s relationship to the SIR epidemiological
model. Our resulting general time-variable BDS process can be fully defined by the
parameter set 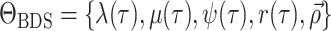 .
.
In order to make inference about the model parameters, we need to calculate the
likelihood, 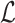 , that an observed
phylogeny,
, that an observed
phylogeny, 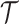 , is the result of a given
BDS process. With respect to the BDS process there are two ways to represent the
information contained in the phylogeny
, is the result of a given
BDS process. With respect to the BDS process there are two ways to represent the
information contained in the phylogeny 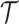 , both of
which have been used in the literature, which we call the “edge” and “critical time”
representations, respectively. We begin by deriving the likelihood in terms of the edge
representation and later demonstrate how to reformulate the likelihood in terms of
critical times. In the edge representation, the phylogeny is summarized as a set of
edges in the mathematical graph that makes up the phylogeny, numbered 1–11 in Figure B.1c, and the types of events that occurred at
each node. We define
, both of
which have been used in the literature, which we call the “edge” and “critical time”
representations, respectively. We begin by deriving the likelihood in terms of the edge
representation and later demonstrate how to reformulate the likelihood in terms of
critical times. In the edge representation, the phylogeny is summarized as a set of
edges in the mathematical graph that makes up the phylogeny, numbered 1–11 in Figure B.1c, and the types of events that occurred at
each node. We define 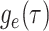 as the probability that
the edge
as the probability that
the edge 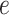 which begins at time
which begins at time
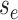 and ends at time
and ends at time
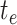 gives rise to the subsequently
observed phylogeny between time
gives rise to the subsequently
observed phylogeny between time 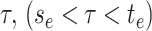 and the present
day. The likelihood of the model for the observed tree is then, is by definition
and the present
day. The likelihood of the model for the observed tree is then, is by definition
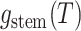 : the probability
density that the stem lineage (
: the probability
density that the stem lineage (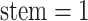 in Figure B.1c) gives rise to the observed phylogeny from
the origin,
in Figure B.1c) gives rise to the observed phylogeny from
the origin, 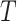 , to the present day. We find that
it is more intuitive to derive the likelihood in terms of the edge representation, as we
show below; from this it is straightforward to derive the critical times formulation
which results in mathematical simplification. Below, we present our five-step technique
for the derivation of the tree likelihood.
, to the present day. We find that
it is more intuitive to derive the likelihood in terms of the edge representation, as we
show below; from this it is straightforward to derive the critical times formulation
which results in mathematical simplification. Below, we present our five-step technique
for the derivation of the tree likelihood.
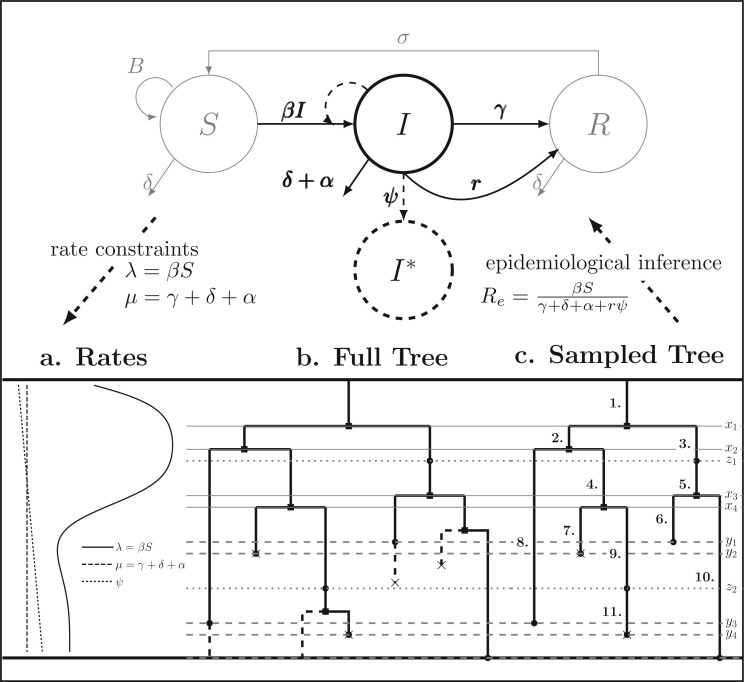
Figure B.1.
BDS-SIR model connection. Top: The SIR epidemiological model. Black (gray) lines and
classes represent rates and variables followed (in)directly by the BDS model. The SIR
model can be used to constrain the rates of the BDS model (a). Simulated forward in time,
the result of the BDS stochastic processes is a full tree (b) giving the
complete genealogy of the viral population. Pruning away extinct and unsampled lineages
produces the sampled tree (c). Arising from a BDS process, this sampled
tree can be summarized in two ways. First by the set of edges (labeled 1–11) or as a set
of critical times (horizontal lines) including: 1) the time of birth events (solid,
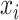 ) 2) terminal sampling times
(dashed,
) 2) terminal sampling times
(dashed, 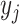 ), and 3) ancestral sampling times
(dotted,
), and 3) ancestral sampling times
(dotted, 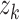 ). Given the inferred rates from a
reconstructed sampled tree, these rates can be used to estimate characteristic parameters
of the SIR model, for example, the basic or effective reproductive number.
). Given the inferred rates from a
reconstructed sampled tree, these rates can be used to estimate characteristic parameters
of the SIR model, for example, the basic or effective reproductive number.
Step 1. Deriving the Initial Value Problem (IVP) for 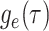
We derive the IVP for the likelihood density 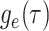 using an
approach first developed by (Maddison et al.,
2007). We begin by deriving the recursion equation for
using an
approach first developed by (Maddison et al.,
2007). We begin by deriving the recursion equation for
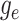 by considering all the possible
events that could occur along edge
by considering all the possible
events that could occur along edge 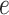 between time
between time
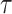 and
and 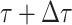 assuming that that
assuming that that
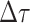 is small enough such that
at most one event is likely to occur.
is small enough such that
at most one event is likely to occur.
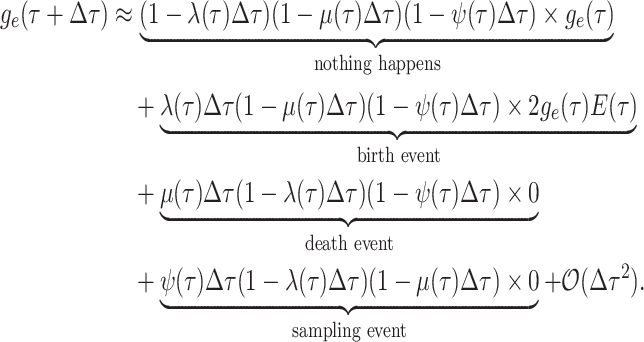 |
(1) |
Here, 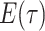 is the probability that a
lineage alive at time
is the probability that a
lineage alive at time 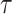 leaves no sampled descendants at
the present day. We will examine this probability in more detail below. Assuming
leaves no sampled descendants at
the present day. We will examine this probability in more detail below. Assuming
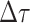 is small, we can
approximate the above recursion equation as the following difference equation.
is small, we can
approximate the above recursion equation as the following difference equation.
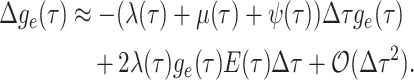 |
(2) |
By the definition of the derivative, we have:
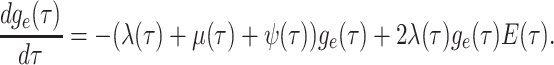 |
(3) |
Equation (3) is known as the
Kolmogorov backward equation of the BDS process (Feller
1949; Louca and Pennell 2020b).
Beginning at time 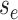 , the initial condition of
, the initial condition of
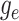 depends on which event occurred
at the beginning of edge
depends on which event occurred
at the beginning of edge 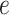 .
.
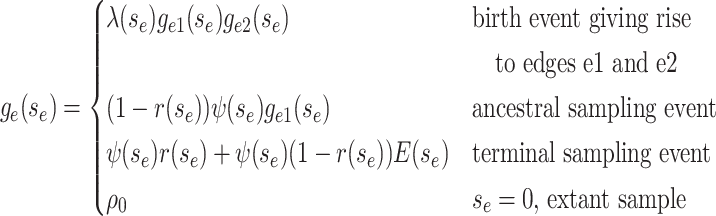 |
(4) |
Together Equations (3) and (4) define the initial value problem for
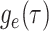 as a function of the
probability
as a function of the
probability 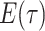 .
.
Because the likelihood density 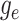 is the solution
to a linear differential equation with initial condition at time
is the solution
to a linear differential equation with initial condition at time
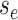 , we can express its solution as
follows:
, we can express its solution as
follows:
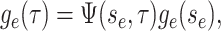 |
(5) |
where the auxiliary function,
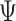 , is given by:
, is given by:
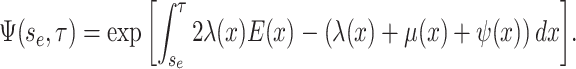 |
(6) |
This function, 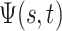 , maps the value of
, maps the value of
 at time
at time 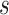 to its value at
to its value at 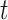 , and hence is known as the probability
“flow” of the Kolmogorov backward equation (Louca and
Pennell 2020b).
, and hence is known as the probability
“flow” of the Kolmogorov backward equation (Louca and
Pennell 2020b).
Step 2. Deriving the IVP for 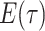
We derive the IVP for 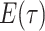 in a similar manner as above,
beginning with a difference equation.
in a similar manner as above,
beginning with a difference equation.
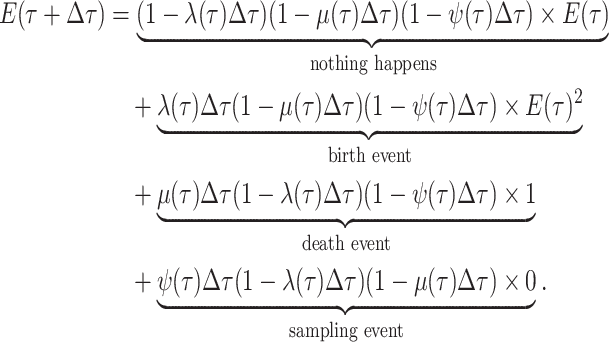 |
(7) |
By the definition of a derivative, we have:
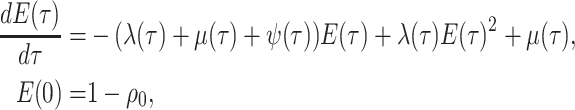 |
(8) |
where 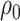 is the
probability a lineage is sampled at the present day. The initial condition at time
is the
probability a lineage is sampled at the present day. The initial condition at time
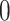 is therefore the probability that a
lineage alive at the present day is not sampled. Given an analytical or numerical
general solution to
is therefore the probability that a
lineage alive at the present day is not sampled. Given an analytical or numerical
general solution to 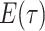 , we can find the likelihood
by evaluating
, we can find the likelihood
by evaluating 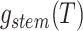 , as follows.
, as follows.
Step 3. Deriving the expression for 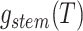 .
.
Given the linear nature of the differential equation for 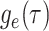 and hence the representation
in Equation (5)), the likelihood
and hence the representation
in Equation (5)), the likelihood
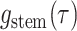 is given by the
product over all the initial conditions times the product over the probability flow for
each edge.
is given by the
product over all the initial conditions times the product over the probability flow for
each edge.
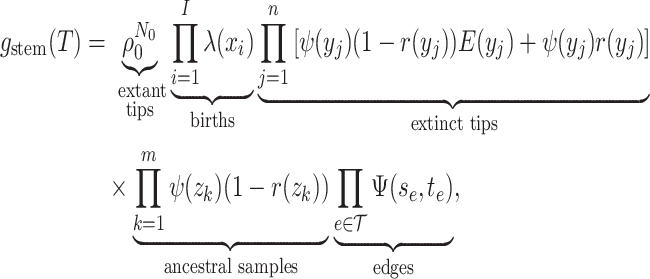 |
(9) |
where 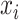 ,
, 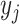 , and
, and 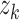 are the times at which individual birth, terminal sampling and ancestral sampling events
occur as we elaborated below.
are the times at which individual birth, terminal sampling and ancestral sampling events
occur as we elaborated below.
Step 4. Representing 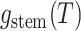 in terms of critical
times.
in terms of critical
times.
Equation (9) can be further
simplified by removing the need to enumerate over all the edges of the phylogeny (the
last term of Equation (9)) and
writing 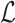 in terms of the tree’s
critical times (horizontal lines in Fig. B.1). The
critical times of the tree are made up of three vectors,
in terms of the tree’s
critical times (horizontal lines in Fig. B.1). The
critical times of the tree are made up of three vectors, 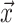 ,
, 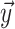 , and
, and 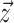 , as well as the time of origin
, as well as the time of origin
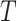 . The vector
. The vector
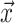 gives the time of each birth
event in the phylogeny and has length
gives the time of each birth
event in the phylogeny and has length 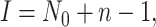 where
where
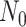 is the number of lineages sampled
at the present day and
is the number of lineages sampled
at the present day and 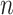 is the number of terminal samples.
Unless noted otherwise the elements of vector
is the number of terminal samples.
Unless noted otherwise the elements of vector 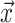 are listed
in decreasing order, such that
are listed
in decreasing order, such that 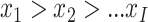 and hence
and hence 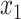 is the time of the most recent
common ancestor
is the time of the most recent
common ancestor 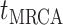 . The vector
. The vector
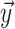 gives the timing of each
terminal sample and hence has length
gives the timing of each
terminal sample and hence has length 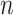 whereas vector
whereas vector
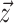 gives the timing of each
ancestral sample and has length
gives the timing of each
ancestral sample and has length 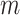 . With respect to
the BDS likelihood then the sampled tree is summarized by
. With respect to
the BDS likelihood then the sampled tree is summarized by 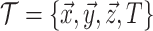 .
We note that the critical times only contain the same information as the edges as a
result of the assumptions of the BDS process but are not generally equivalent
representations of
.
We note that the critical times only contain the same information as the edges as a
result of the assumptions of the BDS process but are not generally equivalent
representations of 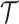 .
.
As a result of the linear nature of 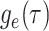 it is
straightforward to rewrite the likelihood in Equation (9) in terms of the critical-time representation of the sampled
tree. Defining
it is
straightforward to rewrite the likelihood in Equation (9) in terms of the critical-time representation of the sampled
tree. Defining
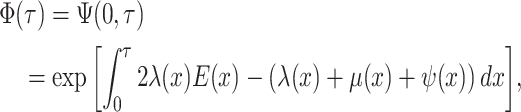 |
(10) |
the probability flow 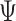 can be rewritten as the following ratio:
can be rewritten as the following ratio:
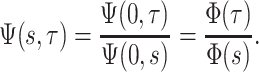 |
(11) |
This relationship allows us to rewrite the likelihood by expressing the product over
the edges as two separate products, one over the start of each edge and the other over
the end of each edge which in turn allows us to rearrange and cancel terms to obtain an
alternative likelihood expression. Edges begin (value of 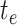 ) at either: 1) the tree origin, 2)
a birth event resulting to two lineages, or 3) an ancestral sampling event. Edges end
(values of
) at either: 1) the tree origin, 2)
a birth event resulting to two lineages, or 3) an ancestral sampling event. Edges end
(values of 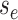 ) at either: 1) a birth event, 2)
an ancestral sampling event, 3) a terminal sampling event, or 4) the present day. Hence
we have:
) at either: 1) a birth event, 2)
an ancestral sampling event, 3) a terminal sampling event, or 4) the present day. Hence
we have:
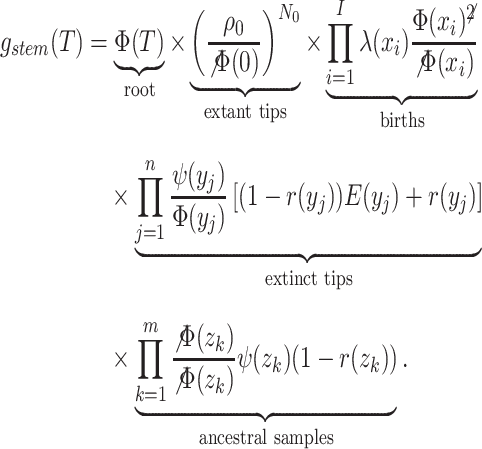 |
(12) |
Note 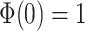 . While Equations (9) and (12) are numerically identical, the critical time expression is
more convenient for application as it requires numerically evaluating only a single
function
. While Equations (9) and (12) are numerically identical, the critical time expression is
more convenient for application as it requires numerically evaluating only a single
function 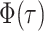 as given by Equation
(10).
as given by Equation
(10).
Step 5. Conditioning the likelihood
While Equation (12) is equal to
the basic model likelihood for the phylogeny 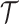 , it is
often appropriate to condition the tree likelihood on the tree exhibiting some property,
for example the condition there being at least sampled lineage. Imposing a condition on
the likelihood is done by multiplying by a factor
, it is
often appropriate to condition the tree likelihood on the tree exhibiting some property,
for example the condition there being at least sampled lineage. Imposing a condition on
the likelihood is done by multiplying by a factor 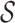 . Various conditioning
schemes are considered in section A4 and listed in Table S3 with the value of
. Various conditioning
schemes are considered in section A4 and listed in Table S3 with the value of
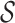 ranging in complexity
from a constant to a general function of the model parameters. The resulting likelihood
expression for the general BDS model is:
ranging in complexity
from a constant to a general function of the model parameters. The resulting likelihood
expression for the general BDS model is:
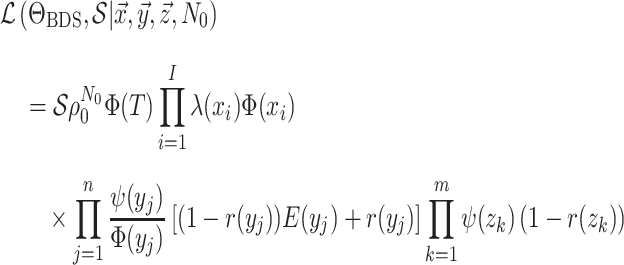 |
(13) |
Many Existing Models are Special Cases of this General BDS Model
A large variety of previously published BDS models in epidemiology and macroevolution are
special cases of the general model presented here (for a summary of the models we
investigated, see Supplementary Table S2.
Indeed, we can obtain the likelihood of these models by adding mathematical constraints
(i.e., simplifying assumptions) to the terms in Equation (13). Our work thus not only provides a consistent notation for
unifying a multitude of seemingly disparate models, it also provides a concrete and
numerically straightforward recipe for computing their likelihood functions. We recognize
that there are many valid approaches for deriving tree likelihoods for BDS models with
share many similarities with our own (e.g., Nee et al.
1994; Maddison et al. 2007; Gernhard 2008; Morlon
et al. 2011; Lambert and Stadler 2013;
Lambert 2018; Laudanno et al. 2020; Louca and Pennell
2020b) and do not claim ours is superior to these; however, we have found our
technique to be intuitive and flexible. We have implemented the single-type BDS likelihood
in the R package  (Louca and Doebeli 2018), including routines for maximum-likelihood
fitting of BDS models with arbitrary functional forms of the parameters given a phylogeny
and routines for simulating phylogenies under the general BDS models (functions
(Louca and Doebeli 2018), including routines for maximum-likelihood
fitting of BDS models with arbitrary functional forms of the parameters given a phylogeny
and routines for simulating phylogenies under the general BDS models (functions
 ,
,
 and
and
 ).
).
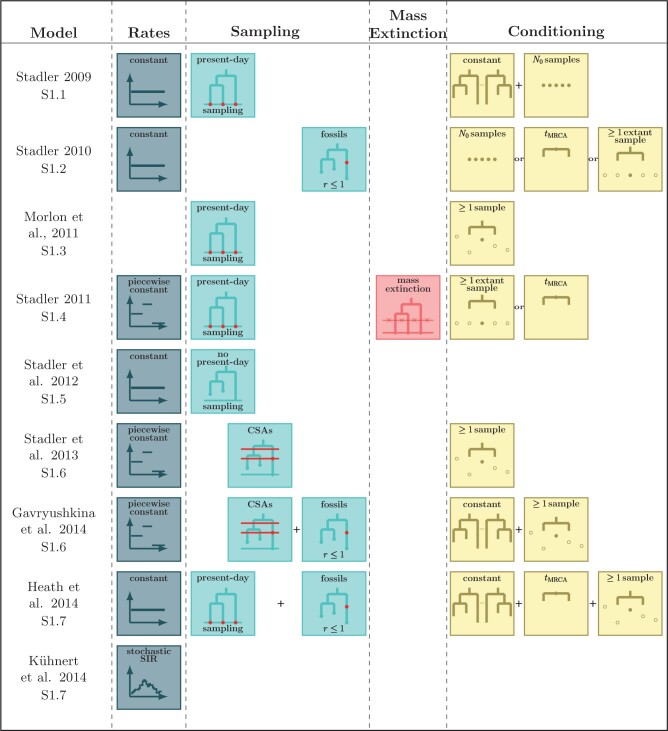
Figure 1 summarizes the simplifying assumptions that
underlie common previously published BDS models; these assumptions generally fall into
four categories: 1) assumptions about the functional form of birth, death, and sampling
rates over time, 2) assumptions pertaining to the sampling of lineages, 3) the presence of
mass-extinction events, and 4) the nature of the tree-conditioning as given by
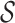 . Here, we provide a brief
overview of the type of previously invoked constraints which are consistent (or not) with
our unified framework; for full details on each specific case, we refer readers to the
Supplementary material.
While we illustrate these constraints within the single-type context, analogous
assumptions can be made within the multitype context examined in the following
section.
. Here, we provide a brief
overview of the type of previously invoked constraints which are consistent (or not) with
our unified framework; for full details on each specific case, we refer readers to the
Supplementary material.
While we illustrate these constraints within the single-type context, analogous
assumptions can be made within the multitype context examined in the following
section.
Figure 1.
Submodel assumptions. Rate, sampling, mass extinction, and conditioning assumptions of existing submodels of the general time-variable BDS process. The key points are that i) each of the previously developed models we considered can be obtained by adding specific combinations of constraints to the various parameters of the general BDS model; and ii) that there are many plausible, and potentially biologically informative combinations of constraints that have not been considered by researchers in epidemiology or macroevolution.
In regards to rate assumptions, many early BDS models (Stadler 2009; Stadler 2010; Stadler et al. 2012) assumed that the birth, death, and
sampling rates remained constant over time. This is mathematically and computationally
convenient since an analytical solution can easily be obtained for
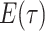 . In the epidemiological case,
holding
. In the epidemiological case,
holding 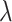 constant, however, implies that
the number of susceptible hosts is effectively constant throughout the epidemic and/or
that the population does not change its behavior over time; this is an unrealistic
assumption given seasonal changes or changes in response to the disease itself. As such,
this assumption is only really valid for small time periods or the early stages of an
epidemic. This is useful for estimating the basic reproductive number,
constant, however, implies that
the number of susceptible hosts is effectively constant throughout the epidemic and/or
that the population does not change its behavior over time; this is an unrealistic
assumption given seasonal changes or changes in response to the disease itself. As such,
this assumption is only really valid for small time periods or the early stages of an
epidemic. This is useful for estimating the basic reproductive number,
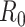 , of the SIR model (Box 1) but not
for the effective reproductive number
, of the SIR model (Box 1) but not
for the effective reproductive number 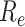 at later time
points (Stadler et al. 2012).
at later time
points (Stadler et al. 2012).
A similarly tractable, but more epidemiologically relevant, model is known as the
“birth–death-skyline” variant (Stadler and Bonhoeffer,
2013; Gavryushkina et al., 2014), in which
rates are piecewise-constant functions through time (like the constant rate model, there
is also an analytical way to calculate the likelihood of this model; see Appendix). The BDS
skyline model has been implemented under a variety of additional assumptions in the
Bayesian phylogenetics software BEAST2 (Bouckaert et al.
2019). The BDS skyline model has also been extended by (Kühnert et al., 2014) to infer the the parameters of an underlying
stochastic SIR model. In this case the diversification model parameters
 are random variables
that emerge from stochastic realizations of the epidemiological model given by
are random variables
that emerge from stochastic realizations of the epidemiological model given by
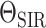 , see Equation (B1). Finally, the birth–death skyline
model with piecewise constant rates can also be applied in the macroevolutionary case when
no sampling occurs through time,
, see Equation (B1). Finally, the birth–death skyline
model with piecewise constant rates can also be applied in the macroevolutionary case when
no sampling occurs through time, 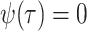 (Stadler 2011).
(Stadler 2011).
In addition to imposing constraints on the temporal variation in the rates, previously
derived submodels have considered a variety of different assumptions about the nature of
the sampling process. Most notably, in macroevolutionary studies, sampling of molecular
data typically occurs only at the present day (Stadler
2009; Stadler 2011; Morlon et al. 2011) whereas past Poissonian sampling can be introduced
to include the sampling of fossil data (Heath et al.
2014). In epidemiology, concerted sampling at the present day is likely
biologically unrealistic (Stadler et al. 2012),
though in some implementations of the models, such a sampling scheme has been imposed.
These concerted sampling attempts prior to the present day as well as mass extinction
events can be incorporated via the inclusion of Dirac distributions in the sampling and
death rates, respectively. Finally, previous models often multiply the likelihood by a
factor 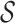 in order to condition on a
particular observation (e.g., observing at least one lineage or exactly
in order to condition on a
particular observation (e.g., observing at least one lineage or exactly
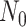 lineages), enumerate
indistinguishable trees (e.g., accounting for possible orientations or unlabeled trees)
(Gavryushkina et al. 2013; Gavryushkina et al. 2014; Stadler
2009), or to reflect known uncertainties. The “fossilized-birth–death” likelihood
derived by Heath et al. (2014) for example,
includes a factor that reflects the uncertainty in the attachment and placement of fossils
on the macroevolutionary tree. This fossilized-birth–death process has been used to
estimate divergence times and to model lineage diversification (Gavryushkina et al. 2017; Landis et al.
2021). Variants of the fossilized-birth–death process, for example including mass
extinction events, are feasible and can be derived using our approach. We also note that
models similar to the time-variable fossilized-birth–death process have been developed for
cases when phylogenetic data is not available (i.e., when only including fossil occurrence
data; see Silvestro et al. 2014; Lehtonen et al. 2017); we have not investigated how
these models relate to our generalized BDS model but we speculate that it would be
possible to also bring these models into a common framework with those that we have
discussed. Supplementary material
demonstrates how these submodels can be re-derived by either imposing the necessary
constraints on the general likelihood formula given in Equation (13) or, alternatively, by starting from
the combinations of assumptions and using the five-step procedure outlined above.
lineages), enumerate
indistinguishable trees (e.g., accounting for possible orientations or unlabeled trees)
(Gavryushkina et al. 2013; Gavryushkina et al. 2014; Stadler
2009), or to reflect known uncertainties. The “fossilized-birth–death” likelihood
derived by Heath et al. (2014) for example,
includes a factor that reflects the uncertainty in the attachment and placement of fossils
on the macroevolutionary tree. This fossilized-birth–death process has been used to
estimate divergence times and to model lineage diversification (Gavryushkina et al. 2017; Landis et al.
2021). Variants of the fossilized-birth–death process, for example including mass
extinction events, are feasible and can be derived using our approach. We also note that
models similar to the time-variable fossilized-birth–death process have been developed for
cases when phylogenetic data is not available (i.e., when only including fossil occurrence
data; see Silvestro et al. 2014; Lehtonen et al. 2017); we have not investigated how
these models relate to our generalized BDS model but we speculate that it would be
possible to also bring these models into a common framework with those that we have
discussed. Supplementary material
demonstrates how these submodels can be re-derived by either imposing the necessary
constraints on the general likelihood formula given in Equation (13) or, alternatively, by starting from
the combinations of assumptions and using the five-step procedure outlined above.
The Multitype Birth–Death-Sampling Model
A common extension of the single-type diversification models explored above is to
consider cases where the diversification rates (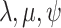 )
and probabilities (
)
and probabilities (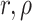 ) vary among lineages as a
function of a categorical “lineage type”. This lineage type can be defined in terms of
specific (Maddison et al. 2007; Rasmussen and Stadler 2019) or unspecified traits
(Beaulieu and O’Meara 2016) or trait combinations
(FitzJohn 2012) (for reviews of these models see
Morlon 2014; Ng
and Smith 2014). Representing these lineage types as colors at nodes and along
branches of the tree, we first extend the single-type model above by deriving the
likelihood of a fully colored tree with topology
) vary among lineages as a
function of a categorical “lineage type”. This lineage type can be defined in terms of
specific (Maddison et al. 2007; Rasmussen and Stadler 2019) or unspecified traits
(Beaulieu and O’Meara 2016) or trait combinations
(FitzJohn 2012) (for reviews of these models see
Morlon 2014; Ng
and Smith 2014). Representing these lineage types as colors at nodes and along
branches of the tree, we first extend the single-type model above by deriving the
likelihood of a fully colored tree with topology 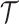 where the states along all
edges of the phylogeny are known as given by
where the states along all
edges of the phylogeny are known as given by 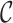 . The
resulting likelihood is an extension of the likelihood first developed by Barido-Sottani et al. (2018), where the diversification
rates and probabilities are allowed to vary arbitrarily through time. To illustrate that
our derivation is indeed quite general, we follow the model developed (independently) by
Magnuson-Ford and Otto (2012) and Goldberg and Igić (2012), where the state of lineages
can change either anagenetically, with a lineage of type
. The
resulting likelihood is an extension of the likelihood first developed by Barido-Sottani et al. (2018), where the diversification
rates and probabilities are allowed to vary arbitrarily through time. To illustrate that
our derivation is indeed quite general, we follow the model developed (independently) by
Magnuson-Ford and Otto (2012) and Goldberg and Igić (2012), where the state of lineages
can change either anagenetically, with a lineage of type 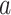 mutating to a type
mutating to a type 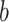 at rate
at rate 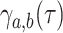 or cladogenetically,
with a lineage of type
or cladogenetically,
with a lineage of type  giving rise to a daughter lineage of
type
giving rise to a daughter lineage of
type 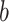 at rate
at rate 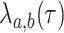 . Lineages go extinct
at a state-dependent rate
. Lineages go extinct
at a state-dependent rate 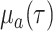 and are sampled at rate
and are sampled at rate
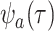 . As in the single-type
model, upon sampling lineages are removed from the population with probability
. As in the single-type
model, upon sampling lineages are removed from the population with probability
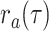 whereas all lineages alive at
the present day are sampled with a probability
whereas all lineages alive at
the present day are sampled with a probability 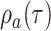 . As
discussed in depth by Goldberg and Igić (2012), the
other discrete variations of state-dependent diversification models (FitzJohn et al. 2009; Goldberg et al.
2011; FitzJohn 2012) fall out as special
cases of this model. (See Ng and Smith 2014; Caetano et al. 2018; Louca and Pennell 2020b for further discussion of the connection between
multitype models.)
. As
discussed in depth by Goldberg and Igić (2012), the
other discrete variations of state-dependent diversification models (FitzJohn et al. 2009; Goldberg et al.
2011; FitzJohn 2012) fall out as special
cases of this model. (See Ng and Smith 2014; Caetano et al. 2018; Louca and Pennell 2020b for further discussion of the connection between
multitype models.)
We use the five-step technique specified above for the single-type case to derive the
probability of observing a given colored tree under a general multitype model (see
Supplementary material
II). We first derive the initial value problem for the probability
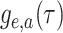 that an edge
that an edge
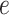 of type
of type 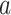 in
the tree at time
in
the tree at time 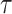 gives rise to the subsequently
observed phylogeny. The edge
gives rise to the subsequently
observed phylogeny. The edge 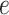 here refers not to an edge in the
topological tree, but to a segment of the tree all of one state between birth, sampling,
or mutation events.
here refers not to an edge in the
topological tree, but to a segment of the tree all of one state between birth, sampling,
or mutation events.
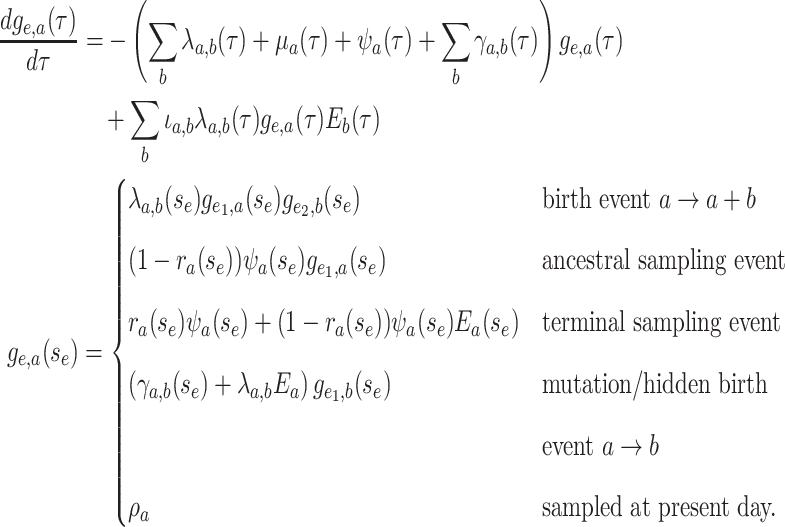 |
(14) |
Equation (16) distinguishes between
multiple types of birth events as pictured in Supplementary Figure S1.
Birth events may be symmetric, with both daughter lineages inheriting the parental type.
The exchangeability of the resulting daughter lineages is reflected in the indicator
variable 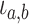 which takes on value of 2
if
which takes on value of 2
if 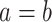 and 1 otherwise. In contrast
asymmetric birth events the resulting daughter lineages differ in type due to caldogenetic
change. Importantly, the differential equation for
and 1 otherwise. In contrast
asymmetric birth events the resulting daughter lineages differ in type due to caldogenetic
change. Importantly, the differential equation for 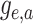 is linear and hence has a known
general solution
is linear and hence has a known
general solution 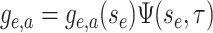 . As
in the single-type model
. As
in the single-type model 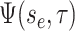 is the probability flow
(Louca and Pennell 2020b) mapping the probability
is the probability flow
(Louca and Pennell 2020b) mapping the probability
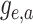 from the initial state at time
from the initial state at time
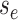 to the probability at time
to the probability at time
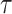 .
.
An analogous initial value problem can be derived for the probability
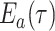 , that a lineage of type
, that a lineage of type
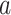 alive at time
alive at time
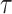 leaves no observed descendants in
the sampled tree.
leaves no observed descendants in
the sampled tree.
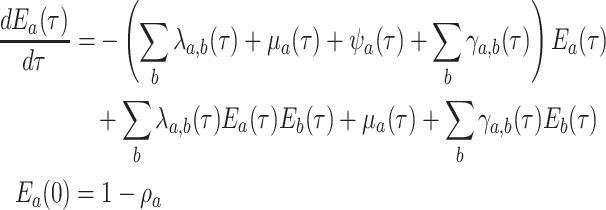 |
(15) |
This is a nonlinear differential equation and must be solved numerically. Given the
solution of 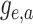 and
and 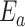 the likelihood for the fully
colored tree is characterized by a series of critical times: first,
the likelihood for the fully
colored tree is characterized by a series of critical times: first,
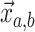 the times at which a
lineage of type
the times at which a
lineage of type 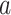 gives birth to a lineage of type
gives birth to a lineage of type
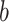 ,
, 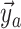 the ages of tip samples of
type
the ages of tip samples of
type 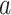 ,
, 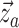 the ages of ancestral samples
of type
the ages of ancestral samples
of type 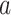 , and
, and 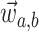 the times at which lineages
are observed to transition events from type
the times at which lineages
are observed to transition events from type 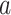 to type
to type
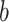 . The resulting likelihood is given
by:
. The resulting likelihood is given
by:
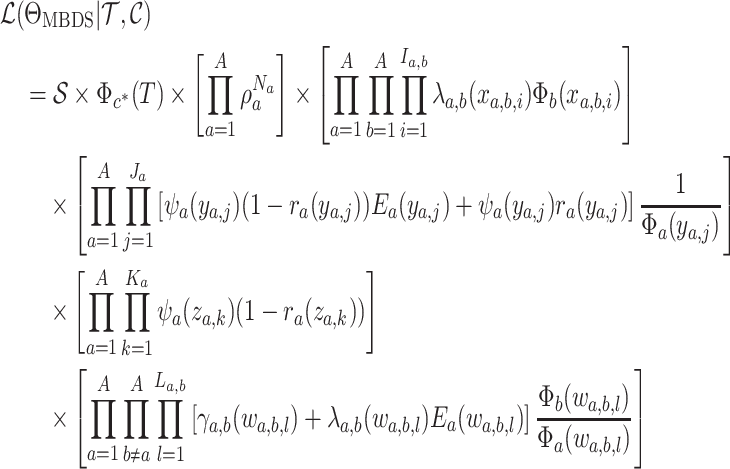 |
(16) |
Here, 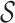 is an arbitrary form of
conditioning as in Equation (13) and
is an arbitrary form of
conditioning as in Equation (13) and
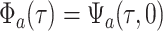 , a complete
list of notation is given in Table
S4.
, a complete
list of notation is given in Table
S4.
Equation (16) gives the likelihood
of a fully colored tree, the tree topology plus the state along each branch and at each
node in the tree. This likelihood is a generalization of that presented by (Barido-Sottani et al., 2018, 2020). Maximizing Equation (16) while incrementally adding and removing changes in state
along the branches of the tree can be used to identify clades with distinct
diversification parameters. This method can be used, for example, to identify transmission
clusters within a disease outbreak (Barido-Sottani et al.
2018). This likelihood is distinct from but related to post-traversal likelihood
methods developed to infer state-dependent diversification rates given the known state of
sampled lineages (e.g., Maddison et al. 2007; Magnuson-Ford and Otto 2012; Stadler and Bonhoeffer 2013). Specifically, these methods give the
likelihood 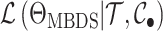 where
where 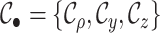 is the state of present-day,
is the state of present-day, 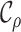 , past
, past
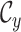 , and ancestral,
, and ancestral,
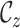 , sampled lineages. The
relationship between the numerically obtained post-traversal likelihood and the
closed-form fully colored likelihood (Equation (16)) is given by:
, sampled lineages. The
relationship between the numerically obtained post-traversal likelihood and the
closed-form fully colored likelihood (Equation (16)) is given by:
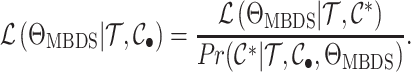 |
(17) |
Here, 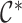 is one specific coloring
of the tree
is one specific coloring
of the tree 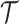 (e.g., a maximum parsimony
ancestral state reconstruction) that is consistent with the observed states. We include
Equation (17) as it clarifies the
relationship between these two different approaches that have been used to calculate
multitype likelihoods in phylogenetics. Whether or not this is useful for inference is an
open question as
(e.g., a maximum parsimony
ancestral state reconstruction) that is consistent with the observed states. We include
Equation (17) as it clarifies the
relationship between these two different approaches that have been used to calculate
multitype likelihoods in phylogenetics. Whether or not this is useful for inference is an
open question as 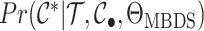 is challenging to compute (the details of which are beyond the scope of the present
paper).
is challenging to compute (the details of which are beyond the scope of the present
paper).
Concluding Remarks
In this article, we have unified a broad class of BDS models that have been widely used both in epidemiology and macroevolution. And in doing so, we have also presented a standardized notation and approach that can be used both for deriving the various submodels that have previously been studied as well as novel combinations of assumptions about the model parameters. The unification of these models clarifies the connections between BDS variants, facilitates the development of new variants tailored to specific scenarios, and provides a structure for understanding how results depend on model assumptions (Kirkpatrick et al. 2002; Lafferty et al. 2015; Louca and Pennell 2020a). And importantly, given the recent discovery of widespread nonidentifiability in birth–death processes fit to extant-only (Louca and Pennell 2020a) and serially sampled (Louca et al. 2021) phylogenetic data, there is a critical need to explore a much broader range of BDS models than were previously considered and the mathematical generalization presented here will be enable this.
Acknowledgments
We would like to thank Sally Otto for her thoughtful comments on this work.
Appendix: Adding assumptions to the general model
In this appendix, we demonstrate how one can obtain the likelihood of submodels with different sets of assumptions by applying constraints to the general likelihood. There are four classes of assumptions that are commonly applied in epidemiological and macroevolutionary studies. First, researchers can make assumptions about the functional form of the birth, death, and sampling rates. Here, we address two such unique assumptions: i) birth, death, and sampling rates are constant (see the Rate Assumptions section, Sections S1.1, S1.2, and S1.5 of the Supplementary material; and ii) birth, death, and sampling rates are piecewise-constant functions of time (see Piecewise-constant Rates section, Section S1.6 of the Supplementary material). The cases where birth, death, and sampling rates are defined by a stochastic or deterministic SIR model are mathematically analogous to the cases of the piecewise-constant and general time-variable models respectively. All additional constraints imposed will depend on the exact compartmental model used and hence we will not discuss them in detail in this section. The second major class of assumptions pertains to sampling. There are four such sampling assumptions: i) sampling happens only at the present day as in a birth–death model (see No Sampling at the Present Section, Sections S1.1, S1.3, and S1.4 of the Supplementary material) or as implemented in the “Birth Death Skyline Contemporary” prior in the BDSKY package in BEAST2; ii) the absence of concerted present-day sampling (see Birth–Death Models section, Section S1.5 of the Supplementary material); iii) the inclusion of ancestral samples with sampled descendants (Sections S1.6 and S1.7 of the Supplementary material; and iv) concerted sampling attempts (CSA) during which all lineages are sampled with a given probability (see Concerted Sampling Attempts section, Section S1.6 of the Supplementary material). The third assumption class considers the presence of mass extinction events (see Mass Extinction section, Section S1.5 of the Supplementary material). The fourth and final major class of assumptions deal with the conditioning of the likelihood. The various conditioning schemes are explored in below and summarized in Supplementary Table S3 available on Dryad.
Rate Assumptions
Constant Rates
Model assumptions: Constant diversification rates:
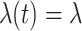 ,
,
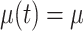 ,
,
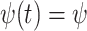 , and constant
removal probability
, and constant
removal probability 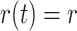 .
.- The IVP for
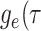 ):
):
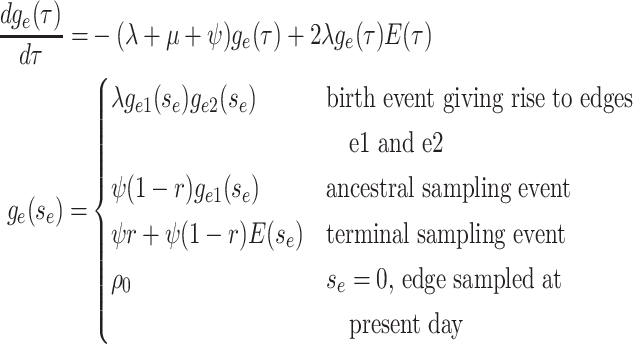
-
The IVP for
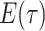 :
:
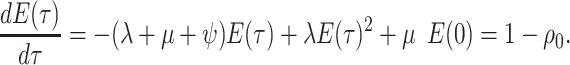 In this case the IVP for
In this case the IVP for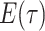 is a
Bernoulli differential equation and has a known analytical solution. As given by
Equation 1 in (Stadler, 2010) this solution
is given by:
is a
Bernoulli differential equation and has a known analytical solution. As given by
Equation 1 in (Stadler, 2010) this solution
is given by: 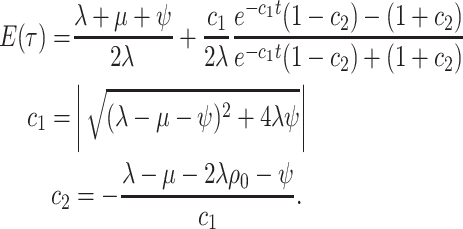
(A1) - The probability flow:
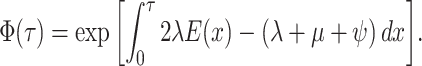
- The likelihood:
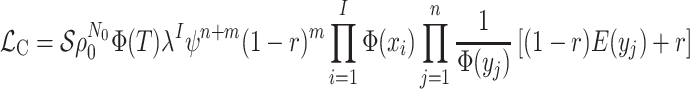
(A2)
Piecewise-Constant Rates
-
Model assumptions: Divide time into
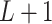 intervals
defined by transition times
intervals
defined by transition times 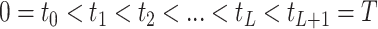 .
Define rates and removal probabilities constant within a given interval.
.
Define rates and removal probabilities constant within a given interval.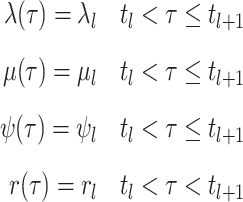
The IVP and solution for
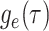 : Given the
definitions of
: Given the
definitions of 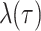 ,
,
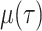 ,
,
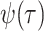 , and
, and
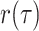 within each time
interval the IVP for
within each time
interval the IVP for 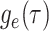 is identical to that
given in Equations (3) and
(4). If
is identical to that
given in Equations (3) and
(4). If
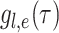 is the
probability density within time interval
is the
probability density within time interval 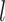 than
than 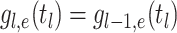 .
.-
The probability flow: We define a probability subflow within each time interval. Specifically, in the
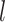 th time interval.
th time interval.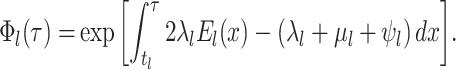 The complete flow can be expressed as a function of the subflows in the following manner:
The complete flow can be expressed as a function of the subflows in the following manner:
where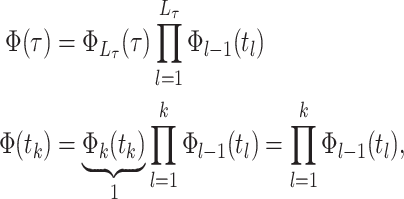
(A3) 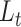 is the
index of the time
is the
index of the time 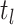 at or after time
at or after time
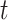 , that is, the largest index
such that
, that is, the largest index
such that 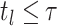 .
. -
The likelihood: Given these piecewise definitions we substitute them into the general BDS likelihood (13).
where we use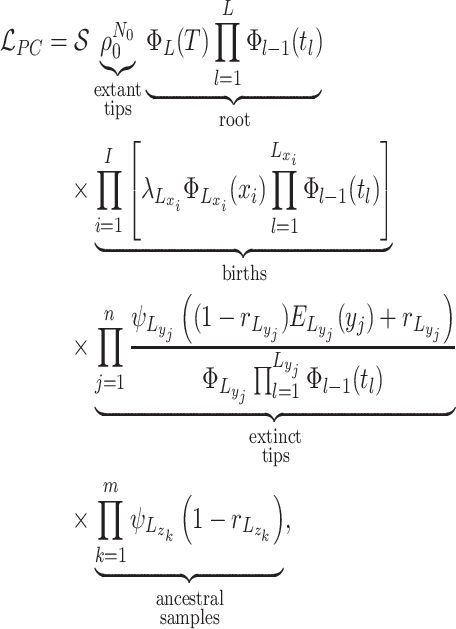
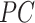 to denote
the piecewise-constant assumption.
to denote
the piecewise-constant assumption.We can simplify several of these products. Let
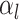 be the number of birth
events
be the number of birth
events 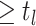 and
and
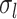 the number of sampling
events
the number of sampling
events 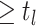 .
.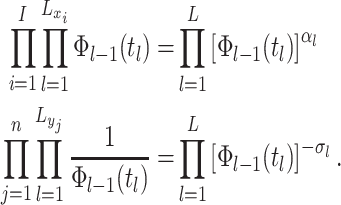
(A4) Let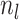 be the number of observed
lineages alive at time
be the number of observed
lineages alive at time 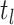 . Because
the number of observed lineages increases with each birth and decreases with each
sampled tip, counting the root we have
. Because
the number of observed lineages increases with each birth and decreases with each
sampled tip, counting the root we have 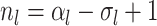 .
Substituting the expressions for the into the likelihood and using the definition
of
.
Substituting the expressions for the into the likelihood and using the definition
of 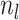 we have:
we have: 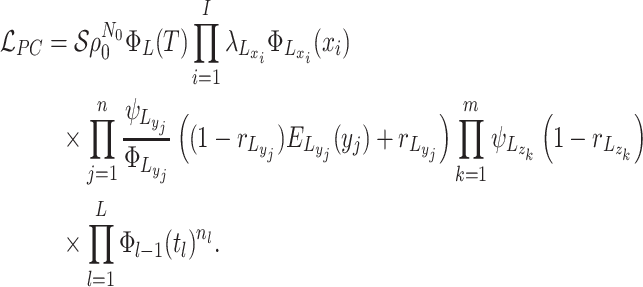
(A5)
Sampling Assumptions
Birth–Death Models
Model assumptions: The birth–death model assumes that
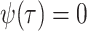 . Note that the
probability of sampling a lineage given it is alive at the present day remains as
. Note that the
probability of sampling a lineage given it is alive at the present day remains as
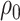 (incomplete
sampling).
(incomplete
sampling).- IVP for
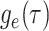 :
:
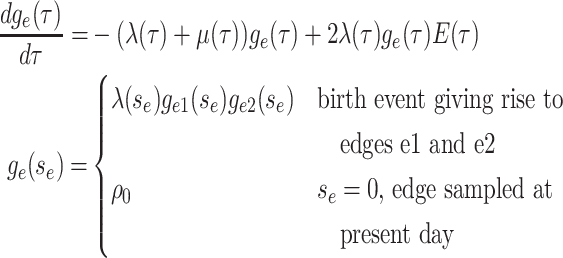
-
IVP for
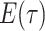 :
:
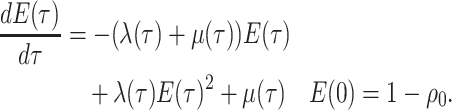 Note in this case
Note in this case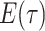 equals
equals
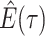 , the probability
a lineage leaves no sampled extant descendants. As demonstrated by (Morlon et al., 2011) there exists a general
solution to this initial value problem, see Section ?? for more
details. This general solution is given by:
, the probability
a lineage leaves no sampled extant descendants. As demonstrated by (Morlon et al., 2011) there exists a general
solution to this initial value problem, see Section ?? for more
details. This general solution is given by: 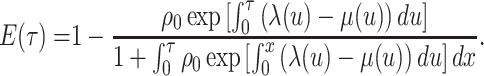
- The probability flow: From (Morlon et al., 2011), the probability flow can be written as the following:
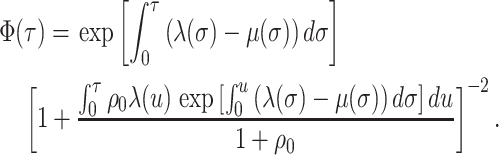
- The likelihood:
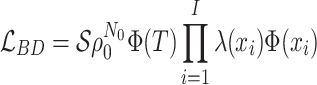
(A6)
No Sampling at the Present
Here, we consider the case when 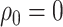 . The
likelihood follows exactly as in the general model case. The resulting likelihood
expression is given by:
. The
likelihood follows exactly as in the general model case. The resulting likelihood
expression is given by:
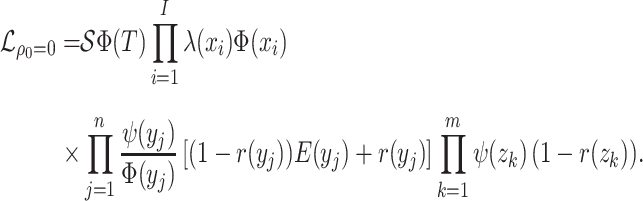 |
(A7) |
Note that in this case 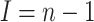 .
.
Concerted Sampling Attempts
- Model assumptions: Here, we introduce
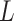 concerted sampling attempts
(CSA) at known points in time,
concerted sampling attempts
(CSA) at known points in time, 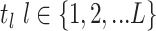 .
Like the CSA at the present day, and in contrast to the background Poissonian
sampling rate, during the CSA at time
.
Like the CSA at the present day, and in contrast to the background Poissonian
sampling rate, during the CSA at time 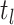 every
lineages is sampled with a fixed probability
every
lineages is sampled with a fixed probability 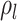 . In the derivation of the
likelihood below, we must distinguish between three different sampling event
types. First, past Poissonian sampling events are those that do
not occur during CSAs. Second, past concerted sampling events are
those that occur during a CSA at time
. In the derivation of the
likelihood below, we must distinguish between three different sampling event
types. First, past Poissonian sampling events are those that do
not occur during CSAs. Second, past concerted sampling events are
those that occur during a CSA at time 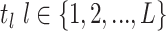 .
Finally, present concerted sampling events are those that occur
at the present day
.
Finally, present concerted sampling events are those that occur
at the present day 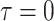 . Past concerted sampling
attempts can be included in the general model above by adding
. Past concerted sampling
attempts can be included in the general model above by adding
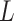 Dirac distributions to the
Poisson sampling rate function. Namely,
Dirac distributions to the
Poisson sampling rate function. Namely,
where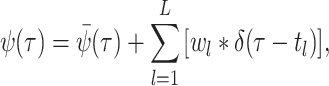
(A8) 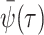 is the
background Poissonian sampling rate and
is the
background Poissonian sampling rate and 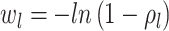 . The
definition of
. The
definition of 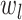 comes from solving the CDF
of the exponentially distribution for the “effective sampling rate” such that the
probability of a lineage being sampled is
comes from solving the CDF
of the exponentially distribution for the “effective sampling rate” such that the
probability of a lineage being sampled is 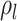 .
. -
IVP for
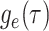 :
:

-
IVP for
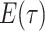 : As with
: As with
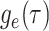 , the IVP for
, the IVP for
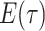 is identical to that
given for the general model in Equation (8). Except in rare cases the IVP must be solved
numerically hence requiring numerical integration over Dirac distributions which
can prove to be problematic.Note however, that when examining the integrals over the CSAs, a priori, it is a matter of convention whether the Dirac distribution should be considered as “integrated over” when located at the upper integration bound
is identical to that
given for the general model in Equation (8). Except in rare cases the IVP must be solved
numerically hence requiring numerical integration over Dirac distributions which
can prove to be problematic.Note however, that when examining the integrals over the CSAs, a priori, it is a matter of convention whether the Dirac distribution should be considered as “integrated over” when located at the upper integration bound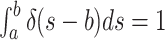 or at
the lower integration bound
or at
the lower integration bound 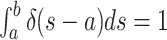 .
Whichever convention we chose, we must rigorously obey it so that the ratio
.
Whichever convention we chose, we must rigorously obey it so that the ratio
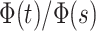 correctly
evaluates to
correctly
evaluates to 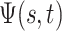 whenever
whenever
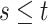 . Using the former
convention, we can rewrite the probability
. Using the former
convention, we can rewrite the probability 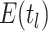 at each concerted sampling
time
at each concerted sampling
time 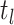 as:
as:
where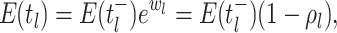
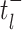 denotes the limit as
time approaches
denotes the limit as
time approaches 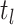 from below. Hence, the
probability
from below. Hence, the
probability 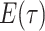 at any time
at any time
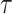 can be evaluated
numerically by considering the dynamics between successive CSAs and at each CSA
separately.
can be evaluated
numerically by considering the dynamics between successive CSAs and at each CSA
separately. -
The probability flow: The probability flow is given by:
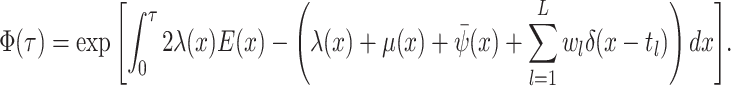
As with
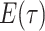 integration over the
dirac distributions can be problematic and hence we rewrite this expression
separating out these terms. Let
integration over the
dirac distributions can be problematic and hence we rewrite this expression
separating out these terms. Let 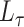 be the
oldest CSA occurring at or after time
be the
oldest CSA occurring at or after time 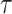 , that
is, the largest index for which
, that
is, the largest index for which 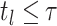 .
.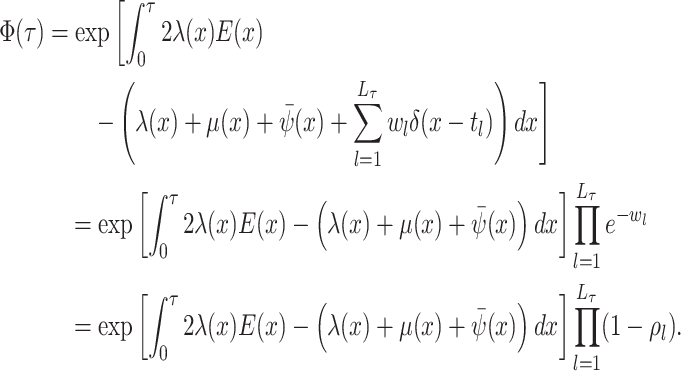
(A9) -
The likelihood: The edge representation of
 is given by:
is given by:
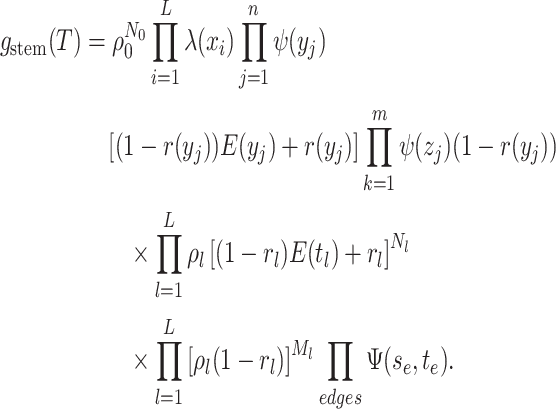 The critical time representation of
The critical time representation of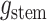 is given by:
is given by: 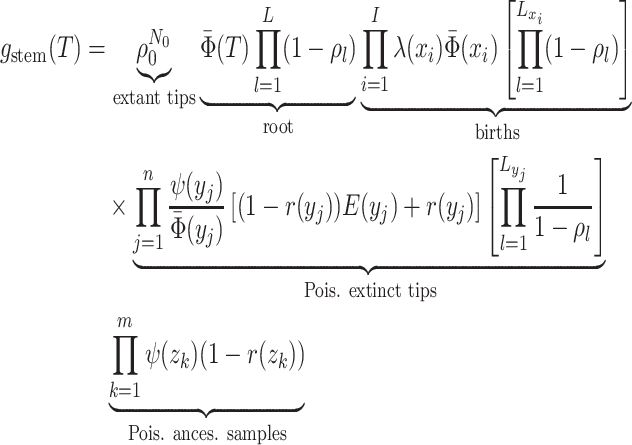
where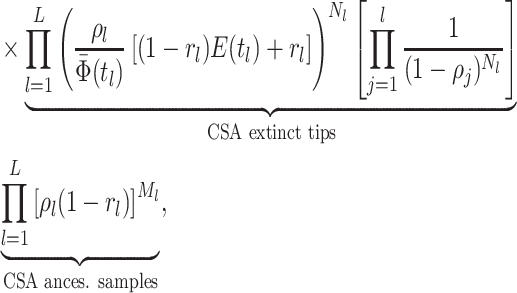
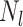 is the
number of tip samples (samples without descendants) obtained during the
is the
number of tip samples (samples without descendants) obtained during the
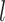 th CSA and
th CSA and
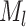 is the number of ancestral
samples (sequences with descendants). By changing how we enumerate birth, death,
and sampling events we can greatly simplify this likelihood. First, let
is the number of ancestral
samples (sequences with descendants). By changing how we enumerate birth, death,
and sampling events we can greatly simplify this likelihood. First, let
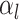 be the number of
branching events at or before the
be the number of
branching events at or before the 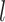 th CSA. In
other words,
th CSA. In
other words, 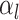 is the number of
branching events if the tree were trimmed at the
is the number of
branching events if the tree were trimmed at the 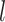 th CSA. Then:
th CSA. Then: 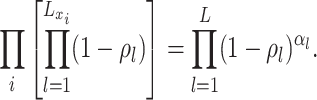
(A12) Second, let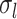 be the number of past
Poissonian sampling events before time
be the number of past
Poissonian sampling events before time 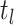 . Then:
. Then:
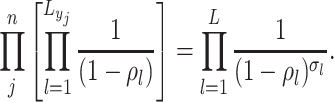
(A13) Finally, let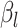 be the number of past
lineages sampled during a CSA at or before the CSA at time
be the number of past
lineages sampled during a CSA at or before the CSA at time
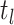 . Hence,
. Hence,
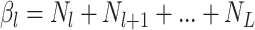 .
Then:
.
Then: 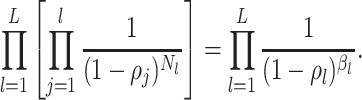
(A14) The likelihood hence simplifies to: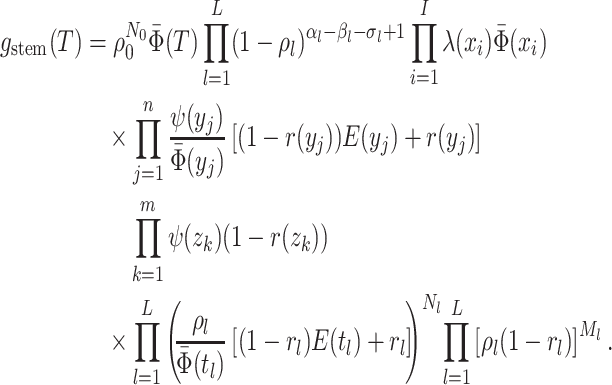
Let
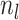 be the number of lineages
that cross
be the number of lineages
that cross 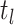 , that is, the number of
lineages alive at time
, that is, the number of
lineages alive at time 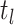 with
sampled descendants at some younger age. Note that by this definition
with
sampled descendants at some younger age. Note that by this definition
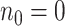 . Then
. Then
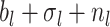 is the number
of tips in the tree had it been trimmed at age
is the number
of tips in the tree had it been trimmed at age 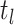 whereas
whereas
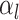 is the number of
branching events. Therefore we must have
is the number of
branching events. Therefore we must have 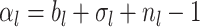 .
This allows us to simplify the conditioned likelihood given below.
.
This allows us to simplify the conditioned likelihood given below.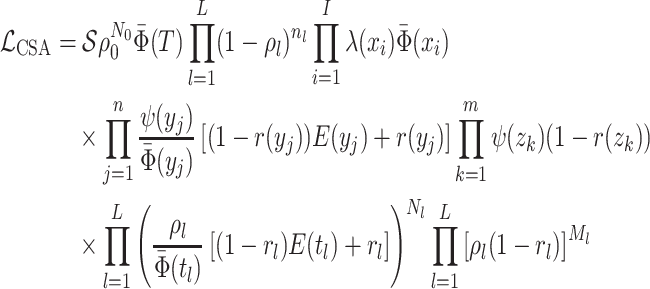
(A15)
Mass Extinction
-
Model assumptions: In addition to the Poisson birth death and sampling events considered in the general model, there are
 mass extinctions events occurring
at times
mass extinctions events occurring
at times  . During the
. During the
 th mass extinction event each
lineage goes extinct with probability
th mass extinction event each
lineage goes extinct with probability 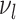 . As with
concerted sampling such mass extinction events can be introduced into the model by
adding a set of dirac-delta functions to the Poisson death rate,
. As with
concerted sampling such mass extinction events can be introduced into the model by
adding a set of dirac-delta functions to the Poisson death rate,
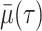 .
.
where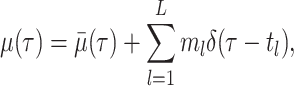
(A16) 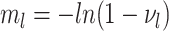 .
. IVP for
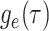 : The initial
value problem for
: The initial
value problem for 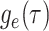 is identical to that
given in equation by Equations (3) and (4) except
that
is identical to that
given in equation by Equations (3) and (4) except
that 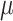 is now includes the mass
extinction events.
is now includes the mass
extinction events.-
IVP for
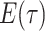 : The IVP for
: The IVP for
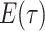 to that given by Equation
(8) except where the
extinction rate is given by Equation (A16). The solution to
to that given by Equation
(8) except where the
extinction rate is given by Equation (A16). The solution to 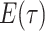 is obtained by numerical
integration. Given the dirac-delta functions this numerical integration can be
carried out in a piecewise manner integrating separately between and over each mass
extinction event. Defining
is obtained by numerical
integration. Given the dirac-delta functions this numerical integration can be
carried out in a piecewise manner integrating separately between and over each mass
extinction event. Defining 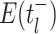 as
the solution up to but not including the mass extinction event at time
as
the solution up to but not including the mass extinction event at time
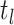 , we have:
, we have: 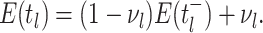
The first term reflects the probability that a lineage that does not go extinct during the
 th mass extinction event
leaves no observable offspring (with probability
th mass extinction event
leaves no observable offspring (with probability 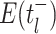 ) whereas the second term
reflects the fact that all lineages that go extinct during the
) whereas the second term
reflects the fact that all lineages that go extinct during the
 th mass extinction leave no
observed descendants with probability 1.
th mass extinction leave no
observed descendants with probability 1. -
The probability flow: The solution to the IVP is once again given by
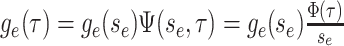 where:
where: 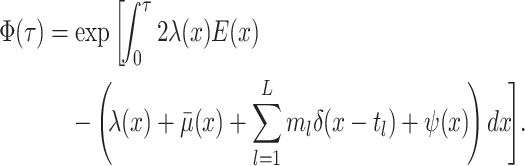
As with the CSAs, let
 be the last index
be the last index
 such that
such that
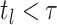 . We can separate out the
mass extinction terms in the following way.
. We can separate out the
mass extinction terms in the following way. -
The likelihood: Given these initial value problems the likelihood follows as in the general model.
Alternative Conditioning
Supplementary Table S3
lists a number of possible conditionings,  that
can be applied to the tree likelihood. First, is the trivial case of no conditioning
that
can be applied to the tree likelihood. First, is the trivial case of no conditioning
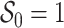 which gives the
probability of the observed tree including the stem edge between time
which gives the
probability of the observed tree including the stem edge between time
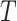 and
and 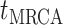 . To obtain the model
likelihood excluding the stem edge, that is, conditioning of the
. To obtain the model
likelihood excluding the stem edge, that is, conditioning of the
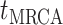 , can be obtained by
setting
, can be obtained by
setting 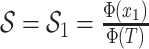 .
Recall that the elements of
.
Recall that the elements of 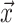 are ordered
such that
are ordered
such that 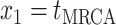 is the first
(oldest) birth event.
is the first
(oldest) birth event.
Acknowledging that one would not reconstruct a phylogeny without any sampled lineages,
we can condition the likelihood on observing at least one sampled lineage (either at or
before the present day) given the time of origin, 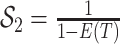 . Or as
with
. Or as
with 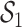 , conditioning on at
least one sampled lineage given the
, conditioning on at
least one sampled lineage given the 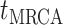 . In
order to have at least one sampled lineage and a most recent common
ancestor, however, each daughter lineage of the common ancestor must have at least one
descendent. Hence, we have
. In
order to have at least one sampled lineage and a most recent common
ancestor, however, each daughter lineage of the common ancestor must have at least one
descendent. Hence, we have  .
The general birth–death-sampling model assumes that all lineages alive at the present
day are sampled with probability
.
The general birth–death-sampling model assumes that all lineages alive at the present
day are sampled with probability  . As with
concerted sampling attempts (CSAs) prior to the present day, this present day CSA may
include the sampling of multiple lineages as well as possibly resulting in no sampled
lineages. As with
. As with
concerted sampling attempts (CSAs) prior to the present day, this present day CSA may
include the sampling of multiple lineages as well as possibly resulting in no sampled
lineages. As with  and
and
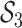 we can condition the
tree likelihood on observing at least one extant lineage at the present day. To do so,
we define
we can condition the
tree likelihood on observing at least one extant lineage at the present day. To do so,
we define 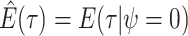 , the
probability that a lineage alive at time
, the
probability that a lineage alive at time 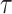 has no extant
descendants. Conditioning on the time of origin we have:
has no extant
descendants. Conditioning on the time of origin we have: 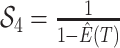 .
Conditioning on the time of the most recent common ancestor we have:
.
Conditioning on the time of the most recent common ancestor we have:
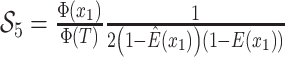 ,
where now at least one of the two daughter lineages of the common ancestor has a present
day sample. In many cases
,
where now at least one of the two daughter lineages of the common ancestor has a present
day sample. In many cases 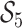 is modified, however,
to condition on both daughter lineages having an extant sampled
descendent:
is modified, however,
to condition on both daughter lineages having an extant sampled
descendent:  .
.
As an alternative to conditioning on at least one extant sampled descent, tree
likelihoods can be conditioned on having exactly
 sampled (extant) descendants. Let
sampled (extant) descendants. Let
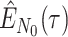 be the
probability a lineage alive at time
be the
probability a lineage alive at time  has exactly
has exactly
 descendants. Although a general
expression for
descendants. Although a general
expression for  is unknown, in
the case of constant birth, death, and sampling rates (the case in which this form of
conditioning has been applied), the expression for
is unknown, in
the case of constant birth, death, and sampling rates (the case in which this form of
conditioning has been applied), the expression for 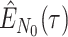 is given by (Gernhard, 2008), (Kendall, 1948) and Theorem 3.3 by (Stadler,
2010):
is given by (Gernhard, 2008), (Kendall, 1948) and Theorem 3.3 by (Stadler,
2010):
 |
where, like  ,
,  is given by Equation (10) evaluated with where
is given by Equation (10) evaluated with where
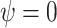 . Given the time of origin we
can condition on observing exactly
. Given the time of origin we
can condition on observing exactly 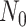 lineages by
setting
lineages by
setting 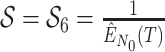 .
When
.
When 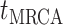 is given instead, then
the number of descendants of the two daughter lineages must add up to
is given instead, then
the number of descendants of the two daughter lineages must add up to
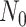 while both daughter lineages must
still have at least one descendant (see (Stadler,
2010) Corollary 3.9).
while both daughter lineages must
still have at least one descendant (see (Stadler,
2010) Corollary 3.9).
 |
While early BDS models often employed such conditioning (Stadler 2009; Stadler 2010), this form of conditioning has not been employed in many later models perhaps because the biological justification for such conditioning is vague.
The final form of conditioning used in the literature, which we will represent simply
as  , is the multiplication
of the BDS likelihood by a constant to account for the enumeration over the possible
indistinguishable representations of a given tree. The value of this constant depends on
whether the tree considered is “labeled” and “oriented” (Gavryushkina et al. 2013) and whether, as in the derivation here, the vector
of birth events,
, is the multiplication
of the BDS likelihood by a constant to account for the enumeration over the possible
indistinguishable representations of a given tree. The value of this constant depends on
whether the tree considered is “labeled” and “oriented” (Gavryushkina et al. 2013) and whether, as in the derivation here, the vector
of birth events,  , is (un)ordered. Inclusion of
such a constant should have no effect on the maximum likelihood inference of the model
parameters given a specified tree. In cases where the constant is a function of the
critical times (Heath et al. 2014), it can
influence the inference when the parameters and the tree are jointly estimated.
, is (un)ordered. Inclusion of
such a constant should have no effect on the maximum likelihood inference of the model
parameters given a specified tree. In cases where the constant is a function of the
critical times (Heath et al. 2014), it can
influence the inference when the parameters and the tree are jointly estimated.
Contributor Information
Ailene MacPherson, Zoology, University of British Columbia, Vancouver V6T 1Z4, Canada; Ecology and Evolutionary Biology, University of Toronto, Toronto M5S 3B2, Canada; Mathematics, Simon Fraser University, Burnaby V5A 1S6, Canada.
Stilianos Louca, Biology, University of Oregon, Eugene 97403, USA; Institute of Ecology and Evolution, University of Oregon, Eugene 97403, USA.
Angela McLaughlin, British Columbia Centre for Excellence in HIV/AIDS, Vancouver V6Z 1Y6, Canada; Bioinformatics, University of British Columbia, Vancouver V6T 1Z4, Canada.
Jeffrey B Joy, British Columbia Centre for Excellence in HIV/AIDS, Vancouver V6Z 1Y6, Canada; Bioinformatics, University of British Columbia, Vancouver V6T 1Z4, Canada; Medicine, University of British Columbia, Vancouver V6T 1Z4, Canada.
Matthew W Pennell, Zoology, University of British Columbia, Vancouver V6T 1Z4, Canada.
Supplementary Material
Data available from the Dryad Digital Repository: https://doi.org/10.5281/zenodo.5028470.
Funding
This work was supported by a Grant for Catalyzing Research Clusters awarded to the UBC Biodiversity Research Centre,NSF DEB Grant [#2028986 award to S.L. and M.W.P.]; a NSERC Discovery Grant to M.W.P.; and in part by the EEB department Postdoctoral Fellowship from the University of Toronto to A.M.; in part by a Canadian Institutes of Health Research (CIHR) doctoral award [#6557] to A.M.c.L..; a Genome Canada Bioinformatics and Computational Biology grant [287PHY], CIHR coronavirus rapid response program grant [440371] to J.B.J. and is grateful to the British Columbia Centre for Excellence in HIV/AIDS for additional funding support.
References
- Barido-Sottani J., Vaughan T.G., Stadler T. 2018. Detection of HIV transmission clusters from phylogenetic trees using a multi-state birth–death model. J. R. Soc. Interface 15:20180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barido-Sottani J., Vaughan T.G., Stadler, T. 2020. A multitype birth–death model for Bayesian inference of lineage-specific birth and death rates. Syst. Biol. 69:973–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J.M., O’Meara B.C. 2016. Detecting hidden diversification shifts in models of trait-dependent speciation and extinction. Syst. Biol. 65:583–601. [DOI] [PubMed] [Google Scholar]
- Boskova V., Bonhoeffer S., Stadler T. 2014. Inference of epidemiological dynamics based on simulated phylogenies using birth-death and coalescent models. PLoS Comput. Biol. 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert R., Vaughan T.G., Barido-Sottani, J., Duchêne S., Fourment M., Gavryushkina A., Heled J., Jones G., Kühnert D., Maio N.D., Matschiner M., Mendes F.K., Müller N.F., Ogilvie H.A., du Plessis, Popinga A., Rambaut A., Rasmussen D., Siveroni I., Suchard M.A., Wu C.-H., Xie D., Zhang C., Stadler T., Drummond A.J. 2019. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 15:e1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano D.S., O’Meara B.C., Beaulieu J.M. 2018. Hidden state models improve state-dependent diversification approaches, including biogeographical models. Evolution 72:2308–2324. [DOI] [PubMed] [Google Scholar]
- Drummond A.J., Pybus O.G., Rambaut A., Forsberg R., Rodrigo A.G. 2003. Measurably evolving populations. Trends Ecol. Evol. 18:481–488. [Google Scholar]
- Drummond A.J., Rambaut A., Shapiro B., Pybus O.G. 2005. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 22:1185–1192. [DOI] [PubMed] [Google Scholar]
- du Plessis L., Stadler T. 2015. Getting to the root of epidemic spread with phylodynamic analysis of genomic data. Trends Microbiol. 23:383–386. [DOI] [PubMed] [Google Scholar]
- Duffy S., Shackelton L.A., Holmes E.C. 2008. Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genetics 9:267–276. [DOI] [PubMed] [Google Scholar]
- Etienne R.S., Haegeman B., Stadler T., Aze T., Pearson P.N., Purvis, A., Phillimore A.B. 2012. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proc. R. Soc. B 279:1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne R.S., Rosindell J. 2012. Prolonging the past counteracts the pull of the present: protracted speciation can explain observed slowdowns in diversification. Syst. Biol. 61:204–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezard T.H.G., Quental T.B., Benton M.J. 2016. The challenges to inferring the regulators of biodiversity in deep time. Philos. Trans. R. Soc. B 371:20150216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller W. 1949. Proceedings of the [First] Berkeley Symposium on Mathematical Statistics and Probability. University of California Press, Berkeley California. [Google Scholar]
- FitzJohn R.G. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 3:1084–1092. [Google Scholar]
- FitzJohn R.G., Maddison W.P., Otto S.P. 2009. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Syst. Biol. 58:595–611. [DOI] [PubMed] [Google Scholar]
- Gavryushkina A., Heath T.A., Ksepka D.T., Stadler T., Welch D., Drummond A.J. 2017. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 66:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavryushkina A., Welch D., Drummond A.J. 2013. Recursive algorithms for phylogenetic tree counting. Algorithms Mol. Biol. 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavryushkina A., Welch D., Stadler T., Drummond A.J. 2014. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comput. Biol. 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernhard T. 2008. The conditioned reconstructed process. J. Theor. Biol. 253:769–778. [DOI] [PubMed] [Google Scholar]
- Goldberg E.E., Igić B. 2012. Tempo and mode in plant breeding system evolution. Evolution 66:3701–3709. [DOI] [PubMed] [Google Scholar]
- Goldberg E.E., Lancaster L.T., Ree R.H. 2011. Phylogenetic inference of reciprocal effects between geographic range evolution and diversification. Syst. Biol. 60:451–465. [DOI] [PubMed] [Google Scholar]
- Grenfell B.T., Pybus O.G., Gog J.R., J. Wood L.N., Daly J.M., Mumford J.A., Holmes E.C. 2004. Unifying the epidemiological and evolutionary dynamics of pathogens. Science 303:327–332. [DOI] [PubMed] [Google Scholar]
- Hagen O., Hartmann K., Steel M., Stadler T. 2015. Age-dependent speciation can explain the shape of empirical phylogenies. Syst. Biol. 64:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath T.A., Huelsenbeck J.P., Stadler T. 2014. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl. Acad. Sci. USA 111:E2957–E2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy J.B., McCloskey R.M., Nguyen T., Liang R.H., Khudyakov Y., Olmstead A., Krajden M., Ward J.W., Harrigan P.R., Montaner J.S.G., Poon A.F.Y. 2016. The spread of hepatitis C virus genotype 1a in North America: a retrospective phylogenetic study. Lancet Infectious Dis. 16:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M.J., Rohani P. 2008. Modeling infectious diseases: in humans and animals. Princeton University Press. [Google Scholar]
- Kendall D.G. 1948. On the generalized “birth-and-death” process. Ann. Math. Stat. 19:1–15. [Google Scholar]
- Kingman J.F.C. 1982. On the genealogy of large populations. J. Appl. Prob. 19:27–43. [Google Scholar]
- Kirkpatrick M., Johnson T., Barton N. 2002. General models of multilocus evolution. Genetics 161:1727–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnert D., Stadler T., Vaughan T.G., Drummond A.J. 2014. Simultaneous reconstruction of evolutionary history and epidemiological dynamics from viral sequences with the birth–death SIR model. J. R. Soc. Interface 11:20131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D., DeLeo G., Briggs C.J., Dobson A.P., Gross T., Kuris A.M. 2015. A general consumer-resource population model. Science 349:854–857. [DOI] [PubMed] [Google Scholar]
- Lambert A. 2018. The coalescent of a sample from a binary branching process. Theor. Popul. Biol. 122:30–35. [DOI] [PubMed] [Google Scholar]
- Lambert A., Stadler T. 2013. Birth–death models and coalescent point processes: the shape and probability of reconstructed phylogenies. Theor. Popul. Biol. 90:113–128. [DOI] [PubMed] [Google Scholar]
- Landis M.J., Eaton D.A.R., Clement W.L., Park B., Spriggs E.L., Sweeney P.W. Edwards E.J., Donoghue M.J. 2021. Joint phylogenetic estimation of geographic movements and biome shifts during the global diversification of viburnum. Syst. Biol. 70:67–85. [DOI] [PubMed] [Google Scholar]
- Laudanno G., Haegeman B., Rabosky D.L., Etienne R.S. 2020. Detecting lineage-specific shifts in diversification: a proper likelihood approach. Syst. Biol. 70:389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen S., Silvestro D., Karger D.N., Scotese C., Tuomisto H., Kessler M., Peña C., Wahlberg N., Antonelli A. 2017. Environmentally driven extinction and opportunistic origination explain fern diversification patterns. Sci. Rep. 7:4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal G.E., Kouyos R., Stadler T., von Wyl V., Yerly S., Böni J., Cellerai C., Klimkait T., Günthard H.F., Bonhoeffer S. 2012. Inferring epidemic contact structure from phylogenetic trees. PLoS Comput. Biol. 8:e1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S. 2020. Simulating trees with millions of species. Bioinformatics. 26:2907–2908. [DOI] [PubMed] [Google Scholar]
- Louca S., Doebeli M. 2018. Efficient comparative phylogenetics on large trees. Bioinformatics 34:1053–1055. [DOI] [PubMed] [Google Scholar]
- Louca S., McLaughlin A., MacPherson A., Joy J.B., Pennell M.W. 2021. Fundamental identifiability limits in molecular epidemiology. Mol. Biol. Evol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S., Pennell M.W. 2020a. Extant timetrees are consistent with a myriad of diversification histories. Nature 580:1–4. [DOI] [PubMed] [Google Scholar]
- Louca S., Pennell M.W. 2020b. A general and efficient algorithm for the likelihood of diversification and discrete-trait evolutionary models. Syst. Biol. 69:545–556. [DOI] [PubMed] [Google Scholar]
- Maddison W.P., Midford P.E., Otto S.P. 2007. Estimating a binary character’s effect on speciation and extinction. Syst. Biol. 56:701–710. [DOI] [PubMed] [Google Scholar]
- Magee A.F., Höhna S. 2021. Impact of K-Pg mass extinction event on crocodylomorpha inferred from phylogeny of extinct and extant taxa. bioRxiv 2021.01.14.426715. Available from: https://www.biorxiv.org/content/10.1101/2021.01.14.426715v1. [Google Scholar]
- Magnuson-Ford K., Otto S.P. 2012. Linking the investigations of character evolution and species diversification. Am. Nat. 180:225–245. [DOI] [PubMed] [Google Scholar]
- Morlon H. 2014. Phylogenetic approaches for studying diversification. Ecol. Lett. 17:508–525. [DOI] [PubMed] [Google Scholar]
- Morlon H., Parsons T.L., Plotkin J.B. 2011. Reconciling molecular phylogenies with the fossil record. Proc. Natl. Acad. Sci. USA 108:16327–16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlon H., Potts M.D., Plotkin J B. 2010. Inferring the dynamics of diversification: a coalescent approach. PLoS Biol. 8:e1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee S. 2006. Birth-death models in macroevolution. Annu. Rev. Ecol. Evol. Syst. 37:1–17. [Google Scholar]
- Nee S., May R.M., Harvey P.H. 1994. The reconstructed evolutionary process. Philos. Trans. R. Soc. B 344:7. [DOI] [PubMed] [Google Scholar]
- Ng J., Smith S.D. 2014. How traits shape trees: new approaches for detecting character state-dependent lineage diversification. J. Evol. Biol. 27:2035–2045. [DOI] [PubMed] [Google Scholar]
- Pybus O.G., Rambaut A., Harvey P.H. 2000. An integrated framework for the inference of viral population history from reconstructed genealogies. Genetics 155:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky D.L., Lovette I.J. 2008a. Density-dependent diversification in North American wood warblers. Proc. R. Soc. B 275:2363–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky D.L., Lovette I.J. 2008b. Explosive evoltuionary radiation: decreasing specaition or increasing extinction through time? Evolution 62:1866–1875. [DOI] [PubMed] [Google Scholar]
- Rasmussen D.A., Stadler T. 2019. Coupling adaptive molecular evolution to phylodynamics using fitness-dependent birth-death models. eLife 8:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raup D.M. 1985. Mathematical models of cladogenesis. Paleobiology 11:42–52. [Google Scholar]
- Romero-Severson E.O., Bulla I., Leitner T. 2016. Phylogenetically resolving epidemiologic linkage. Proc. Natl. Acad. Sci. USA 113:2690–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D., Pennell M.W. 2017. Speciation gradients and the distribution of biodiversity. Nature 546:48–55. [DOI] [PubMed] [Google Scholar]
- Silvestro D., Schnitzler J., Liow L.H., Antonelli A., Salamin N. 2014. Bayesian estimation of speciation and extinction from incomplete fossil occurrence data. Syst. Biol. 63:349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T. 2009. On incomplete sampling under birth-death models and connections to the sampling-based coalescent. J. Theor. Biol. 261:58–66. [DOI] [PubMed] [Google Scholar]
- Stadler T. 2010. Sampling-through-time in birth-death trees. J. Theor. Biol. 267:396–404. [DOI] [PubMed] [Google Scholar]
- Stadler T. 2011. Mammalian phylogeny reveals recent diversification rate shifts. Proc. Natl. Acad. Sci. USA 108:6187–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T. 2013. Recovering speciation and extinction dynamics based on phylogenies. J. Evol. Biol. 26:1203–1219. [DOI] [PubMed] [Google Scholar]
- Stadler T., Bonhoeffer S. 2013. Uncovering epidemiological dynamics in heterogeneous host populations using phylogenetic methods. Philos. Trans. R. Soc. B 368:20120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T., Kouyos R.D., von Wyl V., Yearly S., Böni J. 2012. Estimating the basic reproductive number from viral sequence data. Mol. Biol. Evol. 29:347–357. [DOI] [PubMed] [Google Scholar]
- Stadler T., Kühnert D., Bonhoeffer S., Drummond A.J. 2013. Birth-death skyline plot reveals temporal changes of epidemic spread in HIV and hepatitis C virus (HCV). Proc. Natl. Acad. Sci. USA 110:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T., Vaughan T.G., Gavryushkin A., Guindon S., Kühnert D., Leventhal G.E., Drummond A.J. 2015. How well can the exponential-growth coalescent approximate constant-rate birth-death population dynamics? Proc. Biol. Sci. 282:20150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimmer K., Pybus O.G. 2001. Exploring the demographic history of DNA sequences using the generalized skyline plot. Mol. Biol. Evol. 18:2298–2305. [DOI] [PubMed] [Google Scholar]
- Vaughan T.G., Leventhal G.E., Rasmussen D.A., Drummond A.J., Welch D., Stadler, T. 2019. Estimating epidemic incidence and prevalence from genomic data. Mol. Biol. Evol. 36:1804–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E.M. 2012. Complex population dynamics and the coalescent under neutrality. Genetics 190:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E.M., Frost S.D.W. 2014. Sampling through time and phylodynamic inference with coalescent and birth–death models. J. R. Soc. Interface 11:20140945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E.M., Kosakovsky Pond S.L., Ward M.J., Leigh Brown A.J., Frost S.D.W. 2009. Phylodynamics of infectious disease epidemics. Genetics 183:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]