Abstract
Background
Removal of colorectal polyps during screening could reduce the incidence of colorectal cancer (CRC). However, there is a lack of data on risk factors associated with recurrence of polyps, including conventional adenomas and serrated polyps (SPs). This study aimed to determine risk factors for recurrence of colorectal polyps and their subtypes based on the characteristics of the patients and polyps.
Methods
A total of 1,165 patients diagnosed with conventional adenoma or SP in the Sixth Affiliated Hospital of Sun Yat-sen University between January 2013 and December 2019 were enrolled in this study, including 668 cases with conventional adenomas, 385 with SPs, and 112 with coexistence of adenomas and SPs. Univariate analysis and multivariate logistic regression were used to identify potential risk factors for polyp recurrence. A nomogram was established according to risk factors and the performance was evaluated using calibration plots.
Results
During a median follow-up of 24 months, recurrent polyps were observed in 531 (45.6%) cases. Male, age ≥50 years, body mass index (BMI) ≥24 kg/m2, at least three polyps, smoking, alcohol consumption, family history of polyps, and family history of CRC were independent risk factors for polyp recurrence. The Harrell’s C-index of the nomogram developed with these parameters was 0.69 and the calibration plots showed good agreement between actual polyp recurrence and nomogram-predicted recurrence probability. In the subtype analyses, conventional adenomas had the same risk factors for recurrence as all polyps, while smoking, alcohol consumption, family history of polyps, and family history of CRC were not risk factors for SP recurrence.
Conclusions
We identified several risk factors for recurrence of colorectal polyps and found that some of them could increase the risk of adenoma recurrence but not SP recurrence, including smoking, alcohol consumption, and family history of polyps/CRC, which might help us to understand different etiology and biology between conventional adenomas and SPs.
Keywords: colorectal polyps, recurrence, conventional adenomas, serrated polyps, risk factors
Introduction
Colorectal cancer (CRC) ranks third in the incidence of cancer and is the second leading cause of cancer-related death in the world, accounting for 1.8 million new cases and 881,000 deaths in 2018 [1, 2]. As known, polyps are considered as precancerous lesions. Based on the World Health Organization (WHO) classification, polyps can be divided into four types: adenomas, serrated polyps (SPs), inflammatory polyps, and hamartomas [3]. Conventional adenomas (including tubular, tubulovillous, and villous adenomas) and SPs (including hyperplastic polyps [HPs], sessile serrated adenoma/polyps [SSA/Ps], and traditional serrated adenomas [TSAs]) are considered as two distinct etiologic pathways of carcinogenesis. About two-thirds of CRC cases have been believed to develop through the adenoma–carcinoma sequence for decades [4]. However, accumulated evidence supports that the remaining one-third of CRC cases can originate from the serrated pathway [5]. To be sure, both carcinogenic pathways indicate that the occurrence of CRC is a progressive process. Resection of the polyp in CRC screening is helpful to reduce the incidence and mortality of CRC [6]. Thus, colonoscopic polypectomy and surveillance are important for the prevention of CRC. Guidelines recommend various monitoring intervals after polyp resection according to polyp characteristics [7, 8].
It is worth noting that recurrence rate of polyp is as high as 20%–50% [9, 10]. Some previous studies had paid attention to the risk factors for polyp recurrence and showed that age and being male are risk factors for polyp recurrence [11, 12]. As for living habits, a study from China indicated that smoking status was related to the recurrence of adenomas in the elderly [13], while another study from the USA did not get similar results [14]. However, the sample size of these studies was small and they paid more attention to adenomas rather than SPs. Although some studies have been conducted on the influence of polyp characteristics on polyp recurrence, their conclusions are varied, which may be due to short follow-up time and small sample size [15–20]. Besides, it was reported that poor bowel preparation was associated with a lower polyp detection rate [21, 22]. However, it seems that the aforementioned studies rarely ruled out the influence of this factor on the polyp detection rate, which would confuse the influence of risk factors on polyp recurrence.
In general, although the carcinogenic factors of colorectal polyps have reached a consensus, the factors leading to the recurrence of polyps are still uncertain. Patient demographics (sex, age, lifestyle, etc.), polyp characteristics (growth site, number, size, pathological type, etc.), and procedural factors (follow-up time after polypectomy, quality of polypectomy, etc.) are potential risk factors for polyp recurrence. The purpose of this study was to investigate the risk factors for polyp recurrence and establish a nomogram to predict the risk of polyp recurrence. Besides, we performed a subgroup analysis to explore whether risk factors differed between conventional adenomas and SPs, because they might be genetically different.
Patients and methods
Study design and study subjects
All patients who were diagnosed with conventional adenomas or SPs based on pathology results in the index colonoscopy for any indication or screening purpose and underwent at least one surveillance colonoscopy more than 1 year after index colonoscopy in the Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) between January 2013 and December 2019 were enrolled in this retrospective observational study. Exclusion criteria were as follows: (i) other pathological types of the polyp (e.g. inflammatory polyps and hamartomas) in the index colonoscopies; (ii) personal history of CRC based on International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes; (iii) other histories of colorectal diseases, such as inflammatory bowel disease, familial adenomatous polyposis, Peutz-Jeghers syndrome, or intestinal tuberculosis based on ICD-10 codes; (iv) patients with poor bowel preparation; (v) the polyps were not resected completely during the index colonoscopy.
Based on the results of surveillance colonoscopies, patients who were diagnosed with conventional adenomas or SPs were categorized into the recurrence group, while others were included in the non-recurrence group. The study protocol was approved by the Ethics Review Committee of the Sixth Affiliated Hospital of Sun Yat-sen University without informed consent because we retrieved data anonymously from the electronic databases (No.2021ZSLYEC-264).
Data collection and study definitions
We collected the following clinical data through telephone follow-up and the medical records of the Sixth Affiliated Hospital of Sun Yat-sen University: (i) patient information including sex, age, body mass index (BMI), personal history (e.g. hypertension or diabetes), living habits (e.g. smoking status or alcohol consumption), and family history of CRC or polyps; (ii) polyp characteristics including size, number, anatomical location, pathological diagnosis, and the interval between initial and surveillance colonoscopies. Cigarette-smoking status was defined as smoking at least one cigarette per day for >3 months. Alcohol consumption was defined as three or more drinks per week for 6 consecutive months. Family history of CRC or polyp was defined as positive when at least one relative of that disease was found. Besides, in our study, BMI was divided into <24 and ≥24 kg/m2 according to Chinese classification [23].
All pathological diagnoses were performed by two experienced pathologists separately and only consistent results will be adopted. If there was a disagreement between pathologists, the specimen would be further reviewed by a third pathologist and the final diagnosis would be made by all pathologists together. For patients with multiple polyps, we selected the index polyp by the following standard: (i) for patients with SPs, the largest polyp in size was selected; (ii) for patients with at least one conventional adenoma of ≥10 mm, we selected the largest one; (iii) for patients with all conventional adenomas of <10 mm, the polyp with high-grade dysplasia or the most villous structure was selected. The anatomical location of the polyp was divided into the proximal colon (caecum to splenic flexure), distal colon (descending colon to sigmoid colon), and rectum. In the case of multiple polyps, polyp location was defined as that of the index polyp.
Index colonoscopy was defined as the colonoscopy with the polyp detected first, while surveillance colonoscopy was defined as the colonoscopy performed >1 year later. The guidelines for the intervals of colonoscopy surveillance vary widely. In our study, individuals with one or two small (<10 mm) tubular adenoma were advised to undergo repeated colonoscopy in 5–10 years and those with adenoma of ≥10 mm or more than three small tubular adenomas were advised 3–5 years. For individuals with SPs in our study, we recommend a 5-year surveillance interval for SPs of <10 mm and a 3-year interval for SPs of ≥10 mm. The individuals who suffered from polyps with high-grade dysplasia were advised to undergo repeated colonoscopy in 1 year.
Statistical analyses
A student's t-test or Mann–Whitney U test was used to compare continuous variables according to their respective applicable conditions. Chi-square test was used to compare categorical variables. Multivariate logistic regression was performed to identify risk factors for polyp recurrence. All variables that were predictive at the 0.05 level by using a univariate analysis fit into the multivariate logistic-regression model in an ‘ENTER’ way. We listed the odds ratio (OR) and 95% confidence interval (CI) for each variable. All statistical tests were on two sides and a P-value of <0.05 was considered significant. The statistical analyses mentioned above were performed using SPSS software (Version 22.0).
A nomogram for polyp recurrence was established based on the multivariate logistic-regression model. The performance of the nomogram was evaluated using Harrell’s concordance index (Harrell’s C-index) and calibration plots with bootstrap samples. Harrell’s C-index was calculated using 1,000-fold bootstrap resampling iterations to an initial fitted model in the derivation set. Calibration plots are graphic evaluations of predictive ability that compare observed probabilities with nomogram-predicted probabilities. The same strategy was used for the subgroup analysis. The nomogram-associated statistical analyses were performed using R software (Version 4.0.0).
Results
A total of 1,165 eligible patients were included in the study. There were 739 males and 426 females with a median (interquartile range [IQR]) age of 53 (45, 62) years. The baseline characteristics of these patients are summarized in Table 1. Due to the low compliance, some patients did not strictly follow the above screening strategy. The median (IQR) interval between the index and surveillance colonoscopies was 24.2 (16.0, 35.9) months. Recurrent polyps were observed in 531 (45.6%) cases. The median (IQR) number and size of polyps were 1 (1, 2) and 5 (4, 8) mm, respectively.
Table 1.
Univariate and multivariate logistic-regression analysis on risk factors for polyp recurrence
| Characteristic | No. of patients (n = 1,165) | Polyp recurrence (n = 531) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Age | ||||||
| <50 years | 447 | 167 (37.4) | 1 | 1 | ||
| ≥50 years | 718 | 364 (50.7) | 1.72 (1.36–2.19) | <0.01 | 1.70 (1.31–2.20) | <0.01 |
| Gender | ||||||
| Female | 426 | 149 (35.0) | 1 | 1 | ||
| Male | 739 | 382 (51.7) | 1.99 (1.56–2.54) | <0.01 | 1.42 (1.07–1.90) | 0.02 |
| Body mass index | ||||||
| <24 kg/m2 | 668 | 264 (39.5) | 1 | 1 | ||
| ≥24 kg/m2 | 497 | 267 (53.7) | 1.78 (1.41–2.25) | <0.01 | 1.59 (1.23–2.04) | <0.01 |
| Number of polyps | ||||||
| <3 | 919 | 366 (39.8) | 1 | 1 | ||
| ≥3 | 246 | 165 (67.1) | 3.08 (2.29–4.14) | <0.01 | 1.97 (1.34–2.90) | 0.01 |
| Anatomical location | ||||||
| Proximal colon | 369 | 177 (48.0) | 1 | |||
| Distal colon | 496 | 228 (46.0) | 0.92 (0.70–1.21) | 0.56 | ||
| Rectum | 300 | 126 (42.0) | 0.79 (0.58–1.07) | 0.12 | ||
| Size | ||||||
| <10 mm | 907 | 415 (45.8) | 1 | |||
| ≥10 mm | 258 | 116 (45.0) | 0.97 (0.73–1.28) | 0.97 | ||
| Pathological type | ||||||
| Serrated polyps | 385 | 177 (46.0) | 1 | |||
| Conventional adenomas | 780 | 354 (45.4) | 0.98 (0.76–1.25) | 0.98 | ||
| Coexistence of adenomas and serrated polyps | ||||||
| No | 1,053 | 461 (43.8) | 1 | 1 | ||
| Yes | 112 | 70 (62.5) | 2.14 (1.43–3.20) | <0.01 | 1.28 (0.81–2.02) | 0.28 |
| High-grade dysplasia | ||||||
| No | 1,053 | 479 (45.5) | 1 | |||
| Yes | 112 | 52 (46.4) | 1.04 (0.70–1.54) | 0.85 | ||
| Smoker status | ||||||
| Never | 909 | 373 (41.0) | 1 | 1 | ||
| Current | 256 | 158 (61.7) | 2.32 (1.74–3.08) | <0.01 | 1.52 (1.09–2.13) | 0.01 |
| Alcohol consumption | ||||||
| Never | 1,086 | 476 (43.8) | 1 | 1 | ||
| Current | 79 | 55 (69.6) | 2.94 (1.79–4.81) | <0.01 | 1.84 (1.08–3.16) | 0.03 |
| History of hypertension | ||||||
| No | 916 | 393 (42.9) | 1 | 1 | ||
| Yes | 249 | 138 (55.4) | 1.65 (1.25–2.19) | <0.01 | 1.20 (0.87–1.64) | 0.26 |
| History of diabetes | ||||||
| No | 1,105 | 493 (44.6) | 1 | 1 | ||
| Yes | 60 | 38 (63.3) | 2.14 (1.25–3.67) | 0.01 | 1.47 (0.83–2.63) | 0.19 |
| Family history of polyps | ||||||
| No | 1,068 | 476 (44.6) | 1 | 1 | ||
| Yes | 97 | 55 (56.7) | 1.63 (1.07–2.48) | 0.02 | 1.65 (1.06–2.59) | 0.03 |
| Family history of CRC | ||||||
| No | 1,095 | 486 (44.4) | 1 | 1 | ||
| Yes | 70 | 45 (64.3) | 2.26 (1.36–3.73) | 0.01 | 2.15 (1.26–3.67) | 0.01 |
OR, odds ratio; CI, confidence interval; CRC, colorectal cancer.
Risk factors for recurrence of polyps
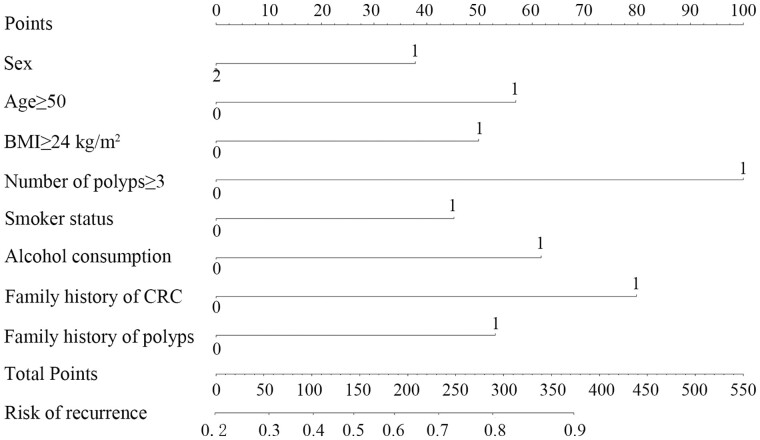
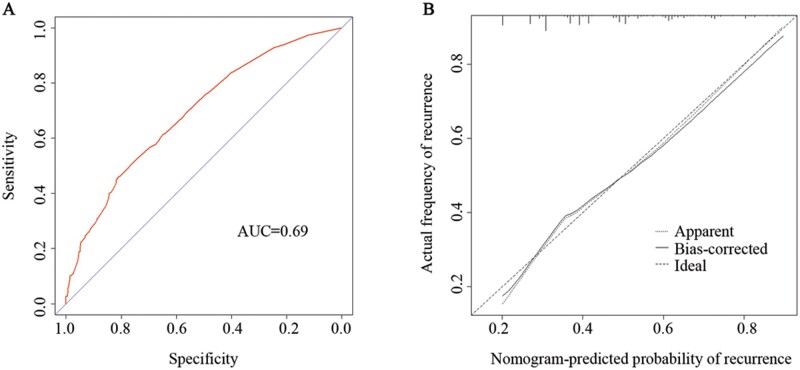
We analysed risk factors for recurrent polyps by univariate and multivariate logistic-regression analysis. Being male, age ≥50 years, BMI ≥24 kg/m2, more than three polyps, smoking, alcohol consumption, family history of polyps, and family history of CRC were independent risk factors for polyp recurrence (Table 1). We established a nomogram for polyp recurrence based on the above independent risk factors (Figure 1). In this way, we can get the risk probability of polyp recurrence. Harrell’s C-index for the derivation set was 0.69 and the nomogram was well calibrated (Figure 2).
Figure 1.
Nomogram for predicting polyp recurrence. For the variable ‘Sex’, 1 = ‘Male’, 2 = ‘Female’. For other variables, 1 = ‘Yes’, 0 = ‘No’. BMI, body mass index; CRC, colorectal cancer; SPs, serrated polyps.
Figure 2.
Receiver-operating characteristic curve (A) and calibration plots (B) of the prediction model for polyp recurrence
Subgroup analyses based on conventional adenomas and SPs
Among the 1,165 patients in our study, 668 and 385 patients, respectively, were diagnosed as conventional adenomas or SPs only, while the remaining 112 patients suffered from both types of polyps and were excluded in the subgroup analyses. Of 668 patients in the conventional adenomas subgroup, 284 cases had polyp recurrence. Risk factors associated with conventional adenomas recurrence did not vary from those of all polyps (Table 2). Similarly, a nomogram for conventional adenoma recurrence was established using a Harrell’s C-index of 0.68 (Supplementary Figure 1). The nomogram was well calibrated (Supplementary Figure 2).
Table 2.
Univariate and multivariate logistic-regression analysis on risk factors for recurrence of conventional adenomas and serrated polyps
| Characteristic | Conventional adenomas |
Serrated polyps |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients (n = 668) | Recurrence (n = 284) | Univariate |
Multivariate |
No. of patients (n = 385) | Recurrence (n = 177) | Univariate |
Multivariate |
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||||
| Age | ||||||||||||
| <50 years | 233 | 79 (33.9) | 1 | 1 | 179 | 67 (37.4) | 1 | 1 | ||||
| ≥50 years | 435 | 205 (47.1) | 1.74 (1.25–2.42) | <0.01 | 1.64 (1.15–2.35) | <0.01 | 206 | 110 (53.4) | 2.49 (1.62–3.84) | <0.01 | 2.06 (1.31–3.23) | <0.01 |
| Gender | ||||||||||||
| Female | 247 | 86 (34.8) | 1 | 1 | 143 | 46 (32.2) | 1 | 1 | ||||
| Male | 421 | 198 (47.0) | 1.66 (1.20–2.30) | <0.01 | 1.33 (0.92–1.93) | 0.13 | 242 | 131 (54.1) | 1.92 (1.27–2.88) | <0.01 | 1.72 (1.02–2.89) | 0.04 |
| Body mass index | ||||||||||||
| <24 kg/m2 | 380 | 147 (38.7) | 1 | 1 | 236 | 88 (37.3) | 1 | 1 | ||||
| ≥24 kg/m2 | 288 | 137 (47.6) | 1.44 (1.05–1.96) | 0.02 | 1.36 (1.03–1.89) | 0.03 | 149 | 89 (59.7) | 2.55 (1.64–3.80) | <0.01 | 2.25 (1.43–3.55) | <0.01 |
| Anatomical location | ||||||||||||
| Proximal colon | 218 | 102 (46.8) | 1 | 101 | 43 (42.6) | 1 | ||||||
| Distal colon | 320 | 130 (40.6) | 0.78 (0.55–1.10) | 0.16 | 130 | 68 (52.3) | 1.48 (0.88–2.50) | 0.14 | ||||
| Rectum | 130 | 52 (40.0) | 0.76 (0.49–1.18) | 0.22 | 154 | 66 (42.9) | 1.01 (0.61–1.68) | 0.96 | ||||
| Number of polyps | ||||||||||||
| <3 | 604 | 244 (40.4) | 1 | 1 | 336 | 145 (43.2) | 1 | 1 | ||||
| ≥3 | 64 | 40 (62.5) | 2.46 (1.45–4.18) | <0.01 | 1.98 (1.14–3.45) | 0.02 | 49 | 32 (65.3) | 2.48 (1.33–4.64) | <0.01 | 1.87 (1.11–3.69) | 0.02 |
| Size (mm) | ||||||||||||
| <10 | 469 | 202 (43.1) | 1 | 358 | 165 (46.1) | 1 | ||||||
| ≥10 | 199 | 82 (41.2) | 0.93 (0.66–1.30) | 0.66 | 27 | 12 (44.4) | 0.84 (0.43–2.06) | 0.87 | ||||
| High-grade dysplasia | ||||||||||||
| No | 571 | 244 (42.7) | 1 | 379 | 172 (45.4) | 1 | ||||||
| Yes | 97 | 40 (41.2) | 0.94 (0.61–1.46) | 0.78 | 6 | 5 (83.3) | 6.02 (0.70–52.00) | 0.10 | ||||
| Smoker status | ||||||||||||
| Never | 538 | 212 (39.4) | 1 | 1 | 291 | 118 (40.6) | 1 | 1 | ||||
| Current | 130 | 72 (55.4) | 1.91 (1.30–2.81) | <0.01 | 1.38 (1.19–2.15) | 0.02 | 94 | 59 (62.8) | 2.47 (1.53–4.00) | <0.01 | 1.38 (0.76–2.49) | 0.29 |
| Alcohol consumption | ||||||||||||
| Never | 625 | 256 (41.0) | 1 | 1 | 357 | 157 (44.0) | 1 | 1 | ||||
| Current | 43 | 28 (61.1) | 2.69 (1.41–5.14) | <0.01 | 1.84 (1.01–3.67) | 0.04 | 28 | 20 (71.4) | 3.19 (1.37–7.42) | <0.01 | 1.73 (0.67–4.46) | 0.26 |
| History of hypertension | ||||||||||||
| No | 534 | 216 (40.4) | 1 | 1 | 308 | 135 (43.8) | 1 | |||||
| Yes | 134 | 68 (50.7) | 1.52 (1.04–2.22) | 0.03 | 1.29 (0.85–1.94) | 0.23 | 77 | 42 (54.5) | 1.54 (0.93–2.54) | 0.09 | ||
| History of diabetes | ||||||||||||
| No | 638 | 270 (42.3) | 1 | 364 | 161 (44.2) | 1 | 1 | |||||
| Yes | 30 | 14 (46.7) | 1.19 (0.57–2.49) | 0.64 | 21 | 16 (76.2) | 4.04 (1.45–11.25) | <0.01 | 2.17 (0.73–6.50) | 0.17 | ||
| Family history of polyps | ||||||||||||
| No | 622 | 258 (41.5) | 1 | 1 | 344 | 155 (45.1) | 1 | |||||
| Yes | 46 | 26 (56.5) | 1.83 (1.00–3.36) | 0.04 | 2.81 (1.42–5.57) | <0.01 | 41 | 22 (53.7) | 1.41 (0.74–2.70) | 0.30 | ||
| Family history of CRC | ||||||||||||
| No | 626 | 256 (40.9) | 1 | 1 | 362 | 165 (45.6) | 1 | |||||
| Yes | 42 | 28 (66.7) | 2.89 (1.49–5.60) | <0.01 | 1.95 (1.04–3.67) | 0.04 | 23 | 12 (52.2) | 1.30 (0.56–3.03) | 0.54 | ||
OR, odds ratio; CI, confidence interval; BMI, body mass index; CRC, colorectal cancer.
Of 385 patients who suffered from SPs, 177 cases had polyp recurrence. It is worth noting that there were only six SSPs and no TSAs in 385 cases. The results of logistic-regression analysis showed that being male, age ≥50 years, BMI ≥24 kg/m2, and more than three SPs were associated with a higher risk for SP recurrence. Differently from conventional adenomas, smoking, alcohol consumption, family history of polyps, and family history of CRC were not risk factors for SP recurrence (Table 2). The nomogram for the recurrence of SPs is shown in Supplementary Figure 3, with a Harrell’s C-index of 0.69. The nomogram was well calibrated (Supplementary Figure 4).
Discussion
In the study, we explored the risk factors for polyp recurrence and performed a subgroup analysis based on the histopathological features of the polyps. We identified that patient characteristics such as being sex, age ≥50 years, BMI ≥24 kg/m2, smoking, alcohol consumption, family history of polyps, and family history of CRC were associated with polyp recurrence. The most highlighted issue in this study was that we excluded participants with poor bowel preparation, as colonoscopy with poor bowel preparation might raise the missing rate of advanced neoplasia to 18%–27% [21, 22], which might make the results more solid.
Given that these precancerous lesions can develop into CRC successively, it is necessary to clarify the risk factors for polyp recurrence and help to adjust the monitoring strategies for CRC [24–26]. However, the risk factors for recurrence of conventional adenomas and SPs seemed to be different. Compared with conventional adenomas, we found that smoking, alcohol consumption, family history of polyps, and family history of CRC were irrelevant with SPs. Considering their carcinogenic mechanism [4, 5], this difference might be explained by distinct etiology and biology between these two types of polyp. Notably, we found that patients with both conventional adenomas and SPs had a significantly higher risk of recurrence than those with conventional adenomas or SPs only, which indicated that a stricter monitoring strategy should be scheduled for patients with coexistent conventional adenomas and SPs.
The current study found that smoking and alcohol consumption were risk factors for conventional adenoma recurrence [13, 27]. The carcinogenic components in cigarettes lead to oxidative stress and DNA damage, producing various carcinogens, which will interrupt cellular replication and inhibit the DNA-repair process [28, 29]. Differently from conventional adenomas, smoking or alcohol consumption did not affect the recurrence of SPs in our study, which was not in line with another study [30]. Since patients who smoke and drink in this study were relatively few, we could not draw a solid conclusion. The association between positive family history of CRC and colorectal neoplasms had been reported prior [31, 32] and we further found that positive family history of polyps or CRC was a risk factor for recurrence of conventional adenomas but not SPs. On the one hand, the rationale for this might be that the etiology was different between these two types of polyp [4, 5]. On the other hand, it was worth noting that limited reports of positive family history in this study would lead to imprecise results. In addition, the risk of polyp recurrence increased along with aging and BMI, which were also proved in previous studies [13, 33, 34]. Advanced age and obesity may be involved in the presence of chronic subclinical inflammatory conditions, which may contribute to polyp recurrence [35]. Also, obesity might elevate the expression level of insulin and insulin-like growth factor-1, which could stimulate the recurrence of polyps [36].
It seemed that the recurrence of polyps was affected by various factors. To assess the influence of risk factors on polyp recurrence more accurately, we put the potential risk factors into the nomogram and calibrated it using calibration plots. When patients with a higher probability of recurrence are identified using the nomogram, they should receive stricter monitoring. However, the power of the nomogram was not enough (AUC = 0.69), which suggested that the factors determined above might lack sufficient ability to predict the recurrence of polyps.
Several limitations need to be noted as well. First, selection bias and recall bias could not be ruled out since our study was a single-center retrospective study. Second, Chinese pathologists did not pay enough attention to the diagnosis of SSPs and TSAs in the past few decades because of the evolving nature and lack of consensus on the diagnostic criteria of SPs. Third, we did not analyse the impact of dietary factors and exercise-related data on polyp recurrence because the related information was hardly achieved in this retrospective study. Multicenter, large-scale, prospective research is needed in the future to solve these problems.
In conclusion, our study illustrated several risk factors for the recurrence of colorectal polyps. Being male, age ≥50 years, BMI ≥24 kg/m2, smoking, alcohol consumption, family history of polyps, and family history of CRC are indicated as risk factors for polyp recurrence. We also found that some risk factors could increase the risk of adenoma recurrence, but not SP, including smoking, alcohol consumption, and positive family history of polyps or CRC.
Supplementary Data
Supplementary data is available at Gastroenterology Report online.
Authors’ Contributions
Conceptualization: Z.C., Y.L., and J.H. Methodology: Z.C. and Y.L. Software: J.C. and X.C. Validation: M.Y.L. and B.Z. Formal analysis: Z.C., Y.L., and J.H. Investigation: Z.C. and J.H. Resources: Y.C. and J.H. Data curation: Z.C. and Y.L. Writing—original draft preparation: Z.C. Writing—review and editing: X.H. and P.L. Visualization: M.Y.L. and X.C. Funding acquisition: X.H. and P.L. Supervision: P.L.
Funding
This work was supported by the National Key R&D Program of China [No. 2017YFC1308800], the National Natural Science Foundation of China [No. 81970482], the Natural Science Foundation of Guangdong Province [No. 2019A1515011313], and National Key Clinical Discipline.
Supplementary Material
Acknowledgements
None.
Conflict of Interest
None declared.
References
- 1. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Xie Y, Shi L, He X. et al. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf) 2021;9:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagtegaal ID, Odze RD, Klimstra D. et al. ; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fearon ER, Vogelstein B.. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- 5. Rex DK, Ahnen DJ, Baron JA. et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dekker E, Rex DK.. Advances in CRC prevention: screening and surveillance. Gastroenterology 2018;154:1970–84. [DOI] [PubMed] [Google Scholar]
- 7. Lieberman DA, Rex DK, Winawer SJ. et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 8. Rutter MD, East J, Rees CJ. et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut 2020;69:201–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hao Y, Wang Y, Qi M. et al. Risk factors for recurrent colorectal polyps. Gut Liver 2020;14:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belderbos TD, Leenders M, Moons LM. et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388–402. [DOI] [PubMed] [Google Scholar]
- 11. Yamaji Y, Mitsushima T, Ikuma H. et al. Right-side shift of colorectal adenomas with aging. Gastrointest Endosc 2006;63:453–8. [DOI] [PubMed] [Google Scholar]
- 12. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C. et al. ; Women's Health Initiative Investigators. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004;350:991–1004. [DOI] [PubMed] [Google Scholar]
- 13. Saiken A, Gu F.. Lifestyle and lifestyle-related comorbidities independently associated with colorectal adenoma recurrence in elderly Chinese people. Clin Interv Aging 2016;11:801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reid ME, Marshall JR, Roe D. et al. Smoking exposure as a risk factor for prevalent and recurrent colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2003;12:1006–11. [PubMed] [Google Scholar]
- 15. Facciorusso A, Di Maso M, Serviddio G. et al. Factors associated with recurrence of advanced colorectal adenoma after endoscopic resection. Clin Gastroenterol Hepatol 2016;14:1148–54. e4. [DOI] [PubMed] [Google Scholar]
- 16. Backes Y, Moons LM, van Bergeijk JD. et al. Endoscopic mucosal resection (EMR) versus endoscopic submucosal dissection (ESD) for resection of large distal non-pedunculated colorectal adenomas (MATILDA-trial): rationale and design of a multicenter randomized clinical trial. BMC Gastroenterol 2016;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamaji Y, Mitsushima T, Ikuma H. et al. Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut 2004;53:568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laiyemo AO, Murphy G, Sansbury LB. et al. Hyperplastic polyps and the risk of adenoma recurrence in the polyp prevention trial. Clin Gastroenterol Hepatol 2009;7:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aniwan S, Orkoonsawat P, Viriyautsahakul V. et al. The secondary quality indicator to improve prediction of adenoma miss rate apart from adenoma detection rate. Am J Gastroenterol 2016;111:723–9. [DOI] [PubMed] [Google Scholar]
- 20. Nusko G, Hahn EG, Mansmann U.. Characteristics of metachronous colorectal adenomas found during long-term follow-up: analysis of four subsequent generations of adenoma recurrence. Scand J Gastroenterol 2009;44:736–44. [DOI] [PubMed] [Google Scholar]
- 21. Chokshi RV, Hovis CE, Hollander T. et al. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012;75:1197–203. [DOI] [PubMed] [Google Scholar]
- 22. Rex DK, Cutler CS, Lemmel GT. et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. [DOI] [PubMed] [Google Scholar]
- 23. Gao M, Lv J, Yu C. et al. ; for the China Kadoorie Biobank (CKB) Collaborative Group. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: a cohort study. PLoS Med 2020;17:e1003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dekker E, Tanis PJ, Vleugels JL. et al. Colorectal cancer. Lancet 2019;394:1467–80. [DOI] [PubMed] [Google Scholar]
- 25. Brenner H, Stock C, Hoffmeister M.. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin JS, Piper MA, Perdue LA. et al. Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA 2016;315:2576–94. [DOI] [PubMed] [Google Scholar]
- 27. Tiemersma EW, Wark PA, Ocke MC. et al. Alcohol consumption, alcohol dehydrogenase 3 polymorphism, and colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2003;12:419–25. [PubMed] [Google Scholar]
- 28. Yamasaki E, Ames BN.. Concentration of mutagens from urine by absorption with the nonpolar resin XAD-2: cigarette smokers have mutagenic urine. Proc Natl Acad Sci USA 1977;74:3555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baron JA, Sandler RS, Haile RW. et al. Folate intake, alcohol consumption, cigarette smoking, and risk of colorectal adenomas. J Natl Cancer Inst 1998;90:57–62. [DOI] [PubMed] [Google Scholar]
- 30. Bailie L, Loughrey MB, Coleman HG.. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology 2017;152:92–104. [DOI] [PubMed] [Google Scholar]
- 31. Wong MC, Ching JY, Chiu HM. et al. Risk of colorectal neoplasia in individuals with self-reported family history: a prospective colonoscopy study from 16 Asia-Pacific regions. Am J Gastroenterol 2016;111:1621–9. [DOI] [PubMed] [Google Scholar]
- 32. He X, Wu K, Ogino S. et al. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology 2018;155:355–73. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwak JY, Kim KM, Yang HJ. et al. Prevalence of colorectal adenomas in asymptomatic young adults: a window to early intervention? Scand J Gastroenterol 2016;51:731–8. [DOI] [PubMed] [Google Scholar]
- 34. Jacobs ET, Martinez ME, Alberts DS. et al. Association between body size and colorectal adenoma recurrence. Clin Gastroenterol Hepatol 2007;5:982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shoelson SE, Herrero L, Naaz A.. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–80. [DOI] [PubMed] [Google Scholar]
- 36. Schatzkin A, Lanza E, Corle D. et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas: Polyp Prevention Trial Study Group. N Engl J Med 2000;342:1149–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.