Brief summary.
Liver cirrhosis is the common end-stage of chronic liver disease from different aetiologies. The worsening of liver disease initiates a cascade of events with intestinal bacterial overgrowth and dysbiosis as central events. Bacterial toxins can directly cause hepatocyte death, while dysbiosis also affects gut barrier function and increases bacterial translocation, which leads to upregulation of systemic inflammation and infections, vasodilation and contributes to acute decompensation and organ failure. Acute decompensation and its dramatic forms pre-acute-on-chronic liver failure (ACLF) and ACLF are sudden deteriorations defined by organ dysfunction and failure and associated with high short-term mortality. The patients with pre-ACLF and ACLF present with high systemic inflammation, which are mainly precipitated by proven bacterial infection and/or severe alcoholic hepatitis. Yet in 30% of these patients no precipitating event is determined and bacterial translocation from the gut microbiota is assumed to be responsible for the high systemic inflammation and decompensation. Different microbiota profiles may influence the rate of decompensation and thereby outcome in these patients. Targeting the microbiota using different tools may be promising strategies to prevent and treat acute decompensation, Pre-ACLF and ACLF. These include use of antibiotics such as rifaximin, faecal microbial transplantation and enterosorbents (e.g. Yaq-001), which bind microbial factors without exerting a direct effect on bacterial growth kinetics. This review focuses on the role of microbiota in the decompensation and strategies targeting microbiota to prevent acute decompensation.
Keywords: Acute-.on-chronic Liver Failure, Acute decompensation, cirrhosis, gut-liver-axis, portal hypertension
1. Introduction
Liver cirrhosis is the result of chronic liver disease (CLD) over many years (1). CLD is silent and slowly changes the liver by restructuring and remodelling the architecture, combining the wound-healing processes, such as remodelling and fibrosis, with a decrease in the functioning parenchymal mass finally leading to cirrhosis (2). Especially in cirrhosis, which is also silent for years, the whole organism (skin, brain, kidneys, gastrointestinal tract, immune system, bone marrow, heart, etc.) is changing and adapting to the diseased liver (1). These changes probably anticipate and prompt the final showdown in the patient’s life, namely the acute decompensating event (3). Also the microbiota, including bacteria (bacteriome), but also fungi (fungome) and viruses (virome), are known to change during the development and progression of liver cirrhosis (4). These changes are due to several factors. First, the aetiology of liver disease seems to be an important factor inducing microbiota, such as alcohol and diet in non-alcoholic fatty liver disease (NAFLD) (5). CLD primarily reduces bile flow and causes cholestasis, which impairs the enterohepatic circulation and majorly affects the microbiota (4, 5). With the progression of CLD itself the changes in microbiota (dysbiosis) are maintained and further aggravated probably by changes in intestinal motility, permeability, barrier function towards the lymphatic and blood compartment, portal hypertension and immune system (6). Yet the role of the microbiota seems to be pivotal in patients with decompensated cirrhosis, as many decompensating events are related to microbes or their interaction with the host (7).
To describe the role of microbiota in cirrhosis and its role in decompensation, we first need to introduce acute decompensation (AD) and its maximal form acute-on-chronic liver failure (ACLF). AD defines the acute development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage or bacterial infections or any combination of these (8). AD is a sudden and fast deterioration in health and is associated with organ dysfunction, the liver and also extrahepatic organs, especially the kidneys and brain (3). The maximal form of AD is the so-called ACLF with extremely high mortality approaching 40% in 28 days (3, 9). Recently, the phenotype of patients with AD without ACLF, have been characterized in the PREDICT study characterizing the group of patients with pre-ACLF, who will develop ACLF in the following 90 days presenting with high systemic inflammation and a very high mortality (10). Pre-ACLF patients can be differentiated from the patients with unstable decompensated cirrhosis (UDC), who will develop complications mainly due to severe portal hypertension (large ascites or bleeding requiring TIPS) and will be readmitted to the hospital within 90 days after their index acute decompensation episode (10). The majority of patients with AD without ACLF in PREDICT, were patients with stable decompensated cirrhosis (10). Yet 10% of these patients went on to develop either ACLF or complications of portal hypertension during one year follow up and die. As highlighted above, different microbiota profiles may either influence beneficially or aggravate the liver phenotype and thereby precipitate decompensation and influence outcome in these patients. These effects support the rationale of targeting the microbiota using different tools (rifaximin, fecal microbiota transplantation) as a promising strategy to prevent and treat decompensation in cirrhosis. This is the topic of the current review focusing on the role of microbiota in the decompensation of cirrhotic patients, as well as strategies targeting microbiota to prevent decompensation of patients or treat AD patients.
2. Alteration of the microbiome and its associated changes in cirrhosis
The large observational prospective studies NACSELD, APASL-AARC, CANONIC and PREDICT identified several precipitating events deriving or possibly deriving from the gut microbiota or their products, which lead to AD and ACLF (9–14). Especially bacterial infection and alcoholic hepatitis are predominantly associated with acute decompensation with recent data from the PREDICT study demonstrating that either of them or the combination account for 90% of identifiable precipitating events (10). However, even in prospective, very detailed investigations in almost one third of the patients, the precipitating event leading to acute decompensation cannot be determined (9, 10). Also in these undetermined cases microbiota and its metabolites may play a role as shown recently (14). The question that arises is, why the cirrhotic microbiota lead to decompensation and which changes in the microbiota during cirrhosis development and progression are relevant for developing the decompensation.
The onset of the changes in microbiota is early in the development of chronic liver disease, even before the damage of the liver is detectable, especially in alcohol-related chronic liver disease and in NAFLD (15). There are different studies showing shifts in the composition of the gut microbiome in different chronic liver diseases (15, 16). Yet in cirrhosis, one common property of these changes, which is easy to assess, represents the diversity, which is decreased massively upon the development of cirrhosis and even more in decompensated cirrhosis (17–19). In addition to the reduced species diversity, there is a bacterial overgrowth in the small bowel so-called small intestine bacterial overgrowth (SIBO) (20), which is due in part to the decreased gut motility (21). It is suspected that due to the sympathetic activation required to regulate the tone of dilated splanchnic vessels in cirrhosis, the motility of the gut is decreased, which leads to an increase in contact time of bacteria, and thereby to fermentation changes of the luminal content (22). This may lead to changes in the microbiota metabolites, which may affect the epithelial cells and the liver itself. Specifically, formation of short chain fatty acids (SCFA) seems to be crucial in the homeostasis of the epithelial layer (23), while different SCFA members may play a pathogenetic role in inflammation (24) and the liver disease itself (25).
Despite the SIBO and decreased richness, several studies have identified cirrhosis-specific profiles of the microbiota (17, 18, 26, 27). These profiles seem to be predominated by Fusobacteria, Proteobacteria, Enterococaceae and Streptococacceae with relative decrease of Bacteroidetes, Ruminococcus, Roseburia, Veillonellaceae and Lachnospiraceae independent of cirrhosis aetiology (17, 18, 26, 27). The similarity of the microbiome changes in cirrhosis is quite important, since it demonstrates that the cirrhotic liver impairs the microbiota. This occurs in patients, in whom the aetiological agent has direct contact with the microbiome (alcohol-related or NASH-cirrhosis), as well as in those, in whom the aetiology of the liver disease is not directly linked with the microbiome, such as hepatitis B and C. In addition to the increase in potential pathogenic taxa, in cirrhosis is observed also a reduction in potential beneficial taxa, such as Akkermansia abundance, which was found to be decreased in cirrhotic patients with different etiology of liver disease (28–30). Yet, these profound changes in the microbiome are at least partly related to the liver disease than direct effects of the etiologic factor. This was confirmed by at least partial restoration of the gut microbiota after liver transplantation (31).
Another reason for the dysbiotic composition of the cirrhotic microbiota is the impairment in the enterohepatic circulation. Cirrhosis is associated with decreased secretion of primary bile acids into the gut lumen (32). The secondary bile acids produced by bacteria are in turn decreased (32–34). Moreover, bile acids are involved in the uptake of fat and fat-soluble proteins, and thereby have a tremendous influence on the metabolism and possibly coagulation (Vitamin K-dependent coagulation factors) as well. Therefore, signs of malnutrition, including increased INR, may be at least partly mediated by the decreased primary and secondary bile acid synthesis and uptake in cirrhosis. Bile acids are also strong modulators of the farsenoid X receptor (FXR)-axis, which is crucial in the homeostasis of epithelial barrier and gut-vascular barrier (35, 36), and its impairment facilitating bacterial translocation. FXR has been also identified as a good target for treatment in cirrhosis demonstrating decreased bacterial translocation after agonism (37, 38). But the bacterial translocation is increased also due to structural changes of the intestinal epithelial layer, resulting from an increase in portal pressure (reviewed elsewhere (22)) and the changes in the type of resident and infiltrating immune cells (38, 39). The changes in the gut-associated immune system include decreased synthesis and release of anti-bacterial peptides, IgA, defensins and hypo- or achlorhydria (40–42). The bacterial translocation, which is facilitated by the above-mentioned changes of the microbiota and its functions, may then induce decompensation of cirrhosis (Figure 1).
Figure 1. Microbiome and decompensated cirrhosis.
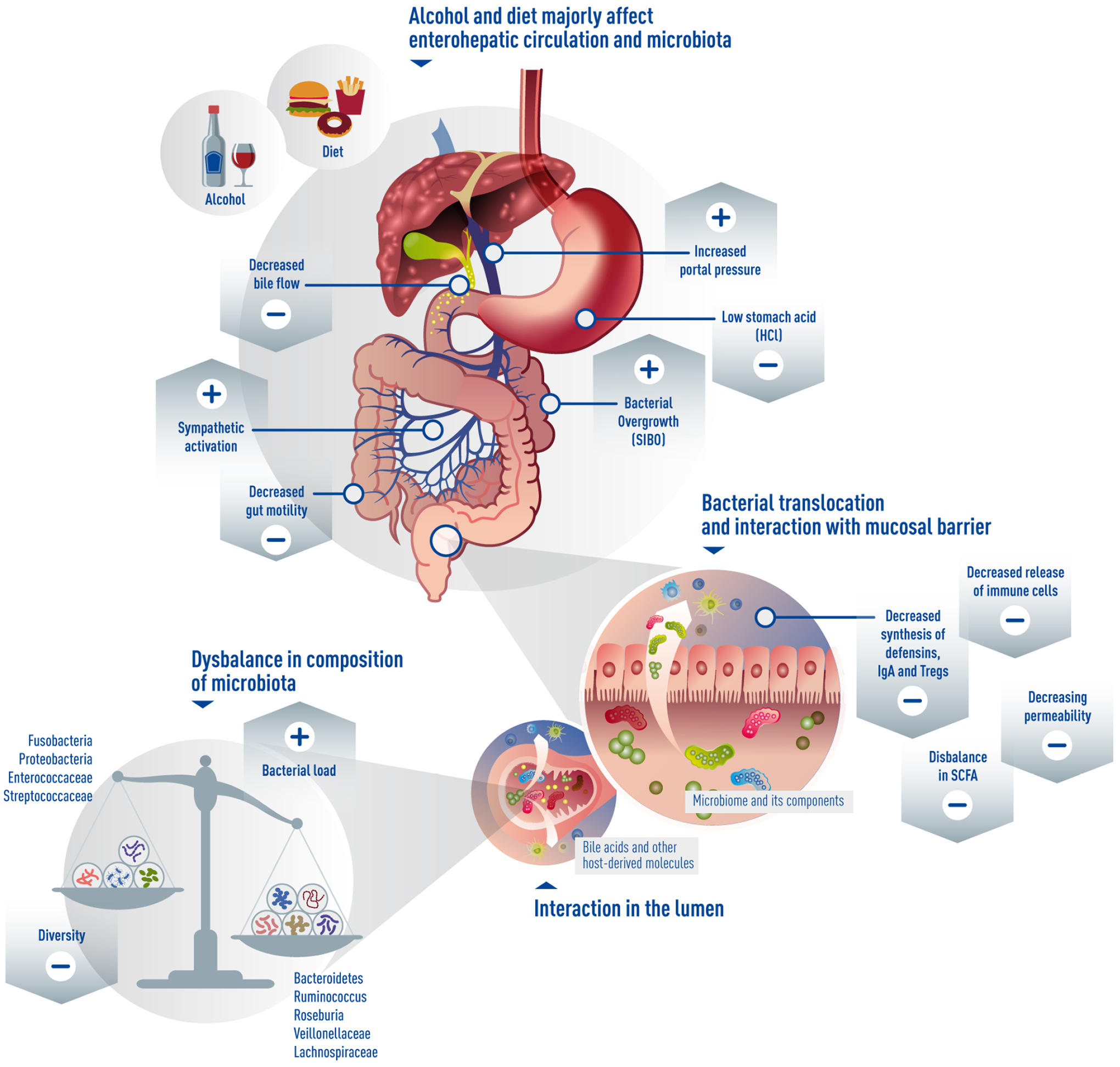
Changes during progression of liver cirrhosis affect to a large extent the microbiota. Especially alcohol and diet, decreased bile flow, portal hypertension and activation of sympathetic nervous system impair gut motility and permeability, lead to decreased diversity, but increased bacterial load and bacterial overgrowth, dysbalance in bacterial species and finally increased bacterial translocation.
3. Microbiome changes and development of decompensation.
Cirrhosis is associated with systemic inflammation as evidenced by increased systemic levels of oxidative stress, inflammatory cytokines, and markers of activated neutrophils and macrophages (43–49). The degree of systemic inflammation increases with liver disease severity, infections (50), renal failure (51), hepatic encephalopathy (52) and ACLF (9). One of the major inducers of systemic inflammation are gut-derived pathogen associated molecular patterns, which translocate through a disrupted gut barrier from the intestinal lumen via the portal vein to the liver and systemic circulation. Decompensation is characterized not only by worsening of this increased paracellular intestinal permeability, but also through translocation of viable bacteria. Bacteria translocate likely through transcytosis from the gut to extraintestinal space and organs (53), where they cause infections such as spontaneous bacterial peritonitis, and contribute to systemic inflammation, arterial vasodilation and organ failure (43, 54, 55). Fungal infections in a cirrhotic inpatient cohort are associated with higher ACLF and worse 30-day survival (56).
Several mechanisms contribute to this additional layer of gut barrier dysfunction, which are all closely connected to intestinal dysbiosis. While intestinal bacterial overgrowth and changes in microbiota composition are common in patients with cirrhosis (20), dysbiosis worsens during decompensation. Fecal microbial gene richness, microbial richness and species diversity decreased in patients with decompensated cirrhosis as compared with compensated cirrhosis (57). A significant reduction in fecal Clostridiales XIV, Ruminococcaceae and Lachnospiraceae with a significant increase in pathogenic taxa such as Enterococcaeae, Staphylococcaceae and Enterobacteriaceae on the family level was found in cirrhotics with worsening liver disease (17). Using metagenomic sequencing, fecal Alistipes indistinctus, Bilophila wadsworthia, Bilophila sp. 4_1_30, Ruminococcus champanellensis, Tannerella sp. 6_1_58FAA_CT1, Clostridium botulinum, Clostridium leptum, Clostridium methylpentosum and Clostridium sp. MSTE9 were lower, while Veillonella atypica, Veillonella sp. ACP1, Veillonella dispar, and Veillonella sp. oral taxon 158 were higher on the species level in patients with decompensated cirrhosis as compared with compensated cirrhosis (57). Changes in microbiota translate into functional metabolic differences (57). Bacterial pathogenicity can be mediated via virulence factors. The toxin cytolysin, secreted by Enterococcus faecalis in the intestinal microbiota, associates with worse clinical outcomes and mortality in patients with alcoholic hepatitis (58). While fungal dysbiosis and decreased fungal diversity is similar between patients with early stages of alcohol-associated liver disease and alcoholic hepatitis, the systemic immune response to fungal products is associated with increased mortality in patients with alcoholic hepatitis likely due to the impaired gut barrier function (59). Candidalysin, a secreted exotoxin of Candida albicans, is associated with liver disease severity and mortality in patients with alcoholic hepatitis (60). Cytolysin and candidalysin can directly damage primary hepatocytes, which might directly contribute to worsening of liver function. Increased viral diversity was observed in fecal samples from patients with alcohol-associated liver disease, with the most significant changes in samples from patients with alcoholic hepatitis. Specific viral taxa, such as Staphylococcus phages and Herpesviridae, were associated with increased disease severity and associated with increased 90-day mortality in patients with alcoholic hepatitis (61). In a recent study of outpatients with cirrhosis, gut virome focused on bacteriophages differentially associated with bacteria over the course of disease were less likely than bacteria to predict 90-day hospitalizations. Phages focused on urease-producing Streptococcus were linked with the action of rifaximin in patients with cirrhosis and hepatic encephalopathy (62). How changes in the intestinal virome contribute to hepatic decompensation is not known.
Dysbiosis causes intestinal inflammation, which in turn contributes to gut barrier dysfunction and pathological bacterial translocation (63). Impaired antimicrobial activity in the intestine is associated with translocation of viable bacteria to the mesenteric lymph nodes in rats with cirrhosis and ascites (42). Intestinal immune surveillance improves following intestinal decontamination with antibiotics in experimental cirrhosis, indicating that the bacterial microbiota contributes to exhausting the mucosal immune response during decompensation (64).
Cholestasis causes a reflux from bile acids from the hepatocytes into the circulation and decreases the bile flow into the biliary system and the intestine. Lower bile flow and less intestinal bile acids will further increase bacterial overgrowth and affect the composition of the gut microbiota during decompensation. Vice versa, dysbiosis changes intestinal bile acid metabolism and reduces the conversion of primary into secondary bile acids, which in turn can affect gut barrier function via modulating FXR activity (38, 65). Patients with advanced cirrhosis had the lowest total fecal bile acids with a reduced ratio of secondary to primary bile acids compared with early cirrhotics and controls, while serum primary bile acids were higher in advanced cirrhotics compared with early cirrhotics and controls (32). Total and conjugated serum bile acids correlate positively with disease severity (MELD) in patients with alcoholic hepatitis (66). In viral hepatitis, the mechanisms may be different. In these patients the hepatic injury may lead via danger-associated molecular pattern (DAMPs) to AD and ACLF (67, 68). Indeed, circulating bacterial DNA as a measure of bacterial translocation was significantly increased in HBV-ACLF patients and correlated to inflammatory markers (69).
Taken together, worsening of liver disease initiates a cascade of events with intestinal bacterial overgrowth and dysbiosis as central events. Bacterial toxins can directly cause hepatocyte death and worsening of liver function. Dysbiosis also affects gut barrier function and increases bacterial translocation, which leads to upregulation of systemic inflammation and infections, vasodilation and contributes to hepatic decompensation and multi organ failure (Figure 2).
Figure 2. Microbiome and hepatic decompensation.
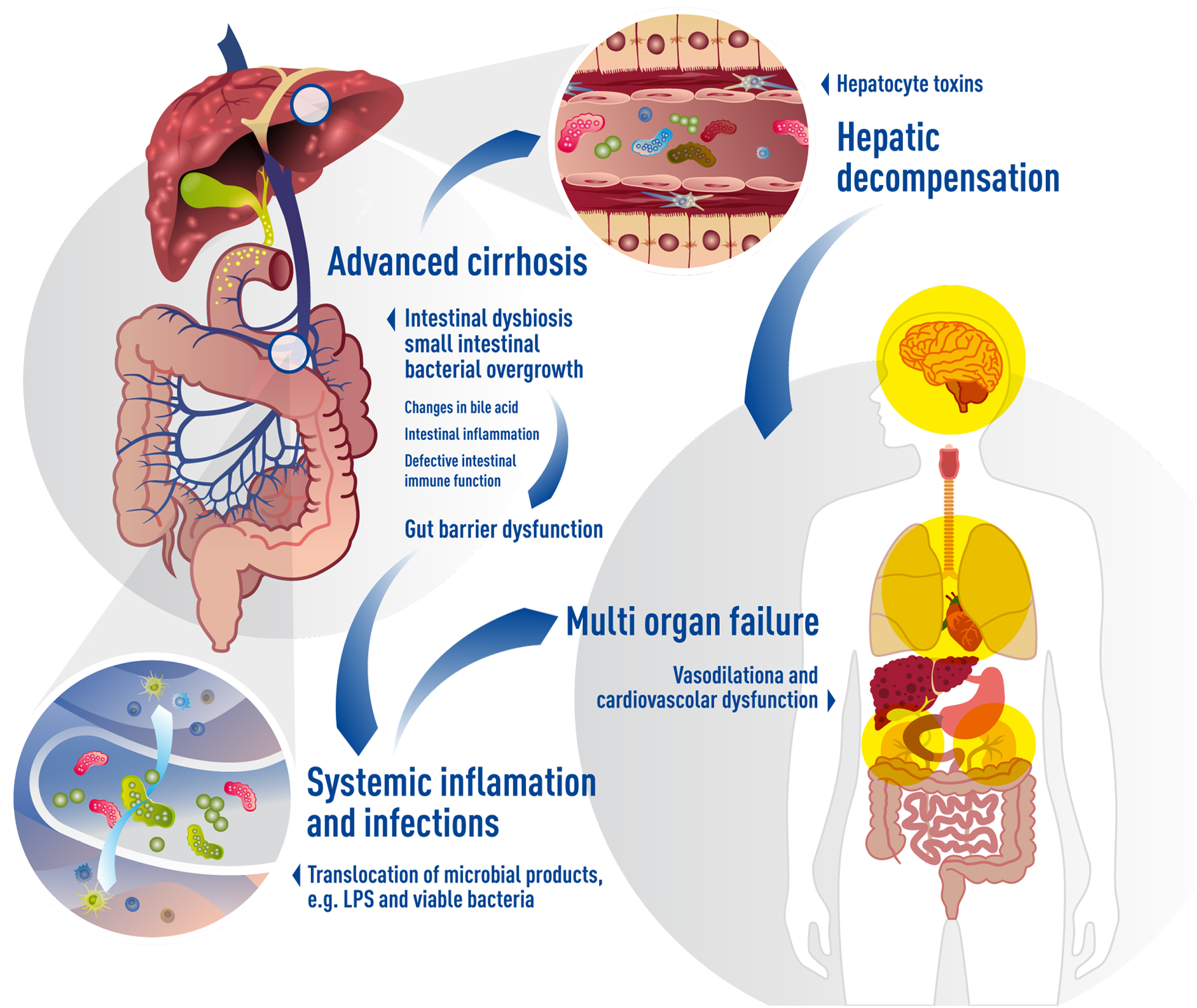
Worsening of liver disease initiates a cascade of events with intestinal bacterial overgrowth and dysbiosis as central events. Intestinal dysbiosis contributes to gut barrier function via several mechanisms. Increases bacterial translocation leads to upregulation of systemic inflammation and infections, vasodilation and contributes to hepatic decompensation and multi organ failure. Toxins produced by the microbiota can directly cause hepatocyte death and worsening of liver function.
4. Lactulose and Nutrition as modulators of the gut microbiome
There are no published data employing untargeted or global culture-independent methodologies assessing the faecal microbiome in healthy individuals receiving lactulose. Lactulose has been shown to increase alpha diversity in healthy mice (70) and pigs (71) and in dogs was shown to increase Veillonellacaeae and Bifidobacteriaceae with a reduction in Bacteroidaceae and Fusobacteriaceae (72). The impact of lactulose in ameliorating dysbiosis in patients with cirrhosis is not clear cut. Studies utilising culture-dependent methodologies in patients with cirrhosis and minimal HE show increased Bifidobacterium, Lactobacillus and Bacteriodaceae colonies and reduced Enterobacteriaceae, Enterococcus and yeasts accompanying plasma ammonia reduction, improved psychometric tests and reduced risk of developing overt HE (73). Furthermore, lactulose leads to a decreased faecal pH with increased aerobic and anaerobic bacterial counts and lactobacilli in patients with cirrhosis without HE (74). Studies utilising 16S rDNA gene sequencing have failed to substantiate any impact of lactulose on the microbiome of cirrhotic patients without HE and have reported only subtle changes in patients with HE (75), including after lactulose withdrawal (76).
The effects of dietary habits on clinical outcomes in patients with cirrhosis has been interrogated and cirrhotic and control groups in the United States compared with groups from Turkey. The Turkish diet, rich in fermented milk products, coffee, tea, and chocolate, was associated with increased microbiotal diversity. Furthermore, it was shown that coffee, tea, vegetables, and cereals were protective against 90-day rehospitalisation rates (77).
5. Potential therapeutic implications of new targets including:
-. Faecal transplantation as a promising tool
The revolving door of hospitalizations, re-hospitalizations, antibiotic and PPI use, multiple instrumentations and inadequate dietary intake contribute to the continued dysbiosis in cirrhosis (78). Resetting this requires a major shift in the gut ecosystem through FMT. Studies in germ-free and specific-pathogen-free mice have shown that FMT from affected human donors can partly replicate the microbial and brain-related injury even without the continued exposure to the toxin(s) that caused the liver injury (79–81). FMT has been extensively used for C. difficile, which is characterized by an acute, major reduction in microbial diversity unlike cirrhosis where there is a consistent gut-liver axis alteration. After FMT in C. difficile there is a recovery of the bile acids moieties indicating functional benefity (82). Moreover, when exposed to liver injury, permissive microbiota were more likely to propagate liver damage (81) but FMT alone did not lead to cirrhosis.
The experience in humans with FMT and cirrhosis spans outpatients with compensated cirrhosis and alcohol use disorder (AUD) for addiction, outpatients with HE on rifaximin and lactulose, and inpatients with alcoholic hepatitis (83) (Table 1). Moreover, it has been successfully used for concomitant C. difficile (84–86). Several more studies are being planned or are in process to leverage this exciting approach (87). Most current studies are small-scale that illustrate the first important step of any investigation, i.e. safety. Of note, there are no data for patients with complications (e.g. variceal bleeding) or decompensated patients. All studies demonstrate that this approach is safe even long-term and does not result in a greater incidence of infections if the donors are screened according to guidelines (88). When these protocols were not followed, donor-derived infections were easily transmitted to the immunosuppressed patients (88). Therefore, it is critical to select donors appropriately and in the era of COVID-19, the challenges of ensuring that FMT is safe is even more important (89, 90).
Table 1:
Studies analyzing fecal microbiota transplantation (FMT) in cirrhosis.
| Study and design | Samples/ Groups compared | Route and Duration of FMT | Findings and Significance | Limitations |
|---|---|---|---|---|
| Alcohol-related disorders | ||||
| Bajaj et al Hepatology 2020 (148) | Men with AUD and cirrhosis who were not successful on abstinence using current therapies | -One-time enema vs placebo -Reduced short-term alcohol craving and consumption with higher SCFA in FMT -Lower AUD-related hospitalizations long-term in FMT vs placebo |
Reduction of addictive behavior resulting in long-term reduction in AUD-related hospitalizations over 6 months | -Small-scale -All men |
| Phillips et al CGH 2017(149) | Men with steroid resistant alcohol-related hepatitis | -One week of daily NJT FMT from many donors - One year open-label study with historical controls |
Higher survival versus controls | -Open-label -Historical controls -All men |
| Phillips et al Indian J Gastro 2018 (150) | Men with alcohol-related hepatitis | -One week of daily NJT FMT from many donors versus standard therapy -Three-month follow-up |
3-month survival higher in FMT group, while 1-month survival was similar | -Open-label -Small numbers -All men |
| Cirrhosis | ||||
| Kao et al Hepatol 2016 (151) | One patient with HE | −1 FMT via colonoscopy followed by 3 weekly enemas -Safe and well-tolerated with improvement in cognitive function |
Case report of FMT in cirrhosis with brain function improvement | -Case report |
| Bajaj et al Hepatol 2017 and 2018 (92, 152) | 20 HE patients on lactulose and rifaximin | -One 90 ml of enema after 5 days of broad-spectrum antibiotics -Safe and well-tolerated, improvement in hospitalizations, dysbiosis and SCFAs post antibiotics after FMT |
First randomized trial to study this in cirrhosis and HE and under Investigational new drug under FDA | -Small-scale -Antibiotics +FMT rather than FMT alone |
| Mehta et al 2018 Indian J Gastro (153) Case series | 10 HE patients open label | -One FMT via colonoscopy -Sustained clinical response at week 20 in 6 patients |
Further evidence about safety and potential efficacy | -Open-label case series |
| Bajaj et al. Hepatology and JCI Insight 2019 (93, 154) | 20 HE patients on lactulose and rifaximin | −15 capsules of FMT vs placebo once -Brain function improved and outcomes got better in those with secondary BA formation |
Oral capsular FMT is also safe in HE and success can be linked to secondary BA formation. | -Small numbers |
As found in trials for C. difficile, FMT did not dramatically change the recipient’s microbial composition or diversity (91). Rather functional changes focused on bile acids, SCFAs and other metabolites were found (92, 93). Currently published trials are not powered for efficacy but nonetheless showed results that support the development and further refinement of FMT.
Hepatic encephalopathy: One case report, one case series and two small randomized controlled trials (RCTs) using enema, colonoscopy, and capsules have shown safety. There were improvements in cognition in the studies that tested for it in the FMT group more than the placebo/standard of care group. Also, there were trends towards lower adverse events in the FMT compared to no-FMT group.
Alcohol use disorder: In a double-blind, placebo-controlled randomized clinical trial of men with AUD who had failed several attempts at pharmacological or behavioral therapy for abstinence, one-time enema FMT was safe over 6 months. There was a short-term reduction in alcohol craving and consumption accompanied by better microbial diversity and SCFA production in FMT patients. Over the long-term, AUD-related serious adverse events significantly reduced in patients randomized to FMT compared to placebo.
Alcohol-related hepatitis: Safety and potential benefit compared to historical controls was found in steroid-ineligible patients over one year, while another open-label trial showed safety compared to usual standard of care
The next steps are (a) defining efficacy (b) dose-response, (c) route of administration and (d) which microbe(s) are essential for potential beneficial impact. The risks and benefits of FMT have to be balanced with the potential risks and absence of a viable therapeutic alternative that can be added. There should be an equipoise when employing FMT for cirrhosis. Given that the underlying liver etiology also needs to be treated, it is important to continue efforts towards correcting the etiology while pursuing FMT.
-. Antibiotics in cirrhosis: A double-edged sword
Perturbations in the gut microbiome underpin the increased susceptibility that patients with cirrhosis have to developing infection which may be asymptomatic in up to 50% of cases (94). Bacterial translocation is a significant driver of cirrhosis-associated immune dysfunction (CAID), although the mechanisms by which intestinal dysbiosis drives immune cell dysfunction remain poorly characterised (53, 95). As infection is a potent precipitant of decompensating events, ACLF, and contributes to high mortality, patients are frequently prescribed broad-spectrum antibiotics (96, 97). Furthermore, approximately 25% of all patients with cirrhosis are on long-term antibiotics for the primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) (98) and prevention of the recurrence of overt hepatic encephalopathy (99). Whilst life-saving on the one hand, antibiotics are very much a double-edged sword exacerbating pre-existing gut dysbiosis, augmenting disruption of the normally symbiotic population of intestinal bacteria and potentially predisposing to further opportunistic infections and small bowel bacterial overgrowth (100–102). This in turn adversely impacts on microbial diversity, composition, activity and gut wall integrity. Moreover, the prevalence of multidrug anti-microbial resistance (AMR) increased from 29 to 38% in culture-positive infections in patients with decompensated cirrhosis and ACLF from 2011 to 2017/18 (97).
Manipulation of the gut microbiome in cirrhosis to reduce hepatic decompensation and improve outcomes.
Rifaximin is an antibacterial drug of the rifamycin class that irreversibly binds the beta sub-unit of the bacterial enzyme DNA-dependent RNA polymerase and consequently inhibits bacterial RNA synthesis. It has a broad antimicrobial spectrum against most of the Gram-positive and negative, aerobic and anaerobic bacteria, including ammonia producing species. It may inhibit the division of urea-deaminating bacteria, thereby reducing the production of ammonia and other compounds that play an important role in the pathogenesis of HE. Rifaximin can down-regulate microbe-induced gut epithelial inflammatory responses by inhibiting activation of the nuclear factor (NF)-KB via the pregnane X receptor and by reducing the expression of the pro-inflammatory cytokines interleukin-1β and tumour necrosis factor-alpha (TNF-α) (103, 104). Rifaximin also has eubiotic effects selecting beneficial bacterial taxa (105, 106). Lactobacilli can grow in response to rifaximin administration, an effect restricted only to this antibiotic and not seen with another poorly absorbed antibiotic, neomycin (107). Rifaximin reduces the risk of recurrence of overt HE and the need for hospitalisation and in conjunction with lactulose has become a mainstay second-line therapy for HE (108). Rifaximin has been associated with significant reductions in hospitalisation, bed days, emergency department attendances and 30-day readmission (109, 110). The specific mechanism of action of rifaximin remains to be elucidated and has not been shown to appreciably low blood ammonia levels in any study. There are changes in microbial function and interaction with bacteriophages focused around urease-producing Streptococcus. Patients with advanced cirrhosis treated with rifaximin have reduced all-cause admissions, episodes of SBP and variceal bleeding, and reduced length of stay (111–114). Furthermore, the use of rifaximin by patients on the liver transplant waiting list has been linked to reduced early allograft dysfunction following transplantation (115). However, considerable concern remains regarding whether long term rifaximin use may contribute to AMR in cirrhosis. Indeed, in a recent study, 50% of patients prescribed rifaximin for HE developed rifaximin-resistant staphylococcal isolates after as little as 1–7 weeks of rifaximin treatment (116).
Antibiotics as modulators of inflammation in cirrhosis.
Rifaximin reduces circulating levels of gut-derived endotoxins such as lipopolysaccharide (117, 118). Studies examining changes in the composition of the faecal microbiome in response to rifaximin have categorically failed to demonstrate any clear changes in microbial abundance by 16S rRNA faecal microbiota profiling (118, 119). A significant increase in serum saturated (myristic, caprylic, palmitic, palmitoleic, oleic and eicosanoic) and unsaturated (linoleic, linolenic, gamma-linolenic and arachnidonic) fatty acids post-rifaximin has however been observed. Rifaximin led to a shift from pathogenic to beneficial metabolite linkages using network connectivity analysis centered on Enterobacteriaceae, Porphyromonadaceae and Bacteroidaceae (118).
There are emerging data to suggest that rifaximin has potent anti-inflammatory actions including reducing anti-tumour necrosis alpha and neutrophil toll-like receptor 4 expression (120). Mucosal-associated invariant T (MAIT) cells are non-conventional T cells that display altered functions during chronic inflammatory diseases. MAIT cells are reduced in patients with alcoholic or non-alcohol fatty liver disease-related cirrhosis while they accumulate in liver fibrotic septa. In two models of chronic liver injury, MAIT cell-enriched mice show increased liver fibrosis and accumulation of hepatic fibrogenic cells, whereas MAIT cell-deficient mice were resistant. Long-term prophylactic antibiotic therapy with norfloxacin or rifaximin was significantly associated with a lower reduction in MAIT cell frequency (121). Antibiotic-exposed cirrhotic patients displayed significant reduction of CD25 expression suggesting long-term antibiotic therapy partially prevents MAIT cell reduction and activation (121).
-. Adsorbents
Manipulation of the gut microbiome may also be achieved via adsorption of intraluminal host or microbial metabolites or ligands. Studies to date have focused on enterosorption of pathogenic factors such as ammonia or endotoxin or modulation of bile acid pathways. The advantage of such an approach is that it does not confer a risk of antimicrobial resistance or introduction of potential pathobionts. Conceptually the enterosorbants act as a ‘sink’ for pathological factors, which drive pathogenesis in liver disease.
Enterosorption of Pathological Bacterial Ligands and Metabolites.
The first carbon-based enterosorbant to be evaluated in cirrhosis was AST-120 (Ocera Therapeutics Inc), a microporous carbon which had been demonstrated to efficiently adsorb ammonia in vitro. Bosoi et al evaluated the capacity of AST-120 to lower blood ammonia, oxidative stress and brain oedema in bile duct ligated (BDL) rats as both a prophylactic and therapeutic strategy (122). Plasma ammonia concentrations in BDL rats were significantly decreased by AST-120 in a dose-dependent manner with normalisation of brain water content and locomotor activity. A multi-centre, double-blind, randomized, placebo-controlled, dose-ranging study of AST-120 was conducted in compensated cirrhotic patients with MELD Score ≤ 25 and covert hepatic encephalopathy (Astute study). AST-120 was found to be well tolerated but failed to achieve its primary endpoint of improvement in covert hepatic encephalopathy (Bajaj et al, personal communication). A Cochrane review concluded that whilst AST-120 lowers blood ammonia concentrations compared to placebo, there was little evidence this translated into clinical benefit (123).
Yaq-001 (Yaqrit Limited, UK) is a more recent carbon-based enterosorbant to be studied in cirrhosis. In contrast to AST-120, Yaq-001 is a non-absorbable synthetic carbon with a tailored bimodal distribution of porous domains within the macroporous range (>50nm) and microporous range (<2nm) and a very large surface area. The biological significance of this is that, in addition to binding smaller mediators such as indoles, acetaldehyde and fMLP, Yaq-001 exhibits rapid adsorption kinetics for larger molecular weight factors such as endotoxin, exotoxins and cytokines (124). Yaq-001 was found to reduce liver injury, portal pressure and LPS-induced reactive oxygen species production in an in vivo model of cirrhosis and ACLF (125). Whilst not exerting a direct effect on bacterial growth kinetics, shifts in microbiome composition were observed in stool (126). Phase 2 clinical studies to evaluate safety and tolerability with secondary efficacy end-points are now completed and due to report later this year.
Modulation of Bile Acid Pathways.
Intraluminal bile acid availability exerts a selection pressure on microbiome composition. Gut microbiota have a reciprocal influence on the biotransformation of bile acids and downstream Farnesoid X Receptor (FXR) and G protein-coupled membrane receptor 5 signaling pathways. Manipulation of these pathways therefore represents a strategy to target to microbiome and impact on clinical outcome and include FXR agonists and intraluminal sequestration of bile acids.
The most well studied compound is synthetic FXR agonist obeticholic acid (OCA) although many other targets of therapy have been developed principally in application to pre-cirrhotic non-alcoholic steatohepatitis. An exhaustive discussion of these studies is beyond the scope of this review. In a rodent model of cirrhosis, OCA was found to significantly reduce bacterial translocation from 78.3% to 33.3% and significantly modulate mucosal microbiota composition (38). Treatment was associated with favourable effects on ileal antimicrobial peptide, tight junction expression, intestinal inflammation and liver fibrosis. Clinical translation however has been hampered by safety concerns over OCA in patients with advanced disease.
Bile acid sequestration.
Bile acid sequestrant Colesevelam has been shown to attenuate cholestatic liver and bile duct injury in Mdr2 −/− mice by modulating composition, signalling and excretion of faecal bile acids. Fuch et al demonstrated that Colesevelam increased faecal bile acid excretion and enhanced conversion towards secondary bile acids, thereby attenuating liver and bile duct injury in Mdr2−/− mice (127).
Phosphate sequestrant sevelamer has been studied in murine models of NASH due to it’s favourable effects on LDL cholesterol attributed to sequestration of hydrophilic bile acids in which it was found to prevent hepatic steatosis, inflammation and fibrosis (128). Sevelamer improved a lower α-diversity and bound intraluminal endotoxin. Takahashi et al demonstrated that in addition to demonstrating efficacy as a prophylactic strategy, sevelamer could reverse liver injury (129). Metabolomic and microbiome analysis revealed that this beneficial effect is associated with changes in the microbiota population and bile acid composition associated with improvements in insulin resistance.
6. Areas of controversy
There remain several areas of controversy in this burgeoning field, which can inform future trials.
The depth of coverage, functional assessment, metabolic activity and host response to microbiota vary between studies(4). These, along with differing geographic areas, dietary practices, sex, ethnic variations, and aetiology differences can potentially alter the microbiome(130, 131). Therefore, these variables need to be controlled for in microbial analyses.
We do not know whether the microbiota are the “chicken or the egg” in human studies. In mice that have been humanized with stools from carefully phenotyped human donors, there is liver injury but not to the extent that is achieved by exposing the mice to the etiological agent or that found in the donor humans(81, 132, 133). Therefore, the complicit nature of the microbes at this stage rather than potential causation needs to be defined.
Microbiota or their products mediating the outcomes are an important source of controversy(14, 32, 58, 60, 134, 135). There are redundancies in microbial function that can cross bacterial taxa that may be more relevant than composition. Studies isolating microbial products and/or dead bacteria offer a controversial insight into these differences.
Modern medical care has had a huge impact on the changes induced in the microbiome. The rampant overuse of antibiotics and PPIs can make the gut milieu in patients with cirrhosis hostile(136, 137). The controversy over routine use of antibiotic prophylaxis in patients with cirrhosis, especially in those who have not experienced SBP is important from a clinical and microbiological perspective(138). Antibiotics such as rifaximin may beneficially select taxa such as Lactobacilli which may be protect against inflammation and hepatic decompensation (107, 114)ref] but others may promote hepatic toxicity. Further mechanistic studies are therefore warranted.
Furthermore, non-antibiotic drugs have a huge effect on the microbiome and may contribute to the development of antibiotic resistance, a growing problem in this patient population (139). Finally, we need to account for the impact of planned and also necessary endoscopic procedures, fasting periods and other interventions (e.g. professional periodontal cleaning) that also may induce at least temporary changes in the microbiome(140, 141). These factors may be crucial in the interpretation of cross-sectional microbiome research and require longitudinal large-scale and in-depth analysis with cautious interpretation that controls for these factors.
In addition, the role of non-bacterial microbiota such as fungi and viruses are important to elucidate since they interact with the bacteria, with each other and their host in a complex ecosystem (59–61, 102). In selected circumstances they can become potentially pathogenic. Virome constituents can potentially be used for treating specific infections or modulating the activity of the target bacteria and their potential products(58, 142).
Microbial changes in easily accessible biofluids such as stool and saliva have been studied but there are relatively rarer reports of microbiota from ascites, mucosal surfaces, liver tissue, bile and other tissues(143–147). Microbial alterations in these tissues may be more linked to organ dysfunction. Therefore, microbial milieu of these organs may be different and should not be conflated with stool or saliva unless there is further evidence.
7. Future perspectives
The field of liver disease still needs more detailed longitudinal large-scale and in depth analysis of the microbiota with cautious interpretation of the results through future trials. There remain several factors that can influence microbiota, such as demographics (geographic area, sex and diet), and disease related (aetiology, drugs, interventions), and finally the sampling compartment. These factors need to be controlled and taken into account for in the interpretation. Moreover, more mechanistic investigations on the role of the microbiota and its components are required to develop treatment strategies that can benefit the patients without negatively influencing the gut ecosystem.
Current understanding on the composition and function of the gut microbiome and how this relates to the progression and outcomes in patients with cirrhosis remains in its infancy and is based on descriptive snapshots afflicted with confounders and lacks robust clinical validation. As perturbations in the gut microbiome are a hallmark of advanced CLD and influence the rate of progression to liver failure, driving the susceptibility to infection, unlocking the potential of the microbiome and developing antibiotic-free therapies such as with faecal microbiota transplantation to tackle these unmet needs becomes a research priority. Further multicentre randomised controlled trials are now needed to prove the efficacy of FMT in larger populations of patients with cirrhosis and to evaluate its mechanisms of action, which remain unclear.
Table 2.
Selected studies of rifaximin to reduce hepatic decompensation and improve outcomes.
| Study and design | Samples/Groups compared | Route and Duration of Therapy | Findings and Significance | Limitations |
|---|---|---|---|---|
| Bass N et al. NEJM 2010;362(12):1071–81 (108) | 299 patients with cirrhosis with 2 or more overt HE episodes in the preceding 6-months. | Rifaximin 550mg twice daily versus placebo for 6 months. 91% of patients were on concomitant lactulose. | Rifaximin significantly reduced risk of HE, as compared with placebo, over a 6-month period (hazard ratio with rifaximin, 0.42; 95% confidence interval [CI], 0.28 to 0.64; P<0.001). A breakthrough episode of HE occurred in 22.1% of patients on rifaximin, as compared with 45.9% of patients on placebo. 13.6% of the patients on rifaximin group had a hospitalization involving HE, as compared with 22.6% of patients on placebo, hazard ratio of 0.50 (95% CI, 0.29 to 0.87; P=0.01). | MELD score 25 or less |
| Bajaj JS et al. PLoS One2013;8(4):e60042 (118) | 20 patients with cirrhosis and minimal HE underwent cognitive testing, endotoxin analysis, urine/serum metabolomics and fecal microbiome assessment (16S rRNA) pre- and post-rifaximin. | Rifaximin 550mg twice daily for 8-weeks | Rifaximin improved cognitive function and endotoxemia accompanied by alteration of gut bacterial linkages with metabolites without significant change in microbial abundance. | - |
| Orr JR et al. Liver Int. 2016;36(9):1295–303. (109) | 326 patients in 10 UK centres. | Patients treated with rifaximin 550mg twice daily. Data on hospital resource utilization collected 12-months pre- and post-rifaximin therapy. | Initiation of treatment with rifaximin-α was associated with a marked reduction in the number of hospital admissions and hospital length of stay. | Retrospective study |
| Goel A et al. Aliment Pharmacol Ther. 2017;46(11–12):1029–36. (112) | Five studies with 555 patients (295 rifaximin, 260 systemic antibiotics) compared rifaximin with systemic antibiotics. | Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. | Rifaximin reduced the risk of SBP by 47% compared to no antibiotics for primary prophylaxis and by 74% compared to systemic antibiotics for secondary prophylaxis. | Systematic review with meta-analysis |
| Ibrahim ES et al. Eur J Gastroenterol Hepatol. 2017;29(11):1247–50 (113) | 80 patients with cirrhosis and ascites. | Randomised to rifaximin 550 mg twice daily for 12 weeks or standard of care. | Hepatorenal syndrome developed more in the control group than the rifaximin group [9 (22.5%) vs. 2 (5%); P=0.048] | No placebo |
| Salehi S et al. Aliment Pharmacol Ther. 2019;50(4):435–41. (114) | 622 patients listed for transplantation; 101 had HE | Outcomes of patients treated with rifaximin 550mg twice daily versus those who were naïve. | Patients on transplant waiting list treated with rifaximin had reduced all-cause admissions, episodes of spontaneous bacterial peritonitis and variceal bleeding. Multivariate regression analysis demonstrated that rifaximin was independently associated with an increase in average days to readmission (adjusted effect estimate 71, 95% CI 3–140 days). | Retrospective study |
Acknowledgements:
This review is supported by MICROB-PREDICT project. The MICROB-PREDICT project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 825694. This reflects only the author’s view, and the European Commission is not responsible for any use that may be made of the information it contains.
J.T. is supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR57, P18 and CRC 1382, A09), European Union’s Horizon 2020 Research and Innovation Programme (Galaxy, No. 668031 and MICROB-PREDICT, No. 825694) and Societal Challenges - Health, Demographic Change and Wellbeing (LIVERHOPE No. 731875), and Cellex Foundation (PREDICT). J.M. is supported by grants from the European Union’s 2020 Research and Innovation Programme (CARBALIVE, No. 634579 and MICROB-PREDICT, No. 825694). B.S. was supported in part by services provided by NIH centers P30 DK120515 and P50 AA011999. D.L.S. is supported by grants from the European Union’s Horizon 2020 Research and Innovation Programme (MICROB-PREDICT, No. 825694) and the National Institute for Health Research (PROFIT Trial No. PB-PG-0215-36070). J.S.B. was supported in part by VA Merit Review 2I0CX001076 and NCATS R21 TR002024 and R21TR003095.
Conflicts of interest:
J.T. has received speaking and/or consulting fees from Gore, Bayer, Alexion, MSD, Gilead, Intercept, Norgine, Grifols, Versantis, and Martin Pharmaceutical. J.M. is a co-founder of Yaqrit Limited and has received speaker fees from Norgine and Yakult Europe. B.S. has been consulting for Ferring Research Institute, Intercept Pharmaceuticals, HOST Therabiomics, Mabwell Therapeutics and Patara Pharmaceuticals. B.S.’s institution UC San Diego has received grant support from BiomX, NGM Biopharmaceuticals, CymaBay Therapeutics, Synlogic Operating Company and Axial Biotherapeutics. D.L.S. has been consulting for Norgine, Kaleido Biosciences, Shionogi and Mallinckrodt Pharmaceuticals and has undertaken paid lectures for Norgine, Alfa Sigma and Falk Pharma. D.S’s institution King’s College London has received grant support from Norgine. J.S.B. has been an advisor to Valeant, Norgine, Kaleido and Takeda and his institution has received support from Bausch Health, Kaleido, Grifols and Mallinckrodt pharmaceuticals.
References:
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–1761. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134:1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med 2020;382:2137–2145. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut 2016;65:2035–2044. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 2018;15:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol 2020;72:558–577. [DOI] [PubMed] [Google Scholar]
- 7.Trebicka J, Reiberger T, Laleman W. Gut-Liver Axis Links Portal Hypertension to Acute-on-Chronic Liver Failure. Visc Med 2018;34:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, et al. PREDICT identifies Precipitating Events Associated with Clinical Course of Acutely Decompensated Cirrhosis. J Hepatol 2020;accepted. [DOI] [PubMed] [Google Scholar]
- 9.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–1437, 1437 e1421–1429. [DOI] [PubMed] [Google Scholar]
- 10.Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo V, Moreau R, Kamath PS, Jalan R, Gines P, Nevens F, Fernandez J, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers 2016;2:16041. [DOI] [PubMed] [Google Scholar]
- 12.Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol 2016;13:131–149. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, Subramanian RM, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367–2374. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Reddy KR, O’Leary JG, Vargas HE, Lai JC, Kamath PS, Tandon P, et al. Serum Levels of Metabolites Produced by Intestinal Microbes and Lipid Moieties Independently Associated With Acute on Chronic Liver Failure and Death in Patients With Cirrhosis. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol 2019;16:235–246. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj JS, Betrapally NS, Gillevet PM. Decompensated cirrhosis and microbiome interpretation. Nature 2015;525:E1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer TM, Steinbruckner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol 2001;96:2962–2967. [DOI] [PubMed] [Google Scholar]
- 21.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology 1998;28:1187–1190. [DOI] [PubMed] [Google Scholar]
- 22.Fukui H, Wiest R. Changes of Intestinal Functions in Liver Cirrhosis. Inflamm Intest Dis 2016;1:24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009;139:1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015;8:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut 2019;68:359–370. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011;54:562–572. [DOI] [PubMed] [Google Scholar]
- 27.Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, Singh S, et al. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addolorato G, Ponziani FR, Dionisi T, Mosoni C, Vassallo GA, Sestito L, Petito V, et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int 2020;40:878–888. [DOI] [PubMed] [Google Scholar]
- 29.Ponziani FR, Putignani L, Paroni Sterbini F, Petito V, Picca A, Del Chierico F, Reddel S, et al. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther 2018;48:1301–1311. [DOI] [PubMed] [Google Scholar]
- 30.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019;69:107–120. [DOI] [PubMed] [Google Scholar]
- 31.Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles H, Heuman D, et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl 2017;23:907–914. [DOI] [PubMed] [Google Scholar]
- 32.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 2013;4:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol 2014;306:G929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res 2008;18:1087–1095. [DOI] [PubMed] [Google Scholar]
- 36.Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, de Gottardi A, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol 2019;71:1126–1140. [DOI] [PubMed] [Google Scholar]
- 37.Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol 2015;185:409–419. [DOI] [PubMed] [Google Scholar]
- 38.Ubeda M, Lario M, Munoz L, Borrero MJ, Rodriguez-Serrano M, Sanchez-Diaz AM, Del Campo R, et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol 2016;64:1049–1057. [DOI] [PubMed] [Google Scholar]
- 39.Munoz L, Borrero MJ, Ubeda M, Conde E, Del Campo R, Rodriguez-Serrano M, Lario M, et al. Intestinal Immune Dysregulation Driven by Dysbiosis Promotes Barrier Disruption and Bacterial Translocation in Rats With Cirrhosis. Hepatology 2019;70:925–938. [DOI] [PubMed] [Google Scholar]
- 40.Shindo K, Machida M, Miyakawa K, Fukumura M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol 1993;88:2084–2091. [PubMed] [Google Scholar]
- 41.Pelletier G, Briantais MJ, Buffet C, Pillot J, Etienne JP. Serum and intestinal secretory IgA in alcoholic cirrhosis of the liver. Gut 1982;23:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology 2012;55:1154–1163. [DOI] [PubMed] [Google Scholar]
- 43.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272–1284. [DOI] [PubMed] [Google Scholar]
- 44.Claria J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amoros A, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249–1264. [DOI] [PubMed] [Google Scholar]
- 45.Sole C, Sola E, Morales-Ruiz M, Fernandez G, Huelin P, Graupera I, Moreira R, et al. Characterization of Inflammatory Response in Acute-on-Chronic Liver Failure and Relationship with Prognosis. Sci Rep 2016;6:32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gronbaek H, Rodgaard-Hansen S, Aagaard NK, Arroyo V, Moestrup SK, Garcia E, Sola E, et al. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF). J Hepatol 2016;64:813–822. [DOI] [PubMed] [Google Scholar]
- 47.Trebicka J, Amoros A, Pitarch C, Titos E, Alcaraz-Quiles J, Schierwagen R, Deulofeu C, et al. Addressing Profiles of Systemic Inflammation Across the Different Clinical Phenotypes of Acutely Decompensated Cirrhosis. Front Immunol 2019;10:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, Klein S, et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Praktiknjo M, Monteiro S, Grandt J, Kimer N, Madsen JL, Werge MP, William P, et al. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int 2020;40:1457–1466. [DOI] [PubMed] [Google Scholar]
- 50.Michelena J, Altamirano J, Abraldes JG, Affo S, Morales-Ibanez O, Sancho-Bru P, Dominguez M, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015;62:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navasa M, Follo A, Filella X, Jimenez W, Francitorra A, Planas R, Rimola A, et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology 1998;27:1227–1232. [DOI] [PubMed] [Google Scholar]
- 52.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 2012;302:G168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taura P, Fuster J, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol 2001;34:32–37. [DOI] [PubMed] [Google Scholar]
- 54.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol 2014;60:197–209. [DOI] [PubMed] [Google Scholar]
- 55.Frances R, Zapater P, Gonzalez-Navajas JM, Munoz C, Cano R, Moreu R, Pascual S, et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 2008;47:978–985. [DOI] [PubMed] [Google Scholar]
- 56.Bajaj JS, Reddy RK, Tandon P, Wong F, Kamath PS, Biggins SW, Garcia-Tsao G, et al. Prediction of Fungal Infection Development and Their Impact on Survival Using the NACSELD Cohort. Am J Gastroenterol 2018;113:556–563. [DOI] [PubMed] [Google Scholar]
- 57.Shao L, Ling Z, Chen D, Liu Y, Yang F, Li L. Disorganized Gut Microbiome Contributed to Liver Cirrhosis Progression: A Meta-Omics-Based Study. Front Microbiol 2018;9:3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang S, Duan Y, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M, Abraldes JG, et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology 2020;71:522–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, Liu J, et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol 2020;72:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang L, Lang S, Duan Y, Zhang X, Gao B, Chopyk J, Schwanemann LK, et al. Intestinal virome in patients with alcoholic hepatitis. Hepatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj J, Sikaroodi M, Shamsaddini A, Henseler Z, Santiago-Rodriguez T, Acharya C, Fagan A, et al. Interaction of Bacterial Metagenome and Virome in Patients with Cirrhosis and Hepatic Encephalopathy. Gut 2020;in press. [DOI] [PubMed] [Google Scholar]
- 63.Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015;61:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munoz L, Jose Borrero M, Ubeda M, Lario M, Diaz D, Frances R, Monserrat J, et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology 2012;56:1861–1869. [DOI] [PubMed] [Google Scholar]
- 65.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018;67:2150–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, Coulter S, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol 2018;69:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 2015;62:232–242. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Chen LY, Zhang NN, Li ST, Zeng B, Pavesi M, Amoros A, et al. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci Rep 2016;6:25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Zhao R, Shi D, Sun S, Ren H, Zhao H, Wu W, et al. Characterization of the circulating microbiome in acute-on-chronic liver failure associated with hepatitis B. Liver Int 2019;39:1207–1216. [DOI] [PubMed] [Google Scholar]
- 70.Zhai S, Zhu L, Qin S, Li L. Effect of lactulose intervention on gut microbiota and short chain fatty acid composition of C57BL/6J mice. Microbiologyopen 2018;7:e00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chae JP, Pajarillo EA, Oh JK, Kim H, Kang DK. Revealing the combined effects of lactulose and probiotic enterococci on the swine faecal microbiota using 454 pyrosequencing. Microb Biotechnol 2016;9:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferreira MDF, Salavati Schmitz S, Schoenebeck JJ, Clements DN, Campbell SM, Gaylor DE, Mellanby RJ, et al. Lactulose drives a reversible reduction and qualitative modulation of the faecal microbiota diversity in healthy dogs. Sci Rep 2019;9:13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziada DH, Soliman HH, El Yamany SA, Hamisa MF, Hasan AM. Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? A neurometabolite study using magnetic resonance spectroscopy. Arab J Gastroenterol 2013;14:116–122. [DOI] [PubMed] [Google Scholar]
- 74.Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, Romiti A, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol 1990;12:433–436. [DOI] [PubMed] [Google Scholar]
- 75.Sarangi AN, Goel A, Singh A, Sasi A, Aggarwal R. Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol 2017;17:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, Schubert CM, et al. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metab Brain Dis 2012;27:205–215. [DOI] [PubMed] [Google Scholar]
- 77.Bajaj JS, Idilman R, Mabudian L, Hood M, Fagan A, Turan D, White MB, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 2018;68:234–247. [DOI] [PubMed] [Google Scholar]
- 78.Bajaj JS, Vargas HE, Reddy KR, Lai JC, O’Leary JG, Tandon P, Wong F, et al. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:756–765 e753. [DOI] [PubMed] [Google Scholar]
- 79.Kang DJ, Hylemon PB, Gillevet PM, Sartor RB, Betrapally NS, Kakiyama G, Sikaroodi M, et al. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol Commun 2017;1:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu R, Kang JD, Sartor RB, Sikaroodi M, Fagan A, Gavis EA, Zhou H, et al. Neuroinflammation in Murine Cirrhosis Is Dependent on the Gut Microbiome and Is Attenuated by Fecal Transplant. Hepatology 2020;71:611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830–839. [DOI] [PubMed] [Google Scholar]
- 82.Weingarden A, Gonzalez A, Vazquez-Baeza Y, Weiss S, Humphry G, Berg-Lyons D, Knights D, et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 2015;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol 2020;72:1003–1027. [DOI] [PubMed] [Google Scholar]
- 84.Pringle PL, Soto MT, Chung RT, Hohmann E. Patients With Cirrhosis Require More Fecal Microbiota Capsules to Cure Refractory and Recurrent Clostridium difficile Infections. Clin Gastroenterol Hepatol 2019;17:791–793. [DOI] [PubMed] [Google Scholar]
- 85.Meighani A, Alimirah M, Ramesh M, Salgia R. Fecal Microbiota Transplantation for Clostridioides Difficile Infection in Patients with Chronic Liver Disease. Int J Hepatol 2020;2020:1874570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng YW, Alhaffar D, Saha S, Khanna S, Bohm M, Phelps E, Ghabril M, et al. Fecal Microbiota Transplantation Is Safe and Effective in Patients With Clostridioides difficile Infection and Cirrhosis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hatton GB, Ran S, Tranah TH, Shawcross DL. Lessons Learned from Faecal Microbiota Transplantation in Cirrhosis. Current Hepatology Reports 2020. [Google Scholar]
- 88.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med 2019;381:2043–2050. [DOI] [PubMed] [Google Scholar]
- 89.Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, Iqbal TH, et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut 2020;69:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kassam Z, Dubois N, Ramakrishna B, Ling K, Qazi T, Smith M, Kelly CR, et al. Donor Screening for Fecal Microbiota Transplantation. N Engl J Med 2019;381:2070–2072. [DOI] [PubMed] [Google Scholar]
- 91.Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol 2014;306:G310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bajaj JS, Kakiyama G, Savidge T, Takei H, Kassam ZA, Fagan A, Gavis EA, et al. Antibiotic-Associated Disruption of Microbiota Composition and Function in Cirrhosis Is Restored by Fecal Transplant. Hepatology 2018;68:1549–1558. [DOI] [PubMed] [Google Scholar]
- 93.Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, et al. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology 2019;70:1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis 2001;33:41–48. [DOI] [PubMed] [Google Scholar]
- 95.Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385–1396. [DOI] [PubMed] [Google Scholar]
- 96.Fernandez J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;67:1870–1880. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol 2019;70:398–411. [DOI] [PubMed] [Google Scholar]
- 98.European Association for the Study of the Liver. Electronic address eee, Clinical Practice Guideline Panel C, Panel m, representative EGB. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol 2019;70:1222–1261.30926241 [Google Scholar]
- 99.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 100.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of Hepatology 2014;60:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Francino MP. Antibiotics and the Human Gut Microbiome: Dysbiosesand Accumulation of Resistances. Frontiers in Microbiology 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bajaj JS, Liu EJ, Kheradman R, Fagan A, Heuman DM, White M, Gavis EA, et al. Fungal dysbiosis in cirrhosis. Gut 2018;67:1146–1154. [DOI] [PubMed] [Google Scholar]
- 103.Hirota SA. Understanding the Molecular Mechanisms of Rifaximin in the Treatment of Gastrointestinal Disorders--A Focus on the Modulation of Host Tissue Function. Mini Rev Med Chem 2015;16:206–217. [DOI] [PubMed] [Google Scholar]
- 104.Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, Distrutti E, Renga B, et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol 2010;80:1700–1707. [DOI] [PubMed] [Google Scholar]
- 105.Brigidi P, Swennen E, Rizzello F, Bozzolasco M, Matteuzzi D. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother 2002;14:290–295. [DOI] [PubMed] [Google Scholar]
- 106.Ponziani FR, Zocco MA, D’Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol 2017;23:4491–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu D, Gao J, Gillilland M 3rd, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014;146:484–496 e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071–1081. [DOI] [PubMed] [Google Scholar]
- 109.Orr JG, Currie CJ, Berni E, Goel A, Moriarty KJ, Sinha A, Gordon F, et al. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-alpha. Liver Int 2016;36:1295–1303. [DOI] [PubMed] [Google Scholar]
- 110.Hudson M, Radwan A, Di Maggio P, Cipelli R, Ryder SD, Dillon JF, Cash WJ, et al. The impact of rifaximin-alpha on the hospital resource use associated with the management of patients with hepatic encephalopathy: a retrospective observational study (IMPRESS). Frontline Gastroenterol 2017;8:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, Zhou H, et al. Rifaximin Exerts Beneficial Effects Independent of its Ability to Alter Microbiota Composition. Clin Transl Gastroenterol 2016;7:e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goel A, Rahim U, Nguyen LH, Stave C, Nguyen MH. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther 2017;46:1029–1036. [DOI] [PubMed] [Google Scholar]
- 113.Ibrahim ES, Alsebaey A, Zaghla H, Moawad Abdelmageed S, Gameel K, Abdelsameea E. Long-term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol 2017;29:1247–1250. [DOI] [PubMed] [Google Scholar]
- 114.Salehi S, Tranah TH, Lim S, Heaton N, Heneghan M, Aluvihare V, Patel VC, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Aliment Pharmacol Ther 2019;50:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito T, Nakamura K, Kageyama S, Korayem IM, Hirao H, Kadono K, Aziz J, et al. Impact of Rifaximin Therapy on Ischemia/Reperfusion Injury in Liver Transplantation: A Propensity Score-Matched Analysis. Liver Transpl 2019;25:1778–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang JY, Kim SE, Kim TH, Woo SY, Ryu MS, Joo YH, Lee KE, et al. Emergence of rifampin-resistant staphylococci after rifaximin administration in cirrhotic patients. PLoS One 2017;12:e0186120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology 2012;55:655–656. [DOI] [PubMed] [Google Scholar]
- 118.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013;8:e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, Krag A, et al. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: A randomized trial. J Gastroenterol Hepatol 2018;33:307–314. [DOI] [PubMed] [Google Scholar]
- 120.Zeng X, Tang XJ, Sheng X, Ni W, Xin HG, Chen WZ, Jiang CF, et al. Does low-dose rifaximin ameliorate endotoxemia in patients with liver cirrhosis: a prospective study. J Dig Dis 2015;16:665–674. [DOI] [PubMed] [Google Scholar]
- 121.Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, Rautou PE, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun 2018;9:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bosoi CR, Parent-Robitaille C, Anderson K, Tremblay M, Rose CF. AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct-ligated rats. Hepatology 2011;53:1995–2002. [DOI] [PubMed] [Google Scholar]
- 123.Zacharias HD, Zacharias AP, Gluud LL, Morgan MY. Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. Cochrane Database Syst Rev 2019;6:CD012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tripisciano C, Kozynchenko OP, Linsberger I, Phillips GJ, Howell CA, Sandeman SR, Tennison SR, et al. Activation-dependent adsorption of cytokines and toxins related to liver failure to carbon beads. Biomacromolecules 2011;12:3733–3740. [DOI] [PubMed] [Google Scholar]
- 125.De Chiara F, Li J, Lu W, Davies N, Mookerjee RP, Jalan R, Macnaughtan J. Characterization of the Protective Effects of Yaq-001 on Organ Injury in Cirrhosis. J Hepatol 2018;68:S463. [Google Scholar]
- 126.Macnaughtan J, Ranchal I, Soeda J, Sawhney R, Oben J, Davies N, Mookerjee R, et al. Oral Therapy with Non-Absorbable Carbons of Controlled Porosity (Yaq-001) Selectively Modulates Stool Microbiome and Its Function and This Is Associated with Restoration of Immune Function and Inflammasome Activation. Journal of Hepatology 2015;62:S240–S240. [Google Scholar]
- 127.Fuchs CD, Paumgartner G, Mlitz V, Kunczer V, Halilbasic E, Leditznig N, Wahlstrom A, et al. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2(−/−) mice by modulating composition, signalling and excretion of faecal bile acids. Gut 2018;67:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsuji Y, Kaji K, Kitade M, Kaya D, Kitagawa K, Ozutsumi T, Fujinaga Y, et al. Bile Acid Sequestrant, Sevelamer Ameliorates Hepatic Fibrosis with Reduced Overload of Endogenous Lipopolysaccharide in Experimental Nonalcoholic Steatohepatitis. Microorganisms 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takahashi S, Luo Y, Ranjit S, Xie C, Libby AE, Orlicky DJ, Dvornikov A, et al. Bile acid sequestration reverses liver injury and prevents progression of nonalcoholic steatohepatitis in Western diet-fed mice. J Biol Chem 2020;295:4733–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johnson AJ, Zheng JJ, Kang JW, Saboe A, Knights D, Zivkovic AM. A Guide to Diet-Microbiome Study Design. Front Nutr 2020;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saboo K, Shamsaddini A, Iyer MV, Hu C, Fagan A, Gavis EA, White MB, et al. Sex is associated with differences in gut microbial composition and function in hepatic encephalopathy. J Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kang DJ, Hylemon PB, Gillevet PM, Sartor RB, Betrapally NS, Kakiyama G, Sikaroodi M, et al. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatology Communications 2017;1:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, Eckmann L, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J 2015;29:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev 2016;30:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, et al. Modulation of the intestinal bile acid-FXR-FGF15 axis improves alcoholic liver disease in mice. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horvath A, Rainer F, Bashir M, Leber B, Schmerboeck B, Klymiuk I, Groselj-Strele A, et al. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci Rep 2019;9:12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, Hylemon PB, et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol 2018;113:1177–1186. [DOI] [PubMed] [Google Scholar]
- 138.Fernandez J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology 2016;63:2019–2031. [DOI] [PubMed] [Google Scholar]
- 139.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018;555:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, Zhang W, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun 2020;11:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bajaj JS, Matin P, White MB, Fagan A, Deeb JG, Acharya C, Dalmet SS, et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol 2018;315:G824–G837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abedon ST, Thomas-Abedon C. Phage therapy pharmacology. Curr Pharm Biotechnol 2010;11:28–47. [DOI] [PubMed] [Google Scholar]
- 143.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 2015;62:1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Santiago A, Pozuelo M, Poca M, Gely C, Nieto JC, Torras X, Roman E, et al. Alteration of the serum microbiome composition in cirrhotic patients with ascites. Sci Rep 2016;6:25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schierwagen R, Alvarez-Silva C, Madsen MSA, Kolbe CC, Meyer C, Thomas D, Uschner FE, et al. Circulating microbiome in blood of different circulatory compartments. Gut 2019;68:578–580. [DOI] [PubMed] [Google Scholar]
- 147.Ye F, Shen H, Li Z, Meng F, Li L, Yang J, Chen Y, et al. Influence of the Biliary System on Biliary Bacteria Revealed by Bacterial Communities of the Human Biliary and Upper Digestive Tracts. PLoS One 2016;11:e0150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M, Patel S, et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 149.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol 2017;15:600–602. [DOI] [PubMed] [Google Scholar]
- 150.Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol 2018;37:215–225. [DOI] [PubMed] [Google Scholar]
- 151.Kao D, Roach B, Park H, Hotte N, Madsen K, Bain V, Tandon P. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 2016;63:339–340. [DOI] [PubMed] [Google Scholar]
- 152.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017;66:1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mehta R, Kabrawala M, Nandwani S, Kalra P, Patel C, Desai P, Parekh K. Preliminary experience with single fecal microbiota transplant for treatment of recurrent overt hepatic encephalopathy-A case series. Indian J Gastroenterol 2018;37:559–562. [DOI] [PubMed] [Google Scholar]
- 154.Bajaj JS, Salzman N, Acharya C, Takei H, Kakiyama G, Fagan A, White MB, et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]


