Abstract

The absorption of SO2 from flue gas and its conversion to chemicals is important in the industry. Functional ionic liquids (ILs) have been broadly used to absorb SO2 in flue gas, but seldom convert it to chemicals. As we know, water is inevitable in a desulfurization process. In this work, three functional ILs (monoethanolaminium lactate-[MEA][Lac], 1,1,3,3-tetramethylguanidinium lactate-[TMG][Lac], tetraethylammonium lactate-[N2222][Lac]) with or without water were used as absorbents to absorb SO2 in flue gas, and then the absorbed SO2 in the absorbents was converted to sulfur via a Claus reaction. The result shows that the three ILs can efficiently absorb SO2 and convert it to sulfur. But the addition of water in the ILs can reduce the conversion of absorbed SO2, and the conversion increases with increasing the acidity of absorbents. To explain this phenomenon, we studied the Claus reaction in H2SO3, NaHSO3 and Na2SO3 aqueous solutions. It turns out that the conversion of the Claus reaction is related to the species of S (IV) in the order of the oxidability: H2SO3 > HSO3– > SO32–, and their proportions dependent on the pH of solutions. On the basis of the absorption mechanism of SO2 in functional ILs aqueous solution, H2S reacts with HSO3– and SO32– with weaker oxidability, resulting in the lower conversion. Importantly, we found that the addition of lactic acid could increase the conversion of SO2 via the Claus reaction.
1. Introduction
The emission of sulfur dioxide (SO2), which is mainly emitted from burning of coal, brings harm to not only the human body but also environmental safety.1,2 Traditional flue gas desulfurization technologies, for example, limestone scrubbing, are deficient in absorbents regeneration and SO2 utilization.3−5 Ionic liquids (ILs), which have many superior properties, have been widely studied in gas separation.6−11 Besides, ILs are promising reaction mediums because of their inherent catalytic reactivity for numerous reactions.12−14 Among them, functional ILs with some special groups can chemically absorb SO2 so that SO2 with low concentrations (like 0.2 vol %) in flue gas can be removed efficiently.15−21 Wang et al. summarized the solubility of low-concentration SO2 in various functional ILs.22 However, the chemical interactions between SO2 and functional ILs are quite strong, which make it difficult to be regenerated. Therefore, the desorption of SO2 from functional ILs by high temperature treatment usually needs high energy consumption.23 Moreover, high temperature treatment also causes decreasing stability of absorbents and increasing difficulty of separation. Huang et al.24 performed good work to use various common ILs as the solvents for the Claus reaction, which could achieve a conversion rate up to 99% at 40 °C. However, more commonly studied ILs can absorb SO2 with high solubility at high SO2 concentrations only by physical interaction. To change the traditional method, our research group put forward a new method to regenerate functional ILs by using the Claus reaction and achieved good results.25 The result shows that the SO2-absorbed functional ILs can be regenerated efficiently under a mild condition, and then SO2 from flue gas can be converted to sulfur for the comprehensive utilization. After several absorption and regeneration cycles, the absorbents still have high absorption capacity of SO2 and the conversion of SO2 via the Claus reaction does not decrease significantly. This new regeneration method is easy to operate and shows a good prospect in application.
In the previous work,25 it has been found that H2O has an effect on the liquid-phase Claus reaction. For monoethanolaminium lactate ([MEA][Lac]) used as an absorbent, the addition of H2O can reduce the conversion of absorbed SO2 via the Claus reaction, which is unfavorable to the regeneration process. However, no matter in the absorption or desorption processes, H2O is an important component. There is about 7 vol % H2O in flue gas at 40 °C, so absorbents contain a certain amount of water.26 In the Claus reaction, H2O is a product of the reaction. H2O is inevitable and always used as an environmentally friendly solvent in ILs to adjust the viscosity of absorbents. Therefore, it is very necessary to study the effect of water on the Claus reaction and explore the mechanism of the Claus reaction in aqueous solutions.
In this study, we used three common functional ILs ([MEA][Lac], 1,1,3,3-tetramethylguanidinium lactate-[TMG][Lac], tetraethylammonium lactate-[N2222][Lac], the structures of which are shown in Scheme 1) mixed with H2O to prepare the absorbents. After absorbing low-concentration SO2, the absorbents were regenerated by using H2S via the Claus reaction. The effect of H2O on the conversion of absorbed SO2 was investigated. To explore the mechanism of the Claus reaction in aqueous solutions more intuitively, we took H2SO3, NaHSO3, and Na2SO3 as examples to analyze whether the existence of S (IV) would affect the conversion of absorbed SO2 via the Claus reaction. After giving a supposition, we further explored the influence of existence of S (IV) to clarify the mechanism of the Claus reaction in the presence of water.
Scheme 1. Chemical Structures of [MEA][Lac], [TMG][Lac], and [N2222][Lac].
2. Results and Discussion
2.1. Absorption Reaction and Claus Reaction in Three Functional ILs and H2O Binary Systems
Three functional ILs ([MEA][Lac], [TMG][Lac], [N2222][Lac]) mixed with H2O were used as absorbents (mIL:mH2O = 1:1). And the absorption capacities of the three absorbents at 40.0 °C are shown in Table 1. It can be seen that the results are consistent with the previous work. For instance, the absorption capacity by [TMG][Lac] is 0.51 mol SO2/mol IL at a SO2 concentration of 2% and a water content of 7.3% in simulated flow gas.26 The absorption capacity by [N2222][Lac] is 0.791 mol SO2/mol IL with 3 vol % SO2 in flue gas at 60.0 °C.27
Table 1. SO2 Absorption Capacity in the Three Functional ILs Aqueous Solutions.
| absorption capacity of SO2a |
|||
|---|---|---|---|
| entry | absorbents (mIL:mH2O = 1:1) | g SO2/g abs | mol SO2/mol IL |
| 1 | [MEA][Lac]+H2O | 0.067 | 0.32 |
| 2 | [TMG][Lac]+H2O | 0.089 | 0.57 |
| 3 | [N2222][Lac]+H2O | 0.103 | 0.71 |
Absorption conditions: temperature, 40.0 °C; SO2 concentration, 2.0%.
According to ref (28), the absorption mechanism of SO2 in these functional ILs is that SO2 is absorbed physically and chemically. The physical absorption follows Henry’s law. The chemical absorption, taking [N2222][Lac] as an example, follows the reaction as shown in eq 1:29
| 1 |
It can be seen from Table 1 that the absorption of SO2 does not reach the stoichiometric ratios because of the low chemical equilibrium constant and the low concentration of SO2.
After that, we used the Claus reaction to regenerate the SO2-absorbed functional ILs aqueous solution by H2S. At 40.0 °C, the pressure of H2S in the chamber as a function of time in the three absorbents are shown in Figure 1a. Ethylene glycol (EG) is a nonaqueous solvent, and it is different from water as a solvent. Therefore, EG was used as a solvent for comparison with water. It is obvious that the reaction rate in aqueous solutions (Figure 1a) is slower than that in an EG solution25 (Figure 1b). Hence, the reaction in aqueous solutions needs a longer time to reach the equilibrium.
Figure 1.
Pressures of H2S as a function of time for the Claus reaction at 40.0 °C in different absorbents. (a) Black square, 50% [MEA][Lac] + 50% H2O; blue circle, 50% [TMG][Lac] + 50% H2O; red triangle, 50% [N2222][Lac] + 50% H2O. (b) Black square, 50% [MEA][Lac] + 50% EG; blue circle, 50% [TMG][Lac] + 50% EG; red triangle, 50% [N2222][Lac] + 50% EG. (b) is adapted with permission from ref (25). Copyright 2019 American Chemical Society.
The conversions of SO2 absorbed in the three absorbents via the Claus reaction are shown in Table 2. Moreover, we compared the conversion rate of SO2 in the three systems, and there was a significant decline in an aqueous solution compared with those in pure ILs and EG solutions. However, compared with EG as the solvent, the dissolution phenomenon of sulfur in aqueous absorbents was very low, and the resulted sulfur could be separated easily by centrifugation and filtration. One possible reason might be the very low solubility of sulfur in aqueous solutions, inhibiting the sulfur dissolution in absorbents to a certain extent.
Table 2. Recovery Ratios of Sulfur in the Three Functional ILs with Different Solventsa.
|
RS |
|||
|---|---|---|---|
| ILs | pure ILs | mIL:mEG = 1:1 | mIL:mH2O = 1:1 |
| [MEA][Lac] | 95.4 ± 1.2 | 96.4 ± 1.1 | 72.4 ± 1.2 |
| [TMG][Lac] | 92.5 ± 1.1 | 91.8 ± 1.1 | 85.7 ± 1.1 |
| [N2222][Lac] | 91.2 ± 1.0 | 90.3 ± 1.2 | 78.7 ± 1.2 |
The conditions of the Claus reaction: temperature, 40.0 °C; initial pressure of H2S, 1.1 MPa.
2.2. Effect of Temperature and H2O Mass Fraction on the Claus Reaction
Taking the [MEA][Lac] aqueous solution as an example, we studied the effect of temperature and H2O mass fraction on the Claus reaction. The conversions of SO2 in [MEA][Lac] + H2O (m[MEA][Lac]:mH2O = 1:1) at 40.0, 50.0, and 60.0 °C are shown in Table 3. As can be seen from Table 3, the conversion decreases as the temperature increases, which is consistent with those in ILs and EG binary systems.25 The results demonstrated that the low temperatures would be beneficial to the liquid-phase Claus reaction. This result can satisfy the desired requirement of using the Claus reaction to regenerate functional ILs, that is, the temperatures of absorption and regeneration are identical, saving energy.
Table 3. Recovery Ratios of Sulfur in [MEA][Lac] + H2O (m[MEA][Lac]:mH2O = 1:1) at Different Temperatures.
| entry | IL | solvent | T/°C | PH2S/MPa | RS/% |
|---|---|---|---|---|---|
| 1 | [MEA][Lac] | H2O | 40.0 | 1.10 | 72.4 ± 1.2 |
| 2 | [MEA][Lac] | H2O | 50.0 | 1.10 | 70.6 ± 1.1 |
| 3 | [MEA][Lac] | H2O | 60.0 | 1.10 | 69.2 ± 1.0 |
In our previous work, the mass fraction of EG had no obvious influence on the conversion of SO2 via the Claus reaction. To investigate whether the content of water has an effect on the conversion, we changed the mass fraction of H2O in the absorbents to do a series of experiments. Table 4 shows the conversion of SO2 using the Claus reaction in [MEA][Lac] aqueous solutions with different water contents. According to the results in Table 4, as water contents increased, the conversion decreased. As reported in the literature,29,30 the chemical equation of the Claus reaction is shown in eq 2. Therefore, a possible reason might be that the Claus reaction can generate water, and the increase of water content can move the reverse direction of the reversible chemical reaction:
| 2 |
Table 4. Recovery Ratios of Sulfur in [MEA][Lac] + H2O with Different Mass Fractions of H2O.
| entry | IL | solvent (mass fraction) | T/°C | PH2S/MPa | RS/% |
|---|---|---|---|---|---|
| 1 | [MEA][Lac] | 40.0 | 1.10 | 95.4 ± 1.2 | |
| 2 | [MEA][Lac] | H2O (25%) | 40.0 | 1.10 | 86.8 ± 1.1 |
| 3 | [MEA][Lac] | H2O (50%) | 40.0 | 1.10 | 72.4 ± 1.2 |
2.3. Study on the Claus Reaction in H2SO3, NaHSO3, and Na2SO3 Aqueous Solutions
In the previous subsections, it can be found that the addition of water can reduce the conversion of SO2 in functional ILs aqueous solution via the Claus reaction. However, water is inevitable in industrial application, and it has a certain influence on the absorption and regeneration stages of functional ILs. As is known, the absorbed SO2 in functional ILs aqueous solution mainly exists as H2SO3, HSO3–, and SO32–. Actually, in the liquid-phase Claus reaction, the three species of S (IV) react with H2S, as shown in eq 3:
| 3 |
To study the effect of water on the Claus reaction, we used H2SO3, NaHSO3, and Na2SO3 aqueous solutions to study the mechanism of the Claus reaction.
The concentration of S (IV) in three aqueous solution is 1.0 mol/L, and the experimental method is the same as that in functional ILs. The conversion was calculated by the ratio of the generated sulfur to the theoretical sulfur. Table 5 shows the conversion of SO2 via the Claus reaction at 40.0 °C. It is very interesting that the conversions have great differences at the same concentrations of S (IV). It should be noted that the conversion is almost zero in the Na2SO3 aqueous solution. We found that the oxidability of three chemical substances varied from pH values of aqueous solutions, and the oxidability order is H2SO3 (SO2) > HSO3– > SO32–, which is in consistence with their standard reduction potentials, 0.45 V, −0.19 V, and −0.90 V, respectively (data were obtained from the refs (31 and 32) and calculated according to the thermodynamics law). The Claus reaction is a redox reaction and the conversion of S (IV) species is directly affected by its oxidability. Therefore, due to its low oxidability, SO32– cannot react with H2S, resulting in its low conversion.
Table 5. Recovery Ratios of Sulfur in H2SO3, NaHSO3, and Na2SO3 Aqueous Solutionsa.
| entry | aqueous solution | concentration of S species (based on SO2)/mol/L | RS/% |
|---|---|---|---|
| 1 | H2SO3 | 1.0 | 78.6 ± 1.1 |
| 2 | NaHSO3 | 1.0 | 37.3 ± 1.0 |
| 3 | Na2SO3 | 1.0 | 0 |
The conditions of the Claus reactions: the total concentration of H2SO3, NaHSO3, and Na2SO3, 1 mol/L, initial pressure of H2S, 1.1 MPa, temperature, 40.0 °C.
As is known, there is an ionization equilibrium in H2SO3, HSO3–, and SO32– aqueous solution, and the pH of the solution is the determining factor. Table 6 shows the mole fractions of H2SO3, HSO3–, and SO32– at different pH, which were calculated by ionization and hydrolysis equilibrium constant.
Table 6. Mole Fractions of H2SO3, HSO3–, and SO32– at Different pH in Aqueous Solution at 25 °C.
| pHa | δ(H2SO3)/% | δ(HSO3–)/% | δ(SO32–)/% |
|---|---|---|---|
| 1 | 87.0 | 13.0 | 0 |
| 2 | 40.0 | 60.0 | 0 |
| 3 | 6.3 | 93.7 | 0 |
| 4 | 0.7 | 99.2 | 0.1 |
| 5 | 0.1 | 98.9 | 1.0 |
| 6 | 0 | 90.9 | 9.1 |
| 7 | 0 | 50.0 | 50.0 |
| 8 | 0 | 9.1 | 90.9 |
The pH values of aqueous solutions were adjusted by HCl and NaOH. The total concentration of H2SO3, HSO3–, and SO32– is 1 mol/L, and the initial pressure of H2S is 1.1 MPa.
As can be seen in Table 6, the mole fraction of H2SO3 decreases as the pH increases or the acidity decreases. When pH value of the aqueous solution is 1, the mole fraction of H2SO3 is 87.0%; when pH value of the aqueous solution is 6 and higher, there is no H2SO3. By the contract, the mole fraction of HSO3– first increases and then decreases as the pH increases or the acidity decreases. The mole fraction of HSO3– is 13.0% at a pH value of 1, and it increases to a maximum value of 99.2% at a pH value of 4 and decreases to 9.1% at a pH value of 8. Moreover, S (IV) mainly exists in the form of HSO3– with mole fractions more than 90% between 3 and 6 of the pH values. Thus, we took the NaHSO3 aqueous solution as an example to study the effect of pH on the Claus reaction. Table 7 shows the pH value of the NaHSO3 aqueous solution before and after the Claus reaction and its recovery ratio of sulfur at 40.0 °C.
Table 7. pH Value of NaHSO3 Aqueous Solution before and after the Claus Reaction and Its Recovery Ratio of Sulfura.
| entry | pH (before the Claus reaction) | pH (after the Claus reaction) | RS/% |
|---|---|---|---|
| 1 | 2.12 | 7.02 | 49.9 ± 1.0 |
| 2 | 3.52 | 7.21 | 37.3 ± 1.2 |
| 3 | 5.05 | 7.96 | 34.8 ± 1.1 |
| 4 | 5.95 | 7.82 | 19.7 ± 1.0 |
| 5 | 7.07 | 7.34 | 0.2 ± 1.1 |
The conditions of the Claus reaction: the concentration of NaHSO3, 1 mol/L; initial pressure of H2S, 1.1 MPa; the temperature, 40.0 °C.
As can be seen in Table 7, the pH values after the reaction are between 7 and 8 no matter the pH value before the Claus reaction. As the pH value before the Claus reaction increases from 2.12 to 7.07, the recovery ratio of sulfur decreases from 49.9% to 0.2%. Two conclusions can be drawn from Table 7. The first one is that the Claus reaction is accompanied by the consumption of H2SO3 and HSO3–, and then the content of SO32– increases after the Claus reaction. Hence, the acidity of the solution decreases as the pH values are between 7.02 and 7.96. The second one is that the stronger the acidity of the solution, the higher the conversion of SO2 via the Claus reaction as the recovery ratio of sulfur is the highest at the lowest pH value of 2.12, which indicates that an acidic aqueous solution is more beneficial to the Claus reaction.
2.4. Effect of pH of Absorbents on the Claus Reaction
According to the above results, it can be found that the pH of the aqueous solution can affect the species of S (IV), which can then further affect the conversion rate of the Claus reaction. Whether the conclusions are applicable to the functional ILs, aqueous solution needs further experimental exploration.
First of all, we investigated the effect of the H2SO4 on the pH of [TMG][Lac] aqueous solution (w[TMG][Lac) = 60%), SO2 absorption capacity, and the conversion of SO2 via the Claus reaction. Table 8 shows the absorption capacity of SO2 (2 vol %) and the conversion of SO2 via the Claus reaction at 40.0 °C. It can be concluded from Table 8 that the conversion increases with the pH value decrease. However, there are two points to which attention should be paid. First, with the increase of H2SO4 content above 2%, the conversion increases to about 91% and remains unchanged. Second, the change of the pH of the absorbents by adding H2SO4 can improve the conversion of SO2 via the Claus reaction but also reduce the absorption capacity of SO2. Therefore, it is necessary to increase the conversion by adding an acid in a proper range.
Table 8. Effect of Amount of Added H2SO4 on SO2 Absorption and the Claus Reaction in [TMG][Lac] Aqueous Solutiona.
| w (H2SO4) | pHabs | absorption capacity (g SO2/g abs) | RS/% |
|---|---|---|---|
| 0 | 8.20 | 0.120 | 86.1 ± 1.0 |
| 2% | 5.85 | 0.101 | 91.2 ± 1.0 |
| 4% | 5.37 | 0.086 | 91.4 ± 1.1 |
| 6% | 5.00 | 0.074 | 91.2 ± 1.2 |
| 8% | 4.59 | 0.055 | 91.6 ± 1.0 |
| 10% | 4.29 | 0.039 | 91.1 ± 1.1 |
The concentration of [TMG][Lac] in aqueous solutions is 60 wt % (w[TMG][Lac] = 60%), the concentration of SO2 is 2 vol %, the temperature of SO2 absorption of is 40 °C, and the temperature of the Claus reaction is 40 °C.
However, H2SO4 is a kind of inorganic strong acid, and it has some interference to the system; thus, we used lactic acid to adjust the acidity of absorbents. Specifically, the functional ILs were synthesized with different mole ratios of MEA (or TMG) and lactic acid. SO2 absorption capacity and recovery ratios of the Claus reaction in [MEA][Lac] and [TMG][Lac] aqueous solutions with different amounts of added lactic acid are shown in Tables 9 and 10. The concentration of SO2 was 2 vol %, and the temperatures of SO2 absorption and the Claus reaction were both 40.0 °C.
Table 9. Effect of nHLac:n[MEA][Lac] on SO2 Absorption and the Claus Reaction in [MEA][Lac] Aqueous Solutiona.
| nHLa:n[MEA][Lac] | absorption capacity (g SO2/g abs) | RS/% |
|---|---|---|
| 0:1 | 0.067 | 72.4 ± 1.1 |
| 0.1:1 | 0.053 | 75.3 ± 1.0 |
| 0.2:1 | 0.050 | 77.2 ± 1.2 |
| 0.3:1 | 0.040 | 78.0 ± 1.0 |
| 0.4:1 | 0.035 | 81.2 ± 1.1 |
The concentration of [MEA][Lac] in aqueous solutions is 50 wt %, the concentration of SO2 is 2 vol %, the temperature of SO2 absorption is 40 °C, and the temperature of the Claus reaction is 40 °C.
Table 10. Effect of nHLac:n[TMG][Lac] on SO2 Absorption and the Claus Reaction in [TMG][Lac] Aqueous Solutiona.
| nHLac:n[TMG][Lac] | absorption capacity (g SO2/g abs) | RS/% |
|---|---|---|
| 0:1 | 0.089 | 85.7 |
| 0.05:1 | 0.081 | 85.7 |
| 0.1:1 | 0.078 | 85.7 |
| 0.2:1 | 0.073 | 87.3 |
| 0.3:1 | 0.069 | 88.1 |
The concentration of [TMG][Lac] in aqueous solutions is 50 wt %, the concentration of SO2 is 2 vol %, the temperature of SO2 absorption is 40.0 °C, and the temperature of the Claus reaction is 40.0 °C.
Through the exploration of effect of pH on the Claus reaction in functional ILs aqueous solutions, it can be found that the addition of lactic acid increases the conversion of SO2 via the Claus reaction, but the increase is limited. Moreover, the SO2 absorption capacity decreases with the addition of lactic acid, but the decrease is not significant compared to the addition of H2SO4.
2.5. Stoichiometry of the Claus Reaction in Functional Ionic Liquids Aqueous Solution
Through the above research, it can be found that the stoichiometry of the Claus reaction is different in the presence of water or the no-water case. We suppose that it is mainly due to the different absorption mechanism in the two cases.
Taking [MEA][Lac] as an example, the stoichiometry of SO2 absorption in absence of water is shown in Figure 2. It is speculated that the Claus reaction mechanism in [MEA][Lac] in the absence of H2O is shown in Figure 3.33
Figure 2.

Stoichiometry of [MEA][Lac] absorbing SO2.
Figure 3.
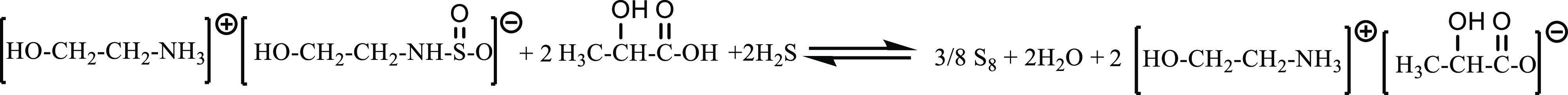
Stoichiometry of the Claus reaction in [MEA][Lac] after absorbing SO2.
In the presence of water, the stoichiometry of [MEA][Lac] absorbing SO2 is shown in eqs 4 and 5:27,29
| 4 |
| 5 |
The stoichiometry of the Claus reaction in [MEA][Lac] aqueous solution is shown in eq 6:
| 6 |
It can be seen from the above analysis whether the species of SO2 after absorption are different in the presence of water or not, which results in a different mechanism of the Claus reaction. In an aqueous solution, the absorbed SO2 exists in species of H2SO3, HSO3–, and SO32–. There is an equilibrium of ionization and hydrolysis between the three substances, which is mainly affected by pH of the solution. With the consumption of H+ during the Claus reaction, the alkalinity of the solution gradually increases, and the absorbed SO2 mainly exists in SO32–, which cannot react with H2S. However, in the absence of water, SO2 is directly absorbed by ILs to form IL-SO2. During the Claus reaction, the chemical bond between SO2 and IL is broken, and the SO2 reacts with H2S. In the process, the existing form of SO2 does not change after being absorbed, and its ability of reacting with H2S does not decrease. Instead, the pure IL environment provides a unique ionic environment, which has certain catalysis effect on the Claus reaction. Therefore, the presence of water influences not only the reaction rate but also the conversion of SO2 via the Claus reaction of H2S with SO2 absorbed in functional ILs. However, it has been found that the addition of lactic acid increases the conversion of SO2 via the Claus reaction. This work provides the information on the Claus reaction for the absorbed SO2 not only by functional ILs but also by functional deep eutectic solvents in the presence of water.
3. Conclusions
In this work, we mixed water with three functional ILs to yield aqueous absorbents of [MEA][Lac] + H2O, [TMG][Lac] + H2O, and [N2222][Lac] + H2O, and studied the effect of water on the Claus reaction in three absorbents due to the presence of water in flue gas and then in absorbents. The result shows that the addition of water into ILs can reduce the conversion of absorbed SO2, and the conversion increases as the acidity of absorbents increases. To explain this phenomenon, the Claus reaction was performed in H2SO3, NaHSO3, and Na2SO3 aqueous solutions. It turns out that the conversion of SO2 via the Claus reaction is related to the species of S (IV), the conversion rate, H2SO3 > HSO3– > SO32–, and their proportions dependent on the pH of solutions. On the basis of the absorption mechanism of SO2 in functional ILs aqueous solution, H2S reacts with HSO3– and SO32– with weaker oxidability, resulting in the lower conversion rate.
4. Experimental Section
4.1. Materials
SO2 (99.95%), H2S (99.95%), and N2 (99.99%) were obtained from Beijing Beiwen Gases Co., Ltd. (Beijing, China). Simulated flue gas with SO2 (2%) was prepared by mixing SO2 and N2 in a 40 dm3 high-pressure cylinder. Analytical reagent 1,1,3,3-tetramethylguanidine (99%), tetraethylammonium hydroxide (25% in water), ethylene glycol (EG), and lactic acid (85%–90% in water) were obtained from Aladdin Chemical Co., Ltd. (Shanghai, China). Monoethanolamine (99%) was purchased from Alfa Aesar (China) Chemicals Co. Ltd., (Beijing, China). NaOH (97%), H2SO3, NaHSO3, and Na2SO3 were purchased from Beijing Chemical Works (Beijing, China). All reactants and solvents were A.R. grade.
[MEA][Lac], [TMG][Lac], and [N2222][Lac] with different mass fractions of water or EG were synthesized and characterized following the literature.34 The water contents in the absorbents were determined by Karl Fischer titration (Leici ZDY-502, China).
4.2. Absorption of Low-Concentration SO2 in Absorbents
The schematic diagram of the apparatus is shown in Figure 4. The simulated flue gas with 2% SO2 with a flow of 100 cm3/min was bubbled through water and absorbents successively to offset the reduction of water contents of absorbents. The concentrations of SO2 in absorbents were analyzed using an iodine titration method (HJ/T 56–2000, a standard method of the State Environmental Protection Administration of China). After a period of absorption, the concentration of SO2 no longer changed, meaning that the absorbents were saturated.
Figure 4.
Schematic diagram of the apparatus for ILs to absorb SO2 from a flue gas stream. 1, Simulated flue gas cylinder; 2, pressure reducing value; 3, rotameter; 4, glass tube with H2O; 5, glass tube with absorbents; 6, tail gas absorption device; 7, water bath.
4.3. Regeneration of Absorbents via the Claus Reaction
The regeneration method was the same as that reported in the literature.25 Briefly, the regeneration reaction was carried out in a stainless-steel chamber (25.641 dm3) equipped with a temperature sensor (±0.1 °C), a pressure sensor (±0.01 MPa), and a magnetic stirrer. A sample of SO2-absorbed functional IL with or without water was loaded in the chamber, and the air in the chamber was removed using N2. H2S was charged into the chamber to a desired pressure, and then the Claus reaction was started. The pressure in the chamber was recorded at certain time intervals until the pressure remained constant, indicating that the reaction reached its equilibrium.
The conversion of SO2 in the Claus reaction was calculated via the recovery ratio of sulfur (RS). After the Claus reaction, pure sulfur was obtained by centrifugation, washing, and drying. Because of the physical absorption of H2S, the residual H2S in the regenerated absorbents was easily removed by a decompression method, and then it could be used for SO2 absorption.
Acknowledgments
The Long-Term Subsidy Mechanism from the Ministry of Finance and the Ministry of Education of PRC (BUCT) is acknowledged. The project is supported financially by the National Natural Science Foundation of China (Nos. 21176020 and 21306007).
The authors declare no competing financial interest.
References
- Chen S.; Li Y.; Yao Q. The health costs of the industrial leap forward in China: Evidence from the sulfur dioxide emissions of coal-fired power stations. China Econom. Rev. 2018, 49, 68–83. 10.1016/j.chieco.2018.01.004. [DOI] [Google Scholar]
- Sousa J. M. M. V.; Sintra T. E.; Ferreira A. G. M.; Carvalho P. J.; Fonseca I. M. A. Solubility of H2S in ammonium-based ionic liquids. J. Chem. Thermodyn. 2021, 154, 106336. 10.1016/j.jct.2020.106336. [DOI] [Google Scholar]
- Lancia A.; Musmarra D.; Prisciandaro M.; Tammaro M. Catalytic oxidation of calcium bisulfite in the wet limestone-gypsum flue gas desulfurization process. Chem. Eng. Sci. 1999, 54, 3019–3026. 10.1016/S0009-2509(98)00483-7. [DOI] [Google Scholar]
- Hansen B. B.; Kiil S.; Johnsson J. E.; Sønder K. B. Foaming in wet flue gas desulfurization plants: the influence of particles, electrolytes, and buffers. Ind. Eng. Chem. Res. 2008, 47, 88–91. 10.1021/ie071660g. [DOI] [Google Scholar]
- Ma X.; Kaneko T.; Tashimo T.; et al. Use of limestone for SO2 removal from flue gas in the semidry FGD process with a powder-particle spouted bed. Chem. Eng. Sci. 2000, 55, 4643–4652. 10.1016/S0009-2509(00)00090-7. [DOI] [Google Scholar]
- Bates E. D.; Mayton R. D.; Ntai I.; et al. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. 10.1021/ja017593d. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Xia G.; Song Y.; Zhu Z.; Li H.; Shi W.; Fang D. Investigation on Protic Ionic Liquids as Physical Solvents for Absorption of NO at Low Pressures. ACS Omega 2021, 6, 28297–28306. 10.1021/acsomega.1c04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Zhao T.; Yang A.; Liu F. Highly Efficient Absorption of CO2 by Protic Ionic Liquids-Amine Blends at High Temperatures. ACS Omega 2021, 6, 34027–34034. 10.1021/acsomega.1c05416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q.; Chen C.; Xing F.; Shi W.; Meng J.; Wan H.; Guan G. Constructing Hierarchically Porous N-Doped Carbons Derived from Poly(ionic liquids) with the Multifunctional Fe-Based Template for CO2 Adsorption. ACS Omega 2021, 6, 7186–7198. 10.1021/acsomega.1c00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao F.-F.; Zhou Y.; Zhu W. S.; SAng X.-Y.; Li Z.-M.; Tao D.-J. Synthesis of Guanidinium-Based Poly(ionic liquids) with Nonporosity for Highly Efficient SO2 Capture from Flue Gas. Ind. Eng. Chem. Res. 2021, 60, 5984–5991. 10.1021/acs.iecr.1c01118. [DOI] [Google Scholar]
- Geng Z. Y.; Xie Q.; Fan Z. J.; Sun W. N.; Zhao W.; Zhang J. H.; Chen J. Q.; Xu Y. Investigation of tertiary amine-based PILs for ideal efficient SO2 capture from CO2. J. Environ. Chem. Eng. 2021, 9, 105824. 10.1016/j.jece.2021.105824. [DOI] [Google Scholar]
- Cole A. C.; Jensen J. L.; Ntai I.; Tran K. L. T.; Weaver K. J.; Forbes D. C.; Davis J. H. Novel Brønsted acidic ionic liquids and their use as dual solvent-catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. 10.1021/ja026290w. [DOI] [PubMed] [Google Scholar]
- Dupont J.; de Souza R. F.; Suarez P. A. Z. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. 10.1021/cr010338r. [DOI] [PubMed] [Google Scholar]
- Ranu B. C.; Banerjee S. Ionic liquid as catalyst and reaction medium. The dramatic influence of a task-specific ionic liquid, [bmIm]OH, in Michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles. Org. Lett. 2005, 7, 3049–52. 10.1021/ol051004h. [DOI] [PubMed] [Google Scholar]
- Ren S. H.; Hou Y. C.; Zhang K.; Wu W. Z. Ionic liquid: functionalization and absorption of SO2. Green Energy Environm. 2018, 3, 179–190. 10.1016/j.gee.2017.11.003. [DOI] [Google Scholar]
- Yan S. R.; Han F.; Hou Q. N.; Zhang S.; Ai S. Recent advances in ionic liquid-mediated SO2 capture. Ind. Eng. Chem. Res. 2019, 31, 13804–13818. 10.1021/acs.iecr.9b01959. [DOI] [Google Scholar]
- Wu W. Z.; Han B. X.; Gao H. X.; Liu Z. M.; Jiang T.; Huang J. Desulfurization of flue gas: SO2 absorption by an ionic liquid. Angew. Chem., Int. Ed. 2004, 43, 2415–2417. 10.1002/anie.200353437. [DOI] [PubMed] [Google Scholar]
- Mao F.-F.; Zhou Y.; Zhu W.; Sang X.-Y.; Li Z.-M.; Tao D.-J. Synthesis of Guanidinium-Based Poly(ionic liquids) with Nonporosity for Highly Efficient SO2 Capture from Flue Gas. Ind. Eng. Chem. Res. 2021, 60 (16), 5984–5991. 10.1021/acs.iecr.1c01118. [DOI] [Google Scholar]
- Cui G. K.; Lyu S. Z.; Wang H. Y.; Li Z. Y.; Zhang R. N.; Wang J. J. Tuning the structure of pyridinolate-based functional ionic liquids for highly efficient SO2 absorption. Fuel 2021, 303, 121311. 10.1016/j.fuel.2021.121311. [DOI] [Google Scholar]
- Li D.; Kang Y.; Li J.; Wang Z. C.; Yan Z. N.; Sheng K. Chemically tunable DILs: Physical properties and highly efficient capture of low-concentration SO2. Sep. Purif. Technol. 2020, 240, 116572. 10.1016/j.seppur.2020.116572. [DOI] [Google Scholar]
- Jiang B.; Hou S.; Zhang L. H.; Yang N.; Zhang N.; Xiao X. M.; Yang X. D.; Sun Y. L.; Tantai X. W. Ether-Linked Diamine Carboxylate Ionic Liquid Aqueous Solution for Efficient Absorption of SO2. Ind. Eng. Chem. Res. 2020, 59, 16786–16794. 10.1021/acs.iecr.0c02877. [DOI] [Google Scholar]
- Jiang L. L.; Mei K.; Chen K. H.; Dao R. N.; Li H. R.; Wang C. M. Design and prediction for highly efficient SO2 capture from flue gas by imidazolium ionic liquids. Green Energy Environ. 2022, 7, 130–136. 10.1016/j.gee.2020.08.008. [DOI] [Google Scholar]
- Wang C. M.; Zheng J. J.; Cui G. K.; Luo X.; Guo Y.; et al. Highly efficient SO2 capture through tuning the interaction between anion-functionalized ionic liquids and SO2. Chem. Commun. 2013, 12, 1166–1168. 10.1039/C2CC37092A. [DOI] [PubMed] [Google Scholar]
- Huang K.; Feng X.; Zhang X. M.; Wu Y. T.; Hu X. B. The ionic liquid-mediated Claus reaction: a highly efficient capture and conversion of hydrogen sulfide. Green Chem. 2016, 18, 1859–1863. 10.1039/C5GC03016A. [DOI] [Google Scholar]
- Zhang Q.; Hou Y. C.; Ren S. H.; Zhang K.; Wu W. Z. Efficient regeneration of SO2-absorbed functional ionic liquids with H2S via the liquid-phase Claus reaction. ACS Sustainable Chem. Eng. 2019, 7, 10931–10936. 10.1021/acssuschemeng.9b01933. [DOI] [Google Scholar]
- Ren S. H.; Hou Y. C.; Wu W. Z.; Chen X. T.; Zhang J. W.; et al. Effect of H2O on the desulfurization of simulated flue gas by an ionic liquid. Ind. Eng. Chem. Res. 2009, 48, 4928–4932. 10.1021/ie9000844. [DOI] [Google Scholar]
- Tian S.; Hou Y.; Wu W.; Ren S.; Zhang C. Absorption of SO2 by thermal-stable functional ionic liquids with lactate anion. RSC Adv. 2013, 3, 3572–3577. 10.1039/c3ra22450c. [DOI] [Google Scholar]
- Jin M. J.; Hou Y. C.; Wu W. Z.; Ren S. H.; Tian S. D.; Xiao L.; Lei Z. Solubilities and thermodynamic properties of SO2 in ionic liquids. J. Phys. Chem. B 2011, 115, 6585–6591. 10.1021/jp1124074. [DOI] [PubMed] [Google Scholar]
- Tian S. D.; Hou Y. C.; Wu W. Z.; Ren S. H.; et al. Absorption of SO2 at high temperatures by ionic liquids and the absorption mechanism. Bull. Korean Chem. Soc. 2014, 9, 2791–2796. 10.5012/bkcs.2014.35.9.2791. [DOI] [Google Scholar]
- Wang H.; Dalla Lana I. G.; Chuang K. T. Kinetics of Reaction between Hydrogen Sulfide and Sulfur Dioxide in Sulfuric Acid Solutions. Ind. Eng. Chem. Res. 2002, 41, 4707–4713. 10.1021/ie020275i. [DOI] [Google Scholar]
- Lide D. R.Hand Book of Phystry and Chemistry, 85th ed.; CRC Press: Boca Raton, FL, 2004. [Google Scholar]
- Zhang Q. L.; Yao F. Y.; Guo D. W.; Gui M. D.. Inorganic Chemistry: Oxygen, Sulfur, and Selenium Group; Science Press: Beijing, China, 1990; Vol. 5. [Google Scholar]
- Zhao J. H.; Ren S. H.; Hou Y. C.; Zhang K.; Wu W. Z. SO2 absorption by carboxylate anion-based task-specific ionic liquids: effect of solvents and mechanism. Ind. Eng. Chem. Res. 2016, 50, 12919–12928. 10.1021/acs.iecr.6b02801. [DOI] [Google Scholar]
- Zhang K.The design of functional deep eutectic solvents and their applications in the absorption of SO2 from flue gas; Beijing University of Chemical Technology: Beijing, China, 2019. [Google Scholar]





