Abstract

Herein, we report a green and efficient synthetic route for the construction of diverse functionalized coumarins in good-to-excellent yields (60–98%) via the Pechmann condensation. The optimized synthetic route involves a biodegradable, reusable, and inexpensive deep eutectic solvent (DES) of choline chloride and l-(+)-tartaric acid in a ratio of 1:2 at 110 °C. Interestingly, phloroglucinol and ethyl acetoacetate, upon reaction, furnished the functionalized coumarin (20) in 98% yield within 10 min. On the other front, the same DES at relatively lower reaction temperature (90 °C) was found to provide the bis-coumarins in decent yields (81–97%) within 20–45 min. Moreover, this particular method was found to be quite effective for large-scale coumarin synthesis without noteworthy reduction in the yields of the desired products. Noticeably, in this versatile approach, the DES plays a dual role as solvent as well as catalyst, and it was effectively recycled and reused four times with no significant drop-down in the yield of the product.
Introduction
Coumarins are notable members of the benzopyrone class of organic compounds, which have recently attracted a great awareness from chemists and biologists across the globe because of their interesting photochemical/photophysical assets1−4 and substantial biological activities such as antioxidant,5 anticoagulant, anti-HIV,6 anticancer,7 antimicrobial,8 antiviral, anti-inflammatory,9 and anticonvulsant.10 Moreover, they have also been employed as additives in food, cosmetics, perfumes, agrochemicals, and pharmaceuticals.11,12 Taking note of the indispensable features of the coumarin backbone, development of new coumarin-based pharmacological drugs and fabrication of optical organic devices (lasers, solar cells, etc.) has become a necessity in the current environment.13−18 On the other hand, coumarin and its congeners by virtue of acting as vital fluorescent probes also exhibit huge potential in diagnostics and drug delivery developments and are thus considered as privileged structures in the biomedical arena.19,20 In fact, their simplicity, peculiar chemical properties, and the efficiency in their synthesis make them highly appealing and interesting for both medicinal and biological chemists.21,22 The structures of some vital biologically active coumarin derivatives are listed here (Figure 1).
Figure 1.
Diverse functionalized coumarins (1–7) of therapeutic significance.
In the recent past, synthesis of the functionalized coumarins has received interest from both synthetic as well as medicinal chemists, which can be underscored by the involvement of diverse named reactions like the Perkin reaction, Claisen rearrangement, Knoevenagel condensation, Reformatsky reaction, Wittig reaction, Kostanecki–Robinson reaction, Pechmann condensation, and so forth in their ease of construction.23−30 Among these methods, the Pechmann condensation discovered by Hans von Pechmann in 1883 is well known, which leads to the acid-catalyzed preparation of substituted coumarins from commercially available starting materials (i.e., substituted phenols and β-ketoesters/acids). The acid catalysts utilized for the Pechmann condensation reaction are concd H2SO4, trifluoroacetic acid (TFA), P2O5, TiCl4, ZrCl4, phosphotungstic acid, Nafion resin, SnCl2·2H2O, HClO4/SiO2, zirconium phosphotungstate, and ionic liquids, etc.31−42 In the context of environmental impact, most of these procedures suffer from one or more serious drawbacks like the use of a stoichiometric amount of costly catalyst, production of large acidic wastes, low yields, no reusability of catalyst, and prolonged reaction time. Consequently, there is an urgent need to develop simple, efficient, high-yielding, and eco-friendly Pechmann condensation tactics, where reusable mild acidic catalysts without involving any hazardous volatile organic solvent could be taken into the consideration.
In the modern era, a major focus of the scientific community is toward the development of green and/or sustainable protocols with the prospect of fostering straightforward yet efficient pathways which not only are economical but also safe for both mankind and the environment. These routes, in turn, avoid the involvement of hazardous volatile organic solvents and corrosive catalysts and also eliminate the generation of nonbiodegradable waste products during the reaction under consideration.43−45 Among various sustainable alternatives,46−51 the deep eutectic solvents (DESs) have gained a the attention of researchers worldwide after their discovery by Abbott and teammates in 2003 and the subsequent decoding of some interesting properties.52 These DESs have been utilized in a myriad of vital synthetic transformations by various research groups. For instance, Tavakol and co-workers have used DESs in the preparation of xanthene derivatives, propargylamines, and 4-aminobenzofurans.53−55 The DESs are generally formed by virtue of complexation between hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs), which are mostly obtained from natural sources and are gifted with numerous interesting characteristics such as being biocompatible, biodegradable, renewable, inexpensive, inflammable, atom economical, thermally stable, etc., besides displaying a dual/triple role (catalyst, solvent, and/or reactant) during the reaction.56−66 The Pechmann condensation for the synthesis of coumarin and its derivatives has already been reported involving ionic liquids (ILs) as reaction media.67−78 Generally, ILs are being considered as the green reaction systems, but actually they are not. This is due to the fact that ILs possesses some serious issues such as huge cost, difficult preparation, and environmental unfriendliness, as they are generally produced from hazardous starting materials involving conventional organic solvents and/or catalysts. Thus, there is an immense urgency to gather diverse functionalized coumarins via Pechmann condensation under environmentally benign conditions having the credentials of green chemistry.
Keeping the remarkable properties of DESs in mind, and also as our major research program in the construction of simple yet architecturally interesting molecules79−86 employing green protocols,87−91 we envisioned involving DES of choline chloride (ChCl) and l-(+)-tartaric acid (TA) for the Pechmann condensation reaction to generate a variety of substituted coumarin derivatives by reacting several substituted phenols with different β-ketoesters under operationally simple reaction conditions. To the best of our knowledge, this is the first report where DESs have been taken into the consideration toward the successful synthesis of coumarins. Hopefully, this versatile protocol will open new opportunities not only to construct diverse interesting coumarin derivatives but also to assemble other heterocycles by means of this simple methodology.
Results and Discussion
In order to test the feasibility of DESs for Pechmann condensation, the reaction of cheap and commercially available resorcinol (8) with a β-ketoester, namely, ethyl acetoacetate (9), was chosen as a model reaction for the synthesis of 7-hydroxy-4-methylcoumarin (10). As can be inspected from Table 1, among the diverse scrutinized DESs, the DES of ChCl/l-(+)-TA (1:2) at 110 °C (Table 1, entry 19) was realized as the best reaction choice. Interestingly, it was observed that increasing the temperature from 60 to 110 °C displays a profound effect not only on the yield of the desired product but also on the reaction time as well (Table 1, entries 13–19). Surprisingly, increasing the reaction temperature from 110 to 120 °C slightly lowered the yield of the required product, but a little charring in the reaction mixture was noticed during the progress of the reaction (Table 1, entry 21). Next, optimization of various amounts of DES (ChCl/l-(+)-TA (1:2)) was tested (Table 1, entries 17–20); from the studies, we observed that increasing the amount of DES from 1.5 to 3 g increases the yield of the target compound (10) (Table 1, entries 17–19). A further increase in the amount from 3 to 4 g does not greatly affect the yield of the required product (Table 1, entry 20). On the other hand, the desired product was also obtained in different ratios of ChCl/l-(+)-TA (entries 11 and 12, Table 1) but in lesser amount of yields due to the formation of some unidentified products. Additionally, an almost identical yield was noticed using ChCl/malonic acid (2:1) (Table 1, entry 22). On the other front, as can be seen from the same table, carbohydrate-based DESs (entries 3–7 and 10) offered the desired product in trace-to-low yields (negligible-29%). In these cases, we noticed the formation of some unidentified complex mixtures along with the desired product. Moreover, almost the same situation happened with other tested DESs (Table 1, entries 1, 2, 8, 9, and 23). However, in a few cases, we also observed some unconsumed starting materials, even though the reactions were kept for extended times. In order to check the recyclability and reusability, the optimized DES of ChCl/l-(+)-TA (1:2) was isolated from the product after a first successful run through liquid–liquid extraction followed by evaporation of the aqueous layer containing optimized DES.92 The recovered DES was then reused for the next run for the same reaction. It has been noticed that even after four runs the efficiency of the DES was not significantly decreased in the preparation of compound 10 under similar reaction conditions (Figure 2).
Table 1. Optimization of Reaction Conditions for the Preparation of 7-Hydroxy-4-methylcoumarin (10).
| entry | DESs | DES amount (g) | reaction temp (° C) | time (h) | yielda (%) |
|---|---|---|---|---|---|
| 1 | DMU/l-(+)-TA (7:3) | 3 | 100 | 24 | 25 |
| 2 | citric acid/DMU (2:3) | 3 | 100 | 12 | 28 |
| 3 | d-(−)-fructose/urea (1:1) | 3 | 100 | 10 | Trace |
| 4 | glucose/urea/NH4Cl (6:3:1) | 3 | 100 | 24 | 23 |
| 5 | citric acid/mannitol/urea (3:2:5) | 3 | 100 | 12 | 29 |
| 6 | lactose/DMU/NH4Cl (5:4:1) | 3 | 100 | 24 | 11 |
| 7 | maltose/DMU/NH4Cl (5:4:1) | 3 | 100 | 24 | 13 |
| 8 | ChCl/DMU (1:2) | 3 | 100 | 24 | Trace |
| 9 | ChCl/urea (1:2) | 3 | 100 | 24 | Trace |
| 10 | ChCl/glucose (1:1) | 3 | 100 | 24 | Trace |
| 11 | ChCl/l-(+)-TA (1:1) | 3 | 100 | 24 | 48 |
| 12 | ChCl/l-(+)-TA (2:1) | 3 | 100 | 24 | 47 |
| 13 | ChCl/l-(+)-TA (1:2) | 3 | 60 | 30 | 45 |
| 14 | ChCl/l-(+)-TA (1:2) | 3g | 80 | 22 | 54 |
| 15 | ChCl/l-(+)-TA (1:2) | 3 | 90 | 17 | 67 |
| 16 | ChCl/l-(+)-TA (1:2) | 3 | 100 | 11 | 74 |
| 17 | ChCl/l-(+)-TA (1:2) | 1.5 | 110 | 12 | 61 |
| 18 | ChCl/l-(+)-TA (1:2) | 2 | 110 | 10 | 66 |
| 19 | ChCl/l-(+)-TA (1:2) | 3 | 110 | 8 | 90 |
| 20 | ChCl/l-(+)-TA (1:2) | 4 | 110 | 8 | 88 |
| 21 | ChCl/l-(+)-TA (1:2) | 3 | 120 | 8 | 83 |
| 22 | ChCl/malonic acid (2:1) | 3 | 100 | 10 | 40 |
| 23 | ChCl/ethylene glycol (1:2) | 3 | 100 | 12 | Trace |
Isolated yields after column chromatography; DMU = N,N′-dimethylurea; ChCl = choline chloride.
Figure 2.
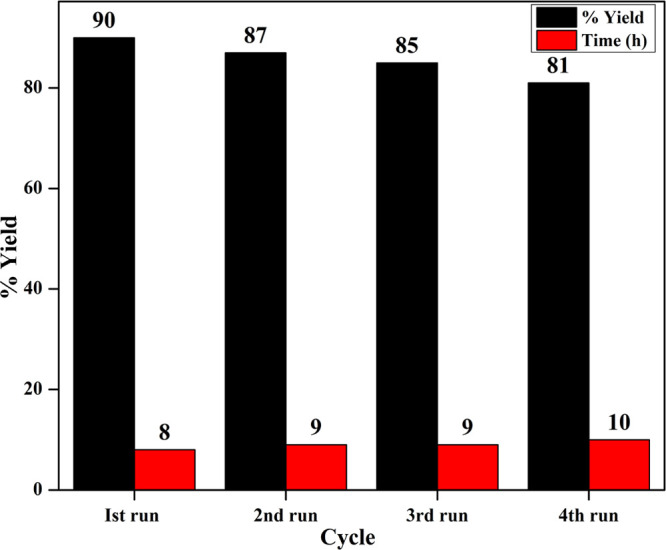
Bar graph showing the recyclability of ChCl/l-(+)-TA (1:2) for the construction of 7-hydroxy-4-methylcoumarin (10).
Encouraged by the above results, we intended to extend the scope of this versatile protocol to assemble a range of other functionalized coumarins under identical reaction conditions. In this line, we have successfully prepared diverse substituted coumarins (20–31) in good-to-excellent yields (60–98%) from various substituted phenols and β-ketoesters (ethyl acetoacetate/ethyl benzoylacetate/ethyl chloroacetoacetate) (Table 2). Strikingly, from our experimentations, we observed that phloroglucinol (11) with ethyl acetoacetate (9) provided the best yield where the reaction proceeded cleanly and rapidly to afford the desired Pechmann condensation product (20) in just 10 min (Table 2, entry 1). More importantly, compound 20 was also obtained on a large scale (5 g, 95%) in just 15 min under similar conditions. To further demonstrate the substrate scope of this method, pyrogallol (12) and catechol (13) upon reaction with 9 also neatly afforded the corresponding coumarins (21 and 22) in respectable yields (Table 2, entries 2 and 3). Noticeably, it has been observed that phenols with electron-releasing groups (EDGs) at the meta-positions efficiently delivered the Pechmann condensation products (23 and 24) with 9 in decent yields (Table 2, entries 4 and 5). It has been observed that phenol derivatives consisting of electron donating groups (EDGs) at the meta-position(s) to phenolic −OH (participating in the cyclization step) increase the rate of the reaction because of the mesomeric effect of these substituents on the attacking benzene ring carbon. This effect can be clearly seen from Table 2, showing that phloroglucinol 11, having two m-OH groups compared to that of phenolic −OH, which participates in the cyclization, or in other words ortho and para with respect to the attacking benzene ring carbon, not only reacts at a faster rate but also furnished the corresponding product in better yield as compared to the pyrogallol 12, having only one such m-hydroxy group. Moreover, we noticed that 3-aminophenol 14 reacted at a faster rate (surprisingly, lower yield obtained) than resorcinol due to the stronger mesomeric effect by the amino group in comparison to the phenolic functionality. On the other hand, a phenol-containing electron-withdrawing group (EWG), for example, 4-chlorophenol (32), failed to undergo the Pechmann condensation reaction, which may be due to low nucleophilicity and/or mild reaction medium under investigation (Table 2, entry 13). Moreover, as shown in the literature,93−95 the parent phenol does not react at all to deliver the corresponding desired product 35 (Table 2, entry 14). As expected, our efforts in this direction were also futile (no starting material was consumed), although we have raised the temperature of the reaction mixture to 140 °C and also stirred the reaction for several days. On the other hand, employing ChCl/l-(+)-TA (1:2) at 110 °C for α/β naphthols (16 and 17) also did not provide the required products (starting materials recovered) even if we attempted the reaction at higher temperatures and for longer times. Pleasingly, use of a catalytic amount of methanesulfonic acid (0.2 equiv) under similar reaction conditions furnished the desired products (25 and 26) from the naphthols (16 and 17) in respectable yields. The use of less reactive ethyl benzoylacetate (18) as a β-ketoester partner with the substituted phenols (8 and 11) furnished the corresponding coumarin derivatives (27 and 28) in comparatively lower yields with protracted reaction times in comparison to the more reactive β-ketoester partner (9) (Table 2, entries 8 and 9). Moreover, significant yields (76–91%) of the functionalized coumarins (29–31) were isolated from the reaction of the substituted phenols (8–11) with ethyl chloroacetoacetate (19) (Table 2, entries 10–12). The phenol systems consisting of two/three phenolic groups were found to be ineffective to deliver the corresponding di- and tricoumarins with 2/3 equiv of β-ketoesters even after the reactions were stirred for longer times at higher temperatures. Surprisingly, in all cases, we were able to isolate only the corresponding monocoumarin derivatives, confirmed both by TLC and 1H NMR spectral data. The reason for the formation of only monocoumarins and not the desired di-/tricoumarins with more equivalents of β-ketoester is still unclear and is under investigation. In order to compare the present green protocol with the already existing ones, we put forth an effort to compile the results of the previously published procedures (Table 3).35,75,96−101 From Table 3, it is clear that our method is not only slightly better from a yield perspective than the others but also involve cheap, biodegradable, and reusable melting mixture. Fascinatingly, bis-coumarins (41–44) were also fruitfully assembled by reacting 7-hydroxycoumarin (36) with aromatic aldehydes (37–40) in respectable yields (81–97%) utilizing ChCl/l-(+)-TA (1:2) at lower temperature (90 °C) (Scheme 1). From these studies, we pointed that aromatic aldehyde containing EWG (38) besides reacting at faster rate with 36 also resulted the desired product (42) in better yield (97%) as compared to the aromatic aldehydes having EDGs (39 and 40) as shown in Scheme 1. More importantly, in most of the cases, the precipitate formation of the desired coumarins by means of adding the water while the reaction was still hot at the end of the reaction limits the unnecessary use of the volatile organic solvents for workup procedures. Interestingly, purification was also not required in some cases of precipitated products, as we obtained reasonably pure compounds for NMR analysis after direct filtration of precipitate.
Table 2. Construction of Vital Coumarin Derivatives (20–31) Utilizing the ChCl/l-(+)-TA (1:2).
NR = no reaction. In these cases, catalytic amount (0.2 equiv) of methane sulfonic acid (MeSO3H) was added.
Table 3. Assessment of the Green Protocol with Previously Reported Ones for the Synthesis of Diverse Coumarin Derivatives (10, 20–31) via Pechmann Condensation.
| entry | catalysts | time/temp/solvent | yield (%) | ref |
|---|---|---|---|---|
| 1 | ZrOCl2·8H2O | 24 h/80 °C/solvent free | 48–94 | (97) |
| 2 | phosphotungstic acid | 30–90 min/90 °C/solvent free | 60–94 | (35) |
| 3 | BiCl3 | 1–2 h/75–110 °C/solvent free | 81–93 | (98) |
| 4 | I2 | 1–2.2 h/85 °C/solvent free | 70–90 | (99) |
| 5 | oxalic acid | 35–50 min/80 °C/solvent free | 91–96 | (100) |
| 6 | sulfamic acid | 20–55 min/130 °C/solvent free | 68–96 | (96) |
| 7 | acidic ionic liquid | 1.5–4 h/85 °C/solvent free | 70–80 | (75) |
| 8 | Zn0.925Ti0.075O | 3–5 h/110 °C/solvent free | 55–89 | (101) |
| 9 | ChCl/l-(+)-TA(1:2) | 10 min–24 h/110 °C/solvent free | 60–98 | This work |
Scheme 1. Synthesis of Diverse Bis-coumarin Derivatives (41–44) Using ChCl/l-(+)-TA (1:2).

In order to expose the reactivity of various substituted phenols (8, 11, and 12) with β-ketoesters (9, 18, and 19), competitive reactions for the generation of corresponding substituted coumarins (10, 20, 21, 28, and 30) were performed involving the optimized reaction conditions (Scheme 2). From these experiments, as detailed in the Scheme 2, while comparing the substituted phenols (8, 11, and 12), phloroglucinol (11) reacted at fastest rate in comparison to the others (11 and 12) in order to deliver the corresponding product (20) in highest yield. On the other hand, as expected, β-ketoester (9) was found to react with high speed than other β-ketoesters (18 and 19). Furthermore, we also compare the reactivity of three aromatic aldehydes in the synthesis of bis-coumarins by reacting the 4-hydroxy coumarin (36) with benzaldehyde (37), 4-nitrobenzaldehyde (38), and anisaldehyde (39) and pointed out that 4-nitrobenzaldehyde reacted at faster rate due to an obvious reason of having high electrophilicity at the carbonyl carbon hence afforded major product as compared to the benzaldehyde (37) (Scheme 2). In the case of anisaldehyde (39), methoxy groups mitigate the reactivity of the carbonyl functionality due to a mesomeric effect. Hence, these findings are in concurrence with the fact that aromatic aldehyde containing EWG reacts at a faster rate with 36 than the aromatic aldehyde containing EDG.
Scheme 2. Competitive Reactions for the Formation of Functionalized Coumarins in ChCl/l-(+)-TA (1:2) Based DES.

The plausible mechanism of 7-hydroxy-4-methylcoumarin (10) via DES of ChCl/l-(+)-TA (1:2) is depicted in the Scheme 3. Initially, the DES (47) is formed via four hydrogen-bonding interactions between hydroxyl groups of l-(+)-TA) and Cl– ions of the ChCl111 which by providing the proton activate the carbonyl group of the ethyl acetoacetate (9). The protonated ethyl acetoacetate 48 then undergoes nucleophilic attack by resorcinol (8) resulting in the generation of 49, which on subsequent aromatization yield the compound 50. Next, attack of the phenolic group to ester functionality followed by the dehydration provided the required compound 7-hydroxy 4-methylcoumarin (10).112
Scheme 3. Plausible Mechanism of the Formation of 7-Hydroxy-4-methylcoumarin (10) via DES of ChCl/l-(+)-TA (1:2).
Conclusions
In summary, Pechmann condensation has been carried out efficiently under the green condition through the utilization of ChCl/l-(+)-TA (1:2) based DES for the construction of diverse privileged coumarins. Moreover, the same DES at relatively lower temperature was also found effective to assemble various bis-coumarin derivatives under similar reaction conditions. Besides the efficient recovery and reutilization of the ChCl/l-(+)-TA (1:2) based DES, the developed green method was also successfully operated for large-scale synthesis. This procedure may be industrialized as functionalized coumarins were cleanly obtained at gram-scale in almost similar time with almost identical yields as in milligram scale. Our methodology is enriched with various salient features like simple-to-use, good-to-excellent yields with mild reaction conditions and no obligation of hazardous volatile organic solvent/external catalyst/inert atmosphere. Thus, the authors believe that the established protocol for coumarins and bis-coumarins synthesis might be beneficial from both environmental as well as industrial viewpoints.
Experimental Section
General Information
All the required chemicals were procured from Sigma-Aldrich, Alfa Aesar, SRL, TCI, GLR, Avra, and Spectrochem. Reaction progress was monitored by thin-layer chromatography (TLC) on silica gel coated aluminum plates using suitable ratio of hexane and EtOAc for development. For the purification of desired compounds, column chromatography using silica gel (100–200 mesh) was carried out with a suitable mixture of EtOAc and petroleum ether. Purified functionalized coumarins and bis-coumarins were characterized by 1H NMR spectra recorded in DMSO-d6 on a Bruker spectrometer (400 and 500 MHz). Melting points of various coumarin derivatives were recorded through Tanco manual melting point apparatus.
General Synthetic Procedure for Functionalized Coumarins (10, 20–31)
In a characteristic experiment, 3 g of ChCl/l-(+)-TA (1:2) mixture was heated at 60–70 °C so as to obtain a transparent melting mixture. Afterward, the temperature of the clear melt was elevated to optimum temperature (110 °C), and at this temperature, substituted phenols (1 mmol) and β-ketoesters (2.1 mmol) were added. The reaction was allowed to stir at 110 °C for 10 min to 24 h. After the completion of the reaction as monitored through consumption of all the starting materials on TLC, 10–15 mL of water was added while still hot. The resulting solid material formed in most of the cases was filtered-off through the sintered glass funnel and washed thoroughly with water. The solid was later dried and purified by column chromatography using appropriate ratio of petroleum ether and ethyl acetate to afford functionalized coumarins (10, 20–31). In the case of no precipitation, workup with ethyl acetate was performed followed by purification through column chromatography.
General Synthetic Procedure for Bis-coumarins 41–44
In an illustrative experiment, 3 g of ChCl/l-(+)-TA (1:2) mixture was heated at 60–70 °C to obtain a clear melting mixture. Later the temperature was raised to 90 °C and 7-hydroxycoumarin (2 mmol) along with appropriate aromatic aldehyde (1 mmol) were added, and the reaction was allowed to stir for 20–45 min. After the completion of the reaction as monitored via consumption of reactants on TLC, 10–15 mL of water was added while the mixture was still hot. The consequent solid material was later filtered through sintered glass funnel and washed thoroughly with water. After proper vacuum drying, purification of crude solid through column chromatography was performed using appropriate ratio of petroleum ether and ethyl acetate to afford functionalized bis-coumarins (41–44).
General Procedure for Recyclability of ChCl/l-(+)-TA (1:2)
After the completion of a particular reaction carried out by using 3 g of ChCl/l-(+)-TA (1:2), water was added to the hot reaction mixture. Wherever precipitation of a product resulted, filtration was carried out via a sintered glass funnel. However, where no precipitation occurred after addition of water, liquid–liquid extraction using ethyl acetate and water was carried out. The aqueous phase containing DES in both cases was evaporated so as to result in a solid DES, which was later dried and reused directly for the next run. The same procedure was followed for further runs.92
7-Hydroxy-4-methyl-2H-chromen-2-one (10):
light pink solid; mp 184–186 °C; yield 90%; 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H), 7.58 (d, J = 8.7 Hz, 1H), 6.80 (d, J = 6.4 Hz, 1H), 6.70 (s, 1H), 6.12 (s, 1H), 2.36 (s, 3H). The spectral data are in accordance with the literature.32,101,102
5,7-Dihydroxy-4-methyl-2H-chromen-2-one (20):
white solid; mp 280–283 °C; yield 98%; 1H NMR (400 MHz, DMSO-d6) δ 10.56 (s, 1H), 10.32 (s, 1H), 6.29 (s, 1H), 6.20 (s, 1H), 5.87 (s, 1H), 2.51 (s, 3H). The spectral data are in accordance with the literature.32,101,102
7,8-dihydroxy-4-methyl-2H-chromen-2-one (21):
light purple solid; mp 240–242 °C; yield 81%; 1H NMR (400 MHz, DMSO-d6) δ 9.72 (s, 2H), 7.08 (d, J = 8.8 Hz, 1H), 6.81 (d, J = 8.4 Hz, 1H), 6.12 (s, 1H), 2.34 (s, 3H). The spectral data are in accordance with the literature.32,101,102
8-Hydroxy-4-methyl-2H-chromen-2-one (22):
light pink solid; mp 169–172 °C; yield 62%; 1H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H), 7.59 (d, J = 8.8 Hz, 1H), 6.80 (d, J = 8.8 Hz, 1H), 6.70 (s, 1H), 6.13 (s, 1H), 2.36 (s, 3H). The spectral data are in accordance with the literature.103,113
7-Amino-4-methyl-2H-chromen-2-one (23):
brown solid; mp 220–223 °C; yield 82%; 1H NMR (400 MHz, DMSO-d6) δ 7.40 (d, J = 8.4 Hz, 1H), 6.57 (d, J = 6.4 Hz, 1H), 6.41 (s, 1H), 6.11 (s, 2H), 5.90 (s, 1H), 2.29 (s, 3H). The spectral data are in accordance with the literature.102,104
7-Methoxy-4-methyl-2H-chromen-2-one (24):
wWhite solid; mp 160–163 °C; yield 65%; 1H NMR (400 MHz, DMSO-d6) δ 7.67 (d, J = 8.4 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 6.20 (s, 1H), 3.85 (s, 3H), 2.39 (s, 3H). The spectral data are in accordance with the literature.101,102,104
4-Methyl-2H-benzo[h]chromen-2-one (25):
light brown solid, mp 154–157 °C; yield 68%; 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 9.6 Hz, 1H), 8.02 (d, J = 7.2 Hz, 1H), 7.84 (d, J = 8.8 Hz, 1H), 7.75 (d, J = 8.8 Hz, 1H), 7.73–7.66 (m, 2H), 6.48 (s, 1H), 2.50 (s, 3H). The spectral data are in accordance with the literature.32,101,102
4-Methyl-2H-benzo[g]chromen-2-one (26):
pale yellow solid; mp 180–183 °C; yield 60%; 1H NMR (400 MHz, DMSO-d6) δ 9.91 (d, J = 10.0 Hz, 1H), 8.28 (d, J = 9.2 Hz, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.81–7.53 (m, 3H), 6.39 (s, 1H), 2.39 (s, 3H). The spectral data are in accordance with the literature.99,105
7-Hydroxy-4-phenyl-2H-chromen-2-one (27)
brown solid; mp 253–256 °C; yield 75%; 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H), 7.52 (dd, J = 15.2 Hz, 3.6 Hz, 5H), 7.26 (d, J = 8.8 Hz, 1H), 6.79–6.76 (m, 2H), 6.13 (s, 1H). The spectral data are in accordance with the literature.32,101,106
5,7-Dihydroxy-4-phenyl-2H-chromen-2-one (28):
light pink solid; mp 240–243 °C; yield 83%; 1H NMR (400 MHz, DMSO-d6) δ 10.42 (s, 1H), 10.14 (s, 1H), 7.37–7.33 (m, 5H), 6.27 (s, 1H), 6.17 (s, 1H), 5.75 (s, 1H). The spectral data are in accordance with the literature.32,101,107
4-(Chloromethyl)-7-hydroxy-2H-chromen-2-one (29):
gray solid; mp 180–183 °C; yield 80%; 1H NMR (400 MHz, DMSO-d6) δ 10.68 (s, 1H), 7.67 (d, J = 8.8 Hz, 1H), 6.84 (dd, J = 8.8, 2.4 Hz, 1H), 6.76 (d, J = 2.4 Hz, 1H), 6.42 (s, 1H), 4.94 (s, 2H). The spectral data are in accordance with the literature.32,108,109
4-(Chloromethyl)-5,7-dihydroxy-2H-chromen-2-one (30):
white solid; mp 185–188 °C; yield 91%; 1H NMR (400 MHz, DMSO-d6) δ 10.90 (s, 1H), 10.52 (s, 1H), 6.28 (s, 1H), 6.25–6.18 (m, 2H), 5.03 (s, 2H). The spectral data are in accordance with the literature.31,32
4-(Chloromethyl)-7,8-dihydroxy-2H-chromen-2-one (31):
light pink solid; mp 131–134 °C; yield 76%; 1H NMR (400 MHz, DMSO-d6) δ 10.17 (s, 1H), 9.54 (s, 1H), 7.18 (d, J = 8.8 Hz, 1H), 6.85 (d, J = 8.8 Hz, 1H), 6.42 (s, 1H), 4.93 (s, 2H). The spectral data are in accordance with the literature.32,97,110
3,3′-(Phenylmethylene)bis(4-hydroxy-2H-chromen-2-one) (41):
white solid; mp 228–231 °C; yield 91%; 1H NMR (400 MHz, DMSO-d6) δ 7.85 (d, J = 7.6 Hz, 2H), 7.54 (t, J = 6.8 Hz, 2H), 7.34–7.23 (m, 4H), 7.22–7.16 (m, 2H), 7.14–7.07 (m, 3H), 6.31 (s, 1H). The spectral data are in accordance with the literature.114,115
3,3′-((4-Nitrophenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (42):
white solid; mp 233–236 °C; yield 97%; 1H NMR (400 MHz, DMSO-d6) δ 8.08 (d, J = 9.2 Hz, 2H), 7.82 (d, J = 8.0 Hz, 2H), 7.58–7.48 (m, 2H), 7.37 (d, J = 8.8 Hz, 2H), 7.32–7.20 (m, 4H), 6.35 (s, 1H). The spectral data are in accordance with the literature.114−116
3,3′-((4-Methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (43):
creamy solid; mp 254–256 °C; yield 85%; 1H NMR (400 MHz, DMSO-d6) δ 7.81 (d, J = 1.6 Hz, 2H), 7.50 (t, J = 8.8 Hz, 2H), 7.24 (q, J = 8.0 Hz, 4H), 7.00 (d, J = 7.6 Hz, 2H), 6.74 (d, J = 8.4 Hz, 2H), 6.21 (s, 1H). The spectral data are in accordance with the literature.114,116,117
3,3′-((4-Hydroxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (44):
Creamy solid; mp 220–223 °C; yield 81%; 1H NMR (400 MHz, DMSO-d6) δ 7.84 (d, J = 7.6 Hz, 2H), 7.52 (t, J = 8.4 Hz, 2H), 7.38–7.16 (m, 4H), 6.89 (d, J = 8.0 Hz, 2H), 6.58 (d, J = 8.8 Hz, 2H), 6.18 (s, 1H). The spectral data are in accordance with the literature.114,116,117
Acknowledgments
We are grateful to DST-SERB, New-Delhi, for financial support (Project File no. ECR/2017/000821) and Jamia Millia Islamia for providing the wonderful infrastructure. I.A.R. also thanks CSIR, New Delhi, for the SRF fellowship award.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00293.
Recyclability test of ChCl/l-(+)-TA (1:2), copies of 1H NMR spectra, and TLC analysis of competitive reactions for various functionalized coumarins (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Christie R. M.; Lui C.-H. Studies of Fluorescent Dyes: Part 2. An Investigation of the Synthesis and Electronic Spectral Properties of Substituted 3-(2′-Benzimidazolyl)Coumarins. Dyes Pigm. 2000, 47 (1–2), 79–89. 10.1016/S0143-7208(00)00066-8. [DOI] [Google Scholar]
- Ayyangar N. R.; Srinivasan K. V.; Daniel T. Polycyclic Compounds Part VII. Synthesis Laser Characteristics and Dyeing Behaviour of 7-Diethylamino-2H-l-Benzopyran-2-Ones. Dyes Pigm. 1991, 16 (3), 197–204. 10.1016/0143-7208(91)85010-6. [DOI] [Google Scholar]
- Chandra A.; Jana K.; Moorthy J. N. One-Pot Synthesis of 4-Carboalkoxy-Substituted Benzo[h]Coumarins from α- and β-Naphthols and their Excited-State Properties. ACS Omega 2020, 5 (1), 207–218. 10.1021/acsomega.9b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasior M.; Kim D.; Singha S.; Krzeszewski M.; Ahn K. H.; Gryko D. T. π-Expanded Coumarins: Synthesis, Optical Properties and Applications. J. Mater. Chem. C 2015, 3 (7), 1421–1446. 10.1039/C4TC02665A. [DOI] [Google Scholar]
- Xiao C.; Luo X.-Y.; Li D.-J.; Lu H.; Liu Z.-Q.; Song Z.-G.; Jin Y.-H. Synthesis of 4-Methylcoumarin Derivatives Containing 4,5-Dihydropyrazole Moiety to Scavenge Radicals and to Protect DNA. Eur. J. Med. Chem. 2012, 53, 159–167. 10.1016/j.ejmech.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Kostova I. Coumarins as Inhibitors of HIV Reverse Transcriptase. Curr. HIV Res. 2006, 4 (3), 347–363. 10.2174/157016206777709393. [DOI] [PubMed] [Google Scholar]
- Wang C.-J.; Hsieh Y.-J.; Chu C.-Y.; Lin Y.-L.; Tseng T.-H. Inhibition of Cell Cycle Progression in Human Leukemia HL-60 Cells by Esculetin. Cancer Lett. 2002, 183 (2), 163–168. 10.1016/S0304-3835(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Vijaya Laxmi S.; Suresh Kuarm B.; Rajitha B. Synthesis and Antimicrobial Activity of Coumarin Pyrazole Pyrimidine 2,4,6(1H,3H,5H)Triones and Thioxopyrimidine 4,6(1H,5H)diones. Med. Chem. Res. 2013, 22 (2), 768–774. 10.1007/s00044-012-0078-y. [DOI] [Google Scholar]
- Grover J.; Jachak S. M. Coumarins as Privileged Scaffold for Anti-Inflammatory Drug Development. RSC Adv. 2015, 5 (49), 38892–38905. 10.1039/C5RA05643H. [DOI] [Google Scholar]
- Annunziata F.; Pinna C.; Dallavalle S.; Tamborini L.; Pinto A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21 (13), 4618. 10.3390/ijms21134618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina F. G.; Marrero J. G.; Macías-Alonso M.; González M. C.; Córdova-Guerrero I.; Teissier García A. G.; Osegueda-Robles S. Coumarin Heterocyclic Derivatives: Chemical Synthesis and Biological Activity. Nat. Prod. Rep. 2015, 32 (10), 1472–1507. 10.1039/C4NP00162A. [DOI] [PubMed] [Google Scholar]
- Calcio Gaudino E.; Tagliapietra S.; Martina K.; Palmisano G.; Cravotto G. Recent Advances and Perspectives in the Synthesis of Bioactive Coumarins. RSC Adv. 2016, 6 (52), 46394–46405. 10.1039/C6RA07071J. [DOI] [Google Scholar]
- Cao D.; Liu Z.; Verwilst P.; Koo S.; Jangjili P.; Kim J. S.; Lin W. Coumarin-based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119 (18), 10403–10519. 10.1021/acs.chemrev.9b00145. [DOI] [PubMed] [Google Scholar]
- Carneiro A.; Matos M. J.; Uriarte E.; Santana L. Trending Topics on Coumarin and its Derivatives in 2020. Molecules 2021, 26 (2), 501. 10.3390/molecules26020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir R.; Stasyuk A. J.; Gryko D. T. Vertically π-Expanded Coumarins: The Synthesis and Optical Properties. J. Org. Chem. 2016, 81 (22), 11104–11114. 10.1021/acs.joc.6b02094. [DOI] [PubMed] [Google Scholar]
- Tasior M.; Poronik Y. M.; Vakuliuk O.; Sadowski B.; Karczewski M.; Gryko D. T. V-Shaped bis-Coumarins: Synthesis and Optical Properties. J. Org. Chem. 2014, 79 (18), 8723–8732. 10.1021/jo501565r. [DOI] [PubMed] [Google Scholar]
- Kielesiński Ł.; Morawski O. W.; Sobolewski A. L.; Gryko D. T. The Synthesis and Photophysical Properties of tris-Coumarins. Phys. Chem. Chem. Phys. 2019, 21 (16), 8314–8325. 10.1039/C9CP00978G. [DOI] [PubMed] [Google Scholar]
- Xue W.; Wang D.; Li C.; Zhai Z.; Wang T.; Liang Y.; Zhang Z. π-Expanded Coumarins: One-Pot Photo Synthesis of 5 H -Benzo[12,1]Tetrapheno[7,6,5- Cde ]Chromen-5-Ones and Photophysical Properties. J. Org. Chem. 2020, 85 (5), 3689–3698. 10.1021/acs.joc.9b03327. [DOI] [PubMed] [Google Scholar]
- Hu S.; Li X.; Wang K.; Wu Q.; Zhang G.; Liu X. Sensor Array Based on Carbon Dots for ATP-Related Physiological Phosphates Detecting and ATP Hydrolysis Monitoring. Sensors Actuators B Chem. 2020, 310, 127851. 10.1016/j.snb.2020.127851. [DOI] [Google Scholar]
- Borges F.; Roleira F.; Milhazes N.; Santana L.; Uriarte E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12 (8), 887–916. 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- Salem M. A.; Helal M. H.; Gouda M. A.; Ammar Y. A.; El-Gaby M. S. A.; Abbas S. Y. An Overview on Synthetic Strategies to Coumarins. Synth. Commun. 2018, 48 (13), 1534–1550. 10.1080/00397911.2018.1455873. [DOI] [Google Scholar]
- Mustafa Y. F. Classical Approaches and their Creative Advances in the Synthesis of Coumarins: A Brief Review. J. Med. Chem. Sci. 2021, 4 (6), 612–625. [Google Scholar]
- Johnson J. R.The Perkin Reaction and Related Reactions. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, 2011; pp 210–265. [Google Scholar]
- Cairns N.; Harwood L. M.; Astles D. P. Tandem Thermal Claisen–Cope Rearrangements of Coumarate Derivatives. Total Syntheses of the Naturally Occurring Coumarins: Suberosin, Demethylsuberosin, Ostruthin, Balsamiferone and Gravelliferone. J. Chem. Soc., Perkin Trans. 1 1994, (21), 3101–3107. 10.1039/P19940003101. [DOI] [Google Scholar]
- Jones G.The Knoevenagel Condensation. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, 2011; pp , 204–599. [Google Scholar]
- Shriner R. L.The Reformatsky Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, 2011; pp 1–37. [Google Scholar]
- Yavari I.; Hekmat-Shoar R.; Zonouzi A. A New and Efficient Route to 4-Carboxymethylcoumarins Mediated by Vinyltriphenylphosphonium Salt. Tetrahedron Lett. 1998, 39 (16), 2391–2392. 10.1016/S0040-4039(98)00206-8. [DOI] [Google Scholar]
- Dittmer D. C.; Li Q.; Avilov D. V. Synthesis of Coumarins, 4-Hydroxycoumarins, and 4-Hydroxyquinolinones by Tellurium-Triggered Cyclizations 1. J. Org. Chem. 2005, 70 (12), 4682–4686. 10.1021/jo050070u. [DOI] [PubMed] [Google Scholar]
- Vekariya R. H.; Patel H. D. Recent Advances in the Synthesis of Coumarin Derivatives via Knoevenagel Condensation: A Review. Synth. Commun. 2014, 44 (19), 2756–2788. 10.1080/00397911.2014.926374. [DOI] [Google Scholar]
- Keshavarzipour F.; Tavakol H. The Synthesis of Coumarin Derivatives Using Choline Chloride/Zinc Chloride as a Deep Eutectic Solvent. J. Iran. Chem. Soc. 2016, 13 (1), 149–153. 10.1007/s13738-015-0722-9. [DOI] [Google Scholar]
- Valizadeh H.; Shockravi A. An Efficient Procedure for the Synthesis of Coumarin Derivatives Using TiCl4 as Catalyst under Solvent-Free Conditions. Tetrahedron Lett. 2005, 46 (20), 3501–3503. 10.1016/j.tetlet.2005.03.124. [DOI] [Google Scholar]
- Sharma G. V. M.; Janardhan Reddy J.; Sree Lakshmi P.; Radha Krishna P. An Efficient ZrCl4 Catalyzed One-Pot Solvent Free Protocol for the Synthesis of 4-Substituted Coumarins. Tetrahedron Lett. 2005, 46 (36), 6119–6121. 10.1016/j.tetlet.2005.06.166. [DOI] [Google Scholar]
- Keri R. S.; Hosamani K. M.; Seetharama Reddy H. R. A Solvent-Free Synthesis of Coumarins Using Phosphotungstic Acid as Catalyst. Catal. Lett. 2009, 131 (1–2), 321–327. 10.1007/s10562-009-9940-z. [DOI] [Google Scholar]
- Hinze R.; Laufer M. C.; Hölderich W. F.; Bonrath W.; Netscher T. The Use of Nafion/Silica Composite Catalysts for Synthesis of Fine Chemicals. Catal. Today 2009, 140 (1–2), 105–111. 10.1016/j.cattod.2008.07.008. [DOI] [Google Scholar]
- Upadhyay K. K.; Mishra R. K.; Kumar A. A Convenient Synthesis of Some Coumarin Derivatives Using SnCl2 · 2H2O as Catalyst. Catal. Lett. 2008, 121 (1–2), 118–120. 10.1007/s10562-007-9307-2. [DOI] [Google Scholar]
- Maheswara M.; Siddaiah V.; Damu G. L. V.; Rao Y. K.; Rao C. V. A Solvent-Free Synthesis of Coumarins via Pechmann Condensation Using Heterogeneous Catalyst. J. Mol. Catal. A Chem. 2006, 255 (1–2), 49–52. 10.1016/j.molcata.2006.03.051. [DOI] [Google Scholar]
- Ghodke S.; Chudasama U. Solvent Free Synthesis of Coumarins Using Environment Friendly Solid Acid Catalysts. Appl. Catal. A Gen. 2013, 453, 219–226. 10.1016/j.apcata.2012.12.024. [DOI] [Google Scholar]
- Kalita P.; Baskar A. V.; Choy J.; Lakhi K. S.; El-Newehy M.; Lawrence G.; Al-deyab S. S.; Balasubramanian V. V.; Vinu A. Preparation of Highly Active Triflic Acid Functionalized SBA-15 Catalysts for the Synthesis of Coumarin under Solvent-Free Conditions. ChemCatChem. 2016, 8 (2), 336–344. 10.1002/cctc.201500884. [DOI] [Google Scholar]
- Kalita P.; Sathyaseelan B.; Mano A.; Zaidi S. M. J.; Chari M. A.; Vinu A. Synthesis of Superacid-Functionalized Mesoporous Nanocages with Tunable Pore Diameters and their Application in the Synthesis of Coumarins. Chem. - A Eur. J. 2010, 16 (9), 2843–2851. 10.1002/chem.200902531. [DOI] [PubMed] [Google Scholar]
- Abbasi Z.; Rezayati S.; Bagheri M.; Hajinasiri R. Preparation of a Novel, Efficient, and Recyclable Magnetic Catalyst, γ -Fe2O3@HAp-Ag Nanoparticles, and a Solvent- and Halogen-Free Protocol for the Synthesis of Coumarin Derivatives. Chin. Chem. Lett. 2017, 28 (1), 75–82. 10.1016/j.cclet.2016.06.022. [DOI] [Google Scholar]
- Ahmed B. A.; Mustafa Y. F.; Ibrahim B. Y. Isolation and Characterization of Furanocoumarins from Golden Delicious Apple Seeds. J. Med. Chem. Sci. 2022, 5, 537–545. [Google Scholar]
- Rezayati S.; Sheikholeslami-Farahani F.; Rostami-Charati F.; Abad S. A. S. One-Pot Synthesis of Coumarine Derivatives Using Butylenebispyridinium Hydrogen Sulfate as Novel Ionic Liquid Catalyst. Res. Chem. Intermed. 2016, 42 (5), 4097–4107. 10.1007/s11164-015-2261-5. [DOI] [Google Scholar]
- Horváth István T.; Anastas P. T. Introduction: Green Chemistry. Chem. Rev. 2007, 107 (6), 2167–2168. 10.1021/cr0783784. [DOI] [PubMed] [Google Scholar]
- Anastas P.; Eghbali N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39 (1), 301–312. 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]
- Li C.-J.; Anastas P. T. Green Chemistry: Present and Future. Chem. Soc. Rev. 2012, 41 (4), 1413. 10.1039/c1cs90064a. [DOI] [PubMed] [Google Scholar]
- Kitanosono T.; Kobayashi S. Reactions in Water Involving the “On-Water” Mechanism. Chem. – A Eur. J. 2020, 26 (43), 9408–9429. 10.1002/chem.201905482. [DOI] [PubMed] [Google Scholar]
- Banerjee B. Recent Developments on Ultrasound-Assisted One-Pot Multicomponent Synthesis of Biologically Relevant Heterocycles. Ultrason. Sonochem. 2017, 35, 15–35. 10.1016/j.ultsonch.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Wencel-Delord J.; Colobert F. A Remarkable Solvent Effect of Fluorinated Alcohols on Transition Metal Catalysed C–H Functionalizations. Org. Chem. Front. 2016, 3 (3), 394–400. 10.1039/C5QO00398A. [DOI] [Google Scholar]
- Oakes R. S.; Clifford A. A.; Rayner C. M. The Use of Supercritical Fluids in Synthetic Organic Chemistry. J. Chem. Soc. Perkin Trans. 1 2001, 9, 917–941. 10.1039/b101219n. [DOI] [Google Scholar]
- Petkovic M.; Seddon K. R.; Rebelo L. P. N.; Silva Pereira C. Ionic Liquids: A Pathway to Environmental Acceptability. Chem. Soc. Rev. 2011, 40 (3), 1383–1403. 10.1039/C004968A. [DOI] [PubMed] [Google Scholar]
- Gawande M. B.; Shelke S. N.; Zboril R.; Varma R. S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47 (4), 1338–1348. 10.1021/ar400309b. [DOI] [PubMed] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 1, 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Shahabi D.; Tavakol H. Synthesis of 14H-Dibenzo Xanthene Derivatives Using Choline Chloride/Tin(II) Chloride Deep Eutectic Solvent and Fe3O4/ƛ-Carrageenan/Zn(II). J. Iran. Chem. Soc. 2017, 14 (1), 135–142. 10.1007/s13738-016-0965-0. [DOI] [Google Scholar]
- Abtahi B.; Tavakol H.. Choline Chloride-urea Deep Eutectic Solvent as an Efficient Media for the Synthesis of Propargylamines via Organocuprate Intermediate. Appl. Organomet. Chem. 2020, 34 ( (11), ). 10.1002/aoc.5895 [DOI] [Google Scholar]
- Abtahi B.; Tavakol H.. CuI-catalyzed, One-pot Synthesis of 3-aminobenzofurans in Deep Eutectic Solvents. Appl. Organomet. Chem. 2021, 35 ( (12), ). 10.1002/aoc.6433 [DOI] [Google Scholar]
- Choi Y. H.; van Spronsen J.; Dai Y.; Verberne M.; Hollmann F.; Arends I. W. C. E.; Witkamp G.-J.; Verpoorte R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology?. Plant Physiol. 2011, 156 (4), 1701–1705. 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; van Spronsen J.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Paiva A.; Craveiro R.; Aroso I.; Martins M.; Reis R. L.; Duarte A. R. C. Natural Deep Eutectic Solvents – Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2 (5), 1063–1071. 10.1021/sc500096j. [DOI] [Google Scholar]
- Faggian M.; Sut S.; Perissutti B.; Baldan V.; Grabnar I.; Dall’Acqua S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21 (11), 1531. 10.3390/molecules21111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Friesen J. B.; McAlpine J. B.; Lankin D. C.; Chen S.-N.; Pauli G. F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81 (3), 679–690. 10.1021/acs.jnatprod.7b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruß C.; König B. Low Melting Mixtures in Organic Synthesis – an Alternative to Ionic Liquids?. Green Chem. 2012, 14 (11), 2969. 10.1039/c2gc36005e. [DOI] [Google Scholar]
- Alonso D. A.; Baeza A.; Chinchilla R.; Guillena G.; Pastor I. M.; Ramón D. J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016 (4), 612–632. 10.1002/ejoc.201501197. [DOI] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114 (21), 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Kotha S.; Ali R. A Simple Approach to Bis-Spirocycles and Spiroindole Derivatives via Green Methods Such as Fischer Indolization, Ring-Closing Metathesis, and Suzuki--Miyaura Cross-Coupling. Turk. J. Chem. 2015, 39, 1190–1198. 10.3906/kim-1502-116. [DOI] [Google Scholar]
- Kotha S.; Chakkapalli C. Application of Fischer Indolization under Green Conditions Using Deep Eutectic Solvents. Chem. Rec. 2017, 17 (10), 1039–1058. 10.1002/tcr.201600138. [DOI] [PubMed] [Google Scholar]
- Kotha S.; Chinnam A. K.; Sreenivasachary N.; Ali R. Design and Synthesis of Polycyclic Indoles under Green Conditions via Fischer Indolization. Indian J. Chem. - Sect. B Org. Med. Chem. 2016, 55B (9), 1107–1111. [Google Scholar]
- Potdar M. K.; Mohile S. S.; Salunkhe M. M. Coumarin Syntheses via Pechmann Condensation in Lewis Acidic Chloroaluminate Ionic Liquid. Tetrahedron Lett. 2001, 42 (52), 9285–9287. 10.1016/S0040-4039(01)02041-X. [DOI] [Google Scholar]
- Gu Y.; Zhang J.; Duan Z.; Deng Y. Pechmann Reaction in Non-Chloroaluminate Acidic Ionic Liquids under Solvent-Free Conditions. Adv. Synth. Catal. 2005, 347 (4), 512–516. 10.1002/adsc.200404316. [DOI] [Google Scholar]
- Zhang Y.; Zhu A.; Li Q.; Li L.; Zhao Y.; Wang J. Cholinium Ionic Liquids as Cheap and Reusable Catalysts for the Synthesis of Coumarins via Pechmann Reaction under Solvent-Free Conditions. RSC Adv. 2014, 4 (44), 22946–22950. 10.1039/C4RA02227K. [DOI] [Google Scholar]
- Shirini F.; Yahyazadeh A.; Mohammadi K. A Solvent-Free Synthesis of Coumarins Using 1,3-Disulfonic Acid Imidazolium Hydrogen Sulfate as a Reusable and Effective Ionic Liquid Catalyst. Res. Chem. Intermed. 2015, 41 (9), 6207–6218. 10.1007/s11164-014-1733-3. [DOI] [Google Scholar]
- Potdar M. K.; Rasalkar M. S.; Mohile S. S.; Salunkhe M. M. Convenient and Efficient Protocols for Coumarin Synthesis via Pechmann Condensation in Neutral Ionic Liquids. J. Mol. Catal. A Chem. 2005, 235 (1–2), 249–252. 10.1016/j.molcata.2005.04.007. [DOI] [Google Scholar]
- Singh V.; Kaur S.; Sapehiyia V.; Singh J.; Kad G. L. Microwave Accelerated Preparation of [Bmim][HSO4] Ionic Liquid: An Acid Catalyst for Improved Synthesis of Coumarins. Catal. Commun. 2005, 6 (1), 57–60. 10.1016/j.catcom.2004.10.011. [DOI] [Google Scholar]
- Dong F.; Jian C.; Kai G.; Qunrong S.; Zuliang L. Synthesis of Coumarins via Pechmann Reaction in Water Catalyzed by Acyclic Acidic Ionic Liquids. Catal. Lett. 2008, 121 (3–4), 255–259. 10.1007/s10562-007-9325-0. [DOI] [Google Scholar]
- Kumar V.; Tomar S.; Patel R.; Yousaf A.; Parmar V. S.; Malhotra S. V. FeCl3 -Catalyzed Pechmann Synthesis of Coumarins in Ionic Liquids. Synth. Commun. 2008, 38 (15), 2646–2654. 10.1080/00397910802219569. [DOI] [Google Scholar]
- Das S.; Majee A.; Hajra A. A Convenient Synthesis of Coumarins Using Reusable Ionic Liquid as Catalyst. Green Chem. Lett. Rev. 2011, 4 (4), 349–353. 10.1080/17518253.2011.572296. [DOI] [Google Scholar]
- Khaligh N. G. Synthesis of Coumarins via Pechmann Reaction Catalyzed by 3-Methyl-1-Sulfonic Acid Imidazolium Hydrogen Sulfate as an Efficient, Halogen-Free and Reusable Acidic Ionic Liquid. Catal. Sci. Technol. 2012, 2 (8), 1633. 10.1039/c2cy20196h. [DOI] [Google Scholar]
- Shaterian H. R.; Aghakhanizadeh M. Ionic-Liquid-Catalyzed Green Synthesis of Coumarin Derivatives under Solvent-Free Conditions. Chin. J. Catal. 2013, 34 (9), 1690–1696. 10.1016/S1872-2067(12)60654-8. [DOI] [Google Scholar]
- Lakouraj M. M.; Bagheri N.; Hasantabar V. Synthesis and Application of Nanocrystalline-Cellulose-Supported Acid Ionic Liquid Catalyst in Pechmann Reaction. Int. J. Carbohydr. Chem. 2013, 2013, 1–8. 10.1155/2013/452580. [DOI] [Google Scholar]
- Rather I. A.; Wagay S. A.; Hasnain M. S.; Ali R. New Dimensions in Calix[4]Pyrrole: The Land of Opportunity in Supramolecular Chemistry. RSC Adv. 2019, 9 (66), 38309–38344. 10.1039/C9RA07399J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather I. A.; Wagay S. A.; Ali R. Emergence of Anion-π Interactions: The Land of Opportunity in Supramolecular Chemistry and Beyond. Coord. Chem. Rev. 2020, 415, 213327. 10.1016/j.ccr.2020.213327. [DOI] [Google Scholar]
- Rather I. A.; Ali R. Indicator Displacement Assays: From Concept to Recent Developments. Org. Biomol. Chem. 2021, 19 (27), 5926–5981. 10.1039/D1OB00518A. [DOI] [PubMed] [Google Scholar]
- Wagay S. A.; Rather I. A.; Ali R. Functionalized Calix[4]Pyrroles: Emerging Class of Ion-Pair Receptors in Supramolecular Chemistry. Mater. Today Proc. 2021, 36, 657–678. 10.1016/j.matpr.2020.04.648. [DOI] [Google Scholar]
- Wagay S. A.; Rather I. A.; Ali R. Functionalized Truxene Scaffold: A Promising Advanced Organic Material for Digital Era. ChemistrySelect 2019, 4 (42), 12272–12288. 10.1002/slct.201903076. [DOI] [Google Scholar]
- Alvi S.; Ali R. Synthetic Approaches to Bowl-Shaped π-Conjugated Sumanene and Its Congeners. Beilstein J. Org. Chem. 2020, 16, 2212–2259. 10.3762/bjoc.16.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R.; Alvi S. The Story of π-Conjugated Isotruxene and Its Congeners: From Syntheses to Applications. Tetrahedron 2020, 76 (35), 131345. 10.1016/j.tet.2020.131345. [DOI] [Google Scholar]
- Alvi S.; Ali R. Design, Synthesis and Photophysical Properties of Novel Star-Shaped Truxene-Based Heterocycles Utilizing Ring-Closing Metathesis, Clauson–Kaas, Van Leusen and Ullmann-Type Reactions as Key Tools. Beilstein J. Org. Chem. 2021, 17, 1374–1384. 10.3762/bjoc.17.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather I. A.; Ali R. A Catalytic and Solvent-Free Approach for the Synthesis of Diverse Functionalized Dipyrromethanes (DPMs) and Calix[4]Pyrroles (C4Ps). Green Chem. 2021, 23 (16), 5849–5855. 10.1039/D1GC01515J. [DOI] [Google Scholar]
- Rather I. A.; Ali R. Investigating the Role of Natural Deep Eutectic Low Melting Mixtures for the Synthesis of Symmetrical Bisamides. ChemistrySelect 2021, 6 (40), 10948–10956. 10.1002/slct.202103104. [DOI] [Google Scholar]
- Alvi S.; Ali R. An Expeditious and Highly Efficient Synthesis of Substituted Pyrroles Using a Low Melting Deep Eutectic Mixture. Org. Biomol. Chem. 2021, 19 (44), 9732–9745. 10.1039/D1OB01618K. [DOI] [PubMed] [Google Scholar]
- Ali R.Low Melting Mixture of L-(+)-Tartaric Acid and N,N′ -Dimethyl Urea: A New Arrival in the Green Organic Synthesis. In Current Topics in Chirality - From Chemistry to Biology; IntechOpen, 2021. [Google Scholar]
- Ahmad Rather I.; Ali R.. Anion-π Catalysis: A Novel Supramolecular Approach for Chemical and Biological Transformations. In Current Topics in Chirality - From Chemistry to Biology; IntechOpen, 2021. [Google Scholar]
- Isci A.; Kaltschmitt M. Recovery and Recycling of Deep Eutectic Solvents in Biomass Conversions: A Review. Biomass Convers. Biorefinery 2021, 10.1007/s13399-021-01860-9. [DOI] [Google Scholar]
- Sethna S.; Phadke R. The Pechmann Reaction. Organic Reactions 2011, 1–58. 10.1002/0471264180.or007.01. [DOI] [Google Scholar]
- Ansary I.; Taher A.. One-Pot Synthesis of Coumarin Derivatives. In Phytochemicals in Human Health; IntechOpen, 2020. [Google Scholar]
- Loncaric M.; Gaso-Sokac D.; Jokic S.; Molnar M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10 (1), 151. 10.3390/biom10010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.; Singh D.; Samant S. Sulphamic Acid - An Efficient and Cost-Effective Solid Acid Catalyst for the Pechmann Reaction. Synlett 2004, 1909–1912. 10.1055/s-2004-830858. [DOI] [Google Scholar]
- Rodríguez-Domínguez J.; Kirsch G. Zirconyl Chloride: A Useful Catalyst in the Pechmann Coumarin Synthesis. Synthesis 2006, 2006 (11), 1895–1897. 10.1055/s-2006-942362. [DOI] [Google Scholar]
- De S. K.; Gibbs R. A. An Efficient and Practical Procedure for the Synthesis of 4-Substituted Coumarins. Synthesis 2005, 8, 1231–1233. 10.1055/s-2005-865282. [DOI] [Google Scholar]
- Prajapati D.; Gohain M. Iodine a Simple, Effective and Inexpensive Catalyst for the Synthesis of Substituted Coumarins. Catal. Lett. 2007, 119 (1–2), 59–63. 10.1007/s10562-007-9186-6. [DOI] [Google Scholar]
- Kokare N. D.; Sangshetti J. N.; Shinde D. B. Oxalic Acid Catalyzed Solvent-Free One Pot Synthesis of Coumarins. Chin. Chem. Lett. 2007, 18 (11), 1309–1312. 10.1016/j.cclet.2007.09.008. [DOI] [Google Scholar]
- Jadhav N. H.; Sakate S. S.; Rasal N. K.; Shinde D. R.; Pawar R. A. Heterogeneously Catalyzed Pechmann Condensation Employing the Tailored Zn0.925Ti0.075O NPs: Synthesis of Coumarin. ACS Omega 2019, 4 (5), 8522–8527. 10.1021/acsomega.9b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi B.; Zareyee D. Design of a Highly Efficient and Water-Tolerant Sulfonic Acid Nanoreactor Based on Tunable Ordered Porous Silica for the von Pechmann Reaction. Org. Lett. 2008, 10 (18), 3989–3992. 10.1021/ol8013107. [DOI] [PubMed] [Google Scholar]
- Heravi M. M.; Khorasani M.; Derikvand F.; Oskooie H. A.; Bamoharram F. F. Highly Efficient Synthesis of Coumarin Derivatives in the Presence of H14[NaP5W30O110] as a Green and Reusable Catalyst. Catal. Commun. 2007, 8 (12), 1886–1890. 10.1016/j.catcom.2007.02.030. [DOI] [Google Scholar]
- Gao S.-T.; Li C.; Wang Y.; Ma J.-J.; Wang C.; Zhang J.-W. NbCl5 -Catalyzed, Solvent-Free, One-Pot Synthesis of Coumarins. Synth. Commun. 2011, 41 (10), 1486–1491. 10.1080/00397911.2010.486514. [DOI] [Google Scholar]
- Takeshi N. NII-Electronic Library Service. Chem. Pharm. Bull. 1977, 57 (534), 364–370. [Google Scholar]
- Timonen J. M.; Nieminen R. M.; Sareila O.; Goulas A.; Moilanen L. J.; Haukka M.; Vainiotalo P.; Moilanen E.; Aulaskari P. H. Synthesis and Anti-Inflammatory Effects of a Series of Novel 7-Hydroxycoumarin Derivatives. Eur. J. Med. Chem. 2011, 46 (9), 3845–3850. 10.1016/j.ejmech.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Jung K.; Park Y.-J.; Ryu J.-S. Scandium(III) Triflate–Catalyzed Coumarin Synthesis. Synth. Commun. 2008, 38 (24), 4395–4406. 10.1080/00397910802369513. [DOI] [Google Scholar]
- Pisani L.; Muncipinto G.; Miscioscia T. F.; Nicolotti O.; Leonetti F.; Catto M.; Caccia C.; Salvati P.; Soto-Otero R.; Mendez-Alvarez E.; Passeleu C.; Carotti A. Discovery of a Novel Class of Potent Coumarin Monoamine Oxidase B Inhibitors: Development and Biopharmacological Profiling of 7-[(3-Chlorobenzyl)Oxy]-4-[(Methylamino)Methyl]-2 H -Chromen-2-One Methanesulfonate (NW-1772) as a Highly Potent, Selective, Reve. J. Med. Chem. 2009, 52 (21), 6685–6706. 10.1021/jm9010127. [DOI] [PubMed] [Google Scholar]
- Wu J.; Diao T.; Sun W.; Li Y. Expeditious Approach to Coumarins via Pechmann Reaction Catalyzed by Molecular Iodine or AgOTf. Synth. Commun. 2006, 36 (20), 2949–2956. 10.1080/00397910600773692. [DOI] [Google Scholar]
- Gumuş A.; Karadeniz S.; Ugras H. İ.; Bulut M.; Çakır U.; Goren A. C. Synthesis, Complexation, and Biological Activity Studies of 4-Aminomethyl-7,8-Dihydroxy Coumarines and Their Crown Ether Derivatives. J. Heterocycl. Chem. 2010, 47 (5), 1127–1133. 10.1002/jhet.435. [DOI] [Google Scholar]
- Koutsoukos S.; Tsiaka T.; Tzani A.; Zoumpoulakis P.; Detsi A. Choline Chloride and Tartaric Acid, a Natural Deep Eutectic Solvent for the Efficient Extraction of Phenolic and Carotenoid Compounds. J. Clean. Prod. 2019, 241, 118384. 10.1016/j.jclepro.2019.118384. [DOI] [Google Scholar]
- Amoozadeh A.; Ahmadzadeh M.; Kolvari E. Easy Access to Coumarin Derivatives Using Alumina Sulfuric Acid as an Efficient and Reusable Catalyst under Solvent-Free Conditions. J. Chem. 2013, 2013, 1–6. 10.1155/2013/767825. [DOI] [Google Scholar]
- Rafiee E.; Fakhri A.; Joshaghani M. Coumarins: Facile and Expeditious Synthesis via Keggin-Type Heteropolycompounds under Solvent-Free Condition. J. Heterocycl. Chem. 2013, 50 (5), 1121–1128. 10.1002/jhet.621. [DOI] [Google Scholar]
- Zavrsnik D.; Muratovic S.; Makuc D.; Plavec J.; Cetina M.; Nagl A.; Clercq E. De; Balzarini J.; Mintas M. Benzylidene-Bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-Ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations. Molecules 2011, 16 (7), 6023–6040. 10.3390/molecules16076023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglari M.; Shirini F.; Mahmoodi N. O.; Zabihzadeh M.; Safarpoor Nikoo Langarudi M.; Alipour Khoshdel M. Taurine/Choline Chloride Deep Eutectic Solvent as a Novel Eco-Compatible Catalyst to Facilitate the Multi-Component Synthesis of Pyrano[2,3- d ]Pyrimidinone (Thione), Hexahydroquinoline, and Biscoumarin Derivatives. Polycycl. Aromat. Compd. 2020, 1–22. 10.1080/10406638.2020.1781212. [DOI] [Google Scholar]
- Khan K. M.; Iqbal S.; Lodhi M. A.; Maharvi G. M.; Ullah Z.; Choudhary M. I.; Rahman A.; Perveen S. Biscoumarin: New Class of Urease Inhibitors; Economical Synthesis and Activity. Bioorg. Med. Chem. 2004, 12 (8), 1963–1968. 10.1016/j.bmc.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Halder B.; Maity H. S.; Nag A. One Pot Synthesis of Biscoumarins and Pyranocoumarins by Coconut Juice as a Natural Catalyst. Curr. Organocatalysis 2019, 6 (1), 20–27. 10.2174/2213337206666190219142408. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






