Abstract
Carbon dioxide (CO2) in enhanced oil recovery (EOR) has received significant attention due to its potential to increase ultimate recovery from mature conventional oil reserves. CO2-enhanced oil recovery (CO2-EOR) helps to reduce global greenhouse gas emissions by sequestering CO2 in subterranean geological formations. CO2-EOR has been exploited commercially over recent decades to improve recovery from light and medium gravity oil reservoirs in their later stages of development. CO2 tends to be used in either continuous flooding or alternated flooding with water injection. Problems can arise in CO2-flooded heterogeneous reservoirs, due to differential mobility of the fluid phases, causing viscous fingering and early CO2 penetration to develop. This study reviews the advantages and disadvantages of the techniques used for injecting CO2 into subsurface reservoirs and the methods adopted in attempts to control CO2 mobility. Recently developed methods are leading to improvements in CO2-EOR results. In particular, the involvement of nanoparticles combined with surfactants can act to stabilize CO2 foam, making it more effective in the reservoir from an EOR perspective. The potential to improve CO2 flooding techniques and the challenges and uncertainties associated with achieving that objective are addressed.
1. Introduction
Gas injection has been used since the early twentieth century to maintain fluid pressure in subsurface oil reservoirs, predating the use of waterflooding. Ultimately, waterflooding became widespread because it was a more effective flushing agent than gas. This is a consequence of the low viscosity of gas, which is 10–15 times less than that of water. This property makes gas highly mobile at reservoir conditions, causing it to quickly break through into production wells via highly permeable reservoir layers. This reduces oil flow and recovery rates and limits the drainage of the reservoirs by fluid displacement.1 To date, many enhanced oil recovery (EOR) methods have been developed and improved. These include gas injection methods applied to certain types of reservoirs.
Under sustainable resource development scenarios, total EOR production is forecast, by some, to increase by 2040 to about 4 million barrels per day (Figure 1).2 However, this figure is highly sensitive to the oil demand and prices that materialize over the next two decades. Additional political support for carbon capture use and storage (CCUS) efforts has substantially increased interest in CO2-EOR in recent years. Mitigating carbon emissions and their negative climate impacts has become a key driver in the selection of EOR technologies. Under suitable geological conditions, CO2-EOR offers an attractive method to reduce the emission intensity of oil supplied to the market and thereby reduces its environmental footprint. For many reservoirs, it can offer improvements both in oil production rates and in expected ultimate oil recovery (EUR).2
Figure 1.
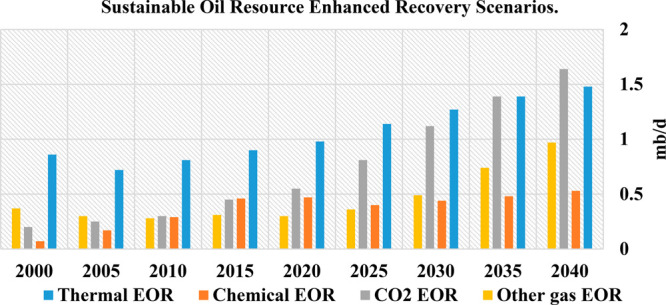
Sustainable oil resource enhanced recovery scenarios, 2000–2040.
Many technologies that require the capture and storage of CO2 remain in the developmental stage. Moreover, there are two distinct approaches to capturing and storing carbon to prevent its accumulation in the atmosphere: natural and industrial. The natural approaches include reforestation, afforestation, and initiating a number of other environmentally friendly land use changes (e.g., rewilding farmland and expansion of wetlands and peat bogs). The industrial approaches require the construction of installations to capture CO2 directly from air and/or from industrial plants (pre- and postfuel combustion) and disposal of the captured CO2 in subsurface reservoirs, either aquifers or depleted oil and gas fields.
In order to stop large amounts of CO2 from being released into the atmosphere from the consumption of various resources, such as fossil fuels used in power generation and in many other industrial and manufacturing processes (e.g., petrochemicals, refining, steel, glass, and cement plants), it needs to be captured, reused, and ultimately stored away (sequestered). One of the most promising ways to do this, at an effective scale, is to capture, transport, and ultimately pump CO2 into underground geological features. This offers the potential to provide long-term (i.e., thousands of years) safe storage of CO2 isolated from the atmosphere, with geological barriers preventing its long-term seepage back into the atmosphere. This subsurface geoengineering sequestration approach can also be coupled with the direct removal of CO2 from ambient air.
Long-term subsurface CO2-EOR is a relatively new concept. The first commercial-scale pilot study was in the Weyburn oil field (Midale, Saskatchewan, Canada) in 2000. Moreover, a comprehensive pilot-scale project involving carbon capture and storage (CCS) was initiated at the Schwarz–Pumpe power plant in eastern Germany in 2008. That project’s objectives were to answer technical questions about the feasibility and the cost-effectiveness of CCS. In that pilot plant, it was observed that CO2 emissions to the atmosphere were reduced by approximately 80–90% compared to plants without (CCS). By 2100, the IPCC projects that the economic potential of CCS may account for 10–55% of the total CO2 emissions. Moreover, oil and gas companies have a substantial competitive advantage to commercially capitalize on this environmental solution since they have abundant sources of CO2 emissions to deal with and multiple mature/partially depleted subsurface reservoirs into which that CO2 could be pumped. That set of circumstances indicates that oil companies could effectively create a new niche market for themselves in large-scale CCUS implementations.3
Theoretically, if enough CO2 could be extracted from plant emissions and/or directly from the atmosphere and stored away for the long term underground, there could be negative carbon emissions from the oil and natural gas sectors. CO2-EOR has the potential to be applied to both conventional and unconventional (shale) oil reservoirs.2 A distinctive feature of the method is that it could be applied or introduced at any stage of oil field development.4 CO2-EOR offers the potential to substantially increase oil production and recovery from certain fields. Moreover, there are associated economic benefits in applying CO2-EOR, such as the low cost of CO2, the possibility of recycling it, and producing high-quality marketable oil.3
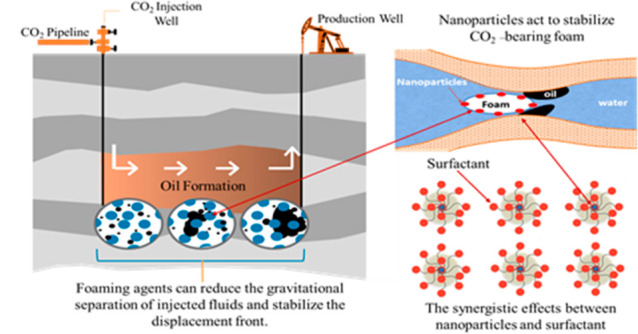
Various studies have evaluated the nature of the interaction of methane, nitrogen, and CO2 with crude oil at reservoir conditions.5 Their results reveal that CO2 behaves in a preferrable manner compared to other gases under EOR reservoir conditions. It is more readily dissolved into oil and water at relatively low-temperature and -pressure conditions, making CO2-EOR a more cost-effective process. The relative dynamic viscosity of CO2 is two to three times higher than the viscosity of the compared gases. This property is of great importance since the ratio of the viscosity of the gas and the formation fluid determines for how long the injected agent will displace oil before it breaks through into the producing wells. The compressibility of CO2 also differs significantly from the compressibility of methane and nitrogen, especially at high pressures.4 The power consumption of the compressors, spent on compressing gases during their transportation and injection into the reservoir, depends on the degree of compressibility of the gas.4 The main problem encountered in CO2-EOR projects tends to be the premature breakthrough of gas into the producing wells. In order to reduce the mobility of CO2 and increase its exposure to displacing reservoir fluids, it is possible to add various chemical agents to the injected fluids. In particular, surfactants laced with nanoparticles (NPs) tend to form stable foams with increased viscosity, thereby improving the flushing efficiency of the CO2-EOR process. The use of foam as the injected media facilitates improved oil displacement compared to waterflooding or simply compressed CO2 gas injection.6 Due to the high chemical stability of NPs, even under harsh reservoir conditions, and their strong selective adsorption at target liquid–liquid interfaces, the use of NPs to form emulsions and foams has received much attention. NP surface treatments can be used to target specific molecules, thereby promoting the formation of a CO2/water foam without the formation of oil/water emulsions.7 In this process, NPs and nanofluids influence the interfacial tension (IFT), rheological properties, and wettability of surfaces. Polymer solutions and surfactants can be modified by adding NPs to alter the rock wettability, reduce IFT, and improve rheological properties. These benefits offer the potential for nanotechnologies to revolutionize the field extraction techniques exploited by the oil and gas industry.8
The mechanism of coinjecting CO2 with dispersed NPs to generate NP-stabilized foam requires a threshold shear rate. In permeable reservoirs, high shear rates tend to occur in the main flow channels typically located in the highest permeability zones.4 These characteristics increase the possibility of creating “self-conducting” fluids that selectively decrease the mobility of CO2 by generating foam only in areas where CO2 flows quickly, such as in fractured zones and/or gravity-impacted areas with relatively low residual oil concentrations. Such foam tends to combine with residual oil, leading to higher oil recovery rates associated with the flowing CO2 foam streams.9
This study reviews the techniques for injecting CO2 into subsurface reservoirs and the advantages and disadvantages of the different techniques (Section 2). The methods for controlling CO2 mobility are explained (Section 3). The described and compared methods are CO2–water alternating gas (WAG) injection (Subsection 3.1), polymer additives for direct thickening of CO2 (Subsection 3.2), in situ polymer gels for CO2 conformance control (Subsection 3.3), preformed polymer gels for CO2 conformance control (Subsection 3.4), surfactant-assisted CO2 mobility control (Subsection 3.5), and NP-enhanced CO2 flooding (Section 4). Recent developments and emerging methods aimed at improving CO2-EOR performance are identified. In particular, the beneficial influences of certain NP properties in stabilizing surfactant foams are recognized, including the size, surface wettability, hydrophilic characteristics, and surface charge of the NP. The findings are summarized, and future requirements for further improving CO2-EOR are recommended (Section 5).
2. Techniques for Injecting CO2 into Subsurface Reservoirs
Displacement of reservoir crude oil by CO2 is a complex process that includes mass transfer, capillary forces, and gravitational effects. Partial or complete miscibility of CO2 with oil entails a change in its rheological properties and contributes to its ability to flush previously immoveable oil from the reservoir. Fluid saturation conditions in the reservoir and prior moveable oil displacement and recovery have significant impacts on the process of displacing additional oil with the aid of CO2.7Table 1 identifies the advantages and disadvantages of injecting CO2 in various forms into subsurface reservoirs.
Table 1. Advantages and Disadvantages of Various Approaches to Injecting CO2 into Subsurface Reservoirs7,9,10.
| approach | definition | advantages | disadvantages | limitations |
|---|---|---|---|---|
| carbonated water injection | carbonated water injection is the simplest way of supplying CO2 to reservoirs and involves the injection of water saturated with CO2 to the maximum of (3–5 wt %) | displacement of oil by carbonated water is accompanied by a significant lag of the front of the concentration of CO2 in water from the front of oil displacement, which depends on the distribution coefficient of CO2 between oil and water, its concentration in water, as well as the pressures and temperatures of the reservoir conditions; this leads to a significant increase in the time taken to obtain beneficial effects from the use of technology | relatively low consumption of CO2 (6–7 times less) during injection into the reservoir compared to other techniques | low permeability or moderately homogeneous reservoirs are the only suitable candidates for this process |
| continuous CO2 injection in the gaseous phase | this method is widely used to displace residual oil in a flooded reservoir by continuous injection of carbon dioxide | it achieves a higher oil displacement ratio than other CO2 technologies; this is due to the fact that in front of the advancing front of CO2 an oil swell is formed, which is characteristic of processes with miscible displacement | rapid breakthroughs of CO2 to production wells through highly permeable formations, gravity separation, and significant reduction in sweep efficiency are possible | after primary recovery, this technique is suitable for reservoirs with light to medium gravity oil and those that are highly flooded or strongly water-wet |
| displacement of oil by CO2 alone requires large expenditures | ||||
| cyclic injection of CO2 into injection wells | this method makes it possible to develop low-permeability, heterogeneous reservoirs by cyclic injection of CO2; this improves upon continuous CO2 injection which tends to be less effective, more costly, and impractical | it is possible to increase both the coefficient of oil displacement by CO2 and the coefficient of reservoir sweep, as well as to minimize the risk of gas breakthrough into the producing wells | displacement of oil by CO2 alone requires large expenditures | suitable for reservoirs exhibiting different zones of permeability. |
| injection of mixtures of captured flue gases | compositions of flue gases vary but typically include air-polluting components, some of which including CO2 could be captured | flue gases are low cost to obtain, and their sequestration would serve to reduce air pollution of the environment | gases other than CO2 mix well only with lighter crude oils | strong dependence of the displacement of oil by gaseous CO2 slugs on the conditions of gravity separation limits the use of this technology in reservoirs with high vertical permeability. |
| alternating injection of CO2 and water | by injecting the required volume of CO2 in small portions, alternately or simultaneously with water, a higher efficiency of CO2 EOR can be achieved; in this case, the duration between cycles depends on the density of the well spacings and patterns of injectors and producers; that duration can vary from several hours to a month or more | effective way of enhancing oil recovery for low-permeability oil reservoirs | low permeability and poor pore space connectivity in some reservoirs limit injection rates | technology can be applied as part of an existing reservoir pressure maintenance system and used both at individual wells and/or across an entire reservoir involving multiple wells |
| in heterogeneous formations with high crude oil viscosity, the quantities of water and gas injected should be lower | ||||
| surface equipment toggling between CO2 and water injection is required | ||||
| CO2-foam injection technology | these methods are based on the use of foams, a process that controls the movement of CO2 by generating foam (dispersing CO2 in a liquid phase) | use of foam helps to achieve better oil displacement compared to waterflooding or the above CO2 injection technologies | CO2-foam injection technology is a lengthy process | this technology is suitable for highly heterogeneous reservoirs with some highly permeable/porous layers and some oil-depleted zones with high water saturations |
| the method reduces the gravitational separation of fluids and stabilizes the displacement front | composition of the surfactant is likely to be unstable under harsh reservoir conditions | |||
| foaming surfactant solutions more strongly reduce the relative permeability of the pore space for gas than water; foam surfactant properties can be improved with NP additives | possibility exists for superficial absorption of the foams by the reservoir rocks; moreover, in some reservoirs, the surfactant around CO2 bubbles might not be effective as foam flows through constricted pore throats | |||
| gas-cyclic injection and production through the same wellbores (“huff-n-puff” process) | this involves the injection of CO2 directly into a production well with its subsequent shutdown for soaking the bottom hole formation zone and subsequent oil production | this technology can significantly reduce capital costs for the implementation of the technology since it does not require the construction of special injection wells | asphaltene dropout | injection of CO2 under supercritical conditions is suitable for deep low-permeability reservoirs and those associated with water drives |
| this method makes it possible to implement the delivery of liquefied CO2 by road, which can be a more profitable option in some cases | possible equipment corrosion | |||
| need to separate and capture recycled CO2 from oil and associated gas ultimately produced | ||||
| stability of equipment seals |
3. Methods for Controlling CO2 Mobility
Controlling CO2 mobility makes it possible to increase oil recovery more cheaply and efficiently, with associated positive effects that mitigate CO2 entering the atmosphere.4 There are several ways to adjust the CO2 phase when injecting it into the reservoir to increase oil recovery:7
-
1.
CO2–water alternating gas (WAG) injection,
-
2.
Polymer additives for direct thickening of CO2,
-
3.
In situ polymer gels for CO2 conformance control,
-
4.
Preformed polymer gels for CO2 conformance control,
-
5.
Surfactant-assisted CO2 mobility control, and
-
6.
NP-enhanced CO2 flooding.
3.1. CO2–WAG Injection
CO2–WAG injection is a combination of conventional waterflooding and CO2 gas injection. The CO2–WAG injection mechanism has its own characteristics for miscible or immiscible displacement conditions. Miscible displacement results in higher oil-displacement ratios than immiscible conditions. Oil sweep efficiency is higher for miscible conditions, where it involves an increase in filtration resistance, with three-phase filtration taking place in the formation. The feasibility of using CO2 immiscible oil displacement, even though it is less effective, is due to its lower process costs and the lower injection pressures involved.4 The process of injecting CO2 into a subsurface reservoir is illustrated schematically in Figure 2.
Figure 2.
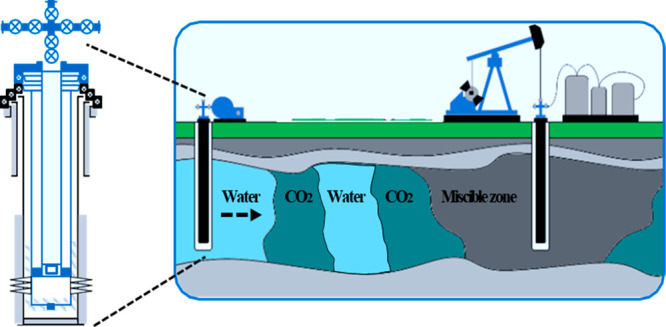
Schematic diagram of oil displacement in a reservoir by water-alternating gas (WAG) CO2 injection.
Unlike water, which occupies small hydrophilic pores and constrictions in the flooded zones of a reservoir formation under the action of capillary forces, the gas injected into the formation, as a nonwetting phase, on the contrary occupies large hydrophobic pores. Under the action of gravitational forces, it also occupies the attic portions of the formation trap. These distinct behaviors of the oil and gas phases in reservoirs and their influences on oil displacement make it more effective to maintain reservoir pressure by injecting both water and gas. This approach tends to flatten the oil displacement profile and increase the reservoir sweep.6 However, technologies for joint water–gas treatment and handling are not yet widely available. The main problem is the limit of existing equipment and technology for effectively toggling the pumping of water and gas in a cyclical manner into injection wells in large volumes.7
3.2. Polymer Additives for Direct Thickening of CO2
Polymers applied as direct CO2 thickeners must be fully dissolved in CO2 under reservoir conditions to achieve an increase in the injected-fluid viscosity. Ideally, the thickener can increase the viscosity by 2–10 times at a concentration of 1 wt % or less.11 Polymers, such as polyfluoro-acrylate-styrene (polyFAST; a direct thickener based on CO2), are easily dissolved in CO2 and significantly increase its viscosity.11 There are two types of polymers used for the thickening of CO2: high molecular weight polymers, such as polydimethyl-siloxane (PDMS) and polyvinyl-acetate (PVAc),12 and low molecular weight polymers, such as polyvinyl-ethyl-ether (PVEE) and poly-1-decene (P1D).12
This method makes it possible to control the mobility of CO2 while directly increasing its viscosity. Some polymers can be dissolved in CO2 to form a single-phase and thermodynamically stable solution, thereby improving the density and viscosity of the injected fluid. The main limitation of this method is that as polymer injection progresses it tends to display a reduction in solubility. The suitability of this method also depends on the prevailing pressure conditions in the reservoir.11
3.3. In Situ Polymer Gels for CO2 Conformance Control
In situ gels go through their formational stage to generate a three-dimensional molecular structure within the reservoir formation following injection. The pre-cross-linked injected gels form a three-dimensional structure, either during synthesis at reservoir temperatures and pressures or during formulation preparation immediately before injection into the formation. The introduction of these molecular structures to the reservoir causes a reduction in its permeability. Since gelation occurs within the formation, there are typically no major constraints associated with their injection capacity or problems resulting from high flow rates within the formation. The suitability of this method also depends on the reservoir formation conditions.13,14
In situ polymer gel formulations consist of two main components: a high molecular weight polymer and a cross-linking agent that is capable of forming bonds with the corresponding fragments of the polymer chain.13 Compounds used as cross-linking agents tend to be derivatives of phenol and formaldehyde (in particular, 2,4,6-hydroxymethylolphenol); a mixture of pyrocatechol, resorcinol, and pyrogallol, which has been successfully tested at a temperature of 150 °C; and polyethylenimine for cross-linking polyacrylamides (PAM) at 130 °C.15
3.4. Preformed Polymer Gels for CO2 Conformance Control
The preformed gel systems involve generating the gels at the surface before injection. The fully formed gels are then injected into the formations without further gel formation within the reservoir. This method tends to display limited sensitivity to the reservoir conditions because the gels are formed before injection and maintain their chemical characteristics according to the polymer gel design. The main limitation of this method is that it requires highly permeable reservoir conditions to work effectively.7
3.5. Surfactant-Assisted CO2 Mobility Control
Foaming agents can reduce the gravitational separation of injected fluids and stabilize the displacement front. Foam-forming surfactant solutions tend to reduce the relative permeability of the pore space for gas ten times more than water. Figure 3 illustrates diagrammatically how surfactants work in a reservoir. Molecules of water and oil repel each other, so it is impossible to completely flush the oil with water alone. Molecules of surfactants are hydrophilic at one end, meaning that they are attracted to water molecules, whereas at the other end, they are hydrophobic and lipophilic, meaning that they repel water but are attracted to fats or oils. This unique property allows them to reduce the surface tension between water and oil. As a result, large drops of oil are broken up by water with a surfactant into even smaller droplets. Detergents, which also contain surfactants, act on the same principle. Water cannot wash away greasy dirt as hydrophobic fat molecules repel water. However, armed with surfactants, water begins to progressively remove small pieces from oil-covered surfaces and carry them away.16 The main beneficial action of surfactants in an oil reservoir with substantial water saturation is that they reduce the surface tension between oil and water and increase the contact angle, causing the wetting tension to decrease 8–10 times.10,17
Figure 3.
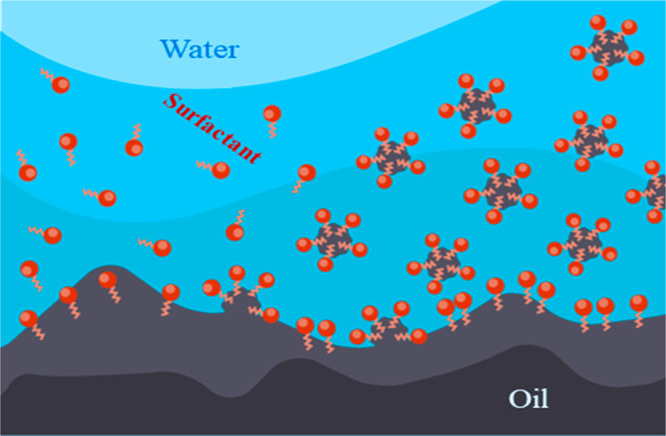
Diagrammatic illustration of the microstructure of foam in porous media.
Surfactant foams can significantly reduce the possibility of CO2 breakthrough and selectively slow down its progress in highly permeable reservoir interlayers. The formation of foam occurs in the pore space following the injection of a foaming agent (surfactant) and CO2. Two methods are distinguished: (1) simultaneous injection of components (coinjection), where the quality of the foam is determined by the fraction of CO2 in the mixture, and (2) surfactant-alternating gas (SAG) injection, where the quality of the foam depends on the proportions of both components (CO2 and surfactant).16 Generally, there are many types of surfactant-stabilized foams used in EOR. These are classified as anionic, cationic, nonionic, biosurfactant, and zwitterionic surfactants. The surfactants commonly used in conjunction with CO2 are described in Table 2.
Table 2. Types of Surfactant-Stabilized Foams Used in EOR16.
| class | structure | soluble T in the water phase | CO2 solubility | adsorption |
|---|---|---|---|---|
| cationic | alkylamines | - | - | |
| quaternary | insoluble | high | ||
| ammonium salts (protonated) | ≤120 °C | insoluble | high | |
| ethoxylated amines | high | low | ||
| anionic | sulfonates | - | insoluble | low |
| nonionic | alkyl ethoxylates | <100 °C | high | low |
| zwitterionic | alkyl betaine | ≤99 °C | insoluble | high |
Tests have shown that multiple SAG cycles make it possible to generate foam with an apparent viscosity of 120 mPa·s, which is almost two times more than with the combined injection of a foaming agent and CO2 (56 mPa·s).18 Those tests also revealed that the additional oil recovery associated with the use of foaming surfactants and CO2 averaged 30%. By applying a combined water–gas treatment model together with foaming surfactants (FAWAG), it is possible to obtain a more stable oil displacement profile without premature breakthroughs and viscous tongues (Figure 4). Experiments using oil displacement technologies with simultaneous injection of CO2 and water rims (SWAG) and FAWAG on a core model showed that a higher oil recovery rate of up to 92% could be achieved.16 These methods, based on the use of foams, can be applied to oil production from highly heterogeneous reservoirs with highly permeable and porous interlayers, as well as sections, including water-saturated intervals.16
Figure 4.
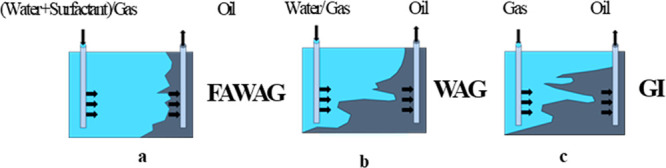
Illustration of the benefits of (a) FAWAG injection in a reservoir, (b) conventional WAG injection, and (c) continuous gas injection. The gray shaded areas represent higher oil-saturated zones; the blue shaded areas represent higher water-saturated zones.
4. NP-Enhanced CO2 Reservoir Flooding
NPs are now widely applied in many fields of research and industrial applications. They offer useful ways to improve the performances of subsurface fluids injected into wellbores and the flow behavior of formation fluids. NPs are adept at penetrating subsurface porous media, enabling them to travel long distances through the pore spaces and flow channels within reservoir formations. Their impacts are assisted by interactions between injection and pore fluids, making it possible for them to target specific zones and influence fluid-flow characteristics deep within oil reservoirs (see Table 4).9
Table 4. Advantages and Disadvantages of Recent Methods in Controlling CO2 Mobility5−7,9,10,12.
| methods | definition | advantages | disadvantages | limitations |
|---|---|---|---|---|
| CO2 flooding enhanced by nanoparticles | liquids and supercritical CO2 have a viscosity enhancement effect and stabilize CO2 foams due to NPs | decreases the IFT between CO2 and oil | NPs can be harmful to health and the environment due to their small size | high cost of NPs, potentially negative health and environmental impacts, and NP agglomeration (if it occurs) |
| improves the viscosity of the CO2 foam | some NPs tend to agglomerate under certain reservoir conditions, which can affect the stability of the injected nanofluids | |||
| certain types of NPs can increase the dispersion of CO2 in aqueous polyacrylamide solutions | ||||
| control of CO2 mobility with surfactants | in liquid, CO2 can be dispersed through foam formation | surfactants have the ability to prevent the free flow of CO2 by reducing CO2 relative permeability and CO2 flow rate | screening surfactants have a lengthy process | surfactant formulations are prone to instability in harsh reservoir conditions if not properly designed |
| eases the impacts of channel separation, viscous fingering, and gravity | possibility of reservoir rock adsorption of surfactants | |||
| reduces IFT and changes wettability | ||||
| flooding CO2 with polymer-enhanced polymers | formation’s permeability can be reduced by injecting preformed gels or in situ gels | high mobility across the formation | there may be injection problems that affect the size of the area the gel reaches | their use is mainly limited to oil and gas reservoirs with extremely high permeability |
| no problems with injection if gelation occurs in the formation | sensitivity of polymer gels to reservoir conditions, which affects gel properties | |||
| if the gel is formed prior to injection, the sensitivity to reservoir conditions is low | ||||
| CO2 polymers for direct thickening | CO2 has its viscosity increased by direct thickening with polymers | a variety of polymers can dissolve in CO2 to create single-phase and stable solutions; this improves the density and viscosity of CO2 and thereby enhances oil recovery | decrease in the solubility of a polymer with an increase in its molecular weight; moreover, sensitivity influences crystallinity formation, chemical nature of the side-chain moieties, and tacticity | solubility of many polymers requires supercritical conditions |
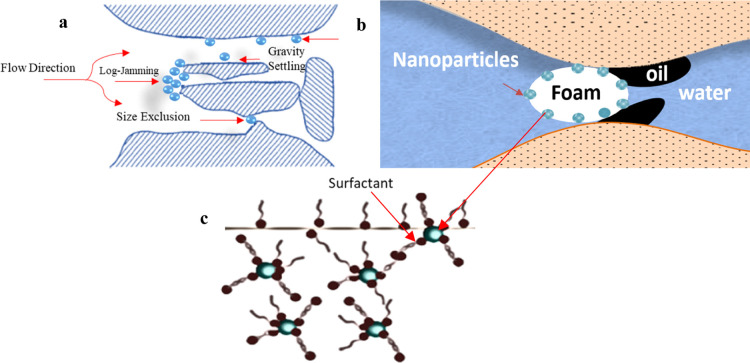
In particular, adding NPs to surfactant foam tends to improve the foam’s stability; this is due to the NP’s beneficial properties such as not being affected by certain characteristic conditions commonly encountered in oil reservoirs, e.g., high temperatures and the presence of a range of hydrocarbons and/or salts. In addition, due to their small size, the NP’s flow through porous media is often not substantially impeded physically by the reservoir matrix, resulting in minimal changes to the formation permeability. Also, the absorption of foam by reservoir rocks is negligible.19 Moreover, the materials from which the required NPs are derived, such as coal ash, can be obtained at low cost. Due to their grafting properties, the wettability of NPs can be relatively easily modified to produce foams of durable stability in the subsurface.17 The degree and durability of a foam’s stability depends on several factors, including the synergy between NP size and the foam, the wettability of NP surfaces, NP concentration, NP surface charge, surfactant charge, and the formation of water salinity; reservoir oil saturation and temperature, crude oil properties, fluid flow rates within the reservoir; and foam absorption by the formation matrix.9
4.1. Effects of NP Size on CO2 Foam Performance
Three sizes of silica NPs (20, 100, and 500 nm) have been used to test foam stability.10 The 20 nm size NP is the most effective at improving foam stability. The 20 nm NPs act to create more coherent layers within the foam to prevent it from coarsening and coalescing. Adding a range of different NPs (SiO2 A300 (hydrophilic), SiO2 R816 (hydrophobic), ZnO, TiO2) to the MFomax surfactant revealed that the stability of foam could be most improved by SiO2 A300 because of its higher surface area to particle size ratio. Also, surfactants can attach to NP surfaces and promote their catalytic activity on the basis of their greater surface area/smaller particle size. As a consequence of surfactant molecules attaching to NP surfaces, they form steric layers of lamellae which tend to resist shrinkage and expansion. This feature assists foams in remaining stable during storage and transport.7
4.2. Effects of NP Surface Wettability on CO2 Foam Performance
NP surface wettability depends on the ratio between the adhesion forces of liquid molecules and molecules (or atoms) of the wetted body/surface (adhesion) and the forces of mutual adhesion of liquid molecules (cohesion).15 The influence of NP surface wettability on the stability of foams has been evaluated using Aerosil SiO2 as a hydrophobic component, showing that the stability of foams increased the contact angle by 26°–56°. Fumed SiO2 AEROSIL816 and SiO2 AEROSIL300 added to sodium dodecyl sulfate surfactant revealed that the wettability of the rock surface tends to determine and control, to a large degree, the location, distribution, and flow of fluids within a particular reservoir.9
4.3. Effects of NP Hydrophilic Concentration on CO2 Foam Performance
It is now well established that the presence of hydrophilic NPs improves the stability of foam. The rheological properties of supercritical CO2 foam at different temperatures, foam qualities, and pressures are informative in this regard.20 The application of the synergy between NPs and polyoxyethylene lauryl ether (C12E23) in stabilizing CO2 foam was evaluated with static-stability tests, pore visualization via microscopic models, and sandbag-flooding tests. NP-C12E23 exhibits high salinity and temperature tolerance. It has excellent profile control and water blocking capabilities, and in the experiments performed, the oil recovery rate increased by 20.1% following water injection. Most studies to date have been limited to one type of NP or surfactant. Static plus dynamic tests that evaluate the relative effectiveness of various NP types and surfactants in stabilizing CO2 foam under subcritical and supercritical conditions have yet to be adequately performed.20
4.4. Interactions between NP Surface Charge and the Net Charge of Surfactants
Oppositely charged components of NP–surfactant systems tend to interact. For instance, the adsorption of surfactant molecules on the surface of NPs is stimulated due to electrostatic attraction. By increasing the surfactant concentrations, the end groups of micelles tend to be more readily adsorbed, leading to an increase in viscosity.21 The interaction effects associated with the surface charge of NPs and the net charge of surfactants are widely studied. For example, Zhao et al.22 used polydimethyl-siloxy (PDMS) coated with SiO2 NPs to test aerosol-OT (AOT; or sodium bis(2-ethylhexyl) sulfosuccinate), which is an anionic surfactant. The surface charge and the net charge interactions between NPs and surfactants improve the stability of foam through different mechanisms, and a longer foam half-life has been reported between NPs and oppositely charged surfactants. This suggests that electrostatic attraction is the key process helping NPs to improve the stability of foam. Determining the optimal concentrations of oppositely charged NPs and surfactants is important in preventing NP accumulation and sedimentation in solution, as the overall charge on the NP surface becomes partially neutralized.7,22
4.5. Effects of Salinity on NP-Enhanced CO2 Foam Performance
Salts can have complex impacts on foam stability. The effects of salts, particularly NaCl and CaCl2, have been evaluated.23 The drainage of NaCl-containing foams is reduced compared to salt-free foams, whereas CaCl2-containing foams did not exhibit a consistent trend. In addition to the type and concentrations of polymer additives and their molecular weights, the type and concentration of salts can also have a great influence on CO2-foam’s rheological properties. High concentrations of NaCl and CaCl2 added to sulfobetaine (LHSB) surfactant tend to reduce the adsorption properties of that surfactant, resulting in less NP adsorption at the gas–liquid interfaces.24 Moreover, for silica dioxide NP–sodium dodecyl sulfate (SiO2–SDS) foam, the half-life of the foam decreases with increasing salt concentration from 0 wt % up to 0.5 wt %. On the other hand, when the salt concentrations exceed 0.5 wt %, the half-life of the foam increases, resulting in lower surface tension and zeta potential. As salt (such as NaCl) is introduced into the foam system, the electric double is layer compressed, allowing more SDS and SiO2 particles to be adsorbed, thereby reducing the surface tension and improving the stability of the foam. However, the adsorption of surfactant onto the kaolinite is controlled by electrostatic forces. Adding salt will decrease electric double layers and zeta potential. Thus, a decreased electric repulsion will lead to increased adsorption on kaolinite.9,17
4.6. Effects of Temperature on NP-Enhanced CO2 Foam Performance
Temperature influences on the stability of NP-enhanced CO2 foam are complex and are associated with a number of competing processes. As temperature rises, the evaporation of solvents and foaming agents tends to increase, and depending on the concentration of the foaming agent and its structure, the stability of the foam could increase or decrease as a consequence. The presence of SiO2 NPs and/or Al2O3 NPs in the foam tends to slow down the rate of release of liquid, thereby slowing down the process of bubble coalescence.25 This impact ultimately increases the half-life and stability of the foam at any temperature. The Al2O3 NP, regardless of the pH value of the system, was found to exhibit a better stabilizing effect than SiO2 NPs at all temperatures tested. Foam stability in the presence of NPs decreases with increasing temperature.17,25
4.7. Effects of Flow Characteristics on NP-Enhanced CO2 Foam Performance
The influence of fluid-flow characteristics on the stability of NP-enhanced CO2 foam has been extensively evaluated. The bulk apparent viscosity of foam can be improved by about 15% in the presence of NPs with modified surfaces.26 The apparent viscosity of foam in capillary-containing porous media was found to be four times higher than that measured in a capillary viscometer. The porous media’s permeability increased as a consequence of the foam’s higher apparent viscosity. The higher apparent viscosity achieved by foam stabilized with surface-modified NPs can enhance pore fluid diversion and pore blocking processes, particularly in heterogeneous reservoirs.26
4.8. Effects of Hydrocarbons on NP-Enhanced CO2 Foam Performance
The effect of petroleum products on NP-enhanced CO2 foam stability is also complex. The defoaming effects of hydrocarbons on foam are usually manifested when their concentrations are very high in comparison to their solubility. If the hydrocarbons present are in a dissolved state, the foam tends to remain reasonably stable. A foam’s oil washing performance can be significantly improved by the addition of NPs. The surface activity of AOT surfactants can be improved by hydrophilic NPA (a sodium-stabilized, commercial colloidal dispersion of SiO2) because of the increase of ionic strength and electrostatic repulsion force.22 Moreover, with a NP mixture containing an adequate proportion of NPB (partially hydrophobic-modified SiO2 NP mixed with sodium dodecyl sulfate), AOT surfactants can effectively stabilize CO2 foam due to the synergistic effects of NPB and AOT.22
4.9. Issues Associated with NP Retention in a Reservoir
The main reasons for NP retention within a porous medium are their adhesion to the pore walls and blocking of the pore channels and pore throats. Pore blockage can be caused by two mechanisms: mechanical plugging and fluid permeability contrasts.27 The continuous sliding of particles into narrow pores is a mechanism that leads to NP retention up to a certain capacity (Figure 5a). Adsorption and surface interaction can generally be thought of as a transient process in which the available surface sites accumulate appropriately charged particles until a certain capacity is reached.9,17 NP retention tends to reduce as the permeability of porous media increases.28 Experiments that reinject NPs recovered (i.e., those NPs that have already flowed through the porous media) back into the formation make comparisons of the properties of injected foam with and without NP retention possible (Figure 5b and 5c).28
Figure 5.
Diagrammatic illustration of the NP-interaction mechanisms with porous media: (a) Issues associated with NP retention in porous reservoir formation; (b) the oil flushing process enhanced by nanoparticle-stabilized foam as applied in EOR; and (c) the synergistic effects that occur between NPs and surfactant. Diagrams modified with permission from refs (9 and 17). Copyright 2021 Elsevier.
4.10. Effects of NP Type on NP-Enhanced CO2 Foam Performance
Table 3 summarizes the effects of NP type on the stability foams with CO2 based on the results of multiple research studies. All the NP types tested exhibit improved stability of NP-enhanced CO2 foams in comparison to surfactant foams without NPs. The increase in foam stability is attributed to multiple characteristics of NPs, including their shape, size, density, surface charge, and wettability. All of those NP characteristics potentially make a contribution to foam stability.29
Table 3. Summary of Studies Testing the Impact of NP Type on the Stability of CO2 Foams.
| nanoparticles/surfactant/polymer | optimum concentration of NP/surfactant/polymer | lab conditions | salinity | half-life time | enhanced oil recovery impacts | main results | ref |
|---|---|---|---|---|---|---|---|
| SiO2/AOS or CTAB/PVA | 0.06 wt %/0.06 wt %/0.15 wt % | 40 °C, 1.0 MPa | 2 g/L of NaCl | 7–10 min | increased crude oil’s sweep efficiency under harsh reservoir conditions; oil has a negative effect on the stability of foam until PVA polymer was introduced because it decreased the contact surface of the foam and the oil; in addition to preventing gas diffusion, the polymer reduced the Gibb’s free energy which is responsible for creating small bubbles | in addition to the two layers formed by surfactants and NPs, the use of polymers also forms a third protective layer; as a result, the strong capillary forces near the plateau border are reduced, allowing the film fluid to remain in the lamellae, resulting in foam stability | (30) |
| Al2O3/AOS or CTAB/PVA | 0.01 wt %/0.06 wt %/0.15 wt % | 8–11 min | |||||
| SiO2/SDS (EH-9, DTAB)/ HEC | 50000 ppm/0.02 wt %/0.02 wt % | 43, 57, 72 °C, 8.96 MPa | 5 wt % of NaCl | 30 min | - | an enhancement in the thermal stability of aqueous CO2 foams at HPHT and an increase in apparent viscosity; as a result of adding polymer to the SiO2-stabilized CO2 foam system, the spatial SiO2 network and foam stability were strengthened; also, the morphologic distribution of NPs at the bubble interface are improved | (31) |
| SiO2/AOS/– | 1 wt %/3.5 wt %/- | ambient temperature, 1 MPa | - | 50 min | enhanced oil recovery by about 13.8–22.4%; this is due to changes in the oil/water IFT, emulsification, wettability, and stability/elasticity of foam films | reduced oil/water IFT, increased the life of foam, and enhanced the adsorption of NPs on the film; the improvement is due to NPB adsorption at the interface between CO2 and water and the aggregation of NPB in the foam films | (22) |
Table 4 summarizes the advantages and disadvantages of the CO2-EOR methods described.
5. Future Research and Recommendations
Extensive research conducted to date addresses the key technical considerations of NP-enhanced CO2 foams adapted for use with surfactants for EOR purposes. However, further research is required to clarify some important uncertainties:
Establishing the key particle interaction mechanisms involved in physical interactions and chemical reactions between NPs, surfactants, and salt ions and their impact on the stability of CO2 foams at reservoir conditions.
The effectiveness of different NP compositions when deployed together with various polymers and surfactants.
The economic challenges/cost effectiveness of using NPs to increase the stability of CO2 foams at the large scales required for effective CCUS.
Field-scale tests injecting NP-enhanced CO2 foams with surfactants to achieve EOR and large-scale, long-term CO2 storage/sequestration. In particular, establishing monitoring methods that definitively establish the effectiveness of CO2 and NP retention in the reservoir.
Surfactant synergies (such as that between AOS and TX-100) are able to enhance the performance of CO2 foam; however, chromatography studies are required to determine the optimal mixing ratios. Foam stability is highly impacted by salinity and temperature under specific conditions, which is why further investigation is necessary.
Polymers, such as polyFAST, are known to be effective CO2 thickeners, but these substances have serious environmental impacts. Therefore, alternative ecofriendly materials to achieve CO2 thickening need to be identified and tested.
Laboratory-based bulk foam stability experiments and core flooding experiments have been mainly used to validate the benefits of NP use in CO2-EOR applications. More simulation studies, large-scale pilot tests, or field trials are required.
Some NPs are capable of delaying bubble coalescence and increasing CO2 foam stability, but more tests are required to identify how these benefits might be optimized.
Further research is needed to develop cost-effective NPs that do not negatively affect health or the environment.
NP agglomeration is one of the major challenges in creating homogeneous and stable nanofluids. More studies are required focused on preventing NP agglomeration at reservoir conditions.
Conclusions
This review of recent research leads to the following conclusions regarding the use of carbon dioxide to enhance oil recovery (CO2-EOR):
•The success of EOR technologies depends on multiple factors, including the physical and chemical properties of oil in the reservoir and structural/stratigraphic conditions of the oil reservoir trap. In addition to reservoir properties, it is also influenced by the stage of field development and the percentage of oil already recovered, water saturation, the volume and composition of displacing agents included in the injected fluids, and the drive mechanism determining fluid flow and oil displacement at reservoir conditions.
•The best EOR results are achieved by displacing oil with CO2, when the process involves miscible displacement, i.e., when unlimited mixing of CO2 with oil is possible at reservoir conditions.
•The choice of EOR technology has to be justified based on careful consideration of the specific reservoir conditions and quantitative characteristics of each field case. That selection cannot be based simply on analogies with other oil fields.
•CO2-EOR is expected to generate high profitability when applied to many types of oil fields. This is because it is possible to use captured and recycled CO2 to inject into the reservoir formation in either liquid, gaseous, or supercritical states.
•High-viscosity (heavy) oils and low-permeability reservoirs, as well as depleted fields with high-water cuts, are all potentially suited to the CO2-EOR technique.
•CO2-EOR offers a potential large-scale solution to help mitigate CO2 emissions and reduce its damaging impacts on the atmosphere.
•Most previous research related to CO2-polymer thickeners primarily addressed the solubility and rheological properties of the thickeners. Studies have shown the beneficial impacts of such polymers on CO2-EOR. However, some polymers, despite their ability to improve CO2 properties, can result in permanent permeability loss in the reservoir.
•Surfactant foam used in CO2-EOR can be effectively stabilized by the addition of nanoparticles (NPs).
•The stability of surfactant foam is inversely proportional to the size of the NP additive. NPs with a size ranging from 7 nm and 10 μm substantially increase the half-life of surfactant foam.
•Both hydrophilic and partially hydrophilic NPs are effective in stabilizing CO2 foam.
•Silica (SiO2) NPs act to change the surface wettability of foam to positively impact its stability.
•CO2 injection methods into subsurface reservoirs are receiving increased attention, primarily because they offer the possibility to provide long-term CO2 storage, isolating it from the atmosphere. The addition of NPs to injected CO2 foam improves its EOR performance. However, more field-scale testing is required to confirm its performance in large-scale, long-term, subsurface CCUS applications.
•Globally, there is an abundance of high-viscosity oils, low-permeability reservoirs, and partially depleted oil fields with high water saturations. CO2-EOR offers a means of increasing productivity and recovery from such reservoirs in an environmentally beneficial way.
Acknowledgments
This research was supported by the Tomsk Polytechnic University development program.
Glossary
Nomenclature/Abbreviations
- SiO2 R816
aerosil silicon dioxide nanoparticle
- AOT
aerosol-OT (sodium bis(2-ethylhexyl) sulfosuccinate)
- AOS
α-olefin sulfonate
- Al2O3
aluminum oxide
- SDS
anionic sodium dodecyl sulfate
- CaCl2
calcium chloride
- CCS
carbon capture and storage
- CCUS
carbon capture use and storage
- CO2
carbon dioxide
- CO2-EOR
carbon dioxide for enhanced oil recovery
- CTAB
cetyltrimethyl ammonium bromide
- DTAB
dodecyl trimethylammonium bromide
- EOR
enhanced oil recovery
- FAWAG
foam-assisted water alternating gas
- GI
gas injection
- SiO2 A300
hydrophilic silicon dioxide nanoparticle
- HEC
hydroxyethyl cellulose (2)
- IFT
interfacial tension
- IPCC
intergovernmental panel on climate change
- LHSB
lauramido propyl hydroxyl sultaine
- NP
nanoparticle
- EH-9
nonionic ecosurf
- NPB
partially hydrophobic nanoparticle
- P1D
poly-1-decene
- PAM
polyacrylamide
- PDMS
polydimethyl siloxane
- PDMS
polydimethyl siloxy
- polyFAST
polyfluoro-acrylate-styrene
- C12E23
polyoxyethylene lauryl ether
- PVAc
polyvinyl acetate
- PVA
poly(vinyl alcohol)
- PVEE
polyvinyl-ethyl-ether
- SiO2
silica dioxide nanoparticle
- SiO2–SDS
silica dioxide nanoparticle–sodium dodecyl sulfate
- SWAG
simultaneous water and gas injection
- NaCl
sodium chloride
- T
soluble temperature in the water phase
- EUR
ultimate oil recovery
- WAG
water alternating gas
Biographies
Shadfar Davoodi is a PhD student at Tomsk Polytechnic University. His research is associated with synthesizing co- and terpolymers, nanotechnology, and nanoparticle applications for drilling fluids and EOR. He holds an MSc degree in Petroleum Engineering from Sharif University of Technology (Iran).
Mohammed Al-Shargabi is a PhD student at Tomsk Polytechnic University. His PhD research is specifically focused on the use of nanoparticles for EOR and drilling fluids applications at harsh conditions. He has an MSc degree from Udmurt State University in the development of oil fields with high viscosity at harsh conditions, with a first-class degree.
David A. Wood is the principal consultant at DWA Energy Limited. He gained his PhD from Imperial College London in 1977 and has worked for many decades in the oil, gas, and energy sectors, collaborating on a wide range of research. In recent years he has published extensively on various applications of nanoparticles and enhanced oil recovery.
Valeriy S. Rukavishnikov is an Associate Professor at Tomsk Polytechnic University, responsible for reservoir management and evaluation, geostatistics, reservoir characterization, drilling, and completion fluids. He holds a PhD in Petroleum Engineering from Heriot-Watt University.
Konstantin M. Minaev is an Associate Professor at Tomsk Polytechnic University. He is engaged in research in chemical methods of enhanced oil recovery, development of new chemical reagents for drilling, and construction of oil and gas wells. He holds a PhD in Chemistry from Tomsk State University.
The authors declare no competing financial interest.
References
- Mohsenatabar Firozjaii A.; Saghafi H. R. Review on Chemical Enhanced Oil Recovery Using Polymer Flooding: Fundamentals, Experimental and Numerical Simulation. Petroleum 2020, 6 (2), 115–122. 10.1016/j.petlm.2019.09.003. [DOI] [Google Scholar]
- IEA . EOR Production in the New Policies Scenario, 2000–2040 Retrieved from International Energy Agency: https://www.iea.org/data-and-statistics/charts/eor-production-in-the-new-policies-scenario-2000-2040.
- Grasso M. A Critical Assessment of the Oil and Gas Industry’s Contribution to Climate Change. E.R. S. Science 2019, 50, 106–115. 10.1016/j.erss.2018.11.017. [DOI] [Google Scholar]
- Fakher S.; Imqam A. Application of Carbon Dioxide Injection in Shale Oil Reservoirs for Increasing Oil Recovery and Carbon Dioxide Storage. Fuel 2020, 265, 116944. 10.1016/j.fuel.2019.116944. [DOI] [Google Scholar]
- Kumar R. S.; Sinha A. S. K.; Sharma H.; Sharma T. High Performance Carbon Dioxide Foams of Nanocomposites of Binary Colloids for Effective Carbon Utilization in Enhanced Oil Recovery Applications. J. Mol. Liq. 2021, 343, 117659. 10.1016/j.molliq.2021.117659. [DOI] [Google Scholar]
- Burrows L. C.; Haeri F.; Cvetic P.; Sanguinito S.; Shi F.; Tapriyal D.; Goodman A.; Enick R. M. A Literature Review of CO2, Natural Gas, and Water-Based Fluids for Enhanced Oil Recovery in Unconventional Reservoirs. Energy Fuels 2020, 34 (5), 5331–5380. 10.1021/acs.energyfuels.9b03658. [DOI] [Google Scholar]
- Massarweh O.; Abushaikha A. S. A Review of Recent Developments in CO2Mobility Control in Enhanced Oil Recovery. Petroleum 2021, 10.1016/j.petlm.2021.05.002. [DOI] [Google Scholar]
- Cheraghian G.; Rostami S.; Afrand M. Nanotechnology in Enhanced Oil Recovery. Processes 2020, Vol. 8, Page 1073 2020, 8 (9), 1073. 10.3390/pr8091073. [DOI] [Google Scholar]
- Ab Rasid S. A.; Mahmood S. M.; Kechut N. I.; Akbari S. A Review on Parameters Affecting Nanoparticles Stabilized Foam Performance Based on Recent Analyses. J. Pet. Sci. Eng. 2022, 208, 109475. 10.1016/j.petrol.2021.109475. [DOI] [Google Scholar]
- Hu N.; Li Y.; Wu Z.; Lu K.; Huang D.; Liu W. Foams Stabilization by Silica Nanoparticle with Cationic and Anionic Surfactants in Column Flotation: Effects of Particle Size. J. Taiwan Inst. Chem. Eng. 2018, 88, 62–69. 10.1016/j.jtice.2018.04.008. [DOI] [Google Scholar]
- Lemaire P. C.; Alenzi A.; Lee J. J.; Beckman E. J.; Enick R. M. Thickening CO2 with Direct Thickeners, CO2-in-Oil Emulsions, or Nanoparticle Dispersions: Literature Review and Experimental Validation. Energy Fuels 2021, 35 (10), 8510–8540. 10.1021/acs.energyfuels.1c00314. [DOI] [Google Scholar]
- Zhang Y.; Zhu Z.; Tang J. Research on Polyether-Based Hydrocarbon Thickener for CO2. Fluid Phase Equilib. 2021, 532, 112932. 10.1016/j.fluid.2020.112932. [DOI] [Google Scholar]
- Amir Z.; Said I. M.; Jan B. M.. In Situ Organically Cross-Linked Polymer Gel for High-Temperature Reservoir Conformance Control: A Review. Polym. Adv. Technol.; John Wiley and Sons Ltd., 2019; pp 13–39. 10.1002/pat.4455. [DOI] [Google Scholar]
- Sun X.; Bai B.; Alhuraishawy A. K.; Zhu D. Understanding the Plugging Performance of HPAM-Cr (III) Polymer Gel for CO2 Conformance Control. SPE J. 2021, 26 (05), 3109–3118. 10.2118/204229-PA. [DOI] [Google Scholar]
- Raza M. A.; Kooij E. S. Toward Superhydrophobic Surfaces. Surf. Interface Sci. 2020, 391–440. 10.1002/9783527680597.ch54. [DOI] [Google Scholar]
- Majeed T.; Kamal M. S.; Zhou X.; Solling T. A Review on Foam Stabilizers for Enhanced Oil Recovery. Energy Fuels 2021, 35 (7), 5594–5612. 10.1021/acs.energyfuels.1c00035. [DOI] [Google Scholar]
- Zhang Y.; Liu Q.; Ye H.; Yang L. L.; Luo D.; Peng B. Nanoparticles as Foam Stabilizer: Mechanism, Control Parameters and Application in Foam Flooding for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2021, 202, 108561. 10.1016/j.petrol.2021.108561. [DOI] [Google Scholar]
- Alcorn Z. P.; Fredriksen S. B.; Sharma M.; Føyen T.; Wergeland C.; Fernø M. A.; Graue A.; Ersland G.. Core-Scale Sensitivity Study of CO2 Foam Injection Strategies for Mobility Control, Enhanced Oil Recovery, and CO2 Storage. In E3S Web Conf.; EDP Sciences, 2020; Vol. 146. 10.1051/e3sconf/202014602002. [DOI] [Google Scholar]
- Yu W.; Kanj M. Y. Review of Foam Stability in Porous Media: The Effect of Coarsening. J. Pet. Sci. Eng. 2022, 208, 109698. 10.1016/j.petrol.2021.109698. [DOI] [Google Scholar]
- Yang K.; Li S.; Zhang K.; Wang Y. Synergy of Hydrophilic Nanoparticle and Nonionic Surfactant on Stabilization of Carbon Dioxide-in-Brine Foams at Elevated Temperatures and Extreme Salinities. Fuel 2021, 288, 119624. 10.1016/j.fuel.2020.119624. [DOI] [Google Scholar]
- Chen Z.; Lu Y.; Nazemi Ashani M.; Manica R.; Feng L.; Liu Q. Modulation of Surface Charge by Mediating Surface Chemical Structures in Nonpolar Solvents with Nonionic Surfactant Used as Charge Additives. J. Phys. Chem. C 2021, 125 (35), 19525–19536. 10.1021/acs.jpcc.1c05088. [DOI] [Google Scholar]
- Zhao J.; Torabi F.; Yang J. The Synergistic Role of Silica Nanoparticle and Anionic Surfactant on the Static and Dynamic CO2 Foam Stability for Enhanced Heavy Oil Recovery: An Experimental Study. Fuel 2021, 287, 119443. 10.1016/j.fuel.2020.119443. [DOI] [Google Scholar]
- Obisesan O.; Ahmed R.; Amani M. The Effect of Salt on Stability of Aqueous Foams. Energies 2021, 14 (2), 279. 10.3390/en14020279. [DOI] [Google Scholar]
- Wang Y.; Wang J.; Fan H.; Du F.; Zhou W.; Yang J. Effect of Inorganic Salt on Foam Properties of Nanoparticle and Surfactant Systems. Tenside, Surfactants, Deterg. 2020, 57 (5), 382–388. 10.3139/113.110698. [DOI] [Google Scholar]
- Chea T. B.; Asyraf N.; Akhir M.; Idris A.; Kamal Idris A.. Investigation on the Effect of Types of Nanoparticles and Temperature on Nanoparticles-Foam Stability. IEOM Society International, 2018.
- Rahim Risal A.; Manan M. A.; Yekeen N.; Mohamed Samin A.; Azli N. B. Rheological Properties of Surface-Modified Nanoparticles-Stabilized CO2 Foam. J. Dispersion Sci. Technol. 2018, 39 (12), 1767–1779. 10.1080/01932691.2018.1462201. [DOI] [Google Scholar]
- Yang X.; Cai J.; Jiang G.; Xie J.; Shi Y.; Chen S.; Yue Y.; Yu L.; He Y.; Xie K. Nanoparticle Plugging Prediction of Shale Pores: A Numerical and Experimental Study. Energy 2020, 208, 118337. 10.1016/j.energy.2020.118337. [DOI] [Google Scholar]
- Sun Q.; Liu W.; Li S.; Zhang N.; Li Z. Interfacial Rheology of Foam Stabilized by Nanoparticles and Their Retention in Porous Media. Energy Fuels 2021, 35 (8), 6541–6552. 10.1021/acs.energyfuels.0c03680. [DOI] [Google Scholar]
- Rezvani H.; Panahpoori D.; Riazi M.; Parsaei R.; Tabaei M.; Cortés F. B. A Novel Foam Formulation by Al2O3/SiO2 Nanoparticles for EOR Applications: A Mechanistic Study. J. Mol. Liq. 2020, 304, 112730. 10.1016/j.molliq.2020.112730. [DOI] [Google Scholar]
- Dehdari B.; Parsaei R.; Riazi M.; Rezaei N.; Zendehboudi S. New Insight into Foam Stability Enhancement Mechanism, Using Polyvinyl Alcohol (PVA) and Nanoparticles. J. Mol. Liq. 2020, 307, 112755. 10.1016/j.molliq.2020.112755. [DOI] [Google Scholar]
- Fu C.; Liu N. Study of the Synergistic Effect of the Nanoparticle-Surfactant-Polymer System on CO2 Foam Apparent Viscosity and Stability at High Pressure and Temperature. Energy Fuels 2020, 34 (11), 13707–13716. 10.1021/acs.energyfuels.0c02435. [DOI] [Google Scholar]