Abstract
A 33-year-old Chinese woman with a history of immune thrombocytopenic purpura presented with heavy menstrual bleeding. She was found to have thrombocytopenia, plasma ADAMTS13 activity of 0%, and positivity for the plasma ADAMTS13 inhibitor. She was diagnosed with the coexistence of thrombotic thrombocytopenic purpura and immune thrombocytopenic purpura. The patient was treated by plasmapheresis, a glucocorticoid, and rituximab. Her platelet level returned to normal, and she was discharged 28 days after admission. The number of plasmapheresis sessions and the timing of rituximab administration may be the key aspects of management of patients with thrombotic thrombocytopenic purpura who have underlying immune dysfunction caused by diseases such as immune thrombocytopenic purpura.
Keywords: Case report, plasmapheresis, rituximab, ADAMTS13, von Willebrand factor, thrombotic thrombocytopenic purpura, immune thrombocytopenic purpura.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is an acute, life-threatening disorder characterized by microangiopathic hemolytic anemia, thrombocytopenia, and organ dysfunction. TTP is specifically related to a severe deficiency in ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin-1-like motif, member 13 of this family of metalloproteases), which is an enzyme that cleaves the blood clotting protein von Willebrand factor (VWF). 1
TTP can be divided into congenital TTP and acquired TTP, the latter of which is usually associated with malignancy, infections, immune disorders, and drugs such as ticlopidine, cyclosporine, and mitomycin C. 2 In women with TTP, it is important to discuss the implications of pregnancy. 3 TTP is classically characterized by the pentad of thrombocytopenia, microangiopathic hemolytic anemia, fever (25%), neurological symptoms (80%), and renal dysfunction (30%). The majority (90%) of patients do not present with all five clinical features. 4 Therefore, the clinical diagnosis of TTP remains a challenge. The diagnosis of TTP is a medical emergency, and therapy should be instituted immediately. We herein present a case of successful treatment of TTP in a woman with immune thrombocytopenic purpura (ITP) caused by viral infection during the coronavirus disease 2019 (COVID-19) pandemic. The patient provided written informed consent for treatment and publication. The requirement for ethics approval was waived because of the nature of this study (case report).
Case Report
Chief complaints
A 33-year-old Chinese woman was admitted to our hospital on 13 February 2020 because of weakness and heavy menstruation for 1 week after she contracted a respiratory infection. The patient also had a low platelet count of 5 × 109/L and petechiae on her skin.
History of present illness
The patient had been diagnosed with ITP nearly 2 years prior. A routine blood examination on 14 May 2018 at a local hospital revealed a white blood cell (WBC) count of 4 × 109/L, a hemoglobin level of 119 g/L, and a platelet count of 18 × 109/L. She denied a history of thyroid gland diseases and other autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis. Antinuclear antibody was positive at a titer of 1:80, but no other specific antibodies were detected, including anti-ENA, anti-U1-RNP, anti-Sm, anti-SS-A, anti-SS-B, ANCA, anti-Ro52, and antiphospholipid antibodies. The patient was also negative for anti-Scl-70, anti-PM-Scl, anti-Jo-1, anti-centromere B, anti-PCNA, anti-dsDNA, anti-nucleosomes, anti-histones, anti-ribosomal P protein, and anti-AMA-M2 antibodies. Urinalysis results were normal. The results of a bone marrow core biopsy revealed a moderate number of megakaryocytes with poor function, confirming the diagnosis of ITP. The patient was treated with 40 mg of dexamethasone daily for 4 days, and her platelet count increased to 70 × 109/L. Approximately 2 weeks later, her platelet count was 115 × 109/L. The disease was well controlled thereafter. One week prior (6 February 2020), the patient had contracted a respiratory infection with rhinorrhea and had no fever, cough, or expectoration. Because of the COVID-19 pandemic, the patient did not go to a hospital to seek further treatment for her illness. On 8 February 2020, the patient experienced heavy menstruation. After a few days, she felt fatigue and dizziness and had developed petechiae on her skin; however, she did not experience headache or any disturbance of consciousness. The patient then went to the local community hospital in Wuyuan Town, Jiangxi Province and underwent a routine blood test, which revealed a decreased platelet count (5 × 109/L). The patient then immediately went to the emergency department of our hospital on the night of 13 February 2020.
History of past illness
The patient had no history of hepatitis, syphilis, or acquired immunodeficiency syndrome. She also had no history of special medications, a family genetic history, or psychosocial illness. She had a 3-year-old son.
Physical examination
On the evening of the first day of admission, the patient’s body temperature was 36.9°C, heart rate was 101 beats/minute, respiratory rate was 16 breaths/minute, and blood pressure was 126/91 mmHg. Physical examination revealed congestion of the mucous membrane of the throat and scattered ecchymosis over the whole body.
Laboratory examinations
Routine bloodwork revealed a WBC count of 4.4 × 109/L, hemoglobin level of 83 g/L, platelet count of 4 × 109/L, C-reactive protein (CRP) level of 2.85 mg/L, alanine aminotransferase level of 175 U/L, aspartate aminotransferase level of 116 U/L, total bilirubin level of 50.3 μmol/L, direct bilirubin level of 22.3 μmol/L, indirect bilirubin level of 28.0 μmol/L, lactate dehydrogenase (LDH) level of 543 U/L, and creatinine level of 44 μmol/L. The patient had liver function impairment, and her platelet count was very low; however, her renal function was normal. The coagulation profile showed a fibrinogen level of 1.84 g/L and a slightly high D-dimer level of 1.08 mg/L FEU. Because of her previous diagnosis of ITP, the patient was given intravenous pulse therapy of dexamethasone (40 mg daily) for 4 days and 20 g (0.4 g/kg) of gamma globulin in an attempt to quickly raise her platelet count.
Imaging examinations
The afternoon after the day of admission, the patient developed an increased body temperature of 37.9°C without chills, cough, or expectoration. Pulmonary computed tomography (CT) examination showed no signs of inflammatory lesions in either lung, and head CT showed no obvious abnormality.
Further diagnostic work-up
On the fourth day of admission (16 February 2020), there was no improvement in the patient’s condition. She had a persistent slight fever of 37.6°C along with fatigue and dizziness; moreover, she complained of new chest tightness. However, the heavy menstrual flow had subsided. Routine bloodwork showed that the WBC count was 7.2 × 109/L, hemoglobin level was 59 g/L, platelet count was 6 × 109/L, CRP level was <1 mg/L, serum alanine aminotransferase level was 206 U/L, serum aspartate aminotransferase level was 111 U/L, total bilirubin level was 31.4 μmol/L, direct bilirubin level was 17 μmol/L, indirect bilirubin level was 14.4 μmol/L, and creatinine level was 52 μmol/L. The reticulocyte proportion was elevated (9.56%), as was the LDH level (481 U/L). The Epstein–Barr virus DNA level was 7.43 × 102 copies/mL, and antinuclear antibody was positive at a 1:80 ratio; however, no other antibodies were detected. A routine urine test was positive for protein. During the hospitalization period, the patient had been treated with the standard treatment regimen for ITP (dexamethasone and gamma globulin), but there was no significant increase in her platelet count. Unexpectedly, the patient’s hemoglobin dropped rapidly with an elevated indirect bilirubin level when her menstrual flow decreased, and there was no massive gastrointestinal bleeding. Moreover, although there was no obvious abnormality on the chest or abdominal CT scans and the CRP level was normal, the patient had an unexplained persistent slight fever. We determined that her PLASMIC score was 6 (platelet count of 4 × 109/L, reticulocytes of 8.2%, no active cancer, no history of solid organ or stem cell transplantation, mean corpuscular volume of 93.6 fL, international normalized ratio of 1.02, and creatinine level of 44 µmol/L). Based on the above findings, we strongly considered the diagnosis of TTP. Thus, we performed more laboratory tests and investigated other causes of thrombocytopenia, including SLE-antiphospholipid antibody syndrome. Peripheral blood smears revealed 1% fragmented red cells (Figure 1), with negative direct and indirect Coombs’ tests. The CD55/CD59 test was normal, and the plasma protamine coagulation test was negative. The patient developed nonimmune hemolytic anemia. The ADAMTS13 activity test was performed; however, it took time for the result to be obtained. One unit of suspended red blood cells was transfused to improve the symptoms of anemia. Meanwhile, bone marrow puncture examination was performed for further diagnosis.
Figure 1.
Peripheral blood smear (Leishman stain, 40×) shows fragmented red blood cells (black arrows).
The next day, the patient’s temperature rose to 38.2°C, and routine blood tests revealed a WBC count of 7.9 × 109/L, hemoglobin level of 62 g/L, and platelet count of 6 × 109/L. The ADAMTS13 test (fluorescence resonance energy transfer assay) indicated that the plasma ADAMTS13 activity was 0% and that the patient’s plasma was positive for the ADAMTS13 inhibitor. The bone marrow puncture results showed hyperplastic anemia and a moderate number of megakaryocytes with poor function. Hence, the diagnosis of coexisting TTP and ITP was confirmed based on the above findings. Unfortunately, caplacizumab was not available in China; therefore, we immediately administered plasmapheresis (40–60 mL/kg), and methylprednisolone was also given at 40 mg every 12 hours for 5 days.
Treatment
On the seventh day of admission (19 February 2020), there was no significant increase in the patient’s platelet count, and routine blood tests showed a platelet count of 7 × 109/L. We further treated the patient with 375 mg/m2 rituximab per week. In addition, plasmapheresis was continued. The next day, the patient had responded to the treatment; her platelet count rose to 17 × 109/L, and we therefore continued the plasmapheresis. On the 10th day, the platelet count had increased to 51 × 109/L, and the total bilirubin, direct bilirubin, indirect bilirubin, and LDH levels had returned to normal.
On the 11th day, however, despite undergoing daily plasmapheresis and treatment with methylprednisolone and rituximab, the patient’s platelet count began to gradually decrease. On the 15th day (27 February 2020), she had a platelet count of 5 × 109/L and hemoglobin level of 77 g/L. Therefore, oral mycophenolate mofetil was started at 250 mg twice daily for immunosuppression.
On 2 March 2020, the patient received the third and final dose of rituximab at 375 mg/m2. Through all the above-described treatment regimens and management, her platelet count and hemoglobin level gradually increased. After 2 March, plasmapheresis was given every other day. On 5 March, routine blood tests showed a WBC count of 4 × 109/L, a hemoglobin level of 89 g/L, and a normal platelet count of 132 × 109/L. The proportion of red blood cell fragments was 1.0%. We remeasured the plasma ADAMTS13 activity and found that it had increased to 100%. The ADAMTS13 inhibitor test was negative, and plasmapheresis was stopped on 8 March 2020. On 12 March, routine blood tests showed that the platelet count was still normal (Figure 2), as was the WBC count, hemoglobin level, and LDH level (Figure 3). The patient was discharged with oral mycophenolate mofetil at 250 mg twice daily and tapered prednisone.
Figure 2.
Platelet counts of the patient after admission.
PEX: plasma exchange; PLT: platelet count.
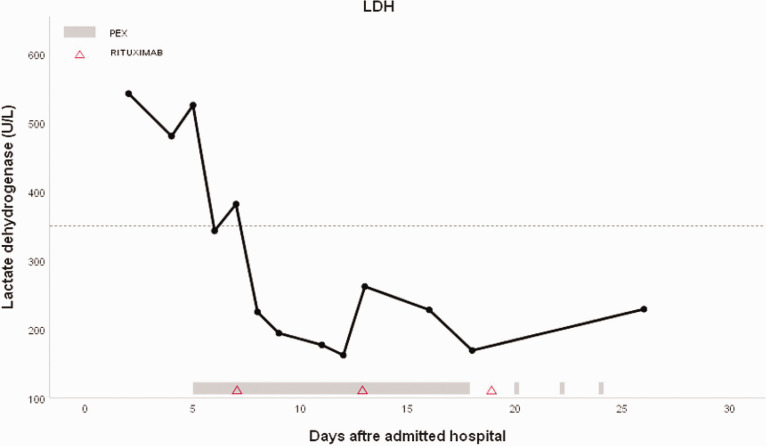
Figure 3.
LDH level after admission.
PEX: plasma exchange; LDH: lactate dehydrogenase.
Results
In August 2021, the patient returned to the hospital for follow-up; her condition was stable and she remained completely asymptomatic. Her routine blood tests were still mostly normal, her plasma ADAMTS13 activity level was 100%, and the plasma ADAMTS13 inhibitor test was negative. The reporting of this study conforms to the CARE guidelines. 5
Discussion
The main management of ITP usually involves high-dose dexamethasone/oral prednisone at 1 mg/kg per day, intravenous immunoglobulin, rituximab, or thrombopoietin receptor agonist. Other options are splenectomy and immunosuppressive agents or antibodies targeting CD40-CD154. 6 Our patient had responded well to corticosteroids when first diagnosed with ITP but failed to respond thereafter. This made us reconsider the patient’s diagnosis.
We recognized that this was a case of infection-triggered TTP in a patient with a prior diagnosis of chronic ITP. However, a limitation in this case is that we did not assess the PLASMIC score in time for early detection of TTP. Initially, we treated the patient with the standard regimen for ITP, but there was no improvement in the patient’s condition. In view of the evidence of thrombocytopenia and microangiopathic hemolytic anemia, the coexistence of TTP and ITP was considered as the diagnosis, although the patient did not have typical renal function impairment or nervous system symptoms. However, the decreased ADAMTS13 activity confirmed the diagnosis of TTP.
Acquired TTP is caused by various conditions, including connective tissue diseases, infections, malignant tumors, and pregnancy. 7 Several reports have described TTP occurring secondary to connective tissue diseases, such as SLE, mixed connective tissue disease, rheumatoid arthritis, scleroderma, and dermatomyositis. 8,9 Our patient had no typical neuropsychiatric symptoms or serious renal impairment. Her heavy menstrual bleeding masked the true cause of her anemia, which interfered with the immediate diagnosis of TTP.
TTP is a life-threatening condition that requires immediate treatment. The first-line therapy for TTP is daily therapeutic plasma exchange supplying deficient ADAMTS13, with or without steroids. The immunomodulators targeting ADAMTS13 autoantibodies that are used in TTP treatment are mainly steroids and the humanized anti-CD20 monoclonal antibody rituximab. 1 One possibility in the present case is that the plasmapheresis may have corrected the hemolysis but not the thrombocytopenia because the patient had underlying ITP, which does not respond to plasmapheresis. Rituximab may have worked for both presentations (ITP and TTP), thus explaining the increase in the platelet count. 10
Plasmapheresis is the first-line treatment for TTP and is often given with corticosteroid pulse therapy. This treatment removes the ADAMTS13 inhibitor, unusually large VWF multimers (UL-VWFM), and inflammatory cytokines that mediate UL-VWFM release from vascular endothelial cells. It also replenishes ADAMTS13 and regular-sized VWFM required for normal hemostasis.
Our patient initially responded well to plasmapheresis; however, the platelet count gradually decreased with the increasing number of plasmapheresis sessions (our patient received 13 rounds of plasmapheresis). Based on our knowledge, we infer that the phenomenon that occurred in our patient was “inhibitor boosting,” in which the plasmapheresis removed the inhibitors of ADAMTS13; however, because of the patient’s autoimmune disorder, more autoantibodies may have been produced by negative feedback. Rituximab is thought to resolve TTP by depleting the B cells that produce ADAMTS13 inhibitors. Our patient received a total of three doses of rituximab, but it takes time for rituximab to work. Combined therapies (plasma exchange and immunosuppressors) were found to be more appropriate than plasma exchange or immunosuppressors alone in treating adults with TTP-SLE and have been shown to achieve a higher remission rate. 8, 11 This may explain why methylprednisolone and mycophenolate mofetil were effective in our patient.
Conclusion
The number of plasmapheresis treatments and the timing of treatment with rituximab be a critical aspect of the management of TTP coexisting with an underlying immune dysfunction disease such as ITP. Diagnosing TTP in patients with a history of autoimmune diseases such as ITP or SLE poses a challenge. The features present in our patient are not known to be associated with typical TTP, and medical staff must be very vigilant with similar cases.
Acknowledgements
The authors express their gratitude to LaiJun Peng and all staff at the Department of Laboratory Support during this work.
Footnotes
Declaration of conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the Zhejiang Province Traditional Chinese Medicine Scientific Research Fund project [grant number 2021ZB092].
ORCID iDs: Hangping Ge https://orcid.org/0000-0002-4718-288X
References
- 1.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood 2017; 129: 2836–2846. [DOI] [PubMed] [Google Scholar]
- 2.Allford SL, Hunt BJ, Rose P, et al. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. Br J Haematol 2003; 120: 556–573. [DOI] [PubMed] [Google Scholar]
- 3.Scully M, Thomas M, Underwood M, et al. Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood 2014; 124: 211–219. [DOI] [PubMed] [Google Scholar]
- 4.Kessler CS Khan BA, andLai-Miller K.. Thrombotic thrombocytopenic purpura: a hematological emergency. J Emerg Med 2012; 43: 538–544. [DOI] [PubMed] [Google Scholar]
- 5.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 6.Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood 2017; 129: 2829–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbeke L, Delforge M, Dierickx D. Current insight into thrombotic thrombocytopenic purpura. Blood Coagul Fibrinolysis 2010; 21: 3–10. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Jiang JJ, Wang CY, et al. Clinical features and prognosis of patients with thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a review of 25 cases. Ital J Pediatr 2019; 45: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998-2008. Intern Med 2010; 49; 7–15. [DOI] [PubMed] [Google Scholar]
- 10.Al-Husban N, Al-Kuran O. Post-partum thrombotic thrombocytopenic purpura (TTP) in a patient with known idiopathic (immune) thrombocytopenic purpura: a case report and review of the literature. J Med Case Rep 2018; 12: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, An X, Li Y, et al. Clinical features and prognostic factors of thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a literature review of 105 cases from 1999 to 2011. Clin Rheumatol 2014; 33: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]