Abstract
Tocilizumab (TCZ) is a humanized immunoglobulin (Ig) G1 monoclonal antibody directed against the interleukin (IL)-6 receptor. We report on two patients with persistent high-grade fever and systemic lupus erythematosus (SLE) who were treated with TCZ. Two female Chinese patients presented with SLE and high-grade fever, with raised inflammatory markers including C-reactive protein, erythrocyte sedimentation rate, and IL-6, but no signs of opportunistic infections. Their fever and other symptoms responded poorly to broad-spectrum antibiotics, antifungals, antivirals, and glucocorticoids. They were both treated with TCZ. Their body temperatures returned to normal after treatment with TCZ, and other symptoms, including arthralgia, gradually improved. Both patients were followed-up and their conditions remained steady to date. TCZ may thus be an alternative treatment for patients with SLE and persistent high-grade fever who fail to respond to initial antibiotics and high-dose glucocorticoids.
Keywords: Tocilizumab, interleukin-6, systemic lupus erythematosus, fever, immunosuppressive agent, arthralgia
Introduction
Systemic lupus erythematosus (SLE) is a multi-systemic inflammatory autoimmune disorder, which is more common in women. Fever due to ongoing infection or to disease activity itself is often the primary reason for hospitalization in patients with SLE. Inflammatory cytokines play an active role in SLE patients with fever. Rheumatologists need to identify the etiology of the fever and treat it accordingly. Recent studies have suggested that the occurrence of fever in SLE patients has declined from 86% in 1950 1 to 42% in 2009, 2 possibly as a result of the increased use of non-steroidal anti-inflammatory drugs, broad-spectrum antibiotics, and glucocorticoids. However, although moderate doses of glucocorticoids can resolve fever, steroid metabolism may be increased when they are stopped. In addition, the frequent and prolonged use of immunosuppressants, on the one hand, increases the risks of opportunistic bacterial, viral, and fungal infections. 1 On the other hand infections, may aggravate and mimic the disease activity of SLE.
Herein, we present two female SLE patients who presented with high-grade fever that responded poorly to antibiotics, antivirals, and antifungals, and to glucocorticoid therapy, in the absence of altered steroid metabolism. There were no signs of infection apart from elevated levels of inflammatory markers. We therefore selected the anti-IL-6 receptor monoclonal antibody tocilizumab (TCZ) as a therapeutic option, resulting in good responses in both patients, including resolution of fever and patient discharge.
Case reports
Case 1
A 46-year-old Chinese woman with SLE was admitted to the rheumatology ward of The Second Affiliated Hospital of Kunming Medical University on 7 August 2020 with complaints of a fever of 39.3°C, chills, and a 2-day history of a sore throat. Her past medical history indicated multiple hospitalizations.
She was initially admitted to another hospital in 2004 for fever, malar rash, and arthralgia. She was positive for anti-nuclear antibody (ANA; titer 1:3200, speckled pattern) and anti-double stranded DNA (anti-dsDNA) antibody, negative for anti-La, anti-Smith (Sm), and anti-U1 ribonucleoprotein (RNP) antibodies, and urinalysis showed mild proteinuria. SLE was considered because she fulfilled both the 1997 American College of Rheumatology (ACR) classification criteria for SLE3,4 and the 2012 Systemic Lupus International Collaborating Clinics (SLICC). 5 She met four (cutaneous, joints, and autoimmune profiles) of the 11 1997 ACR classification criteria for SLE, and four (cutaneous, joints, and autoimmune profiles) of 17 of the 2012 SLICC criteria. She was discharged on maintenance doses of oral prednisone 7.5 mg/day and hydroxychloroquine (HCQ) 400 mg/day.
She was admitted to our hospital ward on 10 February 2020 with high fever, chills, and a sore throat for 3 weeks. Laboratory tests showed high-sensitivity C-reactive protein (hs-CRP) 149.61 mg/L (normal range <10.00 mg/L), IL-6 122 pg/mL (normal range 0.00–7.00 pg/mL), pro-calcitonin (PCT) 0.331 ng/mL (normal range <0.05 ng/mL) and CRP 228.00 mg/L (normal range <10.00 mg/L). She had mild proteinuria. Her autoimmune profile was positive for ANA (titer 1:320, speckled pattern) and anti-Ro52/SSA, and negative for anti-dsDNA, anti-La, anti-Sm, and anti-U1 RNP antibodies. Her lupus anticoagulant was 1.76 U/mL (normal range 0.8–1.2 U/mL). High-resolution chest computed tomography (HRCT) showed mild bronchiectasis in the middle and lower right lung lobe, with bilateral pleural thickening and adhesion.
Intravenous imipenem and cilastatin 3 g/day were administered for 5 days, but her fever continued and they were replaced with fluconazole 0.4 g/day. Meanwhile, her blood, urine, stool, throat swab, and sputum culture reports were negative, ruling out infection. Intravenous methylprednisolone 40 mg/day was therefore commenced to manage ongoing lupus flares. Her symptoms subsequently improved and her inflammatory markers, including hs-CRP, IL-6, and PCT, decreased. Quantitative 24-hour urinary protein analysis showed increased urinary protein of 0.23 g (normal range 0.00–0.15 g) and she was prescribed intravenous cyclophosphamide 0.6 g/day to prevent further transient renal damage due to lupus nephritis. The patient refused a renal biopsy and no further classification could be determined. Oral itraconazole 200 mg/day was given as prophylaxis against opportunistic fungal infections. Her fever and other constitutional symptoms improved and she was discharged with oral prednisone 45 mg/day, gradually tapered to 17.5 mg/day, and HCQ 300 mg/day.
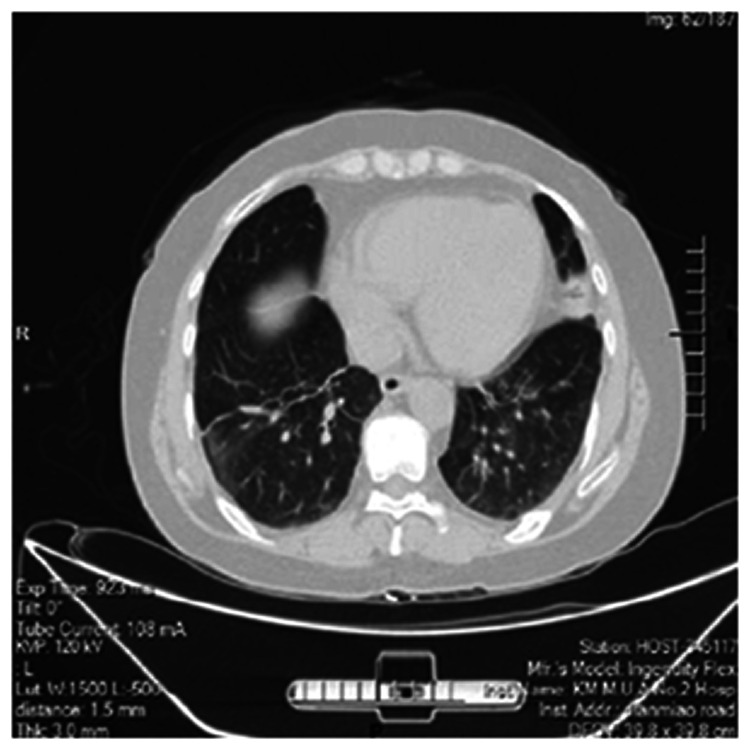
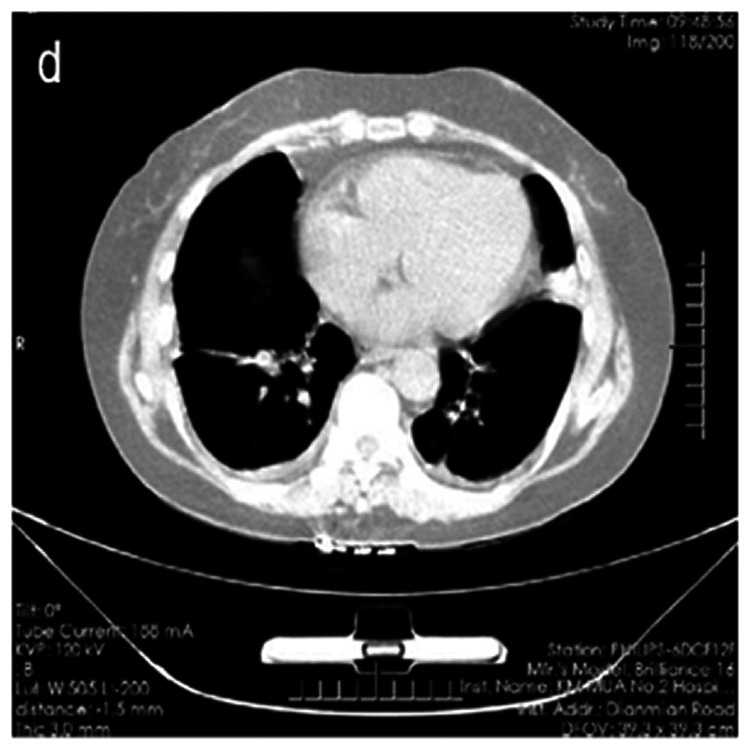
Following her re-admission on 7 August 2020, physical examination revealed non-scarring alopecia. Her blood and sputum culture reports were negative. Chest HRCT showed increased bronchiolar and vascular markings, multiple nodules with linear opacities, atelectasis of the left upper lobe, and thickening of the bilateral pleura (Figure 1). Intravenous piperacillin and tazobactam 4.5 g twice a day was commenced to treat and prevent chest infections. Repeat chest HRCT after 2 days showed improving minimal pleural effusion on both sides of the pleura (Figure 2). She also had effusion and tenderness over both wrist joints and her right knee joint. She scored 7 points (arthritis 4 points, alopecia 2 points, temperature >38°C 1 point) according to the SLE disease activity index 2000 (SLEDAI-2K). 6 Intravenous methylprednisolone 40 mg/day was therefore added to control disease activity and her antibiotic treatment was upgraded with cefoperazone and sulbactam 3.0 g twice a day to treat any on-going bacteremia.
Figure 1.
High-resolution computed tomography scan showing multiple nodular linear opacities with atelectasis and bilateral pleural thickening.
Figure 2.
High-resolution computed tomography scan after 2 days of treatment showing minimal effusion in the pleural cavities.
Despite the treatment, the patient’s fever and joint pain continued, and she showed raised uric acid levels of 412 µmol/L (normal range 115–357 µmol/L). Oral colchicine 0.5 mg/day was administered for acute gout and intravenous fluconazole 0.4 g/day was added for antifungal coverage. Meanwhile, we observed high copy numbers of Epstein–Barr virus (EBV) by quantitative DNA/RNA analysis, and high-output genetic tests conducted by the BGI (Shenzen, China) showed high PCT levels. Blood, sputum, throat swab, urine, and stool cultures were all negative. Her fever ranged from 37.8 to 38.9°C, and intravenous ganciclovir 0.3 g twice a day, immunoglobulin 20 g/day, and cefoperazone sulbactam 1.5 g three times a day were administered for 4 days, and then replaced with imipenem and cilastatin 1 g three times a day. Fluconazole was stopped and oral ursodeoxycholic acid 600 mg/day was prescribed for liver enzyme impairment.
The results of (1-3)-β-D-glucan assay and galactomannan tests for invasive fungal infections and IFN-γ release assay (T-SPOT) for tuberculosis were negative. Her methylprednisolone dose was doubled to 40 mg twice a day. Her detailed laboratory test results are shown in Table 1. However, 2 days later her fever had increased to 39.3°C, together with a severe sore throat and arthralgia. Intravenous vancomycin 1 g/day was continued and methylprednisolone was replaced with dexamethasone 5 mg/day. Her fever continued despite the treatment changes. She had previously received acyclovir and immunoglobulin for 10 days because of the high EBV copy number. Her body temperature remained constant at around 37.2°C for 4 days then re-spiked to 38.0°C, and her other symptoms remained unchanged, even after commencement of several broad-spectrum antibiotics, high-dose glucocorticoids, immunosuppressants, and anti-fungal and antiviral medications.
Table 1.
Laboratory values in Case 1.
| Index (reference range) |
August 2020 |
September 2020 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date | 07 | 10 | 13 | 16 | 20 | 23 | 25 | 27 | 28 | 30 | 03 | 08* |
| WBC (3.5–9.5 × 109/L) | 8.00 | 10.25 | 14.97 | 21.96 | 32.47 | 18.71 | 11.68 | / | 14.70 | 15.45 | 15.59 | 18.51 |
| Neut (40%–75%) | 88.4 | 80.2 | 89.8 | 93.4 | 97.5 | 94.6 | 93.8 | / | 91.4 | 94.6 | 89.1 | 91.4 |
| Neut, n (1.8–6.3 × 109/L) | 7.07 | 8.22 | 13.43 | 20.49 | 31.69 | 17.71 | 10.95 | / | 13.44 | 14.62 | 13.88 | 16.94 |
| Lymph (20%–50%) | 7.60 | 11.80 | 6.10 | 4.00 | 1.70 | 3.70 | 3.50 | / | 5.90 | 2.50 | 6.00 | 4.20 |
| Lymph, n (1.10–3.20 × 109/L) | 0.61 | 1.21 | 0.92 | 0.88 | 0.54 | 0.70 | 0.41 | / | 0.86 | 0.39 | 0.94 | 0.77 |
| Mono (3%–10%) | 3.6 | 6.1 | 4.0 | 2.5 | 0.7 | 1.1 | 1.6 | / | 2.4 | 1.7 | 4.6 | 4.2 |
| Alb% (35–50 g/L) | 37.8 | / | 29.2 | 31.7 | 27.9 | 27.3 | / | 26.2 | / | 25.6 | 26.9 | 28.5 |
| Glo (22–33 g/L) | 21.0 | / | 26.0 | 34.7 | 52.7 | 38.9 | / | 36.4 | / | 31.2 | 63.3 | 47.2 |
| GGT (7–32 U/L) | 90 | / | 136 | 186 | 558 | 559 | / | 275 | / | 200 | 224 | 129 |
| ALP (45–125 U/L) | 80 | / | 103 | 106 | 177 | 158 | / | 111 | / | 96 | 96 | 69 |
| AST (8–40 U/L) | 32 | / | 27 | 30 | 48 | 36 | / | 27 | / | 34 | 19 | 18 |
| TBIL (3.4–20.5 µmol/L) | 16.5 | / | 7.6 | 8.8 | 35.1 | 11.5 | / | 12.3 | / | 13.0 | 12.9 | 11.2 |
| DBIL (0–6.8 µmol/L) | 6.1 | / | 2.8 | 3.4 | 15.8 | 4.9 | / | 3.7 | / | 5.2 | 4.8 | 3.8 |
| IBIL (1.7–13.7 µmol/L) | 10.3 | / | 4.8 | 5.4 | 19.3 | 6.6 | / | 8.5 | / | 7.8 | 8.1 | 7.4 |
| UA (155–357µmol/L) | 412 | / | 242 | / | / | / | / | 209 | / | 193 | / | 214 |
| Crea (53–97 µmol/L) | 51 | / | 43 | / | / | / | / | 45 | / | 53 | / | 38 |
| LDH (109–245 U/L) | 382 | / | / | / | / | / | / | 443 | / | / | / | / |
| HBDH (72–182/L) | 245 | / | / | / | / | / | / | 295 | / | / | / | / |
| CK (26–140 U/L) | 48 | / | / | / | / | / | / | 19 | / | / | / | / |
| Urine-pro (−) | ± | / | / | – | / | / | / | / | / | / | ± | – |
| NT-proBNP (0–125 pg/mL) | 141.0 | / | 4427.0 | 558.3 | 179.1 | / | / | / | / | / | 254.4 | / |
| hs-CRP (<10 mg/L) | 101.28 | 114.62 | 127.79 | 142.17 | 144.80 | / | 133.39 | / | 85.19 | 114.50 | 101.37 | 79.14 |
| IL-6 (0–7 pg/mL) | 62.42 | 136.10 | 23.27 | 49.78 | 93.16 | / | 251.60 | / | 192.80 | 684.00 | 15.90 | 20.30 |
| PCT (<0.05 ng/mL) | 0.222 | 0.317 | 0.353 | 0.436 | 0.529 | / | 2.180 | / | 0.600 | 0.641 | 0.299 | 0.129 |
| CRP (<10 mg/L) | 94.9 | / | / | / | / | / | / | / | / | 133 | / | / |
| IgG (7.00–16.00 g/L) | 10.20 | / | / | / | / | / | / | / | / | / | / | / |
| IgM (0.4–2.8 g/L) | 0.26 | / | / | / | / | / | / | / | / | / | / | / |
| C3 (0.79–1.52 g/L) | 1.00 | / | / | / | / | / | / | / | / | / | / | / |
| C4 (0.1–0.4 g/L) | 0.23 | / | / | / | / | / | / | / | / | / | / | / |
| RF (0.00–20.00 IU/mL) | <20.00 | / | / | / | / | / | / | / | / | / | / | / |
| Ferr (5–223.5 ng/mL) | / | / | / | / | >1000.00 | / | / | / | / | / | / | / |
| ESR (0–20 mm/hour) | 30 | / | / | / | 92 | / | / | / | / | 38 | / | / |
| ANA | 1:1000 titer with speckled/cytoplasm granular patterns | |||||||||||
| Anti-RNP/Sm | + | |||||||||||
| Anti-SM | – | |||||||||||
| Anti-Ro52 | + | |||||||||||
| Anti-SSA/SSB | – | |||||||||||
| Anti-Jo-1 | – | |||||||||||
| Anti-SCI-70 | – | |||||||||||
| Anti-RIB | – | |||||||||||
| Anti-CB | – | |||||||||||
| Anti-M2 | – | |||||||||||
| Lupus anticoagulant (0.8c1.2) | 1.72 | |||||||||||
| ANCA | – | |||||||||||
| Anti-cardiolipin | – | |||||||||||
| Anti-2glycoprotein | – | |||||||||||
| Quantitative determination of EBV DNA (<5.00 × 102 copies/mL) | / | 4.56 × 103 | / | / | / | / | / | / | / | / | / | / |
| Hemoculture | – | / | / | / | / | – | / | – | / | / | / | / |
| Throat swab culture | – | / | / | / | / | / | / | / | / | / | / | / |
| Sputum culture | / | / | – | / | / | / | / | / | / | – | / | / |
| Bone marrow culture | / | / | / | – | / | / | / | / | / | / | / | / |
| Urine cultures | / | / | / | / | / | / | – | / | / | / | / | / |
| Stool culture | / | / | / | / | / | / | – | / | / | / | / | / |
| Anti-Legionella pneumophila IgM antibody | – | |||||||||||
| Anti-Mycoplasma pneumoniae IgM antibody | – | |||||||||||
| Anti-Rickettsia Q IgM antibody | – | |||||||||||
| Anti-adenovirus IgM antibody | – | |||||||||||
| Anti-respiratory syncytial virus IgM antibody | – | |||||||||||
| Anti-influenza A, B virus IgM antibody | – | |||||||||||
| Anti-parainfluenza virus type 1,2,3 IgM | – | |||||||||||
/, not examined; –, negative result; * , time of injection of tocilizumab.
WBC, white blood cells; Neut, neutrophils; Mono, monocytes; C, complement; hs, high sensitivity; CRP, C-reactive protein; IL-6, interleukin 6; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; EBV, Epstein–Barr virus; ANA, antinuclear antibody; ds-DNA, double-stranded DNA; Alb, albumin; GGT, gamma glutamytransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; NT-pro-BNP, N-terminal pro-B natriuretic peptide; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; ANCA, anti-neutrophil cytoplasmic antibody; Ig, immunoglobulin; RF, rheumatoid factor; RNP, ribonucleoprotein; Sm, Smith; Glo, globulin; UA, urea; Crea, creatinine; Ferr, ferritin; HBDH, hydroxybutyrate dehydrogenase; CK, creatine kinase; Urine-pro, urinary protein, SSA: anti-Sjögren syndrome-related antigen A autoantibody; SSB: anti-Sjögren syndrome-related antigen A autoantibody; anti-RIB, anti-ribosomal antibody; anti-M2, anti-mitochondrial M2 antibody; anti-CB, anti-calbindin antibody.
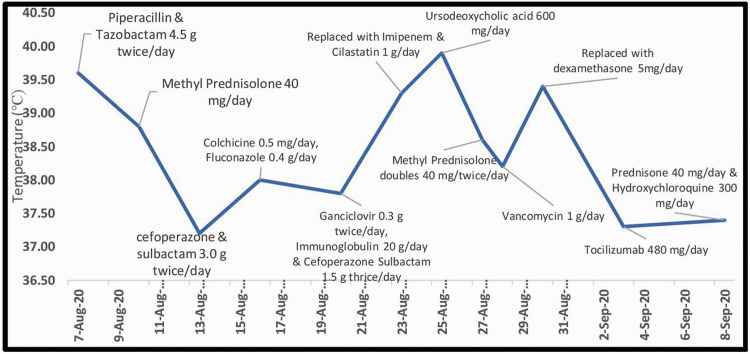
The patient’s IL-6 levels had been elevated since her initial presentation, and re-examination showed IL-6 20.30 pg/mL (normal range 0.00–7.00 pg/mL). Intravenous TCZ 480 mg was therefore administered after appropriate counselling and written consent from the patient. Her body temperature subsequently decreased gradually to normal (36.8°C) and her arthralgia and sore throat improved. Details of her therapies and body temperature are shown in Figure 3. She was discharged after 2 days with maintenance oral prednisone 40 mg/day and HCQ 300 mg/day. At a recent follow-up visit, her inflammatory markers had gradually returned to normal and she remained clinically stable to date.
Figure 3.
Time course of therapies administered with respect to body temperature (°C) in Case 1. TCZ, tocilizumab; MP, methylprednisolone.
Case 2
A 16-year-old Chinese girl was admitted to the rheumatology ward of The Second Affiliated Hospital of Kunming Medical University on 23 October 2017, with a 6-day history of recurrent high-grade fever. Her past medical history included autoimmune hepatitis that was successfully treated in 2015. She had also been admitted to another hospital on 6 March 2017 with a fever of 39°C, malar rash, oral ulcers, and arthralgia in all four joints, along with a productive cough and nausea. Her autoimmune profile was positive for ANA (1:3200 titer) and anti-Ro52/SSA, and negative for anti-dsDNA, anti-La/SSB, anti-Sm, anti-U1 RNP, ANCA, and ACA. SLE was confirmed on the basis of the 1997 ACR classification criteria for SLE3,4 and 2012 SLICC. 5 She met four (cutaneous, joint manifestations, and autoimmune profile) of 11 1997 ACR criteria for SLE, and four (cutaneous, joint manifestations, and autoimmune profile) of 17 2012 SLICC criteria. Her condition was controlled by oral prednisone 60 mg/day and mycophenolate mofetil 2.0 g/day. She had sudden onset of restricted movement of her right limb and facial numbness on 22 March 2017. Brain CT suggested sub-acute cerebral infarction; however, the physicians suspected lupus encephalopathy. During hospitalization, she received intravenous methylprednisolone 80 mg/day, dexamethasone 10 mg, and methotrexate 10 mg. She was later discharged with maintenance doses of oral prednisone 22.5 mg/day and HCQ 200 mg/day.
On admission to our hospital’s rheumatology ward on 23 October 2017, she had a fever of 39.4°C, facial puffiness with erythema, and bilateral ankle and proximal interphalangeal joint pain. Her blood, sputum, throat swab, urine, and stool cultures were negative. Her laboratory values are given in Table 2. Familial Mediterranean fever was provisionally suspected and she was prescribed colchicine 1.0 mg/day. However, her fever spiked to 42°C with chills and arthralgia, which continued to worsen. The colchicine was therefore replaced with cefoperazone and sulbactam 6.0 g/day and an antipyretic was commenced. Meanwhile, her fever continued to spike from 38.5 to 40.0°C and her IL-6 levels were elevated. The patient was counselled regarding possible TCZ treatment as the best option at the time, and she received her first dose of intravenous TCZ 480 mg on 30 October 2017. Her fever and joint pain improved and she was discharged on oral prednisone 22.5 mg/day, HCQ 200 mg/day, and methotrexate 10 mg once a week. She received seven injections of TCZ since her last hospitalization. During one such occasion (20 August 2018), she was re-admitted with fever and pneumonitis; however, her condition improved after commencement of imipenem and she remained stable at recent follow-up visits.
Table 2.
Laboratory values in Case 2.
|
Index (reference range) |
October 2017 |
November 2017 |
December 2017 |
January 2018 |
February 2018 |
|||||||
|
Date |
27 |
30* |
03 |
30 |
01* |
27* |
29 |
05 |
06* |
10 |
||
| WBC (3.5–9.5 × 109/L) | 6.78 | / | 10.54 | 10.02 | / | 6.81 | 14.55 | 12.13 | 9.65 | 13.44 | ||
| Neut (40%–75%) | 77.70 | / | 77.40 | 87.80 | / | 81.60 | 88.50 | 92.10 | 88.10 | 78.70 | ||
| Neut, n (1.8–6.3*109/L) | 5.27 | / | 8.16 | 8.80 | / | 5.55 | 12.88 | 11.18 | 8.50 | 10.58 | ||
| Lymph (20%–50%) | 11.40 | / | 16.10 | 8.70 | / | 9.80 | 4.90 | 4.70 | 10.50 | 14.40 | ||
| Lymph, n (1.10–3.20 × 109/L) | 0.77 | / | 1.70 | 0.87 | / | 0.67 | 6.5 | 0.57 | 1.01 | 1.93 | ||
| Mono (3%–10%) | 10.8 | / | 6.3 | 3.4 | / | 8.2 | 0.94 | 3.0 | 1.3 | 6.7 | ||
| Alb% (35–50 g/L) | 33.2 | / | 39.1 | / | 39.9 | 43.1 | 46.5 | 44.5 | / | 41.5 | ||
| Glo (22–33 g/L) | 27.7 | / | 23.2 | / | 21.7 | 16.7 | 23.4 | 26.8 | / | 24.6 | ||
| GGT (7–32 U/L) | / | / | 101 | / | 100 | 48 | 70 | 119 | / | 105 | ||
| ALP (45–125 U/L) | / | / | 97 | / | 86 | 101 | 156 | 162 | / | 139 | ||
| AST (8–40 U/L) | 64 | / | 34 | / | 26 | 27 | 35 | 31 | / | 29 | ||
| TBIL (3.4–20.5 µmol/L) | 14.8 | / | 9.4 | / | 11.8 | 15.3 | 19.2 | 12.4 | / | 7.5 | ||
| DBIL (0–6.8 µmol/L) | 4.6 | / | 4.0 | / | 5.3 | 6.8 | 10.1 | 6.2 | / | 3.4 | ||
| IBIL (1.7–13.7 µmol/L) | 10.2 | / | 5.4 | / | 6.5 | 8.5 | 9.1 | 6.2 | / | 4.1 | ||
| UA (155–357µmol/L) | 266 | / | 360 | / | 290 | 345 | 343 | 197 | / | 238 | ||
| Crea (53–97 µmol/L) | 66 | / | 48 | / | 44 | 49 | 62 | 66 | / | 43 | ||
| LDH (109–245 U/L) | 1619 | / | / | / | 445 | 398 | 406 | / | / | / | ||
| HBDH (72–182/L) | / | / | / | / | 382 | 308 | 299 | / | / | / | ||
| CK (26–140 U/L) | 23 | / | 23 | / | / | 27 | 26 | 32 | / | / | ||
| Urine-pro (−) | ± | / | / | / | / | ± | ± | ± | / | / | ||
| NT-proBNP (0–125 pg/mL) | / | / | / | / | / | / | / | / | / | / | ||
| IgG (7.00–16.00 g/L) | 8.78 | / | / | / | 8.25 | 7.33 | / | / | / | / | ||
| IgM | 0.11 | / | / | / | 0.12 | 0.09 | / | / | / | / | ||
| hs-CRP (<10 mg/L) | 23.65 | / | 6.98 | 21.08 | / | 1.26 | / | 15.55 | 67.19 | 8.52 | ||
| IL-6 (0–7 pg/mL) | 127.80 | / | 262.90 | 14.75 | / | 220.30 | / | 259.30 | 977.50 | 18.56 | ||
| PCT (<0.05 ng/mL) | 0.252 | / | 0.052 | 0.087 | / | 0.061 | / | 0.180 | 4.090 | 0.479 | ||
|
Index (reference range) |
October 2017 |
November 2017 |
December 2017 |
January 2018 |
February 2018 |
|||||||
|
Date |
27 |
30* |
03 |
30 |
01* |
27* |
29 |
05 |
06* |
10 |
||
| CRP (<10 mg/L) | 23.10 | / | / | / | 21.20 | 3.10 | / | / | / | / | ||
| C3 (0.79–1.52 g/L) | 1.3 | / | / | / | 1.21 | 0.67 | / | / | / | / | ||
| C4 (0.1–0.4 g/L) | 0.21 | / | / | / | 0.16 | 0.08 | / | / | / | / | ||
| RF (0.00–20.00 IU/mL) | <20.00 | / | / | / | / | / | / | / | / | / | ||
| Ferr (5–223.5 ng/mL) | / | / | 384.06 | / | / | / | 107.40 | / | / | / | ||
| ESR (0–20 mm/hour) | 41 | / | / | / | / | / | / | / | / | / | ||
| ANA | 1:1000 titer with speckled/cytoplasm granular pattern | |||||||||||
| Anti-RNP/Sm | / | / | / | / | / | / | – | / | / | / | ||
| Anti-SM | / | / | / | / | / | / | – | / | / | / | ||
| Anti-Ro52 | / | / | / | / | / | / | + | / | / | / | ||
| Anti-SSA/SSB | / | / | / | / | / | / | – | / | / | / | ||
| Anti-Jo-1 | / | / | / | / | / | / | – | / | / | / | ||
| Anti-SCI-70 | / | / | / | / | / | / | – | / | / | / | ||
| Anti-RIB | / | / | / | / | / | / | – | / | / | / | ||
| Anti-CB | / | / | / | / | / | / | – | / | / | / | ||
| Anti-M2 | / | / | / | / | / | / | – | / | / | / | ||
| Lupus anticoagulant (0.8c1.2) | / | / | / | / | / | / | 1.21 | / | 1.21 | / | ||
| ANCA | / | / | – | / | / | / | / | / | / | / | ||
| Anti-cardiolipin | / | / | / | / | / | / | / | / | / | / | ||
| Anti-2glycoprotein | / | / | / | / | / | / | / | / | / | / | ||
| Hemoculture | – | / | / | – | / | / | – | / | – | / | ||
| Urine cultures | – | / | / | / | / | / | / | / | / | / | ||
| Anti-Legionella pneumophila IgM antibody | / | |||||||||||
| Anti-Mycoplasma pneumoniae IgM antibody | / | |||||||||||
| Anti-Rickettsia Q IgM antibody | / | |||||||||||
|
Index (reference range) |
October 2017 |
November 2017 |
December 2017 |
January 2018 |
February 2018 |
|||||||
|
Date |
27 |
30* |
03 |
30 |
01* |
27* |
29 |
05 |
06* |
10 |
||
| Anti-adenovirus IgM antibody | / | |||||||||||
| Anti-respiratory syncytial virus IgM antibody | / | |||||||||||
| Anti-influenza A, B virus IgM antibody | / | |||||||||||
| Anti-parainfluenza virus type 1,2,3 IgM | / | |||||||||||
| Quantitative determination of EBV DNA (<5.00 × 102copies/mL) | / | / | / | / | / | / | – | / | / | / | ||
|
Index (reference range) |
March 2018 |
April 2018 |
May 2018 |
June 2018 |
July 2018 |
July 2020 |
August 2020 |
|||||
|
Date |
05* |
10* |
27 |
28 |
29 |
01* |
10 |
25 |
27 |
29 |
01 |
04 |
| WBC (3.5–9.5 × 109/L) | 9.31 | 10.21 | 10.48 | 7.89 | / | 10.40 | 8.32 | / | 14.68 | 14.27 | 21.41 | 28.39 |
| Neut (40%–75%) | 77.50 | 74.80 | 82.50 | 82.70 | / | 83.20 | 74.60 | / | 78.90 | 83.60 | 89 | 86.60 |
| Neut, n (1.8–6.3*109/L) | 7.21 | 7.64 | 8.65 | 6.52 | / | 8.65 | 6.21 | / | 11.58 | 11.92 | 19.05 | 24.57 |
| Lymph (20%–50%) | 14.30 | 16.50 | 10.30 | 9.00 | / | 9.50 | 14.70 | / | 10.60 | 8.90 | 7.70 | 7.90 |
| Lymph, n (1.10–3.20 × 109/L) | 1.33 | 1.68 | 1.08 | 0.71 | / | 0.99 | 1.22 | / | 1.55 | 1.27 | 1.64 | 2.24 |
| Mono (3%–10%) | 7.9 | 8.2 | 6.8 | 8.1 | / | 7.3 | 10.1 | / | 9.6 | 6.9 | 3.0 | 5.1 |
| Alb% (35–50 g/L) | 46.4 | 44.2 | 42.9 | / | / | / | 38.8 | 35.2 | / | 40.3 | 40.4 | / |
| Glo (22–33 g/L) | 36.1 | 16.6 | 25.9 | / | / | / | 21.3 | 37.6 | / | 33.2 | 37.5 | / |
| GGT (7–32 U/L) | 78 | 50 | 91 | / | / | / | 79 | 232 | / | 227 | 183 | / |
| ALP (45–125 U/L) | 73 | 57 | 74 | / | / | / | 77 | 326 | / | 268 | 219 | / |
|
Index (reference range) |
March 2018 |
April 2018 |
May 2018 |
June 2018 |
July 2018 |
July 2020 |
August 2020 |
|||||
|
Date |
05* |
10* |
27 |
28 |
29 |
01* |
10 |
25 |
27 |
29 |
01 |
04 |
| AST (8–40 U/L) | 19 | 17 | 22 | / | / | / | 15 | 142 | / | 18 | 15 | / |
| TBIL (3.4–20.5 µmol/L) | 16.4 | 14.5 | 12.2 | / | / | / | 9.8 | 9.7 | / | 8.0 | 7.0 | / |
| DBIL (0–6.8 µmol/L) | 6.1 | 6.0 | 5.0 | / | / | / | 5.2 | 6.5 | / | 4.1 | 3.4 | / |
| IBIL (1.7–13.7 µmol/L) | 10.3 | 8.5 | 7.2 | / | / | / | 4.6 | 3.2 | / | 3.9 | 3.6 | / |
| UA (155–357µmol/L) | 379 | 303 | 300 | / | / | / | 375 | 182 | / | 192 | 255 | / |
| Crea (53–97 µmol/L) | 50 | 51 | 48 | / | / | / | 47 | 50 | / | 42 | 47 | / |
| LDH (109–245 U/L) | 330 | 306 | 355 | / | / | / | 279 | 298 | / | 228 | / | / |
| HBDH (72–182/L) | 289 | 245 | 281 | / | / | / | 199 | 166 | / | 142 | / | / |
| CK (26–140 U/L) | 23 | 35 | 27 | / | / | / | 30 | 12 | / | 19 | / | / |
| Urine-Pro (−) | – | ± | / | ± | / | / | – | / | / | / | / | / |
| NT-proBNP (0–125 pg/mL) | / | / | / | / | / | / | / | / | / | 84.3 | / | / |
| IgG (7.00–16.00 g/L) | 6.15 | 5.75 | 9.02 | / | / | / | 7.89 | 18.20 | / | / | / | / |
| IgM | 0.11 | 0.08 | <0.07 | / | / | / | 0.1 | 0.49 | / | / | / | / |
| hs-CRP (<10 mg/L) | <0.30 | <0.30 | / | 10.09 | / | 9.84 | 5.81 | / | 40.27 | 30.20 | 20.77 | / |
| IL-6 (0–7 pg/mL) | 56.05 | 33.05 | / | 50.42 | / | 15.77 | 7.25 | / | 103.70 | 9.21 | 3.71 | / |
| PCT (<0.05 ng/mL) | 0.022 | <0.020 | / | 0.084 | / | 0.069 | 0.025 | / | 0.170 | 0.108 | 0.076 | / |
| CRP (<10 mg/L) | 1.40 | 1.24 | 9.09 | / | / | / | 5.79 | 129 | / | / | / | / |
| C3 (0.79–1.52 g/L) | 0.79 | 0.07 | 1.12 | / | / | / | 0.93 | 1.12 | / | / | / | / |
| C4 (0.1–0.4 g/L) | 0.08 | 0.09 | 0.2 | / | / | / | 0.18 | 0.19 | / | / | / | / |
| RF (0.00–20.00 IU/mL) | / | <20.00 | <20.00 | / | / | / | <20.00 | <20.00 | / | / | / | / |
| Ferr (5–223.5 ng/mL) | 33.02 | 26.70 | / | / | 69.74 | / | 24.53 | / | / | / | / | / |
| ESR (0–20 mm/hour) | 4 | / | / | / | / | / | / | / | / | / | / | / |
| ANA | At 1:320∼1:1000 titer with cytoplasm/granular speckled pattern | |||||||||||
| Anti-RNP/Sm | – | – | / | – | / | / | / | – | / | / | / | / |
| Anti-SM | – | – | / | – | / | / | / | – | / | / | / | / |
| Anti-Ro52 | + | + | / | + | / | / | / | + | / | / | / | / |
| Anti-SSA/SSB | – | – | / | – | / | / | / | – | / | / | / | / |
|
Index (reference range) |
March 2018 |
April 2018 |
May 2018 |
June 2018 |
July 2018 |
July 2020 |
August 2020 |
|||||
|
Date |
05* |
10* |
27 |
28 |
29 |
01* |
10 |
25 |
27 |
29 |
01 |
04 |
| Anti-Jo-1 | – | – | / | – | / | / | / | + | / | / | / | / |
| Anti-SCI-70 | – | – | / | – | / | / | / | – | / | / | / | / |
| Anti-RIB | – | – | / | – | / | / | / | – | / | / | / | / |
| Anti-CB | – | – | / | – | / | / | / | – | / | / | / | / |
| Anti-M2 | – | – | / | – | / | / | / | – | / | / | / | / |
| Lupus anticoagulant (0.8c1.2) | / | 0.99 | / | 1.10 | / | / | / | 1.55 | / | / | / | / |
| ANCA | / | / | / | – | / | / | – | – | / | / | / | / |
| Anti-cardiolipin | / | / | / | / | / | / | – | – | / | / | / | / |
| Anti-2glycoprotein | / | / | / | / | / | / | – | – | / | / | / | / |
| Hemoculture | / | / | / | / | / | / | / | / | / | / | / | / |
| Urine cultures | / | / | / | / | / | / | / | / | / | / | / | / |
| Anti-Legionella pneumophila IgM antibody | – | |||||||||||
| Anti-Mycoplasma pneumoniae IgM antibody | – | |||||||||||
| Anti-Rickettsia Q IgM antibody | – | |||||||||||
| Anti-adenovirus IgM antibody | – | |||||||||||
| Anti-respiratory syncytial virus IgM antibody | – | |||||||||||
| Anti-influenza A, B virus IgM antibody | – | |||||||||||
| Anti-parainfluenza virus type 1,2,3 IgM | – | |||||||||||
|
Index (reference range) |
March 2018 |
April 2018 |
May 2018 |
June 2018 |
July 2018 |
July 2020 |
August 2020 |
|||||
|
Date |
05* |
10* |
27 |
28 |
29 |
01* |
10 |
25 |
27 |
29 |
01 |
04 |
| Quantitative determination of EBV DNA (<5.00 × 102copies/mL) | / | / | / | / | – | / | / | – | 5.01 × 103 | / | / | / |
/, not examined; -, negative result; *, time of injection of tocilizumab.
WBC, white blood cells; Neut, neutrophils; Mono, monocytes; C, complement; hs, high sensitivity; CRP, C-reactive protein; IL-6, interleukin 6; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; EBV, Epstein–Barr virus; ANA, antinuclear antibody; ds-DNA, double-stranded DNA; Alb, albumin; GGT, gamma glutamytransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; NT-pro-BNP, N-terminal pro-B natriuretic peptide; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; ANCA, anti-neutrophil cytoplasmic antibody; Ig, immunoglobulin; RF, rheumatoid factor; RNP, ribonucleoprotein; Sm, Smith; Glo, globulin; UA, urea; Crea, creatinine; Ferr, ferritin; HBDH, hydroxybutyrate dehydrogenase; CK, creatine kinase; Urine-pro, urinary protein, SSA: anti-Sjögren syndrome-related antigen A autoantibody; SSB: anti-Sjögren syndrome-related antigen A autoantibody; anti-RIB, anti-ribosomal antibody; anti-M2, anti-mitochondrial M2 antibody; anti-CB, anti-calbindin antibody.
Discussion
Fever is common in patients with SLE and must be diagnosed promptly and accurately to prevent further organ damage and fatality. However, despite ongoing research into the mechanism of fever in SLE, there is currently no consensus and no guidelines have been made available. Fever in patients with active SLE is generally associated with a low white cell count, normal levels of complement (C3 and C4), moderately high levels of CRP, and anti-dsDNA positivity. The lack of particularly high CRP levels is due to the development of anti-CRP antibody, except in cases with serositis.1,8,9 A recent study found that, except in Asian populations, 10 PCT could not be used as an infection-related marker in SLE patients with fever.
SLE patients with fever are usually sensitive to glucocorticoids and most respond well to even a moderate dose. Occasionally, however, the dose may need to be doubled in patients taking simultaneous anti-epileptic drugs such as phenobarbital and carbamazepine, or anti-bacterial drugs such as rifampicin. These cases require higher doses of glucocorticoids because of accelerated glucocorticoid metabolism during simultaneous treatment with these agents.
A previous study suggested that continued fever despite treatment with oral prednisone 20 to 40 mg/day was highly indicative of infection, rather than SLE disease activity. 1 It is therefore essential to rule out infection and commence timely empirical antibiotic therapy in such clinical circumstances. In addition, high-dose glucocorticoids or immunosuppressants can sometimes be fatal when considering active lupus flares. 1 In both the current cases, leukocytosis and peripheral blood smears initially suggested infections, and several antibiotics and antifungals, and in one case an antiviral, was administered simultaneously with glucocorticoids, but with poor responses in both patients. In addition, their blood, urine, sputum, and stool cultures were negative. Notably however, examination of their inflammatory cytokine markers during the latter course of treatment showed high IL-6 levels in the blood, and TCZ accordingly had good effects in both patients.
IL-6 is a pleiotropic cytokine produced by immunocytes, hepatocytes, myocytes, and adipose tissues. It has two receptors, IL-6 receptor α (IL-Rα) subunit and membrane-anchored glycoprotein (gp) 130. IL-6 executes its biological functions via a hexameric complex composed of IL-6, IL-6Rα, and gp 130, which activates gp 130 and triggers the activation of downstream signaling pathways, such as the Janus-activated kinase-signal transducer and activator of transcription 3 and mitogen-activated protein kinase pathways.11,12 IL-6 levels are very low under physiological conditions, but play an important role in maintaining homeostasis. However, IL-6 synthesis is promptly induced under inflammatory conditions resulting from infections or injuries.11,13 This significant increment in IL-6 level is implicated as a pathogenic mechanism of autoimmune disease. It is the strongest CRP inducer, and in synergy with transforming growth factor (TGF)-β, IL-6 also preferentially induces the differentiation of naïve CD4+ T cells into T-helper (Th) 17 cells, simultaneously inhibiting the growth of regulatory T-cells induced by TGF-β. 14 Th17 cells can further release cytokines including tumor necrosis factor (TNF) and IL-6, as well as IL-23 and IL-17, which are involved in inflammation in SLE patients. 15 As an endogenous pyrogen, TNF can cause fever through direct stimulation of the hypothalamus thermoregulatory center, and further stimulates other cells to produce IL-6, resulting in amplification of inflammatory signals. 12
Meanwhile, IL-6 can stimulate the secretion of the neuropeptide, substance P, which is involved in lipopolysaccharide-dependent or prostaglandin-dependent fever, and induces the synthesis of prostaglandin E2 to influence the thermoregulatory center located in the hypothalamus.16,17 A study in animals found that IL-6 also induced fever through IL-6Rα located in the brain. IL-6 is thus an important mediator of fever induction. 18
SLE is considered as a disease with an “IFN signature”. IFN-α levels are higher in SLE patients than in healthy people, and increased serum IFN-γ levels in patients during the development of SLE were reported to correlate with the development of lupus-specific autoantibody. 19 Synergy of the breakpoint cluster region, Toll-like receptor, and CD40 signals with IFN-γ can promote B cells to produce IL-6. A lack of B cell-derived IL-6 protected against lupus nephritis in a mouse study. 20 In addition, serum IL-6 levels were elevated concurrently with or prior to autoantibody positivity in SLE, suggesting a key role for IL-6 signals in initiating breaks in B and/or T cell tolerance. 19 Furthermore, high levels of serum IL-6 impaired the autophagic degradation of macrophages via augmentation of high-level expression of IL-6Rs, 21 and high levels of IL-6 were observed in the pericardial fluid in SLE patients. 22 The above mechanisms have led to the wide use of the IL-6R antagonist, TCZ, to treat fever of unknown origin in various inflammatory diseases, such as Still’s disease and juvenile idiopathic arthritis, but seldom in SLE patients to date.23–25 In a recent case report, a 41-year-old woman with refractory SLE who suffered from intractable high fever, massive pericarditis, macrophage activation syndrome, and glomerulonephritis showed dramatic improvement after TCZ therapy. 22 Likewise, a pilot study in 16 SLE patients and a trial in 15 SLE patients treated with TCZ showed better curative outcomes and significantly reduced levels of IgG and anti-dsDNA.26,27 We had already treated the current patients aggressively with several antibiotics and other immunosuppressants on multiple occasions, with no response, and TCZ was the remaining best option. We accordingly chose to administer TCZ due to its targeted mechanism, less immunosuppression, and fewer side effects compared with other available biologics at that time.
In conclusion, further studies are needed to clarify the role of IL-6 in the pathogenesis of SLE and the therapeutic role of TCZ in SLE patients with high fever. These results will further our understanding of the pathogenesis of the disease, leading to alternative treatment strategies.
Supplemental Material
Supplemental material, sj-jpg-1-imr-10.1177_03000605221088558 for Tocilizumab therapy for persistent high-grade fever in systemic lupus erythematosus: two cases and a literature review by Ma Chaoyi, Bikash Shrestha, Li Hui, Ding Qiujin and Fu Ping in Journal of International Medical Research
Supplemental material, sj-jpg-2-imr-10.1177_03000605221088558 for Tocilizumab therapy for persistent high-grade fever in systemic lupus erythematosus: two cases and a literature review by Ma Chaoyi, Bikash Shrestha, Li Hui, Ding Qiujin and Fu Ping in Journal of International Medical Research
Acknowledgements
The authors would like to thank Mrs. Sunita Shakya for English language editing, and for the graph.
Ethics statement: The study protocol was approved by the ethics review committee of the Second Affiliated Hospital of Kunming Medical University. The reporting of this study conforms to CARE guidelines.7 Both patients provided verbal and written informed consent for the submission and publication of the manuscript and the included images.
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by a grant from the National Nature Science Foundation of China [grant number 81860287].
ORCID iD: Ma Chaoyi https://orcid.org/0000-0003-3417-313X
References
- 1.Rovin BH, Tang Y, Sun J, et al. Clinical significance of fever in the systemic lupus erythematosus patient receiving steroid therapy. Kidney Int 2005; 68: 747–759. [DOI] [PubMed] [Google Scholar]
- 2.Zhou WJ, Yang CD. The causes and clinical significance of fever in systemic lupus erythematosus: a retrospective study of 487 hospitalized patients. Lupus 2009; 18: 807–812. [DOI] [PubMed] [Google Scholar]
- 3.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus (letter). Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288–291. [PubMed] [Google Scholar]
- 7.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz HM, Pieterse L, Ruter T, et al. Fever in systemic lupus erythematous: disease exacerbation or infection? Z Rheumatol 2020; 79: 325–331. (Article in German). [DOI] [PubMed] [Google Scholar]
- 9.Suh CH, Chun HY, Ye YM, et al. Unresponsiveness of C-reactive protein in the non-infectious inflammation of systemic lupus erythematosus is associated with interleukin 6. Clin Immunol 2006; 119: 291–296. [DOI] [PubMed] [Google Scholar]
- 10.Liu LN, Wang P, Guan SY, et al. Comparison of plasma/serum levels of procalcitonin between infection and febrile disease flare in patients with systemic lupus erythematosus: A meta-analysis. Rheumatol Int 2017; 37: 1991–1998. [DOI] [PubMed] [Google Scholar]
- 11.Rose-John S. Interleukin-6 signaling in health and disease. F1000Res 2020; 9: F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfi F, Franza L, Carusi V, et al. Interleukin-6 in rheumatoid arthritis. Int J Mol Sci 2020; 21: 5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol 2003; 149: 1–38. [DOI] [PubMed] [Google Scholar]
- 14.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17balamce. Eur J Immunol 2010; 40: 1830–1835. [DOI] [PubMed] [Google Scholar]
- 15.Koga T, Ichinose K, Tsokos GC. T cells and IL-17 in lupus nephritis. Clin Immunol 2017; 185: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X, Zhang M, Xiao Y, et al. Interleukin-6 producing pheochromocytoma as a reason for fever of unknown origin: A retrospective study. Endocr Pract 2018: 24: 507–511. [DOI] [PubMed] [Google Scholar]
- 17.Brito HO, Barbosa FL, Reis RCD, et al. Evidence of substance P autocrine circuitry that involve TNF-alpha, IL-6, PGE-2 in endogenous pyrogen-induced fever. J Neuroimmunol 2016; 293: 1–7. [DOI] [PubMed] [Google Scholar]
- 18.Egecioglu E, Anesten F, Schele E, et al . Interleukin-6 is important for regulation of core body temperature during long-term cold exposure in mice. Biomed Rep 2018; 9: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munroe ME, Lu R, Zhao YD, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis 2016; 75: 2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arkatkar T, Du SW, Jacobs HM, et al. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 2017; 214: 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu HC, Chen YH, Lin TS, et al. Systemic lupus erythematosus is associated with impaired autophagic degradation via interleukin-6 in macrophages. Biochim Biophys Acta Mol Basis Dis 2021; 1867: 166027. [DOI] [PubMed] [Google Scholar]
- 22.Iwai A, Naniwa T, Tamachika S, et al. Short-term add-on tocilizumab and intravenous cyclophosphamide exhibited a remission-inducing effect in a patient with systemic lupus erythematosus with refractory multiorgan involvements including massive pericarditis and glomerulonephritis. Mod Rheumatol 2017; 27: 529–532. [DOI] [PubMed] [Google Scholar]
- 23.Iriki H, Ouchi T, Ito H, et al. Case of lamotrigine-induced drug adverse reaction under tocilizumab treatment with clinical and virological features of drug induced hypersensitivity syndrome. J Dermatol 2018; 45: 738–741. [DOI] [PubMed] [Google Scholar]
- 24.Nihira H, Nakagawa K, Izawa K, et al. Fever of unknown origin with rashes in early infancy is indicative of adenosine deaminase type 2 deficiency. Scand J Rheumatol 2018; 47: 170–172. [DOI] [PubMed] [Google Scholar]
- 25.Wawrzycki B, Krasowska D, Pietrzak A, et al. Urticarial rash, fever, and arthritis: A case of refractory adult-onset Still’s disease with good response to tocilizumab. Dermatol Ther 2019; 32: e13041. [DOI] [PubMed] [Google Scholar]
- 26.Shirota Y, Yarboro CH, Fischer R, et al. Impact of interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann Rheum Dis 2013; 72: 118–128. [DOI] [PubMed] [Google Scholar]
- 27.Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 2010; 62: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-imr-10.1177_03000605221088558 for Tocilizumab therapy for persistent high-grade fever in systemic lupus erythematosus: two cases and a literature review by Ma Chaoyi, Bikash Shrestha, Li Hui, Ding Qiujin and Fu Ping in Journal of International Medical Research
Supplemental material, sj-jpg-2-imr-10.1177_03000605221088558 for Tocilizumab therapy for persistent high-grade fever in systemic lupus erythematosus: two cases and a literature review by Ma Chaoyi, Bikash Shrestha, Li Hui, Ding Qiujin and Fu Ping in Journal of International Medical Research