Abstract
Some new pyridinethione and thienopyridine derivatives have been synthesized and evaluated for their antiproliferative activity using the MTT assay. Nicotinamide derivatives 3 have been synthesized and used for the preparation of new condensed thieno [2,3-b]pyridines by their reactions with active halo compounds. Finally the synthesized thienopyridine underwent ring closure whenever possible through boiling in a solution of sodium ethoxide. The antiproliferative evaluation against (HCT-116, HepG-2, and MCF-7) human cancer cells and one human healthy cell line (BJ-1) revealed that compounds 3b, 4c–5d, 7b–12a, 10d, and 13b have interesting antitumor activity specifically as antihepatocellular and anticolon cellular carcinoma agents. Besides, the docking results for most active derivatives were in agreement with the in vitro antitumor results.
1. Introduction
Development of newer, safer, and more efficient anticancer agents has a significant interest, especially the use of available chemotherapeutics is frequently limited due to unwanted side effects. Moreover, drug resistance to cancer chemotherapeutic is becoming increasingly prevalent.1
The pyridine nucleus is predominant in numerous natural products as vitamins, coenzymes, and alkaloids (Figure 1). Additionally, the pyridine moiety is incorporated in many drugs, including anticancer, antiviral, antimicrobial, anti-inflammatory, antihypertensive, analgesic, antimalarial and antidiabetic antioxidants, psychopharmacological antagonists, and insecticidal and antiamoebic agents.2−14
Figure 1.
Natural products containing the pyridine nucleus.
Nicotinonitriles15 and pyridinethione16 were used lately by many research groups as a scaffold for synthesis of compounds with several biological activities especially as anticancer agents. For example, 4,6-diaryl-2-oxo-1,2-dihydropyridine-3- carbonitriles have been prescribed as inhibitors of the oncogenic serine/threonine kinase PIM-1 that plays a role in the survival of cancer cells.17 In addition, an anticancer effect against MCF7, SK-OV-3 (ovarian adenocarcinoma cells), and CCRF-CEM (blood cancer cells) was detected from pyridine-2(1H)-thione derivatives.18 Also, 3-cyano-2-thioxopyridines are of great interest since they have potential synthetic and biological abilities.19
According to druglike molecules build up approach, an improvement in the pharmacological profiles may be obtained through fused analogues or by combination with other heterocycles.20 Among these heterocyclic systems, thienopyridines are considered as the most active and important ones, where a number of drug containing thienopyridine nucleus are available in the market. For example, prasugrel,21 and ticlopidine and clopidogrel22 (Figure 2) were reported as antiplatelet drugs. Potent biological activities such as antileishmanial, antimicrobial, anti-inflammatory, anticancer, and antiplatelet agents were also observed from thienopyridine and their derivatives.23−29
Figure 2.
Marketed drugs containing a thienopyridine nucleus.
Prompted by these and as an extension of our preceding effort in the chemistry of pyridine, pyridinethione, and thienopyridine,30−33 we describe here the synthesis of a new series of substituted and condensed pyridines.
2. Results and Discussion
2.1. Chemistry
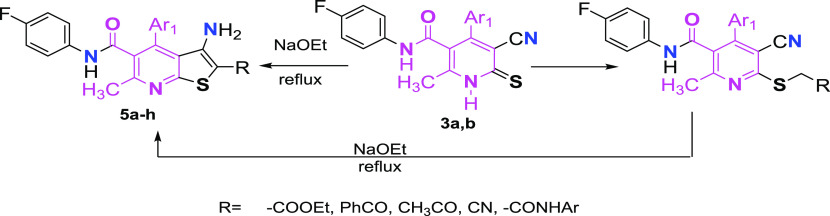
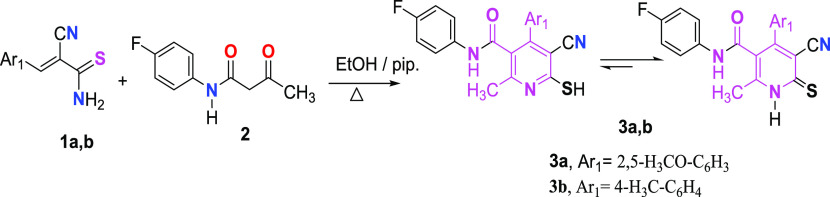
3-Aryl-2-cyano-prop-2-enethioamide derivatives 1a,b were refluxed with N-(4-fluorophenyl)-3-oxobutanamide 2 in ethanol, in the presence of a few drops of piperidine to give cyanopyridinethione derivatives 3a,b (Scheme 1).
Scheme 1. Synthesis of New Cyanopyridinethione Derivatives.
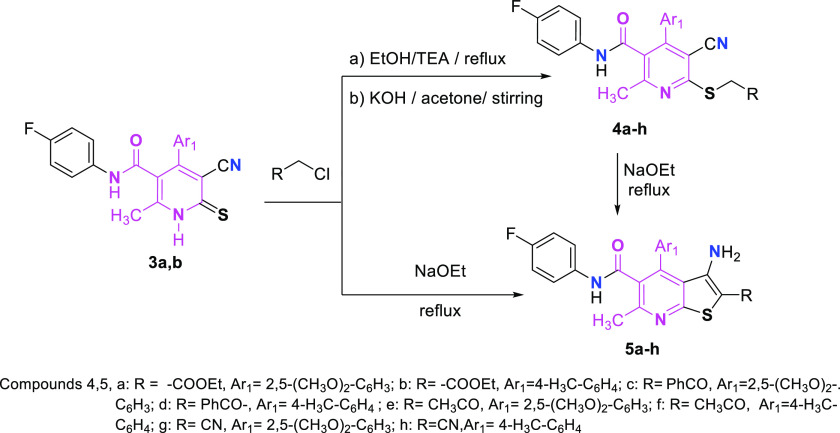
To explore the synthetic potentiality of pyridinethiones 3a,b, reactions with several reagents as ethyl chloroacetate, phenacyl chloride, chloroacetone, and chloroacetonitrile were performed, resulting in the formation of the corresponding S-alkylated derivatives 4a–4h, respectively (Scheme 2).
Scheme 2. Synthesis of S-Alkylated Cyanopyridines and Their Thienopyridines.
Former compound 4a–4h underwent intramolecular cyclization when refluxed in sodium ethoxide solution to provide an additional series of thieno[2,3-b]pyridine derivatives 5a–5h, respectively. However, thieno[2,3-b]pyridine derivatives 5a–5h could also be synthesized directly through the action of sodium ethoxide on pyridinethione 3 with α-halocarbonyl compounds. Structures of derivatives (5a–5h) were confirmed using both the elemental analysis plus spectral data.
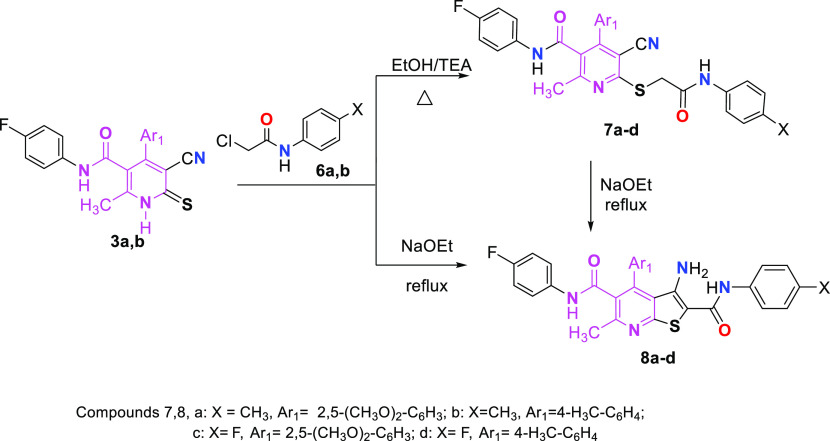
When 2-thioxopyridine-3-carbonitrile 3a,b was refluxed with derivatives of 2-chloro-N-arylacetamide 6a,b as 2-chloro-N-(4-fluorophenyl)acetamide or 2-chloro-N-(p-tolyl)acetamide34 in EtOH containing a catalytic amount of Et3N, the corresponding 2-(N-aryl)-carboxamidomethylthiopyridine derivatives 7a–7d were obtained, respectively (Scheme 3).
Scheme 3. Synthesis of 2-(N-Aryl)carboxamidomethylthiopyridine Derivatives 7a–7d and Their Condensed Products 8a–8d.
Derivatives of thieno[2,3-b]pyridine-2,5-dicarboxamide 8a–8d can be obtained by the cyclization of compounds 7a–7d or directly from compounds 3a,b with 2-chloro-N-arylacetamide derivatives 6a,b, in sodium ethoxide solution (Scheme 3). The structures of compound 8a–8d were proved through elemental analysis in addition to spectral data. Where the 1H NMR spectra for 8a–8d derivatives were characterized by the absence of the CH2 group singlet aforementioned in compounds 7a–7d and the appearance of a peak near 6 ppm corresponding to NH2.
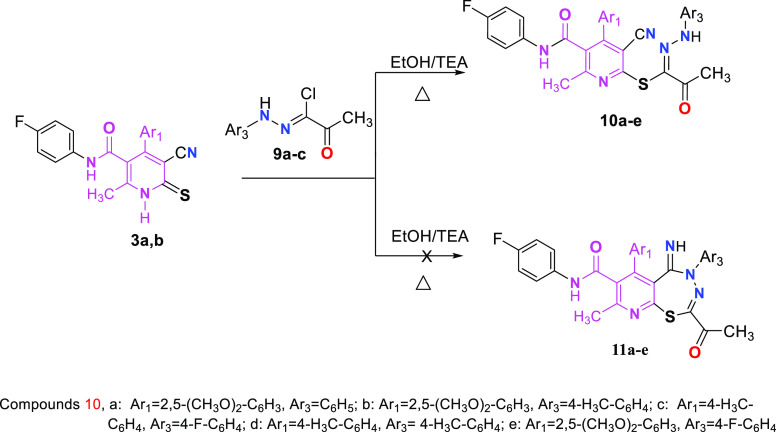
The study was concerned also in examining the activity of thiopyridine derivative 3 toward (C-acetyl)-N-arylhydrazonoyl chlorides 9a–9c through refluxing them in ethanol containing drops of trimethylamine, and the results showed that derivative 10 (open structure) and not derivative 11 (fused structure) was isolated from the reaction mixture (Scheme 4).
Scheme 4. Reaction of Pyridinethions 3a,b with Hydrazoyl Halides 9a–9c.
The structure of compounds 10a–10e were determined by their elemental analysis and spectral data. Hence, the IR spectra showed bands owing to the CN group.
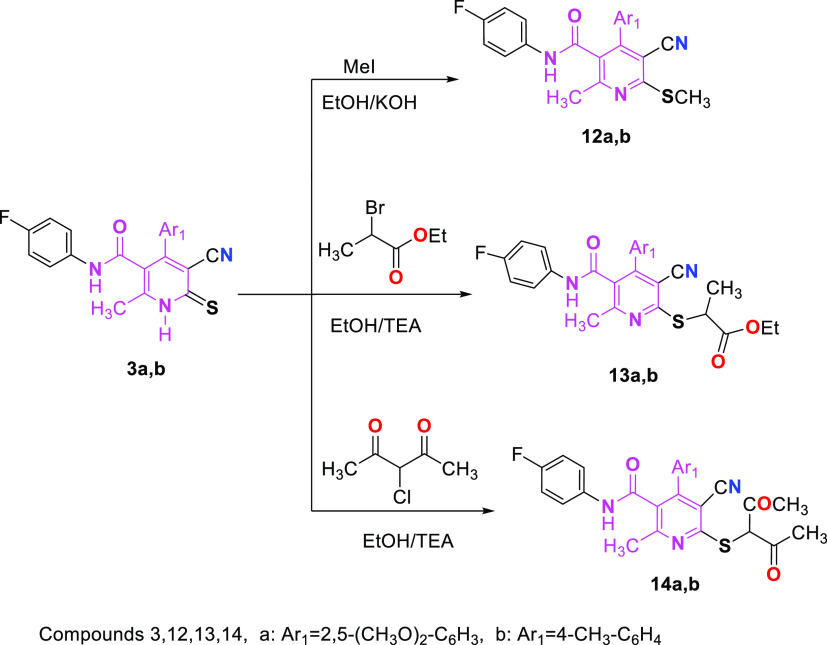
Finally, pyridinethione derivatives 3a,b were reacted with various halogenated compounds such as (methyl iodide, 2-bromopropionate or 3-choloro-2,4-pentandione) and KOH or TEA under reflux to afford S-alkylated compounds 12, 13, and 14, respectively (Scheme 5). The products structures 12a,b, 13a,b, and 14a,b were established based on their correct spectral data and elemental analyses. In general, the IR spectra were characterized by one, two, and three bands assigned for the C=O group, respectively.
Scheme 5. Reaction of Thienopyridines 3a,b with Halogenated Compounds.
2.2. Antiproliferative Activity
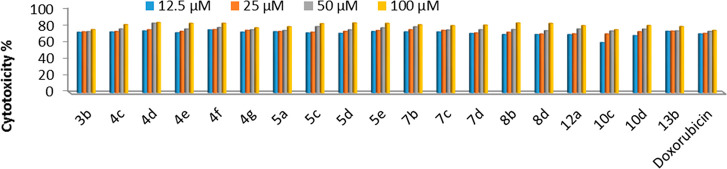
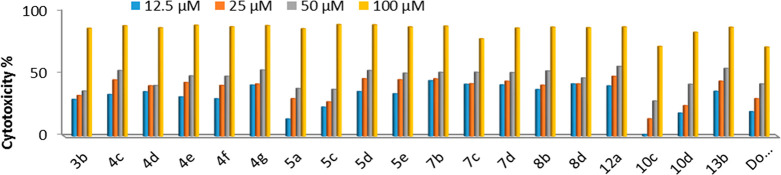
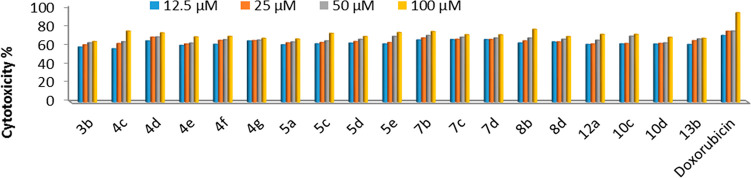
The in vitro screening of a series of 19 derivatives for their activity against HCT-116, HepG-2, and MCF-7 human cancer cells and one human healthy cell line (BJ-1) were performed by the MTT assay. Intact cells percentages were calculated and compared with those of the control. Compounds activities against the three cell lines were compared to the activity of doxorubicin. A suppression in all cancer cells was observed in a dose-dependent manner (Figures 3 and 4). For HCT-116 human colorectal carcinoma cells, both Figure 3 and Table 4 show that 17 compounds (5d, 4d, 4f, 7b, 5c, 4e, 4c, 7c, 8b, 13b, 4g, 5a, 7d, 10d, 8d, 12a, and 3b, respectively) have more potent cytotoxic activities; two compounds (10c and 5e, respectively) have significantly less cytotoxic activity against HCT-116 relative to that of doxorubicin. In the case of MCF-7 human breast cancer cells, two compounds (7b and 4c, respectively) have slightly less cytotoxic activities; the rest of the compounds have essentially less cytotoxic activities against MCF-7 relative to the reference drug (Figure 5 and Table 1). In the case of HepG2 human liver cancer cells, 18 compounds (7b, 12a, 4g, 5d, 7d, 13b, 4c, 8b, 5e, 8d, 4e, 7c, 4f, 4d, 3b, 5c, 10d, and 5a, respectively) have strong activities; one compound (10c) has altogether less cytotoxic activity against HepG2 relative to that of doxorubicin (Figure 4 and Table 1). From the above results, it is possible to conclude that compounds 3b, 4c–5d, 7b–12a, 10d, 13b are selectively active on both human liver and colon cancer types but not active on the human breast cancer type. Compound 5e is specifically active on only the human liver cancer type but not active on both the human colon and breast cancer types. Compound 10c is selectively active on only human colon cancer but not active on both the human liver and breast cancer types. All compounds were tested against the nontumor fibroblast-derived cells line (BJ-1) and demonstrated very low cytotoxicity.
Figure 3.
Dose dependent antiproliferative data of the 19 compounds against HCT-116 cancer cells according to the MTT assay after 48 h of exposure.
Figure 4.
Dose dependent antiproliferative data of the 19 compounds against HepG2 cancer cells according to the MTT assay after 48 h of exposure.
Table 4. Docking Results of 4d, 5e, 7c, 7d, 8b, and 10c with the Receptor 6p05.
| cmpd | ligand moiety | receptor site | interacting residues (type of interaction) | distance (Å) | E (kcal/mol) | docking score (kcal/mol) |
|---|---|---|---|---|---|---|
| 4d | –7.3447 | |||||
| 5e | –7.5894 | |||||
| 7c | –7.1441 | |||||
| 7d | –7.5635 | |||||
| 8b | 6-ring | CD1 LEU 92 (A) | π–H | 4.00 | –0.6 | –6.7931 |
| 6-ring | OH TYR 97 (A) | π–H | 4.45 | –0.8 | ||
| 10c | –7.5544 |
Figure 5.
Dose dependent antiproliferative data of the 19 compounds against MCF-7 cancer cells according to the MTT assay after 48 h of exposure.
Table 1. Antiproliferative IC50 Values against the Three Cancer Cell Lines (MTT Assay).
| IC50 (μM) ± SD |
|||
|---|---|---|---|
| compound code | HCT-116 | HepG-2 | MCF-7 |
| 3b | 19.7 ± 2.8 | 52.9 ± 5.1 | 38.7 ± 4.1 |
| 4c | 15.4 ± 1.9 | 36.1 ± 3.9 | 32.8 ± 3.6 |
| 4d | 10.3 ± 1.1 | 45.1 ± 4.6 | 24.8 ± 3.1 |
| 4e | 15.2 ± 1.3 | 40.4 ± 4.1 | 34.7 ± 2.9 |
| 4f | 12.3 ± 1.5 | 43.6 ± 4.3 | 30.9 ± 3.5 |
| 4g | 17.2 ± 2.1 | 33.3 ± 3.1 | 30.8 ± 2.7 |
| 5a | 17.3 ± 2.1 | 60.7 ± 5.8 | 34.5 ± 4.1 |
| 5c | 14.8 ± 2.5 | 55.8 ± 5.2 | 30.6 ± 2.5 |
| 5d | 9.5 ± 1.1 | 33.6 ± 3.7 | 30.8 ± 3.5 |
| 5e | 26.7 ± 3.1 | 37.5 ± 3.8 | 28.3 ± 1.9 |
| 7b | 13.7 ± 1.8 | 30.4 ± 3.2 | 23.2 ± 2.5 |
| 7c | 15.9 ± 2.1 | 41.7 ± 4.1 | 26.3 ± 1.7 |
| 7d | 17.5 ± 2.4 | 35.4 ± 4.1 | 26.5 ± 3.5 |
| 8b | 16.3 ± 1.9 | 37.4 ± 3.9 | 26.3 ± 3.1 |
| 8d | 18.1 ± 2.6 | 37.8 ± 4.3 | 30.4 ± 3.7 |
| 12a | 18.4 ± 3.1 | 31.6 ± 3.1 | 31.9 ± 2.9 |
| 10c | 25.1 ± 3.5 | 85.1 ± 5.9 | 29.6 ± 2.5 |
| 10d | 17.8 ± 2.5 | 59.3 ± 5.1 | 34.3 ± 3.5 |
| 13b | 17.1 ± 2.4 | 35.9 ± 3.5 | 31.8 ± 2.8 |
| doxorubicin | 21.8 ± 2.9 | 63.2 ± 5.8 | 16.7 ± 1.5 |
2.3. Molecular Docking
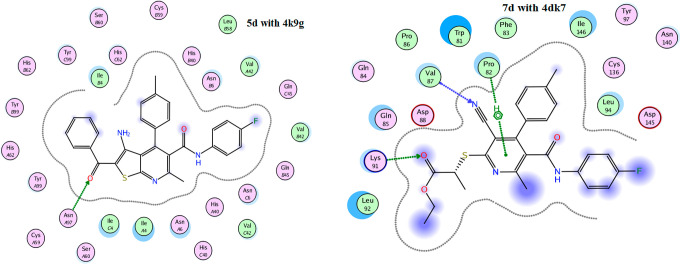
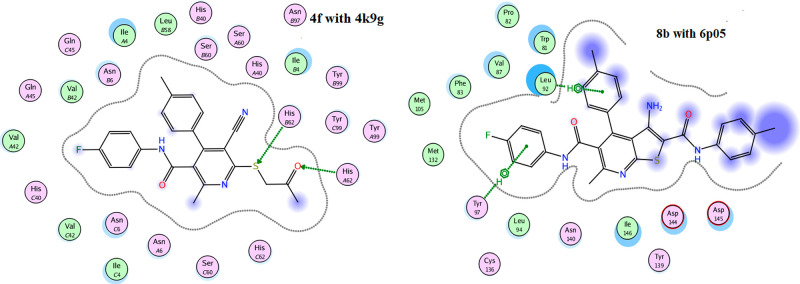
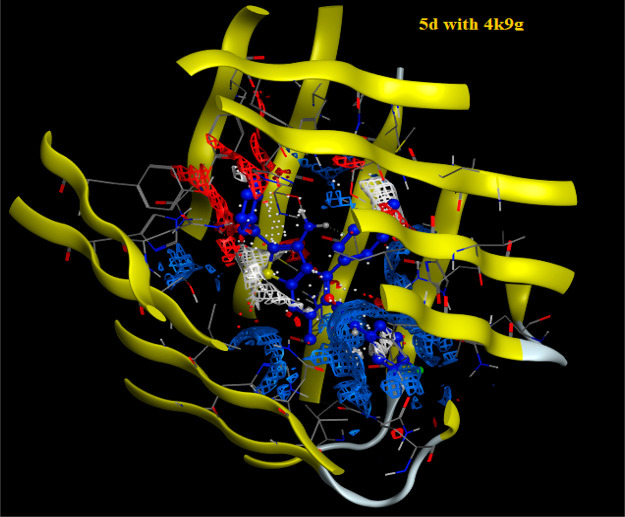
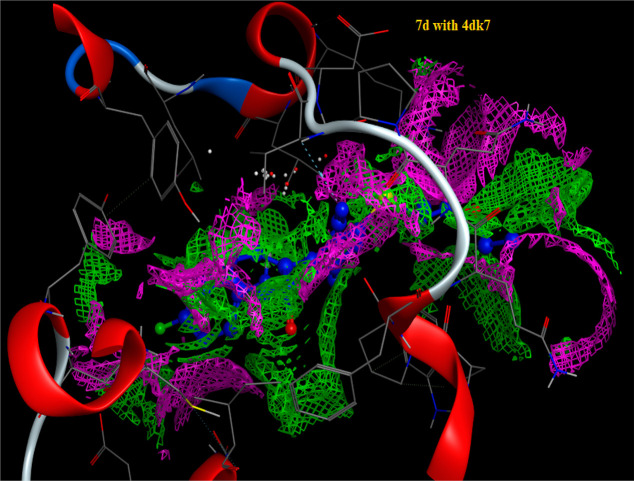
Molecular docking has become a widespread tool for drug discovery.35,36 To illustrate the potential binding mode of pyridine derivatives into the active sites of the three types of tumor cells we investigated (HCT-116, HepG-2 and MCF-7), a docking study of the most active derivatives against each cell type using MOE (2014.0901) (Molecular Operating Environment software). The molecular docking studies were performed using (4k9g), (4dk7), and (6p05), the cocrystal structures of proteins for colon, human liver, and breast cancer cells, respectively. The results are presented in Tables 2–4 and Figures 6–9. From the results of docking study, we noticed that the highest binding interactions were found between the pyridine derivatives 4d, 4f, 5c, 5d, and 7b with 4k9g of colon cancer (−9.4430 to −8.0733 kcal/mol) which is compatible with experimental results of the highest activity against colon cancer HCT-116 (Table 4). The most reactive derivative 5d showed H-acceptor interaction toward the 4k9g protein with fit docking paths (bond length ≤3.5 Å) (Figures 6 and 7). In the case of 4dk7 for human liver, all tested pyridine derivatives showed interactions with docking scores ranging from −7.6825 to −4.5118 (Table 3). The two more reactive pyridine derivatives 7b and 7d showed a π–H interaction of the pyridine ring toward TYR97 and PRO82 amino acid residues. The pyridine derivative 7d showed two other H-acceptor interactions with the amino acid residues VAL87 and LYS91due to the presence of C≡N and C=O groups (Figure 4). The H-acceptor interaction with HIS-62 is the common type of interaction in derivatives 4f and 7b (Table 4 and Figure 8). The pyridine derivatives exert low anticancer activity on the MCF-7 cell line. This is consistent with the data shown in Table 4 as all derivatives did not interact with the amino acids of 6p05 protein, except derivative 8b (Figure 9). The latter incurred π–H interaction of the 6-ring with LEU92 and TYR97 amino acids.
Table 2. Docking results of 4d, 4f, 5c, 5d and 7b with receptor of 4k9g.
| cmpd | ligand moiety | receptor site | interacting residues (type of interaction) | distance (Å) | E (kcal/mol) | docking score (kcal/mol) |
|---|---|---|---|---|---|---|
| 4d | –9.3347 | |||||
| 4f | S 1 | NE2 HIS 62 (B) | H-acceptor | 3.33 | –1.2 | –8.2076 |
| O 45 | NE2 HIS 62 (A) | H-acceptor | 3.06 | –3.1 | ||
| 5c | –8.0733 | |||||
| 5d | O 54 | ND2 ASN 97 (A) | H-acceptor | 3.27 | –2.2 | –9.4430 |
| 7b | O 37 | NE2 HIS 62 (C) | H-acceptor | 2.76 | –6.0 | –8.8233 |
Figure 6.
2D docked model of compound 5d and 7d, respectively, into the active site of 4k9g and 4dk7.
Figure 9.
2D docked model of compounds 4f and 8b, respectively, into the active site of 4k9g and 6p05.
Figure 7.
Electrostatic map of 5d on the active site of 4k9g
Table 3. Docking Results of 4c, 5d, 4g, 7b, 7d, 12a, and 13b with the Receptor of 4dk7.
| cmpd | ligand moiety | receptor site | interacting residues (type of interaction) | distance (Å) | E (kcal/mol) | docking score (kcal/mol) |
|---|---|---|---|---|---|---|
| 4c | S 1 | O ALA 359 (A) | H-donor | 3.91 | –0.7 | –5.9269 |
| 6-ring | CB MSE 423 (A) | π–H | 4.20 | –1.0 | ||
| 5d | S 15 | OE1 GLU 355 (A) | H-donor | 3.56 | –2.2 | –4.5118 |
| 4g | –6.3130 | |||||
| 7b | 6-ring | OH TYR 97 (A) | π–H | 4.70 | –0.8 | –7.6102 |
| 7d | O 40 | NZ LYS 91 (A) | H-acceptor | 2.87 | –3.0 | –7.6825 |
| N 58 | CA VAL 87 (A) | H-acceptor | 3.18 | –1.2 | ||
| 6-ring | CB PRO 82 (A) | π–H | 4.21 | –0.6 | ||
| 12a | –7.2737 | |||||
| 13b | –7.2510 |
Figure 8.
Contact performance of 7d on the active site of 4dk7.
3. Structure Activity Relationships
By correlation of the afforded activity results against the tested cancer cell lines with the characteristic structural features (SAR) of the most active compounds, taking into account the docking results, the effect of aromatic subistitution in hydrazonyl chlorides, 6a,b and also (C-acetyl)-N-arylhydrazonylchlorides 9a–9c on the activities of the synthesized compounds 7a–7d and 10a–10e is more evident. Thus, in the light of our current results (Figures 3–5), we can deduce that the presence of the methyl group or fluoro atom on the aromatic rings lead to an increased inhibitory behavior. It is conspicuous that the efficiency was minimized in other compounds which do not include such structural functional moieties in their structural skeleton.
The structures are usually diverse from what we have seen in comparative structures in which the methyl group and fluoro atom are linked to aromatic ring in the pyridine ring system. Within the current work, the foremost successful structures were the substituted pyridine derivatives 7b, 7c, 7d, and 10d when tested considering doxorubicin as a standard drug. The contrast in action between the newly synthesized compounds may be credited to the shown connections of the molecule’s pyridine ring.
4. Conclusions
This report presents a convenient and efficient synthesis of novel pyridinethione and thienopyridine derivatives. The new products were screened for their cytotoxic activity against human cancer cell lines. Several compounds were selectively active on both human liver and colon cancer cells but not active on human breast cancer cells. All compounds demonstrated low cytotoxicity when screened against a nontumor fibroblast-derived cell line thereby providing a good safety profile as potential anticancer agents. Molecular docking analysis illustrated that the synthesized compounds are predicted to fit into the binding sites of the target 4k9g and 4dk7 proteins associated with specific cancer cell types.
5. Experimental Section
5.1. Synthesis
All melting points were measured on a Gallenkamp Melting point apparatus and are uncorrected. The IR spectra were recorded on a Shimadzu FT-IR 8101 PC infrared spectrophotometer (Shimadzu, Tokyo, Japan) using KBr disks. The NMR spectra were preserved on a Varian Mercury VX-400 NMR spectrometer (Varian, Palo Alto, CA). 1H NMR spectra were run at 400 MHz and 13C NMR spectra were run at 100 MHz in deuterated chloroform (CDCl3) or dimethyl sulfoxide (DMSO-d6) as specified in individual compound characterizations. Chemical shifts are given in parts per million and were referenced to those of the solvents. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX mass spectrometer at 70 eV. Elemental analyses were registered on an Elementar-Vario EL (Germany) automatic analyzer. The molecular docking studies have been performed using MOE (2014.0901) (Molecular Operating Environment software). The molecular docking studies were obtained using (4k9g), (4dk7), and (6p05), the cocrystal structures of proteins for colon, human liver, and breast cancer cells, respectively, using MOE (2014.0901) (Molecular Operating Environment software).
5.1.1. Preparation of Pyridine-2(1H) Thione Derivatives (3a, 3b)
In absolute ethanol (15 mL), a mixture of the appropriate 3-aryl-2-cyano-prop-2-enethioamide derivative 1a or 1b (10 mmol), N-(4-fluorophenyl)-3-oxobutanamide 2 (10 mmol), and a few drops of piperidine were heated under reflux for 5–8 h. The reaction mixture was then allowed to cool before the precipitate was filtered, dried, and crystallized from ethanol to get the pyridine-2(1H) thione derivatives.
5.1.1.1. 5-Cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-6-mercapto-2-methylnicotin-amidemethylnicotinamide (3a)
Orange solid (81%); mp: 170–172 °C (EtOH); IR (KBr, v, cm–1): 3454 (NH), 3252 (NH), 2229 (CN), 1644 (C=O); 1H NMR (DMSO-d6): δ 2.51 (s, 3H, CH3), 3.76 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 7.13–8.32 (m, 7H, CH-Ar), 9.60 (bs, 1H, NH), 10.10 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 23.21, 56.03, 56.81, 113.11, 113.37, 113.56, 116.79, 120.36, 121.32, 121.82, 121.87, 143.12, 153.16, 153.46, 192.65; MS m/z (%): 423 [M+] (95). Anal. calcd for C22H18FN3O3S: C, 62.40; H, 4.28; N, 9.92. Found: C, 62.42; H, 4.25; N, 9.90.
5.1.1.2. 5-Cyano-N-(4-fluorophenyl)-2-methyl-6-thioxo-4-(p-tolyl)-1,6-dihydropyridine-3-carboxamide (3b)
Yellow solid (86%); mp: 228–230 °C (EtOH); IR (KBr, v, cm–1): 3448 (NH), 3221 (NH), 2222 (CN), 1650 (C=O); 1H NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.51 (s, 3H, CH3), 6.95–7.89 (m, 8H, CH-Ar), 9.65 (bs, 1H, NH), 10.42 (bs, 1H, NH); MS m/z (%): 377 [M+] (29.55), 267 (100), 110 (13), 359 (16.38), 82 (33). Anal. calcd for C21H16FN3OS: C, 66.83; H, 4.27; N, 11.13. Found: C, 66.85; H, 4.26; N, 11.15.
5.1.2. Preparation of Compounds 4a–4h
5.1.2.1. Method A
A few drops of triethylamine were added to a mixture of 3a or 3b (10 mmol) and the appropriate α-haloketone (namely, ethyl chloroacetate, phenacyl chloride, chloroacetone, or chloroacetonitrile) (10 mmol) in absolute ethanol (15 mL). The reaction mixture was allowed to cool after being refluxed for 3–5 h. Compounds 4a–4h were obtained by filtering off the produced precipitate, drying, and crystallizing it from the proper solvent.
5.1.2.2. Method B
A white to pale yellow precipitate was noticed after stirring a mixture of 3a or 3b (10 mmol), the appropriate α-haloketone (namely, ethyl chloroacetate, phenacyl chloride, chloroacetone, or chloroacetonitrile) (10 mmol) and potassium hydroxide (10 mmol) in acetone (15 mL) for 10 h. Compounds 4a–4h were obtained by filtering the generated precipitate, drying, and crystallizing it from ethanol.
5.1.2.3. Ethyl-2-((3-cyano-4-(2,5-dimethoxyphenyl)-5-((4-fluorophenyl)-carbamoyl)-6-methylpyridin-2-yl)thio)acetate (4a)
White solid [A] (90%), [B] (65%); mp: 201–204 °C (EtOH); IR (KBr, v, cm–1): 3240 (NH), 2221 (CN), 1747 (C=O), 1636 (C=O); 1H NMR (DMSO-d6): δ 1.26 (t, 3H, CH3), 2.55 (s, 3H, CH3), 3.70 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 4.17 (s, 2H, CH2), 4.19 (q, 2H, CH2), 6.95–7.46 (m, 7H, CH-Ar), 10.52 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 14.56, 23.28, 33.05, 55.27, 56.32, 61.55, 104.65, 113.41, 115.47, 116.13, 116.51, 121.72, 123.82, 130.08, 134.94, 148.89, 150.23, 153.06, 158.57, 159.31, 160.66, 163.80, 169.02; MS m/z (%): 509 [M+] (45.32), 478 (12.15), 399 (100), 311 (15.40). Anal. calcd for C26H24FN3O5S: C, 61.29; H, 4.75; N, 8.25. Found: C, 61.31; H, 4.77; N, 8.22.
5.1.2.4. Ethyl-2-((3-cyano-5-((4-fluorophenyl)carbamoyl)-6-methyl-4-(p-tolyl)pyridin-2-yl)-thio)acetate (4b)
Yellow solid [A] (93%), [B] (70%); mp: 166–168 °C (EtOH); IR (KBr, v, cm–1): 3232 (NH), 2229 (CN), 1741 (C=O), 1642 (C=O); 1H NMR (DMSO-d6): δ 1.20 (t, 3H, CH3), 2.31 (s, 3H, CH3), 2.50 (s, 3H, CH3), 4.13 (s, 2H, CH2), 4.15 (q, 2H, CH2), 7.07–7.38 (m, 8H, CH-Ar), 10.43 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 14.10, 21.20, 21.70, 31.90, 61.10, 105,30, 113.30, 116.20, 123.10, 126.50, 127.40, 130.20, 132.60, 134.70, 155.10, 162.50, 163.20, 164.80, 168.20, 170.10; MS m/z (%): 463 [M+] (25), 353 (15), 307 (36), 110 (85), 83(100). Anal. calcd for C25H22FN3O3S: C, 64.78; H, 4.78; N, 9.07. Found: C, 64.80; H, 4.77; N, 9.10.
5.1.2.5. 5-Cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-2-methyl-6-((2-oxo-2-phenyl- ethyl)thio)nicotinamide (4c)
Pale yellow solid [A] (90%); [B] (72%); mp: 193–195 °C (EtOH). IR (KBr, v, cm–1): 3272 (NH), 2225 (CN), 1696 (C=O), 1655 (C=O); 1H NMR (DMSO-d6): δ 2.51 (s, 3H, CH3), 3.63 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 4.92 (s, 2H, CH2), 6.93–8.14 (m, 12H, CH-Ar), 10.47 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 22.60, 37.53, 56.33, 56.99, 105.02, 113.75, 115.86, 116.89, 121.73, 123.15, 129.04, 129.72, 134.28, 135.30, 136.64, 149.19, 150.60, 153.72, 158.27, 159.62, 161.42, 164.17, 193.43; MS m/z (%): 541 [M+] (6), 510 (16), 105 (100), 77 (62). Anal. calcd for C30H24FN3O4S: C, 66.53; H, 4.47; N, 7.76. Found: C, 66.55; H, 4.49; N, 7.73.
5.1.2.6. 5-Cyano-N-(4-fluorophenyl)-2-methyl-6-((2-oxo-2-phenylethyl)thio)-4-(p-tolyl)-nicotinamide (4d)
White solid [A] (89%); [B] (73%); mp: 205–207 °C (EtOH); IR (KBr, v, cm–1): 3434 (NH), 2222 (CN), 1705 (C=O), 1656 (C=O); 1H NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 2.32 (s, 3H, CH3), 4.95 (s, 2H, CH2), 7.09–8.14 (m, 13H, CH-Ar), 10.46 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 21.31, 22.86, 39.73, 103.95, 115.45, 115.84, 115.97, 121.87, 121.91, 128.70, 128.74, 129.31, 129.53, 131.51, 133.99, 136.87, 139.89, 151.77, 158.29, 161.29, 164.12, 193.84; MS m/z (%): 495 [M+] (65), 463 (5), 384 (100), 105 (55). Anal. calcd for C29H22FN3O2S: C, 70.29; H, 4.47; N, 8.48. Found: C, 70.31; H, 4.44; N, 8.46.
5.1.2.7. 5-Cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-2-methyl-6-((2-oxopropyl)-thio)nicotinamide (4e)
Yellow solid [A] (88%); [B] (75%); mp: 187–190 °C (EtOH); IR (KBr, v, cm–1): 3272 (NH), 2220 (CN), 1668 (C= O), 1649 (C=O); 1H NMR (DMSO-d6): δ 2.37 (s, 3H, CH3), 2.56 (s, 3H, CH3), 3.69 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 4.62 (s, 2H, CH2), 6.95–7.45 (m, 7H, CH-Ar), 10.78 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 23.29, 29.55, 39.79, 55.65, 56.32, 105.69, 112.33, 114.79, 115.88, 116.13, 123.15, 130.08, 134.94, 149.15, 150.22, 153.07, 158.24, 158.94, 160.65, 163.79, 202.06; MS m/z (%): 479 [M+] (47), 448 (69), 369 (100), 82 (35). Anal. calcd for C25H22FN3O4S: C, 62.62; H, 4.62; N, 8.76. Found: C, 62.64; H, 4.59; N, 8.77.
5.1.2.8. 5-Cyano-N-(4-fluorophenyl)-2-methyl-6-((2-oxopropyl)thio)-4-(p-tolyl)nicotinamide (4f)
White solid [A] (91%); [B] (78%); mp: 198–200 °C (EtOH). IR (KBr, v, cm–1): 3235 (NH), 2215 (CN), 1736 (C=O), 1644 (C=O); 1H NMR (DMSO-d6): δ 2.32 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.54 (s, 3H, CH3), 4.28 (s, 2H, CH2), 7.13–7.41 (m, 8H, CH-Ar), 10.49 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 21.31, 23.19, 29.76, 39.98, 104.04, 115.40, 115.87, 116.00, 121.87, 128.74, 129.33, 129.52, 131.52, 134.95, 139.89, 151.78, 158.30, 158.45, 161.39, 201.97; MS m/z (%): 433 [M+] (30), 390 (25), 323 (100), 281 (83), 110 (44). Anal. calcd for C24H20FN3O2S: C, 66.50; H, 4.65; N, 9.69. Found: C, 66.53; H, 4.62; N, 9.67.
5.1.2.9. 5-Cyano-6-((cyanomethyl)thio)-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-2-methylnicotinamide (4g)
Yellow solid [A] (89%); [B] (73%); mp: 140–144 °C (EtOH); IR (KBr, v, cm–1): 3451 (NH), 2229 (CN), 1684 (C=O); 1H NMR (DMSO-d6): δ 2.68 (s, 3H, CH3), 3.64 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 4.44 (s, 2H, CH2), 6.98–7.49 (m, 7H, CH-Ar), 10.59 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 19.31, 22.98, 55.43, 56.51, 105.78, 113.08, 115.40, 116.36, 117.41, 118.37, 122.33, 123.33, 123.67, 130.98, 134.93, 149.86, 150.51, 154.10, 158.43, 159.48, 164.09; MS m/z (%): 462 [M+] (98), 352 (90), 69 (100). Anal. calcd for C24H19FN4O3S: C, 62.33; H, 4.14; N, 12.11. Found: C, 62.30; H, 4.12; N, 12.15.
5.1.2.10. 5-Cyano-6-((cyanomethyl)thio)-N-(4-fluorophenyl)-2-methyl-4-(p-tolyl)nicotin-amide (4h)
Yellow solid [A] (87%); [B] (71%); mp: 230–232 °C (EtOH); IR (KBr, v, cm–1): 3403 (NH), 2226 (CN), 1649 (C=O); 1H NMR (DMSO-d6): δ 2.26 (s, 3H, CH3), 2.58 (s, 3H, CH3), 4.39 (s, 2H, CH2), 7.02–7.46 (m, 8H, CH-Ar), 10.52 (bs, 1H, NH); 13C NMR (DMSO-d6: δ 17.30, 21.10, 21.80, 114,60, 115.80, 117.20, 124.30, 127.60, 128.10, 131.20, 132.70, 135.10, 154.80, 162.70, 163.20, 164.20, 169.30, 171.30; MS m/z (%): 416 [M+] (5); Anal. calcd for C23H17FN4OS: C, 66.33; H, 4.11; N, 13.45. Found: C, 66.36; H, 4.09; N, 13.42.
5.1.3. Preparation of Compounds 5a–5h
5.1.3.1. Method A
Compounds 4a–4h (10 mmol) were refluxed for 3–6 h in sodium ethoxide solution (0.23 g Na in 100 mL of EtOH). After cooling the mixture, a white to pale yellow solid precipitated in each case, which was then filtered out, dried, and crystallized from the specified solvent to provide the thieno[2,3-b]pyridine derivatives 5a–5h.
5.1.3.2. Method B
For 3 h, a mixture of compound 3 (10 mmol) and the appropriate α-haloketone (10 mmol) (namely, ethyl chloroacetate, phenacyl chloride, chloroacetone, or chloroacetonitrile) was refluxed in sodium ethoxide solution (0.23 g Na in 100 mL of EtOH). The produced precipitate was then filtered out, dried, and crystallized from the specified solvent, yielding the thieno[2,3-b]pyridine derivatives (5a–5h).
5.1.3.3. Ethyl-3-amino-4-(2,5-dimethoxyphenyl)-5-((4-fluorophenyl)-carbamoyl)-6-methyl-thieno[2,3-b]pyridine-2-carboxylate (5a)
White solid [A] (89%); [B] (75%); mp: 175–178 °C (EtOH); IR (KBr, v, cm–1): 3481–3311 (NH2), 3260 (NH), 1730 (C=O), 1674 (C=O); 1H NMR (DMSO-d6): δ 1.30 (t, 3H, CH3), 2.55 (s, 3H, CH3), 3.65 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 4.24 (q, 2H, CH2), 6.90–7.44 (m, 9H, 7CH-Ar + NH2), 10.37 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 13.89, 23.65, 55.65, 56.69, 60.12, 104.65, 113.38, 115.48, 116.89, 120.02, 121.36, 123.15, 129.71, 131.13, 135.31, 140.83, 148.86, 150.58, 152.69, 155.82, 157.51, 158.57, 159.33, 164.85, 207.02; MS m/z (%): 509 [M+] (23), 437 (8), 399 (25), 110 (65), 83 (100). Anal. calcd for C26H24FN3O5S: C, 61.29; H, 4.75; N, 8.25. Found: C, 61.31; H, 4.72; N, 8.29.
5.1.3.4. Ethyl-3-amino-5-((4-fluorophenyl)carbamoyl)-6-methyl-4-(p-tolyl)thieno[2,3-b]-pyridine-2-carboxylate (5b)
Yellow solid; [A] (92%); [B] (70%); mp: 250–252 °C (EtOH); IR (KBr, v, cm–1): 3446–3311 (NH2), 3257 (NH), 1710 (C=O), 1647 (C=O); 1H NMR (DMSO-d6): δ 1.03 (t, 3H, CH3), 2.31 (s, 3H, CH3), 2.57 (s, 3H, CH3), 4.37 (q, 2H, CH2), 5.23 (bs, 2H, NH2), 6.90–7.44 (m, 8H, CH-Ar), 10.40 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 15.10, 20.80, 21.40, 61.33, 116.40, 119.30, 123.60, 126.10, 126.70, 127.40, 130.20, 131.20, 132.20, 135.40, 136.30, 157.10, 161.50, 163.60, 164.80, 165.30, 168.40; MS m/z (%): 463 [M+] (2), 391 (35), 280 (100), 82 (63). Anal. calcd for C25H22FN3O3S: C, 64.78; H, 4.78; N, 9.07. Found: C, 64.74; H, 4.80; N, 9.11.
5.1.3.5. 3-Amino-2-benzoyl-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-6-methylthieno-[2,3-b]pyridine-5-carboxamide (5c)
Yellow solid [A] (86%); [B] (73%); mp: 165–168 °C (EtOH); IR (KBr, v, cm–1): 3465–3314 (NH2), 3280 (NH), 1721 (C=O), 1656 (C=O); 1H NMR (DMSO-d6): δ 2.51 (s, 3H, CH3), 3.66 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 7.03–7.79 (m, 14H, 12CH-Ar + NH2), 10.39 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 23.21, 56.03, 56.70, 103.49, 113.75, 115.82, 115.95, 117.12, 120.16, 121.76, 121.81, 122.44, 127.76, 129.03, 131.10, 131.74, 141.16, 142.11, 150.43, 151.00, 153.31, 157.47, 158.17, 161.14, 164.93, 189.46; MS m/z (%): 541 [M+] (90). Anal. calcd for C30H24FN3O4S: C, 66.53; H, 4.47; N, 7.76. Found: C, 66.55; H, 4.48; N, 7.73.
5.1.3.6. 3-Amino-2-benzoyl-N-(4-fluorophenyl)-6-methyl-4-(p-tolyl)thieno-[2,3-b]pyridine-5-carboxamide (5d)
Pale yellow solid [A] (91%); [B] (72%); mp: 284–286 °C (EtOH); IR (KBr, v, cm–1): 3471–3283 (NH2), 3147 (NH), 1699 (C=O), 1644 (C=O); 1H NMR (DMSO-d6): δ 2.36 (s, 3H, CH3), 2.65 (s, 3H, CH3), 7.11–7.78 (m, 15H, 13CH-Ar + NH2), 10.47 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 21.41, 23.14, 103.58, 115.80, 115.93, 119.57, 121.91, 121.95, 127.78, 128.72, 129.03, 129.76, 130.56, 130.93, 131.75, 139.45, 141.13, 145.16, 150.82, 157.28, 158.23, 159.60, 161.35, 165.06, 189.49; MS m/z (%): 495 [M+] (9), 350 (18), 216 (100), 111 (42), 77 (82). Anal. calcd for C29H22FN3O2S: C, 70.29; H, 4.47; N, 8.48. Found: C, 70.27; H, 4.44; N, 8.51.
5.1.3.7. 2-Acetyl-3-amino-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-6-methylthieno- [2,3-b]pyridine-5-carboxamide (5e)
Yellow solid [A] (88%); [B] (73%); mp: 200–202 °C (EtOH); IR (KBr, v, cm–1): 3467–3297 (NH2), 3142 (NH), 1674 (C=O), 1652 (C=O); 1H NMR (DMSO-d6): δ 2.37 (s, 3H, CH3), 2.65 (s, 3H, CH3), 3.63 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 6.99–7.46 (m, 9H, CH-Ar + NH2), 10.38 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 23.14, 29.55, 56.00, 56.65, 105.08, 115.80, 115.93, 116.96, 120.59, 121.74, 121.78, 122.48, 131.03, 135.21, 141.88, 148.36, 150.45, 153.21, 157.02, 158.15, 159.52, 159.71, 164.99, 192.16; MS m/z (%): 479 [M+] (41), 248 (25), 217 (100), 60 (93). Anal. calcd for C25H22FN3O4S: C, 62.62; H, 4.62; N, 8.76. Found: C, 62.64; H, 4.59; N, 8.77.
5.1.3.8. 2-Acetyl-3-amino-N-(4-fluorophenyl)-6-methyl-4-(p-tolyl)thieno[2,3-b]pyridine-5-carboxamide (5f)
Yellow solid [A] (92%); [B] (71%); mp: 250–253 °C (EtOH); IR (KBr, v, cm–1): 3473–3387 (NH2), 3304 (NH), 1659 (C=O), 1619 (C=O); 1H NMR (DMSO-d6): δ 2.30 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.60 (s, 3H, CH3), 6.36–7.40 (m, 10H, CH-Ar + NH2), 10.45 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.60, 21.60, 53.10, 115.40, 119.30, 123.20, 126.10, 126.80, 127.50, 130.40, 131.20, 132.20, 135.20, 136.40, 157.30, 160.70, 163.40, 164.90, 165.50, 168.10; MS m/z (%): 433 [M+] (7), 323 (9), 110 (98), 83 (100). Anal. calcd for C24H20FN3O2S: C, 66.50; H, 4.65; N, 9.69. Found: C, 66.52; H, 4.62; N, 9.72.
5.1.3.9. 3-Amino-2-cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-6-methylthieno[2,3-b]pyridine-5-carboxamide (5g)
Yellow solid [A] (89%); [B] (72%); mp: 190–194 °C. IR (KBr, v, cm–1): 3450–3263 (NH2), 3148 (NH), 2228 (CN), 1647 (C=O); 1H NMR (DMSO-d6): δ 2.66 (s, 3H, CH3), 3.68 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 6.88–7.48 (m, 9H, 7CH-Ar + NH2), 10.54 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 23.01, 55.46, 56.04, 105.30, 112.96, 114.57, 115.19, 115.64, 116.28, 116.53, 117.50, 121.18, 121.37, 123.50, 126.36, 130.32, 149.03, 150.71, 152.21, 156.85, 158.66, 161.82, 163.18, 177.57 MS m/z (%): 462 [M+] (12), 423 (15), 352 (50), 118 (100). Anal. calcd for C24H19FN4O3S: C, 62.33; H, 4.14; N, 12.11. Found: C, 62.30; H, 4.12; N, 12.14.
5.1.3.10. 3-Amino-2-cyano-N-(4-fluorophenyl)-6-methyl-4-(p-tolyl)thieno[2,3-b]pyridine-5-carboxamide (5h)
Yellow solid [A] (93%); [B] (74%); mp: 183–185 °C. IR (KBr, v, cm–1): 3396–3209 (NH2), 3139 (NH), 2228 (CN), 1653 (C=O); 1H NMR (DMSO-d6): δ 2.33 (s, 3H, CH3), 2.53 (s, 3H, CH3), 7.15–7.36 (m, 10H, 8CH-Ar + NH2), 10.54 (bs, 1H, NH); 13C NMR (DMSO-d6): δ: 20.30, 21.40, 114.10, 115.50, 119.40, 123.60, 126.30, 126.60, 127.50, 130.60, 131.40, 132.30, 135.30, 136.50, 157.40, 163.50, 164.80, 165.60, 167.10; MS m/z (%): 416 [M+] (8). Anal. calcd for C23H17FN4OS: C, 66.33; H, 4.11; N, 13.45. Found: C, 66.35; H, 4.08; N, 13.43.
5.1.4. Preparation of Compounds 7a–7d
A few drops of triethylamine were added to a mixture of compounds 3a or 3b (10 mmol) and 2-chloro-N-arylacetamide derivatives 6a or 6b (10 mmol) in absolute ethanol (15 mL), and the mixture was refluxed for 2 h. The reaction was allowed to cool before the precipitate was filtered, dried, and crystallized from ethanol to produce the compounds 7a–7d.
5.1.4.1. 5-Cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-2-methyl-6-((2-oxo-2-(p-tolylamino)ethyl)thio)nicotinamide (7a)
Yellow solid (82%); mp: 268–270 °C (EtOH); IR (KBr, v, cm–1): 3468 (NH), 3328 (NH), 2221 (CN), 1675 (C=O), 1627 (C=O); 1H NMR (DMSO-d6): δ 2.34 (s, 3H, CH3), 2.61 (s, 3H, CH3), 3.68 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 4.17 (s, 2H, CH2), 6.95–7.87 (m, 11H, CH-Ar), 9.50 (bs, 1H, NH), 10.45 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.90, 21.50, 40.10, 56.80, 57.10, 105.20, 112.60, 113.50, 113.90, 115.90, 116.10, 122.50, 123.10, 127.30, 129.20, 129.60, 134.60, 135.10, 136.80, 150.40, 154.30, 155.60, 162.30, 163.10, 165.30, 168.60, 170.20; MS m/z (%): 570 [M+] (40). Anal. calcd for C31H27FN4O4S: C, 65.25; H, 4.77; N, 9.82. Found: C, 65.28; H, 4.79; N, 9.80.
5.1.4.2. 5-Cyano-N-(4-fluorophenyl)-2-methyl-6-((2-oxo-2-(p-tolylamino)ethyl)-thio)-4-(p-tolyl)nicotinamide (7b)
White solid (81%); mp: 235–237 °C. (EtOH); IR (KBr, v, cm–1): 3271 (NH), 3141 (NH), 2220 (CN), 1667 (C=O), 1649 (C=O); 1H NMR (DMSO-d6): δ 2.36 (s, 3H, CH3), 2.39 (s, 3H, CH3), 2.65 (s, 3H, CH3), 4.20 (s, 2H, CH2), 6.09–7.45 (m, 12H, CH-Ar), 9.43 (bs, 1H, NH), 10.62 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.90, 21.30, 21.50, 40.10, 105.20, 112.40, 113.50, 113.80, 115.70, 116.10, 122.40, 123.20, 127.40, 129.20, 129.90, 134.50, 135.30, 136.10, 150.20, 154.40, 155.10, 162.10, 163.40, 165.20, 168.80, 170.10; MS m/z (%): 524 [M+] (90). Anal. calcd for C30H25FN4O2S: C, 68.68; H, 4.80; N, 10.68. Found: C, 68.65; H, 4.82; N, 10.66.
5.1.4.3. 5-Cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-6-((2-((4-fluorophenyl) amino)-2-oxoethyl)thio)-2-methylnicotinamide (7c)
Yellow solid (88%); mp: 230–233 °C. (EtOH); IR (KBr, v, cm–1): 3351 (NH), 3277 (NH), 2206 (CN), 1668 (C=O), 1644 (C=O); 1H NMR (DMSO-d6): δ 2.65 (s, 3H, CH3), 3.68 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 4.21 (s, 2H, CH2), 7.01–7.92 (m, 11H, CH-Ar), 9.45 (bs, 1H, NH), 10.39 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.90, 39.10, 56.10, 56.80, 104.20, 112.50, 113.40, 113.90, 115.60, 116.20, 122.30, 123.10, 127.20, 128.30, 129.50, 134.20, 135.50, 136.70, 150.20, 153.90, 155.20, 161.40, 163.10, 164.90, 167.80, 169.80; MS m/z (%): 574 [M+] (47). Anal. calcd for C30H24F2N4O4S: C, 62.71; H, 4.21; N, 9.75. Found: C, 62.69; H, 4.22; N, 9.73.
5.1.4.4. 5-Cyano-N-(4-fluorophenyl)-6-((2-((4-fluorophenyl)amino)-2-oxoethyl)thio)-2-methyl-4-(p-tolyl)nicotinamide (7d)
Pale yellow solid (84%); mp: 243–245 °C. (EtOH); IR (KBr, v, cm–1): 3446 (NH), 3336 (NH), 2225 (CN), 1661 (C=O), 1641 (C=O); 1H NMR (DMSO-d6): δ 2.36 (s, 3H, CH3), 2.54 (s, 3H, CH3), 4.15 (s, 2H, CH2), 7.11–7.97 (m, 12H, CH-Ar), 9.46 (bs, 1H, NH), 10.63 (bs, 1H, NH); MS m/z (%): 528 [M+] (95). Anal. calcd for C29H22F2N4O2S: C, 65.90; H, 4.20; N, 10.60. Found: C, 65.92; H, 4.19; N, 10.63.
5.1.5. Preparation of Compounds 8a–8d
5.1.5.1. Method A
After 3 h of refluxing compound 7a–7d (10 mmol) in sodium ethoxide solution (0.23 g Na in 100 mL EtOH), a yellow precipitate was detected. The reaction was then allowed to cool before the produced precipitate was filtered, dried, and crystallized in the appropriate solvent to yield compounds 8a–8d.
5.1.5.2. Method B
For 3 h, a combination of compound 3a or 3b (10 mmol) was refluxed in sodium ethoxide solution ethoxide (0.23 g Na in 100 mL of EtOH) with the appropriate derivative of 2-chloro-N-arylacetamide (10 mmol). After observing a yellow precipitate, the mixture was allowed to cool. The produced solid was filtered out, dried, and crystallized from ethanol.
5.1.5.3. 3-Amino-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-6-methyl-N-(p-tolyl) thieno[2,3-b]pyridine-2,5-dicarboxamide (8a)
Yellow solid [A] (91%), [B] (79%); mp: 236–238 °C (EtOH). IR (KBr, v, cm–1): 3477–3397 (NH2), 3340 (NH), 3318 (NH), 1650 (C=O), 1633 (C=O). 1H NMR (CDCL3): δ 1.87 (s, 3H, CH3), 2.72 (s, 3H, CH3), 3.71 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 5.84 (bs, 2H, NH2), 6.95–7.87 (m, 11H, CH-Ar), 9.52 (bs, 1H, NH), 10.24 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.80, 21.20, 55.90, 56.20 112.40, 113.50,,114.80, 116.10, 122.50, 123.40, 126.90, 127.80, 128.20, 129.30, 132.50, 134.50, 135.50, 136.70, 150.90, 154.10, 155.60, 157.10, 161.80, 163.90, 168.10, 170.80; MS m/z (%): 570 [M+] (58). Anal. calcd for C31H27FN4O4S: C, 65.25; H, 4.77; N, 9.82. Found: C, 65.28; H, 4.79; N, 9.80.
5.1.5.4. 3-Amino-N-(4-fluorophenyl)-6-methyl-N,4-di-p-tolylthieno[2,3-b]pyridine-2,5-dicarboxamide (8b)
Pale yellow solid [A] 92%, [B] 75%; mp: 245–247 °C (EtOH); IR (KBr, v, cm–1): 3532–3468 (NH2), 3342 (NH), 3315 (NH), 1655 (C=O), 1631 (C=O); 1H NMR (DMSO-d6): δ 2.27 (s, 6H, 2CH3), 2.61 (s, 3H, CH3), 5.76 (bs, 2H, NH2), 7.13–7.61 (m, 12H, CH-Ar), 9.53 (bs, 1H, NH), 10.38 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.80, 21.20, 21.60, 112.20, 113.70, 115.40, 116.30, 122.30, 123.20, 127.60, 128.30, 129.10, 129.80, 133.60, 134.40, 135.60, 136.30, 151.10, 153.90, 155.30, 158.90, 163.50, 164.80, 167.90, 171.20; MS m/z (%): 524 [M+] (16). Anal. calcd for C30H25FN4O2S: C, 68.68; H, 4.80; N, 10.68. Found: C, 68.70; H, 4.78; N, 10.71.
5.1.5.5. 3-Amino-4-(2,5-dimethoxyphenyl)-N,N-bis(4-fluorophenyl)-6-methylthieno[2,3-b]pyridine-2,5-dicarboxamide (8c)
Yellow solid [A] 91%, [B] 72%; mp: 240–243 °C (EtOH); IR (KBr, v, cm–1): 3467–3369 (NH2), 3328 (NH), 3146 (NH), 1673 (C=O), 1626 (C=O); 1H NMR (DMSO-d6): δ 2.79 (s, 3H, CH3), 3.71 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.73 (bs, 2H, NH2), 6.79–7.83 (m, 11H, CH-Ar), 9.66 (bs, 1H, NH), 10.56 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 22.33, 56.08, 56.72, 97.69, 115.42, 115.80, 115.97, 116.00, 116.01, 116.03, 121.85, 121.92, 123.79, 123.85, 131.01, 135.30, 135.31, 135.33, 135.55, 135.57, 135.58, 140.90, 147.67, 150.76, 153.20, 155.97, 158.95, 159.88, 164.30, 165.30; MS m/z (%): 574 [M+] (7), 464 (18), 137 (100), 109 (65), 82 (35). Anal. calcd for C30H24F2N4O4S: C, 62.71; H, 4.21; N, 9.75. Found: C, 62.72; H, 4.23; N, 9.73.
5.1.5.6. 3-Amino-N,N-bis(4-fluorophenyl)-6-methyl-4-(p-tolyl)thieno[2,3-b]pyridine-2,5-dicarboxamide (8d)
Yellow solid [A] 90%, [B] 74%; mp: 225–228 °C (EtOH); IR (KBr, v, cm–1): 3526–3469 (NH2), 3343 (NH), 3305 (NH), 1654 (C=O), 1632 (C=O); 1H NMR (DMSO-d6): δ 2.33 (s, 3H, CH3), 2.63 (s, 3H, CH3), 5.77 (bs, 2H, NH2), 7.07–7.66 (m, 12H, CH-Ar), 9.57 (bs, 1H, NH), 10.39 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 21.41, 22.98, 97.77, 115.41, 115.58, 115.77, 115.95, 116.00, 116.01, 116.03, 122.0, 122.07, 123.79, 123.85, 129.0, 129.48, 130.98, 131.01, 135.30, 135.33, 135.55, 135.57, 135.58, 143.98, 147.44, 155.51, 159.19, 164.27, 165.44; MS m/z (%): 528 [M+] (16). Anal. calcd for C29H22F2N4O2S: C, 65.90; H, 4.20; N, 10.60. Found: C, 65.92; H, 4.23; N, 10.57.
5.1.6. Preparation of Compounds 10a–10e
A few drops of trimethylamine were added to a combination of 3a or 3b (10 mmol) and ethyl 2-(2-phenylhydrazono)-2-chloroacetate 9a or 9b (10 mmol) in absolute ethanol (15 mL). After 3 h of reflux, the reaction mixture was allowed to cool. Compounds 10a–10e were obtained by filtering the produced precipitate, drying, and crystallizing it in the appropriate solvent.
5.1.6.1. 3-Cyano-4-(2,5-dimethoxyphenyl)-5-((4-fluorophenyl)carbamoyl)-6-methylpyridin-2-yl-2-oxo-N-phenylpropanehydrazonothioate (10a)
Yellow solid (90%); mp 190–192 °C (EtOH); IR (KBr, v, cm–1): 3461 (NH), 3277 (NH), 2221 (CN), 1695 (C=O), 1650 (C=O); 1H NMR (DMSO-d6): δ 2.51 (s, 3H, CH3), 2.66 (s, 3H, CH3), 3.66 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 7.04–7.79 (m, 12H, CH-Ar), 9.66 (bs, 1H, NH), 10.40 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.50, 54.80, 56.20, 104.50, 113.70, 114.40, 115.60, 115.90, 116.60, 121.90, 122.80, 127.50, 129.50, 130.10, 135.40, 136.70, 139.10, 150.20, 154.20, 155.90, 158.30, 162.60, 163.10, 165.10, 170.00, 193.50; MS m/z (%): 583 [M+] (91). Anal. calcd for C31H26FN5O4S: C, 63.80; H, 4.49; N, 12.00. Found: C, 63.79; H, 4.51; N, 11.99.
5.1.6.2. 3-Cyano-4-(2,5-dimethoxyphenyl)-5-((4-fluorophenyl)carbamoyl)-6-methylpyridin-2-yl-2-oxo-N-(p-tolyl)propanehydrazonothioate (10b)
Deep yellow solid (90%); mp 216–218 °C (EtOH); IR (KBr, v, cm–1): 3361 (NH), 3270 (NH), 2217 (CN), 1709 (C=O), 1653 (C=O); 1H NMR (DMSO-d6): δ 2.48 (s, 3H, CH3), 2.59 (s, 3H, CH3), 2.67 (s, 3H, CH3), 3.65 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 7.23–7.67 (m, 11H, CH-Ar), 9.71 (bs, 1H, NH), 10.92 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.70, 21.50, 55.90, 56.70, 104.20, 113.80, 114.60, 115.20, 115.70, 116.20, 122.80, 123.40, 128.10, 129.30, 129.90, 135.30, 136.90, 139.80, 150.50, 155.30, 156.50, 158.70, 162.40, 163.60, 165.50, 170.20, 194.80; MS m/z (%): 597 [M+] (62). Anal. calcd for C32H28FN5O4S: C, 64.31; H, 4.72; N, 11.72. Found: C, 64.29; H, 4.74; N, 11.73.
5.1.6.3. 3-Cyano-5-((4-fluorophenyl)carbamoyl)-6-methyl-4-(p-tolyl)pyridin-2-yl-2-oxo-N-phenylpropanehydrazonothioate (10c)
Yellow solid (90%); mp 205–207 °C (EtOH); IR (KBr, v, cm–1): 3461 (NH), 3277 (NH), 2218 (CN), 1718 (C=O), 1654 (C=O); 1H NMR (DMSO-d6): δ 2.39 (s, 3H, CH3), 2.53 (s, 3H, CH3), 2.62 (s, 3H, CH3), 6.95–7.64 (m, 13H, CH-Ar), 9.68 (bs, 1H, NH), 10.84 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.80, 21.70, 102.60, 113.20, 114.10, 115.80, 116.20, 118.20, 121.30, 123.10, 127.80, 129.10, 134.80, 136.10, 138.90, 148.20, 154.60, 155.20, 157.30, 158.40 160.50, 162.50, 165.40, 169.90, 194.90; MS m/z (%): 537 [M+] (54). Anal. calcd for C30H24FN5O2S: C, 67.02; H, 4.50; N, 13.03. Found: C, 67.04; H, 4.48; N, 13.05.
5.1.6.4. 3-Cyano-5-((4-fluorophenyl)carbamoyl)-6-methyl-4-(p-tolyl)pyridin-2-yl-2-oxo-N-(p-tolyl)propanehydrazonothioate (10d)
Deep yellow solid (86%); mp 200–201 °C (EtOH); IR (KBr, v, cm–1): 3461 (NH), 3277 (NH), 2218 (CN), 1718 (C=O), 1654 (C=O); 1H NMR (DMSO-d6): δ 2.34 (s, 3H, CH3), 2.52 (s, 3H, CH3), 2.55 (s, 3H, CH3), 2.64 (s, 3H, CH3), 7.13–7.89 (m, 12H, CH-Ar), 9.42 (bs, 1H, NH), 10.91 (bs, 1H, NH); MS m/z (%): 551 [M+] (32). Anal. calcd for C31H26FN5O2S: C, 67.50; H, 4.75; N, 12.70; Found: C, 67.53; H, 4.73; N, 12.72.
5.1.6.5. 3-Cyano-4-(2,5-dimethoxyphenyl)-5-((4-fluorophenyl)carbamoyl)-6-methylpyridin-2-yl(E)-N-(4-fluorophenyl)-2-oxopropanehydrazonothioate (10e)
Yellow solid (87%); mp 220–222 °C (EtOH); IR (KBr, v, cm–1): 3259 (NH), 3235 (NH), 2221 (CN), 1676 (C=O), 1650 (C=O); 1H NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.66 (s, 3H, CH3), 3.63(s, 3H, OCH3), 3.71 (s, 3H, OCH3), 7.12–7.76 (m, 11H, CH-Ar), 9.51 (bs, 1H, NH), 10.62 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 20.80, 56.10, 57.30, 103.40, 113.70, 114.10, 115.10, 115.80, 117.10, 123.20, 124.30, 127.50, 128.90, 129.30, 134.50, 136.30, 138.70, 150.00, 155.90, 157.10, 158.10, 160.40, 162.50, 164.30, 169.50, 195.50; MS m/z (%): 601 [M+] (85), 538 (62), 405 (100). Anal. Calcd for C31H25F2N5O4S: C, 61.89; H, 4.19; N, 11.64. Found: C, 61.85; H, 4.21; N, 11.67.
5.1.7. Preparation of Compounds 12a or 12b
5.1.7.1. Method A
After adding methyl iodide, compound 3a or 3b (10 mmol) in sodium ethoxide solution (0.23 g Na in 100 mL of EtOH) was refluxed for 3–6 h (15 mmol). The methylthio nicotinamide derivatives 12a and 12b were obtained by filtering the solid generated after cooling, drying, and crystallizing it from the specified solvent.
5.1.7.2. Method B
Methyl iodide (15 mmol) was added to a stirred solution of compound 3a or 3b (10 mmol) and potassium hydroxide (10 mmol) in dichloromethane (75 mL) and stirred for another 6 h at room temperature. The resultant precipitate was filtered and recrystallized from ethanol to provide the methylthio nicotinamide derivatives 12a and 12b, respectively.
5.1.7.3. 5-Cyano-4-(2,5-dimethoxyphenyl)-N-(4-fluorophenyl)-2-methyl-6-(methylthio)-nicotinamide (12a)
Yellow solid [A] 91%; [B] 88%; mp: 190–193 °C (EtOH); IR (KBr, v, cm–1): 3245 (NH), 2222 (CN), 1638 (C=O); 1H NMR (DMSO-d6): δ 2.62 (s, 3H, CH3), 2.69 (s, 3H, CH3), 3.96 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.94–7.48 (m, 7H, CH-Ar), 10.49 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 13.38, 23.58, 55.91, 56.53, 105.59, 113.39, 115.88, 116.01, 116.72, 121.73, 121.78, 123.61, 129.62, 135.09, 148.95, 150.45, 153.05, 158.23, 158.67, 159.60, 162.03, 164.06; MS m/z (%): 437 [M+] (15), 327 (22), 312 (60), 281 (100). Anal. calcd for C23H20FN3O3S: C, 63.15; H, 4.61; N, 9.61. Found: C, 63.17; H, 4.59; N, 9.63.
5.1.7.4. 5-Cyano-N-(4-fluorophenyl)-2-methyl-6-(methylthio)-4-(p-tolyl)-nicotinamide (12b)
Pale yellow solid [A] 93%, [B] 89%; mp: 95–99 °C (EtOH); IR (KBr, v, cm–1): 3245 (NH), 2222 (CN), 1638 (C = O); 1H NMR (DMSO-d6): δ 2.32 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.63 (s, 3H, CH3), 7.06–7.40 (m, 8H, CH-Ar), 10.42 (bs, 1H, NH); 13C NMR (DMSO-d6): δ 13.38, 21.10, 22.10, 105.40, 113.40, 115.90, 116.20, 116.60, 121.60, 122.10, 123.20, 128.50, 134.10, 146.80, 149.50, 152.90, 157.30, 159.80, 160.10, 163.20, 164.50; MS m/z (%): 391 [M+] (5), 352 (10), 281 (100), 83 (57). Anal. calcd for C22H18FN3OS: C, 67.50; H, 4.63; N, 10.73. Found: C, 67.51; H, 4.60; N, 10.71.
5.1.8. Preparation of Compounds 13 and 14
Compound 3a or 3b (10 mmol) was refluxed with 2-bromopropionate or 3-choloro-2,4-pentandione (10 mmol) in the presence of absolute ethanol (75 mL) and few drops of TEA as a catalyst for 3–6 h. The solid formed after cooling was filtered off, dried, and crystallized from the specified solvent to afford the corresponding nicotinamide derivatives 13 and 14 respectively.
5.1.8.1. Ethyl-2-((3-cyano-4-(2,5-dimethoxyphenyl)-5-((4-fluorophenyl)carbamoyl)-6-methylpyridin-2-yl)thio)propanoate (13a)
Pale yellow solid (81%); mp 240–244 °C (EtOH); IR (KBr, v, cm–1): 3243 (NH), 2221 (CN), 1742 (C=O), 1652 (C=O); 1H NMR (DMSO-d6): δ 1.26 (t, 3H, CH3), 2.16 (s, 3H, CH3), 2.55 (s, 3H, CH3), 3.70 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 4.19 (q, 2H, CH2), 4.62 (q, 1H, CH), 6.95–7.46 (m, 7H, CH-Ar), 10.52 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 13.90, 21.10, 56.10, 56.90, 61.30, 103.83, 115.30, 115.90, 116.70, 120.90, 121.80, 127.30, 128.50, 129.80, 129.50, 130.10, 131.50, 135.10, 138.90, 150.70, 157.40, 158.90, 159.60, 161.20, 164.50, 170.90; MS m/z (%): 523 [M+] (63). Anal. calcd for C27H26FN3O5S: C, 61.94; H, 5.01; N, 8.03. Found: C, 61.92; H, 4.99; N, 8.06.
5.1.8.2. Ethyl-2-((3-cyano-5-((4-fluorophenyl)carbamoyl)-6-methyl-4-(p-tolyl)pyridin-2-yl)thio)propanoate (13b)
Yellow solid (88%); mp 210–212 °C (EtOH); IR (KBr, v, cm–1): 3238 (NH), 2217 (CN), 1725 (C=O), 1648 (C=O); 1H NMR (DMSO-d6): δ 1.23 (t, 3H, CH3), 2.18 (s, 3H, CH3), 2.44 (s, 3H, CH3), 2.59 (s, 3H, CH3), 4.20 (q, 2H, CH2), 4.65 (q, 1H, CH), 7.09–7.48 (m, 8H, CH-Ar), 10.47 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 14.50, 18.99, 21.28, 23.10, 61.72, 103.92, 115.20, 115.86, 115.97, 121.92, 121.97, 128.24, 128.74, 129.37, 129.49, 129.52, 131.46, 134.90, 139.93, 151.86, 158.33, 158.58, 159.70, 160.96, 164.13, 171.87; MS m/z (%): 477 [M+] (78), 433 (58), 323 (100), 281 (48); MS m/z (%): 477 [M+] (78), 433 (58), 323 (100), 281 (48). Anal. calcd for C26H24FN3O3S: C, 65.39; H, 5.07; N, 8.80. Found: C, 65.43; H, 5.09; N, 8.79.
5.1.8.3. 5-Cyano-4-(2,5-dimethoxyphenyl)-6-((2,4-dioxopentan-3-yl)thio)-N-(4-fluorophenyl)-2-methylnicotinamide (14a)
Pale yellow solid (81%); mp 188–192 °C (EtOH); IR (KBr, v, cm–1): 3342 (NH), 2219 (CN), 1728 (C=O), 1688 (C=O), 1650 (C=O); 1H NMR (DMSO-d6): δ 2.34 (s, 6H, 2CH3), 2.61 (s, 3H, CH3), 3.65 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 4.65 (s, 1H, CH), 7.11–7.82 (m, 7H, CH-Ar), 10.50 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 21.30, 26.90, 55.90, 56.20, 78.10, 103.83, 112.90, 113.30 113.60, 115.90, 116.70, 123.10, 127.30, 133.60, 150.40, 153.20, 153.80, 154.90, 159.60, 162.30, 163.20, 164.80, 170.10, 205.90; MS m/z (%): 521 [M+] (42). Anal. calcd for C27H24FN3O5S: C, 62.18; H, 4.64; N, 8.06. Found: C, 62.15; H, 4.66; N, 8.08.
5.1.8.4. 5-Cyano-6-((2,4-dioxopentan-3-yl)thio)-N-(4-fluorophenyl)-2-methyl-4-(p-tolyl)nicotinamide (14b)
Yellow solid (85%); mp 212–216 °C (EtOH); IR (KBr, v, cm–1): 3258 (NH), 2223 (CN), 1712 (C=O), 1696 (C=O), 1641 (C=O); 1H NMR (DMSO-d6): δ 2.34 (s, 6H, 2CH3), 2.51 (s, 3H, CH3), 2.63 (s, 3H, CH3), 4.65 (s, 1H, CH), 6.14–7.49 (m, 8H, CH-Ar), 10.47 (bs, 1H, NH); 13C NMR (DMSO-d6) δ: 20.90, 27.10, 77.90, 103.60, 112.20, 113.100 113.80, 115.50, 116.80, 123.50, 127.40, 133.20, 150.60, 153.10, 153.60, 154.80, 160.10, 162.50, 163.90, 165.70, 169.10, 206.10, MS m/z (%): 476 [M++1] (39), 462 (45), 367 (100), 293 (100). Anal. calcd for C26H22FN3O3S: C, 65.67; H, 4.66; N, 8.84. Found: C, 65.64; H, 4.69; N, 8.80.
5.2. Antiproliferative Activity
5.2.1. Cell Culture Conditions
Human colorectal carcinoma (HCT-116), human liver carcinoma (HepG-2), human breast adenocarcinoma (MCF-7), and normal human skin fibroblast (BJ-1) cell lines were purchased from the American Type Culture Collection (Rockville, MD) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U mL–1 penicillin, and 100 U mL–1 streptomycin. The cells were grown at 37 °C in a humidified atmosphere of 5% CO2.
5.2.2. MTT Antiproliferative Assay
The antiproliferative properties of the compounds were assessed using the 3-[4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) test against HepG-2, HCT-116, MCF-7, and BJ-1 cells. MTT cleavage by mitochondrial dehydrogenases in live cells is used in this test.37−39 Cells were seeded in a 96-well sterile microplate (5 × 104 cells well–1) and incubated for 48 h at 37 °C in serum-free media containing dimethyl sulfoxide (DMSO) and either a series of different concentrations of each test compound or doxorubicin (as a positive control) before the MTT assay. After incubation, the medium was withdrawn and each well was filled with 40 μL of MTT (2.5 mg mL–1). The incubation period was extended for another 4 h. The purple formazan dye crystals that resulted were solubilized in 200 μL of DMSO. In a Spectra Max Paradigm Multi-Mode microplate reader, absorbance was measured at 590 nm (Molecular Devices, LLC, San Jose, CA). The mean proportion of viable cells compared to untreated control cells was used to calculate relative cell viability. Technical triplicates and three biological duplicates were used in all studies. All data was presented as a mean standard deviation. SPSS Inc. determined the IC50 values. Probit analysis was used to determine the IC50 values (IBM Corp., Armonk, NY).
Acknowledgments
The authors offered their thanks to the National Research Center, Egypt, for funding this work as a part of a Ph.D. thesis (Project No. 1/6/14).
The authors declare no competing financial interest.
References
- Elansary A. K.; Moneer A. A.; Kadry H. H.; Gedawy E. M. Synthesis and anticancer activity of some novel fused pyridine ring system. Arch. Pharmacal Res. 2012, 35 (11), 1909–1917. 10.1007/s12272-012-1107-6. [DOI] [PubMed] [Google Scholar]
- Altaf A. A.; Shahzad A.; Gul Z.; Rasool N.; Badshah A.; Lal B.; Khan E. A review on the medicinal importance of pyridine derivatives. J. Drug Des. Med. Chem. 2015, 1 (1), 1–11. 10.11648/j.jddmc.20150101.11. [DOI] [Google Scholar]
- Elkanzi N. A. A; El-Sofany W. I.; Gaballah S. T.; Mohamed A. M.; Kutkat O.; El-Sayed W. A. Synthesis, molecular modeling, and antiviral activity of novel triazole nucleosides and their analogs. Russ. J. Gen. Chem. 2019, 89 (9), 1896–1904. 10.1134/S1070363219090263. [DOI] [Google Scholar]
- Abdelgawad M. A.; Mohamed A. M.; Musa A.; Mostafa E. M.; Awad H. M. Synthesis, chromatographic separation and antimicrobial evolution of new azoquinoline-8-ol. J. Pharm. Sci. Res. 2018, 10 (6), 1314–1318. [Google Scholar]
- Ibrahim A. A.; Mohamed A. M.; Amr A. E.-G. E.; Al-Omar M. A. Synthesis of chiral linear and macrocyclic candidates: II. Synthesis and investigation of 3, 5-bis-linear and macrocyclic tetrapeptide Schiff base pyridine derivatives. Russ. J. Gen. Chem. 2015, 85 (6), 1506–1512. 10.1134/S1070363215060250. [DOI] [Google Scholar]
- Mohamed A. M.; El-Sayed W. A.; Alsharari M. A.; Al-Qalawi H. R.; Germoush M. O. Anticancer activities of some newly synthesized pyrazole and pyrimidine derivatives. Arch. Pharmacal Res. 2013, 36 (9), 1055–1065. 10.1007/s12272-013-0163-x. [DOI] [PubMed] [Google Scholar]
- Mohamed A. M.; Amr A.-G. E.; Alsharari M. A.; Al-Qalawi H. R.; Germoush M. O.; Al-Omar M. A. Anticancer activities of some new synthesized thiazolo [3, 2-a] pyrido [4, 3-d] pyrimidine derivatives. Am. J. Biochem. Biotechnol. 2011, 7 (2), 43–54. 10.3844/ajbbsp.2011.43.54. [DOI] [Google Scholar]
- Rashad A. E.; El-Sayed W. A.; Mohamed A. M.; Ali M. M. Synthesis of new quinoline derivatives as inhibitors of human tumor cells growth. Arch. Pharm. 2010, 343 (8), 440–448. 10.1002/ardp.201000002. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif N. A.; Sabry N. M.; Mohamed A. M.; Abdulla M. M. Synthesis, analgesic, and antiparkinsonian profiles of some pyridine, pyrazoline, and thiopyrimidine derivatives. Monatsh. Chem. 2007, 138 (7), 715–724. 10.1007/s00706-007-0656-8. [DOI] [Google Scholar]
- Amr A.-G. E.; Mohamed A. M.; Mohamed S. F.; Abdel-Hafez N. A.; Hammam A. E.-F. G. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg. Med. Chem. 2006, 14 (16), 5481–5488. 10.1016/j.bmc.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Patil P.; Sethy S.; Sameena T.; Shailaja K. Pyridine and its biological activity: a review. Asian J. Chem. 2013, 6 (10), 888–899. [Google Scholar]
- El-Dean A. M. K.; Abd-Ella A. A.; Hassanien R.; El-Sayed M. E. A.; A. Abdel-Raheem S. A. Design, synthesis, characterization, and insecticidal bioefficacy screening of some new pyridine derivatives. ACS Omega 2019, 4 (5), 8406–8412. 10.1021/acsomega.9b00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelaziz M. E.; El-Miligy M. M.; Fahmy S. M.; Mahran M. A.; Hazzaa A. A. Design, synthesis and docking study of pyridine and thieno [2, 3-b] pyridine derivatives as anticancer PIM-1 kinase inhibitors. Bioorg. Chem. 2018, 80, 674–692. 10.1016/j.bioorg.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Helal M.; El-Awdan S.; Salem M.; Abd-Elaziz T.; Moahamed Y.; El-Sherif A.; Mohamed G. Synthesis, biological evaluation and molecular modeling of novel series of pyridine derivatives as anticancer, anti-inflammatory and analgesic agents. Spectrochim. Acta, Part A 2015, 135, 764–773. 10.1016/j.saa.2014.06.145. [DOI] [PubMed] [Google Scholar]
- Asokan C.; Anabha E.; Thomas A. D.; Jose A. M.; Lethesh K.; Prasanth M.; Krishanraj K. A facile method for the synthesis of nicotinonitriles from ketones via a one-pot chloromethyleneiminium salt mediated three-component reaction. Tetrahedron Lett. 2007, 48 (32), 5641–5643. 10.1016/j.tetlet.2007.06.032. [DOI] [Google Scholar]
- Peruzyńska M.; Piotrowska K.; Tkacz M.; Kurzawski M.; Struk Ł.; Borzyszkowska A.; Idzik T. J.; Sośnicki J. G.; Droździk M. Comparative evaluation of new dihydropyrimidine and dihydropyridine derivatives perturbing mitotic spindle formation. Future Med. Chem. 2018, 10 (20), 2395–2410. 10.4155/fmc-2018-0094. [DOI] [PubMed] [Google Scholar]
- Cheney I. W.; Yan S.; Appleby T.; Walker H.; Vo T.; Yao N.; Hamatake R.; Hong Z.; Wu J. Z. Identification and structure–activity relationships of substituted pyridones as inhibitors of Pim-1 kinase. Bioorg. Med. Chem. Lett. 2007, 17 (6), 1679–1683. 10.1016/j.bmcl.2006.12.086. [DOI] [PubMed] [Google Scholar]
- Hayakawa I.; Shioya R.; Agatsuma T.; Furukawa H.; Sugano Y. Thienopyridine and benzofuran derivatives as potent anti-tumor agents possessing different structure–activity relationships. Bioorg. Med. Chem. Lett. 2004, 14 (13), 3411–3414. 10.1016/j.bmcl.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Lebedyeva I. O.; Dotsenko V. V.; Turovtsev V. V.; Krivokolysko S. G.; Povstyanoy V'y. M.; Povstyanoy M. V. The Thorpe–Ziegler-type reaction of 3-cyanopyridine-2 (1H)-thiones with Biginelli 6-bromomethyl-3, 4-dihydropyrimidin-2 (1H)-ones: cascade assembling of tetra-and pentacyclic heterocyclic scaffolds. Tetrahedron 2012, 68 (47), 9729–9737. 10.1016/j.tet.2012.09.041. [DOI] [Google Scholar]
- Litvinov V.; Dotsenko V.; Krivokolysko S. The chemistry of thienopyridines. Adv. Heterocycl. Chem. 2007, 93, 117–178. 10.1016/S0065-2725(06)93003-7. [DOI] [Google Scholar]
- Angiolillo D. J.; Bates E. R.; Bass T. A. Clinical profile of prasugrel, a novel thienopyridine. Am. Heart J. 2008, 156 (2), 16S–22S. 10.1016/j.ahj.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Hankey G. J.; Sudlow C. L.; Dunbabin D. W. Thienopyridines or aspirin to prevent stroke and other serious vascular events in patients at high risk of vascular disease? A systematic review of the evidence from randomized trials. Stroke 2000, 31 (7), 1779–1784. 10.1161/01.STR.31.7.1779. [DOI] [PubMed] [Google Scholar]
- Aly A. A.; Ramadan M.; Mohamed A. M.; Ishak E. A. Thieno [2, 3-d] pyrimidines in the Synthesis of New Fused Heterocyclic Compounds of Prospective Antitumor and Antioxidant Agents (Part II). J. Heterocycl. Chem. 2012, 49 (5), 1009–1018. 10.1002/jhet.843. [DOI] [Google Scholar]
- Abdel-Hafez N. A.; Mohamed M.; Amr A. E.-G. E.; Abdalla M. M. Antiarrhythmic activities of some newly synthesized tricyclic and tetracyclic thienopyridine derivatives. Sci. Pharm. 2009, 77 (3), 539–554. 10.3797/scipharm.0905-06. [DOI] [Google Scholar]
- Sangshetti J.; Zambare A.; Khan F.; Gonjari I.; Zaheer Z. Synthesis and biological activity of substituted-4, 5, 6, 7-tetrahydrothieno pyridines: a review. Mini-Rev. Med. Chem. 2014, 14 (12), 988–1020. 10.2174/1389557514666141106131425. [DOI] [PubMed] [Google Scholar]
- Madivada L. R.; Anumala R. R.; Gilla G.; Kagga M.; Bandichhor R. An efficient and large scale synthesis of clopidogrel: antiplatelet drug. Der Pharma Chemica 2012, 4 (1), 479–488. [Google Scholar]
- Samala G.; Devi P. B.; Nallangi R.; Sridevi J. P.; Saxena S.; Yogeeswari P.; Sriram D. Development of novel tetrahydrothieno [2, 3-c] pyridine-3-carboxamide based Mycobacterium tuberculosis pantothenate synthetase inhibitors: molecular hybridization from known antimycobacterial leads. Bioorg. Med. Chem. 2014, 22 (6), 1938–1947. 10.1016/j.bmc.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Gomha S. M.; Dawood K. M. Synthetic Utility of Pyridinium Bromide: Synthesis and Antimicrobial Activity of Novel 2, 4, 6-Trisubstituted Pyridines Having Pyrazole Moiety. J. Heterocycl. Chem. 2017, 54 (3), 1943–1948. 10.1002/jhet.2790. [DOI] [Google Scholar]
- Gomha S. M.; Abdelrazek F. M.; Abdelrahman A. H.; Metz P. Synthesis of some new Pyridine-based Heterocyclic Compounds with Anticipated Antitumor Activity. J. Heterocycl. Chem. 2018, 55 (7), 1729–1737. 10.1002/jhet.3210. [DOI] [Google Scholar]
- Amr A. E.-G. E.; Mohamed S. F.; Abdel-Hafez N. A.; Abdalla M. M. Antianexiety activity of pyridine derivatives synthesized from 2-chloro-6-hydrazino-isonicotinic acid hydrazide. Monatsh. Chem. 2008, 139 (12), 1491–1498. 10.1007/s00706-008-0949-6. [DOI] [Google Scholar]
- Amr A. E.-G. E.; Abdel-Hafez N. A.; Mohamed S. F.; Abdalla M. M. Synthesis, reactions, and antiarrhythmic activities of some novel pyrimidines and pyridines fused with thiophene moiety. Turk. J. Chem. 2009, 33 (3), 421–432. [Google Scholar]
- Elsayed M. A.; Abdel Hafez N. A.; Elshahawi M. M.; Ali K. A. Design, Synthesis, and Structural Elucidation of New Pyridine and Thienopyridine Derivatives. J. Heterocycl. Chem. 2019, 56 (1), 172–179. 10.1002/jhet.3392. [DOI] [Google Scholar]
- Elgemeie G. H.; El-Naggar D. H. Novel dihydropyridine thioglycosides and their corresponding dehydrogenated forms as potent anti-hepatocellular carcinoma agents. Nucleosides Nucleotides Nucleic Acids 2018, 37 (4), 199–216. 10.1080/15257770.2018.1457161. [DOI] [PubMed] [Google Scholar]
- Sh. Beheshtia Y.; Khorshidi M.; M. Heravi M.; Baghernejad B. A catalytic method for the synthesis of 4-alkyl (aryl)-6-aryl-3-cyano-2 (1H)-pyridinones and their 2-imino isosteres as nonsteroidal cardiotonic agents. Bull. Chem. Soc. Ethiop. 2010, 24 (3), 433–438. 10.4314/bcse.v24i3.60691. [DOI] [Google Scholar]
- Berry M.; Fielding B.; Gamieldien J. Practical considerations in virtual screening and molecular docking. Emerging trends in computational biology, bioinformatics, and systems biology 2015, 487. 10.1016/B978-0-12-802508-6.00027-2. [DOI] [Google Scholar]
- Perez S.; Tvaroška I. Carbohydrate–protein interactions: Molecular modeling insights. Adv. Carbohydr. Chem. Biochem. 2014, 71, 9–136. 10.1016/B978-0-12-800128-8.00001-7. [DOI] [PubMed] [Google Scholar]
- Abuelizz H. A.; Marzouk M.; Bakheit A. H.; Awad H. M.; Soltan M. M.; Naglah A. M.; Al-Salahi R. Antiproliferative and Antiangiogenic Properties of New VEGFR-2-targeting 2-thioxobenzo [g] quinazoline Derivatives (In Vitro). Molecules 2020, 25 (24), 5944. 10.3390/molecules25245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem A. F.; Nassar I. F.; Abdel-Aal M. T.; Awad H. M.; El-Sayed W. A. Synthesis and anticancer activity of new ((Furan-2-yl)-1, 3, 4-thiadiazolyl)-1, 3, 4-oxadiazole acyclic sugar derivatives. Chem. Pharm. Bull. 2019, 67 (8), 888–895. 10.1248/cpb.c19-00280. [DOI] [PubMed] [Google Scholar]
- Abd-El-Maksoud M.; El-Hussieny M.; Awad H.; Mossa A.-T.; Soliman F. Chemistry of Phosphorus Ylides. Part 47. Synthesis of Organophosphorus and Selenium Pyrazolone Derivatives, Their Antioxidant Activity, and Cytotoxicity against MCF7 and HepG2. Russ. J. Gen. Chem. 2020, 90 (12), 2356–2364. 10.1134/S1070363220120208. [DOI] [Google Scholar]