Abstract

This study emphasizes tuning the synthesis conditions of MFI zeolites to achieve better catalytic properties by optimizing the mesoporosity, the balance between Brønsted and Lewis sites, and the zeolite particle sizes. The MFI zeolites were hydrothermally synthesized at various temperatures employing different silica sources. The synthesis temperature was varied between 110 to 180 °C at constant synthesis time (15 h). Different silicon sources led to variations in structure, morphology, and size of the MFI zeolite along with tuned Lewis and Brønsted acid sites in parallel correlation with shape selectivity of the reaction. The catalytic activities of synthesized zeolites were investigated in the catalytic cracking of n-dodecane to produce value-added chemicals. The zeolite synthesized at 180 °C using fumed silica presented the highest catalytic conversion (96.6%), while maximum light olefin gaseous products (73.1%) were obtained for the sample synthesized at 140 °C using tetraethyl orthosilicate as the silica source. The MFI zeolite synthesized at 180 °C employing tetraethyl orthosilicate as a silica source facilitated the formation of both naphthenes and aromatics (71.3%) as major liquid products.
1. Introduction
Light olefins and aromatics have been facing a huge demand–supply gap in recent years, and this gap will surge exponentially in the future due to the large consumption of these chemicals for the production of plastics, textiles, drug intermediates, and fine chemicals. Due to the importance of these chemicals, there is a growing interest in the utilization of heavy oil feedstocks to produce refined fuels and petrochemicals by upgrading the heavy oil to lighter hydrocarbons.1−4 Catalytic cracking of n-dodecane to chemicals is among these processes which have been performed to produce light olefins and aromatics. n-Dodecane, with a molecular weight of 170.3 g/mol, boiling point of 216.3 °C, and specific gravity of 0.7524, is one of the typical model compounds suitable to represent heavy naphtha for catalytic experiments and for model-based simulations.5n-Dodocane originates from crude oil and also from lignocellulose-based biomass sources. The production of these long-chain hydrocarbons through plant or vegetable oil has the potential to offer an alternate solution to the threat of the depletion of fossil fuels.6 Alkanes such as n-dodecane (C12) are one of the main products from the catalytic conversion of furfural which is derived from lignocellulosic biomass.7−10
n-Dodecane has been studied in steam catalytic conversion over zeolite-based catalysts to produce lighter hydrocarbons.1,11−15 The importance of this process encouraged us to further explore the catalytic and steam catalytic cracking of n-dodecane. Numerous catalysts such as MFI, BEA, and FAU zeolites are used for the catalytic cracking process.16−18 ZSM-5 zeolites with an MFI topology were developed for the first time by Exxon Mobil.19 The catalytic activity of MFI zeolite made it one of the most versatile catalysts for steam catalytic cracking, heavy oil upgrading, and methanol to olefin reactions.20−23 The topology of MFI zeolite features a 3-dimensional channel system with straight and zigzag channels,24 which makes it one of the best catalysts for aromatization25 and cyclization reactions.26 Due to its versatile nature to catalyze large number of industrially important reactions, there is a strong desire to enhance its catalytic properties by modifying the physical and chemical properties.27−29
MFI zeolite is an aluminosilicate material mainly synthesized, in general, by employing silica, alumina, and alkaline sources along with an organic structure-directing agent. Any change in the source of these entities can alter the physicochemical properties of the produced zeolites. It can also play an essential role in fine-tuning the acidity, textural properties, structure, and morphology and, hence, the catalytic performance.30−32 The optimization of zeolite properties employing various aluminum, silicon, and template sources and heating conditions has been largely studied and reported in the literature. Specifically, many zeolites such as MFI, NaX, zeolite beta, MCM-22, and LTA were extensively studied.33−36 There are various reports on the synthesis of MFI zeolite employing different silica sources.30,37−41 Reda et al. studied the influence of sodium metasilicate, tetraethyl orthosilicate (TEOS), fumed silica, and colloidal silica on ZSM-5 crystallization with a synthesis time of 45 h at 230 °C.30 Kalipcilar et al. synthesized a free-template ZSM-5 zeolite at 200 °C and with a synthesis time between 24 and 168 h, using silicic acid and two different sources of colloidal silica.37 Kalita et al. synthesized nanocrystalline MFI zeolite at 200 °C for 7 h of synthesis time in the presence of tetrapropylammonium bromide (TPABr) using TEOS, fumed silica, colloidal silica, and Aerosil 200.39 These studies showed a significant influence of silica sources on the physicochemical properties of zeolites produced due to differences in synthesis media. Hence, the variation in silica source can highly affect the primary unit of the zeolite framework and the assembly of each pentasil unit to form MFI zeolite with different pore structures.
Additionally, different particle sizes of the same silica source can alter the physical properties and hence the catalytic properties as was observed by Zhang et al.41 Hamilton et al. also reached a similar conclusion that the morphology and particle size of zeolite X were affected by the type of silica source used during the synthesis process.33
Although extensive studies investigating the effect of various parameters on the synthesis and catalytic performance of MFI zeolite are available, a study investigating the structure–property correlation resulting from various silica sources is desired. Herein, various silica sources (fumed silica, silica gel, silicic acid, and tetraethyl orthosilicate) were used to synthesize the MFI zeolite at various temperatures (110, 140, and 180 °C), and properties like particle size, morphology, acidity, and crystal growth rate of the obtained zeolites are compared. The reason behind using these silica sources was because they are different in their physical and chemical properties. Fumed silica and silica gel have different particle sizes and different methods of production which can affect the morphology of the synthesized zeolite. TEOS, on the other hand, can produce ethanol during its hydrolysis in deionized water. Ethanol can then act as a cosolvent in the synthesis and highly affect the synthesis results. Silicic acid is a silica source that is acidic in nature and can reduce the pH of the synthesis precursor and affect the final product. The influence of these changes was observed on the physical properties of the final zeolitic products. The catalytic efficiency of the synthesized zeolites for the steam catalytic cracking of n-dodecane was evaluated in a fixed bed reactor, and the reaction products are analyzed in detail.
2. Experimental Section
2.1. Reagents and Chemicals
The following chemicals are used as received without any further purification and are used for the synthesis: aluminum sulfate octadecahydrate [Al2(SO4)3·18H2O, Acros], tetrapropylammonium hydroxide solution (TPAOH, 1.0 M in H2O, Sigma-Aldrich), TEOS (99%, Sigma-Aldrich), silica gel (Sigma-Aldrich), silicic acid (Sigma-Aldrich), and fumed silica (Sigma-Aldrich). All of the solutions were prepared with deionized water (DIW) with resistivity >18.2 MΩ cm.
2.2. Synthesis Procedures
The MFI samples were synthesized using different silicon sources with the following molar composition: 1.0 SiO2/0.100 TPAOH/0.01 Al2O3/58.57 H2O. The silicon to aluminum ratio (Si/Al) of the sol–gel mixture was designed to be 50. For a typical synthesis of MFI zeolite using fumed silica, 0.351 g of Al2(SO4)3·18H2O was added under continuous stirring to 50.14 g of DIW to maintain a water to silica ratio of 58.576. Subsequently, 6.34 g of 1.0 M TPAOH was added as a template, and finally, 3.17 g of fumed silica was added to the synthesis mixture. The slurry was aged for 90 min to allow the nucleation process followed by hydrothermal treatment in a Teflon reactor for 15 h at the desired temperature (110–180 °C). After 15 h, the reactor was quenched to cool the mixture, and the product was separated using a high-speed centrifuge. The collected powder was dried overnight followed by calcination at 550 °C for 5 h. The calcined sample was ion-exchanged with 2.0 M ammonium nitrate solution at 85 °C under microwave irradiation for 10 min. The weight ratio of zeolite to ammonium nitrate solution was kept at 1.0 to 20.0. The process was repeated twice to ensure complete ion exchange. The sample was dried and calcined again (550 °C for 5 h) to form proton-exchanged MFI zeolites (H-ZSM-5). Other zeolite samples were also prepared in a similar way using the respective silica sources in place of fumed silica in the above case. The following names were assigned to the synthesized zeolite samples: fumed silica-140 °C (FS-140), fumed silica-180 °C (FS-180), silicic acid-140 °C (SE-140), silicic acid-180 °C (SE-180), silica gel-140 °C (SG-140), silica gel-180 °C (SG-180), TEOS-140 °C (TE-140), and TEOS-180 °C (TE-180).
2.3. Zeolite Characterization
2.3.1. Structure and Morphology
The phase purity and crystallinity of the synthesized zeolite powders were determined by X-ray diffraction (XRD, Rigaku Miniflex) with Cu Kα radiation (λ = 0.154 06 nm). The samples were scanned in the range 2θ = 5–50° at a speed of 3° min–1 with a step size of 0.02. The particle size and morphology of the synthesized zeolites were obtained using field-emission scanning electron microscopy (FE-SEM, LYRA 3 Dual Beam, Tescan). A detailed structural analysis was carried out by transmission electron microscopy (TEM) and high-resolution TEM (HR-TEM; JEM-2100F operated at 200 kV).
The type and nature of the obtained coordination systems of the produced zeolite samples were investigated using high-resolution 27Al MAS NMR spectra (Bruker Ultrashield 400WB plus NMR spectrometer). The solid-state 27Al MAS NMR spectra of the synthesized samples were evaluated at a resonance frequency of 104.3 MHz. The total collected accumulated scans were 1910, and the rate of spinning was kept at 13 kHz.
2.3.2. Surface Area and Acidity
The nitrogen adsorption–desorption isotherm was utilized to evaluate the BET surface area, external surface area, and pore, micropore, and mesopore volumes. Surface concentrations of acidic and basic sites were determined by temperature-programmed desorption of ammonia by performing an NH3-TPD analysis on temperature-programmed desorption (BELCAT II, MicrotracBel). For the analysis, 50 mg of zeolite sample was preheated to 500 °C (10 °C min–1) for 60 min under the flow of He (50 mL min–1). Thereafter, the sample was cooled to 100 °C, and ammonia gas was introduced with a flow rate of 30 mL min–1. The sample was flushed with He for 45 min to remove the excess ammonia, and the temperature was ramped again to 600 °C at a ramping rate of 10 °C min–1; the TCD signal was recorded simultaneously. Pyridine FTIR was performed to determine the Lewis and Brønsted acid sites of the synthesized zeolites on a Nicolet FTIR-6700 spectrometer. The sample (30 mg) was pressed into a pellet (self-supported) and placed in a custom-made cell. The sample was heated to 550 °C under a vacuum to remove moisture. After 1 h, the temperature was reduced to 150 °C, and spectra were recorded to evaluate OH stretching in the range 3200–4000 cm–1. Then, pyridine vapor was introduced at 150 °C for 30 min to determine Lewis and Brønsted acid sites. The physisorbed pyridine was evacuated at 150 °C, and subsequently, spectra were recorded. Both Brønsted and Lewis acid sites can be quantitatively evaluated by the following formula:42
where 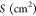 refers to the area of the sample pellet,
refers to the area of the sample pellet, 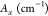 refers to the area under the peak of the
IR band at the requested peak,
refers to the area under the peak of the
IR band at the requested peak, 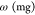 is the weight of the sample pellet, and
is the weight of the sample pellet, and  is the extinction coefficient. The extinction
coefficient is 1.28 and 1.13 cm μmol–1 for
Lewis and Brønsted acid sites, respectively.
is the extinction coefficient. The extinction
coefficient is 1.28 and 1.13 cm μmol–1 for
Lewis and Brønsted acid sites, respectively.
2.4. Catalytic Evaluation
The synthesized catalysts were evaluated for steam-assisted n-dodecane cracking in a fixed bed reactor (PID Microactivity-Effi reactor) at atmospheric pressure and a 350 °C reaction temperature. N2 gas with a flow rate of 15 cm3 min–1 was used as the inert gas for the reaction. The ratio of steam to n-dodecane was kept at 1.0 to 9.0, and the weight hourly space velocity (WHSV) was fixed at 4.5 h–1. The reactant mixture was fed over the catalyst using HPLC liquid pumps. The products were cooled to separate the gaseous and liquid products. The collected liquid products were quantified and analyzed using an offline GC-MS instrument (Agilent J&W DB, 5 ms column), and the gaseous products were analyzed using online GC (Agilent GasPro column, 30 m length and 0.32 mm diameter).
3. Results and Discussion
3.1. Zeolite Characterization
MFI zeolite was extensively studied in our previous works to improve the physicochemical properties.1,43 However, further modifications were performed in this study using different silica sources. Figure 1 shows the XRD patterns of ZSM-5 samples synthesized with different silicon sources and at different temperatures. Apparently, only amorphous matter is obtained by crystallization at 110 °C, whereas crystalline MFI zeolites can be achieved at 140 and 180 °C, and the crystallinity increases with crystallization temperature. The main characteristic XRD peaks observed at 2θ = 7.98°, 8.86°, 23.12°, 23.96°, and 24.44° associated with the plane of the MFI pentasil framework were observed in all crystalline samples.44
Figure 1.
XRD patterns of modified MFI zeolite synthesized under hydrothermal conditions with different silica sources and different synthesis temperatures (Ts): (A) fumed silica, (B) silicic acid, (C) silica gel, and (D) tetraethyl orthosilicate.
The particle sizes and zeolite morphology were investigated employing field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy. It is widely discussed in the literature that particle shape, size, morphology, and crystallite size have a significant role in the catalytic activity of ZSM-5 zeolites for catalytic reactions.45−47 The variation of silica source and synthesis temperature profoundly affected the zeolite size and morphology. Irregularly shaped agglomerated particles were observed with all of the silica sources at a synthesis temperature of 140 °C as shown in Figure 2. At higher synthesis temperature, both the morphology and particle size changed, and the particles acquired a certain degree of regular morphology. FS-140 and FS-180 zeolites (ZSM-5 samples synthesized using fumed silica as the silica source) produced irregular microsized secondary MFI particles which are made up of primary aggregated nanosized particles. The size of agglomerated particles varied in the range 2.0–9.0 μm. Silicic acid yielded irregular, large, and agglomerated particles at 140 °C. A close observation of the micrographs revealed that the agglomerated particles are primarily composed of nanosized particles arranged into irregularly shaped particles of 4.5–18.0 μm (SE-140). When the synthesis temperature was increased to 180 °C (SE-180), these particles evolved into more regularly shaped particles possessing a nuts-and-bolts morphology. The size of these particles ranged between 7.0 and 19.0 μm. The use of silica gel as a silica source produced zeolite particles with a close morphology, and the particles produced from silicic acid showed a similar shape but with a slight variation in particle size. Irregularly shaped agglomerated particles (6.5–10.0 μm) were observed for 140 °C batches (SG-140). The particles become semiregularly agglomerated by increasing the synthesis temperature to 180 °C (SG-180). The size of the agglomerated irregular particles is in the range 10–30.0 μm. TE-140 resulted in irregularly shaped particles (1.0–11.0 μm). A mixture of nuts-and-bolts shape particles (12.0–20.0 μm) and nanosized particles was observed (TE-180) when the synthesis temperature was increased to 180 °C.
Figure 2.
FE-SEM micrographs of modified MFI zeolite synthesized under hydrothermal conditions with different silica sources.
Transmission electron microscopy (TEM) using a multipurpose field emission gun was utilized to obtain high-resolution images as shown in Figure 3. These results support our claim suggesting that the silica source contributed to producing MFI zeolite with nanosized primary particles that are agglomerated to produce secondary micron-sized MFI particles. The influence of silicon sources on zeolite particle size can be classified into two cases as follows: (i) The zeolites FS-140, FS-180, SE-140, SG-140, and TE-140 are confirmed to have irregular nanosized particles; (ii) SE-180, SG-180, and TE-180 showed a combination of regular and irregular particles. The regular particles resulted from the continuous growth of the irregular particles and led to shaping the final particle morphology, accompanied by the process of particle growth. Longer synthesis time or higher synthesis temperature can lead to faster ordering and faster shaping of the irregular particles to form regular particles.
Figure 3.
TEM microstructure characterizations of modified MFI zeolite synthesized under hydrothermal conditions with different silica sources.
The MFI zeolite samples synthesized with different silica sources were further characterized by nitrogen adsorption–desorption isotherms to evaluate the textural properties. As shown in Figure 4A, samples prepared using fumed silica displayed a combination of type I and type IV isotherms. Furthermore, the hysteresis loop was observed at a relative pressure higher than 0.90. However, the presence of the type IV isotherm was more limited when the synthesis temperature was increased from 140 to 180 °C as shown in Figure 4A. The same findings were obtained when the silica source was changed from fumed silica to silicic acid, silica gel, or tetraethyl orthosilicate as shown in Figure 4B–D. It is interesting to note that a hysteresis loop was present in the isotherms of samples synthesized at a temperature of 140 °C. This indicates the high mesoporous volume for these samples as confirmed from Table 1.48 Moreover, both FE-SEM and TEM images confirm the growth of primary particles with an increase in synthesis temperature to form the regular secondary particle. This growth at a higher synthesis temperature led to the reduction of intraparticle space between the primary particles and a decrease in the void spaces. On the other hand, when the synthesis temperature increased from 140 to 180 °C, the isotherm uptake increased which indicates the ability of these samples to adsorb more N2 due to the availability of larger microporous areas. This increase in N2 uptake due to an increase in crystallization temperature from 140 to 180 °C (Figure 4) was observed in all silica sources used in this study.
Figure 4.
BET isotherm of modified MFI zeolite synthesized under hydrothermal conditions with different silica sources: (A) fumed silica, (B) silicic acid, (C) silica gel, and (D) tetraethyl orthosilicate.
Table 1. Textural Properties of the MFI Zeolite Samples Synthesized with Different Silicon Sources.
| sample name | modification type | synthesis temperature (°C) | Qm (cm3/g STP) | SBET (m2/g) | Smicroa (m2/g) | Sextb (m2/g) | Vtotalc (cm3/g) | Vmicrod (cm3/g) | Vmesoe (cm3/g) |
|---|---|---|---|---|---|---|---|---|---|
| FS-140 | fumed silica | 140 | 67 | 291 | 129 | 162 | 0.472 | 0.055 | 0.417 |
| FS-180 | fumed silica | 180 | 70 | 307 | 151 | 155 | 0.332 | 0.064 | 0.268 |
| SE-I40 | silicic acid | 140 | 68 | 296 | 93 | 203 | 0.695 | 0.041 | 0.655 |
| SE-180 | silicic acid | 180 | 82 | 355 | 167 | 188 | 0.258 | 0.072 | 0.186 |
| SG-140 | silica gel | 140 | 60 | 259 | 84 | 178 | 0.637 | 0.036 | 0.601 |
| SG-180 | silica gel | 180 | 69 | 300 | 133 | 167 | 0.566 | 0.057 | 0.509 |
| TE-140 | TEOS | 140 | 75 | 326 | 58 | 268 | 0.727 | 0.026 | 0.701 |
| TE-180 | TEOS | 180 | 83 | 361 | 145 | 216 | 0.298 | 0.062 | 0.236 |
t-plot micropore surface area.
t-plot external surface area.
p/p0 = 0.988 866.
t-plot micropore volume.
Vmeso = Vtotal – Vmicro.
The textural properties of the produced MFI zeolites are summarized in Table 1. We observed that the BET surface areas, microporous areas, and microporous volumes increased with all silica sources as the synthesis temperature was increased. The increase in the BET surface area is due to the transformation of the remaining small amorphous phases to well-crystalline materials as confirmed by XRD. On the other hand, the external surface area, total pore volume, and mesoporous area decreased as the synthesis temperature increased in all silica source cases as shown in Table 1. These decreases in external surface area, total pore volume, and mesoporous area were related to the increase in particle sizes which led to reductions of the intraparticle spaces due to the zeolite channel growth.
Acidity is one of the most important properties of zeolite materials and can be classified based on its strength or its nature. One of the most important ways to evaluate the strength of zeolite acidity is through temperature-programmed desorption of ammonia (NH3-TPD). The NH3-TPD signals obtained for the samples synthesized at 140 and 180 °C using different silica sources are shown in Figure 5A,B. It is clear from the profiles that the MFI zeolites synthesized at 140 and 180 °C have both weak and strong acid sites. Furthermore, silicon sources also have an influence on the strength of both weak and strong acid sites. For samples synthesized at 140 °C, the strong acid site increased from 0.010 mmol/g for silicic acid (SE-140) to 0.073 mmol/g for silica gel (SG-140). Both TEOS and fumed silica sources showed strong acid sites of 0.054 and 0.055 mmol/g, respectively, as shown in Figure 5C. Furthermore, the weak acid sites of samples synthesized at 140 °C showed a trend analogous to that of strong acid sites. In addition, the order of both weak and strong acid sites was different for samples synthesized at 180 °C. Fumed silica (FS-180) and silicic acid (SE-180) produced zeolites with the least amount of weak acidity of 0.043 mmol/g each. The amount of weak acidity for all silica sources was in the order SE-180 = FS-180 < TE-180 < SG-180. Furthermore, the strong acid sites were in the order SG-180 > TE-180 > SE-180 > FS-180 as shown in Figure 5D.
Figure 5.
NH3-TPD measurement of the MFI zeolite synthesized with different silica sources and at different synthesis temperatures: (A) TCD signal at 140 °C, (B) TCD signal at 180 °C, (C) acidity at 140 °C, and (D) acidity at 180 °C.
The nature of zeolite acidity was further probed through pyridine-FTIR which is an important technique employed for the quantitative and qualitative estimation of the type of acidity in heterogeneous catalysis. Lewis and Brønsted acid sites are present at band positions of 1455 and 1545 cm–1, respectively, in zeolites. The strength of both Lewis and Brønsted acid sites can vary depending on the number of active sites in these zeolites. The first peak at band 1455 cm–1 appears due to pyridine association to Lewis acid sites while the other band at 1545 cm–1 results from the adsorption of the pyridinium ion on Brønsted acid sites.49 As shown in Figure 6A,B, the zeolites synthesized with different silica sources as well as different synthesis temperatures showed different acidity concentrations. We noticed that all silica sources can produce zeolites possessing both Lewis and Brønsted acid sites. Due to the variation in morphology and particle size, the variation in the concentration of both Lewis and Brønsted acid sites differs from one sample to the other.
Figure 6.
Pyr-FTIR analysis of the MFI zeolite synthesized with different silica sources and at different synthesis temperatures: (A) absorbance at 140 °C, (B) absorbance at 180 °C, (C) acidity at 140 °C, and (D) acidity at 180 °C.
Figure 6C,D summarizes the quantitative analysis of acidity using Pyr-FTIR. The influence of silica source in varying the nature and strength of both Lewis and Brønsted acid sites is shown in Figure 6A–D. As the synthesis temperature increases, the amount of Brønsted acid sites increases as observed from Figure 6C,D. This trend was observed with all silica sources, except for fumed silica which showed the opposite trend. Furthermore, the amount and type of acidic sites vary from one silica source to another at each synthesis temperature. The Brønsted site acidity value was 0.036 mmol/g for SE-140 and 0.082 mmol/g for FS-140. TEOS (TE-140) and silica gel (SG-140) sources at 140 °C showed Brønsted acid sites of 0.040 and 0.059 mmol/g, respectively, as shown in Figure 6C. The strengths of the Lewis acid sites were found to be in the following order: fumed silica source > silicic acid source > TEOS source > silica gel source. The order of both Brønsted and Lewis acid sites was different for syntheses conducted at 180 °C. The silicic-acid-based sample (SE-180) was found to have the least total acidity with Brønsted and Lewis acid site values of 0.044 and 0.018 mmol/g, respectively. The concentration of both Brønsted and Lewis acid sites increased as the silicon source changed from silicic acid to TEOS, fumed silica, and silica gel in their order as shown in Figure 6D. The general sequence of the Brønsted acid strength with respect to the type of used silica source can be sorted from maximum to minimum as follows: silica gel > fumed silica > TEOS > silicic acid.
The OH-stretching region was evaluated by FTIR to estimate the presence of zeolite functionalities such as the silanol, silanol nest, and hydrogen-bonded Si–OH groups. The measurements were collected after sufficient evacuation of the samples to ensure that all water moisture was removed. The influence of silica sources on the OH-stretching region of the zeolites synthesized at two different temperatures is shown in Figure 7. It was observed that all synthesized samples have an intense band with a shoulder at 3741 cm–1 which corresponds to the silanol group. However, comparing this band among different silica sources at 140 °C can emphasize that the TEOS source showed the most intense band. The other silica sources have similar intensities. Moreover, the influence of silica sources on the band intensities located at 3741 cm–1 becomes more pronounced when the synthesis temperature decreases from 180 to 140 °C. In general, this band was more pronounced at lower synthesis temperatures (140 °C) than at high synthesis temperatures (180 °C) as shown in Figure 7A–D.
Figure 7.
IR spectra of OH bonds of the MFI zeolite synthesized with different silica sources at 140 and 180 °C: (A) fumed silica, (B) silica gel, (C) silicic acid, (D) tetraethyl orthosilicate, and (E) comparison between silica sources at different temperatures.
Another band was observed in some of the synthesized samples at 3600 cm–1 which corresponds to Al–O(H)–Si hydroxyls.50 This band was observed in the cases of FS-140 and FM-180 and also for SG-180 and TE-180 as shown in the overlapped spectra in Figure 7E. Another visible broadband between 3300 and 3550 cm–1 was assigned to H-bonded Si–OH groups.51,52 This band was more pronounced for SE-180 and TE-180 samples.
The coordination system of MFI zeolite samples synthesized with different silica sources was characterized by NMR MAS spectra. Both 27Al and 29Si NMR analyses were performed to understand the nature of the coordination of both aluminum and silicon in the zeolite framework. As shown in Figure 8, 27Al MAS NMR showed that all MFI samples have a peak located around 53.0 ppm, which corresponds to the tetrahedral coordination system. Furthermore, another small peak around 0 ppm was also observed, which corresponds to the extra-framework (octahedral coordination system) of aluminum. When we analyze the effect of synthesis temperature, it is observed that aluminum association to the zeolite framework becomes more pronounced at a higher synthesis temperature (180 °C). This can be observed from the intensity of the tetrahedral coordination system, which was observed with all used silica sources. The ratio of tetrahedral to octahedral coordination also changed when moving from one silica source to another. With all of the used silicon sources, it was observed that a more octahedral coordination system can be obtained at a lower synthesis temperature as shown in Table 2.
Figure 8.
27Al MAS NMR of the MFI zeolite synthesized under hydrothermal conditions with different silica sources: (A) fumed silica, (B) silicic acid, (C) silica gel, and (D) tetraethyl orthosilicate.
Table 2. Distribution of Tetrahedral and Octahedral Coordination Systems of the MFI Zeolite Synthesized at Different Synthesis Temperatures with Different Silica Sources.
| sample name | octahedral [%] | tetrahedral [%] |
|---|---|---|
| FS-140 | 7.60 | 92.40 |
| FS-180 | 3.60 | 96.40 |
| SE-140 | 5.14 | 94.86 |
| SE-180 | 2.67 | 97.33 |
| SG-140 | 9.93 | 90.07 |
| SG-180 | 6.50 | 93.50 |
| TE-140 | 7.20 | 92.80 |
| TE-180 | 0.87 | 99.13 |
3.2. Catalytic Evaluation
MFI zeolite samples synthesized with different silica sources were evaluated for steam-assisted catalytic cracking of n-dodecane, which is a model reaction for upgrading heavy oils. Results of steam catalytic conversion over the zeolites synthesized using different silica sources and at different synthesis temperatures are shown in Figure 9. All of the zeolite catalysts prepared at 180 °C showed higher conversion than those synthesized at 140 °C irrespective of the silica source used. The conversions increased to 96.6% from 83.1% (for fumed-silica-based), to 92.9% from 68.0% (for silicic acid-based), to 87.3 from 85.7% (for silica-gel-based), and to 91.5% from 69.5% (for TEOS-based) for zeolites when the synthesis temperature changed to 180 from 140 °C as shown in Figure 9A–D, respectively. A higher conversion was achieved for the fumed-silica-based zeolite compared to zeolites prepared with other silica sources. The increase in catalytic conversion was minimum for silica-gel-based samples when we changed the synthesis temperature from 180 to 140 °C. Silicic-acid-based and TEOS-based zeolites demonstrated a significant drop in conversion when we changed the synthesis temperature.
Figure 9.
Steam catalytic conversion over the MFI zeolite synthesized with different silica sources and at different synthesis temperatures. Reaction conditions: 4.5 WHSV (h–1), 350 °C, 10 wt % water, N2 = 15 mL/min. (A) Fumed silica, (B) silicic acid, (C) silica gel, and (D) tetraethyl orthosilicate.
Catalytic activity, product distributions, and zeolite properties need to be correlated in order to understand the effect of silica sources. Better catalytic activity was observed for all samples prepared at 180 °C irrespective of silica source due to various advantages discussed as follows. Better zeolite crystallinity together with larger surface area and larger micropores were obtained at a higher synthesis temperature. Brønsted acid sites observed from Pyr-FTIR are more pronounced for all of the samples at the higher synthesis temperature except for the fumed-silica-based sample. Lewis acidic sites do not experience significant changes among the samples prepared and characterized in this study whereas Brønsted sites do. Lewis sites can accept hydrogen, leading to dehydrogenation and the formation of olefins,53 and, in combination with enough mesoporosity of ZSM-5 zeolites, can produce aromatics as well.54 Brønsted sites are critical to initiate various catalytic reactions, as proven by previous researchers,53 typically cracking in this case. The presence and proximity of both Lewis and Brønsted sites have also been discussed in the literature. In such cases, Brønsted acidic sites enhance the cracking function by means of creating a proximal polarization of C–H bonds of alkanes.55 In fumed-silica-based MFI zeolite, the Brønsted acid values were inversely proportional to the synthesis temperature. However, we considered that this relation could be caused by the irregular morphological nature of fumed-silica-based zeolites. The size of the secondary agglomerated particles might affect the adsorption of the pyridinium ion on Brønsted acid sites. Moreover, it was observed from NH3-TPD that as the strong acidity increases the catalytic conversion is also enhanced as shown in Table 3. We understand that there is no big difference in the conversion between 180 and 140 °C for silica gel and fumed silica, and these small differences could be in the range of the error bar. However, we would like to highlight that some silica sources such as tetraethyl orthosilicate and silicic acid have a higher difference in the catalytic activity as the strong acid site increases in the zeolite. Also, the percentage of the tetrahedral coordination system increased as the synthesis temperature increased which is also attributed to enhancing the acidic sites as confirmed by NH3-TPD.
Table 3. Relation between Strong Acid Sites and Catalytic Conversion of the MFI Zeolites Synthesized at Different Temperatures and Using Different Silica Sources.
| sample name | strong acid site, mmol/g | difference in strong acidity,a mmol/g | conversion [%] | difference in conversionb [%] |
|---|---|---|---|---|
| SG-140 | 0.073 | 0.002 | 84.8 | 2.5 |
| SG-180 | 0.075 | 0.002 | 87.3 | 2.5 |
| FS-140 | 0.055 | 0.008 | 93.1 | 3.5 |
| FS-180 | 0.063 | 0.008 | 96.6 | 3.5 |
| TE-140 | 0.054 | 0.019 | 69.5 | 22.0 |
| TE-180 | 0.073 | 0.019 | 91.5 | 22.0 |
| SE-140 | 0.010 | 0.062 | 68.0 | 23.9 |
| SE-180 | 0.072 | 0.062 | 91.9 | 23.9 |
Difference in strong acidity for each silica source = strong acid site at synthesis temperature of 180 °C – strong acid site at synthesis temperature of 140 °C.
Difference in conversion for each silica source = conversion at synthesis temperature of 180 °C – conversion at synthesis temperature of 140 °C.
Deep observation and careful judgment are required to link the catalytic activity, catalytic stability, catalytic selectivity, and zeolite properties. Generally, products were classified into gaseous products and liquid products as shown in Figure 10A denoted as the average product distribution for liquid and gaseous products. The reported values are based on the average product distribution for a total of 8.0 h of reaction on stream. The catalytic selectivities for both liquid and gaseous products vary depending on the synthesis conditions of the zeolite samples. When silica gel was used as the precursor, the liquid products were 33.5% and 38.7% for synthesis temperatures of 140 °C (SG-140) and 180 °C (SG-180), respectively. FS-140 and FS-180 showed 28.9% and 44.1% of liquid products, respectively. SE-140 and SE-180 generated liquid products of 25.6% and 31.6% for 140 and 180 °C, respectively. These three silica sources showed an increase in liquid products as the synthesis temperature increased from 140 to 180 °C. However, TEOS-based zeolites produced lesser liquid products (reduced to 24.8% from 28.0%) as the synthesis temperature increased from 140 to 180 °C as shown in Figure 10A.
Figure 10.
(A) Average product distribution for liquid and gaseous products. (B) Product distribution (after 1 h) of liquid products over the MFI zeolites synthesized with different silica sources and at different synthesis temperatures. Reaction condition: 4.5 WHSV (h–1), 350 °C, 10 wt % water, N2 = 15 mL/min.
Liquid products varied in product selectivity as shown in Figure 10B. The detailed hydrocarbons analysis (DHA) was performed by offline GC to determine the PIONA (paraffins, isoparaffins, olefins, naphthenes, and aromatics) after 1.0 h of reaction on stream using ASTM D6729. For silica-gel-based samples, liquid product distributions were almost similar for both zeolite samples synthesized at 140 and 180 °C (that is, SG-140 and SG-180). Naphthenes and aromatics for these two cases were 60.8% and 65.3% at 140 and 180 °C, respectively. The remaining products were distributed between paraffins, isoparaffins, and olefins. The liquid product distribution of fumed-silica-based zeolites clearly varied based on the synthesis temperature. When the synthesis temperature was 180 °C (FS-180), the product distribution was comparable with silica-gel-based samples (SG-140 and SG-180) with a total naphthenes and aromatics selectivity of 65.1%. For the synthesis temperature of 140 °C (FS-140), the liquid product distribution dramatically changed, and the selectivity toward naphthenes and aromatics reduced to 37.3%. For the silicic acid-based MFI zeolite (SE-140 and SE-180), product selectivity toward naphthenes and aromatics increased from 30.1% (for SE-140) to 61.6% (for SE-180) as the synthesis temperature increased from 140 to 180 °C, respectively. The TEOS-based MFI zeolite also showed a varied product distribution depending on the synthesis temperature. When the synthesis temperature was 140 °C (TE-140) the selectivity toward both naphthenes and aromatics was 8.1% only. This selectivity of both naphthenes and aromatics significantly increased to 71.3% by increasing the synthesis temperature to 180 °C (for TE-180).
The liquid product distribution was mainly affected by the nature of active sites in both straight and zigzag channels of the synthesized zeolites which contribute to the primary and secondary reforming reactions. The primary reaction mainly involves cracking which produces olefins and paraffins. The secondary reaction which involves cyclization and isomerization can produce mainly naphthenes and aromatics while passing through the zigzag path of the zeolite channels.54 Product distribution varied depending on the type of silica source and synthesis temperature used. Both of these parameters (that is, silica source and synthesis temperature) contributed to the formation of zeolite pentasil units which in turn affects the localization of Al and Si atoms within the straight and zigzag channels; this in turn has a profound effect on the catalytic activity.56 Selectivities toward naphthenes and aromatics were very small for samples synthesized using fumed silica, silicic acid, and tetraethyl orthosilicate and at 140 °C. In each of these three samples, the conversion was found to be lower compared to the other samples which produce more naphthenes and aromatics. This emphasizes the weaker ability of these catalysts to crack and convert the feed to intermediates which in turn appear for the secondary reaction. The product distributions of SG-140, SG-180, FS-180, SE-180, and TE-180 had predominantly naphthenic and aromatic products due to the effect of both straight and zigzag channels. Hence, both primary and secondary reactions were taking place thus leading to a higher cyclization reaction.
The gaseous products found in our analysis include hydrogen, paraffins (C1–C4), and olefins (C2–C4). Furthermore, the selectivity to gaseous products was also affected depending on the silica source as shown in Figure 11A. The cumulative values of light olefins (C2= , C3= , and C4=) for each sample are also shown in Figure 11B. As discussed earlier, we have used the average product distribution for a total of 8.0 h of reaction on stream in all of the results presented in this paper. Silica-gel-based zeolites showed 65.0% (SG-140) and 60.2% (SG-180) selectivity toward light olefins as we increased the synthesis temperature from 140 to 180 °C, respectively. The selectivity toward light olefins was 60.4% and 51.5% for FS-140 and FS-180 whereas it was 67.4% and 58.7% for SE-140 and SE-180 samples, respectively. The highest production of light olefin was observed when TEOS was used as the silica source. The selectivity to light olefins was 73.1% (for TE-140) and 67.1% (for TE-180) at a synthesis temperature of 140 and 180 °C, respectively. Generally, the lower synthesis temperature (140 °C) showed higher selectivity toward light olefins as compared to the higher synthesis temperature (180 °C) suggesting that secondary reactions will facilitate further cyclization and isomerization of the formed olefins to higher fractions and aromatics.
Figure 11.
(A) Average product distribution of gaseous products. (B) Average product distribution for light olefins (C2=, C3=, and C4=) over MFI zeolites synthesized with different silica sources and at different synthesis temperatures. Reaction condition: 4.5 WHSV (h–1), 350 °C, 10 wt % water, N2 = 15 mL/min.
4. Conclusions
Mesoporous MFI zeolites were hydrothermally synthesized at various temperatures employing different silica sources to understand their effect on the resulting catalyst properties and to evaluate their catalytic properties and analyze the structure–property correlations. The variation of silicon sources presented zeolites with different physicochemical properties. The catalytic performances of the synthesized MFI zeolites were evaluated for steam-assisted catalytic cracking of n-dodecane. The highest catalytic conversion was observed for the fumed-silica-based sample synthesized at 180 °C (FS-180) which showed 96.6% conversion. The highest gaseous product (62.5%) was observed when TEOS was employed as the silica source and at a synthesis temperature of 180 °C (TE-180) which made it a choice for the production of gaseous hydrocarbons. Furthermore, it is also noted that 71.3% of the liquid product formed is naphthenes and aromatics. Also, the maximum light olefin (73.1%) in the gaseous products was obtained when TEOS was used as the silica source and at a synthesis temperature of 140 °C (TE-140), which made it a choice for the production of light olefins. The differences in selectivity to light olefins between the synthesized samples are understood to be majorly due to the effect of mesoporosity, the presence of a sufficient concentration of Lewis and Brønsted sites, and also the effective particle size of the zeolites.
Acknowledgments
The authors are thankful for the financial support to research projects funded by Saudi Aramco with Contract 6510877551 under Project Code CENT2207. The authors would like to acknowledge the Interdisciplinary Research Center for Hydrogen and Energy Storage (IRC-HES) at King Fahd University of Petroleum and Minerals in the Kingdom Saudi Arabia for hosting and supporting the research activities.
The authors declare no competing financial interest.
References
- Sanhoob M. A.; Muraza O.; Shafei E. N.; Yokoi T.; Choi K.-H. Steam Catalytic Cracking of Heavy Naphtha (C12) to High Octane Naphtha over B-MFI Zeolite. Appl. Catal. B Environ. 2017, 210, 432. 10.1016/j.apcatb.2017.04.001. [DOI] [Google Scholar]
- Khalil U.; Muraza O.; Kondoh H.; Watanabe G.; Nakasaka Y.; Al-Amer A.; Masuda T. Robust Surface-Modified Beta Zeolite for Selective Production of Lighter Fuels by Steam-Assisted Catalytic Cracking from Heavy Oil. Fuel 2016, 168, 61–67. 10.1016/j.fuel.2015.11.085. [DOI] [Google Scholar]
- Muraza O.; Galadima A. Aquathermolysis of Heavy Oil: A Review and Perspective on Catalyst Development. Fuel 2015, 157, 219–231. 10.1016/j.fuel.2015.04.065. [DOI] [Google Scholar]
- Corma A.; Mengual J.; Miguel P. J. Steam Catalytic Cracking of Naphtha over ZSM-5 Zeolite for Production of Propene and Ethene: Micro and Macroscopic Implications of the Presence of Steam. Appl. Catal. A Gen. 2012, 417–418, 220–235. 10.1016/j.apcata.2011.12.044. [DOI] [Google Scholar]
- Al-Shammari A. A.; Ali S. A.; Al-Yassir N.; Aitani A. M.; Ogunronbi K. E.; Al-Majnouni K. A.; Al-Khattaf S. S. Catalytic Cracking of Heavy Naphtha-Range Hydrocarbons over Different Zeolites Structures. Fuel Process. Technol. 2014, 122, 12–22. 10.1016/j.fuproc.2014.01.021. [DOI] [Google Scholar]
- Galadima A.; Muraza O. Catalytic Upgrading of Vegetable Oils into Jet Fuels Range Hydrocarbons Using Heterogeneous Catalysts: A Review. J. Ind. Eng. Chem. 2015, 29, 12–23. 10.1016/j.jiec.2015.03.030. [DOI] [Google Scholar]
- Huber G. W.; Chheda J. N.; Barrett C. J.; Dumesic J. A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science (80-.) 2005, 308 (5727), 1446–1450. 10.1126/science.1111166. [DOI] [PubMed] [Google Scholar]
- Chheda J. N.; Dumesic J. A. An Overview of Dehydration, Aldol-Condensation and Hydrogenation Processes for Production of Liquid Alkanes from Biomass-Derived Carbohydrates. Catal. Today 2007, 123 (1), 59–70. 10.1016/j.cattod.2006.12.006. [DOI] [Google Scholar]
- Liu D.; Chen E. Y.-X. Integrated Catalytic Process for Biomass Conversion and Upgrading to C12 Furoin and Alkane Fuel. ACS Catal. 2014, 4 (5), 1302–1310. 10.1021/cs500058p. [DOI] [Google Scholar]
- Deneyer A.; Renders T.; Van Aelst J.; Van den Bosch S.; Gabriëls D.; Sels B. F. Alkane Production from Biomass: Chemo-, Bio- and Integrated Catalytic Approaches. Curr. Opin. Chem. Biol. 2015, 29, 40–48. 10.1016/j.cbpa.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Xian X.; Liu G.; Zhang X.; Wang L.; Mi Z. Catalytic Cracking of N-Dodecane over HZSM-5 Zeolite under Supercritical Conditions: Experiments and Kinetics. Chem. Eng. Sci. 2010, 65 (20), 5588–5604. 10.1016/j.ces.2010.08.004. [DOI] [Google Scholar]
- Zhao J.; Guo W.; Liu G.; Zhang X.; Wang L. Cracking of N-Dodecane during Supercritical State on HZSM-5 Membranes. Fuel Process. Technol. 2010, 91 (9), 1090–1097. 10.1016/j.fuproc.2010.03.019. [DOI] [Google Scholar]
- Diao Z.; Wang L.; Zhang X.; Liu G. Catalytic Cracking of Supercritical N-Dodecane over Meso-HZSM-5@Al-MCM-41 Zeolites. Chem. Eng. Sci. 2015, 135, 452–460. 10.1016/j.ces.2014.12.048. [DOI] [Google Scholar]
- Ji Y.; Yang H.; Zhang Q.; Yan W. Phosphorus Modification Increases Catalytic Activity and Stability of ZSM-5 Zeolite on Supercritical Catalytic Cracking of n-Dodecane. J. Solid State Chem. 2017, 251, 7–13. 10.1016/j.jssc.2017.03.023. [DOI] [Google Scholar]
- Ishihara A.; Inui K.; Hashimoto T.; Nasu H. Preparation of Hierarchical β and Y Zeolite-Containing Mesoporous Silica–Aluminas and Their Properties for Catalytic Cracking of n-Dodecane. J. Catal. 2012, 295, 81–90. 10.1016/j.jcat.2012.07.027. [DOI] [Google Scholar]
- Hopkins P. D. Cracking Activity of Some Synthetic Zeolites and the Nature of the Active Sites. J. Catal. 1968, 12 (4), 325–334. 10.1016/0021-9517(68)90116-4. [DOI] [Google Scholar]
- Sanhoob M. A.; Khalil U.; Shafei E. N.; Choi K.-H.; Yokoi T.; Muraza O. Steam Cracking of Green Diesel (C12) to BTX and Olefins over Silane-Treated Hierarchical BEA. Fuel 2020, 263, 116624. 10.1016/j.fuel.2019.116624. [DOI] [Google Scholar]
- Al-Absi A. A.; Al-Khattaf S. S. Conversion of Arabian Light Crude Oil to Light Olefins via Catalytic and Thermal Cracking. Energy Fuels 2018, 32 (8), 8705–8714. 10.1021/acs.energyfuels.8b01932. [DOI] [Google Scholar]
- Argauer R. J.; Landolt G. R.. Crystalline Zeolite Zsm-5 and Method of Preparing the Same. US Patent US3702886A, 1972.
- Borade R. B.; Hegde S. G.; Kulkarni S. B.; Ratnasamy P. Active Centres over Hzsm5 Zeolites for Paraffin Cracking. Appl. Catal. 1984, 13 (1), 27–38. 10.1016/S0166-9834(00)83325-4. [DOI] [Google Scholar]
- Bizreh Y. W.; Gates B. C. Butane Cracking Catalyzed by the Zeolite H-ZSM-5. J. Catal. 1984, 88 (1), 240–243. 10.1016/0021-9517(84)90071-X. [DOI] [Google Scholar]
- Kanai J.; Inui T. Aromatization of N-Hexane Over Ga–H–Zsm–5 Catalysts. Stud. Surf. Sci. Catal. 1989, 44, 211–217. 10.1016/S0167-2991(09)61295-7. [DOI] [Google Scholar]
- Lugstein A.; Jentys A.; Vinek H. Hydroisomerization and Cracking of N-Octane and C8 Isomers on Ni-Containing Zeolites. Appl. Catal. A Gen. 1999, 176 (1), 119–128. 10.1016/S0926-860X(98)00231-2. [DOI] [Google Scholar]
- Baerlocher C.; McCusker L. B.; Olson D. H.. Atlas of Zeolite Framework Types; Elsevier Science, 2007. [Google Scholar]
- Ummer A. C.; Akhtar M. N.; Alnaimi E.; Ding L.; Alasiri H. S. Aromatization of Commercial Full Range Naphtha Over Modified Hierarchical ZSM-5 Catalyst. ChemistrySelect 2021, 6 (42), 11541–11550. 10.1002/slct.202103033. [DOI] [Google Scholar]
- Cejka J.; van Bekkum H.. Zeolites and Ordered Mesoporous Materials: Progress and Prospects: The 1st FEZA School on Zeolites; Gulf Professional Publishing: Prague, Czech Republic, 2005; Vol. 157. [Google Scholar]
- Inagaki S.; Sato K.; Hayashi S.; Tatami J.; Kubota Y.; Wakihara T. Mechanochemical Approach for Selective Deactivation of External Surface Acidity of ZSM-5 Zeolite Catalyst. ACS Appl. Mater. Interfaces 2015, 7 (8), 4488–4493. 10.1021/am507982n. [DOI] [PubMed] [Google Scholar]
- Grand J.; Talapaneni S. N.; Aleksandrov H. A.; Vayssilov G. N.; Mintova S. Hydrophobic Tungsten-Containing MFI-Type Zeolite Films for Exhaust Gas Detection. ACS Appl. Mater. Interfaces 2019, 11 (13), 12914–12919. 10.1021/acsami.8b17626. [DOI] [PubMed] [Google Scholar]
- Wang S.; Bai P.; Wei Y.; Liu W.; Ren X.; Bai J.; Lu Z.; Yan W.; Yu J. Three-Dimensional-Printed Core–Shell Structured MFI-Type Zeolite Monoliths for Volatile Organic Compound Capture under Humid Conditions. ACS Appl. Mater. Interfaces 2019, 11 (42), 38955–38963. 10.1021/acsami.9b13819. [DOI] [PubMed] [Google Scholar]
- Mohamed R. M.; Aly H. M.; El-Shahat M. F.; Ibrahim I. A. Effect of the Silica Sources on the Crystallinity of Nanosized ZSM-5 Zeolite. Microporous Mesoporous Mater. 2005, 79 (1–3), 7–12. 10.1016/j.micromeso.2004.10.031. [DOI] [Google Scholar]
- Li M.; Wang Y.; Bai L.; Chang N.; Nan G.; Hu D.; Zhang Y.; Wei W. Solvent-Free Synthesis of SAPO-34 Nanocrystals with Reduced Template Consumption for Methanol-to-Olefins Process. Appl. Catal. A Gen. 2017, 531, 203–211. 10.1016/j.apcata.2016.11.005. [DOI] [Google Scholar]
- Lateef S. A.; Bakare I. A.; Mayoral A.; Sebastian V.; Muraza O. Selective Catalytic Cracking of N-Hexane to Olefins over SSZ-54 Fabricated by Facile and Novel Dual Templating Method. Fuel 2018, 227, 48–58. 10.1016/j.fuel.2018.03.161. [DOI] [Google Scholar]
- Hamilton K. E.; Coker E. N.; Sacco A.; Dixon A. G.; Thompson R. W. The Effects of the Silica Source on the Crystallization of Zeolite NaX. zeolites 1993, 13 (8), 645–653. 10.1016/0144-2449(93)90137-R. [DOI] [Google Scholar]
- Güray I.; Warzywoda J.; Baç N.; Sacco A. Synthesis of Zeolite MCM-22 under Rotating and Static Conditions. Microporous Mesoporous Mater. 1999, 31 (3), 241–251. 10.1016/S1387-1811(99)00075-X. [DOI] [Google Scholar]
- Ge Q.; Shao J.; Wang Z.; Yan Y. Effects of the Synthesis Hydrogel on the Formation of Zeolite LTA Membranes. Microporous Mesoporous Mater. 2012, 151, 303–310. 10.1016/j.micromeso.2011.10.019. [DOI] [Google Scholar]
- Schmidt W.; Toktarev A. V.; Schüth F.; Ione K. G.; Unger K.. 02-P-23 - The Influence of Different Silica Sources on the Crystallization Kinetics of Zeolite Beta. In Stud. Surf. Sci. Catal.; Galarneau A., Fajula F., Di Renzo F., Vedrine J., Eds.; Elsevier, 2001; Vol. 135, p 190. 10.1016/S0167-2991(01)81362-8. [DOI] [Google Scholar]
- Kalipcilar H.; Culfaz A. Influence of Nature of Silica Source on Template-Free Synthesis of ZSM-5. Zeitschrift für experimentelle und technische Kristallographie 2001, 36 (11), 1197–1207. . [DOI] [Google Scholar]
- Kordatos K.; Gavela S.; Ntziouni A.; Pistiolas K. N.; Kyritsi A.; Kasselouri-Rigopoulou V. Synthesis of Highly Siliceous ZSM-5 Zeolite Using Silica from Rice Husk Ash. Microporous Mesoporous Mater. 2008, 115 (1), 189–196. 10.1016/j.micromeso.2007.12.032. [DOI] [Google Scholar]
- Kalita B.; Talukdar A. K. An Efficient Synthesis of Nanocrystalline MFI Zeolite Using Different Silica Sources: A Green Approach. Mater. Res. Bull. 2009, 44 (2), 254–258. 10.1016/j.materresbull.2008.06.014. [DOI] [Google Scholar]
- Shaikh I. R.; Shaikh R. A.; Shaikh A. A.; War J. A.; Hangirgekar S. P.; Shaikh A. L.; Shaikh P. R.; Shaikh R. R. H-ZSM-5 Zeolite Synthesis by Sourcing Silica from the Wheat Husk Ash: Characterization and Application as a Versatile Heterogeneous Catalyst in Organic Transformations Including Some Multicomponent Reactions. J. Catal. 2015, 2015, 1–14. 10.1155/2015/805714. [DOI] [Google Scholar]
- Zhang J.; Li X.; Liu J.; Wang C. A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance. Catalysts 2019, 9 (1), 13. 10.3390/catal9010013. [DOI] [Google Scholar]
- Konan K. L.; Peyratout C.; Smith A.; Bonnet J. P.; Magnoux P.; Ayrault P. Surface Modifications of Illite in Concentrated Lime Solutions Investigated by Pyridine Adsorption. J. Colloid Interface Sci. 2012, 382 (1), 17–21. 10.1016/j.jcis.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Sanhoob M. A.; Muraza O. Synthesis of Silicalite-1 Using Fluoride Media under Microwave Irradiation. Microporous Mesoporous Mater. 2016, 233, 140. 10.1016/j.micromeso.2016.01.004. [DOI] [Google Scholar]
- Treacy M. M.; Higgins J. B.. Collection of Simulated XRD Powder Patterns for Zeolites; Elsevier, 2007. [Google Scholar]
- Sugimoto M.; Katsuno H.; Takatsu K.; Kawata N. Correlation between the Crystal Size and Catalytic Properties of ZSM-5 Zeolites. Zeolites 1987, 7 (6), 503–507. 10.1016/0144-2449(87)90087-X. [DOI] [Google Scholar]
- Shiralkar V. P.; Joshi P. N.; Eapen M. J.; Rao B. S. Synthesis of ZSM-5 with Variable Crystallite Size and Its Influence on Physicochemical Properties. Zeolites 1991, 11 (5), 511–516. 10.1016/S0144-2449(05)80127-7. [DOI] [Google Scholar]
- Rownaghi A. A.; Rezaei F.; Hedlund J. Yield of Gasoline-Range Hydrocarbons as a Function of Uniform ZSM-5 Crystal Size. Catal. Commun. 2011, 14 (1), 37–41. 10.1016/j.catcom.2011.07.015. [DOI] [Google Scholar]
- Cejka J.; van Bekkum H.; Corma A.; Schueth F.. Introduction to Zeolite Molecular Sieves; Elsevier Science, 2007. [Google Scholar]
- Heitmann G. P.; Dahlhoff G.; Hölderich W. F. Modified Beta Zeolites as Catalysts for the Beckmann Rearrangement of Cyclohexanone Oxime. Appl. Catal. A Gen. 1999, 185 (1), 99–108. 10.1016/S0926-860X(99)00126-X. [DOI] [Google Scholar]
- Mihaylova A.; Hadjiivanov K.; Dzwigaj S.; Che M. Remarkable Effect of the Preparation Technique on the State of Cobalt Ions in BEA Zeolites Evidenced by FTIR Spectroscopy of Adsorbed CO and NO, TPR and XRD. J. Phys. Chem. B 2006, 110 (39), 19530–19536. 10.1021/jp0634398. [DOI] [PubMed] [Google Scholar]
- Dzwigaj S.; Massiani P.; Davidson A.; Che M. Role of Silanol Groups in the Incorporation of V in β Zeolite. J. Mol. Catal. A Chem. 2000, 155 (1–2), 169–182. 10.1016/S1381-1169(99)00332-5. [DOI] [Google Scholar]
- Sadowska K.; Wach A.; Olejniczak Z.; Kuśtrowski P.; Datka J. Hierarchic Zeolites: Zeolite ZSM-5 Desilicated with NaOH and NaOH/Tetrabutylamine Hydroxide. Microporous Mesoporous Mater. 2013, 167, 82–88. 10.1016/j.micromeso.2012.03.045. [DOI] [Google Scholar]
- Schüßler F.; Schallmoser S.; Shi H.; Haller G. L.; Ember E.; Lercher J. A. Enhancement of Dehydrogenation and Hydride Transfer by La3+ Cations in Zeolites during Acid Catalyzed Alkane Reactions. ACS Catal. 2014, 4 (6), 1743–1752. 10.1021/cs500200k. [DOI] [Google Scholar]
- Weitkamp J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem. 2012, 4 (3), 292–306. 10.1002/cctc.201100315. [DOI] [Google Scholar]
- Rane N.; Kersbulck M.; Van Santen R. A.; Hensen E. J. M. Cracking of N-Heptane over Brønsted Acid Sites and Lewis Acid Ga Sites in ZSM-5 Zeolite. Microporous mesoporous Mater. 2008, 110 (2–3), 279–291. 10.1016/j.micromeso.2007.06.014. [DOI] [Google Scholar]
- Schuring D.; Koriabkina A. O.; De Jong A. M.; Smit B.; Van Santen R. A. Adsorption and Diffusion of N-Hexane/2-Methylpentane Mixtures in Zeolite Silicalite: Experiments and Modeling. J. Phys. Chem. B 2001, 105 (32), 7690–7698. 10.1021/jp010158l. [DOI] [Google Scholar]













