Abstract
Fifty-three patients with chronic hepatitis B and active viral replication were studied for 4 weeks while on treatment and for 12 weeks after treatment with the oral nucleoside analogue lamivudine. Children aged 2 to 12 years were randomized to receive twice-daily doses of 0.35, 1.5, or 4 mg of lamivudine solution per kg of body weight or once-daily doses of 3 mg of lamivudine solution per kg. Adolescents aged 13 to 17 years received lamivudine at 100 mg (as tablets). Blood samples for pharmacokinetic assay were taken on days 1 and 28. Lamivudine was rapidly absorbed following oral administration, with the maximum concentration in serum being reached 0.5 to 1 h postdosing. Apparent oral clearance (CL/F) was higher in younger children and decreased with age, with CL/F values for adolescents reaching those seen for adults by the age of 12. All doses produced a dramatic fall in serum hepatitis B virus (HBV) DNA levels, with a median reduction of ≥99.5% after 4 weeks of treatment and with the levels returning to the baseline levels posttreatment. The correlation of dose, area under the concentration-time curve (AUC), and changes in HBV DNA levels, as measured by the Chiron Quantiplex assay, showed maximal antiviral effects (99.9% inhibition and a reduction of the amount of HBV DNA of approximately 3 log10) at 3 mg/kg/day, with no discernible increase in effect seen whether the drug was given at 4 mg/kg twice daily or whether it was given once daily or twice daily. The limit of detection of the assay (2.5 pg/ml) was reached for some but not all patients across the dose ranges, with the smallest number (n = 2) of those having values negative by the Chiron Quantiplex assay being in the lowest-dose group. The 13- to 17-year-olds showed a similar overall response in terms of the HBV DNA level reduction compared to that for patients younger than age 13. Analysis of the same samples by PCR, which has a lower limit of sensitivity than the Chiron Quantiplex assay, also showed average drops in HBV DNA levels of about 3 log10 at 4 weeks for patients for which the AUC was ≥4,000 ng · h/ml, confirming the conclusions given above. Lamivudine treatment was well tolerated at all doses, with no significant adverse events or laboratory data changes. On the basis of pharmacokinetic and pharmacodynamic data, a 3-mg/kg/day dose in children (ages 2 to 12 years) with chronic hepatitis B provides levels of exposure and trough concentrations similar to those seen in adults following the receipt of doses of 100 mg. The 100-mg dose is being evaluated in a large phase III study with HBV-infected pediatric patients.
More than 350 million individuals around the world, or approximately 5% of the world's population, have chronic hepatitis B virus (HBV) infection (20). In areas where HBV is endemic, it is most frequently transmitted vertically from the mother to her newborn or between close contacts during early childhood. After acute infection, the virus is eliminated in 90 to 95% of infected adults, while in the remaining 5 to 10% a chronic infection persists (19). The progression to chronic hepatitis after an acute infection is inversely related to age. Infants and young children who acquire HBV infection are most at risk for chronic infection and consequently are at highest risk for eventual cirrhosis and primary hepatocellular carcinoma (15).
Alpha interferon has recently been approved for use in the treatment of chronic hepatitis B in children. However, in children, as in adults, two-thirds to three-quarters of the patients do not respond to treatment, stressing the need for alternative and/or more effective treatments (4, 21, 22). In addition, the need for repeated and prolonged parenteral administration, as well as the numerous side effects of interferon, is an important obstacle to treatment acceptance and compliance.
Lamivudine, (−)-β-2′,3′-dideoxy-3′-thiacytidine (also known as 3TC and GR109714X), is an oral nucleoside analogue that inhibits viral DNA replication and that has in vitro values for inhibiting viral replication by 50% (IC50) and 90% (IC90) in hepatoma cell lines of about 0.02 μM (5 ng/mL) and 0.3 μM (70 ng/ml), respectively (data on file). Studies with adults have shown that 52 weeks of lamivudine treatment resulted in a significantly higher histologic response rate compared to that achieved with placebo (P < 0.001) and a significant reduction in the progression of fibrosis compared to that achieved with placebo (P = 0.01) (12). In two placebo-controlled studies lamivudine was associated with a significantly higher rate of hepatitis B e antigen (HBeAg) seroconversion (loss of HBeAg and production of anti-HBe antibody), sustained HBV DNA suppression, and sustained normalization of alanine aminotransferase (ALT) levels compared to those achieved with placebo (12; J. Dienstag, E. Schiff, T. Wright, R. Perillo, H.-W. Hann, L. Crowther, M. Woessner, M. Rubin, and N. Brown, Gastroenterology 114:A1235, 1998, abstr. L0148). HBeAg seroconversion in lamivudine-treated patients occurred at a rate similar to that in patients receiving alpha interferon and appeared to be durable for at least 2 to 12 months posttreatment (Dienstag et al., abstr. L0148). Lamivudine is approved for use in human immunodeficiency virus (HIV)-infected children and adults, as well as HBV-infected adults in some countries.
Pharmacokinetic studies conducted with HIV- or HBV-infected patients and healthy volunteers have shown that lamivudine is extensively and rapidly absorbed and exhibits linear kinetics. Renal impairment requires dose modification when creatinine clearance (CLCR) falls below 50 ml/min, whereas hepatic impairment and transplantation have no significant impact on the level of lamivudine exposure (6–9, 16, 18, 24, 25). A previous pharmacokinetic study with HIV-infected children (13, 17) showed that lamivudine was similarly well absorbed but that the apparent bioavailability was lower and the clearance was higher relative to those for adults. Consequently, the recommended pediatric dose of lamivudine for treatment of HIV infection is twice the adult dose, in milligram-per-kilogram terms.
The study described here was carried out to evaluate the safety profile, antiviral activity, and pharmacokinetics of lamivudine over 4 weeks in order to establish the most appropriate dose for children with chronic hepatitis B.
MATERIALS AND METHODS
Patients.
Eligible patients included males and females (ages, 2 to 17 years) with detectable hepatitis B surface antigen (HBsAg) in the serum at screening and for 6 months before screening, HBeAg and HBV DNA (determined by the Chiron Quantiplex assay) in the serum at the time of screening, and serum ALT and aspartate aminotransferase (AST) levels below 300 IU/liter. Patients were excluded if they had hepatitis C virus or HIV infection or decompensated liver disease (defined by a serum bilirubin level more than twice the upper limit of the reference range, a serum albumin level less than 32 g/liter, or a history of ascites, variceal hemorrhage, or hepatic encephalopathy). Patients were also excluded if they had received an investigational drug within 30 days before enrollment in the study, any antiviral therapy, biologic modifiers, immunomodulators, or systemic corticosteroids within 6 months of screening.
The study was approved by the ethics committees at the participating centers, and all the patients' guardians or the patients themselves gave written informed consent before enrollment in the study.
Study design.
Patients aged 2 to 12 years were centrally randomized to receive one of the following doses of lamivudine oral solution: 0.35 mg/kg of body weight twice daily (b.d.), 3 mg/kg once daily (o.d.), 1.5 mg/kg b.d., or 4 mg/kg b.d. The 13- to 17-year-olds all received 100-mg lamivudine tablets orally o.d. Patients were instructed to avoid fatty foods and dairy products on the mornings of days 1 and 28. They were permitted a light breakfast but were instructed to refrain totally from food and drink for 1 h before dosing. They were also asked to refrain from fluids for 1 h after dosing and from food for 4 h after dosing. The patients were stratified into the age groups 2 to 6 years (stratum 1), 7 to 12 years (stratum 2), and 13 to 17 years (stratum 3) to ensure the inclusion of adequate numbers of subjects in each dose group within each age group. The aim was to recruit into the study a total of 50 patients from four centers.
Treatment was for 4 weeks, and follow-up was for 12 weeks. After the screening visit, patients returned at the baseline (day 1), days 14 and 28 during treatment, and 4, 8, and 12 weeks after treatment completion.
Drug assay and pharmacokinetic evaluations.
Samples of blood were collected and processed to provide serum for lamivudine assays. Urine was also collected and was analyzed for lamivudine concentrations but was collected only from the individuals in the group receiving the highest dose (4 mg/kg b.d.). A single group was chosen, as collection of urine from small children is notoriously difficult and the 4-mg/kg b.d. group would be the group most likely to yield practically useful results because large enough amounts of drug would be given to quantify the amount excreted. The assay with serum used at Glaxo Wellcome, •••, N.C., was a modification of the published high-pressure liquid chromatography assay (5) and used a gas chromatography system with tandem mass spectrometry detection validated to good clinical practice guidelines. The assay with urine was a straightforward application of the high-pressure liquid chromatography method in which direct injection following solid-phase extraction on Bond-Elut cartridge was used. The lower limits of detection were 2 to 5 ng/ml for serum and 1 μg/ml for urine.
On days 1 and 28 a full pharmacokinetic profile was prepared. Blood samples were taken predosing and at 0.5, 1, 2, 4, 8, and 12 h after dosing. An extra blood sample was taken at 24 h after dosing on day 28 from patients in the 3-mg/Kg o.d. and 100-mg o.d. (tablet) groups only. On day 1 only, urine was collected from the 4-mg/kg b.d. group at the baseline and at 0 to 6 and 6 to 12 h postdosing. On day 14 one blood sample was taken predosing for pharmacokinetic (trough) comparisons.
The maximum drug concentration in serum (Cmax) and the time to Cmax (Tmax) were obtained directly from the concentration-time data. The terminal rate constant for lamivudine (λz) and the corresponding half-life (t1/2) were calculated by linear least-squares regression (using WinNonlin, version 1.0; SCI Software, Cary, N.C.). The number of points included in the terminal phase was decided by visual inspection. Noncompartmental pharmacokinetic analysis was used to calculate parameter estimates. The area under the concentration-time curve (AUC) to time t (AUCt), which was 12 or 24 h for the b.d. and o.d. dosing schedules, respectively, was calculated by using the trapezoidal rule and was extrapolated to infinite time to achieve the AUC at infinity (AUC∞). For day 7, the accumulation ratio (R; where R = AUCτ/AUCt, where t is 24 h and τ is the dosing interval at steady state) was obtained, but AUC∞ was not calculated. The apparent oral clearance (CL/F) was also determined.
The amount of lamivudine recovered in the urine was used to calculate the renal clearance (CLR) on day 1. CLR was calculated as follows: the amount excreted to time t (where t is the last time of complete collection) was divided by the area under the curve of the serum lamivudine concentration over the same time (AUCt).
Clinical evaluations.
Serum was assayed for HBV DNA, HBeAg and anti-HBe antibody, HBsAg and anti-HBs antibody, and ALT levels at each clinic visit. Hematology and biochemistry tests were also performed to determine the safety of the treatment, and the adverse events that had occurred since the previous visit were recorded.
Assays for HBV DNA.
The samples were assayed for HBV DNA at the Public Health Laboratory Service, Heartlands Hospital, Birmingham, United Kingdom. The HBV DNA in serum was quantified by the Quantiplex HBV DNA assay (Chiron Corporation, Emeryville, Calif.) (10), which has a lower limit of detection of 2.5 pg/ml and a ≤25% interassay coefficient of variation. The day 1 and day 28 samples were also assayed by the Roche Amplicor PCR assay (3), which has a lower limit of detection of 400 copies/ml and a 7 to 13% interassay coefficient of variation. HBeAg and anti-HBe antibody were assessed semiquantitatively and qualitatively by the Amerlite Assay (Ortho Clinical Diagnostics, Amersham, United Kingdom). HBsAg was assessed quantitatively by the Amerlite assay (Ortho Clinical Diagnostics), and anti-HBs antibody was assessed quantitatively by the Amerlite Anti-HBs assay (Ortho Clinical Diagnostics).
Statistical analysis.
No statistical comparisons of the efficacy or safety parameters were made in this study because of the small numbers of patients. The modified intent-to-treat population (ITTm) was used for the efficacy analyses and was defined as all patients with confirmed chronic hepatitis B who were randomized. The as-treated population was used for the safety analyses and was defined as all patients for whom no clear evidence of failure to take study medication was available.
The percent change in HBV DNA levels from the baseline levels was calculated. In addition, as the actual HBV DNA levels were of a much higher magnitude than those obtained by previous assays, the data were log transformed and the change from the baseline level was presented, as is common practice for patients with diseases caused by viruses.
Summary statistics of pharmacokinetic parameters were calculated for each treatment: median, maximum, minimum, arithmetic mean, standard deviation, and coefficient of variation for all pharmacokinetic parameters and geometric mean and 95% confidence interval of logarithmically transformed data for Cmax, AUC∞, t1/2, AUCt, R, and CL/F for each dose and age group.
Evidence of dose proportionality (for AUC and Cmax) across the full dose range was investigated by using the power model, which was applied by using PROC MIXED in SAS, version 6.12 (SAS Institute), where log (parameter) = a + [b · log (dose)], where a is the intercept and b is the slope. When assessing dose proportionality, data for all dose groups were included in the analysis. In order to make valid comparisons, the dose for the 100-mg-dose group was normalized by patient weight by dividing the 100-mg dose by each patient's individual weight recorded at screening. A repeat analysis was carried out after removing the data for the low-dose group (0.35-mg/kg b.d.), as the estimates confounded the proportionality assessment.
RESULTS
Study population.
The study was conducted between June 1997 and January 1998. A total of 58 patients were screened for entry into the study, and of these, 53 patients were considered to meet the study criteria and were randomized at the baseline to a study treatment (as-treated population). The ITTm population comprised 52 patients because 1 patient did not have evidence of HBsAg for 6 months before enrollment. These 52 patients were stratified by age, with 17 patients in stratum 1, 23 patients in stratum 2 and 12 patients in stratum 3.
The four treatment groups, which were stratified by age, were well matched with regard to baseline characteristics (Table 1), except that the 4-mg/kg b.d. group had 82% males, whereas the other groups had approximately 50% males. There was a wide range of median HBV DNA levels between the treatment groups at the baseline. Any minor differences in groups were probably due to the small numbers.
TABLE 1.
Baseline characteristics
| Characteristic | Dose group
|
||||
|---|---|---|---|---|---|
| 0.35 mg/kg b.d. | 3 mg/kg o.d. | 1.5 mg/kg b.d. | 4 mg/kg b.d. | 100 mg o.d. | |
| No. of patients | 8 | 11 | 10 | 11 | 12 |
| Mean (SD) age (yr) | 7.8 (4.4) | 6.7 (2.2) | 6.7 (3.2) | 7.6 (3.4) | 14.6 (1.4) |
| No. (%) of males | 4 (50) | 5 (45) | 5 (50) | 9 (82%) | 7 (58) |
| Ethnicity (no. [%] of subjects) | |||||
| Asian | 4 (50) | 5 (45) | 7 (70) | 6 (55) | 8 (67) |
| Negroid (black) | 0 | 1 (9) | 0 | 1 (9) | 0 |
| Caucasian (white) | 4 (50) | 5 (45) | 3 (30) | 4 (36) | 4 (33) |
| Median (range) baseline HBV DNA level (pg/ml) | 3,900 (2,000–20,000) | 6,300 (710–20,000) | 9,800 (800–20,000) | 9,000 (110–20,000) | 4,300 (480–17,000) |
| ALT (×ULN) | |||||
| Median | 1.1 | 2.2 | 1.4 | 1.0 | 1.3 |
| Range | 0.2–2.3 | 0.3–12.5 | 0.2–5.9 | 0.3–4.6 | 0.3–3.8 |
Pharmacokinetics.
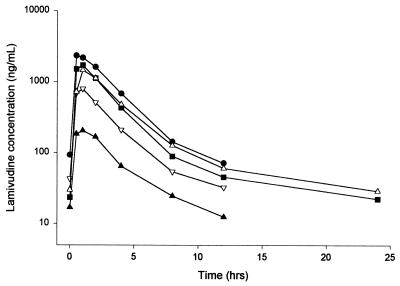
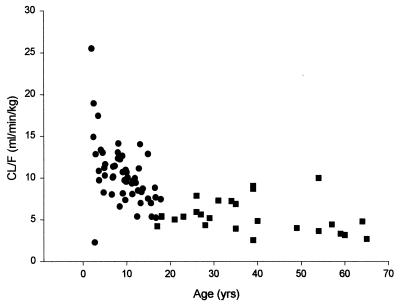
All doses of lamivudine, whether given by solution or tablet, showed rapid absorption, with tmax values being, on average, 0.5 to 1 h (Fig. 1). Steady-state parameter estimates showed a small degree of accumulation (AUC and Cmax values on day 28 were generally a little higher than those on day 1). The data were generally consistent with profiles seen in adults, although CL/F is increased in pediatric patients relative to that in adults. This function between lamivudine CL/F and age can be seen in Fig. 2. Pharmacokinetic parameter estimates for the different dose and age groups are shown in Tables 2 and 3.
FIG. 1.
Steady-state median serum lamivudine concentration-time profiles following the administration of different oral doses of lamivudine. Lamivudine was given as a solution to provide doses of 0.35 mg/kg b.d. (▴), 1.5 mg/kg b.d. (▿), 3 mg/kg o.d. (■), and 4 mg/kg b.d. (●) and as a 100-mg tablet (▵). Note that sampling was continued to 24 h for the o.d. dose cohorts only.
FIG. 2.
Relationship between lamivudine clearance and age. CL/F values are for adults (■) and pediatric or adolescent (●) patients.
TABLE 2.
Lamivudine pharmacokinetic parameters by dose groupa
| Parameter | Day | Dose group
|
||||
|---|---|---|---|---|---|---|
| 0.35 mg/kg b.d. (n = 8) | 1.5 mg/kg b.d. (n = 11) | 3 mg/kg o.d. (n = 10) | 4 mg/kg b.d. (n = 11) | 100-mg tablet (n = 12) | ||
| Tmax (h)b | 1 | 0.51 (0.51–1.02) | 0.51 (0.51–2.03) | 0.51 (0.51–2.03) | 0.51 (0.51–4.07) | 1.02 (0.51–4.07) |
| 28 | 1.02 (0.51–2.03) | 1.02 (0.51–2.03) | 0.51 (0.51–2.03) | 1.02 (0.51–1.02) | 1.02 (0.51–2.03) | |
| Cmax (ng/ml) | 1 | 257 (192–345) | 971 (753–1,253) | 1,573 (1,173–2,110) | 2,177 (1,862–2546) | 1,392 (1,073–1,804) |
| 28 | 286 (166–492) | 798 (637–999) | 1,855 (1,440–2,390) | 2,760 (2,338–3,259) | 1,496 (1,233–1,816) | |
| AUCt (ng · h/ml) | 1 | 660 (491–886) | 2,296 (1,969–2,678) | 4,0577 (3,247–5,068) | 6,399 (5,788–7,075) | 4,504 (3,951–5,133) |
| 28 | 857 (708–1,039) | 2,616 (2,217–3,085) | 5,784 (4,899–6,829) | 7,562 (6,062–9,432) | 5,414 (4,656–6,297) | |
| AUC∞ (ng · h/ml) | 1 only | 675 (503–905) | 2,353 (2,019–2,743) | 4,160 (3,343–5,177) | 6,540 (5,911–7,236) | 4,639 (4,072–5,286) |
| t1/2 (h) | 1 | 2.09 (1.92–2.27) | 2.16 (1.95–2.39) | 2.14 (1.78–2.58) | 1.95 (1.75–2.17) | 2.70 (2.03–2.39) |
| 28c | 3.40 (2.87–4.03) | 2.985 (2.12–4.11) | 7.56 (5.13–11.15) | 2.52 (2.14–2.97) | 6.45 (4.75–8.77) | |
| Cmin (trough) (ng/ml)d | 28 | 13 (9–36) | 33 (24–58) | 24 (13–73) | 72 (59–203) | 29 (26–37) |
| CLR (ml/min) | 1 | NCe | NC | NC | 256 (205–318) | NC |
| Rb | 28 | 1.32 (0.87–1.91) | 1.17 (0.81–1.57) | 1.19 (0.94–3.50) | 1.19 (0.78–1.79) | 1.11 (0.67–1.63) |
Values are geometric means (95% confidence intervals) unless indicated otherwise.
Tmax and R are shown as median (minimum value/maximum value).
Day 28 values for t are greater when sampling is to 24 h, i.e., in the o.d. regimen groups.
Cmin, minimum concentration in serum. Values are medians (ranges).
NC, not calculated.
TABLE 3.
Steady-state (day 28) lamivudine dose-normalized pharmacokinetic parameters versus agea
| Parameter | Age group (yr)
|
||
|---|---|---|---|
| 2–6 (n = 18) | 7–12 (n = 23) | 13–17 (n = 12) | |
| Tmax (h)b | 0.51 (0.51–2.03) | 0.51 (0.51–4.07) | 1.02 (0.51–4.07) |
| Cmax (ng/ml) | 1,215 (791–1866) | 1,138 (763–1,699) | 1,496 (1,233–1,816) |
| AUCτ (ng · h/ml) | 3,474 (2,365–5,103) | 3,622 (2,486–5,278) | 5,414 (4,656–6,297) |
| t1/2 (h) | 3.92 (2.80–5.49) | 3.49 (2.75–4.44) | 6.45 (4.25–8.77) |
| CL/F (ml/min/kg) | 9.83 (8.53–11.33) | 7.78 (7.00–8.66) | 6.64 (5.50–8.02) |
Values are geometric means (95% confidence intervals) unless indicated otherwise.
Values are medians (ranges).
AUCt on day 1 generally showed proportionality between the dose levels, although the results of the statistical analysis for dose proportionality showed some inconsistency depending on the approach. With all data included, dose proportionality could not be concluded; however, removal of the data for the 0.35-mg/kg b.d. group resulted in dose proportionality between the other dose levels by either statistical method. The results of the power model analysis are shown in Table 4.
TABLE 4.
Dose proportionality power model results (with and without data for 0.35-mg/kg dose group)
| Parameter | Day | Adjusted mean slope | 90% CIa
|
|
|---|---|---|---|---|
| Lower | Upper | |||
| Including data for 0.35-mg/kg dose group | ||||
| AUC∞ (ng · h/ml) | 1 | 0.902 | 0.822 | 0.982 |
| AUC24 (ng · h/ml)b | 28 | 0.894 | 0.807 | 0.981 |
| Cmax (ng/ml) | 1 | 0.839 | 0.718 | 0.960 |
| 28 | 0.916 | 0.791 | 1.041 | |
| Removing data for 0.35-mg/kg dose group | ||||
| AUC∞ (ng · h/mL) | 1 | 0.907 | 0.737 | 1.077 |
| AUC24 (ng · h/mL)b | 28 | 0.967 | 0.801 | 1.132 |
| Cmax (ng/ml) | 1 | 0.696 | 0.432 | 0.960 |
| 28 | 1.115 | 0.873 | 1.358 | |
CI, confidence interval.
For the 0.35-mg/kg b.d., 1.5-mg/kg b.d., and 4-mgkg b.d. treatment groups, AUC24 was calculated as AUClast multiplied by 2.
For the adolescent group (ages 13 to 17 years), pharmacokinetic parameter estimates were in line with those seen in previous studies with adults. A Cmax of 1,400 to 1,500 ng/ml was reached within an hour of dosing. The geometric mean steady-state AUCt was 5,414 ng · h/ml, again with a small degree of accumulation (R = 1.1) from the first dose.
Estimates of t1/2 were generally on the order of 2 to 3 h for all children and adolescent patients when sampling was carried out to 12 h postdosing, although when examined by age, t1/2 estimates were generally shorter in children (ages 2 to 12 years). The mean R ranged from 1.2 to 1.3 for b.d. dosing across the doses. The extended blood sampling to 24 h on day 28 in the two o.d. dose groups showed that the elimination t1/2 values were on the order of 6 to 8 h. This value for t1/2 determines the observed level of accumulation.
CL/F estimates, corrected for weight, were generally similar across the dose groups on day 1, with similar values seen at steady state. Values for CL/F on day 1 were 11.87, 9.58, and 7.75 ml/min/kg for the 2- to 6-year-old, 7- to 12-year-old, and 13- to 17-year-old patients, respectively, with similar values detected at day 28.
Trough values for all patients were about the same between day 14 and day 28, indicating that steady state had been achieved. There were also detectable lamivudine concentrations in all samples. The median trough value for patients in the 3-mg/kg o.d. group was 24 ng/ml (range, 13 to 73 ng/ml).
CLR, which was calculated only for the 4-mg/kg b.d. dose group on day 1, was 255 ml/min (95% confidence interval, 205 to 318 ml/min), with 44 to 72% of the administered drug recovered in urine over 12 h.
HBV DNA levels.
By week 2 all treatments showed considerable antiviral activity, and there was some differentiation of activity by dose, as indicated by rapid falls in median HBV DNA levels. Median percent reductions in HBV DNA levels of at least 99.1 and 99.5% were seen at week 4 (Fig. 3). At week 4, the number of patients that became HBV DNA negative varied across the treatment groups, ranging from 17% (2 of 12) in the 100-mg group to 64% (7 of 11) in the 4-mg/kg b.d. group. By week 8 (4 weeks posttreatment) all patients had again become HBV DNA positive.
FIG. 3.
Mean change from baseline in log10 HBV DNA level (in picograms per milliliter) obtained by the Chiron Quantiplex assay for the ITTm population. Lamivudine was given as a solution to provide doses of 0.35 mg/kg b.d. (▴), 1.5 mg/kg b.d. (▿), 3 mg/kg o.d. (■), and 4 mg/kg b.d. (●) and as a 100-mg tablet (▵).
The 0.35-mg/kg b.d. groups and the 100-mg group showed mean reductions of 2.6 and 2.3 log10, respectively, at week 4. The other dose groups (1.5 mg/kg b.d., 3 mg/kg o.d., and 4 mg/kg b.d.) showed mean reductions of approximately 3 log10 at week 4. Formal statistical evaluation of the pharmacokinetic and pharmacodynamic responses did not yield significant findings, as all doses produced significant and almost equally maximal effects; however, the lowest dose definitely had the smallest effect, with doses of 3 mg/kg/day and greater having equal effects. Quantitative PCR (Roche Amplicor) analysis of the day 1 and day 28 samples gave results similar to those obtained by the Chiron Quantiplex assay (Table 5). Although individual samples gave slightly different viral load measurements (possibly as a result of the freezing-thawing of samples for reanalysis) than that seen by the Chiron Quantiplex assay, the overall magnitudes of the response and the differentiation of activity by dose were very similar.
TABLE 5.
Baseline HBV DNA level and mean change from baseline log10 HBV DNA level determined by PCRa for the ITTm population
| Characteristic | Dose group
|
||||
|---|---|---|---|---|---|
| 0.35 mg/kg b.d. | 3 mg/kg o.d. | 1.5 mg/kg b.d. | 4 mg/kg b.d. | 100 mg o.d. | |
| No. of patients | 8 | 11 | 10 | 11 | 12 |
| HDV DNA level (log10)b | |||||
| Baseline | 8.4 (0.7) | 8.4 (0.5) | 8.6 (0.6) | 8.3 (0.9) | 8.2 (0.7) |
| Wk 4 | −2.6 (0.3) | −2.7 (0.5) | −3.3 (0.4) | −2.8 (0.5) | −2.1 (0.6) |
The Roche Amplicor PCR was used to determine HBV DNA levels.
Values are means (standard deviations).
In the 29 patients with elevated ALT levels at the baseline, no substantial effect on ALT level normalization was seen during the 4-week treatment period, although for two patients in the 1.5-mg/kg b.d. group and one patient in each of the 0.35-mg/kg b.d. and the 100-mg o.d. groups ALT levels were reduced to less than the upper limit of the reference range. There was no adverse effect of stopping lamivudine after 4 weeks, although patients whose ALT levels had normalized returned to the baseline levels during follow-up.
One patient lost HBeAg at week 12, while no patients gained anti-HBe antibody. No patients lost HBsAg or gained anti-HBs antibody.
Pharmacokinetics and pharmacodynamics.
The relationship between dose, systemic exposure, and antiviral response, assessed as the log10 change in HBV DNA levels measured by the Chiron Quantiplex assay, is shown in Fig. 4. Although some of the samples became negative for HBV DNA by the Chiron Quantiplex assay, outstretching the limits of the assay, this response was also evaluated by a PCR assay, by which a similar functional relationship between dose and response could be seen (Fig. 5). In both Fig. 4 and Fig. 5 it is apparent that beyond a systemic exposure (AUC) of about 4,000 ng · h/ml, assuming that trough levels (i.e., the concentration just before the next dose) are well above the IC50 (4 to 7 ng/ml), there is no appreciable difference in response.
FIG. 4.
Pharmacokinetics and pharmacodynamics of lamivudine in pediatric patients (ages 2 to 12 years) as steady-state exposure versus HBV DNA suppression (as determined by the Chiron Quantiplex assay). All pediatric data are by dose group, with group means and standard deviations given. Lamivudine was given as a solution to provide doses of 0.35 mg/kg b.d. (▴), 1.5 mg/kg b.d. (▿), 3 mg/kg o.d. (■), and 4 mg/kg b.d. (○).
FIG. 5.
Pharmacokinetics and pharmacodynamics of lamivudine as steady-state exposure versus HBV DNA suppression (as determined by the Roche PCR assay). All data are by dose group. Lamivudine was given as a solution to provide doses of 0.35 mg/kg b.d. (▴), 1.5 mg/kg b.d. (▿), 3 mg/kg o.d. (■), and 4 mg/kg b.d. (○). A 100-mg dose (tablet) was given to adolescents (■). The line of best fit (y = 0.13lnx + 1.5). is also shown.
Safety.
The number of patients experiencing adverse events was similar across all five treatment groups (36 of 53 [68%] overall). The 2- to 6-year-olds receiving 4 mg/kg b.d. all had at least one adverse event. The percentage of patients experiencing adverse events categorized as drug related was much lower, with an overall incidence of 21% (11 of 53). No patient in the 100-mg group (13- to 17-year-olds) had an adverse event considered by the investigators to be drug related.
The most common adverse events were ear, nose, and throat symptoms (22 of 53; 42%), gastrointestinal symptoms (18 of 53; 34%), non-site-specific adverse events (20 of 53 [38%], 15 of which were malaise and fatigue), and lower respiratory symptoms (12 of 53; 23%). Table 6 shows the adverse events which were seen in at least 15% of the patients.
TABLE 6.
Most common adverse events (experienced by more than 15% of patients) in the as-treated population
| Characteristic | Dose group
|
||||
|---|---|---|---|---|---|
| 0.35 mg/kg b.d. | 3 mg/kg o.d. | 1.5 mg/kg b.d. | 4 mg/kg b.d. | 100 mg o.d. | |
| No. of patients | 7 | 11 | 12 | 11 | 12 |
| No. (%) of patients with the following adverse events: | |||||
| Malaise and fatigue | 2 (29) | 3 (27) | 4 (33) | 3 (27) | 3 (25) |
| ENTa infections | 2 (29) | 1 (9) | 4 (33) | 0 | 2 (17) |
| Cough | 0 | 2 (18) | 2 (17) | 3 (27) | 1 (8) |
| Headache | 1 (14) | 0 | 3 (25) | 0 | 2 (17) |
| Temp regulation disturbance | 0 | 2 (18) | 1 (8) | 3 (27) | 0 |
| Nausea and vomiting | 0 | 0 | 3 (25) | 1 (9) | 1 (8) |
| Viral ENT infections | 1 (14) | 0 | 1 (8) | 2 (18) | 1 (8) |
| Ear signs and symptoms | 0 | 0 | 3 (25) | 1 (9) | 0 |
| Pharyngitis | 1 (14) | 0 | 0 | 2 (18) | 0 |
| Gastroenteritis | 1 (14) | 0 | 2 (17) | 0 | 0 |
A comparison of the incidence of laboratory abnormalities across treatment groups did not indicate any unexpected abnormalities. There were no discernible effects on a selection of predefined hepatic function tests (ALT and bilirubin levels), clinical chemistry (albumin, amylase, creatinine phosphokinase, or lipase levels), or hematology (total numbers of white blood cells, neutrophils, or platelets or hemoglobin concentration) parameters. Only two patients had serious adverse events in the study, and both of these occurred during the follow-up period and were not considered by the investigator to be related to the study medication. Lamivudine was well tolerated by all treatment groups and individuals across all age ranges, with no patients withdrawing from the study either while on treatment or during follow-up.
DISCUSSION
Lamivudine is a well-established part of combination therapy for HIV (as Epivir) and has been extensively studied in adults with HBV infection. This study has shown that similar antiviral efficacy can be seen in pediatric and adolescent patients and that modification of the daily adult dose is required, as for the treatment of HIV infection, to achieve similar levels of systemic exposure in these patients.
All doses of lamivudine, whether given by solution or tablet, showed rapid absorption, with most pharmacokinetic parameter values generally in line with those from previous studies with either adults, adolescents, or children. When broken out by age (Table 3) most of the pharmacokinetic parameters are broadly similar; the exception is CL/F. A small difference in exposure (AUC) between the pediatric population in this and a previous study with HIV-infected children who received the same dose level (13) was seen: Lewis et al. (13) found values of 4,457 ng · h/ml (AUC∞ from the first dose) following the administration of 4-mg/kg b.d. doses. In the present study a comparable dose provided values of 6,540 ng · h/ml. The AUC values in the study of Lewis et al. (13) are predicted from data obtained after administration of a single dose, which probably underestimates the steady-state AUC by about 30%, as their estimate of t1/2, 1.85 h, is truncated by more limited sampling (see below in Discussion). The AUC values found in the present study are still slightly higher than expected, probably reflecting the difference in disease state and potential effects on absorption, since the HIV-infected children in the study of Lewis et al. (13) had advanced HIV disease and may have absorbed less lamivudine.
The extent of exposure (AUC) was generally proportional to the dose, as seen in previous studies with both adults and (HIV-infected) children.
CL/F estimates were similar to those seen in previous studies (9) and, when stratified by age, demonstrate the same relationship as that seen in HIV-infected patients. CL/F values are at a peak at 2 years of age and decline with age to about 12 years of age, when the values are similar to those seen in adults. This is mirrored by a slightly shorter t1/2 (Table 3), is independent of disease (HBV or HIV infection), and is consistent with the ratio of organ clearance function and body size, which decreases with age (i.e., organ function increases less rapidly than body size as the child matures) (11). The increase in CL/F observed in children is consistent with these changes and has been observed with many compounds with pharmacokinetic profiles similar to those of lamivudine (2, 23).
The majority of renally cleared lamivudine (44 to 72%) was excreted within 12 h, although a small underestimation of CLR is possible, as lamivudine was present in the final collection. CLR values, although they displayed a high degree of variability, were similar to those seen in previous studies in which urine was also analyzed for the primary metabolite, lamivudine sulfoxide (8). This metabolite is present in small quantities (5 to 10%) in urine from adult patients with healthy renal function, but the quantities increase in the urine of patients with significant renal dysfunction (7). Some of the unaccounted for drug material in this study is likely to be present in urine as the sulfoxide metabolite, but this was not ascertained.
In HBV-infected adults, 100 mg of lamivudine (i.e., about 1.4 mg/kg, based on an average 70-kg adult) provides an average AUC at steady state of 4,400 ng · h/ml, which correlates with maximal viral suppression (9). In this study estimates of AUC for the 3-mg/kg/day dose groups were comparable to those for adults. This demonstrates that, as seen in HIV-infected patients, the adult dose (in milligram-per-kilogram terms) should be doubled for children to achieve exposures comparable to those achieved in adults.
A small degree of accumulation (R, ∼ 1.2) caused steady-state values to be slightly higher than those on day 1 for AUC and Cmax. This degree of accumulation is expected on the basis of t1/2 values of 6 to 8 h, which was seen in the o.d. dose groups when sampling was continued to 24 h postdosing. The shorter t1/2 of about 2 h was seen in all groups when sampling was to 12 h postdosing and reflects an underestimate because of the use of points likely to be in the distribution phase to establish the terminal slope. This is consistent with previous data, in which sampling to 12 h generally yielded t1/2 estimates of 2 h for children (13) and 3 to 4 h for adults (19).
Trough values at steady-state, assessed at day 14 and day 28, showed that for all doses detectable lamivudine concentrations were present in serum. This means that trough concentrations can be maintained at the required level, i.e., above 4 to 7 ng/ml (equivalent to the in vitro IC50) with o.d. dosing. The long t1/2 of the triphosphate moiety established in vitro (17 to 19 hours [9]) contributes to the duration of the effect and the lack of an observed difference between o.d. and b.d. dosing intervals.
Lamivudine treatment results in a rapid decline in HBV DNA levels in children at all doses studied. The lowest dose used in this study was 0.35 mg/kg b.d., which was by practical necessity when using a 5-mg/ml solution. This equates in exposure (following the doubling of a dose to account for the increased clearance in children) to a 20- to 25-mg dose for adults. At this dose over 90% suppression is seen in adults, and therefore, large differences in response between the doses in this study were not expected or seen. However, plotting of exposure (AUC) against log reduction from the baseline HBV DNA level (Fig. 4) revealed a slightly lower response (∼2.4 log10) in the 0.35-mg/kg b.d. group, whereas the effects were equally maximal (∼3 log10) in the 1.5- and or 4-mg/kg b.d. groups. At the lowest measured AUC only a 1-log reduction was seen. Once AUC values are 4,000 ng · h/ml or more, i.e., those achieved with doses of 3 mg/kg/day or more, little difference in viral response is observed by the Chiron Quantiplex assay. However, the lower limit of detection (2.5 pg/ml) of the Chiron Quantiplex assay was reached for some of the patients, with the lowest number (n = 2) becoming undetectable by the Chiron Quantiplex assay for the 0.35-mg/kg group, i.e., the lowest-dose group. Consequently, confirmation of the lack of differentiation of activity by dose and the overall magnitude of the drop in log10 achievable at 4 weeks by Roche Amplicor PCR analysis of the same samples is fundamental in understanding the dose-response.
The 100-mg o.d. group (the 13- to 17-year-olds) did not appear to respond as well as the younger groups in this study, with a median drop of 2.1 log10 (range, 1.6 to 3.6 log10). This magnitude of drop has, however, been seen in studies with adults, in whom changes of 2 to 3 log10 at 4 weeks are commonplace (12). The reasons for the difference between the groups in this study are unknown but could likely be due to the variability caused by the small numbers of patients in the study. If it is a true difference, then physiological factors such as hormonal change and immunological status may have had some influence. Compliance may also have been an issue in the group of adolescent patients in the present study, as the younger children were more likely to have been supervised while taking their medication. However, no firm evidence, such as from the tablet count, of this was seen. Initial (baseline) HBV DNA levels were also slightly lower in the adolescent group, which would mean that they had the potential for only a 2 to 2.5 log10 reduction before the limit of detection of the assay was reached.
The effect of lamivudine has not previously been studied in children with HBV infection, so it has not been established whether HBV DNA suppression will correlate to liver histology improvements in the long term. However, studies with adults (12) have shown an association between HBV DNA suppression and improvements in liver histology. Histological abnormalities are usually less severe in children than in adults, probably because of the shorter duration of the disease, the fact that the children may be in an immunotolerant phase, and the absence of superimposed causes of liver damage such as alcohol consumption. On the basis of studies with adults it can be expected that lamivudine will prevent histological progression in children with chronic hepatitis B, active viral replication, and chronic cytolysis. Rapid increases in HBV DNA levels to the baseline levels were seen after the cessation of treatment; this was expected on the basis of results from short-term treatment studies with adults. This increase in HBV DNA levels was not associated with a rise in serum ALT levels with the short, 4-week treatment period, which is also not sufficient to have any effect on HBeAg or HBsAg levels or on seroconversion. It is probable that a longer treatment period would have an impact on HBeAg loss and seroconversion, as seen in adults (12; Dienstag et al., abstr. L0148). Longer-term studies with lamivudine in this age group will also need to address whether rises in ALT levels are seen after the cessation of long-term treatment. A higher proportion of lamivudine-treated adult patients than placebo-treated adult patients have ALT elevations after the cessation of treatment, but this has not been associated with an increased risk of decompensation (N. Leung, J. Deinstag, E. Schiff, M. Sullivan, M. Atkins, R. Grice, M. Woessner, N. Brown, and C. M. Hunt, Hepatology 28:587A, 1998, abstract 1698). Isolated reports of posttreatment flares have, however, been reported (P. Honkoop, R. A. de Man, R. Heijtink, and S. W. Schalm, Letter, Lancet, 346:1156–1157, 1995) but not usually in patients who have seroconverted. In addition, future studies will need to investigate the development of YMDD variant virus in this age group. Among adults who have been studied to date, after 52 weeks of treatment 16 to 32% developed YMDD mutant HBV, which was usually first detected after week 36 M. Atkins, C. M. Hunt, and N. Brown, Hepatology 28:319A, 1998, abstr. 625). These patients nonetheless exhibited continued suppression of HBV DNA (median decrease, 80%) and significantly improved sustained ALT normalization and histologic responses compared to those for the placebo-treated controls.
Lamivudine has previously been shown to be well tolerated by adults, and this study has also shown that a short-term course of lamivudine is well tolerated by children and adolescents infected with HBV. A daily dose of 3 mg/kg is suitable for pediatric patients aged 2 to 12 years.
ACKNOWLEDGMENTS
This study was supported by Glaxo Wellcome Research and Development.
We thank J. Avolio, Hospital for Sick Children, Toronto, Ontario, Canada; A. Bourgeois, Cliniques Universitaires Saint-Luc; P. D'Silva, King's College Hospital; N. McGee, Hospital for Sick Children, Toronto, Ontario, Canada; N. Molam, Birmingham Children's Hospital; E. Mullen, King's College Hospital; J. Sira, Birmingham Children's Hospital; P. Wallmaqc, Cliniques Universitaires Saint-Luc; and J. Workman, Heartlands Hospital, Birmingham, United Kingdom.
REFERENCES
- 1.Angel J B, Hussey E K, Hall S T, Donn K H, Morris D M, McCormack J P, Montaner J S G, Ruedy J. Pharmacokinetics of 3TC (GR109714X) administered with and without food to HIV-infected patients. Drug Invest. 1993;6:70–74. [Google Scholar]
- 2.Butler D R, Kuhn R J, Chandler M H H. Pharmacokinetics of anti-infective agents in paediatric patients. Clin Pharmacokinet. 1994;26:374–395. doi: 10.2165/00003088-199426050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gerken G, Gomes J, Lampertico P, Colombo M, Rothaar T, Trippler M, Colucci G. Clinical evaluation and applications of the Amplicor HBV Monitor test, a quantitative HBV DNA PCR assay. J Virol Methods. 1998;74:155–165. doi: 10.1016/s0166-0934(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 4.Gregorio G V, Jara P, Hierro L, Diaz C, de la Vega A, Vegnente A, Iorio R, Bortolotti F, Crivellaro C, Zancan L, Daniels H, Portmann B, Miele-Vergani G. Lymphoblastoid interferon alfa with or without steroid pre-treatment in children with chronic hepatitis B: a multicenter controlled trial. Hepatology. 1996;23:700–707. doi: 10.1002/hep.510230407. [DOI] [PubMed] [Google Scholar]
- 5.Harker A J, Evans G L, Hawley A E, Morris D M. High-performance liquid chromatographic assay for 2′-dideoxy-3′ thiacytidine in human serum. J Chromatogr B Biomed Appl (Netherlands) 1994;657:227–232. doi: 10.1016/0378-4347(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 6.Heald A E, Hsyu P H, Yuen G J, Robinson P, Mydlow P K, Bartlett J A. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob Agents Chemother. 1996;40:1514–1519. doi: 10.1128/aac.40.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson M A, Horak J, Breuel P B. The pharmacokinetics of lamivudine in patients with impaired hepatic function. Eur J Clin Pharmacol. 1998;54:363–366. doi: 10.1007/s002280050476. [DOI] [PubMed] [Google Scholar]
- 8.Johnson M A, Verpooten G A, Daniel M J, Plumb R, Moss J, van Caesbroeck D, de Broe M E. Single-dose pharmacokinetics of lamivudine in subjects with impaired renal function and effect of hemodialysis. Br J Clin Pharmacol. 1998;46:21–27. doi: 10.1046/j.1365-2125.1998.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson M A, Moore K M, Yuen G, Bye A. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66. doi: 10.2165/00003088-199936010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kapke G F, Watson G, Sheffler S, Hunt D, Frederick C. Comparison of the Chiron Quantiplex branched DNA (bDNA) assay and the Abbott Genostics solution hybridisation assay for quantification of hepatitis B viral DNA. J Viral Hepatitis. 1997;4:67–75. doi: 10.1046/j.1365-2893.1997.00127.x. [DOI] [PubMed] [Google Scholar]
- 11.Kearns G L, Reed M D. Clinical pharmacokinetics in infants and children. Clin Pharmacokinet. 1989;17(Suppl. 1):29–67. doi: 10.2165/00003088-198900171-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lai C L, Chien R N, Leung N, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray D F. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 13.Lewis L L, Venzon D, Church J, Farley M, Wheeler S, Keller A, Rubin M, Yuen G, Mueller S, Sloas M, Wood L, Balis F, Shearer G M, Brouwers P, Goldsmith J, Pizzo P A. Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. J Infect Dis. 1996;174:16–25. doi: 10.1093/infdis/174.1.16. [DOI] [PubMed] [Google Scholar]
- 14.Maynard J E. Hepatitis B: global importance and need for control. Vaccine. 1990;8(Suppl.):S18–S20. doi: 10.1016/0264-410x(90)90209-5. [DOI] [PubMed] [Google Scholar]
- 15.McMahon B J, Alward W L, Hall D B, Heyward W L, Bender T R, Francis D P, Maynard J E. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 16.Moore K H, Yuen G J, Raasch R H, Eron J J, Martin D, Mydlow P K, Hussey E K. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin Pharmacol Ther. 1996;59:550–558. doi: 10.1016/S0009-9236(96)90183-6. [DOI] [PubMed] [Google Scholar]
- 17.Mueller B U, Lewis L L, Yuen G J, Farley M, Keller A, Church J A, Goldsmith J C, Venzon D J, Rubin M, Pizzo P A, Balis F M. Serum and cerebrospinal fluid pharmacokinetics of intravenous and oral lamivudine in human immunodeficiency virus infected children. Antimicrob Agents Chemother. 1998;42:3187–3192. doi: 10.1128/aac.42.12.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluda J M, Cooley T P, Montaner J S G, Shay L E, Reinhalter N E, Wartham S N, Ruedy J, Hirst H M, Vicary C A, Quinn J B, Yuen G J, Wainberg M A, Rubin M, Yachoan R. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J Infect Dis. 1995;171:1438–1447. doi: 10.1093/infdis/171.6.1438. [DOI] [PubMed] [Google Scholar]
- 19.Seef L B, Koff R S. Evolving concepts of hepatitis B virus infection. Semin Liver Dis. 1986;6:11–22. doi: 10.1055/s-2008-1040788. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro C N. Epidemiology of hepatitis B. Pediatr Infect Dis J. 1993;12:433–437. doi: 10.1097/00006454-199305000-00036. [DOI] [PubMed] [Google Scholar]
- 21.Sokal E M, Wirth S, Goyens P, Depreterre A, Cornu C. Interferon alfa 2b therapy in children with chronic hepatitis B. Gut. 1993;34(Suppl. 2):S87–S90. doi: 10.1136/gut.34.2_suppl.s87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokal E M, Conjeevaram H S, Roberts E A, Alvarez F, Bern E M, Goyens P, Rosenthal P, Lachaux A, Shelton M, Sarles J. Interferon alpha therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114:988–995. doi: 10.1016/s0016-5085(98)70318-x. [DOI] [PubMed] [Google Scholar]
- 23.Stoeckel K, Tam Y K, Kneer J. Pharmacokinetics of oral cefetamet pivoxil (Ro 15-8075) and intravenous cefetamet (Ro 15-8074) in humans: a review. Curr Med Res Opin. 1989;11:432–441. doi: 10.1185/03007998909115930. [DOI] [PubMed] [Google Scholar]
- 24.van Leeuwen R, Lange J M A, Hussey E K, Donn K H, Hall S T, Harker A J, Jonker P, Danner S V. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS. 1992;6:1417–1425. doi: 10.1097/00002030-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Yuen G J, Morris D M, Mydlow P K, Haider S, Hall S T, Hussey E K. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35:1174–1180. doi: 10.1002/j.1552-4604.1995.tb04043.x. [DOI] [PubMed] [Google Scholar]