Abstract
Two new families of glycerol-based dendrimers (glyceroladendrimers (GADs) and glyceroclickdendrimers (GCDs)) have been synthesized. Three generations have been isolated for each family with good yields and were fully analyzed. The encapsulation of essential oils (citronella and cinnamon) in GADs, GCDs, and also in previously described glycerodendrimers GD-PAMAMs and GD-PPIs has been studied by dynamic-headspace gas chromatography coupled to mass spectrometry. The retention rates obtained were from −35.8 to 26.65% for citronella essential oil and from 2.14 to 38.84% for the cinnamon essential oil. In addition, the best results were obtained with GD-PAMAMs and GD-PPIs of higher generation. The interaction study between essential oils or more precisely their major components have been performed through NMR spectroscopy (1H NMR and DOSY NMR). No direct interactions between dendrimers and essential oils have been observed, but a surprising behavior of compression of the dendrimer in stable emulsions was observed. Indeed, the hydrodynamic radius of GD-PPI-3 has been reduced in the presence of cinnamon essential oil.
1. Introduction
Dendrimers are macromolecules synthesized from monomers in a three-dimensional structure following divergent or convergent approaches.1−3 Due to their multivalent and monodisperse character, dendrimers have stimulated wide interest in the field of chemistry, physics, and biology.4 Some recent applications have been reported in the literature especially in the fields of drug delivery,5−7 photodynamic therapy,8 electronics,9 catalysis,10−12 and water purification.13
In the past few years, some glycerol-based dendrimers derived from polypropylenimine (PPI) and polyamidoamine (PAMAM) dendrimers were developed and used in catalysis and encapsulation.14−17 Dendritic ionic entities were also performed to encapsulate metallic species.18
Glycerol is a very know by-product of the biodiesel industry.19 As this compound is expensive to purify for food applications, its valorization toward value-added products was largely developed for around 20 years through essentially catalytic processes.20−25
Next, concerning the use of essential oils (EOs), in a global context of the willingness of a return to green and clean agriculture to feed the population growing very quickly, the research about the creation of an herbicide containing essential oil is in full swing.26 Cinnamon and citronella EOs are part of these natural products. Concerning the citronella oil, therapeutic27 and pesticide applications have been reported in the literature.28 Similar properties have been also revealed for cinnamon oil.29
However, EOs are very volatile products that is why they need to be retained and protected to allow an effective use of their biological properties. Many matrices can be used to encapsulate these EOs in order to preserve their applicative potential.30,31 Some are solid, others are liquid, and their release can be very quick or very slow. For the creation of a pesticide, a liquid product with a slow release seems to be a good choice.
In this work, three or four generations of four families of glycerol-based dendrimers have been screened as potential encapsulation matrices of cinnamon and citronella EOs. Among these families, two are new and their synthesis will be described. The encapsulation capacities have been demonstrated through the technique of headspace chromatography coupled to mass spectroscopy.32 Moreover, NMR (1D, 2D, and DOSY33) investigations were realized to understand potential interactions between major compounds of cinnamon oils and dendrimer moieties.
2. Results and Discussion
2.1. Dendrimers Synthesis
Four families of glycerol-based dendrimers have been employed for our study: first of all, the glycerodendrimers (GD)-PPIs and GD-PAMAMs, then the glyceroclickdendrimers (GCDs), and next the glyceroladendrimers (GADs). The two first family syntheses have already been described in the literature.14,16 The synthesis of GCDs and GADs are presented here following the procedures described respectively in two patents.34,35
2.1.1. Glycerol-Based PPIs and PAMAMs
The four generations of functionalized PPIs (polypropyleneimines) and PAMAMs (polyamidoamines) (Figures 1 and 2) were synthesized following previously described works.14,16 Commercial dendrimers (PPIs or PAMAMs) react with glycerol carbonate in methanol with Et3N as a catalyst. Products were characterized by NMR and elemental analysis and then used for EO encapsulations.
Figure 1.
Three generations of GD-PAMAMs.
Figure 2.
Four generations of GD-PPIs.
2.1.2. Glyceroclickdendrimers (GCDs)
The starting material for GCD family is a glycerol derivate, the solketal. This compound is classically obtained from glycerol through various methods largely described in the literature.36 Compound I.3 (Scheme 1) is prepared in two steps starting from solketal: the mesylation of the hydroxyl group is followed by substitution by sodium azide.37,38 The expected azide was obtained with good yields, without purification steps by simple phase separation and especially without the use of organic solvents such as DMSO or DMF. Next, compound I.4 synthesis was carried out from glycerol in the presence of propargyl bromide and a base, in DMF as a solvent, for 24 h at 50 °C.39 KOH was chosen as base instead of NaH to avoid its disadvantages (explosive in contact with H2O, flammable in humid air). During this synthesis, several by-products were observed and identified by NMR as mono- and di-substitution products. Different reaction conditions were tested in order to reduce the formation of these compounds (increase in the number of base and propargyl bromide equivalents, addition of these reagents during the reaction, increase of temperature, and reaction time), but they did not help to promote the tripropynylation. Nevertheless, these various by-products are eliminated easily by flash chromatography on silica gel and the yields in trialcynyl compounds are good.
Scheme 1. Synthesis of GCD-1 (Compound I.6).
On the 1H NMR spectrum of I.4, the 4.15 ppm peak integrates twice as much as the 4.30 ppm peak, confirming the symmetry of only two branches of the molecule. Moreover, the signal integration at 2.42 ppm is low, which is explained by a different relaxation of the protons of the terminal alkyne group. A ″click″ reaction was then performed between compound I.3 and compound I.4 to obtain the first generation of this new family of dendrimers from glycerol, compound I.5. The conditions used for the click reaction are classical (catalytic amounts of CuSO4·5H2O (0.1 equiv), sodium ascorbate (0.2 equiv), tBu4NBr (0.1 equiv) in a CH2Cl2/NaHCO3 (aq) mixture (1:1) at 50 °C for 24 h).40,41 After washing with ammonia and precipitation with petroleum ether, the expected compound I.5 is obtained with an isolated yield of 72%. The 1H NMR spectrum of I.5 showed two signals at 4.65 and 4.79 ppm corresponding to protons H3 and H12. In the molecule, two branches are thus equivalent two by two. Nevertheless, it should be noted that contrary to compound I.4, this partial symmetry is only observed for the protons of the pyrazole rings. Beyond the cycle, the protons are all equivalent on the three branches. This can be explained by the size of the molecule, which becomes more important. The deprotection of I.5 is then realized in an acid medium and led to the formation of I.6 (GCD-1).
Once compound I.5 is obtained, a last step is necessary to obtain the first generation of this family (I.6): the deprotection of acetal groups. I.5 is dissolved in a minimum of water and one equivalent per protected function of HCl is added at room temperature. The advantage of this method is to obtain very good yields, even quantitative ones.42 The reaction is monitored by 1H NMR by observing the disappearance of the signals corresponding to the CH3 groups.
The reacting sequence was repeated one time to furnish the second generation of GCD (compound I.9, GCD-2, Scheme 2) and another time too for the third one (compound I.12, GCD-3, Scheme 3).
Scheme 2. Synthesis of GCD-2 (Compound I.9).

Scheme 3. Synthesis of GCD-3 (Compound I.12).
Three generations of new glyceroclickdendrimers from glycerol have been synthesized and purified with good yields. These glyceroclickdendrimers present respectively 6, 12, and 24 OH terminal functions and are soluble in water.
2.1.3. GlycerolADdendrimers (GADs)
GADs are a new family of glycerodendrimers presenting only the pattern glycerol as the core with OH functions at the periphery (6, 12, or 24 for generations 1, 2, and 3, respectively).
At first, the synthesis of glycerol triallyl was carried out from glycerol in the presence of allyl bromide and potassium hydroxyde in DMF (Scheme 4).
Scheme 4. Synthesis of GAD-1 (Compound II.2), GAD-2 (Compound II.4), and GAD-3 (Compound II.6).

Compound II.1 is obtained with an isolated yield of 46%. This yield can be explained by the formation, during the synthesis, of by-products from mono- and disubstitution, which can be easily removed by flash chromatography on silica gel. To reduce the formation of these compounds, we varied the reaction conditions. An increase of the number of equivalents of potassium hydroxide or allyl bromide, the reaction temperature, or even the reaction time unfortunately did not improve the yields. Likewise, a successive addition of the different reagents during the reaction did not increase the reaction yield either.
The 1H NMR of II.1 at room temperature curiously showed the presence of signals with a complex multiplicity (Figure 3a). For a better understanding of the structure, a homo-nuclear J-resolved NMR (Figure 3b) was realized. Thanks to NMR and more particularly to J-resolved NMR, we were thus able to attribute each signal of the molecule and to observe that compound II.1 presented, just like compound I.4, a central symmetry along the C1-C6 axis. The various results obtained by J-resolved NMR complement those already described in 2006 by Kuźnik et al. concerning the isomerization of allyl alkyls and allyl silyl ethers catalyzed by ruthenium complexes.43 The J-resolved NMR analysis completed this first analysis by determining the multiplicity of all protons (Table 1).
Figure 3.
(a) 1H NMR of II.1. (b) J-resolved NMR of II.1 in CDCl3.
Table 1. Data Relative to the 1H NMR of II.1.
| proton | signal (ppm) | coupling constants |
|---|---|---|
| H2 (not equivalent) | two duplicated doublets (3.52 and 3.56) | JH2b-H2a = 10.4 Hz |
| JH2b-H1 = 5.5 Hz | ||
| JH2a-H2b = 10.4 Hz | ||
| JH2a-H1 = 4.7 Hz | ||
| H1 | split quintuplet (3.69) | JH1-H2a = 4.7 Hz |
| JH1-2b = 5.5 Hz | ||
| H3 | two doublets of doublets (4.01 and 4.15) | JH5a-H3a = JH5a-H3b = 1.2 Hz |
| JH4-H3a = JH4-H3b = 5.6 Hz | ||
| H6 | doublet of doublet of doublet (4.15) | JH8a-H6a = JH8a-H6b = 1.2 Hz |
| JH8b-H6a = JH8b-H6b = 1.6 Hz | ||
| JH7-H6a = JH7-H6b = 5.6 Hz | ||
| H5a and H8a (with H4 in cis position) | two doublets of doublets of doublets (5.15 and 5.17) | JH8a-H7 = 10.4 Hz |
| JH8a-H8b = 3.07 Hz | ||
| JH8a-H6a = JH8a-H6b = 1.2 Hz | ||
| JH5a-H4 = 10.4 Hz | ||
| JH5a-H5b = 3.07 Hz | ||
| JH5a-H3a = JH5a-H3b = 1.2 Hz | ||
| H5b and H8b (with H4 in trans position) | two doublets of doublets (5.27 and 5.28) | JH5b-H4 = 17.2 Hz |
| JH5b-H5a = 3.5 Hz | ||
| JH5b-H3a = JH5b-H3b = 1.6 Hz | ||
| JH8b-H7 = 17.2 Hz | ||
| JH8b-H8a = 3.5 Hz | ||
| JH8b-H6a = JH8b-H6b = 1.6 Hz | ||
| H4 and H7 | two doublets of triplets (5.90 and 5.92 ppm) | JH4-H3a = JH4-H3b = 5.6 Hz; JH4-H5a = 10.4 Hz; JH4-H5b = 17.2 Hz; JH7-H6a = JH7-H6b = 5.6 Hz; JH7-H8a = 10.4 Hz; JH7-H8 = 17.2 Hz) |
Next, in order to obtain a first generation of ″poly glycerol″ dendrimers comprising six alcohol functions at the periphery, we realized a dihydroxylation of the three double bonds of compound II.1. We used the classical conditions of Sharpless.44 The dihydroxylation is carried out in the presence of the AD-mix-β (1.4 g for 1 mmol olefin) in a tBuOH/H2O mixture (1:1) at 0 °C. After 24 h, Na2SO3 was added to reduce K2OsO2(OH)4 to OsO2.
The same reaction scheme was applied to prepare the second and then the third generation of this glycerol-based dendrimer family (Scheme 4).
Compound II.3 was obtained with a yield of 89%. Up to this generation, the purification was simplified by simple precipitation of the impurities, which were insoluble in diethyl ether, and no silica gel chromatography was necessary. Compounds II.4 and II.6 with 12 and 24 OH functions, respectively, at the periphery were similarly obtained with yields of 65 and 75%.
The first three generations of ″poly glycerol″ dendrimers were synthesized with a glycerol core. The synthesis of each generation is carried out easily in two steps: allylation of the OH functions and then dihydroxylation of the double terminal links. The presence of alcohol functions on the surface of the dendrimer allows increasing its water solubility and then considering the use of these glycerodendrimers for aqueous catalysis or stabilization of nanoparticles. In addition, the presence of double bonds at the periphery of the ″half generations″, easily isolatable, also allows for the possibility of considering for these molecules the transformations by metathesis or ″click″.
2.2. Essential Oils Encapsulations
For retention experiments, the same amount of each EO was used to prepare solutions with or without dendrimers, which are then analyzed by DHS-GC–MS. EO retention by GDs was quantified by comparing the sum of the chromatographic peaks areas of EO components in the presence of GDs to that of the blank experiment. The percentage of retention (r) of EOs by GDs was calculated by the equation and listed in Table 2.31,45
where ∑AD is the sum of peak areas of EO component in the presence of dendrimers and ∑A0 is the sum of the peak of the EO component in the free EO solution (control).
Table 2. Essential Oils Encapsulation Results for Two Essential Oils, Citronella and Cinnamon, inside the Three or Four First Generations of Four Families of Dendrimers (GD-PPI, GD-PAMAM, GCD, and GAD).
| dendrimers | i (%) citronella EO | r (%) cinnamon EO |
|---|---|---|
| GD-PAMAM-0 | 9.83 ± 0.44 | 12.17 ± 0.41 |
| GD-PAMAM-1 | 6.49 ± 0.72 | 29.01 ± 0.68 |
| GD-PAMAM-2 | 24.88 ± 4.80 | 38.84 ± 0.57 |
| GD-PAMAM-3 | 20.39 ± 2.38 | 32.97 ± 1.13 |
| GD-PPI-1 | –12.37 ± 0.97 | 24.35 ± 4.23 |
| GD-PPI-2 | 3.09 ± 1.95 | 14.15 ± 3.77 |
| GD-PPI-3 | 26.65 ± 5.77 | 25.99 ± 4.36 |
| GD-PPI-4 | 10.55 ± 3.53 | 24.21 ± 3.95 |
| GCD-1 | –35.80 ± 0.09 | 13.67 ± 1.57 |
| GCD-2 | –28.60 ± 3.13 | 9.37 ± 2.54 |
| GCD-3 | –4.74 ± 0.85 | 2.14 ± 0.50 |
| GAD-1 | 3.89 ± 1.27 | 16.23 ± 5.30 |
| GAD-2 | –16.64 ± 1.58 | 23.38 ± 2.85 |
| GAD-3 | 8.28 ± 1.45 | 4.31 ± 1.30 |
Depending on the family and generation of dendrimers, the peak areas of all EO components decreased in the presence of GDs. This decay was attributed to the retention of EOs upon encapsulation formation.45 Citronella EO in GCDs, GD-PPI-1, and GAD-2 did not show a remarkable retention capacity. The main volatile compounds present in this EO are limonene, geraniol, citronellal, and citronellol. These terpenes are very apolar such as GCD, which could induce repulsion instead of attraction as a salting-out effect of volatile EO components from the GD solution to the vapor phase. Negative retentions were also reported in the literature.46,47 For the other cases, retention values showed that EOs were retained by GDs. This reflected the tendency of their components to form encapsulation with GDs. The comparison of the retention capacities of GDs toward the two EOs showed that GD-PPI and GD-PAMAM were more efficient than GCD and GAD; the GD retention capacities can be ranked for each family following the generation order G3 > G4 > G2 > G1. These results suggested that GDs, particularly GD-PPI-3, GD-PAMAM-2, GCD-2, and GAD-1, could efficiently retain EOs and consequently protect them from evaporation. Thus, GDs might be considered as efficient materials to encapsulate and improve the release efficiency of volatile compounds enlarging the applications of EOs for pesticides. The rate of retention obtained is comparable to the Lippia sidoides essential oil nanocapsule produced for agricultural purposes.48
2.3. Interaction Analyses
1D NMR of dendrimers GD-PPI-3, GD-PAMAM-2, GCD-2, and GAD-1 have been recorded alone and in the presence of citronella and cinnamon EOs. No real shift or interaction could be remarked with GD-PAMAM-2, GCD-2, and GAD-1. On the contrary, considering the solution of GD-PPI-3 and cinnamon EO, a shoulder of the peak relative to the CH2-N around 2.5 ppm is observed suggesting an interaction between cinnamon EO or more particularly its major compound, the trans-cinnamaldehyde and this part of the dendrimer (Figure 4).
Figure 4.
1H NMR in D2O of (A) GD-PPI-3 and cinnamon essential oil, (B) GD-PPI-3, (C) focus on 1.5 to 3 ppm for GD-PPI-3 and cinnamon EO, and (D) focus on 1.5 to 3 ppm for GD-PPI-3.
A similar behavior has been already reported in previous works.16 So, we decided to realize DOSY experiments to finalize this interaction.
As the evaluation of the ability of glycerodendrimers to encapsulate essential oils is the main goal of this work, the hydrodynamic radii of these macromolecules were determined by DOSY NMR because it was already found that the encapsulation efficiency depends on the dendrimer size. In addition, a variation of this size when dendrimers are in the presence of EOs should demonstrate that encapsulation modifies dendrimer organization.
The determination of the hydrodynamic radius was based on the Stokes–Einstein equation and relied on the measurement of the diffusion coefficient of the dendrimer.15 For each solution, the diffusion coefficients of the solute and of the solvent were extracted from the 2D DOSY spectra by using the TopSpin software (Bruker) and the solvent viscosity was not changed by the presence of the dendrimer because of their low concentration. DOSY NMR (Figure 5) was realized for GD-PPI-3, GD-PAMAM-2, GCD-2, and GAD-1, but more attention has been devoted for the emulsion GD-PPI-3 with cinnamon EO where an interaction was noticed in 1H NMR.
Figure 5.
DOSY NMR of the emulsion GD-PPI-3 and cinnamon EO.
The diffusion coefficients for this study are detailed in Table 3.
Table 3. Diffusion Coefficients of GD-PPI-3 Alone and in Emulsion with Cinnamon EO.
| solution | Dsolvant (HOD dans D2O) 10–10 (m2 s–1) | Ddendrimer (D2O) 10–10 (m2 s–1) |
|---|---|---|
| GD-PPI-3 | 18.4 | 1.07 |
| GD-PPI-3 with cinnamon EO | 18.9 | 0.83 |
The diffusion coefficient of the dendrimer is higher in the presence of the cinnamon EO, which suggests that its radius is lower in this solution than alone. This observation led us to think that the dendrimer is compressed with the essential oil and that the latter is even more stabilized. To our knowledge, this behavior has not been reported in the literature, the size of the dendrimers being always bigger when various species (metallic complexes or organic compounds) are encapsulated inside. These studies should be continued by microscopic studies to confirm this phenomenon and preliminary results seemed to confirm this phenomenon.
3. Conclusions
Three generations of two new family of dendrimers based on glycerol have been successfully synthetized. Their EO encapsulation abilities have been studied at the same time for two others, more known, and already described glycerol-based dendrimers, GD-PPI and GD-PAMAM. GD-PPI-3, GD-PAMAM-2, GCD-2, and GAD-1 showed good retention properties, so the interaction existing between EOs and GDs had been thoroughly analyzed. The NMR experiments (1D and DOSY) showed an interaction between the GD-PPI-3 and the cinnamon EO, and furthermore, the dendrimer is compressed in a stable emulsion containing this dendrimer and the essential oil. Investigations have to be pursued with GD-PPI-3 using microscopic analyses to confirm this surprising phenomenon.
4. Experimental Part
4.1. Materials
The PPI dendrimers were purchased from SyMO-Chem, Netherlands (no CAS numbers available), and the PAMAM dendrimers from Merck (Sigma-Aldrich), PAMAM-1 (CAS: 142986-44-5), PAMAM-2 (CAS: 93376-66-0), and PAMAM-3 (CAS: 153891-46-4). Other compounds were used as received.
1H and 13C NMR spectra were recorded at room temperature with a Bruker AC 500 spectrometer (500 or 600 MHz for 1H, 62.5 or 150 MHz for 13C). High resolution mass spectra were recorded on a Q TOF micro (Micromass) with an electrospray source (injection by infusion: 5 L/min, solvent used: MeOH +0.2% (by volume) of formic acid, source temperature: 80 °C and drying gas: nitrogen at 100 °C). The processing of the spectra is done with the Masslynx software.
4.2. Dendrimers Synthesis
4.2.1. PPI and PAMAM Decorations
The four
first generations of PPI and PAMAM had been synthesized following
the previous method. Here, only the characteristics of GD-PPI-3 are
mentioned again.
4.2.2. GCDs Synthesis
The synthesis of three first generations of glyceroclickdendrimer is protected by the patent PCT/EP2019/070097.
4.2.2.1. Synthesis of I.2
Solketal (1 equiv, CAS: 100-79-8) is dissolved in CH2Cl2 (100 mL, CAS: 75-09-2). Et3N (1.2 equiv, CAS: 121-44-8) is then added, and the mixture is stirred at 0 °C for 1 h. Then, MeSCl (1.15 equiv, CAS: 124–63-0) is added dropwise at 0 °C followed by a stirring for 30 min at 0 °C and 18 h at room temperature. After that, the mixture is washed three times with a saturated NaHCO3 solution and two times with water. The organic phase is then dried on MgSO4 and filtered, and the solvent is removed under pressure. The product is obtained as a yellow oil with 81% yield.
4.2.2.2. Synthesis of I.3
To I.2 (1 equiv) is added Et3BnNCl (0.2 equiv) and NaN3 (aq) (2 equiv, 30 mL). After stirring the mixture under reflux for 48 h, the product is extracted with diethyl ether and obtained as a yellow oil with 92% yield.
4.2.2.3. General Procedure for the Synthesis of I.4, I.7, and I.10
KOH (1.2 equiv per −OH function) is dried under vacuum for 10 min, and then I.3, I.6, or I.9 (1 equiv) dissolved in DMF is added under argon. After 18 h of stirring at room temperature, propargyl bromide (1.2 equiv per −OH function, CAS: 106-96-7) is added dropwise at 0 °C. The mixture is then stirred for 48 h at 4 °C under argon.
I.4. Purification
After addition of water,
the aqueous phase is extracted three times with CH2Cl2. Organic phases are collected and dried on MgSO4; the solvent is then removed under pressure, and the crude mixture
is purified on silica gel chromatography with the mixture petroleum
ether/AcOEt (7:3) as an eluting mixture. I.4 is obtained
as a yellow oil with 80% yield.
I.7. and I.10 Purification
After the addition of water, the aqueous phase is extracted three times with CH2Cl2. Organic phases are collected and dried on MgSO4; the solvent is then removed under pressure. After verification by NMR 1H, a simple purification by precipitation in CH2Cl2 with an excess of petroleum ether can be performed.
I.7 and I.10 are obtained as yellow oils
with respectively 70% and 52% yields.
4.2.2.6. General Procedure for the “Click” Reaction (Synthesis of I.5, I.8, and I.11)
I.3 (1.2 equiv per alkyne function), TBAB (0.2 equiv, CAS: 1643-19-2), and sodium ascorbate (0.2 equiv, CAS: 134-03-2) are mixed under argon. I.4, I.7, or I.10 (1 equiv) is dissolved in a mixture of CH2Cl2/NaHCO3 (aq) and added to the reaction followed by an aqueous solution of CuSO4 5.H2O (1 M, 0.5 equiv, CAS: 7759-99-8) at 0 °C. The mixture is stirred at 4 °C under argon for 48 h. After an extraction with CH2Cl2, the organic phase is washed with NH4OH solution (0.8 M) until the aqueous phase gets uncolored and then two times with water. Organic phases are collected and dried on MgSO4, and the solvent is removed under pressure. The resulting mixture is dissolved in a minimum of CH2Cl2, and the addition of an excess of petroleum ether allows the precipitation of the required products (I.5, I.8, and I.11).
The desired compounds are
obtained as a brown resin with 70, 70, and 43% yields.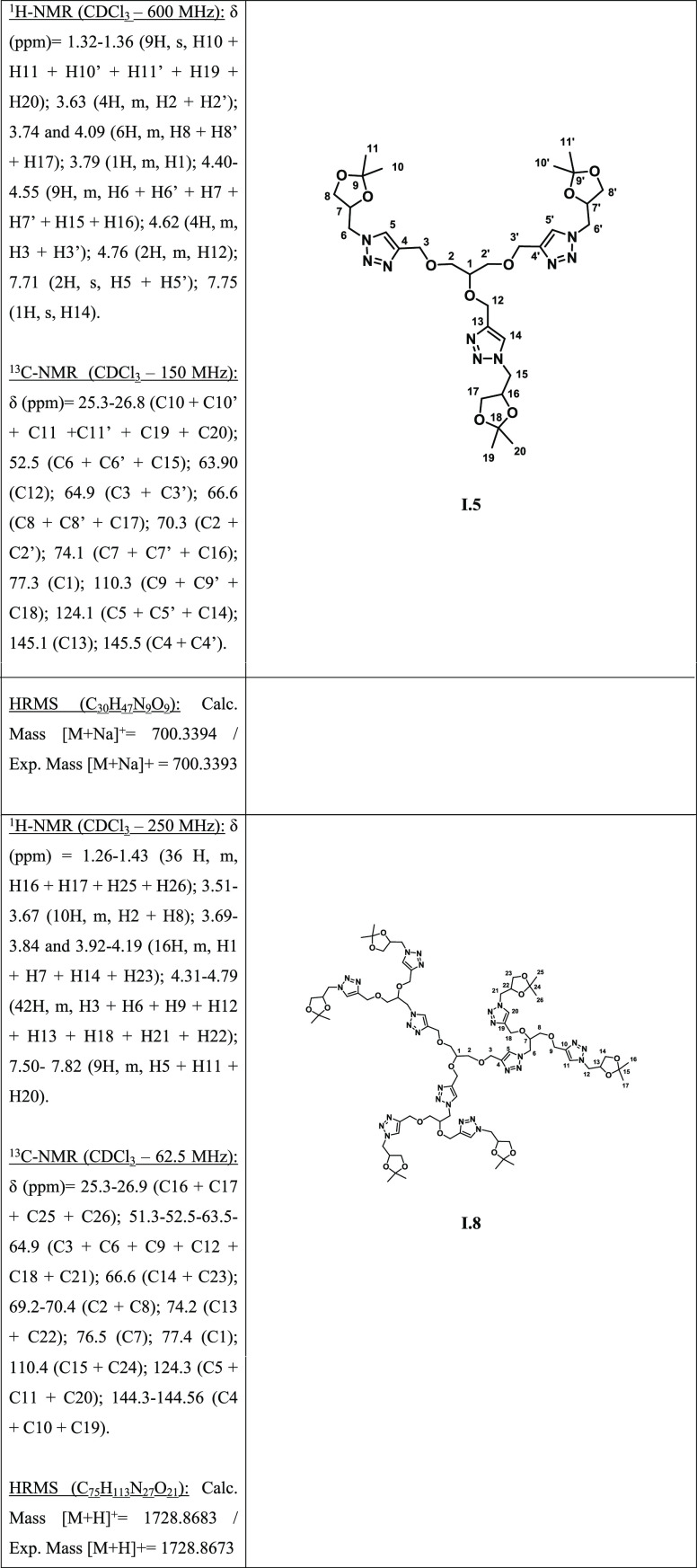

4.2.2.7. General Procedure for the Removing of Protection (Synthesis of I.6, I.9, and I.12)
I.5, I.8, or I.11 are dissolved with minimum water, and then a HCl solution
(1 M, 1.2 equiv per branch) is added. The mixture is stirred at room
temperature until the total deprotection (followed by 1H NMR). Then, a NaOH solution (1 M, 1 equiv per equiv HCl) is added.
The mixture is filtered on celite, and water is removed under reduced
pressure. The products are obtained as brown wax with quantitative
yields.

4.2.3. Glyceroladendrimer Synthesis
The synthesis of three first generations of glyceroladendrimer is protected by the patent PCT/EP2019/070092.
4.2.3.1. General Procedure for II.1, II.3, and II.5 Synthesis
KOH (1.2 equiv per OH function) is dried under vacuum for 10 min, and then II.1, II.3, or II.5 (1 equiv) dissolved in DMF is added under argon. After 18 h of stirring at room temperature, allyl bromide (1.2 equiv per OH function, CAS: 106-95-6) is added dropwise at 0 °C. The mixture is then stirred for 48 h at 4 °C under argon.
4.2.3.2. II.1 Purification
Excess of KOH is neutralized with water, and then the aqueous phase is extracted three times with CH2Cl2. Organic phases are assembled and dried on MgSO4, and the solvent is removed under pressure. The crude material is purified on silica gel chromatography with the mixture petroleum ether/AcOEt (7:3) as the eluting mixture. II.1 is obtained as a yellow oil with 65% yield.
4.2.3.3. II.3 and II.5 Purification
After the addition of water, the aqueous phase is extracted three times with CH2Cl2. Organic phases are collected and dried on MgSO4; the solvent is removed under pressure. After verification by 1H NMR, a purification by precipitation in CH2Cl2 with the excess of petroleum ether is realized. II.3 and II.5 are obtained as yellow oils with 77 and 74% yields, respectively.
4.2.3.4. General Procedure for the Terminal Alkene Dihydroxylation (Synthesis of II.2, II.4, and II.6)
AD-mix-β ((1.4 g per mmol
olefin) × number of double bonds) is added to a mixture of tBuOH/H2O (1:1) and stirred at room temperature
until obtaining two clear phases. The reaction is cooled to 0 °C
and after precipitation of the salts, II.1, II.3, or II.5 is added in one time. The mixture is stirred
at 4 °C for 48 h, then Na2SO3 (1.5 g per
1.4 g d’AD-mix, CAS: 7757-83-7) is added, and the mixture is
stirred until the temperature reaches room temperature. The solvents
are removed under pressure and the products extracted with acetone.
The organic phase is filtered and evaporated under pressure. II.2, II.4, and II.6 are obtained
as white waxes with 60, 69, and 72% respectively.

4.3. Essential Oils Encapsulations
EOs were placed in 22 mL headspace glass vials with 7.5 mL of EtOH and 2.5 mL of water or aqueous dendrimers solutions (8 mM). Vials were then sealed by using a silicone septa and aluminum foil and stirred at 750 rpm at room temperature for 1 h.
4.4. Dynamic-Headspace Chromatography–Mass Spectrometry (DHS-GC–MS) Analysis
The percentage of retention (r) of EOs by GDs was determined by dynamic head-space sampling (DHS, Gerstel, Germany) coupled to a thermal desorption unit (TDU, Gerstel, Germany), a gas chromatograph (Agilent Technologies 7890A), and a mass spectrometer (MS, Agilent Technologies 5975C). During treatment in the DHS unit, the vials were conditioned at 25 °C for 30 min with agitation (500 rpm). The head-space sampling was performed on Gerstel TDU desorption tubes (OD 6.00 mm, filled with 60 mg of Tenax TA, Gerstel, Germany), for 200 mL at 20 mL/min, followed by 200 mL at 60 mL/min of the drying phase. Desorption then occurred for 10 min at 300 °C and coupled to a cooled injection system (CIS, Gerstel, Ger- many) set at −80 °C. EOs were then transferred to the GC column (VF-WAXms, Agilent technologies USA; 30 m length, 0.250 mm I.D, 0.25 μm film thickness) for separation with temperature programs as follows: citronella, from 70 °C (5 min) to 100 °C at a rate of 8 °C/min, then 2 °C/min to 160 °C, and then 20 °C/min to 260 °C (10 min); cinnamon, from 40 °C (4 min) to 80 °C at a rate of 3.5 °C/min, then 5 °C/min to 160 °C, and then 20 °C/min to 220 °C (10 min) with helium as carrier gas at a flow rate of 1.5 mL/min. The MS were recorded in electron ionization mode at 70 eV (scanned mass range: 35 to 300 m/z); source and quadrupole temperature at 230 and 150 °C, respectively. The component identification was performed by comparison of the recorded spectra with two data libraries (Pal 600 K and Wiley 275).
4.5. Nuclear Magnetic Resonance Analyses
4.5.1. One-Dimension NMR
1H and 13C NMR spectra were recorded on an AVANCE III 500 MHz Bruker in D2O, MeOH-d4 or CDCl3 as solvents purchased from Eurisotop. The NMR spectrometer is equipped with a 5 mm BBFO+ probe and using Topspin software (Rheinstetten Germany).
4.5.2. DOSY Experiments
Samples (30 mg) were dissolved in D2O or MeOH-d4 and placed in NMR tubes.
The 2D 1H DOSY NMR experiments were carried out using the Bruker sequence ledbpgp2s, at 298 K. The gradient value G = 0.535 T·m–1 was generated by a 10A amplifier. The strength of the pulsed-field gradient was linearly increased from 2 to 95% in 24 steps. The diffusion time (Δ) and the gradient duration (δ/2) were set at 180 ms and 800 us. The longitudinal eddy current delay and the spoil gradient delay were fixed at 5 and 0.2 ms, respectively. Spectral data were processed via dynamics center version 2.6.2 software (Bruker) or from topspin software for DOSY experiments.
Acknowledgments
We are grateful to the Universities of Reims Champagne-Ardenne (France) and Liège (Belgium) for material funds and the doctoral position to ChloëMaes.
The authors declare no competing financial interest.
References
- Buhleier E.; Wehner W.; Vögtle F. “Cascade”- And “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. 10.1055/s-1978-24702. [DOI] [Google Scholar]
- Morgenroth F.; Reuther E.; Müllen K. Polyphenylene Dendrimers: From Three-Dimensional to Two-Dimensional Structures. Angew. Chem. Int. Ed. 1997, 36, 631–634. 10.1002/anie.199706311. [DOI] [Google Scholar]
- Hawker C. J.; Fréchet J. M. J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 2002, 112, 7638–7647. 10.1021/ja00177a027. [DOI] [Google Scholar]
- Azzouz A.; Roy R. Dendrimers: Syntheses, Toxicity, and Applications toward Catalysis, Environmental Sciences, and Nanomedecine. Can. J. Chem. 2017, 95, v–vii. 10.1139/cjc-2017-0537. [DOI] [Google Scholar]
- Gogulapati N.; Manalan B. V.; Nadendla R. R. Poly (Propylene Imine) Dendrimer: Synthesis, Characterization and Applications in Various Drug Delivery. Asian J. Pharm. Pharmacol. 2020, 6, 190–203. 10.31024/ajpp.2020.6.3.10. [DOI] [Google Scholar]
- Gupta L.; Sharma A. K.; Gothwal A.; Khan M. S.; Khinchi M. P.; Qayum A.; Singh S. K.; Gupta U. Dendrimer Encapsulated and Conjugated Delivery of Berberine: A Novel Approach Mitigating Toxicity and Improving in Vivo Pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. 10.1016/j.ijpharm.2017.04.073. [DOI] [PubMed] [Google Scholar]
- Mandal A. K. Dendrimers in Targeted Drug Delivery Applications: A Review of Diseases and Cancer. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 287–297. 10.1080/00914037.2020.1713780. [DOI] [Google Scholar]
- Klajnert B.; Rozanek M.; Bryszewska M. Dendrimers in Photodynamic Therapy. Curr. Med. Chem. 2012, 19, 4903–4912. 10.2174/0929867311209024903. [DOI] [PubMed] [Google Scholar]
- Ghann W.; Kang H.; Uddin J.; Gonawala S. J.; Mahatabuddin S.; Ali M. M. Dendrimer-Based Nanoparticle for Dye Sensitized Solar Cells with Improved Efficiency. J. Nanomed. Nanotechnol. 2018, 09, 496. 10.4172/2157-7439.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A. M.; Shon Y. S. Water-Soluble Noble Metal Nanoparticle Catalysts Capped with Small Organic Molecules for Organic Transformations in Water. ACS Appl. nano Mater. 2021, 4, 3294–3318. 10.1021/acsanm.1c00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami O.; Laurent R.; Majoral J. P.; El Brahmi N.; El Kazzouli S.; Caminade A. M. Copper Complexes of Phosphorus Dendrimers and Their Properties. Inorg. Chim. Acta 2021, 517, 120212. 10.1016/j.ica.2020.120212. [DOI] [Google Scholar]
- Astruc D.; Chardac F. Dendritic Catalysts and Dendrimers in Catalysis. Chem. Rev. 2001, 101, 2991–3024. 10.1021/cr010323t. [DOI] [PubMed] [Google Scholar]
- Militello M. P.; Ramírez R. E. H.; Lijanova I. V.; Previtali C. M.; Bertolotti S. G.; Arbeloa E. M. PAMAM Dendrimers with a Porphyrin Core as Highly Selective Binders of Li+ in an Alkaline Mixture. A Spectroscopic Study. New J. Chem. 2019, 43, 16246. 10.1039/C9NJ04088A. [DOI] [Google Scholar]
- Balieu S.; El Zein A.; De Sousa R.; Jérôme F.; Tatibouët A.; Gatard S.; Pouilloux Y.; Barrault J.; Rollin P.; Bouquillon S. One-Step Surface Decoration of Poly(Propyleneimines) (PPIs) with the Glyceryl Moiety: New Way for Recycling Homogeneous Dendrimer-Based Catalysts. Adv. Synth. Catal. 2010, 352, 1826–1833. 10.1002/adsc.201000229. [DOI] [Google Scholar]
- Balieu S.; Cadiou C.; Martinez A.; Nuzillard J. M.; Oudart J.-B.; Maquart F.-X.; Chuburu F.; Bouquillon S. Encapsulation of Contrast Imaging Agents by Polypropyleneimine-Based Dendrimers. J. Biomed. Mater. Res., Part A 2013, 101A, 613–621. 10.1002/jbm.a.34359. [DOI] [PubMed] [Google Scholar]
- Menot B.; Stopinski J.; Martinez A.; Oudart J.-B.; Maquart F.-X.; Bouquillon S. Synthesis of Surface-Modified PAMAMs and PPIs for Encapsulation Purposes: Influence of the Decoration on Their Sizes and Toxicity. Tetrahedron 2015, 71, 3439–3446. 10.1016/j.tet.2015.03.078. [DOI] [Google Scholar]
- Menot B.; Salmon L.; Bouquillon S. Platinum Nanoparticles Stabilized by Glycerodendrimers: Synthesis and Application to the Hydrogenation of α,β-Unsaturated Ketones under Mild Conditions. Eur. J. Inorg. Chem. 2015, 2015, 4518–4523. 10.1002/ejic.201500530. [DOI] [Google Scholar]
- Hayouni S.; Robert A.; Maes C.; Conreux A.; Marin B.; Mohamadou A.; Bouquillon S. New Dendritic Ionic Liquids (DILs) for the Extraction of Metallic Species from Water. New J. Chem. 2018, 42, 18010–18020. 10.1039/C8NJ01921E. [DOI] [Google Scholar]
- Abdul Raman A. A.; Tan H. W.; Buthiyappan A. Two-Step Purification of Glycerol as a Value Added by Product From the Biodiesel Production Process. Front. Chem. 2019, 7, 774. 10.3389/fchem.2019.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev K. L.; Vodyankina O. V. Selective Oxidation of Bio-Based Platform Molecules and Their Conversion Products over Metal Nanoparticle Catalysts: A Review. React. Chem. Eng. 2021, 6, 418–440. 10.1039/D0RE00352B. [DOI] [Google Scholar]
- da Silva Ruy A. D.; de Brito Alves R. M.; Reis Hewer T. L.; de Aguiar Pontes D.; Gomes Teixeira L. S.; Magalhães Pontes L. A. Catalysts for Glycerol Hydrogenolysis to 1,3-Propanediol: A Review of Chemical Routes and Market. Catal. Today 2021, 381, 243–253. 10.1016/j.cattod.2020.06.035. [DOI] [Google Scholar]
- Abdullah R.; Mohamed Saleh S. N.; Embong K.; Abdullah A. Z. Recent Developments and Potential Advancement in the Kinetics of Catalytic Oxidation of Glycerol. Chem. Eng. Commun. 2020, 207, 1298–1328. 10.1080/00986445.2019.1641699. [DOI] [Google Scholar]
- Holade Y.; Tuleushova N.; Tingry S.; Servat K.; Napporn T. W.; Guesmi H.; Cornu D.; Kokoh K. B. Recent Advances in the Electrooxidation of Biomass-Based Organic Molecules for Energy, Chemicals and Hydrogen Production. Catal. Sci. Technol. 2020, 10, 3071–3112. 10.1039/C9CY02446H. [DOI] [Google Scholar]
- Checa M.; Nogales-Delgado S.; Montes V.; Encinar J. M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279–1320. 10.3390/catal10111279. [DOI] [Google Scholar]
- Nda-Umar U. I.; Ramli I.; Taufiq-Yap Y. H.; Muhamad E. N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and Other Bio-Based Chemicals. Catalysts 2019, 9, 15. 10.3390/catal9010015. [DOI] [Google Scholar]
- Pravallika B. K.; Parimala S.; Sumakanth M. A Review on Natural Pesticides. World J. Pharm. Pharm. Sci. 2017, 6, 731–742. [Google Scholar]
- Sharma R.; Rao R.; Kumar S.; Mahant S.; Khatkar S. Therapeutic Potential of Citronella Essential Oil: A Review. Curr. Drug Discovery Technol. 2019, 16, 330–339. 10.2174/1570163815666180718095041. [DOI] [PubMed] [Google Scholar]
- Fierascu R. C.; Fierascu I. C.; Dinu-Pirvu C. E.; Fierascu I.; Paunescu A. The Application of Essential Oils as a Next-Generation of Pesticides: Recent Developments and Future Perspectives. Z. Naturforsch. C 2020, 75, 183–204. 10.1515/znc-2019-0160. [DOI] [PubMed] [Google Scholar]
- Ashakirin S. N.; Tripathy M.; Patil U.; Majeed A. Chemistry and Bioactivity of Cinnamaldehyde: A Natural Molecule of Medicinal Importance. Int. J. Pharm. Sci. Res. 2017, 8, 2333–2340. [Google Scholar]
- Maes C.; Bouquillon S.; Fauconnier M.-L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539–2554. 10.3390/molecules24142539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C.; Brostaux Y.; Bouquillon S.; Fauconnier M.-L. Use of New Glycerol-Based Dendrimers for Essential Oils Encapsulation: Optimization of Stirring Time and Rate Using a Plackett—Burman Design and a Surface Response Methodology. Foods 2021, 10, 207–222. 10.3390/foods10020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C. F.; Cooke M.; Wilson I. D.. Encyclopedia of Separation Science; Ten-Volume Set; 2000.
- Barjat H.; Morris G. A.; Smart S.; Swanson A. G.; Williams S. C. R. High-Resolution Diffusion-Ordered 2D Spectroscopy (HR-DOSY) - A New Tool for the Analysis of Complex Mixtures. J. Magn. Reson. Ser. B 1995, 108, 170–172. 10.1006/jmrb.1995.1118. [DOI] [Google Scholar]
- Hayouni S.; Menot B.; Bouquillon S.. PCT/EP2019/070092: Dendrimer-Type Compounds, Methods for Producing Same and Uses Thereof. FR1856990. 2019.
- Hayouni S.; Menot B.; Bouquillon S.. PCT/EP2019/070097: Dendrimer-Type Compounds, Methods for Producing Same and Uses Thereof. FR1856989. 2019.
- Nascimento J. A. C.; Pinto B. P.; Calado V. M. A.; Mota C. J. A. Synthesis of Solketal Fuel Additive from Acetone and Glycerol Using CO2 as Switchable Catalyst. Front. Energy Res. 2019, 7, 58. 10.3389/fenrg.2019.00058. [DOI] [Google Scholar]
- Gibson F. S.; Park M. S.; Rapoport H. Bis[[4-(2,2-Dimethyl-1,3-Dioxolyl)]Methyl]-Carbodiimide (BDDC) and Its Application to Residue-Free Esterifications, Peptide Couplings, and Dehydrations. J. Org. Chem. 1994, 59, 7503–7507. 10.1021/jo00103a054. [DOI] [Google Scholar]
- Albanese D.; Foschi F.; Penso M. A Practical Approach to the Synthesis of Enantiomerically Pure 2,6-Disubstituted Morpholines under Phase Transfer Catalysis Conditions. Catal. Today 2009, 140, 100–104. 10.1016/j.cattod.2008.07.005. [DOI] [Google Scholar]
- André S.; Lahmann M.; Gabius H.-J.; Oscarson S. Glycocluster Design for Improved Avidity and Selectivity in Blocking Human Lectin/Plant Toxin Binding to Glycoproteins and Cells. Mol. Pharmaceutics 2010, 7, 2270–2279. 10.1021/mp1002416. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. . [DOI] [PubMed] [Google Scholar]
- Wu P.; Feldman A. K.; Nugent A. K.; Hawker C. J.; Scheel A.; Voit B.; Pyun J.; Fréchet J. M. J.; Sharpless K. B.; Fokin V. V. Efficiency and Fidelity in a Click-Chemistry Route to Triazole Dendrimers by the Copper(I)-Catalyzed Ligation of Azides and Alkynes. Angew. Chem Int. Ed. 2004, 43, 3928–3932. 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- Nieschalk J.; Hamilton J. T. G.; Murphy C. D.; Harper D. B.; O’Hagan D. Biosynthesis of Fluoroacetate and 4-Fluorothreonine by Streptomyces Cattleya. The Stereochemical Processing of Glycerol. Chem. Commun. 1997, 799–800. 10.1039/a700498b. [DOI] [Google Scholar]
- Krompiec S.; Kuźnik N.; Urbala M.; Rzepa J. Isomerization of Alkyl Allyl and Allyl Silyl Ethers Catalyzed by Ruthenium Complexes. J. Mol. Catal. A: Chem. 2006, 248, 198–209. 10.1016/j.molcata.2005.12.022. [DOI] [Google Scholar]
- Sharpless K. B.; Amberg W.; Bennani Y. L.; Crispino G. A.; Hartung J.; Jeong K. S.; Kwong H. L.; Morikawa K.; Wang Z. M.; Xu D.; Zhang X. L. The Osmium-Catalyzed Asymmetric Dihydroxylation: A New Ligand Class and a Process Improvement. J. Org. Chem. 1992, 57, 2768–2771. 10.1021/jo00036a003. [DOI] [Google Scholar]
- Kfoury M.; Auezova L.; Greige-Gerges H.; Fourmentin S. Promising Applications of Cyclodextrins in Food: Improvement of Essential Oils Retention, Controlled Release and Antiradical Activity. Carbohydr. Polym. 2015, 131, 264–272. 10.1016/j.carbpol.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Jouquand C.; Ducruet V.; Giampaoli P. Partition Coefficients of Aroma Compounds in Polysaccharide Solutions by the Phase Ratio Variation Method. Food Chem. 2004, 85, 467–474. 10.1016/j.foodchem.2003.07.023. [DOI] [Google Scholar]
- Reineccius T. A.; Reineccius G. A.; Peppard T. L. The Effect of Solvent Interactions on α-, β-, and γ-Cyclodextrin/Flavor Molecular Inclusion Complexes. J. Agric. Food Chem. 2005, 53, 388–392. 10.1021/jf0488716. [DOI] [PubMed] [Google Scholar]
- de Pinto N. O. F.; Rodrigues T. H. S.; de Pereira R. C. A.; e Silva L. M. A.; Cáceres C. A.; de Azeredo H. M. C.; Muniz C. R.; de Brito E. S.; Canuto K. M. Production and Physico-Chemical Characterization of Nanocapsules of the Essential Oil from Lippia Sidoides Cham. Ind. Crops Prod. 2016, 86, 279–288. 10.1016/j.indcrop.2016.04.013. [DOI] [Google Scholar]










