Abstract
Cancer patients often use cannabinoids for alleviating symptoms induced by cancer pathogenesis and cancer treatment. This use of cannabinoids can have unexpected effects in cancer patients depending on the cancer type, resulting in either beneficial (e.g., anticancer) or adverse (e.g., oncogenic) effects. While cannabinoids can enhance the growth and progression of some cancers, they can also suppress the growth and progression of other cancers. However, the underlying mechanisms of such differential effects are poorly understood. miRNAs have been shown to be involved in driving the hallmarks of cancer, affecting cancer growth and progression as well as cancer therapy response. Although the understanding of the effects of cannabinoids and miRNAs as they relate to cancer continues to improve, the interplay between cannabinoid system and miRNAs in cancer pathogenesis and cancer treatment response is poorly understood. Investigation of such interactions between the cannabinoid system and miRNAs could provide novel insights into the underlying mechanisms of the differential effects of cannabinoids in cancer and can help predict and improve the prognosis of cancer patients.
1. Cannabinoid System in Cancer
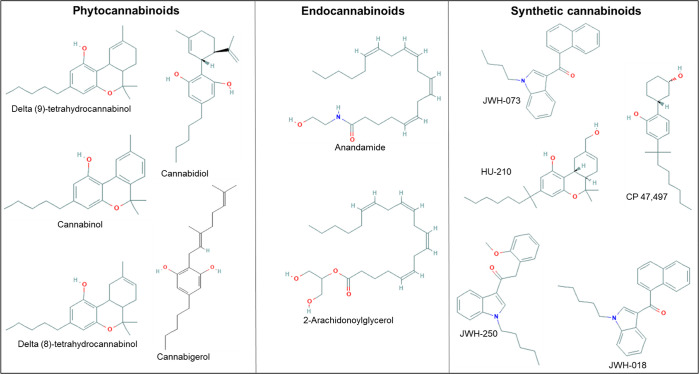
Cannabinoids are classified into phytocannabinoids, synthetic cannabinoids or cannabimimetics, and endocannabinoids (Figure 1). Phytocannabinoids include cannabidiol (CBD), delta-9-tetrahydrocannabinol (Δ9-THC), delta-8-tetrahydrocannabinol (Δ8-THC), cannabigerol (CBG), and cannabinol. While endocannabinoids include anandamide and 2-arachidonoylglycerol [2-AG], synthetic cannabinoids include HU-210, CP 47,497, JWH-018, JWH-073, and JWH-250. Cannabinoids mediate their actions by activation or inhibition of the cannabinoid receptors (CBs), CB1 and CB2, as well as through putative cannabinoid receptors such as G protein-coupled receptor 55 (GPR55).1 CB1 receptors are primarily expressed in the central nervous system (CNS), while CB2 receptors are predominantly located in the immune system.1 GPR55 is widely found in the brain.1
Figure 1.
Examples of natural and synthetic cannabinoids with their chemical structures.
Receptors that are not canonically associated with the cannabinoid system have also been reported to be modulated by endogenous and exogenous cannabinoids. An example is the transient receptor potential vanilloid receptor (TRPV).2 The endogenous cannabinoid anandamide and the exogenous cannabinoids CBD and CBG have been shown to activate TRPV1.2 Likewise, exogenous cannabinoids such as Δ9-THC, CBD, and CBG act on TRPV2.2 TRPV1 is primarily expressed in the CNS, while TRPV2 is expressed in the digestive tract as well as in the pancreas and liver.2 Studies have also reported that cannabinoids interact with a variety of other receptors, such as G protein-coupled receptor 18 (GPR18), and other well-known GPCRs, such as the opioid or serotonin receptors. Additionally, cannabinoids have been shown to modulate nuclear receptors such as peroxisome proliferator-activated receptors.3
Cancer patients use cannabinoids to alleviate cancer-induced symptoms such as loss of appetite, pain, and anxiety. Cannabinoids are also used by cancer patients to mitigate the cancer treatment associated symptoms such as nausea and vomiting. For example, Dronabinol and Nabilone are cannabinoid-based therapeutics approved by the FDA for management of nausea and vomiting associated with cancer chemotherapy.4 Similarly, Sativex, a cannabinoid-derived prescription drug, is approved in several countries as an analgesic, for the treatment of cancer-associated pain.4 Several clinical trials are being conducted to evaluate the use of cannabinoids in the management of cancer.4
Multiple studies showed a potential positive correlation between the endogenous cannabinoid system and cancer growth and progression. That is, elevated levels of endocannabinoids and cannabinoid receptors were found to be correlated with an increased risk of cancer growth and progression. For example, the endocannabinoids 2-AG and anandamide were shown to be increased in glioma and colorectal cancer patients. Increased levels of CB1 and CB2 receptors were evident in the biopsied samples from human cancer patients such as glioma, lung cancer, pancreatic cancer, thyroid cancer, mantle cell lymphoma, B-cell non-Hodgkin lymphoma, and human melanoma.1 Expression of CB1 and CB2 receptors was also found to be upregulated in cancer cell lines such as breast, pancreatic, and prostate.1 TRPV1, a noncanonical cannabinoid receptor, was also reported to be upregulated in several cancers such as in human breast cancer tissues5 and in human glioma cells such as U373.6 While it is possible for an upregulated endogenous cannabinoid system to exert tumor suppressor effects in some cancer patients, it remains to be determined whether such a correlation exists in human cancer patients.
Exogenous cannabinoids have been shown to exert oncogenic effects. For example, treatment of the U373-MG human glioblastoma cells and NCI-H292 human lung carcinoma cells with Δ9-THC, a partial agonist of the CB1 receptor, led to accelerated proliferation of the glioblastoma and lung carcinoma cells.7 Exogenous cannabinoids also exhibit tumor suppressor effects. For instance, CBD displayed anti-invasive effects in the human primary lung cancer cells as well as in the human lung adenocarcinoma xenografts.1 Similarly, treatment with CBD resulted in induction of apoptosis in the U87MG and U118MG human glioma8 and MDA-MB-231 breast carcinoma cells.9 CBG was shown to protect against colon tumorigenesis by inhibiting cell growth in a mouse model of colon cancer.2 Together, these studies suggest that the effects of the cannabinoid system are both context and cancer specific. Therefore, future studies are warranted to determine the underlying mechanisms of the differential effects of cannabinoids in cancer.
As cannabinoids are sometimes used in combination with chemotherapy drugs, interactions between cannabinoids and anticancer drugs should be considered, as such interactions can lead to either beneficial (chemosensitive) or adverse (chemoresistant or toxic) effects. For example, since CBD is mainly metabolized by the drug-metabolizing enzyme CYP3A4, administration of CBD in combination with chemotherapeutics that are also CYP3A4 substrates, such as the androgen receptor inhibitor enzalutamide, can lead to decreased bioavailability of either enzalutamide or CBD.10 Decreased bioavailability of enzalutamide can result in reduced therapeutic efficacy of enzalutamide. On the other hand, administration of CBD with chemotherapeutics that are CYP3A4 inhibitors such as imatinib can lead to increased CBD bioavailability.10 Higher bioavailability of CBD may induce adverse effects such as increased heart rate.4 Additionally, CBD was also reported to have an inhibitory effect on the drug-metabolizing enzyme UGT1A9. Therefore, administration of chemotherapeutics that are also UGT1A9 substrates with CBD, such as tyrosine kinase inhibitor sorafenib, could lead to decreased excretion and increased bioavailability of sorafenib resulting in adverse effects such as hypertension.10
2. miRNAs in Cancer
microRNAs (miRNAs) are small noncoding RNAs which are involved in post-transcriptional regulation of gene expression. miRNAs are involved in exhibiting oncogenic or tumor-suppressor activities through the regulation of genes involved in oncogenic and tumor suppressor pathways. It has been well established that miRNAs are involved in all hallmarks of cancer pathogenesis: self-sufficiency in growth, insensitivity to antigrowth signals, apoptosis evasion, infinite replicative potential, angiogenesis, invasion, and metastases.11 As a result, miRNAs are not only involved in cancer pathogenesis but also involved in affecting the outcome of cancer therapy.11
3. Interplay between the Cannabinoid System and miRNAs in Cancer
The involvement of cannabinoid systems or miRNAs alone in cancer has been widely studied. Although the cannabinoid system is regulated by and regulates epigenetic mechanisms, the interactions between the cannabinoid system and miRNAs are poorly understood. Recent studies suggest an interplay between the cannabinoid system and miRNAs in cancers. Therefore, the actions of cannabinoid system in cancers may also be due, in part, to the downregulation or upregulation of miRNAs by cannabinoids or vice versa. That is, bidirectional regulation between cannabinoid system and miRNAs may in part contribute to the differential effects of cannabinoids in cancer.
3.1. Oncogenic and Tumor Suppressor Effects of Cannabinoid System via miRNAs
Cannabinoids can drive their anticancer effects by modulation of miRNAs (Table 1). For example, CBD, a phytocannabinoid and antagonist of CB1 and CB2 receptors, displayed anticancer actions in the SH-SY5Y and IMR-32 human neuroblastoma cells by modulating miRNA expression. Specifically, a CBD-mediated increase in apoptosis and decrease in invasiveness was found to be associated with a downregulation of the miRNA hsa-let-7a, as well as an upregulation of hsa-miRNA-197212 (Table 1). However, it remains to be determined whether the CBD-mediated anticancer actions in neuroblastoma require changes in these two miRNAs.
Table 1. Effect of Cannabinoid Receptor Midulators on miRNA and Hallmark of Cancer.
| cannabinoid receptor modulator | effected miRNA | effected hallmark of cancer | ref |
|---|---|---|---|
| CBD | Downregulation of hsa-let-7a | Increased cell death in neuroblastoma | (12) |
| WIN | Upregulation of miRNA-29b1 | Decreased invasion and metastasis in osteosarcoma | (13) |
| WIN | Downregulation of miRNA-27a | Inhibition of cancer cell growth and apoptosis induction in colon cancer | (14) |
| CBD | Upregulation of hsa-miRNA-1972 | Inhibition of replicative immortality in neuroblastoma | (12) |
| Betulinic acid | Downregulation of miRNA-27a | Increased cell death in breast cancer | (15) |
In another study, treatment of WIN 55,212–2 (WIN), a synthetic cannabinoid and a CB1 agonist, in the MG63 human osteosarcoma cells, resulted in increased expression of miRNA-29b1. This increase in miRNA-29b1 levels was associated with a decrease in cell migration by WIN13 (Table 1). In another study, treatment of WIN downregulated miRNA-27a in the human SW480 colon carcinoma cells leading to inhibition of cancer cell growth and apoptosis induction14 (Table 1). Crosstalk between miRNA-27a and cannabinoid receptors was discovered in the BT474 and MDA-MB-453 human breast cancer cells treated with betulinic acid, which is an agonist of CB1 and CB2 receptors. Treatment with betulinic acid led to induction of apoptosis and inhibition of tumor growth. This anticancer action of betulinic acid was associated with a downregulation of miRNA-27a15 (Table 1).
The above studies suggest that cannabinoids exert their anticancer actions by crosstalking with miRNAs. However, future studies are warranted to demonstrate that such crosstalk is required to drive the anticancer actions of cannabinoids. Currently, it is unknown whether the cannabinoid system can promote tumor growth and progression via miRNAs, and future studies are expected to explore whether cannabinoid system interacts with miRNAs to promote cancer growth and progression.
3.2. Oncogenic and Tumor Suppressor Effects of miRNAs via Cannabinoid System
Aside from the cannabinoid system modulating miRNA expression, miRNAs can also regulate the cannabinoid system contributing to either promotion or inhibition of cancer growth and progression. For example, in the LoVo human colon cancer cells, miRNA-1273g-3p was shown to regulate CB1 receptors. Specifically, this study showed that miRNA-1273g-3p promotes proliferation, migration, and invasion of the human colon cancer cells by targeting the CB1 receptors16 (Table 2).
Table 2. Effect of miRNA on Cannabinoid Receptor and Hallmark of Cancer.
| miRNA | effected cannabinoid receptor | effected hallmark of cancer | ref |
|---|---|---|---|
| Upregulation of miRNA-1273g-3p | Decrease in CB1 expression | Increased invasion and metastasis in colon cancer | (16) |
| Downregulation of miRNA-675-5p | Increase in GPR55 expression | Increased proliferative signaling in nonsmall cell lung cancer | (17) |
| Downregulation of miRNA-7116-5p | Decrease in GPR55 expression | Decreased angiogenesis in nonsmall cell lung cancer | (18) |
| Downregulation of hsa-miRNA-29b-3 | Increase in CB1 expression | Spontaneous involution of the pediatric low grade glioma after subtotal surgical removal | (19) |
miRNAs can also show their anticancer actions by modulating the cannabinoid system. For instance, in nonsmall cell lung cancer (NSCLC) patients, low expression of the tumor suppressor miRNA-675-5p was correlated with the progression and metastasis of NSCLC, while overexpression of miRNA-675-5p inhibited proliferation and invasion of NSCLC.17 miRNA-675-5p accomplished these tumor suppressor actions by downregulating the GPR55 receptor17 (Table 2). Additionally, another study reported that GPR55 is also a target of miRNA-7116-5p, resulting in suppression of IL-10-mediated malignant pleural effusion formation as well as tumor angiogenesis and tumor growth18 (Table 2). In pediatric patients with low-grade glioma, CB1 expression was reported to be significantly higher in the spontaneously involuted tumors after subtotal surgical removal compared to the relapsed tumors. In contrast, hsa-miRNA-29b-3 was higher in the relapsed tumors when compared to the spontaneously involuted tumors after subtotal surgical removal. Based on this inverse association between CB1 and hsa-miRNA-29b-3, the authors predicted that CB1 and hsa-miRNA-29b-3 may crosstalk in the pediatric low-grade glioma19 (Table 2).
Together, the above studies suggest that cannabinoid system and miRNAs crosstalk with each other to exert their oncogenic or tumor suppressor effects. However, future studies are required to demonstrate that such crosstalk is required to drive their oncogenic or suppressor effects in a wide range of cancers. Along the same lines, investigation is also needed to determine whether such interactions between the cannabinoid system and miRNAs affect the outcome of cancer treatment.
4. Future Studies
Due to the immense inter- and intramolecular diversity existing between both patients and cancers, the observed cannabinoid-induced events of one patient or cancer type may not always correlate to similar cases. This fact is highlighted by the variable patient care outcomes seen with standardized cancer therapies.20 It is therefore important to determine the potential variation of cannabinoid-induced cancer responses for a multitude of cancers and more varied patient populations. As both recreational and medicinal use of cannabinoids increases, pharmacovigilance studies must also increase to determine potential adverse responses and/or increased therapeutic responses. Aside from the most studied cannabinoids such as CBD and THC, other types of cannabinoids also need to be explored, as they can have varied effects on cancers.
Moreover, although much work has been done in vitro to further the understanding of cannabinoids, many of those studies have yet to be explored in an in vivo model. It is important that future studies focus on relevant models to determine how in vitro effects will translate to a living organism. It has also been well established that cannabinoids can interact with non CB1/CB2 receptors.2,3 However, the currently known receptors that interact with cannabinoids are limited. Thus, potential adverse events arising from these off-target interactions are difficult to predict. Future studies will be needed to determine the capacity of cannabinoids to interact with other receptors, and to further delve into the resulting phenotypes produced.
Additionally, cannabinoids have been shown to produce undesirable effects in combination with clinical drugs.21,22 These resulting interactions range in severity from reduced therapy efficacy to complete therapy failure, toxicity, and even death.21 As cannabinoid usage continues to increase, future studies of the potential of cannabinoids to induce drug interactions must be conducted.
Future studies must continue to explore interactions with cannabinoids and non-miRNA epigenetic regulators. Other noncoding RNAs such as long noncoding RNAs and epigenetic regulators such as histones are responsible for regulation of gene expression. Similar to miRNA, alterations in these epigenetic regulators can induce disease states such as cancer.23 Recent studies indicate that cannabinoids can induce epigenetic changes, resulting in dysregulation of homeostasis. Much of this work has focused on the epigenetic dysregulation of the endocannabinoid system itself and the resulting disease states.21 Future studies must continue to explore interactions with cannabinoids and non-miRNA epigenetic regulators. This work could determine the extent of cannabinoid system interactions in driving a pro-oncogenic environment, as well as the potential use of cannabinoids in the repression of oncogenic genetic regulators.
Lastly, the effects of cannabinoids on miRNA expression are also shown to be responsible for the modulation of the immune as well as inflammatory systems. For example, Δ9-THC treatment in the normal lymph node cells and CD4+ T cells resulted in a decreased expression of miRNA-17, miRNA-92, miRNA-421, and miRNA-374b24 (Table 3). It is known that these miRNAs play an important role in tumor growth and promotion.25 However, it is not known whether Δ9-THC treatment regulates these miRNAs to induce oncogenic effects. Another study reported the beneficial influence of cannabinoids on immune response such as neuroprotection and immunosuppression by modulation of miRNAs. In this study, the results gathered indicated a link between the effects of cannabinoids on inflammatory signaling pathways. CBD inhibited inflammation-stimulated expression of miRNA-146a and miRNA-155. Furthermore, CBD upregulated miRNA-34a (Table 3). miRNA-155 and miRNA-34a are involved in immune response, cell cycle regulation, as well as cellular stress, and redox homeostasis.26 Two studies reported that endocannabinoid anandamide alters the expression of miRNAs that are involved in immunosuppressive pathways.27,28 Specifically, treatment with anandamide in the mice resulted in downregulation of miRNA-23a-3p and miRNA-34a-5p as well as upregulation of miRNA-125a-5p, miRNA-301a, miRNA-30e, and miRNA-151, leading to suppression of inflammation.27,28 Therefore, the relationship between cannabinoids, miRNA, and inflammation should be considered, as numerous causes and risk factors of cancer are linked with some form of chronic inflammation. Inflammation plays a vital part at various stages of tumorigenesis such as in initiation, malignant transformation, invasion, and metastasis. Inflammatory responses also impact cancer treatment response, and the immune cells that invade the tumors are involved in substantial crosstalk with cancer cells.29
Table 3. Effect of Cannabinoids on miRNA and Inflammation.
| cannabinoid | effect on miRNA | effect on inflammation | ref |
|---|---|---|---|
| Δ9-THC | Downregulation of miRNA-17, miRNA-92, miRNA-421, and miRNA-374b | Increased inflammation | (24) |
| CBD | Downregulation of miRNA-146a, miRNA-34a and miRNA-155 | Decreased inflammation | (26) |
| CBD | Upregulation of miRNA-34a | Decreased inflammation | (26) |
| Anandamide | Downregulation of miRNA-23a-3p, miRNA-34a-5p | Decreased inflammation | (27) |
| Anandamide | Upregulation of miRNA-125a-5p, miRNA-301a, miRNA-30e, and miRNA-151 | Decreased inflammation | (28) |
5. Conclusion
As mentioned above, several studies have explored the effects of cannabinoids in cancer pathology and treatment, and they showed conflicting results. A comprehensive understanding of the underlying mechanisms of the differential effects of cannabinoids in cancer would help predict and improve the prognosis of cancer patients. However, it remains to be demonstrated whether crosstalk between cannabinoid system and miRNAs occur in the majority of cancers and whether such an interplay contributes to cancer prognosis and cancer treatment outcomes. Therefore, it is important that future studies be focused on interactions between the cannabinoid system and miRNAs in a variety of cancers.
Acknowledgments
This work is supported by Auburn University Research Initiative in Cancer Grant and Animal Health and Disease Research Grant to S. R.P.
Biographies
Julia M. Salamat is currently a PhD student in the Biomedical Science Program at Auburn University under the mentorship of Dr. Satyanarayana R. Pondugula. Julia earned her Bachelor of Science in Biology degree at University of Tennessee at Chattanooga and her Master of Science in Biology degree at East Tennessee State University. Her research interests are in anticancer drug discovery and anticancer drug metabolism.
Kodye L. Abbott is currently a postdoctoral fellow at the Salk Institute for Biological Studies under the mentorship of Dr. Edward Stites. Kodye earned his bachelor’s degree in Cellular, Molecular, and Microbial Biology and his PhD in Biomedical Science from Auburn University. His research interests focus on the discovery of novel drug strategies to overcome chemotherapy resistance arising from dysregulation in the RAS/RAF/MEK/ERK (MAPK) pathway.
Patrick C. Flannery is currently a third year Medical Student at the Rocky Vista University College of Osteopathic Medicine in Parker, Colorado. He received his undergraduate degree in Microbiology and his Masters in Biomedical Sciences at Auburn University. His prior research focused on drug–drug interactions and pediatric high-grade gliomas.
Elizabeth L. Ledbetter is currently an undergraduate student pursuing her Bachelor of Science in Biomedical Sciences and Neuroscience Degrees with a pre-medical concentration at Auburn University. She is an Honors undergraduate researcher in Dr. Satyanarayana R. Pondugula’s lab. She is graduating in May 2023, and is planning to go to medical school. Her research interests are in anticancer drug discovery.
Dr. Satyanarayana R. Pondugula is currently an associate professor in the Department of Anatomy, Physiology, and Pharmacology at Auburn University. He earned his DVM from the College of Veterinary Science in Tirupati, India, and his PhD from Kansas State University in Manhattan, KS. He continued his training as a postdoctoral fellow at St. Jude Children’s Research Hospital in Memphis, TN. His research interests are focused on adverse drug interactions, anticancer drug resistance, and anticancer drug discovery.
Author Contributions
# J.M.S. and K.L.A. contributed equally. S.R.P. conceived the ideas, outlined the manuscript, and revised the manuscript. J.M.S., K.L.A., P.C.F, and. E.L.L. collected the literature and drafted the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
References
- Bifulco M.; Laezza C.; Pisanti S.; Gazzerro P. Cannabinoids and cancer: pros and cons of an antitumour strategy. Br. J. Pharmacol. 2006, 148 (2), 123–135. 10.1038/sj.bjp.0706632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli F.; Pagano E.; Romano B.; Panzera S.; Maiello F.; Coppola D.; De Petrocellis L.; Buono L.; Orlando P.; Izzo A. A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35 (12), 2787–2797. 10.1093/carcin/bgu205. [DOI] [PubMed] [Google Scholar]
- Vuckovic S.; Srebro D.; Vujovic K. S.; Vucetic C.; Prostran M. Cannabinoids and Pain: New Insights From Old Molecules. Front Pharmacol 2018, 9, 1259. 10.3389/fphar.2018.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott K. L.; Gill K. S.; Flannery P. C.; Boothe D. M.; Dhanasekaran M.; Pondugula S. R. Nothing Ventured, Nothing Gained: Regulations Cripple Potentially Life-Saving Research of Illicit Substances. ACS Chem. Neurosci. 2020, 11 (10), 1382–1384. 10.1021/acschemneuro.0c00241. [DOI] [PubMed] [Google Scholar]
- Weber L. V.; Al-Refae K.; Wolk G.; Bonatz G.; Altmuller J.; Becker C.; Gisselmann G.; Hatt H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer (Dove Med. Press) 2016, 8, 243–252. 10.2147/BCTT.S121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantini C.; Mosca M.; Nabissi M.; Lucciarini R.; Caprodossi S.; Arcella A.; Giangaspero F.; Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem 2007, 102 (3), 977–990. 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- Hart S.; Fischer O. M.; Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004, 64 (6), 1943–1950. 10.1158/0008-5472.CAN-03-3720. [DOI] [PubMed] [Google Scholar]
- Ivanov V. N.; Wu J.; Hei T. K. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, gamma-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget 2017, 8 (43), 74068–74095. 10.18632/oncotarget.18240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A.; Kuzontkoski P. M.; Groopman J. E.; Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther 2011, 10 (7), 1161–1172. 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- Brown J. D.; Winterstein A. G. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin Med. 2019, 8 (7), 989. 10.3390/jcm8070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik D.; Campbell N. R.; Karikari C.; Chivukula R.; Kent O. A.; Mendell J. T.; Maitra A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. cancer ther 2011, 10 (8), 1470–1480. 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharris E.; Singh N. P.; Nagarkatti P. S.; Nagarkatti M. Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 2019, 10 (1), 45–59. 10.18632/oncotarget.26534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaro A.; Emanuele S.; Geraci F.; D’Anneo A.; Lauricella M.; Calvaruso G.; Giuliano M. WIN55,212–2-Induced Expression of Mir-29b1 Favours the Suppression of Osteosarcoma Cell Migration in a SPARC-Independent Manner. Int. J. Mol. Sci. 2019, 20 (20), 5235. 10.3390/ijms20205235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan S.; Safe S. The cannabinoid WIN 55,212–2 decreases specificity protein transcription factors and the oncogenic cap protein eIF4E in colon cancer cells. Mol. cancer ther 2013, 12 (11), 2483–2493. 10.1158/1535-7163.MCT-13-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Jutooru I.; Lei P.; Kim K.; Lee S. O.; Brents L. K.; Prather P. L.; Safe S. Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a:ZBTB10 in breast cancer. Mol. cancer thers 2012, 11 (7), 1421–1431. 10.1158/1535-7163.MCT-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Qian X.; Zhu M.; Li A.; Fang M.; Zhu Y.; Zhang J. miR1273g3p promotes proliferation, migration and invasion of LoVo cells via cannabinoid receptor 1 through activation of ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Mol. Med. Rep 2018, 17 (3), 4619–4626. 10.3892/mmr.2018.8397. [DOI] [PubMed] [Google Scholar]
- He D.; Wang J.; Zhang C.; Shan B.; Deng X.; Li B.; Zhou Y.; Chen W.; Hong J.; Gao Y.; et al. Down-regulation of miR-675–5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol. Cancer 2015, 14, 73. 10.1186/s12943-015-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai K.; Shi X.-Y.; Yi F.-S.; Huang Z.-Y.; Wu X.-Z.; Dong S.-F.; Wang W.; Wu M.-T.; Shi H.-Z. IL-10 promotes malignant pleural effusion by regulating TH1 response via an miR-7116–5p/GPR55/ERK pathway in mice. Eur. J. Immunol. 2020, 50 (11), 1798–1809. 10.1002/eji.202048574. [DOI] [PubMed] [Google Scholar]
- Sredni S. T.; Huang C. C.; Suzuki M.; Pundy T.; Chou P.; Tomita T. Spontaneous involution of pediatric low-grade gliomas: high expression of cannabinoid receptor 1 (CNR1) at the time of diagnosis may indicate involvement of the endocannabinoid system. Childs Nerv Syst 2016, 32 (11), 2061–2067. 10.1007/s00381-016-3243-7. [DOI] [PubMed] [Google Scholar]
- Lee W.; Huang D. S.; Han K. Constructing cancer patient-specific and group-specific gene networks with multi-omics data. BMC Med. Genomics 2020, 13 (S6), 81. 10.1186/s12920-020-00736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadou E.; Jacob L. S.; Slack F. J. Non-coding RNA networks in cancer. Nature reviews. Cancer 2018, 18 (1), 5–18. 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamat J. M.; Abbott K. L.; Flannery P. C.; Pondugula S. R. Dysregulation of Endobiotic Homeostasis Mechanisms: Novel Insights into Adverse Pharmacokinetic Interactions between Illicit Substances and Clinical Drugs. ACS Chem. Neurosci. 2020, 11 (24), 4021–4023. 10.1021/acschemneuro.0c00726. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; He C.; Wang M.; Ma X.; Mo F.; Yang S.; Han J.; Wei X. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther 2019, 4, 62. 10.1038/s41392-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Bam M.; Nagarkatti P. S.; Nagarkatti M. RNA-seq Analysis of delta9-Tetrahydrocannabinol-treated T Cells Reveals Altered Gene Expression Profiles That Regulate Immune Response and Cell Proliferation. J. Biol. Chem. 2016, 291 (30), 15460–15472. 10.1074/jbc.M116.719179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H.; Zhou Y.; Zhou D.; Zhang Y.; Shang D.; Qi J. The latest progress on miR-374 and its functional implications in physiological and pathological processes. J. Cell Mol. Med. 2019, 23 (5), 3063–3076. 10.1111/jcmm.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juknat A.; Gao F.; Coppola G.; Vogel Z.; Kozela E. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-Effect of cannabinoids. PloS one 2019, 14 (2), e0212039. 10.1371/journal.pone.0212039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan M.; Alghetaa H.; Mohammed A.; Abdulla O. A.; Wisniewski P. J.; Singh N.; Nagarkatti P.; Nagarkatti M. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating miRNA that Target Inflammatory Pathways. Front Pharmacol 2021, 12, 644281. 10.3389/fphar.2021.644281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. R.; Nagarkatti P.; Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS One 2014, 9 (4), e93954 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I.; Greten F. R.; Karin M. Immunity, inflammation, and cancer. Cell 2010, 140 (6), 883–899. 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]