Abstract
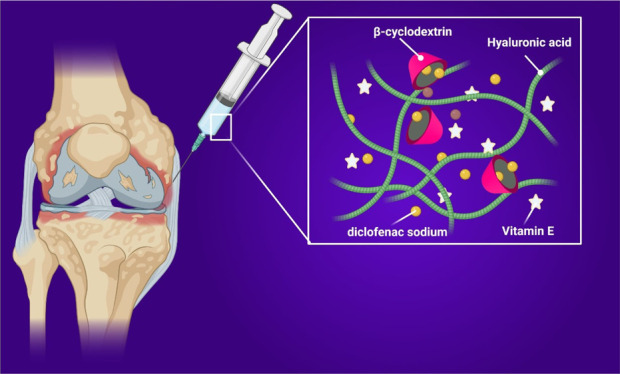
Hyaluronic acid (HA) and its derivatives are widely used for intra-articular injection to augment compromised viscoelastic properties of damaged synovial fluid. Combining HA-based devices with anti-inflammatory drugs or bioactive principles in order to provide an additional benefit to the viscosupplementation is emerging as a new promising approach to improve the clinical outcome. Here, we aim to design a novel active viscosupplementation agent that can load and release hydrophobic drugs and at the same time possessing antioxidant properties. Optimized ternary systems named HCV based on HA, (2-hydroxypropyl)-β-cyclodextrin (CD), and vitamin E (VE), without being engaged in formal chemical bonding with each other, showed the best viscoelastic and lubrication properties along with antioxidant capabilities, able to solubilize and release DF. The physical–chemical characterization suggested that the HCV system displayed rheological synergism and higher thermal stability because of the presence of VE and its antioxidant activity, and the loading of hydrophobic drugs was improved by the presence of CD and VE. Cell morphology and viability tests on L929 cells exhibited high biocompatibility of the HCV system with higher level expression of anti-inflammatory interleukin-10.
1. Introduction
Hyaluronic acid (HA) is a naturally occurring polysaccharide, which is the major component of the extracellular matrix (ECM) in mammalian connective tissues, where it fulfills important physicochemical and biological functions. HA, indeed, controls viscoelastic properties of soft tissues, cell growth, proliferation, and tissue remodeling.1 In the articular joints, HA confers to the synovial fluid (SF) highly viscoelastic properties because of its capability to retain water and to act as an expanded random coil, occupying a large hydrodynamic volume. This causes neighboring molecules to overlap to form a transient network structure with marked viscoelasticity.2 In the case of trauma, pathologies such as osteoarthritis (OA), and aging, the SF loses its rheological properties because of the decrease of HA concentration, molecular weight, and HA distribution within the joint, causing the decline of viscoelastic features of the endogenous SF.3 The current therapeutic approaches for restoring SF properties consist of intra-articular injection of HA and its derivatives (viscosupplementation agents) to joints thus to augment the compromised SF viscoelastic properties.4 In OA, which is a destructive joint disease causing ECM degradation, deterioration of cartilage, and bone and joint inflammation, oral therapy based on nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., diclofenac sodium (DF)) is often necessary, in combination or as an alternative to HA viscosupplementation, to reduce inflammation and help the homeostasis of the joint.5 However, the prolonged use of such drugs causes important systemic adverse effects. One of the major concerns in the use of viscosupplementation agents is how to reduce the number of injections by increasing the residence time of HA. To this aim, chemical modification and intra and intermolecular chemical crosslinking are performed to stabilize the HA network and improve the viscoelastic properties.6 These methods often can impair HA biological properties. At the same time, local and prolonged administration of NSAIDs is desirable to minimize the amount of orally administered drug along with maximizing its concentration at the joint.7 Thus, new approaches are emerging combining HA with drugs or bioactive principles in a single injection, to provide an additional benefit to the viscosupplementation. Mixtures based on the combination of DF mechanically mixed with a highly crosslinked HA have been developed.8 However, drugs such as DF possess a short half-life (of approximately 2 h for DF), and conventional dosage forms do not provide a prolonged release of the drug. Moreover, the solubility of lipophilic drugs as DF in the water-based HA viscosupplementation agents is low.9 During OA, chondrocytes release higher levels of reactive oxygen species in response to partial oxygen pressure fluctuations, mechanical stress, and inflammatory mediators, resulting in oxidative damage to various components of the joint, which include collagen, proteoglycans, and hyaluronan and by interfering in matrix synthesis and inducing cell apoptosis.10 To overcome these drawbacks, some viscosupplements containing antioxidants, such as mannitol and sorbitol, which are known to be very well tolerated, have been previously studied,11 to further add an antioxidant character to HA systems and to reduce their in situ degradation, increasing their time of contact with damaged tissue. Among antioxidant molecules, significantly lower amounts of vitamin E (VE) in the SF of osteoarthritic knees in a study comparing the levels of antioxidants in the osteoarthritic knees with severe cartilage damage with the knees having intact cartilage have been reported.12 Intra-articular administration of VE resulted in the preservation of the joint surface displaying a potential chondroprotective effect,13 but restoring the impaired SF viscoelastic properties was lacking. In this frame, the aim of this study was to design an active viscosupplementation agent able to load and prolong release of hydrophobic drugs at the same time possessing antioxidant properties. As for the ability to improve the drug loading and release, (2-hydroxypropyl)-β-cyclodextrin (CD) has been employed because of its ability to form inclusion complexes with a variety of drugs; in particular, CD is known to improve the solubility of hydrophobic drugs, such as DF and to protect against physicochemical and enzymatic degradation.14,15 Indeed, CD is chemically and physically stable under physiological conditions, with excellent biocompatibility and nonimmunogenicity properties. During the formation of an inclusion complex, the drug molecules are partially or completely entrapped inside its hydrophobic cavity with no covalent bonding.15 VE has been used to confer antioxidant ability to the systems. VE is referring to a group of lipid-soluble bioactive compounds, including tocopherols (a-, b-, g -, and d-tocopherol) and tocotrienols (a-, b-, g-, and d-tocotrienol), with potent antioxidant activities, as they scavenge lipid peroxyl radicals by donating hydrogen from the phenolic group on the chromanol ring.16 In general, lipid peroxidation is increased because of the diminished antioxidant defense mechanisms. Thanks to its antioxidant activity, VE has been used to improve the thermal stability of the formulations. Here, formulations based on HA, CD, and VE, without introducing chemical bonds among those components, have been produced and characterized for the delivery of NSAIDs, such as DF, and for the simultaneous viscosupplementation of the impaired SF. The thermal properties of the systems were analyzed through differential scanning calorimetry (DSC). Then the formulations were also characterized by rheological and tribological analysis and solubility and drug release studies. In vitro biological responses, such as cytotoxicity, cell morphology, and anti-inflammatory level expression of interleukin-10 (IL-10), have been evaluated.
2. Results and Discussion
2.1. Antioxidant Activity
The ability of the HCV formulations to scavenge the stable radical DPPH17 was estimated to have information about the antioxidant activity of the samples. DPPH is a stable radical, which adsorbs at 515 nm, commonly used in the literature in the quantitative analysis of the antioxidant activity. In Figure 1, the absorbance of DPPH as the control and a mixture of HCV samples at different concentrations, plus DPPH, in the wavelength range of 450–600 nm is reported. The ability of the formulations to scavenge DPPH is shown by the lowering of the maximum absorption as the concentration of the systems increases. The SA of HCV was also evaluated. SA increases as concentration increases; indeed, it was 8.4, 9, and 14% for HCV at a concentration of 25 and 300 μg/mL, respectively.
Figure 1.
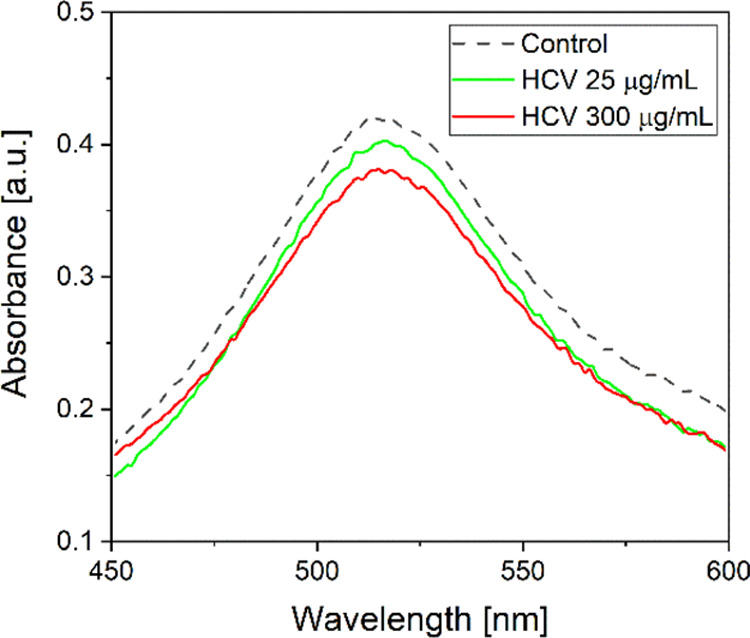
Effect of concentration of HCV on the maximum absorbance peak of DPPH.
2.2. Thermal Properties
The DSC thermograms of the samples are reported in Figure 2. This kind of analysis allows distinguishing the different behavior of water in polymeric systems: nonfreezing water (NFW) and freezing water (FW).18 NFW is related to water molecules that, due to strong interactions with the polymer, are not able to freeze, and consequently, they do not show a peak in DSC thermograms. FW is related to the water not bound to polymer molecules, which acts as in the absence of other components with a melting point at about 0 °C. The amount of FW in the sample was calculated by dividing the endothermic peak area for the water melting enthalpy (333.5 mJ mg–1), and the amount of NFW was calculated by subtracting FW from the total water in the systems. The percentages of NFW and FW are reported in Table 1. In the DSC thermograms, there is one endothermic peak at about 0 °C. This peak is associated with the melting of FW. The area of the endothermic peak of HA was 65 mJ mg–1 while HV materials exhibit a reduction in melting water (37 mJ mg–1) compared to HA, probably because of the formation of water clusters around the hydrophobic VE.19 Thermograms after the addition of CD to the HC system show a reduction in melting enthalpy (26 mJ mg–1) of FW, probably because of the hydration of CD, which reduces the amount of water capable of freezing. In fact, in the absence of hydrophobic molecules, water tends to arrange in the CD cavity.20 On the contrary, in HCV the addition of VE brings to an increase in the water melting enthalpy up to 78 mJ mg–1, probably caused by the formation of CD-VE inclusion complexes and less availability of the CD cavity to water molecules.21 DSC results confirm that the addition of VE to the ternary HCV system allows a strong interaction between CD and VE.
Figure 2.
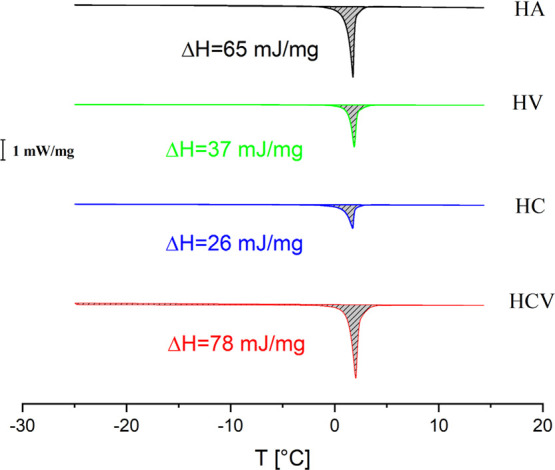
DSC thermograms for the samples HA, HV, HC, and HCV at 2.5 °C/min.
Table 1. Percentage of NFW and FW Calculated by DSC Analysis.
| sample | NFW (%) | FW (%) |
|---|---|---|
| HA | 80 | 20 |
| HV | 89 | 11 |
| HC | 92 | 8 |
| HCV | 75 | 25 |
2.3. Rheological and Tribological Properties
Figure 3 shows the comparison of mechanical spectra, that is, G′ and G″ as a function of frequency, of HCV and of a commercially available viscosupplementation agent used in the clinical practice. As it can be seen, HCV exhibited both G′ and G″ values, higher than those of the commercial product over all the frequency ranges analyzed. Both systems exhibit a rheological behavior that is typical of an entangled network, namely, viscous at low frequency (G″ > G′) and prevalently elastic at high frequencies (G′ > G″). The limit between the two regions is represented by the crossover frequency, fc, which was about 1.77 Hz for the commercial one and 0.6 Hz for HCV systems. At low frequency, the molecular chains can release stress by disentanglement and molecular rearrangement during the period of oscillation, and hence, the solution shows viscous behavior (G″ > G′). At high frequency, however, molecular chains cannot disentangle during this short period of oscillation, and therefore, they behave as a temporary crosslinked network, and the elastic behavior (G′ > G″) is prevalent.2 Crossover frequency is inversely related to the relaxation time of the system. This behavior indicates that the polymeric chains are linked with each other, and their possibility to disentangle and flow (and, hence, their relaxation time) depends on the frequency of the applied deformation.22 HCV has crossover frequency similar to the healthy knee SF, which is around 0.3 Hz.2,23 These data indicated that HCV behaves as a more elastic system than the commercial one. This prevalent elastic character is particularly desirable for a product suitable for viscosupplementation devices in OA applications. This is not only due to its ability to reduce the mechanical energy applied to the cartilage, but also because it has been seen that the higher sample systems’ elasticity results in higher analgesic capacity.2 Substances with enhanced elastic characteristics have been reported to reduce the effect of the nociceptive stimulus on the activity of the medial articular nerve and decrease the sensory response to passive movements of the inflamed knee joints. Hypothetically, this positive effect is due to their ability to absorb a significant part of the mechanical energy of the stimulus, thus reducing transmission to the mechanical-transducer apparatus where the pain signal originates.6
Figure 3.
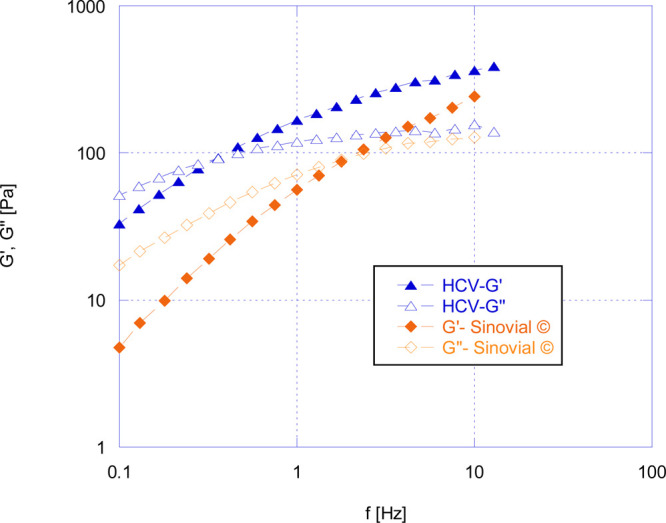
Comparison of mechanical spectra of HCV and a commercially available product in the market at 37 °C.
Figure 4A shows the G′ and G″ curves at 37 °C, as0 a function of frequency for HCV and for the binary formulations HC and HV and HA solution as comparison, while Figure 4B shows the representative curve of both moduli at 37 °C after AC at 121 °C. As previously reported, before the AC process all materials exhibit the mechanical spectra of an entangled polymer solution. Mechanical spectra registered at 37 °C for HA, HC, and HV are almost stackable, sign that the addition of CD and VE does not alter the properties of entangled HA solution. As reported in Table 2, the HCV formulation had improved rheological properties (both G′ and G″) in comparison with other binary systems HC and HV and HA solution. Elastic and viscous moduli at f = 1 of HCV were 126 and 98 Pa, respectively, before AC. After AC, HCV systems had G′ and G″ values of 47 and 67 Pa (Table 2). The mechanical spectra of HA and HV show a collapse of the mechanical properties of the materials, and the disappearance of the crossover after AC indicated the loss of the elastic response because of the reduction of the entanglement among the chains, which occurs due to the breaking of the chains caused by thermal treatment. Contrariwise, HC systems undergo a much lower decrease in the value of viscoelastic moduli, and their crossover point is just shifted to higher frequency values, which indicated the interaction of CD and HA stabilizing the network. The reduction of viscoelastic properties when VE was added into the HCV systems is even less, and the crossover value is shifted from 0.45 to 4.7 Hz. These results highlight how the presence of CD and VE prevents the thermal degradation of HA, contributing to maintaining the molecular weight of the polymer after sterilization and to avoid the reduction of the mechanical properties in HCV systems. The ratio of G′/G′AC reported in Table 2 was calculated by dividing elastic moduli before and after the sterilization at 10 Hz, and it allows to express quantitatively the effect of CD and VE on the degradation of HA. It is possible to observe that the ratio is 9.66, 2.99, 5.04, and 1.57 for HA, HC, HV, and HCV, respectively; this clearly indicated that the simultaneous presence of CD and VE contributed to preventing the loss of the viscoelastic properties after the sterilization process. The results suggested that the addition of VE and CD in HCV systems contributed to the formation of a cooperative system, probably because of the inclusion complex between CD and VE according to DSC data, and by the interaction between CD and HA according to rheological data. This improvement in the viscoelastic properties of HCV indicated that a rheological synergism was established, because of the physico-chemical interactions among the three entities. The rheological synergy can be quantified by the interaction parameter, which is the difference between the dynamic modulus values of the mixture evaluated by the rheological test and the theoretical one given by summing the dynamic modulus values of the primary components. G′ values were used to calculate the synergistic parameter (ΔG′synergistic) according to eq 1:
| 1 |
G′CD is the elastic modulus of a CD solution at the same concentration used to prepare HC and HCV and is 0.013 Pa. The values of ΔG′synergistic are reported in Table 3, where 77 and 40 are the values before and after AC, respectively. These strongly positive values of ΔG′synergistic indicated the presence of robust synergism, and thus the addition of both CD and VE to a solution of HA plays an important role in creating a network that stabilized the HCV system and led to an improvement of the viscoelastic properties. These data are corroborated also by the DSC analyses, previously reported in which the interaction between CD and VE, known to form an inclusion complex, was highlighted. Furthermore, rheological characterization has demonstrated the cooperative system in which the three entities HA, CD, and VE synergistically cooperate with one another, without being engaged in formal chemical binding with each other, to achieve an increased viscoelastic profile and an elevated degree of protection of the same, obtaining a product suitable to withstand high-impact treatments like, for example, thermal sterilization.
Figure 4.
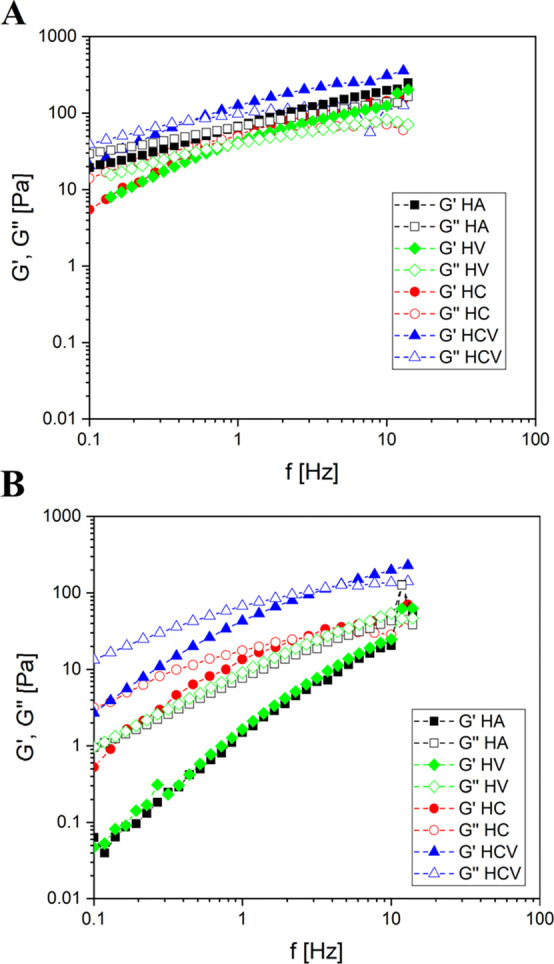
Mechanical spectra of HC, HA, HV, and HCV formulations before AC (A) and after AC (B) at 37 °C.
Table 2. Viscoelastic Properties before and after Autoclaving (AC) with the Values of G′ and G″ Obtained at 1 Hz at 37 °C.
| before AC at 37 °C | after
AC at 37 °C |
G′/G′AC 37 °C |
|||
|---|---|---|---|---|---|
| 1 Hz | 10 Hz | ||||
| G′ (Pa) | G″ (Pa) | G′ (Pa) | G″ (Pa) | ||
| HA | 72 | 68 | 2 | 11 | 9.66 |
| HC | 53 | 49 | 15 | 20 | 2.99 |
| HV | 57 | 50 | 2 | 9 | 5.04 |
| HCV | 126 | 98 | 43 | 67 | 1.57 |
Table 3. Synergistic Effect of Formulation Viscoelastic Parameters at 1 Hz.
| condition | G′HCV (Pa) | G′HA+VE (Pa) | G′CD (Pa) | ΔG′Synergistic (Pa) |
|---|---|---|---|---|
| 37 °C | 130 | 53 | 0.013 | +77 |
| 37 °C after AC | 48 | 8 | 0.013 | +40 |
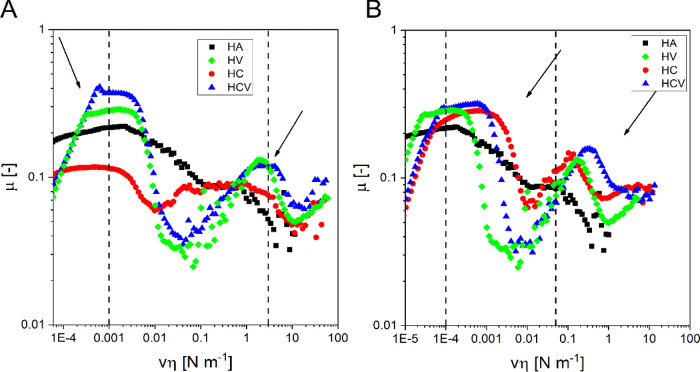
Figure 5 shows the Stribeck curves before (A) and after AC (B), which represent the friction coefficient (μ) as a function of the product of sliding velocity (v) and viscosity (η).
Figure 5.
Stribeck curves of materials HA, HV, HC, and HCV before (A) and after (B) AC and physiological range of vη (between dotted lines).
According to the behavior described in the literature for shear-thinning lubricants, all the curves exhibited peaks associated with two transition regions in which the lubrication was dominated respectively by surface–liquid asperity (mixed regime) and liquid–liquid asperity interactions, respectively.24 The curves were constructed using the zero-shear viscosity for each sample (data not shown).25 The Stribeck curves of HA and HC, qualitatively, show the same trend, which differs from the theoretical one reported in the literature24 because it shows two vη ranges (first range from to 2 × 10–5 to 2 × 10–3 N m–1 and second range from 0.01 to 1 N m–1) in which the μ remains constant (constant region). This behavior is associated with an inefficient lubrication because of the elastoviscous response of HA chains, and this causes the rubbing of the surfaces (boundary regime).26 This regime is responsible for the wear of cartilage,27 and therefore, it should be avoided that a viscosupplementation device operates in that regime under the physiological sliding speeds (0.1–50 mm s–1); quantitatively, the μ of HC in the first constant region is lower compared to mu of HA indicating that CD interacts with the asperities of the surface, resulting in a μ decrease. Contrariwise, in the second constant region, the HC curve overlaps the HA curve, showing that for both systems this second region is dominated by HA–HA interactions. The Stribeck curve of HV and HCV instead exhibited a trend similar to that described in the literature, indicating that the addition of VE improved the lubrication of the systems, by improving the interaction of the systems with the surfaces (I peak, vη = 1.3 × 10–3 N m–1) and with other components of the formulation (II peak, vη = 2.5 N m–1). As reported above, the ability to avoid the friction between the surfaces is fundamental for a viscosupplementation device, and the addition of VE represents an effective way to improve the tribology properties of these materials.
The Stribeck curves for the AC samples are reported in Figure 5B. As well as for the viscoelastic properties, the sterilization process brought a reduction in the viscosity of the materials, and consequently, the curves were shifted to lower vη values. After sterilization, a quantitative reduction in μ is not observed, which remains in the order of magnitude of 0.01–0.1. In the HA curve, the constant region is narrower. According to rheological data, this is probably due to the loss of viscoelastic properties because of the thermal degradation, with a consequent lower resistance to lubrication.
The curves of the HCV and HV samples exhibit a qualitatively similar trend after AC, with the first transition at about 1 10–4 Nm–1 and the second transition at about 0.1 Nm–1, vμ values lower than those before AC. This suggests that the presence of VE in HV allows the material to maintain its lubrication properties also after the AC. The HC curve exhibits narrower peaks in correspondence of the two transitions, indicating that, probably, the partial thermal degradation of HA reduces the lubrication resistance of the material. Finally, for the HCV that possesses the best viscoelastic properties also after AC, the curve has a lubrication behavior analogous to that of HV, and moreover in vμ values corresponding to the walking and running velocity, it presents a minimum of μ suggesting that HCV presents optimum features from both viscoelastic and lubrication points of view.
2.4. Drug Solubility and Release Kinetics
The solubility of DF in phosphate-buffered saline (PBS) was 5.15 mg/mL in accordance with the literature.28 The solubility of DF in HCV systems was 16 mg/mL, which was three times higher than that of DF in physiological solution.
Experimental and simulated in vitro fractional release profiles of DF in phosphate buffer are shown in Figure 6. It can be seen that after 10 h only 48% of the drug in the medium is released, and after 24 h, there is complete release. Prominently, no significant differences among the three samples were observed, which indicated a homogeneous solubilization in the prepared composition. Data were fitted with the Korsmeyer–Peppas kinetic model (eq 4), which gave a better fit with a high correlation coefficient (R2 = 0.96) for the composition. From the fitting, the values of the kinetic release constant, Kk (0.046 h–0.9682), and of the release exponent, n (0.97), were obtained. In particular, the value of n provides information about the release mechanism: n = 0.5 is related to Fickian diffusion (Higuchi matrix), 0.5 < n < 1.0 indicates anomalous (non-Fickian) diffusion, and n = 1.0 indicates case II transport (zero-order release), associated with swelling and release of polymeric chains, and n > 1.0 indicates super case II transport.29 The value of n obtained from the model (n ≈ 1) let us to hypothesize that the release of DF was mainly due to the swelling of the material and/or to the relaxation of polymer chains, and so related to the structures of the proposed systems.
Figure 6.
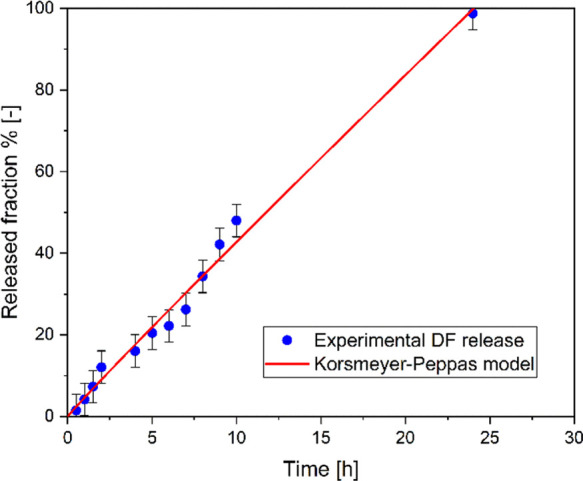
Drug release kinetics fitted the kinetic equations. It can be seen that after 10 h, only 48% of the drug was released in the medium, and after 24 h, there is the complete release of the drug, which shows the controlled release of the composition.
2.5. Biological Response
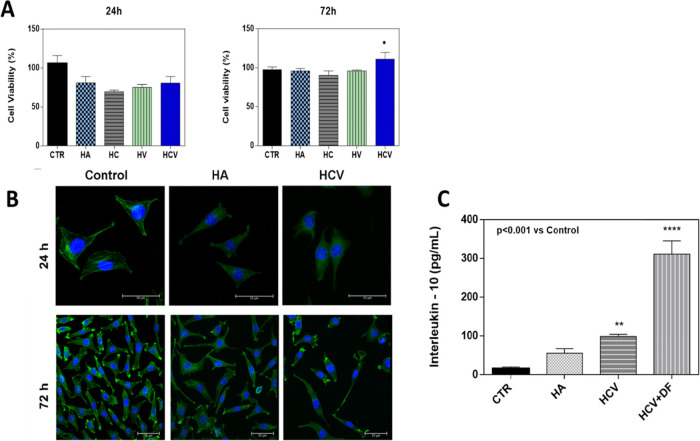
Cell viability and morphological analysis are a primary feature that must be evaluated in the design of viscosupplementation systems able to prolong the release of an anti-inflammatory drug into the joint cavity. Indeed, it has been widely demonstrated that the viscoelastic characteristics of the materials influence cell behavior, affecting the biocompatibility of the cells.30 L929 cell viability was evaluated by Alamar blue (AB) assay, and the prepared formulations exhibited good in vitro biocompatibility. As reported in Figure 7A, both HCV systems and binary formulations (HC and HV) showed good safety after 24 and 72 h of incubation with L929 cells, compared to the untreated control and HA control. In particular, after 24 h of incubation with both formulations, L929 cell viability is around 80%; note that, at 72 h, the viability increases around 100% and in particular over 100% (about 110%) for the sample with HCV. It is noteworthy that cooperative HCV systems, with improved viscoelastic properties, after 72 h of exposure with L929 cells, have demonstrated a significant enhancement in cell metabolic activities directly related to the percentage of cell viability, probably because the nutrient recirculation was more efficient in these systems.31 Biocompatibility results were also confirmed by cell morphology. Actin filaments, a constituent of the cytoskeleton, were stained with FITC phalloidin after 24 h of incubation with systems. L929 cells, indeed, exhibited a typical no cytotoxic fibroblast-like morphology after the incubation with the formulations (Figure 7B). Their morphology was alike to the characteristic in vitro L929 morphology that is spread-shaped, often characterized by several extending processes, which consists of cell protrusions adhering at the flat surface. Overall, these results indicated that the devices have good biocompatibility properties and that the HCV formulation, containing HA, CD, and VE, improved the viability of the cells after 72 h of incubation, suggesting that the combination of these three compounds positively affects the cell metabolism. Synovial inflammation in OA diseases appears to be fundamental to the progression of cartilage lesions. This is due to the secretion of inflammatory mediators in OA joint tissue. The anti-inflammatory cytokines, such as IL-10, serve to counterbalance the activities of proinflammatory cytokines involved in OA.32 For this reason, the expression of IL-10 levels has been tested to prove the anti-inflammatory potential of HCV systems per se and, in particular, after the incorporation of DF drugs into them (Figure 7C). Results demonstrated that cooperative HCV systems, after inflammatory stimulation of cells with lipopolysaccharides (LPSs), induced higher production of IL-10 levels (100 pg/mL), compared to untreated cell controls (15 pg/mL) and HA-controls (55 pg/mL). Interestingly, levels of IL-10 were found to be three times higher (about 300 pg/mL) when the DF drug was incorporated into the HCV system, confirming an efficient release of the drug in cells in vitro tests and suggesting a potential remarkable action of the loaded systems for OA pathologies. The HA anti-inflammatory action has already been widely correlated with its high molecular weight,33 and the ability of VE to promote the production of anti-inflammatory cytokines has been also reported;34 moreover, among the anti-inflammatory effects, DF promotes the production of IL-10.35 HCV DF-embedded systems revealed significant IL-10 expression, indicating that cooperative systems with improved viscoelastic properties were suitable for viscosupplementation and simultaneous local delivery of drugs.
Figure 7.
(A) Percentage viability of binary systems HC, HV, HA solution, and HCV systems at 24 and 72 h compared to the control untreated cells. The data are representative of three repeated experiments in triplicate. *p < 0.05. (B) Cell morphology of the control, HA, and HCV systems after 24 h. Actin filaments, stained by phalloidin-FITC (green) and DAPI (blue)-stained nuclei cells. Images were acquired with a resolution of 1024 × 1024 pixel with a 63× oil immersion objective. (C) Effects of HCV and HCV + DF systems on anti-inflammatory IL-10 expression on L929 cells, after 24 h of LPS (1 μg/mL) inflammatory stimulation. Results are mean ± of three experiments. *p < 0.001 vs Control.
3. Conclusions
Here, we successfully developed HA-based devices able simultaneously to act as viscosupplementation agents and to delivery locally hydrophobic drugs, such as diclofenac. Ternary systems named HCV, based on HA, CD, and VE have been optimized. The formulation exhibited improved rheological properties along with lower friction, compared to the HA solution, indicating the feasibility of the device for viscosupplementation applications potentially able to restore the viscoelastic features of the pathologic SF. Moreover, the HCV system displayed antioxidant activity, along with thermal stability and the ability to solubilize and release diclofenac. The HCV system showed high biocompatibility as a result of natural-based components and optimal expression levels of anti-inflammatory IL-10.
4. Materials and Methods
4.1. Materials
HA with a weight-average molecular weight (Mw) of 1490 kDa, DF, CD (128446-35-5), and VE acetate (Tocopherol acetate 7695-91-2) were kindly provided by Altergon Italia. PBS was purchased from Sigma-Aldrich.
4.2. Formulation Preparation
Formulations were prepared by mixing the dry powder of CD, HA, and liquid VE with each other according to the sample to be obtained; subsequently, PBS solution was added to the mixture with maintaining stirring for at least 8 h at room temperature (RT). Several formulations based on HA, CD, and VE were prepared and investigated by rheological properties and thermal stability. The final ternary optimized formulation, named HCV, has a concentration of 2% (w/w) of HA, CD, and VE, respectively. The compositions of binary formulations, named, HC, HV, and HA solution were used as comparison; HC at a concentration of 2% (w/w) of HA and CD, and HV at a concentration of 2% (w/w) of HA and VE, and HA solution at a concentration of 2% (w/w).
4.3. Antioxidant Activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method is widely used to evaluate the free radical scavenging ability of natural antioxidants. In the radical form, the DPPH molecule has an absorbance at 517 nm, which disappears after acceptance of an electron or hydrogen radical from an antioxidant agent to become a stable diamagnetic molecule.36 For this purpose, in 3 mL of EtOH containing HCV at different concentrations (25 and 300 μg/mL) 1 mL of ethanolic solution of DPPH (25.0 μg/mL) was added. The resultant mixture was shaken thoroughly and allowed to stand at RT in a dark place for 1 h. Subsequently, the absorbance of the samples was measured using a UV–visible spectrophotometer (UV–VIS JASCO Mod. V570) to measure optical density at 517 nm. Ethanol and DPPH solution were used as the blank and negative control, respectively. The following equation was used to calculate the percentage of scavenging ability (SA):
| 2 |
where Asample is the absorbance in the presence of the samples and standard and Acontrol is the absorbance of the control.
4.4. Thermal Properties
The thermal behavior of the samples was determined using DSC, using a TA Instruments (Discovery series, USA) under N2 flow of 20 mL min–1 at a heating rate of 2.5 °C min–1 from −20 to 40 °C. DSC is traditionally employed to study the hydration of carbohydrate polymers, through freezing/thawing experiments.37
4.5. Rheological Properties
Small-amplitude oscillatory shear tests were performed to evaluate the time-dependent response of the systems. The measurements were carried out through a rotational rheometer (Mars III, HAAKE Rheometer, USA), using a parallel-plate geometry. The tests were carried out at the controlled temperatures of 20 and 37 °C using a thermostatic bath. To identify the linear viscoelastic response range of the materials, preliminary strain sweep tests were performed on the samples, at an oscillation frequency of 1 Hz. The tests were repeated at least three times on each sample. The frequency was in the range from 0.01 to 15 Hz. From the test, it was possible to evaluate the dependence of the storage or elastic modulus (G′) and the loss or viscous modulus (G″) upon the frequency, the so-called mechanical spectra. G′ gives information about the elasticity or the energy stored in the material during deformation, whereas G″ describes the viscous character or the energy dissipated as heat. In particular, the elastic modulus gives information about the capability of the sample to sustain load and return in the initial configuration after an imposed stress or deformation.
4.6. Rotational Tribology
The lubrication ability of the optimized ternary systems was evaluated through tribology experiments using a rotational rheometer (Physica MCR 302, Austria) with a ball-on-three-pin configuration. The instrument was equipped with a tribology glass measuring ball (BC12.7, diameter = 12.7 cm) and a sample holder in which three cylindrical shaped pins of polydimethylsiloxane were loaded, to simulate the behavior of the cartilage surface.38 These cylinders were fully immersed by adding 1 mL of the formulations. During the measurements, a normal force of 6 N was applied, resulting in a contact pressure of approximately 0.1 MPa. The friction coefficient μ, that expresses the interaction between two sliding surfaces, was measured as a function of the sliding velocity in the range of 0.1–1000 mm/s. The lubrication properties of the materials were evaluated before and after the sterilization process in an autoclave by reporting the friction coefficient as a function of the product vη, where v is the sliding velocity and η is the viscosity of the material. This represents a simplified form of the Stribeck curve that is commonly used to study the performances of a lubricant.39
4.7. Drug Solubility and Release Kinetics
To perform drug solubility and release kinetic tests, 20 mg/mL of DF were added to the HCV formulation and stirred until being completely homogenized. To carry out the solubility test, the formulation was kept for 24 h and centrifuged (6000 rpm for 15 min), and the supernatant was analyzed by UV spectroscopy. The tests were performed in triplicate, and the wavelength used for the detection of DF was 276 nm. Moreover, a calibration curve was constructed by plotting absorbance against the predetermined concentration of DF in PBS. Then, linear regression was used to determine the calibration curve. The solubilized fraction percentage (SF%) was expressed by eq 3:
| 3 |
To perform the release test, 1 g of the formulation containing DF at 1% w/w was inserted in a dialysis membrane (cut off 500 to 1000 Da) and immersed in PBS (18 mL) at a temperature of 37 °C. At predetermined time intervals, 50 μL aliquots of the medium were withdrawn, and the same volume of fresh medium was replaced. The drug concentration released into the PBS buffer was detected using a UV spectrophotometer as a function of time.
Experimental data were analyzed using the Korsmeyer–Peppas kinetic model reported in the literature (eq 4):
| 4 |
where Qt and Q∞ are the amounts of drug released at the time (t) and at equilibrium, respectively. kk is the release rate constant that considers the geometric and structural features of the carrier, and n is the release or diffusional exponent, which is related to the drug release mechanism.29
4.8. Cell Culture
To evaluate the biological response of the formulations, L929 cells derived from mouse C34/An connective tissues (Sigma-Aldrich, USA) were used. L929 cells were grown in a T-75 cell culture flask (Falcon, Italy), in cell culture medium Dulbecco’s Modified Eagle’s medium (Hyclone, USA) supplemented with 10% fetal bovine serum and antibiotics (penicillin G sodium 100 U/mL, streptomycin 100 μg/mL) at 37 °C and 5% CO2. The medium was changed every 3–4 days.
4.8.1. Cell Viability and Morphology Assay
To understand the cell viability, L929 cells were seeded at a density of 8 × 104 cells/mL on a 96-well plate (World Precision Instruments, Inc.). The formulations were sterilized by steam AC at 121 °C for 20 min. The cells were incubated with 5 μL of the formulations for each well in triplicate up to 72 h, and then AB assay was performed by adding AB reagent to the samples (at 10% v/v with respect to the medium) and incubated at 37 °C for 4 h. The absorbance of the samples was measured using a spectrophotometer plate reader (1420 Victor, PerkinElmer) at 570 nm and 600 nm. AB is an indicator dye that incorporates an oxidation–reduction indicator that changes color in response to the chemical reduction in the growth medium, resulting from cell viability. L929-seeded wells were used as a control. Data are expressed as the percentage difference between the treated and control cells to evaluate the percentage of reduction (Reduction %), which is calculated with the following formula (eq 5):
| 5 |
where O1 and O2 are the molar extinction coefficient (E) of oxidized AB at 570 and 600 nm; A1 is the absorbance of test wells at 570 nm; A2 is the absorbance of test wells at 600 nm; P1 is the absorbance of the control well at 570 nm; and P2 is the absorbance of the control well at 600 nm. The percentage of reduction for each sample was normalized to the percentage of reduction for the control to obtain the cell viability percentage.40
For the cell morphology assay, cells were seeded at a density of 1 × 104 cells/mL on fluorodish-35 mm (World Precision Instruments, Inc), and 5 μL of the formulations were incubated for 24 h. Then, the samples were washed two times with PBS and fixed with 10% formaldehyde for 1 h at 4 °C. The fixed cells were permeabilized with Triton X-100 0.1% in PBS for 3–5 min. The actin filaments were stained with FITC phalloidin in PBS for 30 min at RT. Finally, after two washes with PBS to remove the unbound phalloidin conjugate, cell nuclei were stained with 4′,6-diamidino-2-phenylindole, DAPI, (Sigma-Aldrich). The samples were observed using a confocal microscope system (Leica TCS SP8) with a 63X oil immersion objective. Images were acquired with a resolution of 1024 × 1024 pixel.
4.8.2. IL-10 Anti-Inflammatory Expression
Anti-inflammatory efficacy, in terms of IL-10 expression, was tested by putting in contact HA, HCV, and HCV + DF systems to L929 cells, seeded at a density of 2 × 104 in a 96-multiwall plate, using untreated cells as the control. Inflammation was induced by exposing cells to 1 μg/mL LPSs41 for 24 h, and then the expression levels of IL-10 in cell supernatants were quantified using a commercial ELISA kit (Elabscience) according to the manufacturer protocol.
4.9. Statistical Analysis
All experiments were performed independently at least three times and reported as mean ± standard deviation. Statistical analyses were performed using GraphPad Prism, version 6.00, and data were analyzed using ordinary one-way analysis of variance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05622.
A table with a complete list of acronyms and their full form, sample calculation with each of the equations presented in the paper, summary of statistical results, and methods used (PDF)
Author Contributions
⊥ P.K. and F.D.S. equally contributed to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Makvandi P.; Ali G. W.; Della Sala F.; Abdel-Fattah W. I.; Borzacchiello A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023–115034. 10.1016/j.carbpol.2019.115023. [DOI] [PubMed] [Google Scholar]; b Della Sala F.; di Gennaro M.; Lista G.; Messina F.; Ambrosio L.; Borzacchiello A. Effect of Hyaluronic Acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes. Polymers 2021, 13, 2928. 10.3390/polym13172928. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Della Sala F.; Fabozzi A.; di Gennaro M.; Nuzzo S.; Makvandi P.; Solimando N.; Pagliuca M.; Borzacchiello A. Advances in Hyaluronic acid-based (nano) devices for Cancer Therapy. Macromol. Biosci. 2021, e2100304 10.1002/mabi.202100304. [DOI] [PubMed] [Google Scholar]
- Borzacchiello A.; Mayol L.; Schiavinato A.; Ambrosio L. Effect of hyaluronic acid amide derivative on equine synovial fluid viscoelasticity. J. Biomed. Mater. Res., Part A 2010, 92, 1162–1170. 10.1002/jbm.a.32455. [DOI] [PubMed] [Google Scholar]
- Altman R. D.; Manjoo A.; Fierlinger A.; Niazi F.; Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskeletal Disord. 2015, 16, 321. 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duymus T. M.; Mutlu S.; Dernek B.; Komur B.; Aydogmus S.; Kesiktas F. N. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 485–492. 10.1007/s00167-016-4110-5. [DOI] [PubMed] [Google Scholar]
- Sulistio A.; Mansfeld F. M.; Reyes Ortega F.; D’Souza A. M.; Ng S. M.; Birkett S.; Blencowe A.; Qiao G. G.; Little C. B.; Shu C. C. Intra-articular treatment of osteoarthritis with diclofenac-conjugated polymer reduces inflammation and pain. ACS Appl. Bio Mater. 2019, 2, 2822–2832. 10.1021/acsabm.9b00232. [DOI] [PubMed] [Google Scholar]
- Mayol L.; Biondi M.; Russo L.; Malle B. M.; Schwach-Abdellaoui K.; Borzacchiello A. Amphiphilic hyaluronic acid derivatives toward the design of micelles for the sustained delivery of hydrophobic drugs. Carbohydr. Polym. 2014, 102, 110–116. 10.1016/j.carbpol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Altman R.; Bosch B.; Brune K.; Patrignani P.; Young C. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri B.; Rottigni V.; Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug. Des. Devel. Ther. 2013, 7, 7. 10.2147/DDDT.S37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin N.; Hops C.; Lucke A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Delivery Rev. 2006, 58, 226–242. 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Suantawee T.; Tantavisut S.; Adisakwattana S.; Tanavalee A.; Yuktanandana P.; Anomasiri W.; Deepaisarnsakul B.; Honsawek S. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. J. Clin. Diagn. Res. 2013, 7, 1855. 10.7860/JCDR/2013/5802.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes-Quero G. M.; García-Fernández L.; Aguilar M. R.; San Román J.; Cano J. P.; Vázquez-Lasa B.. Active viscosupplements for osteoarthritis treatment. In Seminars in arthritis and rheumatism; Elsevier, 2019; Vol. 49, pp 171–183. [DOI] [PubMed] [Google Scholar]
- Sutipornpalangkul W.; Morales N. P.; Charoencholvanich K.; Harnroongroj T. Lipid peroxidation, glutathione, vitamin E, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int. J. Rheum. Dis. 2009, 12, 324–328. 10.1111/j.1756-185X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- Ozkan F. U.; Uzer G.; Türkmen I.; Yildiz Y.; Senol S.; Ozkan K.; Turkmensoy F.; Ramadan S.; Aktas I. Intra-articular hyaluronate, tenoxicam and vitamin E in a rat model of osteoarthritis: evaluation and comparison of chondroprotective efficacy. Int. J. Clin. Exp. Med. 2015, 8, 1018. [PMC free article] [PubMed] [Google Scholar]
- Challa R.; Ahuja A.; Ali J.; Khar R. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech 2005, 6, E329–E357. 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidwani B.; Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed Res. Int. 2015, 2015, 198268 10.1155/2015/198268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A. Many tocopherols, one vitamin E. Mol. Aspects Med. 2018, 61, 92–103. 10.1016/j.mam.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Xie J.; Schaich K. Re-evaluation of the 2, 2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. 10.1021/jf500180u. [DOI] [PubMed] [Google Scholar]
- Kučerík J.; Průšová A.; Rotaru A.; Flimel K.; Janeček J.; Conte P. DSC study on hyaluronan drying and hydration. Thermochim. Acta 2011, 523, 245–249. 10.1016/j.tca.2011.04.034. [DOI] [Google Scholar]
- Grdadolnik J.; Merzel F.; Avbelj F. Origin of hydrophobicity and enhanced water hydrogen bond strength near purely hydrophobic solutes. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 322–327. 10.1073/pnas.1612480114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereva S.; Nikolova V.; Angelova S.; Spassov T.; Dudev T. Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. 10.3762/bjoc.15.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebioglu A.; Uyar T. Antioxidant vitamin E/cyclodextrin inclusion complex electrospun nanofibers: enhanced water solubility, prolonged shelf life, and photostability of vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. 10.1021/acs.jafc.7b01562. [DOI] [PubMed] [Google Scholar]
- Maltese A.; Borzacchiello A.; Mayol L.; Bucolo C.; Maugeri F.; Nicolais L.; Ambrosio L. Novel polysaccharides-based viscoelastic formulations for ophthalmic surgery: rheological characterization. Biomaterials 2006, 27, 5134–5142. 10.1016/j.biomaterials.2006.05.036. [DOI] [PubMed] [Google Scholar]; From NLM
- Bhuanantanondh P.; Grecov D.; Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J. Med. Electron. Biol. 2012, 32, 12–16. 10.5405/jmbe.834. [DOI] [Google Scholar]
- Xu Y.; Stokes J. R. Soft lubrication of model shear-thinning fluids. Tribol. Int. 2020, 152, 106541 10.1016/j.triboint.2020.106541. [DOI] [Google Scholar]
- Bonnevie E. D.; Galesso D.; Secchieri C.; Bonassar L. J. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS One 2019, 14, e0216702 10.1371/journal.pone.0216702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie E. D.; Galesso D.; Secchieri C.; Cohen I.; Bonassar L. J. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS One 2015, 10, e0143415 10.1371/journal.pone.0143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. W.; Banquy X.; Israelachvili J. N. Stick-slip friction and wear of articular joints. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E567–E574. 10.1073/pnas.1222470110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincl M.; Meleh M.; Veber M.; Vrecer F. Study of physicochemical parameters affecting the release of diclofenac sodium from lipophilic matrix tablets. Acta Chim. Slov. 2004, 51, 409–425. [Google Scholar]
- a Korsmeyer R. W.; Gurny R.; Doelker E.; Buri P.; Peppas N. A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]; b Monjezi J.; Jamaledin R.; Ghaemy M.; Moeini A.; Makvandi P. A Performance Comparison of Graft Copolymer Hydrogels Based on Functionalized-Tragacanth Gum/Polyacrylic Acid and Polyacrylamide as Antibacterial and Antifungal Drug Release Vehicles. Am. J. Nanotechnol. Nanomed. 2018, 1 (1), 10–15. [Google Scholar]
- a Della Sala F.; Biondi M.; Guarnieri D.; Borzacchiello A.; Ambrosio L.; Mayol L. Mechanical behavior of bioactive poly (ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885 10.1016/j.jmbbm.2020.103885. [DOI] [PubMed] [Google Scholar]; b Panzetta V.; Guarnieri D.; Paciello A.; Della Sala F.; Muscetti O.; Raiola L.; Netti P.; Fusco S. ECM mechano-sensing regulates cytoskeleton assembly and receptor-mediated endocytosis of nanoparticles. ACS Biomater. Sci. Eng. 2017, 3, 1586–1594. 10.1021/acsbiomaterials.7b00018. [DOI] [PubMed] [Google Scholar]
- a Li Q.-L.; Wang L.; Qiu X.-L.; Sun Y.-L.; Wang P.-X.; Liu Y.; Li F.; Qi A.-D.; Gao H.; Yang Y.-W. Stimuli-responsive biocompatible nanovalves based on β-cyclodextrin modified poly (glycidyl methacrylate). Polym. Chem. 2014, 5, 3389–3395. 10.1039/C4PY00041B. [DOI] [Google Scholar]; b Vaz S.; Silva R.; Amaral M.; Martins E.; Lobo J. S.; Silva A. Evaluation of the biocompatibility and skin hydration potential of vitamin E-loaded lipid nanosystems formulations: In vitro and human in vivo studies. Colloids Surf., B 2019, 179, 242–249. 10.1016/j.colsurfb.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Alaaeddine N.; Di Battista J. A.; Pelletier J. P.; Kiansa K.; Cloutier J. M.; Martel-Pelletier J. Inhibition of tumor necrosis factor α–induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: Distinct targeting in the signaling pathways. Arthritis Rheum. 1999, 42, 710–718. . [DOI] [PubMed] [Google Scholar]
- Ruppert S.; Hawn T.; Arrigoni A.; Wight T.; Bollyky P. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 2014, 58, 186–192. 10.1007/s12026-014-8495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lira F. S.; Rosa J. C.; Cunha C. A.; Ribeiro E. B.; do Nascimento C. O.; Oyama L. M.; Mota J. F. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011, 10, 1–5. 10.1186/1476-511X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Belisle S. E.; Hamer D. H.; Leka L. S.; Dallal G. E.; Delgado-Lista J.; Fine B. C.; Jacques P. F.; Ordovas J. M.; Meydani S. N. IL-2 and IL-10 gene polymorphisms are associated with respiratory tract infection and may modulate the effect of vitamin E on lower respiratory tract infections in elderly nursing home residents. The Am. J. Clin. Nutr. 2010, 92, 106–114. 10.3945/ajcn.2010.29207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdy A.; Galley H. F.; Abdel-Wahed M.; El-Korny K.; Sheta S.; Webster N. R. Differential modulation of interleukin-6 and interleukin-10 by diclofenac in patients undergoing major surgery. Br. J. Anaesth. 2002, 88, 797–802. 10.1093/bja/88.6.797. [DOI] [PubMed] [Google Scholar]
- Liu D.; Shi J.; Ibarra A. C.; Kakuda Y.; Xue S. J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT–Food Sci. Technol. 2008, 41, 1344–1349. 10.1016/j.lwt.2007.08.001. [DOI] [Google Scholar]
- Talik P.; Hubicka U. The DSC approach to study non-freezing water contents of hydrated hydroxypropylcellulose (HPC). J. Therm. Anal. Calorim. 2018, 132, 445–451. 10.1007/s10973-017-6889-9. [DOI] [Google Scholar]
- Läuger J.; Pondicherry K.. New Insights into the Use of a Rotational Rheometer as Tribometer. Annual Transactions of the Nordic Rheology Society; 2017; pp 1–10.
- Selway N.; Chan V.; Stokes J. R. Influence of fluid viscosity and wetting on multiscale viscoelastic lubrication in soft tribological contacts. Soft Matter 2017, 13, 1702–1715. 10.1039/C6SM02417C. [DOI] [PubMed] [Google Scholar]
- a Makvandi P.; Ali G. W.; Della Sala F.; Abdel-Fattah W. I.; Borzacchiello A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng., C 2020, 107, 110195 10.1016/j.msec.2019.110195. [DOI] [PubMed] [Google Scholar]; b Makvandi P.; Caccavale C.; Della Sala F.; Zeppetelli S.; Veneziano R.; Borzacchiello A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. 10.3390/polym12081847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Ruan X. Z.; Powis S. H.; Fernando R.; Mon W. Y.; Wheeler D. C.; Moorhead J. F.; Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-γ–dependent mechanism. Kidney Int. 2005, 67, 867–874. 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.