Abstract
We adapted an in vitro pharmacodynamic model of infection to incorporate infected fibrin clots. The bactericidal activities of various fluoroquinolones against two strains of penicillin-resistant Streptococcus pneumoniae were studied over a 48-h period. Bacteria were prepared in Muller-Hinton broth by using colonies from a 24-h tryptic soy agar plus 5% sheep blood plate and were added to a mixture of cryoprecipitate (80%) and thrombin (10%) to achieve approximately 106 CFU of organism per fibrin clot. The fibrin clots were suspended into the models and removed, in triplicate, at various time points over 48 h. Control models were also conducted to characterize the growth of S. pneumoniae in the growth medium without antibiotic. Trovafloxacin, gatifloxacin, clinafloxacin, sparfloxacin, levofloxacin, and ciprofloxacin were administered to simulate their pharmacokinetic profiles in humans. Fibrin clot samples were also plated onto antibiotic-containing tryptic soy agar plus 5% lysed horse blood to detect resistance. The newer fluoroquinolones demonstrated better activity than ciprofloxacin against both isolates. In conclusion, the newer quinolones demonstrated significant activity against penicillin-resistant S. pneumoniae, with standard dosing resulting in area under the concentration-time curve/MIC ratios and peak concentration/MIC ratios that resulted in 99.9% killing against these isolates.
Streptococcus pneumoniae remains the leading cause of bacterial pneumonia and the most common pathogen associated with otitis media, sinusitis, and meningitis. The emergence of resistant S. pneumoniae over the past two decades has become a worldwide problem (2, 4). Based on a surveillance study conducted from 1996 to 1997 in the United States, the average prevalence of penicillin-resistant pneumococci has increased to approximately 33.4% (17). Even though the majority of the reports focus primarily on penicillin-resistant S. pneumoniae, resistance to macrolides, trimethoprim-sulfamethoxazole, and other therapeutic alternatives is also becoming commonplace (9, 12). The global increase in strains of S. pneumoniae resistant to penicillin and other antimicrobials increases the need for alternative therapeutic agents. Fluoroquinolones appear to be an appropriate alternative; however, their activity against gram-positive organisms has been variable. More recent quinolones, such as trovafloxacin, gatifloxacin, levofloxacin, clinafloxacin, and sparfloxacin, have demonstrated increased activity against S. pneumoniae, which may enhance the clinical capacity of these agents. The newer fluoroquinolones also offer many advantages over the older quinolone compounds, such as a long half-life, once-a-day dosing, an increased spectrum of activity, and enhanced bioavailability. In addition, these newer agents have been found to be very effective in the treatment of upper respiratory infections, including community-acquired pneumonia, otitis media, sinusitis, and bronchitis (5, 19).
Even though these fluoroquinolones exhibit greater activity against gram-positive organisms, their pharmacokinetic and pharmacodynamic properties, such as the peak concentration/MIC ratio (peak/MIC), area under the concentration time curve (AUC)/MIC ratio (AUC/MIC), and postantibiotic effect, are not well characterized as they are for gram-negative organisms. Utilizing our past experiences, we conducted this experiment to further evaluate and compare the activities and pharmacodynamics of various newer quinolones, including trovafloxacin, gatifloxacin, clinafloxacin, sparfloxacin, and levofloxacin, to those of ciprofloxacin against S. pneumoniae in an infection model over a 48-h period.
MATERIALS AND METHODS
Bacterial strains.
Two clinical isolates of penicillin-resistant (MIC, >2 μg/ml) and macrolide-resistant (MIC, >6 μg/ml) S. pneumoniae (isolates 68 and 79) were obtained from two patients treated at Detroit Receiving Hospital.
Antibiotics.
Trovafloxacin (lot no. 25381-086-02) was supplied by Pfizer Inc, Groton, Conn. Gatifloxacin (lot no. 331) was supplied by Bristol-Myer Squibb Pharmaceutical, New Brunswick, N.J. Clinafloxacin (lot no. PD127391-0002 Lot J) was supplied by Parke-Davis Pharmaceutical Research, Ann Arbor, Mich. Sparfloxacin (lot no. 721A) was supplied by Rhone-Poulenc Rorer, Collegeville, Pa. Levofloxacin (lot no. N-8018) was supplied by Ortho-McNeil Pharmaceutical, Raritan, N.J. Ciprofloxacin for injection (lot no. 851640) was supplied by Bayer Corporation, West Haven, Conn.
E-strips.
Trovafloxacin (lot no. B62424), gatifloxacin (lot no. B72030), clinafloxacin (lot no. B72330), sparfloxacin (lot no. B52046), levofloxacin (lot no. B71753), and ciprofloxacin (lot no. B52049) E-strips were supplied by AB Biodisk North America Inc., Piscataway, N.J.
Media.
Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB) plus 5% lysed horse blood (LHB) (Rockland, Inc., Gilbertsville, Pa.) was used for all susceptibility testing. Todd-Hewitt broth (THB) (Difco Laboratories) supplemented with calcium (6 mg/liter) and 0.5% yeast extract (Difco Laboratories) was used for susceptibility testing and in the in vitro infection models.
Susceptibility testing.
MICs and minimal bactericidal concentrations (MBCs) of all of the antibiotics were determined in quadruplicate by broth microdilution in SMHB plus 5% LHB according to National Committee for Clinical Laboratory Standards guidelines (16). MICs and MBCs were also determined in THB supplemented with yeast. The trovafloxacin MIC and MBC were determined in presence of albumin (4 g/dl) to account for protein binding of the drug. Samples (5 μl) from clear wells were plated onto tryptic soy agar (TSA) plates with 5% sheep blood (SB) to determine MBCs. All plates were incubated in candle jars (approximately 3% CO2) at a temperature of 37°C for 24 h. MICs were also determined by E-test. Development of resistance to fluoroquinolones was evaluated at each time point by placing 100 μl of the sample on antibiotic-containing plates (5% LHB) at four and eight times the MIC.
Infected fibrin clots.
A 0.5 McFarland suspension of organisms was prepared in 0.9% saline by using colonies from a 24-h TSA–5% SB plate. A 1:10 dilution was then made by adding 1 ml of the 0.5 McFarland solution to 9 ml of SMHB. Infected fibrin clots were then prepared by mixing 0.5 ml of human cryoprecipitate from volunteer donors (American Red Cross, Detroit, Mich.) and 0.1 ml of organism suspension (initial inoculum of 106 CFU/clot) in 1.5-ml siliconized tubes. Bovine thrombin (5,000 U) was added to each tube (50 μl) after insertion of a sterile monofilament line into the mixture. Clots were then removed with a sterile 21-gauge needle and placed in the models.
In vitro infection model.
A one-compartment in vitro infection model (500 ml) which allows for the simulation of the pharmacokinetics of drugs in human was used. Infected fibrin clots simulating a sequestered infection site were suspended on a monofilament line (15). The apparatus was prefilled with sterile THB plus 0.5% yeast extract. To assure the sterility of the model, each port was sealed with a rubber stopper. Antibiotics were administered as boluses over a 48-h period into the central compartment via an injection port. The model apparatus was placed in a 37°C water bath throughout the procedure, and a magnetic stir bar was placed in the medium for thorough mixing of the drug. Fresh medium was continuously supplied and removed from the compartment along with the drug via a peristaltic pump set to achieve the half-lives of the antibiotics. Fibrin clots were then removed over a 48-h period to be homogenized. Samples of the homogenized clots were plated onto TSA with 5% SB and incubated in candle jars at 37°C. Samples taken at 0, 24, and 48 h were collected at trough levels and diluted one- to twofold to avoid antibiotic carryover. Samples obtained at 8 and 32 h were diluted two- to threefold to avoid antibiotic carryover.
Models without antibiotic were used for each isolate to characterize growth kinetics. Experimental regimens included ciprofloxacin administered to simulate 400 mg twice daily (peak concentration in serum of 5 μg/ml), levofloxacin at 400 mg daily (peak concentration in serum of 6 μg/ml), sparfloxacin at 300 mg daily (peak concentration in serum of 1 μg/ml), trovafloxacin at 300 mg daily (peak concentration in serum of 3 μg/ml), gatifloxacin at 400 mg daily (peak concentration in serum of 3.5 μg/ml), and clinafloxacin at 200 mg twice daily (peak concentration in serum of 2 μg/ml). Using a 500-ml model, the pump was set at 1.0, 2.0, 1.0, 0.4, 0.7, and 0.6 ml/min to simulate half-lives of levofloxacin (6 h), ciprofloxacin (3 h), clinafloxacin (6 h), sparfloxacin (14 h), gatifloxacin (8 h), and trovafloxacin (10 h), respectively. All infection model experiments were performed in duplicate.
Pharmacodynamic analysis.
Three fibrin clots were removed from each model (a total of six clots per time point) at 0, 8, 24, 32, and 48 h. The clots were weighed and placed in a vial containing 3-mm-diameter glass beads, 1.25% trypsin (1:250 powder, lot no. 26H71305; Sigma), and normal saline. Each vial was then placed in a minibeater grinder for 30 s or until a homogenized sample was obtained. Suitable 10-fold dilutions of the homogenized samples were made in 0.9% sodium chloride and plated onto TSA with 5% SB in triplicate. Plates were incubated at 37°C in candle jars for 24 h, at which time the colonies were counted. The total reduction in log10 CFU per gram over 48 h was then determined by plotting time-kill curves based on the number of remaining organisms over the 48-h time period. The time to achieve a 99.9% bacterial load reduction was determined by linear regression if r2 was ≥0.95 or by visual inspection.
Pharmacokinetic analysis.
Samples (1.0 ml) were obtained from the central compartment, through the injection port, at 0, 0.5, 1, 2, 4, 8, 24, 32, and 48 h to determine the antibiotic concentrations. Samples were stored in Eppendorf tubes at −70°C until analysis. Concentrations of the fluoroquinolones were determined by microbioassay utilizing Klebsiella pneumoniae ATCC 10031. Blank 1/4-in. disks were spotted with 20 μl of the standards or samples. Each standard was tested in triplicate by placing the disk on Mueller-Hinton agar plates, which were preswabbed with a 0.5 McFarland suspension of the test organism. Plates were incubated for 18 to 24 h at 37°C, at which time the zone sizes were measured. The correlation coefficient of ≥0.98 was achieved for all plates. Concentrations of 5.0, 1.25, and 0.3125 μg/ml were used as standards, and the coefficient of variation was <10% for each standard. The half-lives, AUCs, and peak concentrations of the antibiotics were determined by trapezoidal methods utilizing RStrip software (Micromath, Salt Lake City, Utah).
Statistical analysis.
The time to 99.9% reduction in log10 CFU per gram for each regimen was assessed by linear regression (if r2 was ≥0.95) or by visual inspection. Changes in CFU with respect to AUC/MIC, peak/MIC, and time above the MIC (T > MIC) at 24 and 48 h were compared by two-way analysis of variance with Tukey's post-hoc test. P values of ≤0.05 were considered significant.
RESULTS
Susceptibility testing.
MIC and MBC results for the two isolates are summarized in Table 1. The two strains appear to have similar susceptibility patterns, with greater sensitivity to trovafloxacin and clinafloxacin, followed by gatifloxacin, sparfloxacin, ciprofloxacin, and levofloxacin. The MICs obtained by broth microdilution were similar to those obtained by E-strip. Trovafloxacin MICs/MBCs obtained in MHB plus LHB and THB plus yeast supplemented with albumin were 0.125/0.125 and 0.25/0.5 μg/ml for isolate 68 and 0.25/0.25 and 0.125/0.25 μg/ml for isolate 79, respectively.
TABLE 1.
Antibiotic susceptibilities of the two clinical isolates of S. pneumoniae determined by microdilution
| Antimicrobial agent |
S. pneumoniae 68
|
S. pneumoniae 79
|
||||
|---|---|---|---|---|---|---|
| MIC (μg/ml) (E-strip) | MIC/MBC (μg/ml) (microdilution)
|
MIC (μg/ml) (E-strip) | MIC/MBC (μg/ml) (microdilution)
|
|||
| THB-yeast | MHB-LHB | THB-yeast | MHB-LHB | |||
| Trovafloxacin | 0.064 | 0.25/0.5 | 0.06/0.125 | 0.094 | 0.125/0.25 | 0.06/0.125 |
| Gatifloxacin | 0.25 | 0.125/0.5 | 0.125/0.5 | 0.25 | 0.25/0.5 | 0.25/0.5 |
| Clinafloxacin | 0.064 | 0.25/0.5 | 0.03/0.03 | 0.064 | 0.03/0.06 | 0.06/0.125 |
| Sparfloxacin | 0.19 | 0.125/0.25 | 0.125/0.25 | 0.25 | 0.25/0.25 | 0.25/0.5 |
| Levofloxacin | 0.75 | 0.5/1.0 | 1/1 | 0.75 | 1.0/1.0 | 1/1 |
| Ciprofloxacin | 1.0 | 0.5/1.0 | 0.5/0.5 | 1.0 | 1.0/1.0 | 0.5/0.5 |
Pharmacodynamic and pharmacokinetic studies.
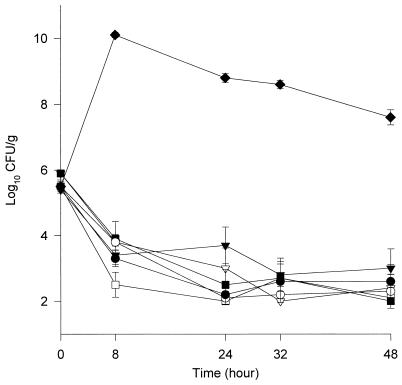
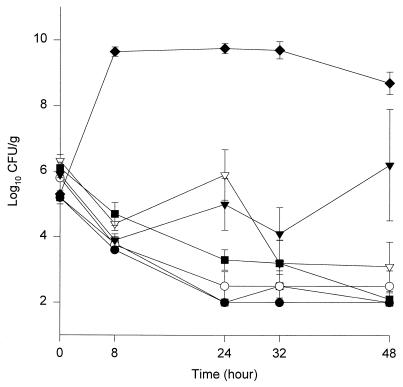
The activities of the different agents against the two isolates are shown in Fig. 1 and 2. By 48 h all fluoroquinolones except ciprofloxacin resulted in 99.9% killing (≥3-log10-unit reduction in CFU per gram) against both isolates, and there were no significant differences in the activities of these agents. In the presence of levofloxacin and ciprofloxacin, regrowth occurred by 24 h with isolate 79, which was statistically significant compared to the other fluoroquinolones evaluated (P < 0.05). The ciprofloxacin experiments were repeated three additional times to verify the results. The times to 99.9% killing against isolates 68/79 were 24/24, 32/32, 8/24, 24/24, and 24/48 h for trovafloxacin, levofloxacin, clinafloxacin, gatifloxacin, and sparfloxacin, respectively.
FIG. 1.
Activities of trovafloxacin (○), gatifloxacin (●), clinafloxacin (□), sparfloxacin (■), levofloxacin (▿), and ciprofloxacin (▾) against S. pneumoniae isolate 68. ⧫, growth control. Error bars indicate standard deviations.
FIG. 2.
Activities of trovafloxacin (○), gatifloxacin (●), clinafloxacin (□), sparfloxacin (■), levofloxacin (▿), and ciprofloxacin (▾) against S. pneumoniae isolate 79. ⧫, growth control. Error bars indicate standard deviations.
The peak/MIC ratios ranged from 3.8:1 to 27.2:1, with trovafloxacin having the highest peak/MIC, followed by clinafloxacin, gatifloxacin, ciprofloxacin, levofloxacin, and sparfloxacin. The AUC/MIC ratio ranged from 40.8 to 405.9, with trovafloxacin having the highest ratio, followed by clinafloxacin and gatifloxacin. The T > MIC ranged from 55 to 100%, with levofloxacin and ciprofloxacin being the only two fluoroquinolones with a T > MIC of less than 100%. The mean peak, trough, half-life, and AUC0–24 for each fluoroquinolone can be seen in Table 2. The average AUC0–24/MIC, peak/MIC, and T > MIC for each isolate can be seen in Table 3. Resistance to fluoroquinolones was not detected at any time point up to 48 h.
TABLE 2.
Peak, trough, half-life, and AUC0–24 for various fluoroquinolonesa
| Antimicrobial agent | Peak (μg/ml) | Trough (μg/ml) | Half-life (h) | AUC (μg/ml/h) |
|---|---|---|---|---|
| Trovafloxacin | 2.98 ± 0.5 | 0.99 ± 0.2 | 14.97 ± 1.5 | 43.4 ± 8.3 |
| Gatifloxacin | 4.21 ± 0.4 | 0.62 ± 0.2 | 8.9 ± 2.3 | 44.6 ± 4.9 |
| Clinafloxacin | 1.75 ± 0.8 | 0.38 ± 0.1 | 5.9 ± 1.4 | 21.5 ± 7.8 |
| Sparfloxacin | 1.08 ± 0.2 | 0.31 ± 0.1 | 13.15 ± 3.0 | 14.34 ± 3.4 |
| Levofloxacin | 7.15 ± 1.1 | 0.62 ± 0.3 | 6.86 ± 1.5 | 62.84 ± 6.5 |
| Ciprofloxacin | 6.42 ± 1.0 | 0.4 ± 0.3 | 2.92 ± 0.7 | 50.84 ± 14.0 |
All results are means and standard deviations.
TABLE 3.
AUC0–24/MIC, peak/MIC, and T > MIC of each fluoroquinolone against each isolatea
| Antimicrobial agent | AUC0–24/MIC
|
Peak/MIC
|
T > MIC (%)
|
|||
|---|---|---|---|---|---|---|
| 68 | 79 | 68 | 79 | 68 | 79 | |
| Trovafloxacin | 405.9 (5.9) | 292.6 (1.4) | 27.2 (0.41) | 20.7 (2.0) | 100 | 100 |
| Gatifloxacin | 167.1 (4.5) | 189.8 (12.6) | 15.4 (22.6) | 183. (10.8) | 100 | 100 |
| Clinafloxacin | 215.1 (32.0) | 526.9 (4.9) | 18.8 (33.6) | 37.9 (3.3) | 100 | 100 |
| Sparfloxacin | 137 (11.2) | 46.7 (6.2) | 9.6 (12.8) | 3.8 (0.74) | 100 | 100 |
| Levofloxacin | 135 (9.2) | 58.2 (2.4) | 15.4 (19.7) | 6.6 (1.3) | 86 (5.8) | 76 (9.3) |
| Ciprofloxacin | 121.8 (12.6) | 40.8 (27.5) | 14.5 (5.9) | 5.6 (3.6) | 100 | 55 (15.7) |
Results are means, with the percent coefficient of variation given in parentheses.
DISCUSSION
The incidence of pneumococcal isolates with multidrug resistance has increased dramatically worldwide (3). Older fluoroquinolones such as ciprofloxacin have demonstrated poor activity against gram-positive pathogens, including S. pneumoniae, with MICs close to the breakpoint. The more recent quinolones have demonstrated better activity against these organisms. Their potent and broad spectra of activity and relative safety render these agents an appropriate alternative in the treatment of community or nosocomial infections, including those caused by multidrug-resistant S. pneumoniae. Our study demonstrates that newer quinolones have activity against penicillin-resistant S. pneumoniae, and at 48 h there was no statistically significant difference in the activities of these agents. However, all agents were superior to ciprofloxacin against isolate 79 (P < 0.05). In our models, at 48 h there was a gradual decline in the growth control curve for isolate 68, which could have overestimated the activity of the quinolones against this isolate. Also, other factors such as leukocytes, which contribute to the killing and thus the efficacy of the antimicrobial agents, were not present in the models.
Previous studies have suggested that certain pharmacodynamic parameters may correlate with the therapeutic efficacy of fluoroquinolones (1, 3, 6, 7, 8, 10, 11, 13, 14, 17). Forrest et al. identified the AUC/MIC, peak/MIC, T > MIC, and several other parameters of fluoroquinolones to be associated with outcome (7, 8). In a report evaluating pharmacodynamic parameters of fluoroquinolones in different experimental models of endocarditis, an AUC/MIC of ≥100 was better correlated with response (r2 = 0.45), followed by T > MIC (r2 = 0.43) and a peak/MIC of >8 (r2 = 0.41) (1). The pharmacodynamic predictors for fluoroquinolones have been characterized in the literature against gram-negative pathogens; however, limited data are available to support these endpoints for efficacy against gram-positive organisms. In a recent study the activities of four different fluoroquinolones against S. pneumoniae were compared in an in vivo model of experimental pneumonia (3). The authors concluded that higher AUC/MIC ratios (>100) are required to achieve sufficient activity against S. pneumoniae. In an in vitro study by Wright et al., the pharmacodynamics of ciprofloxacin and levofloxacin against three isolates of S. pneumoniae with variable susceptibility to ciprofloxacin (MIC = 1, 2, and 4 μg/ml) were compared (D. H. Wright, M. L. Peterson, L. B. Havde, G. Brown, and J. C. Rotschafer, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-111a, 1998). In this study bacterial regrowth was not observed with T > MIC values of greater than 12 h. Ciprofloxacin AUC/MIC ratios of 17.4 and 8.7 were associated with regrowth at 24 h, while no regrowth was seen with a value of 34.8 against the penicillin-resistant strain (ciprofloxacin MIC = 1.0 μg/ml). Although a limited number of S. pneumoniae isolates were evaluated, there may be a correlation between the bacterial regrowth and certain pharmacodynamic parameters of fluoroquinolones. Zhanel et al. demonstrated that a ciprofloxacin AUC/MIC of 20 did not result in 99.9% killing, while a levofloxacin AUC/MIC of 100 was associated with 3.8-log10-unit decrease in CFU/ml (G. G. Zhanel, N. Laing, J. Karlowsky, and D. Hoban, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A24, 1998). In our investigation, the AUC0–24/MIC, peak/MIC, and T > MIC ranged from 40.8 to 405.9, 3.8 to 27.2, and 55 to 100%, respectively. A 99.9% kill was not achieved with ciprofloxacin, and no significant relationship was demonstrated between the time to 99.9% killing and any pharmacodynamic parameter of ciprofloxacin, trovafloxacin, gatifloxacin, or clinafloxacin. Sparfloxacin and levofloxacin had the second lowest AUC/MIC ratios, of 47 and 58, against isolate 79, resulting in 99.9% killing by 32 and 48 h, respectively, which is slightly longer than the time achieved by the other newer quinolones (8 to 24 h). Ciprofloxacin resulted in bacterial regrowth at 48 h when used against isolate 79 (AUC/MIC = 40.8 and T > MIC = 55%), although no change in susceptibility was detected. The large standard deviation with ciprofloxacin against isolate 79 at 48 h was associated with regrowth of only one of the two models. These models were repeated, and the results were similar. The only significant pharmacodynamic differences between the two models were in AUC/MIC (33 versus 49) and T > MIC (43 versus 66%). The peak/MIC ratios were 5.5 and 5.7, which were not significantly different. This may suggest that a lower AUC/MIC or a T > MIC of <55% may be associated with bacterial regrowth. The lack of polymorphonuclear leukocytes in the models may have also contributed to the regrowth with ciprofloxacin. Trovafloxacin, clinafloxacin, gatifloxacin, levofloxacin, and sparfloxacin achieved the highest AUC/MIC and peak/MIC against the two isolates, respectively, and the T > MIC was 100% for all of these quinolones except levofloxacin (76 to 86%).
In conclusion, the newer fluoroquinolones demonstrate greater activity against penicillin-resistant S. pneumoniae than older fluoroquinolones such as ciprofloxacin. Although no clear association could be identified between pharmacodynamic parameters and bacterial killing, an AUC/MIC of ≤40 or a T > MIC of ≤55% appeared to be associated with decreased killing and significant regrowth. It also appears that AUC/MIC and T > MIC may be better predictors of activity than peak/MIC, since sparfloxacin resulted in greater killing than ciprofloxacin regardless of the lower peak/MIC. Further experiments with isolates demonstrating a wider variety of fluoroquinolones MICs, (i.e., close to or greater than the susceptibility breakpoints) are required to better define the pharmacodynamic parameters which may best predict the efficacy of these agents against S. pneumoniae.
ACKNOWLEDGMENTS
This project was supported by a grant from Pfizer Pharmaceuticals and a partial grant from Bristol-Myers Squibb Pharmaceuticals.
REFERENCES
- 1.Andes D R, Craig W A. Pharmacodynamics of fluoroquinolones in experimental models of endocarditis. Clin Infect Dis. 1998;27:47–50. doi: 10.1086/514624. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaun P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Bedos J P, Rieux V, Bauchet J, Muffat-Joly M, Carbon C, Azoulay-Dupuis E. Efficacy of trovafloxacin against penicillin-susceptible and multiresistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1998;42:862–867. doi: 10.1128/aac.42.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiman R F, Butler J C, Tenover F C, Elliot J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 5.Carbon C. Quinolones in the treatment of lower respiratory tract infections in adult patients. Drugs. 1993;45(Suppl. 3):91–97. doi: 10.2165/00003495-199300453-00016. [DOI] [PubMed] [Google Scholar]
- 6.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 7.Forrest A, Chodosh S, Amantea M A, Collins D A, Schentag J J. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1997;40:45S–57S. doi: 10.1093/jac/40.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 8.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofman J O, Cetron M S, Farley M M, Baughman W S, Facklam R R, Elliott J A, Deaver K A, Breiman R F. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 10.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antimicrobial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–39. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 12.Lonks J R, Medeiros A A. The growing threat of antibiotic-resistant Streptococcus pneumoniae. Med Clin N Am. 1995;79:523–535. doi: 10.1016/s0025-7125(16)30054-2. [DOI] [PubMed] [Google Scholar]
- 13.Madaras-Kelly K J, Alison J L, Rotschafer J C. J. Antimicrob Chemother. 1996;37:703–710. doi: 10.1093/jac/37.4.703. [DOI] [PubMed] [Google Scholar]
- 14.Madaras-Kelly K J, Ostergaard B E, Hovde L B, Rotschafer J C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40:627–632. doi: 10.1128/aac.40.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath B J, Kang S L, Kaatz G W, Rybak M J. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob Agents Chemother. 1994;38:2034–2040. doi: 10.1128/aac.38.9.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 17.Thornsberry C, Ogilvie P, Kahn J, Mauriz Y. Surveillance of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States in 1996–1997 respiratory season. Diagn Microbiol Infect Dis. 1997;29:249–257. doi: 10.1016/s0732-8893(97)00195-8. [DOI] [PubMed] [Google Scholar]
- 18.Trautmann M, Ruhnke M, Borner K, Wagner J, Koeppe P. Pharmacokinetics of sparfloxacin and serum bactericidal activity against pneumococci. Antimicrob Agents Chemother. 1996;40:776–779. doi: 10.1128/aac.40.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker R C, Wright A J. The fluoroquinolones. Mayo Clin Proc. 1991;66:1249–1259. doi: 10.1016/s0025-6196(12)62477-x. [DOI] [PubMed] [Google Scholar]