Abstract
Objective:
To investigate the desirable healing time of micro-screws by histomorphologic and histomorphometric evaluations of osseointegration after immediate and early loading.
Materials and Methods:
Fifty-four micro-screws were bilaterally placed in the maxillary premolar regions of nine beagles. Then the micro-screws with various healing time of 0 day (0D group), 2 weeks (2W group), and 4 weeks (4W group) were loaded with an orthodontic force (100 g) for 8 weeks. The direction of the orthodontic force was vertical to the long axis of the micro-screws. Hard tissue sections containing micro-screws were prepared for histomorphologic and histomorphometric evaluations.
Results:
The survival rate of the micro-screws in this study was 100%. Bone remodeling, close contact bone-implant interface, and endochondral ossification were observed in all osseous specimens. Activated osteoblasts aggregated to the bone-implant interface of the 4W group, and lamellar bone was found in the peri-implant regions. Micro-screws of the 2W group were partially surrounded by collagen fibers; and neonatal lines of bone, woven bone, and osteoclasts were found in the peri-implant regions. Micro-screws of the 0D group were surrounded by more collagen fibers compared with the other two groups. Bone implant contact ratios of the three groups were 43.74% (0D group), 66.26% (2W group), and 73.28% (4W group), respectively and statistical differences were significant (ANOVA, P < .01).
Conclusion:
All micro-screws in the three groups can provide stable orthodontic anchorage. However, to obtain improved stationary anchorage, a 4-week healing time is recommended before orthodontic loading.
Keywords: Micro-screw, Anchorage, Healing time, Osseointegration
INTRODUCTION
Anchorage, which is defined as resistance to unwanted tooth movement caused by the reacting force of orthodontic loading, plays a very important role in orthodontic treatment.1 Endosseous implants have been successfully used in orthodontic treatment to provide absolute anchorage2–4 since the theory of osseointegration (the close contact between bone and implant) was established by Branemark and colleagues.5–8 However, the large size of a conventional endosseous implant limits its usage as anchorage. To overcome this limitation, the micro-screw has been introduced because of its advantages compared with conventional implants. These advantages include smaller size, simpler implantation and removal, the possibility of immediate or early loading, and so on.3,9–14
Osseointegration is a critical determinative factor of the performance of endosseous implant,14–18 and the bone implant contact ratio (BIC) reflects the degree of osseointegration.5–8 Healing time has a considerable impact on osseointegration.5–8,13 Osseointegration of the micro-screw at various healing times was evaluated in many studies.10,19,20 Subsequently, researchers have questioned how long the desirable healing time should be before the orthodontic loading.
Authors presented various answers to this question. Saito19 suggested that orthodontic force should be loaded on the micro-screw after 18 weeks of healing. Roberts21 concluded that micro-screws could stand orthodontic loading of 100 g after 6 weeks of healing. Studies by Costa22 indicated that micro-screws could provide stable anchorage after 4 weeks of healing. Melsen10 reported that osseointegration could be observed on the immediately loaded bone-implant interface. Healing time is becoming shorter and shorter with clinical appliances,23–25 and immediate loading is upheld by some clinicians.26
Although many rigorous studies have been done on healing time, the desirable healing time remains unclear. The present study aimed to investigate the desirable healing time of micro-screws by histomorphologic and histomorphometric evaluations of osseointegration.
MATERIALS AND METHODS
Micro-screws and Animals
Fifty-four drill-free commercially pure titanium micro-screws (Aarhus Microscrew, Medicon Company, Tuttlingen, Germany) measuring 1.6 mm in diameter and 6 mm in length and nine adult (18 months old) male beagles (West China Medical Laboratory Animal Center, Sichuan University, Sichuan, PR China) were used in this study. All micro-screws and beagles were divided into three groups: the immediate loading group (0D group), 2-week healing group (2W group), and 4-week healing group (4W group) (Figure 1). There were 18 micro-screws and three beagles in each group. The 0D group, 2W group, and 4W group represented the three pathological periods of bone healing after the insertion of implant, that is, the traumatic period, granulation period, and callus period, respectively.27
Figure 1.
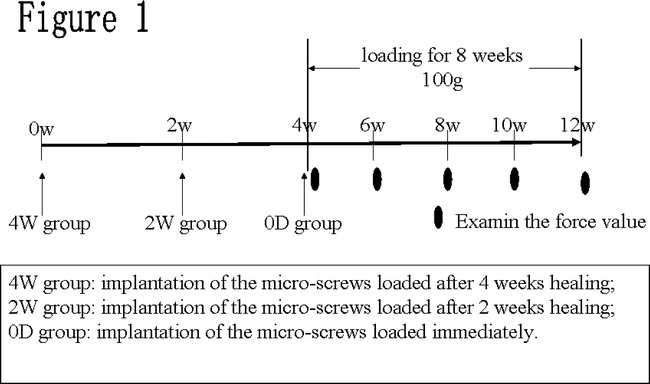
Experimental method sequence of surgical procedure and loading. W: time in weeks.
Surgical Procedures and Loading
Under the approval of the Institutional Committee for Animal Care, Sichuan University, Sichuan, China, surgical procedures were carried out in sterile conditions and under systemic anesthesia (5 mg/kg ketamine and 2 mg/kg xylazine intramuscularly [IM], Xi'an Bodyguard, Xi'an, China). To avoid drilling in the mucosa, 4- to 5-mm incisions were made at the keratinized mucosa in the regions between the mesial and distal roots of the second (P2), third (P3), and fourth (P4) premolars on both sides of the maxilla. Micro-screws were then implanted vertically to the surface of maxilla at the sites of P2, P3, and P4 by a drill-free method (Figure 2A). The incisions were sutured on both sides of the micro-screws after the implantation.
Figure 2.
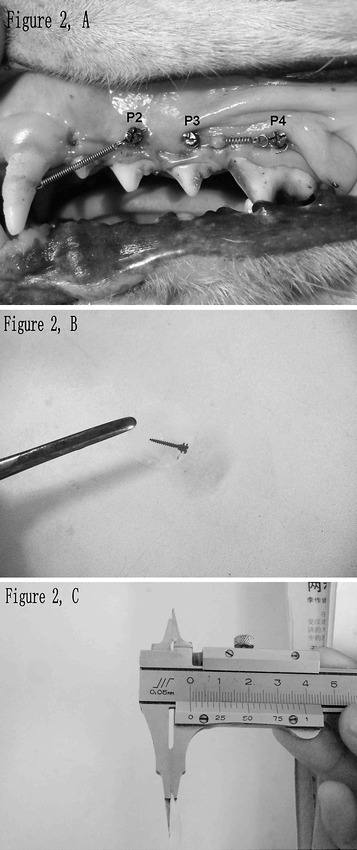
Implantation sites on maxilla and hard tissue sections containing micro-screw. (A) Micro-screws were implanted between the mesial and distal roots of the second (P2), third (P3) and fourth (P4) premolar and loaded using Ni-Ti coil springs. The micro-screws of P3 and P4 were loaded with each other. The micro-screw of P2 was loaded with the canine. (B) A hard tissue section containing micro-screw. (C) Measuring the thickness (60–80 µm) of a hard tissue section with a sliding caliper.
After the surgical operations, all beagles received a mixture of penicillin G procaine and penicillin G benzathine (300,000 U/mL, 0.2 mL/5 kg IM, once a day, Xi'an Bodyguard, Xi'an, China) for 3 days and a soft diet for 7 days.20 To ensure good oral hygiene, the beagles' oral cavities were locally rinsed with 2% chlorhexidine solution twice a day during the study.
A constant low-magnitude force of 100 g was loaded on the micro-screws vertically to their long axis using Ni-Ti coil springs at day 0 (0D group), 2 weeks (2W group), or 4 weeks (4W group) after the implantation, respectively. The micro-screws of P3 and P4 were loaded with each other. The micro-screw of P2 was loaded with the canine. To ensure the stability of the force, every Ni-Ti coil spring was examined with a force meter and reactivated every 2 weeks during the loading.
Histomorphologic and Histomorphometric Evaluations
After the 8-week loading, beagles of the three groups were euthanized by anesthesia overdose under the approval of the Institutional Committee for Animal Care, Sichuan University. The maxillas were retrieved with sharp dissection and subsequently chipped into individual osseous specimens (2×1×1 cm3) using a cutting disc under continuous saline flush. Every osseous specimen contained one micro-screw in its central region.
All osseous specimens were fixed with 4% paraformaldehyde for 1 week, dehydrated with gradient alcohol and chloroform by turn, and then embedded with methyl methacrylate.28 According to Donath's method,29 the embedded osseous specimens were sectioned in longitudinal direction parallel to the long axis of the micro-screw by a section cutter (SP 1600, Leica Instruments, Nussloch, Germany) at a low speed (0.4 mm/min). Three to four hard tissue sections, 60- to 80-µm thick (Figure 2B,C), with the micro-screw's long axis in the force vector plane, were obtained from each micro-screw.
The hard tissue sections were stained with 1% toluidine blue for 48 hours. Histomorphologic and histomorphometric observations were performed using an image-analyzing system (Em120 and ACT-1, Nikon, Tokyo, Japan). Image Pro-Plus software (v 4.5, Media Cybernetic Inc, Bethesda, MD) was used to evaluate the bone-implant interfaces and calculate BICs according to the following formula30:
 |
Statistical Analysis
To calculate the mean BIC of every osseous specimen, three hard tissue sections derived from the same osseous specimen were evaluated. The mean BIC of every group was then calculated based on the data of all osseous specimens in the corresponding group. Statistical analysis was carried out using the Statistical Package for Social Sciences (Windows v11.0, SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) was used to evaluate the difference in BICs of the three groups. The Student-Newman-Keuls test (S-N-K) was used to investigate the differences in BICs between groups. A difference was considered statistically significant if P < .05.
RESULTS
Histomorphologic Observation
No postoperative infection occurred during the study. After the application of Ni-Ti coil springs, slight peri-implant soft tissue inflammation, caused by food impaction, was observed around several micro-screws. But the peri-implant inflammation could be controlled by regular oral rinsing with 2% chlorhexidine solution. The survival rate of micro-screws was 100%.
After the 8-week orthodontic loading, all hard tissue sections of the 0D group (Figure 3A), 2W group (Figure 3B), and 4W group (Figure 3C) showed different degrees of osseointegration and bone remodeling. Though close contact between bone and micro-screw was observed in all groups, the micro-screws of 0D group were surrounded by more collagen fibers than those of the other two groups (Figure 3A).
Figure 3.
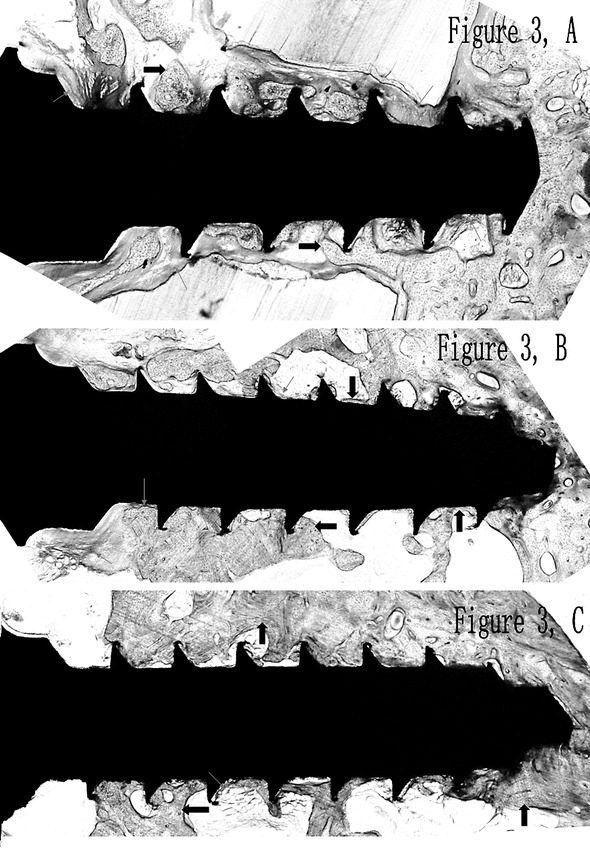
Osseointegration of the 0D group (A), the 2W group (B), the 4W group (C). Collagen fibers and bone are indicated by red arrows and black arrows, respectively. (A) The micro-screws of the 0D group were surrounded by more collagen fibers than the other two groups. (B) The micro-screws of the 2W group were partially surrounded by collagen fibers. (C) Close contact between bone and micro-screw was found surrounding the micro-screws of the 4W group.
The 0D group showed crescent-shaped bone trabeculae, evidence of bone remodeling caused by osteoclast phagocytosis. Large amount of collagen fibers were observed in the peri-implant regions (Figure 4A).
Figure 4.
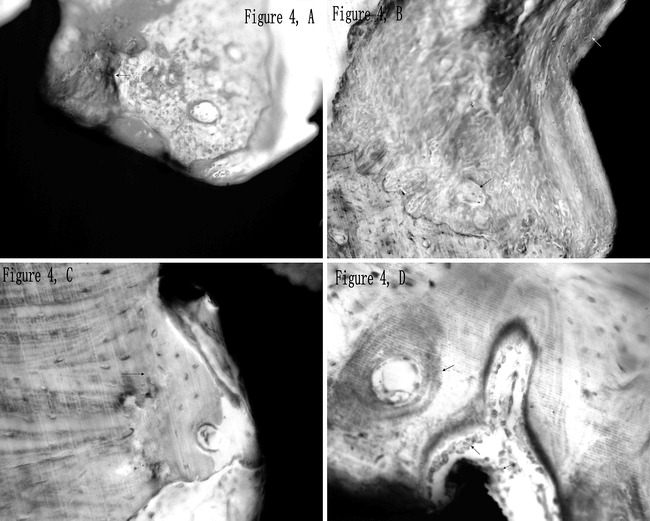
Bone remodeling in the peri-implant region. (A) There were many collagen fibers (red arrow) and crescent-shaped bone trabeculae (black arrow) surrounding the micro-screws of the 0D group. (B) Serrated edge of bone resorption (red arrow) caused by activated osteoclasts (black arrow) was observed in the peri-implant region, and the newly formed woven bone attaching to the bone-implant interface is indicated by the white arrow (2W group). (C) A neonatal line (arrow) was found alongside with newly formed bone near the micro-screw (2W group). (D) Osteoblasts (black arrow) aggregated and arranged in a line attaching to the bone-implant interface and bone trabecula (4W group). The endochondral ossification with osteoblasts aggregate and lamellar bone with Haversian system are indicated by the red arrow and blue arrow, respectively.
In the 2W group, the micro-screws were partially surrounded by collagen fibers (Figure 3B). Activated osteoclasts and serrated edges of bone resorption were observed in the peri-implant regions; newly formed woven bone was found attaching to the surface of the micro-screw (Figure 4B). A neonatal line, evidence of bone neoformation, was found alongside newly formed bone near the micro-screw (Figure 4C).
Remodeling of bone trabeculae was prominent in the peri-implant regions of the 4W group, where osteoblasts aggregated and arranged in a line attaching to the surface of bone trabecula and the bone-implant interface (Figure 4D). Endochondral ossification with osteoblasts aggregated and lamellar bone with Haversian system were found in the peri-implant regions.
Histomorphometric Evaluation
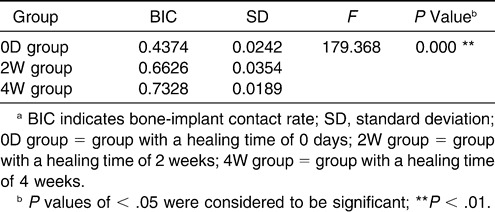
Mean BICs of the 0D group, 2W group, and 4W group were 43.74%, 66.26% and 73.28%, respectively (Table 1), and the statistical difference among groups was significant (ANOVA, P < .01). The results of S-N-K showed that the statistical differences between every two groups were significant, which indicated that BIC increased significantly with prolonged healing time.
Table 1.
Mean values ± standard deviation of BIC for every groupa
DISCUSSION
Various healing times were designed in this study according to the principle of Albrektsson et al27: the 0D group, 2W group, and 4W group represented traumatic period, granulation period, and callus period of bone healing after the insertion, respectively.
Miyawaki12 observed an 85% success rate of micro-screws and evaluated the relative risk (4.6) of the failure of micro-screw anchorage in subjects with peri-implant inflammation; he then concluded that preventing peri-implant inflammation is important for preventing failure of the implant anchor. According to Freudenthaler11 and Roberts,14 the major factor causing the losses of the loaded micro-screws is peri-implant soft-tissue inflammation, rather than the application of the orthodontic loading. To prevent postoperative infection in long term, two long-acting antibiotics, penicillin G procaine and penicillin G benzathine, were applied to the dogs in this study, and the peri-implant inflammation was effectively controlled by regular oral rinsing with 2% chlorhexidine solution. Therefore, one possible reason for the 100% survival rate of the micro-screws was the decreased peri-implant soft tissue inflammation. The 100% survival rate indicated that micro-screws can provide stable anchorage, even under immediate loading, if good oral hygiene is ensured.
Bone remodeling is a simultaneous process of resorption and formation of bone.31,32 It plays an important role in maintaining osseointegration of the implant by replacing the previously existing bone and repairing fatigue damage of bone.33 When mechanical stimulation is applied on implant, bone neoformation and remodeling can be found in the peri-implant regions.34 Therefore, mechanical stimulation can initiate and promote bone remodeling.35 After the 8-week loading, a neonatal line was observed at the border of the previously existing bone and the neoformative bone, which indicated that neoformation of the bone around the micro-screws had already occurred; activated osteoclasts and osteoblasts, crescent-shaped bone trabeculae, and serrated edge of bone resorption reflected the progressing bone remodeling (Figure 4). The results were consistent with those of the study by Deguchi et al,13 which showed that orthodontic loading did not hinder bone remodeling and osseointegration once the screw was implanted in bone. Interestingly, along with the prolonged healing time, collagen fibers around the micro-screws decreased significantly (Figure 3) and the BICs increased significantly (Table 1); woven bone and lamellar bone were found in the 2W group and 4W group, respectively. The results reflected the significantly different degrees of osseointegration among all groups and were consistent with previous studies, which indicated that the woven bone will be replaced by lamellar bone13 and the degree of osseointegration will become higher5–8,20 with prolonged healing time.
Osseointegration shows an ankylosis-like interface between living tissues and implant biomaterial,5,6 which could resist the force loaded on the implant, such as bite force in prosthetic treatment and orthodontic loading.18,36,37 According to Branemark and colleagues,5–8 sufficient osseointegration leads to improved stability of dental implants. The anchorage stability of the micro-screw is closely related to the degree of osseointegration.38 BIC is a quantitative parameter that reflects the degree of osseointegration.39 A higher BIC means more favorable distribution of stress caused by loading.40 Therefore, the significantly different BICs of the 0D group, 2W group, and 4W group (Table 1) suggest that longer healing time results in improved stability of micro-screw anchorage.
CONCLUSIONS
The 100% survival rate indicates that micro-screws implanted in maxilla can support both immediate and early orthodontic loading.
The three groups had significantly different degrees of osseointegration, and the 4W group showed the highest degree of osseointegration. Therefore, a 4-week healing time should be considered before orthodontic loading to improve stationary anchorage.
Acknowledgments
This project was supported by grants from the National Nature Science Foundation of China (No. 30470436, No. 10572160).
REFERENCES
- 1.Proffit W. Mechanical principles in orthodontic force control. In: Proffit W, Fields H. W, editors. Contemporary Orthodontics. St Louis, MO: Mosby; 1993. pp. 289–315. [Google Scholar]
- 2.Melsen B, Lang N. K. Biological reactions of alveolar bone to orthodontic loading of oral implants. Clin Oral Implant Res. 2001;12:144–152. doi: 10.1034/j.1600-0501.2001.012002144.x. [DOI] [PubMed] [Google Scholar]
- 3.Gray J. B, Steen M. E, King G. J, Clark A. E. Studies on the efficacy of implants as orthodontic anchorage. Am J Orthod. 1983;83:311–317. doi: 10.1016/0002-9416(83)90226-9. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi K. W, Slack J. M. The use of titanium fixtures for intraoral anchorage to facilitate orthodontic tooth movement. Int J Oral Maxillofac Implants. 1991;63:338–344. [PubMed] [Google Scholar]
- 5.Branemark P. I, Adell R, Breine U, et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81–100. doi: 10.3109/02844316909036699. [DOI] [PubMed] [Google Scholar]
- 6.Branemark P. I, Hansson B. O, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg. 1977;16(suppl):1–132. [PubMed] [Google Scholar]
- 7.Albrektsson T, Branemark P. I, Hansson H. A, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 8.Adell R, Lekholm U, Rockler B, Branemark P. I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 9.Kanomi R. Mini-implant for orthodontic anchorage. J Clin Orthod. 1997;31:763–767. [PubMed] [Google Scholar]
- 10.Melsen B, Costa A. Immediate loading of implants used for orthodontic anchorage. Clin Orthod Res. 2000;3:23–28. doi: 10.1034/j.1600-0544.2000.030105.x. [DOI] [PubMed] [Google Scholar]
- 11.Freudenthaler J. W, Hass R, Bantleon H. P. Bicortical titanium screws for critical orthodontic anchorage in the mandible: a preliminary report on clinical applications. Clin Oral Implant Res. 2001;13:358–363. doi: 10.1034/j.1600-0501.2001.012004358.x. [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki S, Koyama I, Inoue M, et al. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2003;124:373–378. doi: 10.1016/s0889-5406(03)00565-1. [DOI] [PubMed] [Google Scholar]
- 13.Deguchi T, Takamo-Yamamoto T, Kanomi R, Hartsfield J. K. The use of small titanium screws for orthodontic anchorage. J Dent Res. 2003;82:377–381. doi: 10.1177/154405910308200510. [DOI] [PubMed] [Google Scholar]
- 14.Roberts W. E, Marshall K. J, Mozsary P. G. Rigid endosseous implants for orthodontic and orthopedic anchorage. Angle Orthod. 1990;59:247–256. doi: 10.1043/0003-3219(1989)059<0247:REIFOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Roberts W. E. Bone dynamics of osseointegration, ankylosis, and tooth movement. J Indiana Dent Assoc. 1999;78:24–32. [PubMed] [Google Scholar]
- 16.Roberts W. E, Smith R. K, Zilberman Y, et al. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod. 1984;86:95–111. doi: 10.1016/0002-9416(84)90301-4. [DOI] [PubMed] [Google Scholar]
- 17.Roberts W. E. Orthodontic anchorage with osseointegrated implants: bone physiology, metabolism, and biomechanics. In: Higuchi K. W, editor. Orthodontic Applications of Osseointegrated Implants. Chicago, IL: Quintessence Publishing; 2000. pp. 161–190. [Google Scholar]
- 18.Roberts W. E. Bone tissue interface. Int J Oral Implantol. 1988;5:71–74. [PubMed] [Google Scholar]
- 19.Saito S, Sugimoto N, Morohashi T, et al. Endosseous titanium implants as anchors for mesiodistal tooth movement in the beagle dog. Am J Orthod Dentofacial Orthop. 2001;18:601–607. doi: 10.1067/mod.2000.110636. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Deng F, Wang Z, Zhao Z, Wang J. Biomechanical and histomorphometric analyses of the osseointegration of microscrews with different surgical techniques in beagle dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:644–650. doi: 10.1016/j.tripleo.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Roberts W. E, Marshall K. J, Mozsary P. G. Rigid endosseous implant utilized as anchorage to protract molars and close an atrophic extraction site. Angle Orthod. 1990;60:135–152. doi: 10.1043/0003-3219(1990)060<0135:REIUAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Costa A, Raffaini M, Melsen B. Miniscrews as orthodontic anchorage: a preliminary report. Int J Adult Orthod Orthognath Surg. 1998;13:201–209. [PubMed] [Google Scholar]
- 23.Park H. S, Kwon O. W, Sung J. H. Micro-implant anchorage for forced eruption of impacted canines. J Clin Orthod. 2004;38:297–302. [PubMed] [Google Scholar]
- 24.Chung K. R, Kim S. H, Kook Y. A. The C-orthodontic micro-implant. J Clin Orthod. 2004;38:478–486. [PubMed] [Google Scholar]
- 25.Giancotti A, Greco M, Mampleri G, Arcuri C. Treatment of ectopic maxillary canines using a palatal implant for anchorage. J Clin Orthod. 2005;39:607–611. [PubMed] [Google Scholar]
- 26.Garfinkle J. S, Cunningham L. L, Beeman C. S, Thomas Kluemper G, Preston Hicks E, Kim M. Evaluation of orthodontic mini-implant anchorage in premolar extraction therapy in adolescents. Am J Orthod Dentofacial Orthop. 2008;133:642–653. doi: 10.1016/j.ajodo.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 27.Albrektsson T, Johansson C, Sennerby L. What is osseointegration? In: Worthington P, Evans J. R, editors. Controversies in Oral & Maxillofacial Surgery. Philadephia, PA: WB Saunders Company; 1994. pp. 436–450. [Google Scholar]
- 28.Qin L, Hung L, Leung K. S, et al. Staining intensity of individual osteons correlated with elastic properties and degrees of mineralization. J Bone Miner Metab. 2001;19:359–364. doi: 10.1007/s007740170005. [DOI] [PubMed] [Google Scholar]
- 29.Donath K. Die Trenn-Dünnschlifftechnik zur Herstellung histologischer Präparate von nicht schneibaren Geweben und Materialen. Der Präparator. 1988;34:197–206. [Google Scholar]
- 30.Buys R, Dogterom A. A. An improved method for embedding hard tissue in polymethacrylate. Stain Technol. 1983;58(2):135–141. doi: 10.3109/10520298309066774. [DOI] [PubMed] [Google Scholar]
- 31.Misch C. E, Bidez M. W, Sharawy M. A bioengineered implant for a predetermined bone cellular response to loading forces. A literature review and case reports. J Periodontol. 2001;72:1276–1286. doi: 10.1902/jop.2000.72.9.1276. [DOI] [PubMed] [Google Scholar]
- 32.Misch C. E. Implant design considerations for the posterior regions of the mouth. Implant Dent. 1999;8:376–386. doi: 10.1097/00008505-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Minkin C, Marinho V. C. Role of the osteoclast at the bone-implant interface. Adv Dent Res. 1999;13:49–56. doi: 10.1177/08959374990130011401. [DOI] [PubMed] [Google Scholar]
- 34.Romanos G. E, Toh C. G, Siar C. H, et al. Histological and histomorphometric evaluation of peri-implant bone subjected to immediate loading: an experimental study with Macaca fascicularis. Int J Oral Maxillofac Implants. 2002;17:44–51. [PubMed] [Google Scholar]
- 35.Tanck E, Homminga J, Van Lenthe G. H, Huiskes R. Increase in bone volume fraction precedes architectural adaptation in growing bone. Bone. 2001;28:650–654. doi: 10.1016/s8756-3282(01)00464-1. [DOI] [PubMed] [Google Scholar]
- 36.Roberts W. E. Bone physiology of tooth movement, ankylosis, and osseointegration. Semin Orthod. 2000;6:173–182. [Google Scholar]
- 37.Smalley W. M, Shapiro P. A, Hohi T. H, Kokich V. G, Branemark P. I. Osseointegrated titanium implants for maxillofacial protaction in monkeys. Am J Orthod Dentofacial Orthop. 1988;94:285–295. doi: 10.1016/0889-5406(88)90053-4. [DOI] [PubMed] [Google Scholar]
- 38.Homolka P, Beer A, Birkfellner W, et al. Bone mineral density measurement with dental quantitative CT prior to dental implant placement in cadaver mandibles: pilot study. Radiology. 2002;224:247–252. doi: 10.1148/radiol.2241010948. [DOI] [PubMed] [Google Scholar]
- 39.Johansson C. B, Albrektsson T, Thomesen P, et al. Tissue reaction to titanium-6-aluminium-4-randiunm alloy. Ear J Exp Musculoskel Res. 1992;1(2):161–169. [Google Scholar]
- 40.David E, Steflik M. S, Francis T, et al. Histologic observation of bone remodeling adjacent to endosteal dental implant. J Oral Implant. 1995;21:96–106. [PubMed] [Google Scholar]


