Abstract
Objective:
Patients with OSAS (obstructive sleep apnea syndrome) demonstrate decreased upper airway dimension and craniofacial skeletal abnormalities. The study was performed to analyze whether upper airway dimensions differed among Chinese nonsnoring children of different sagittal and vertical skeletal facial morphologies.
Materials and Methods:
Lateral cephalometric records were used to measure the dimensions of the upper airway. Two groups of subjects were studied. A group of subjects with a normodivergent facial pattern (n = 190; FH-MP angle between 23.5° and 30.5°) was divided into three subgroups according to ANB angle (Class I, II, or III). A second group of subjects with a normal sagittal facial pattern (n = 180; ANB angle between 0.7° and 4.7°) was divided into three subgroups according to the FH-MP angle (low angle, normal angle, or high angle). All subgroups were matched for age and sex.
Results:
In the group of subjects with a normodivergent facial pattern, a significant tendency for reduced upper airway dimension in the inferior part (palatopharyngeal and hypopharynx) was found in the Class III, Class I, and Class II subgroups, in that order. In the group of subjects with a normal sagittal facial pattern, the superior part of the airway (nasopharyngeal and palatopharyngeal) decreased with increasing mandibular plane angle.
Conclusion:
The sagittal and vertical skeletal patterns may be contributory factors for the variation of the inferior and superior part of the upper airway, respectively. Skeletal deficiency of nonsnoring Chinese children may predispose them to upper airway obstruction.
Keywords: Nonsnoring children, Lateral cephalometry, Inferior part of upper airway, Superior part of upper airway, Sagittal skeletal facial morphology, Vertical skeletal facial morphology
INTRODUCTION
The pathogenesis of obstructive sleep apnea (OSA) has been investigated for many years. Previous studies of different samples have shown an association between craniofacial skeletal morphology and upper airway dimension in OSA patients.1–4 The conclusions noted variables that may affect airway size or ventilation. These included mandibular deficiency, bimaxillary retrusion, steep occlusal plane, increased mandibular plane angle, and a more caudally positioned hyoid bone.5–8
According to the close relationship between the pharyngeal structures and dentofacial structures in OSA patients, a mutual association is expected to exist between the pharyngeal structures and the dentofacial pattern in the common population. Mergen and Jacobs9 reported that the midsagittal nasopharyngeal area and the nasopharyngeal depth are significantly larger in subjects with normal occlusion than in those with Class II malocclusion. Solow et al10 presumed that airway adequacy was related to the size and position of the mandible rather than maxillary variables. Ceylan and Oktay11 demonstrated that the pharyngeal structures were not affected by changes in the ANB angle. De Freitas et al12 reported that malocclusion type did not influence upper pharyngeal airway width; however, Class II and Class III patients with vertical growth patterns had significantly narrower upper pharyngeal airways than those with a normal growth pattern.
The present study sought to assess the effect of craniofacial morphology on the upper airway dimension with a sufficient sample size and to control for interactions between sagittal and vertical patterns. In addition, few studies of Chinese subjects have been reported to date.13,14 Because cephalometry is a useful and inexpensive clinical tool to evaluate Chinese patients with OSA,15 we chose it for the measurement of Chinese nonsnoring children. The aim of the present study was to investigate whether the upper airway dimensions of Chinese nonsnoring children were affected by sagittal and vertical skeletal variables, respectively.
MATERIALS AND METHODS
The sample for this study was taken from the Department of Orthodontics, School and Hospital of Stomatology, Peking University, China. A total of 370 subjects, ages 11 to 16 years, was selected. All subjects were informed of the research content, and a consent form was signed by each child's parents. The study did not proceed without the approval of both the institutional review board and the participants' parents. No subjects had a history of previous orthodontic/orthopedic treatment or any palatal/lip cleft symptom. The parents were questioned about their children's medical history to exclude any children with chronic mouth breathing, permanent snoring, and tonsillectomy or adenoidectomy. Subjects with obvious hyperplasia of tonsils and adenoids on cephalometric films were excluded from further analysis.
The subjects were divided into two groups: a normodivergent facial pattern group and a normal sagittal facial pattern group. The selection criteria for the normodivergent facial pattern group were FH-MP between 23.5° and 30.5° (mean 26.6°). This group was divided into three subgroups according to the ANB angle.
Subgroup 1: Class III, ie, ANB angle smaller than 0.7° (54 subjects)
Subgroup 2: Class I, ie, ANB angle between 0.7° and 4.7° (82 subjects)
Subgroup 3: Class II, ie, ANB angle larger than 4.7° (54 subjects)
The selection criteria for the normal sagittal facial pattern group were ANB angle between 0.7° and 4.7° (mean 2.9°). This group was divided into three subgroups according to the FH-MP angle:
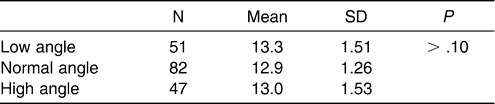
Subgroup 1: Low angle, ie, FH-MP angle smaller than 23.5° (51 subjects)
Subgroup 2: Normal angle, ie, FH-MP angle between 23.5° and 30.5° (82 subjects)
Subgroup 3: High angle, ie, FH-MP angle larger than 30.5° (47 subjects)
Lateral cephalometric radiographs were exposed with the patients seated in an upright position with Frankfort horizontal plane paralleled to the floor. Patients were instructed to breathe at ease with the teeth in centric occlusion. The radiographs were obtained with a UNTPANOCP-80 cephalometer with a linear magnification of 11%. All the films were digitized with a MICROTEK ScanMaker4 scanner and Photoshop 9.0 CS software. The cephalometric landmarks and analysis were based on the methods described previously by Lowe et al,16 Tangugsorn et al,17 and Liu et al.18 The landmarks and measurements identified in this study with OPAIM software are outlined in Figures 1 and 2 and Table 1. One operator measured 10 films twice 1 week apart to determine repeatability of the measurements, and intraclass correlation coefficients were calculated.
Figure 1.
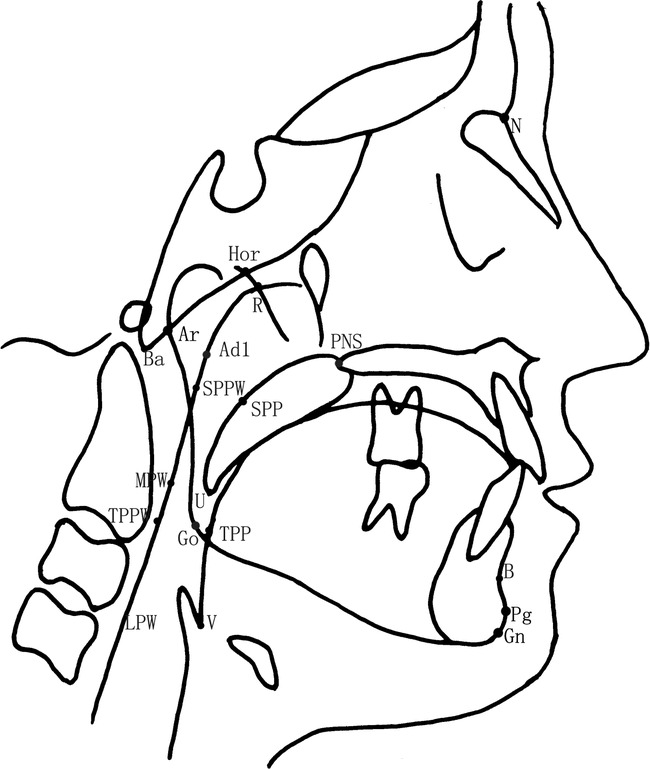
Cephalometric landmarks.
Figure 2.
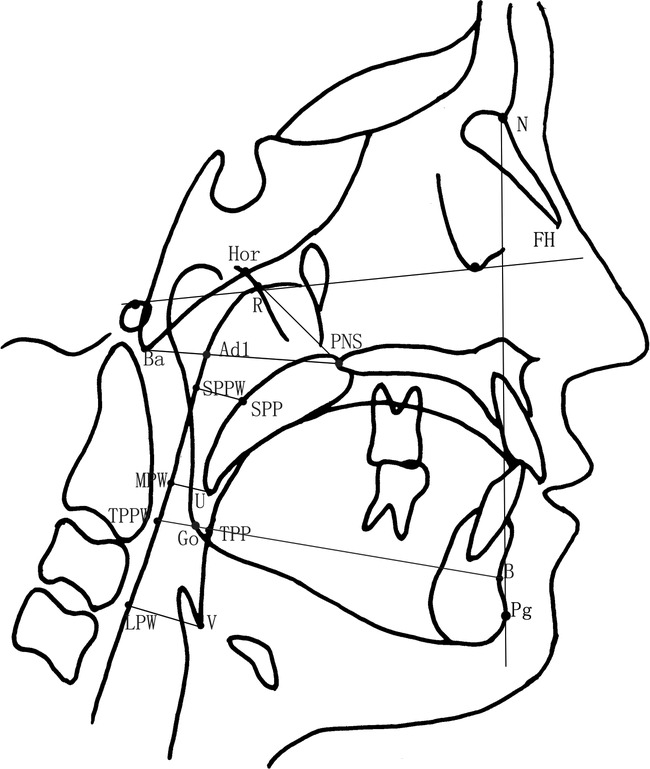
Cephalometric measurements.
Table 1.
Statistical procedures were performed on the recorded data using SPSS 11.0 software. One-way analysis of variance (ANOVA) and least significant difference (LSD) test for cephalometric comparison were performed among the subgroups.
To control distribution of sex and age in balance and to eliminate the interaction between the sagittal and vertical skeletal patterns among the three subgroups, the following analyses were performed for both the normodivergent facial pattern group and the normal sagittal facial pattern group. A chi-square test was performed to examine the sex distribution in each subgroup of the normodivergent facial pattern group. No significant difference was detected (Table 2). Age distribution was compared among the three subgroups (Table 3), and no statistical difference was observed. For the normal sagittal facial pattern group, the same analyses were performed. No significant differences with regard to sex or age distribution were detected (Tables 4 and 5).
Table 2.
Sex Distribution in the Subgroups of Subjects with a Normodivergent Facial Pattern
Table 3.
Age Distribution in the Subgroups of Subjects with a Normodivergent Facial Pattern
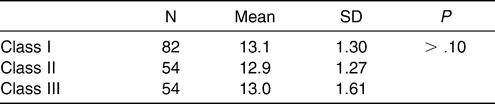
Table 4.
Sex Distribution in the Subgroups of Subjects with a Normal Sagittal Facial Pattern
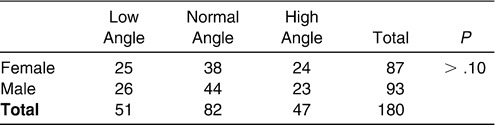
Table 5.
Age Distribution in the Subgroups of Subjects with a Normal Sagittal Facial Pattern
RESULTS
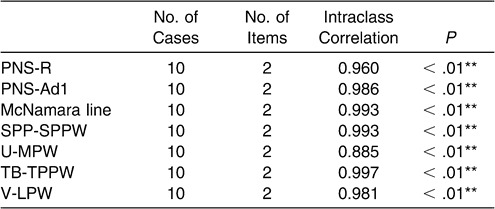
Intraclass correlation coefficients for the two separate measurements on the cephalometric radiographs ranged between 0.885 and 0.997 (Table 6).
Table 6.
Intraclass Correlation Coefficients of Cephalometric Measurements
Part A: Normodivergent Facial Pattern Group
There was no significant difference in the nasopharynx as measured from the PNS-R to SPP-SPPW levels among the subgroups of normodivergent facial pattern subjects (one-way ANOVA). The sagittal dimension of the inferior part of the upper airway (palatopharyngeal and hypopharynx) decreased from Class III to Class I to Class II, and these differences were significant. The most significant difference existed at the TB-TPPW level of the low oropharynx (P < .01) (Table 7, Figure 3).
Table 7.
Cephalometric Comparison of Normodivergent Subgroupsa
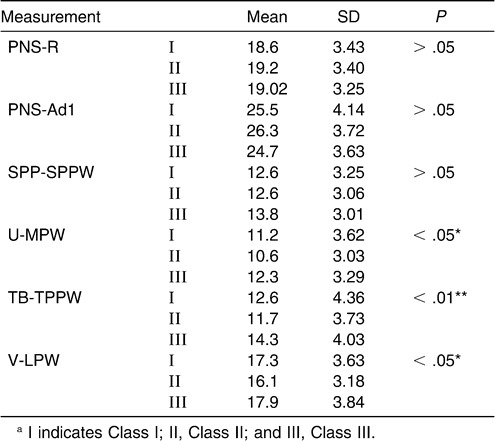
Figure 3.
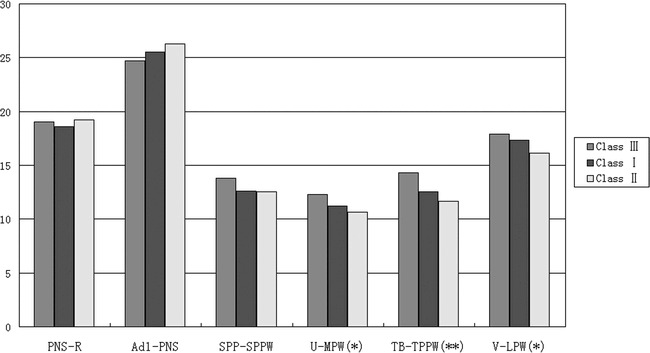
Cephalometric comparison of normodivergent subgroups (Class I, Class II, and Class III).
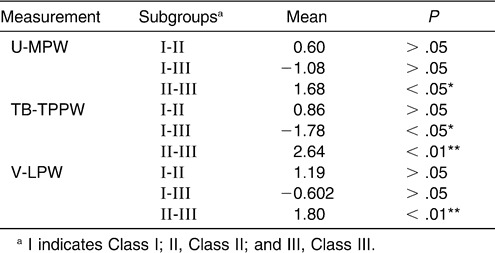
Pairwise comparisons among subgroups were done via the LSD test (Table 8). The data demonstrated a significant difference between Class II and Class III subgroups at the levels of the uvula, low oropharynx, and hypopharynx. At the level of TB-TPPW there was a significant difference between the Class I and Class III subgroups.
Table 8.
Multiple Comparison of Inferior Part of Upper Airway with LSD Test in the Normodivergent Subgroups
The literature suggests that there is a reduced tendency from Class III to Class I to Class II for the dimension of the inferior part of the upper airway, and this may contribute to the different mandibular sizes and positions. The LSD test was applied to the measurements of the mandibular size and position, and the results showed a significant difference (Table 9).
Table 9.
Cephalometric Comparison of Mandible Size and Position in the Normodivergent Subgroups
Part B: Normal Sagittal Facial Pattern Group
A statistically significant difference was found among the subgroups in the nasopharynx, as measured on the PNS-R and PNS-Ad1 levels and in the palatopharyngeal area as measured on the SPP-SPPW level with (one-way ANOVA). The superior part of the upper airway dimension decreased with an increasing FH-MP angle. However, no significant difference could be found in the inferior part of the upper airway, in contrast to the normodivergent group (Table 10, Figure 4).
Table 10.
Cephalometric Comparison of Normal Sagittal Subgroups
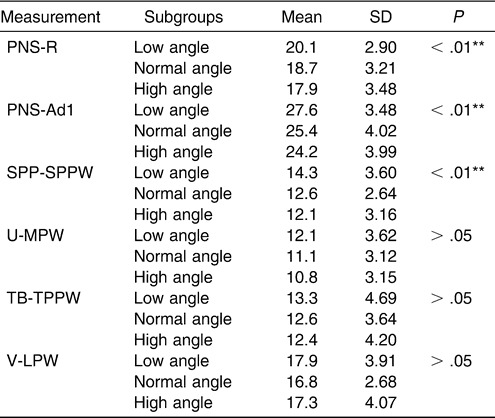
Figure 4.
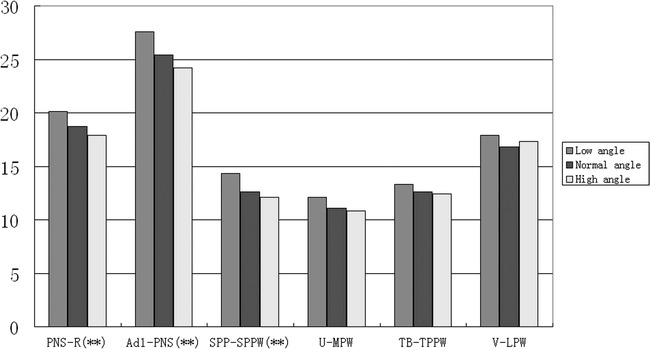
Histogram plots of cephalometric comparison of normal sagittal subgroups (low-angle, normal-angle, and high-angle).
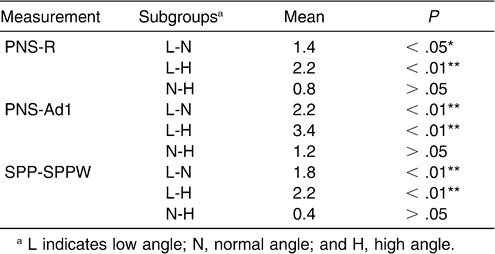
The LSD test for pairwise comparisons was performed to determine whether there were differences in the superior upper airway among the subgroups (Table 11). The data demonstrated a significant difference, except between the normal-angle and high-angle subgroups.
Table 11.
Multiple Comparison of Superior Part of Upper Airway Depth with LSD Test of Normal Sagittal Subgroups
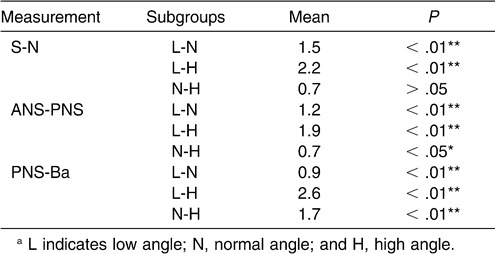
We tested for significant differences in the size of the craniomaxillary complex among the subgroups. There were significant differences in S-N, ANS-PNS, and PNS-Ba (Table 12).
Table 12.
Cephalometric Comparison of Craniomaxillary Complex Among Normal Sagittal Subgroups
DISCUSSION
Normal respiration is dependent on sufficient anatomical dimensions of the airway. In recent years, many studies have contributed to the knowledge that variations in the skeletal pattern could predispose persons to upper airway obstruction. Bacon et al19 reported that lower face height was the leading factor affecting the pharynx. Liu et al20,21 demonstrated that OSA patients with more retrognathic mandibles showed a significantly higher apnea index and respiratory disturbance index. They also reported that OSA patients showed a Class II and hypodivergent skeletal pattern compared with a normal population. In 1999, Gao et al22 reported that nasopharyngeal size is closely associated with OSA syndrome.
More recently, scholars have focused on assessing the intrinsic relationship between craniofacial morphology and upper airway morphology in orthodontic populations to predict the occurrence of OSA and to facilitate treatment for OSA patients. Because of inconsistencies in the conclusion of these studies, we tried to enhance the knowledge in the field by controlling for known factors. We expanded the sample size and controlled the distribution of sex and age, without significant differences among the three subgroups of each group. The influence of tonsil and adenoid hypertrophy was also limited. The influence of a vertical skeletal pattern was excluded in the normodivergent group to detect the effects of a sagittal skeletal pattern on upper airway structure and the influence of a sagittal skeletal pattern in a group of subjects with normal ANB angle. Therefore, the interference of many interrelated and confounding variables was minimized, since the selection criteria were defined strictly.
In this study, the sagittal skeletal pattern did not affect the dimensions of the nasopharynx in the normodivergent facial pattern group. In agreement with our findings, Sosa et al23 could find no clear-cut relationship between the nasopharyngeal area and Class I or Class II, division 1, malocclusions. Wenzel et al24 reported no correlations between airway size and mandibular morphology, although a significant relationship was observed between changes in nasopharyngeal airway size and maxillary prognathism. However, Kerr25 reported that Class II malocclusion subjects showed smaller nasopharyngeal dimensions compared with Class I and normal occlusion subjects. However, in his study, control of vertical skeletal pattern was not emphasized.
With respect to the inferior part of the upper airway, the results of this study seemed to suggest that the dimension of the oropharynx decreased markedly, from Class III to Class I to Class II subgroups, in the normodivergent facial pattern group. A significant difference was found between the Class II and Class III subgroups at all levels, from U-MPW to V-LPW. At the level of TB-TPPW, a significant difference existed between the Class I and Class III subgroups. However, no significant differences were found between the Class I and Class II subgroups for all the levels checked. Ceylan and Oktay11 claimed that the pharyngeal structures were not affected by the ANB angle, although they found a significant difference in the oropharyngeal area between Class I and Class III, as well as between Class II and Class III. Also, no vertical role could be found in his study. Most recently, Freitas et al12 measured the dimensions of the upper and lower oropharynx and found no significant difference between Class I and Class II malocclusions. Although that study classified its sample by molar relationships, which differed from the present classification by skeletal pattern subgroups, the results were similar.
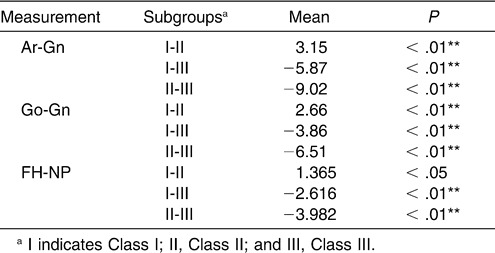
It seems that the sagittal skeletal pattern predisposes palatopharynx and hypopharynx obstruction as a result of the decreased size and posterior position of the mandible. Lam et al26 found that retroposition of the mandible was associated with severe OSA in Chinese subjects. Similarly, the research of Hou et al27 showed that mandibular body length was a significant predictor for OSA. Ang et al28 suggested that the mandible tended to be more retrognathic in their moderate to severe sample of OSA patients. When we detected a difference in mandible size and position among the subgroups, the same significant reduced tendency was seen with the dimension of the inferior part of the upper airway, from the Class III to the Class I to the Class II subgroups.
The pharyngeal cephalometric analysis in this study revealed significant dimensional differences in the superior part of the upper airway among hypodivergent, normodivergent, and hyperdivergent subgroups of normal sagittal facial pattern subjects. Dimensions at the PNS-R and PNS-Ad1 levels in the nasopharynx decreased with an increasing Frankfort mandibular plane angle. So did the dimension at SPP-SPPW in the middle of the palatopharynx. From the U-MPW level to the V-LPW level of the hypopharynx, no significant dimensional difference could be found among the subgroups. Since no significant difference in the ANB angle distribution existed in the subgroups, the impact of a different sagittal skeletal pattern on the superior part of the upper airway was excluded.
The finding is in agreement with Kerr,25 who suggested that the depth of the nasopharynx correlates with face height. Joseph et al29 also reported that the nasopharyngeal airway in hyperdivergent individuals was significantly narrower than in normodivergent individuals. However, they suggested that the difference occurred because of the relative bimaxillary retrusion exhibited by the hyperdivergent group. Their selection criteria of an experimental group included lip incompetence of 4 mm and no medical history regarding adenoids and tonsils; moreover, there was no restriction of sagittal skeletal pattern in the subjects in their study. Freitas et al12 also demonstrated that vertical growth patterns influence the upper oropharyngeal airways but have no impact on the lower oropharyngeal airways in Class I and Class II malocclusion. Interestingly, our findings in the normal sagittal group are in agreement with this.
The relationship between the superior part of the upper airway and the vertical facial pattern may be a result of deficient development of the craniomaxillary complex. Hou et al27 suggested that normal-weight OSA patients had a significantly shorter anterior cranial base and maxillary length. Paoli et al30 had found that patients of OSA with a body mass index <30 had a shorter anterior floor of cranial base. In this study, the analysis of the craniofacial skeleton demonstrated that the dimensions of S-N, ANS-PNS, and PNS-Ba also decreased with increases in the Frankfort mandibular plane angle. The identical tendency may imply that the deficient development of the craniomaxillary complex caused the decrease in the superior part of the upper airway dimensions in hyperdivergent patients.
As a result, we suggest sagittal facial pattern as the potential explanation for the discrepancy in the depth of the inferior part of the upper airway as a result of mandibular size and position. A vertical facial pattern was responsible for the deficiency in depth of the superior part of the upper airway because of the craniomaxillary complex. We believe that the upper airway obstruction of OSA patients could be traced back to their juvenile stage, with a susceptible craniofacial morphology as the potential mechanism. The present study partially demonstrated that a skeletal deficiency might predispose a person to upper airway narrowing, which in turn might predispose the person to obstruction in a population without permanent snoring. A longitudinal study of craniofacial morphology as a potential pathogenic factor is warranted in the future.
CONCLUSIONS
The present study confirms the association between pharyngeal structure and sagittal or vertical craniofacial skeletal pattern in nonsnoring Chinese children.
The sagittal skeletal pattern may be a contributory factor in variations in the inferior part of the upper airway.
Variations in the superior part of the upper airway may be attributable to a vertical skeletal pattern.
Acknowledgments
This study was supported by the National Natural Sciences Foundation of the Peoples Republic of China Grants 30872915.
REFERENCES
- 1.Lowe A. A, Santamatia J. D, Fleetham J. A, et al. Facial morphology and obstructive sleep apnoea. Am J Orthod Dentofacial Orthop. 1986;90:484–491. doi: 10.1016/0889-5406(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 2.Bacon W. H, Turbot J. C, Krieger J, et al. Craniofacial characteristics in patients with obstructive sleep apnoeas syndrome. Cleft Palate J. 1988;25:374–378. [PubMed] [Google Scholar]
- 3.Hui D. S, Ko F. W, Chu A. S, et al. Cephalometric assessment of craniofacial morphology in Chinese patients with obstructive sleep apnea. Respir Med. 2003;97:640–646. doi: 10.1053/rmed.2003.1494. [DOI] [PubMed] [Google Scholar]
- 4.Battagel J. M, L'Estrange P. R. The cephalometric morphology of patients with obstructive sleep apnoea. Eur J Orthod. 1996;18:557–569. doi: 10.1093/ejo/18.6.557. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson A, Guilleminault C, Partinen M, Quera-Salva M. A. Obstructive sleep apnea patients have craniomandibular abnormalities. Sleep. 1986;9:469–477. doi: 10.1093/sleep/9.4.469. [DOI] [PubMed] [Google Scholar]
- 6.Tangugsorn V, Skatvedt O, Krogstad O, Lyberg T. Obstructive sleep apnea: a cephalometric study. Part I. Cervicocraniofacial skeletal morphology. Eur J Orthod. 1995;17:45–56. doi: 10.1093/ejo/17.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya M, Lowe A. A, Pae E. K, Fleetham J. A. Obstructive sleep apnea subtypes by cluster analysis. Am J Orthod Dentofacial Orthop. 1992;101:533–542. doi: 10.1016/0889-5406(92)70128-W. [DOI] [PubMed] [Google Scholar]
- 8.Andersson L, Brattatrom V. Cephalometric analysis of permanently snoring patients with and without obstructive sleep apnea syndrome. Int J Oral Maxillofac Surg. 1991;20:159–162. doi: 10.1016/s0901-5027(05)80007-4. [DOI] [PubMed] [Google Scholar]
- 9.Mergen D. C, Jacobs R. M. The size of nasopharynx associated with normal occlusion and Class II malocclusion. Nasopharynx. 1970;40:342–346. doi: 10.1043/0003-3219(1970)040<0342:TSONAW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Solow B, Siersbæk-Nielsen S, Greve E. Airway adequacy, head posture, and craniofacial morphology. Am J Orthod. 1984;86:214–223. doi: 10.1016/0002-9416(84)90373-7. [DOI] [PubMed] [Google Scholar]
- 11.Ceylan I, Oktay H. A study on the pharyngeal size in different skeletal patterns. Am J Orthod Dentofacial Orthop. 1995;108:69–75. doi: 10.1016/s0889-5406(95)70068-4. [DOI] [PubMed] [Google Scholar]
- 12.De Freitas M. R, Alcazar N. M, Janson G, et al. Upper and lower pharyngeal airways in subjects with Class I and Class II malocclusions and different growth patterns. Am J Orthod Dentofacial Orthop. 2006;130:742–745. doi: 10.1016/j.ajodo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Shen G. F, Samman N. Cephalometric studies on the upper airway space in normal Chinese. Int J Oral Maxillofac Surg. 1994;23:243–247. doi: 10.1016/s0901-5027(05)80379-0. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X. L, Forsberg C. M, Linder A. S. Craniofacial morphology in Chinese and Swedish Children with Angle Class I and Class II occlusion relation. Aust Orthod J. 1998;15:168–176. [PubMed] [Google Scholar]
- 15.Chang E. T, Shiao G. M. Craniofacial abnormalities in Chinese patients with obstructive and positional sleep apnea. Sleep Med. 2008;9:403–410. doi: 10.1016/j.sleep.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Lowe A. A, Ono T, Fleetham J. A, et al. Cephalometric comparisons of craniofacial and upper airway structure by skeletal subtype and gender in patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1996;110:653–664. doi: 10.1016/s0889-5406(96)80043-6. [DOI] [PubMed] [Google Scholar]
- 17.Tangugsorn V, Skatvedt O, Krogstad O, et al. Obstructive sleep apnea: a cephalometric study. Part II. Uvulo-glossopharyngeal morphology. Eur J Orthod. 1995;17:57–67. doi: 10.1093/ejo/17.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Zeng X, Lowe A. A, et al. Effects of a mandibular repositioner on obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2000;118:248–256. doi: 10.1067/mod.2000.104831. [DOI] [PubMed] [Google Scholar]
- 19.Bacon W. H, Krieger J, Stierle J. L, et al. Cephalometric evaluation of pharyngeal obstructive factors in patients with sleep apneas syndrome. Angle Orthod. 1989;60:115–121. doi: 10.1043/0003-3219(1990)060<0115:CEOPOF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zeng X, Fu M, Huang X. A comparative study on the severity of respiratory disturbance among the OSAS patients with different craniofacial morphology. J Clin Stomatology. 1998;14:20–21. [Google Scholar]
- 21.Liu Y, Lowe A. A, Zeng X, Fu M, Fleetham J. A. Cephalometric comparisons between Chinese and Caucasian patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2000;117:479–485. doi: 10.1016/s0889-5406(00)70169-7. [DOI] [PubMed] [Google Scholar]
- 22.Gao X, Zeng X, Fu M, Huang X. Nasopharygeal size in obstructive sleep apnea syndrome. Chin J Otorhinolaryngol. 1999;34:37–40. [PubMed] [Google Scholar]
- 23.Sosa F. A, Graber T. M, Muller T. P. Postpharyngeal lymphadenoid tissue in Angle Class I and Class II malocclusions. Am J Orthod. 1982;81:299–309. doi: 10.1016/0002-9416(82)90216-0. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel A, Williams S, Ritzau M. Relationships of changes in craniofacial morphology, head posture, and nasopharyngeal airway size following mandibular osteotomy. Am J Orthod Dentofacial Orthop. 1989;96:138–143. doi: 10.1016/0889-5406(89)90254-0. [DOI] [PubMed] [Google Scholar]
- 25.Kerr W. J. The nasopharynx, face height, and overbite. Angle Orthod. 1985;55:31–36. doi: 10.1043/0003-3219(1985)055<0031:TNFHAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Lam B, Ooi C. G, Peh W. C, Lauder I, Tsang K. W, Lam W. K, Ip M. S. Computed tomographic evaluation of the role of craniofacial and upper airway morphology in obstructive sleep apnea in Chinese. Respir Med. 2004;98:301–307. doi: 10.1016/j.rmed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Hou H. M, Hägg U, Sam K, Rabie A. B, Wong R. W, Lam B, Ip M. S. Dentofacial characteristics of Chinese obstructive sleep apnea patients in relation to obesity and severity. Angle Orthod. 2006;76:962–969. doi: 10.2319/081005-273. [DOI] [PubMed] [Google Scholar]
- 28.Ang P. K, Sandham A, Tan W. C. Craniofacial morphology and head posture in Chinese subjects with obstructive sleep apnea. Semin Orthod. 2004;10:90–96. [Google Scholar]
- 29.Joseph A. A, Elbaum J, Cisneros G. J, Eisig S. B. A cephalometric comparative study of the soft tissue airway dimensions in persons with hyperdivergent and normodivergent facial patterns. J Oral Maxillofac Surg. 1998;56:135–139. doi: 10.1016/s0278-2391(98)90850-3. [DOI] [PubMed] [Google Scholar]
- 30.Paoli J. R, Lauwers F, Lacassagne L, et al. Craniofacial differences according to the body mass index of patients with obstructive sleep apnoea syndrome: cephalometric study in 85 patients. Br J Oral Maxillofac Surg. 2001;39:40–45. doi: 10.1054/bjom.2000.0551. [DOI] [PubMed] [Google Scholar]


