Abstract
Objectives
To compare flow cytometric minimal residual disease (MRD) data obtained using the EuroFlow approach, including the CD38-multiepitope (ME) antibody or the VS38c antibody.
Methods
We evaluated 29 bone marrow samples from patients with multiple myeloma (MM), of whom 15 had received daratumumab within the past 6 months. We evaluated MRD data and fluorescence intensities.
Results
Qualitative MRD data were 100% concordant between the 2 approaches. In MRD-positive samples (n = 14), MRD levels showed an excellent correlation (R2 = 0.999). Whereas VS38c staining was strong in both normal plasma cells and MM cells, independent of daratumumab treatment, staining intensities for CD38 were lower in MM cells compared with normal plasma cells, and on both cell types CD38 expression was significantly reduced in daratumumab-treated patients.
Conclusions
Both CD38-ME and VS38c allow reliable MRD detection in MM patients, but the high expression of VS38c allows easier identification of MM cells, especially in daratumumab-treated patients.
Keywords: VS38c, CD38-multiepitope, EuroFlow, MM, MRD
KEY POINTS.
The EuroFlow multiple myeloma (MM) minimal residual disease (MRD) assay, including the CD38-multiepitope antibody, reliably detects MRD in patients with MM, even in daratumumab-treated patients.
VS38c is an alternative marker for identification of MM cells that is strongly expressed and not affected by treatment with daratumumab.
Daratumumab treatment results in reduced expression of CD38 on MM cells.
INTRODUCTION
With the introduction of immunotherapies, such as the CD38 monoclonal antibody daratumumab, outcomes for patients with multiple myeloma (MM) have significantly improved.1 Patient response can be evaluated by measuring minimal residual disease (MRD) by using molecular or flow cytometric assays. In flow cytometry, CD38 is frequently used as a gating marker for plasma cells.2-5 In patients treated with daratumumab (or other CD38 antibodies), however, the therapeutic antibody may interfere with the diagnostic antibody, thereby hampering appropriate identification of plasma cells in the diagnostic bone marrow aspirate.6
To overcome this interference, 2 recent studies evaluated the use of the VS38c antibody for detection of plasma cells.7,8 VS38c recognizes an intracellular protein that is strongly expressed in plasma cells as well as in melanoma cells, osteoblasts, and neuroendocrine tumors. It has been used in immunohistochemistry for a long time,9 but only recently has it been used more widely in flow cytometry. Mizuta and colleagues7 evaluated VS38c in 15 patients with MM and showed that this antibody enabled detection of plasma cells in all cases, including in 3 patients who had received daratumumab. In the latter patients, the regular CD38 antibody used (clone HIT2) did not detect the MM cells. Subsequently, Courville and colleagues8 evaluated VS38c in 38 patients with MM; they showed that the percentage of MM cells calculated using VS38c and standard panels (including CD38, clone HB7) did not differ but that identification and quantitation using the VS38c antibody was easier. Of note, in 1 daratumumab-treated patient, VS38c was not able to detect MM cells (probably because of downregulation of p63). These 2 studies show that VS38c is a reliable marker for plasma cell identification in patients with MM, including in patients treated with daratumumab.
Another approach to overcome interference of the therapeutic and diagnostic antibody is the application of a multiepitope (ME) CD38 antibody, as was included in the EuroFlow MM MRD panel.2 In this study of 29 patients with MM, of whom 15 had been treated with daratumumab within the past 6 months, we investigated whether MRD data, as defined by the EuroFlow panel with the CD38-ME, were comparable to MRD data obtained with the VS38c antibody.
MATERIALS AND METHODS
Bone marrow aspirates were obtained from 29 patients with MM during or after therapy. All patients provided written informed consent before enrollment. The study was approved by the medical ethics committee of the Erasmus MC (MEC-2016-606) and was conducted in accordance with the Declaration of Helsinki.
Bone marrow aspirates were processed using bulk lysis according to EuroFlow standard operating procedures2,10 and subsequently stained using the EuroFlow MM MRD panel (which consists of CD45/CD19/CD138/CD56/CD27/CD38-ME combined with CD117 and CD81 in tube 1 or cytoplasmatic [cy] immunoglobulin [Ig] κ and CyIgλ in tube 2).2 In parallel, 1 tube was stained using the EuroFlow MM MRD tube 2, in which CD38-ME was replaced with the fluorescein isothiocyanate (FITC)–conjugated VS38c antibody, according to the manufacturer’s instructions (Agilent Technologies). Both the original EuroFlow tube 2 (containing CyIgκ and CyIgλ) and the modified tube 2 (containing VS38c, targeting an intracellular epitope) were processed according to the protocol for intracellular stainings.11 Samples were acquired on a FACSLyric flow cytometer (BD Biosciences) using EuroFlow settings11,12; data were analyzed using Infinicyt 2.0 software (Cytognos). The person conducting the analysis was blinded to the therapy the patient received. In all cases, the 2 EuroFlow tubes were analyzed first; only after that was the VS38c tube analyzed, which implies that knowledge of VS38c staining was not used for the interpretation of the CD38-ME EuroFlow data.
RESULTS
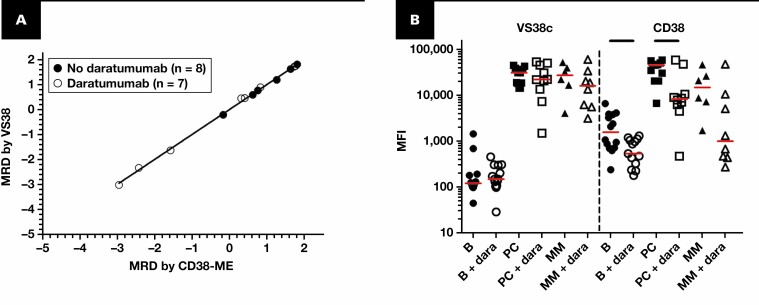
We analyzed bone marrow aspirates of 29 patients with MM obtained during follow-up using the CD38-ME and VS38c approaches. Using both approaches, 14 of 29 samples (48%) were MRD positive, and 15 samples (52%) were MRD negative, with 100% concordance between approaches. Further analysis of the 14 MRD-positive samples showed a highly significant correlation between the 10log-transformed MRD data of both measurements (R2 = 0.999, P < .0001; slope = 1.0) FIGURE 1A). Overall, these data indicate that MRD analysis in patients with MM using CD38-ME and VS38c provide highly comparable data, both qualitative as quantitative.
FIGURE 1.
A, Minimal residual disease (MRD) data (10log-transformed percentage of MRD cells) obtained using the EuroFlow approach, including CD38-multiepitope (ME) vs MRD data obtained using VS38c. The correlation coefficient for all samples together was 0.998 (P < .001); similar numbers were obtained if the 2 groups (with and without daratumumab) were analyzed separately. The samples obtained from patients treated without (n = 14) or with daratumumab (n = 15) are shown in closed and open circles, respectively. Fifteen samples (8 from patients not treated with daratumumab and 7 from patients treated with daratumumab) were MRD negative by both approaches and are shown in the bottom left this plot. B, Mean fluorescence intensities (MFIs) of CD38-ME and VS38c on B cells (B), normal plasma cells (PC), and multiple myeloma (MM) cells (if present at levels equal to or above 0.01%) in patients without (n = 14) or with daratumumab treatment (+ dara; n = 15). For VS38c, no significant differences were observed for the MFI values of PC and MM cells, independent of daratumumab treatment. For CD38, expression was highest on normal PC from patients not treated with daratumumab; MM cells from such patients showed a tendency for lower CD38 expression levels (P = .06). In patients treated with daratumumab, CD38 expression on B cells and normal cells was significantly lower compared with these cells in patients not treated with daratumumab (P < .05), and the same tendency was present for MM cells (P = .09). All P values were based on 2-tailed Mann-Whitney tests. The red lines represent the median MFI value of the group. The black lines on top of the figure indicate statistically significant differences between the 2 groups.
Because VS38c has been reported to be easier for MM cell identification than using CD38, we next evaluated whether this approach is also valid for the CD38-ME. As shown in FIGURE 1B, CD38-ME and VS38c both showed high and comparable mean fluorescence intensities (MFIs) on normal plasma cells from patients not treated with daratumumab. In contrast, in these patients, VS38c showed significantly lower MFI values on normal B cells compared with CD38. Consequently, the ratio between normal plasma cells and B cells was significantly higher for VS38c than for CD38-ME (VS38c: median [range], 202 [12.8-364]; CD38-ME: median [range], 24.5 [8.6-67.6]; P < .01). In daratumumab-treated patients, VS38c expression levels on B cells and normal plasma cells were comparable with those in patients not treated with daratumumab, whereas CD38 expression on B cells and normal plasma cells was significantly lower FIGURE 1B. The ratio between normal plasma cells and B cells in daratumumab-treated patients was significantly higher for VS38c compared with CD38-ME (VS38c: median [range], 148 [3.3-511]; CD38-ME: median [range], 19.2 [1.4-55.7]; P < .01). VS38c was expressed in MM cells at similar levels as on normal plasma cells, without a clear effect of daratumumab. In contrast, CD38 expression in MM cells tended to be lower than in normal plasma cells (P = .056) and decreased even more in the majority of daratumumab-treated patients FIGURE 1B. An example of a patient with virtually complete loss of CD38 expression is shown in FIGURE 2. Despite the differences in expression levels between VS38c and CD38-ME, identification of MM cells was felt to be comparably easy for both approaches in the context of the other markers.
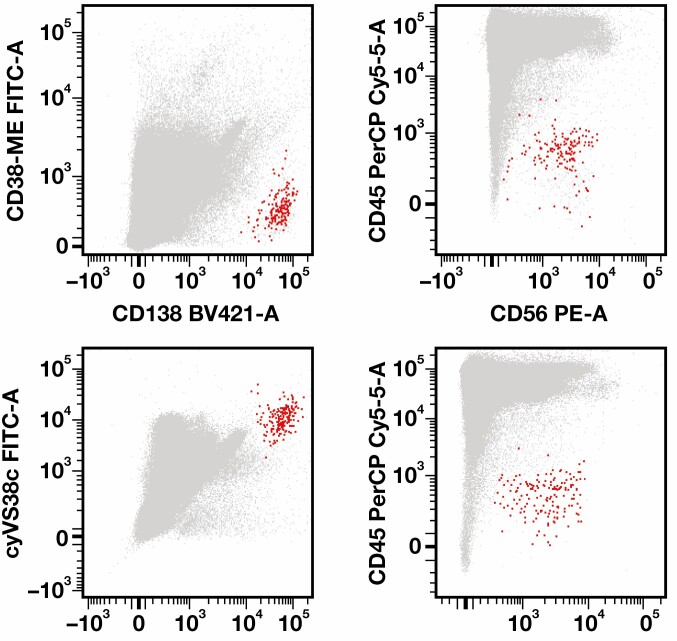
FIGURE 2.
Flow cytometric data from a patient with multiple myeloma (MM) treated with daratumumab. Upper row: Data obtained using the EuroFlow MM minimal residual disease (MRD) tube, including the CD38-multiepitope (ME) antibody. Whereas CD138 is brightly expressed by the MM cells (in red), CD38 expression is hardly detectable. Bottom row: Data obtained using the EuroFlow MM MRD tube, in which the CD38-ME antibody has been replaced by the VS38c antibody. Both CD138 and VS38c are brightly expressed by the MM cells (in red). In both tubes, the MM cells could easily be distinguished by low CD45 expression in combination with CD56 expression. FITC, fluorescein isothiocyanate; PerCP Cy5.5, peridinin-chlorophyll-protein complex-cyanine 5.5.
DISCUSSION
There have been concerns that current MM MRD assays do not work well in patients treated with targeted therapies, such as daratumumab. Within the EuroFlow consortium, a CD38-ME was developed and included in the MM MRD panel.2 Alternatively, other markers for identification of MM cells can be used, and VS38c so far seems most promising.7,8 In this study, we show that both approaches provide similar MRD data and that both analysis approaches are equally easy. The latter logically is subjective and dependent on knowledge and experience, but we felt that even in patients with relatively low or no CD38 expression of MM cells, appropriate identification is possible by taking the remaining 7 markers present in the panel into account, as well FIGURE 2. Note that VS38c and CD38-ME both gave similarly high MFI values on normal plasma cells but that more B cells were positive for CD38 compared with VS38c. This finding is not unexpected, because naive B cells do express variable CD38 levels, depending on their proliferative history.13
In patients treated without daratumumab, both approaches (using CD38-ME or VS38c) worked well. The strong correlation in MRD data obtained using either VS38c or CD38-ME on these patient samples indicates that the 2 approaches identify a similar population of MM cells. Importantly, in the patients treated with daratumumab, MRD data were also highly comparable between both approaches. In addition, no samples were MRD positive using the VS38c approach but missed using the CD38-ME approach.
Our data are in line with those by Courville and colleagues,8 who found similar MRD values through analysis by VS38c or by a single-epitope CD38 antibody. These authors also reported lower CD38 MFI values in plasma cells and natural killer cells from patients treated with daratumumab in the past 6 months and indicated competition as opposed to antigen loss as the mechanistic pathway. The CD38-ME antibody also detects lower CD38 expression levels in daratumumab-treated patients, but antigenic loss does seem to occur, as well. Virtual complete downregulation of CD38 on MM cells as an escape mechanism seems rare14 but can be observed, as exemplified by some patients in our study. The virtual loss of CD38 in some daratumumab-treated patients with measurable residual disease may be a warning that more extensive downregulation will occur with the increase in use of daratumumab or other CD38-targeting therapies. Obviously, in such cases, CD38 is of limited value, and the availability of VS38c will facilitate MM cell identification.
We conclude that VS38c and CD38-ME produce highly comparable MRD data in patients with MM, including patients receiving daratumumab. Given the therapy-induced downregulation of CD38, it might be beneficial to expand the 8-color EuroFlow MM MRD tubes with VS38c. Doing so, however, is not yet feasible because VS38c is currently available only as an FITC or peridinin-chlorophyll-protein complex-cyanine 5.5 conjugate.
Acknowledgments
We gratefully acknowledge the technicians of the Leukemia and Lymphoma Diagnostics Group (Department of Immunology) for their contribution in sample processing and data analysis.
Funding: This work was supported by a nonrestrictive grant from Agilent Technologies.
Disclosure: J.K. is an employee of Agilent. V.H.J.vdV. is one of the inventors on the EuroFlow-owned patent PCT/NL2013/0505420 (methods, reagents and kits for detecting minimal residual disease). The related patent is licensed to Cytognos, which company pays royalties to the EuroFlow Consortium. V.H.J.vdV. reports a laboratory services agreement with Agilent Technologies and with BD Biosciences.
Contributor Information
Annemiek Broijl, Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Augustinus C M de Jong, Department of Immunology, Laboratory Medical Immunology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Mark van Duin, Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Pieter Sonneveld, Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Jesper Kühnau, Reagent Partnership Division, Diagnostics and Genomics Group, Agilent Technologies, Glostrup, Denmark.
Vincent H J van der Velden, Department of Immunology, Laboratory Medical Immunology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
REFERENCES
- 1. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29-38. [DOI] [PubMed] [Google Scholar]
- 2. Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Dongen JJ, Lhermitte L, Bottcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26:1908-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Royston DJ, Gao Q, Nguyen N, et al. Single-tube 10-fluorochrome analysis for efficient flow cytometric evaluation of minimal residual disease in plasma cell myeloma. Am J Clin Pathol. 2016;146:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stetler-Stevenson M, Paiva B, Stoolman L, et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom. 2016;90:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oberle A, Brandt A, Alawi M, et al. Long-term CD38 saturation by daratumumab interferes with diagnostic myeloma cell detection. Haematologica. 2017;102:e368-e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizuta S, Kawata T, Kawabata H, et al. VS38 as a promising CD38 substitute antibody for flow cytometric detection of plasma cells in the daratumumab era. Int J Hematol. 2019;110:322-330. [DOI] [PubMed] [Google Scholar]
- 8. Courville EL, Yohe S, Shivers P, et al. VS38 identifies myeloma cells with dim CD38 expression and plasma cells following daratumumab therapy, which interferes with CD38 detection for 4 to 6 months. Am J Clin Pathol. 2020;153:221-228. [DOI] [PubMed] [Google Scholar]
- 9. Turley H, Jones M, Erber W, et al. VS38: a new monoclonal antibody for detecting plasma cell differentiation in routine sections. J Clin Pathol. 1994;47:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theunissen P, Mejstrikova E, Sedek L, et al. ; EuroFlow Consortium . Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 2017;129:347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalina T, Flores-Montero J, van der Velden VH, et al. ; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708) . EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26:1986-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glier H, Novakova M, Te Marvelde J, et al. Comments on EuroFlow standard operating procedures for instrument setup and compensation for BD FACS Canto II, Navios and BD FACS Lyric instruments. J Immunol Methods. 2019;475:112680. [DOI] [PubMed] [Google Scholar]
- 13. Theunissen PMJ, van den Branden A, Van Der Sluijs-Gelling A, et al. Understanding the reconstitution of the B-cell compartment in bone marrow and blood after treatment for B-cell precursor acute lymphoblastic leukaemia. Br J Haematol. 2017;178:267-278. [DOI] [PubMed] [Google Scholar]
- 14. Minarik J, Novak M, Flodr P, et al. CD38-negative relapse in multiple myeloma after daratumumab-based chemotherapy. Eur J Haematol. 2017;99:186-189. [DOI] [PubMed] [Google Scholar]