Abstract
Background
Open fractures of the major long bones are complex limb‐threatening injuries that are predisposed to deep infection. Treatment includes antibiotics and surgery to debride the wound, stabilise the fracture and reconstruct any soft tissue defect to enable infection‐free bone repair. There is a need to assess the effect of timing and duration of antibiotic administration and timing and staging of surgical interventions to optimise outcomes.
Objectives
To assess the effects (risks and benefits) of the timing of antibiotic administration, wound debridement and the stages of surgical interventions in managing people with open long bone fractures of the upper and lower limbs.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and clinical trial registers in February 2021. We also searched conference proceedings and reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs that recruited adults with open fractures of the major long bones, comparing: 1) timings of prophylactic antibiotic treatment, 2) duration of prophylactic antibiotic treatment, 3) timing of wound debridement following injury or 4) timing of the stages of reconstructive surgery.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We aimed to collect data for the following outcomes: limb function, health‐related quality of life (HRQoL), deep surgical site infection, delayed or non‐union, adverse events (in the short‐ and long‐term course of recovery), and resource‐related outcomes.
Main results
We included three RCTs of 613 randomised participants with 617 open fractures. Studies were conducted in medical and trauma centres in the USA and Kenya. Where reported, there was a higher proportion of men and a mean age of participants between 30 and 34 years old. Fractures were in the upper and lower limbs in one study, and were tibia fractures in two studies; where reported, these were the result of high‐energy trauma such as road traffic accidents. No studies compared the timing of antibiotic treatment or wound debridement.
Duration of prophylactic antibiotic treatment (1 study, 77 participants available for analysis)
One study compared antibiotic treatment for 24 hours with antibiotic treatment for five days. We are very uncertain about the effects of different durations of antibiotic treatment on superficial infections (risk ratio (RR) 1.19, 95% CI 0.49 to 2.87, favours 5 day treatment; 1 study, 77 participants); this was very low‐certainty evidence derived from one small study with unclear and high risks of bias, and with an imprecise effect estimate. This study reported no other review outcomes.
Reconstructive surgery: timing of the stages of surgery (2 studies, 458 participants available for analysis)
Two studies compared the timing of wound closure, which was completed immediately or delayed. In one study, the mean time of delay was 5.9 days; in the other study, the time of delay was not reported. We are very uncertain about the effects of different timings of wound closure on deep infections (RR 0.82, 95% CI 0.37 to 1.80, favours immediate closure; 2 studies, 458 participants), delayed union or non‐union (RR 1.13, 95% CI 0.83 to 1.55, favours delayed closure; 1 study, 387 participants), or superficial infections (RR 6.45, 95% CI 0.35 to 120.43, favours delayed closure; 1 study, 71 participants); this was very low‐certainty evidence. We downgraded the certainty of the evidence for very serious risks of bias because both studies had unclear and high risks of bias. We also downgraded for serious imprecision because effect estimates were imprecise, including the possibility of benefits as well as harms, and very serious imprecision when the data were derived from single small study. These studies reported no other review outcomes.
Authors' conclusions
We could not determine the risks and benefits of different treatment protocols for open long bone fractures because the evidence was very uncertain for the two comparisons and we did not find any studies addressing the other possible comparisons. Well‐designed randomised trials with adequate power are needed to guide surgical and antibiotic treatment of open fractures, particularly with regard to timing and duration of antibiotic administration and timing and staging of surgery.
Plain language summary
The timing of treatment for broken bones in the legs and arms that also have open wounds
Key messages
We are uncertain whether the different timings of treatments (antibiotics, wound cleaning and whether to do all the surgery in one operation or more) might affect how well people recover from open long bone fractures. We found very few studies, and they did not provide reliable evidence.
What is an open fracture?
Some broken bones include open skin wounds around the break. These are called open fractures and are most common in high impact injuries such as road traffic accidents or falls from a height. In this review, we were interested in the treatment of open fractures of long bones (in the thigh, shin, lower leg, upper arm and forearms). These fractures can be very serious injuries. Because of the open wound, people are at serious risk of infection. A 'deep infection' involves the bone and will require further surgery; it may be limb‐threatening and affect long‐term recovery.
What are the treatments?
‐ Antibiotics (to fight infection) may be started early (within an hour of injury) or later; they may be given for a short time (until the wound is surgically closed) or a longer time.
‐ Debridement: surgically removing any dirt, foreign objects, or dead and damaged tissue from inside the wound: this may be done early (within 12 hours of injury) or later.
‐ Treatment also includes surgery to fix the bone and close the wound (this may involve moving skin or muscle, or both, from another body area to cover the wound). All stages (debridement, fixing the bone, wound closure) may be completed during a single operation or in multiple operations. If this is done in multiple operations, the wound may be closed early (within 72 hours of injury) or later.
What did we do?
We searched for studies that compared one or more of these treatments for adults who had broken bones with open skin wounds. We wanted to find out the benefits and harms of these different treatments. If we found similar studies, of the same treatments, we combined the findings from the studies to see if we could find out if some treatment resulted in better outcomes.
What did we find?
We found three studies with 613 adults, who had 617 open fractures. Most fractures were in men, and the average age was 30 to 34 years. In two studies, the open fractures were in the shin bone (a common place for this type of injury because there is not much skin and muscle between the bone and skin surface). In the other study, injuries were in arms and legs.
One study compared giving antibiotics for 24 hours (short course) with five days of antibiotics (long course). It is unclear if these treatment times had an effect on the number of people who developed superficial infections within 14 days. Superficial infections are in the skin around the wound, but not deep enough to affect the bone; they may occur early in the recovery period and can be easily treated with antibiotics only.
Two studies compared closing the wound immediately after fixing the bone with a delay to closing the wound. It is unclear if timing of wound closure had an effect on the number of people who developed deep infections, superficial infections, or had delayed bone union (when the broken parts of the bone heal together more slowly than expected) or non‐union (when the bone does not heal).
None of the studies looked at how well a person can move their injured arm or leg, or the health‐related quality of life of people six to 12 months after treatment.
We found no studies that compared starting antibiotics immediately or later, whether wound cleaning started within 12 hours of the injury or later, or whether the different stages of treatment happened at the same time or separately.
Are we confident in what we found?
We are not very confident in these findings because:
‐ we only had information from one small study about how long antibiotics are given, and from two studies about when the wound is closed;
‐ the studies were not well reported, and one was only reported in a short summary;
‐ all of the findings included the possibility of benefit (for example, fewer infections with a short course of antibiotics) as well as the possibility of harm (for example, more infections with a short course of antibiotics).
How up to date is this review?
The evidence is up to date to February 2021.
Summary of findings
Summary of findings 1. Duration of prophylactic antibiotic treatment for open long bone fractures of the upper and lower limbs.
| Duration of prophylactic antibiotic treatment for open long bone fractures of the upper and lower limbs | ||||||
| Population: adults with open long bone fractures; study in the review included only Gustilo II open tibia fractures Setting: single centre in Kenya Intervention: prophylactic antibiotic treatment given for 24 hours Comparison: prophylactic antibiotic treatment given for 5 days | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 5 days of treatment | Risk with 24 hours of treatment | |||||
| Limb function at 6 or 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| HRQoL at 6 or 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| Deep surgical site infection at 30 days or 90 days, or time points reported by study authors | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| Delayed or non‐union at time points reported by study authors | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| Unplanned operations at 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| Adverse events: superficial infection Follow‐up in the included study was at 14 days |
Study population | RR 1.19 (0.49 to 2.87) | 77 (1 study) | Very lowb | ||
| 189 per 1000a | 225 per 1000 (93 to 543) | |||||
| Return to work by 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HRQoL; health‐related quality of life; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aDerived from the data in the 5‐day treatment group of the included study. bDowngraded by three levels: one level for serious risk of bias because the included study had unclear and high risks of bias, and two levels for very serious imprecision because evidence derived from few participants and the wide CI indicated possible benefits as well as harms.
Summary of findings 2. Timing of wound closure (immediate versus delayed) for open long bone fractures of the upper and lower limbs.
| Immediate versus delayed wound closure for open long bone fractures of the upper and lower limbs | ||||||
| Population: adults with open long bone fractures of the upper and lower limbs Setting: medical and trauma centres in the USA Intervention: immediate wound closure Comparison: delayed wound closure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with delayed closure | Risk with immediate closure | |||||
| Limb function at 6 or 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| HRQoL at 6 or 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| Deep surgical site infection Follow‐up time points were not clearly reported in the included studies. In one study, overall mean follow‐up was 296 daysa, but time for follow‐up was not reported in the other study. |
Study population | RR 0.82 (0.37 to 1.80) | 458 (2 studies) | Very lowc | ||
| 89 per 1000b | 73 per 1000 (33 to 161) | |||||
| Delayed union or non‐union Follow‐up time was not clearly reported. Overall mean follow‐up was 296 daysa |
Study population | RR 1.13 (0.83 to 1.55) | 387 (1 study) | Very lowc | ||
| 274 per 1000b | 309 per 1000 (227 to 424) | |||||
| Unplanned operations at 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| Adverse events ‐ superficial infection Follow‐up time point was not reported |
Study population | RR 6.45 (0.35 to 120.43) | 71 (1 study) | Very lowe | ||
| 0 out of 34b | 3 out of 37d | |||||
| Return to work by 12 months | ‐ | ‐ | Not estimable | ‐ | ‐ | No studies reported this outcome. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aWe could not be certain at what time point up to 296 days this outcome was measured. bDerived from the data reported in the included studies for the delayed closure group, or from the pooled estimate of the delayed closure group when data were available from more than one study. cWe downgraded the certainty of the evidence by three levels: by two levels for very serious risks of bias because the included studies were at unclear and high risks of bias; and by one level for serious imprecision because the effect estimate indicated possible benefits as well as harms. dData as reported in the included study for the immediate closure group. eWe downgraded the certainty of the evidence by four levels: by two levels for very serious risks of bias because the included studies were at unclear and high risks of bias; and by two levels for very serious imprecision because the effect estimate indicated possible benefits as well as harms.
Background
Description of the condition
Long bones are relatively long and narrow bones, typically consisting of a shaft (diaphysis) with widenings and growth plates (epiphyses) at either end. This review considers the major long bones of the lower and upper limbs. These include the femur (thigh‐bone), tibia (shin‐bone), fibula (the other lower leg bone), humerus (upper arm bone), and the radius and ulna (the two forearm bones). The small bones of the hand, wrist, toes and forefoot are excluded from this review because they tend not to have the same clinical problems as the long bones. For open fractures of these small bones, the risks of deep infection and need for surgery are less, particularly for 'open tuft' fractures of the distal phalanges (end bones) of the fingers, which are often treated non‐operatively.
Open fractures tend to be high‐energy injuries, often due to road traffic accidents, and are associated with extensive damage to the bone and overlying soft tissues. Around 15% of people with these injuries are severely injured (defined as an Injury Severity Score of 18 or more) (Pollak 2000), and 30% to 50% may also have other injuries (Costa 2018; Court‐Brown 2012). These open fractures are complex limb‐threatening injuries, and they are predisposed to deep infection. Although the definition of infection differs in the literature, in this review we use the internationally recognised Centers for Disease Control and Prevention (CDC) definition of a deep surgical site infection (SSI) (Horan 2008), i.e. a wound infection involving the tissues below the skin that occurs within 30 days of injury.
National epidemiological data for open fracture incidence rates are scarce. The annual incidence of open long bone fractures in the UK, after excluding open fractures of the phalanges, is estimated to be 30.7 per 100,000 adults. The tibia is the most commonly injured long bone (Court‐Brown 2012). There is a lack of data from lower‐income countries (Chen 2017). However, we expect wide variations between high‐ and low‐income countries due to socioeconomic factors and differences in health and safety regulations. Young men of working age are the most commonly affected group. However, there is a growing subgroup of older people in high‐income countries who sustain open fragility fractures secondary to osteoporosis and poor‐quality soft tissues (Costa 2018).
Open fractures vary in their severity. The most widely used open fracture classification is the Gustilo‐Anderson classification system shown below (Gustilo 1976; Gustilo 1984).
Type I: open fracture with a clean wound that is less than 1 cm in length.
Type II: open fracture, without extensive soft tissue damage, flaps, avulsions with a wound greater than 1 cm but less than 10 cm in length.
-
Type III: an open fracture with extensive soft tissue damage; a traumatic amputation; or an open segmental fracture. It can also include specific categories of open fractures, such as those caused by farm injuries, fractures requiring vascular repair, or fractures that have been open for eight hours before treatment.
IIIA: type III fracture with adequate coverage of the fractured bone, despite extensive soft tissue damage.
IIIB: type III fracture with extensive soft tissue loss and periosteal stripping and bone damage (usually associated with massive contamination).
IIIC: type III fracture associated with an arterial injury requiring repair.
Court‐Brown 2012 reported that 44.6% of tibia and fibula fractures were type III fractures, whereas only 2.2% of open distal radius fractures were of this type.
Open fractures are emergencies, and the open nature of the injury means that the fracture is at risk of deep infection. Treatment of major long bone fractures includes antibiotics and surgery to clean the wound and debride (surgically remove) dead tissues. The fracture will usually require the fractured bone(s) to be stabilised and reconstruction of any soft tissue defect to encourage infection‐free bone repair. The most severe injuries require limb salvage, which involves major reconstructive surgery of the bone and overlying soft tissues.
For people with open fractures, and in particular open lower limb fractures, outcomes are often poor. Recovery is lengthy, with an average time to fracture union (consolidation of a fracture resulting from bone healing) for tibial fractures of 41 to 43 weeks (Eccles 2020; Gopal 2004; Keating 2000). Complications relating to fracture healing occur in 10% to 13% of people and include non‐union (failure of bone healing), malunion (consolidation of a fracture in a position of deformity) or delayed union (Audige 2005; Harris 2009). Despite soft tissue reconstruction, deep infection rates of up to 27% have been reported, even in specialist trauma centres (Glass 2011; Pollak 2000). Treatment for deep infection is challenging, usually comprising multiple surgeries and long courses of antibiotics. This typically continues for years after the initial injury, with the risk of antibiotic resistance in chronic wounds and even amputation in extreme cases. Following open lower limb fracture, 20% to 50% of people are permanently disabled, and 40% cannot return to work (Seekamp 1996). Hence, these injuries create enormous health, social and economic costs for individuals and healthcare systems (Bondurant 1988; Parker 2018). Unfortunately, there is currently limited guidance or published data on the outcomes of equivalent injuries affecting the upper limb.
Description of the intervention
The management principles of open fractures are to debride the wound and provide stability of the bone and soft tissue cover to promote infection‐free bone repair. While clinical practice varies, many countries have adopted the British Orthopaedic Association & British Association of Plastic, Reconstructive & Aesthetic Surgeons Audit Standards for Trauma on Open Fracture (British Orthopaedic Association 2017) and National Institute for Health and Care Excellence (NICE) guidance (NICE 2016).
The British Orthopaedic Association recommends that people with open long bone fractures be treated at a specialist centre to provide combined orthopaedic and plastic surgical (orthoplastic) care (British Orthopaedic Association 2017). It stipulates that injuries posing the greatest threat to life, including airway problems and haemorrhage, should be treated immediately and sequentially. Once life‐threatening emergencies have been stabilised, the injured limb should then be attended to.
The recommended steps are outlined in Figure 1. Following antibiotic administration, the injured limb should be examined, including assessing and documenting the vascular and neurological status before realignment of the fracture and splinting. Re‐examination must be completed systematically and with each reduction manoeuvre or splinting of the fracture. The open wound should only be handled to remove gross contaminants and, following medical photography, covered with saline‐soaked gauze and an occlusive dressing.
1.
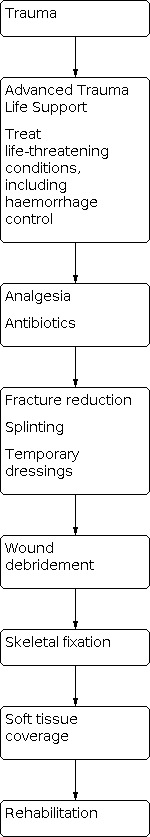
Flow diagram showing key stages in the management of open fractures.
Antibiotic administration
The British Orthopaedic Association recommends that prophylactic intravenous broad‐spectrum antibiotics be administered within an hour of injury to reduce the risk of deep SSI as part of a standard management protocol for open fractures of long bones (British Orthopaedic Association 2017). While there is a wide variation in clinical practice shown in the literature (Chang 2019), most studies report gram‐positive and gram‐negative coverage administered for two to three days. Most publications recommend immediate prophylactic systemic antibiotics delivered intravenously, providing gram‐positive coverage, administered for up to three days for less severe injuries, but broad antimicrobial coverage for two to three days for more severe injuries.
Limb reconstruction surgery
Limb reconstruction surgery consists of three distinct steps: surgical wound debridement, skeletal fixation and soft tissue closure.
Wound debridement
Wound debridement involves the surgical removal of debris (foreign material such as road material, stones, leaves and glass) and excision of dead or irreparably damaged tissues (such as skin, muscle, bone). It is critical that a thorough debridement is undertaken, as this step is key to reducing the contaminant load and hence the risk of an infection developing in the wound. However, it remains uncertain how soon this procedure should be undertaken after the injury. It is also unclear whether any delay increases the risk of infection and, if so, by how much. According to NICE guidance, the initial surgical debridement should be performed immediately for highly contaminated open fractures or where there is vascular compromise, within 12 hours of the injury for high‐energy open fractures that are not highly contaminated or within 24 hours for all other open fractures (NICE 2016).
Reconstruction
Skeletal fixation
Skeletal fixation provides stability across the fracture site to promote bone repair. This can either be temporary or definitive and can be in the form of an intramedullary nail, screws, plate(s) and screws, or an external fixator with pins, wires or both. External fixators (pins through the bone on either side of the fracture, connected by a bar) or plaster casts are commonly used as temporary stabilisation devices to protect soft tissues and reduce pain until definitive fixation can be achieved. Definitive skeletal fixation is the final planned orthopaedic fixation, with any further return for skeletal reconstruction categorised as an unplanned return to surgery. In cases where definitive skeletal fixation is not performed immediately following debridement under the same anaesthetic, the fracture can be temporarily stabilised and fixed definitively at a later time point. There are numerous reasons for delayed definitive fixation. These include delayed hospital transfer, stabilisation in a multiply‐injured person, and surgical preference when the surgeons feel that it would be beneficial to perform multiple debridements for a heavily contaminated wound. There can also be logistical difficulty in arranging for joint plastic surgery input if definitive skeletal fixation and soft tissue reconstruction are planned to be undertaken in a single stage (so‐called 'fix‐and‐flap', see below).
Soft tissue coverage
Soft tissue coverage is critical to create a barrier against microorganisms causing deep infection and provide the cytokine and cellular environment for bone repair (Chan 2012). At the end of wound debridement, the wound can be closed primarily, skin grafted or reconstructed using a flap if these are not possible. Skin grafts cannot be applied to exposed cortical bone or bare tendons as they will not survive. Flap coverage is a procedure whereby the soft‐tissue defect is covered by bringing in vascularised soft tissues (skin and fascia or muscle, or both) from another anatomical region of the body. Depending on the location of the flap harvest site, it may or may not require detachment from its blood supply and joining to local recipient blood vessels. These are termed 'free flaps' and 'pedicled flaps', respectively.
In cases where the skin wound can be closed primarily or skin grafted, this will usually be done in the same operation as the skeletal fixation. For cases that require flap reconstruction, skeletal fixation and soft tissue reconstruction can be performed either as a single procedure, ‘fix‐and‐flap’, or a two‐stage procedure, 'fix‐then‐flap', whereby skeletal fixation and soft tissue reconstruction are performed in two separate operative sessions.
How the intervention might work
Antibiotic treatment and surgery aim to promote infection‐free fracture union, leading to a healthy functional limb. Wound debridement aims to remove all contaminants and non‐viable tissues. Skeletal fixation promotes fracture union in optimal alignment, while soft tissue coverage provides a vascularised barrier between the external environment and the fracture, and hence the biological environment for the fracture to heal (Chan 2012).
The administration of antibiotics is part of the standard management protocol for open limb fractures. A previous Cochrane Review found that antibiotics effectively reduce the incidence of wound infections compared with no antibiotics or placebo in open limb fractures (Gosselin 2004). However, the review excluded trials that compared different antibiotics, antibiotic dosages, routes of administration and differences in timing or duration of administration. For fixation of closed fractures, there is evidence that antibiotic prophylaxis significantly reduces both deep and superficial site infections (Gillespie 2010). This is likely to be pertinent to open fractures as superficial site infection can lead to wound breakdown, fracture exposure, and subsequent deep infection. There is currently no international consensus on antibiotic treatment in open fractures regarding the optimal timing of administration, route, duration, and type (Chang 2019). Whitehouse 2017 reported evidence of reduced infection rates with delivery of antibiotics within 66 minutes of open limb fractures, however this review included only cohort studies and the evidence of early delivery was found in only one trial. If early antibiotic administration is effective, routine prehospital treatment for people with open fractures may become the standard treatment protocol. Furthermore, evidence‐based duration of antibiotic administration is important to improve antibiotic stewardship and reduce the emergence of antimicrobial resistance.
There is also uncertainty about the optimal timing of surgical intervention and whether performing these steps (wound debridement and reconstruction) in a single stage or as separate procedures affects the outcome.
The British Orthopaedic Association 2017 and NICE 2016 recommend wound debridement within 12 hours for wounds that are not highly contaminated and, if necessary, flap coverage immediately at the time of debridement or within 72 hours after injury (Eccles 2020; NICE 2016). However, these timings are controversial and require a major trauma network (England and Wales), allowing people to be brought to specialist centres that can provide this care within the recommended time frames. These time frames are currently not achievable in most countries, including some parts of the UK.
Several studies have shown that the main micro‐organisms in deep infections are nosocomial (hospital‐acquired) (Glass 2011; Sheehy 2010). In addition, there is evidence from retrospective cohort studies to suggest that the time from definitive fixation to flap coverage is related to subsequent wound infection (Kuripla 2021), and that early surgical intervention and the 'fix‐and‐flap' approach are associated with lower deep infection rates (Godina 1986; Gopal 2004; Liu 2012; Mathews 2015). Earlier surgical wound debridement, antibiotic treatment and soft tissue cover may reduce the deep infection rate due to the shorter time interval during which the fracture is exposed to nosocomial microorganisms. A single‐stage 'fix‐and‐flap' approach, compared with a two‐stage technique whereby soft tissue coverage is delayed, may also reduce the risk of bacterial contamination and biofilm formation within the wound.
In some situations, particularly for people with multiple injuries, it may be necessary to perform wound debridement and delay surgical reconstruction for the affected limbs until the person is stabilised. Furthermore, flap reconstruction may need to be delayed in some settings and performed as a separate stage. It may be safer to perform this long and complex surgery during office hours when the appropriately skilled surgical, anaesthetic and nursing staff are available. In these situations, a temporary skeletal external fixator and temporary dressings are often used. The effect of temporary dressings, such as negative pressure wound therapy (NPWT), has been investigated. According to a recent Cochrane Review (Iheozor‐Ejiofor 2018), there is no clear difference in healing rates when comparing NPWT with standard care in treating open fracture wounds. The review also demonstrated uncertainty regarding whether NPWT may reduce the risk of infection and found no clear evidence that NPWT improves pain, adverse events or the experience of receiving therapy. Furthermore, it found moderate‐certainty evidence that NPWT is not a cost‐effective treatment for open fracture wounds. A recent randomised controlled trial (RCT) also found that NPWT did not improve self‐rated disability at 12 months, compared with standard wound dressing, among people with severe open lower limb fracture (Costa 2018).
Furthermore, the wound must heal satisfactorily following soft tissue coverage. Failure of wound healing may lead to chronic ulceration and deep infection. Wound healing may be compromised for many reasons, including excessive tension across the wound, infection and failure of the flap procedure so that part or the whole of the flap dies. It remains unclear whether the timing and staging of the different surgical procedures and cases requiring soft tissue reconstruction and the type of flap used affect patient outcomes.
Why it is important to do this review
Despite improvements in the management of open limb fractures, there remains an unacceptably high complication rate, particularly deep infection. Improving the management of open fractures will have a large clinical and socioeconomic impact. In England, the introduction of a multidisciplinary approach (involving orthopaedic and plastic surgeons together with microbiologists), prompt surgical intervention and a major trauma network have been associated with improved outcomes and reduced infections rates. Based on UK data, a recent RCT that compared NPWT with standard wound management in severe open fracture of the lower limb found no statistically significant difference in deep surgical site infections: 7.1% in the NPWT group and 8.1% in the standard dressing group (Costa 2018). A prospective cohort study found a 4.3% rate of further operations for early infection (Young 2019). Whilst these rates are encouraging, there remains uncertainty about whether the timings of surgical intervention and antibiotic administration affect the outcome (Young 2019).
Evidence from RCTs on the timing and staging of surgical intervention and antibiotic administration is important to guide practice. We have not identified any previous systematic reviews; therefore, this review is warranted. It may also provide a basis for the design of future multicentre clinical trials. Our findings may also have important implications regarding the future design of care pathways for people with open long bone fractures.
Objectives
To assess the effects (risks and benefits) of the timing of antibiotic administration, wound debridement and the stages of surgical interventions in treating people with open long bone fractures of the upper and lower limbs.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (where the method of allocating participants to treatment is not strictly random, e.g. by hospital number), as well as cluster‐RCTs and cross‐over trials. We included studies irrespective of publication status or language.
Types of participants
We included adults with open fractures of the major long bones, as described in the primary study reports. We excluded studies that focused on open fractures of the hand, wrist, toes and forefoot. We expected the population for each comparison to vary according to the study question of interest. For example, not all open fracture wounds require flap coverage; generally, cases that require flap coverage tend to be more severe injuries and may have poorer outcomes.
If trials studied populations with a mix of demographics or injury patterns, we planned to extract data by these subgroups where possible, contacting study authors for more information if necessary, and seeking statistical advice if the design was complex.
The biological process of bone repair differs between adults and prepubertal children (age under 12 years), as such children experience earlier bone union and much lower incidences of complications. Therefore, the results from prepubertal children will not be generalisable to the adult population. We excluded studies of all children (< 18 years of age) from the review. Since the incidence of open long bone fractures is much higher in adults than children, it was anticipated that the adult numbers would substantially outweigh those of children in mixed population trials. Where studies reported data separately for subgroups, we planned to only extract the adult data. If the studies did not present data separately for an adult subgroup, we included reports where the proportion of adults exceeds (or was likely to exceed) 80% of the sample.
Types of interventions
This review included studies that evaluated multidisciplinary management, including surgical intervention and antibiotic administration, for adults with open long bone fractures of the upper and lower limbs.
We included the following comparisons.
1. Timing of the start of prophylactic antibiotic treatment
Early (≤ 1 hour from injury) versus late (> 1 hour from injury).
We defined early commencement of prophylactic antibiotics as administering one dose of any antibiotic(s) via any route (oral, enteral or parenteral), within one hour of injury, for all participants in the intervention group. Comparator groups exclusively included participants who started taking antibiotics one hour after injury. In either group, there may have been a change in choice of antibiotics or route of administration, discontinuation of antibiotics after the first dose, or discontinuation after a multi‐dose course of any duration.
We considered other definitions of 'early' used in otherwise eligible studies.
2. Duration of prophylactic antibiotic treatment
Terminated at soft tissue closure (any type: primary closure, skin graft or flap reconstruction) versus continued.
We considered other time points which were short duration versus long duration in otherwise eligible studies.
3. Timing of wound debridement following injury
Early (< 12 hours from injury) versus late (> 12 hours from injury).
We defined early surgical debridement as the first surgical intervention to decontaminate the wound through the removal of debris and excision of non‐viable tissues at any point within 12 hours of injury. Comparator groups exclusively included participants whose initial surgical debridement was undertaken more than 12 hours after the time of injury. Participants may have undergone immediate reconstruction or waited for varying lengths of time after debridement until reconstruction.
As above, we considered other definitions of 'early', such as under six hours, used in otherwise eligible studies.
4. Reconstructive surgery: timing of the stages of surgery
Single‐stage surgery (including wound debridement, skeletal fixation, plus soft tissue closure not requiring flap reconstruction) versus multi‐stage surgery. In terms of the staging of surgical procedures, wound debridement, skeletal fixation and soft tissue closure can either be undertaken under the same anaesthetic or continued under another one (or more). We considered participants who remained under general anaesthesia but spent the period between any two surgeries in a critical care setting to have had multi‐stage surgery.
For participants who required definitive fixation and flap reconstruction: one‐stage 'fix‐and‐flap' versus two‐stage 'fix‐then‐flap'. Single‐stage reconstruction is often termed ‘fix‐and‐flap’ (Gopal 2000), i.e. skeletal fixation, such as via an intramedullary nail and flap cover under a single anaesthetic. Comparator groups exclusively included participants who underwent two‐stage reconstruction, or 'fix‐then‐flap', i.e. when skeletal fixation and flap cover were delivered as two separate operations.
For participants who underwent 'fix‐then‐flap' reconstruction: wound closure within 72 hours versus after 72 hours. Selection of the 72 hours threshold was based on the British Orthopaedic Association 2017 and NICE 2016 recommendation that definitive soft tissue cover should be performed within 72 hours of injury if it cannot be performed at the time of debridement. As above, we considered other thresholds if used in otherwise eligible studies.
Types of outcome measures
The primary focus of this review is on functional recovery and quality of life. However, we anticipated that most trials would not include patient‐reported outcome measures (PROMs), and we have therefore included fracture healing outcomes.
Primary outcomes
Limb function using validated PROMs at six months and 12 months; for example, the Lower Extremity Functional Scale (Binkley 1999) and Enneking Score (Enneking 1993).
Health‐related quality of life (HRQoL) using PROMs at six months and 12 months; for example, the patient‐reported EuroQol 5 Dimensional Score (EQ‐5D) (Brooks 1996; Dolan 1997), Disability Rating Index (Salen 1994), Sickness Impact Profile (Bergner 1981; de Bruin 1994) and the 36‐Item Short‐Form Health Survey (SF‐36) (Brazier 2002; Jenkinson 1999).
Deep surgical site infection (SSI). In preference, we used the internationally recognised Centers for Disease Control and Prevention (CDC) definition of a deep SSI (Horan 2008), i.e. a wound infection involving the tissues below the skin that occurs within 30 days of injury. We considered any infection that required continuing medical intervention for 30 days after surgery without an implant, or 90 days after surgery using an implant, to be a deep infection. In the event that study authors did not define the type of infection, we made judgements about whether it was a deep infection from other information in the study report.
Secondary outcomes
Delayed or non‐union. There is no consensus on the definition of fracture union, and therefore on the definition of delayed or non‐union. The widely accepted definition of a healed fracture in the literature is when a callus bridges three of four cortices on orthogonal radiographs, or an absence of pain and movement at the fracture site, or both. For this review, we used author definitions of non‐union and delayed union. We anticipated that most studies will record time to union for each participant, but it is possible that some studies might present a proportional analysis of healed fractures at several fixed time points after treatment.
-
Adverse events, including:
death within 12 months of injury date, from any cause;
amputation following failed reconstruction within 24 months;
number of participants undergoing unplanned operations within three months;
number of participants undergoing unplanned operations between three months and 12 months;
flap failure – partial or total. Clinically diagnosed necrosis of part or all of the soft tissue flap that leads to exposed fracture or any internal metalwork, or necessitates further procedures (including NPWT or further surgery) to achieve soft‐tissue coverage;
chronic pain condition: diagnosed by an appropriate clinician (e.g. lower limb surgeon, pain specialist, family doctor), i.e. not self‐reported;
superficial wound infection. In the event that study authors did not define the type of infection, we made judgements about whether it was a superficial infection from other information in the study report;
wound dehiscence;
non‐primary wound‐related infections (chest, urinary);
thromboembolic events;
pressure sores.
-
Resource‐related outcomes:
length of hospital stay for treatment of acute injury as well as all subsequent episodes related to open fracture;
readmission;
the number of participants returning to independence by 12 months;
the number of participants returning to employment by 12 months.
We recognise that the selection of many of the time thresholds and cut‐offs is arbitrary. We therefore used time points presented by study authors. If time points were not reported, and attempts to contact study authors were unsuccessful, we included the outcome without a time point.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to find reports of relevant RCTs and quasi‐RCTs:
Cochrane Central Register of Controlled Trials (CENTRAL; 4 February 2021, Issue 1);
MEDLINE ‐ Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 1 February 2021);
Embase (1980 to 4 February 2021);
BIOSIS Citation Index (Web of Science; 1900 to 11 November 2020);
National Technical Information Service (NTIS, for technical reports; ntrl.ntis.gov/NTRL/; 11 November 2020);
OpenGrey (11 November 2020);
Grey Literature Report (11 November 2020);
ClinicalTrials.gov (4 February 2021);
The WHO International Clinical Trials Registry Platform (4 February 2021).
At the time of the search, CENTRAL was fully up‐to‐date with all records from the Bone, Joint and Muscle Trauma (BJMT) Group’s Specialised Register, so it was not necessary to search this separately.
In MEDLINE, we combined subject‐specific terms with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2021). Details of the search strategies can be found in Appendix 1.
We applied no restrictions based on language or date of publication.
Searching other resources
We also searched the reference lists of short‐listed articles and relevant systematic reviews to identify additional suitable studies.
Data collection and analysis
Selection of studies
Two review authors (JC, AA) independently screened all titles and abstracts to identify potential studies for review using Covidence. We compared lists of potential studies and agreed on a shortlist. We obtained copies of the full‐text articles on the shortlist and reviewed them, resolving any disagreements by discussion and by referral to a third review author (JN or XG). If uncertainties had remained about eligibility, we would have corresponded with the study authors of the reports.
We did not mask titles of journals or names of study authors and supporting institutions.
Data extraction and management
Two review authors (JC, AA or SL) independently used a piloted form to extract data from each trial regarding sources, study design, population, interventions and other care, and outcomes (for template data extraction form, see Appendix 2). This review aimed to investigate the effects of multiple variables that may contribute to patient outcome, and hence serve as potential confounders (such as differences in clinical management other than of the interventions relevant in this review, e.g. negative pressure wound therapy). We extracted the data for these variables either from the reported studies or, if not available, we attempted to contact the study authors; in the event that we were unable to contact study authors, we made judgements about clinical management protocols from other information in the reports. We resolved any disagreements by consensus after a third author undertook an additional review (JN or XG). Where necessary and practical, we contacted trialists for additional data and clarification.
Assessment of risk of bias in included studies
Two review authors (JC, AA or SL) independently used the risk of bias tool developed by Cochrane (Higgins 2017). We assessed seven domains, including sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. We considered subjective and functional outcomes (physical function, pain, satisfaction) and objective outcomes (complications, treatment failure) separately in our assessment of blinding and completeness of outcome data. We considered other sources of bias, such as performance bias from imbalance in clinician training in the treatment groups, as well as biases that we did not anticipate. We classified studies as 'high risk of bias', 'low risk of bias' or 'unclear risk of bias' for each domain (Appendix 3). We resolved any disagreements by discussion and referral to a third review author (JN or XG).
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for binary outcomes, such as delayed union, non‐union, amputation, chronic pain (which may indicate osteomyelitis), flap failure and death.
Continuous data
Where pooled studies reported a single measure for continuous outcomes, such as duration of inpatient stay, we planned to report mean differences with 95% CIs. Where pooled studies within a single meta‐analysis used different measurement scales, such as for different HRQoL measures, we planned to use standardised mean differences (SMDs) (Hedges 1982). For continuous outcomes, we planned to present final scores in preference to change scores.
Unit of analysis issues
We expected that the participant would be the unit of randomisation in most of the studies. One study included some participants with more than one open fracture (Benson 1983), and reported outcome data at the participant level as well as the injury level; for analysis, we used participant‐level data for consistency with other studies in the review. Given the limited studies in the review, we did not perform sensitivity analysis to investigate this decision.
Where there were multiple observations for the same outcome over time, we planned to use the data nearest to the upper limits of the time periods stipulated for individual outcomes in our analyses, such as six and 12 months for HRQoL measures. There may have been events that reoccurred or multiple treatment attempts per participant: we planned to ensure that we used the number of participants randomised, and not the number of treatment attempts, to calculate the effect estimate and confidence intervals.
We planned to be alert to the unit‐of‐analysis issues relating to composite outcomes, such as total adverse events, where participants can have more than one individual outcome; in these instances, we planned to report the number of participants rather than the number of outcomes.
For cross‐over designs, we planned to only include data from the first phase, and for cluster‐randomised trials, we planned to seek statistical support.
Dealing with missing data
We anticipated several types of missing data: unreported data, participants who withdrew or dropped out, and missing statistics. If the included studies did not report data, we attempted to contact the study authors to request information. Where the studies reported the number of participants who provided data for any particular outcome, we used these data. For studies that reported several events, but the denominator was unclear, we planned to use numbers randomised or alive at follow‐up. Where feasible, we planned to calculate missing standard deviations from other data (standard errors, 95% CIs, exact P values). We did not plan to perform imputation for missing data. However, we planned to perform sensitivity analysis to assess the impact of missing data on the results.
Assessment of heterogeneity
The decision to pool the results of individual studies depended on assessing clinical and methodological heterogeneity. If we considered studies sufficiently homogeneous for data pooling, we planned to assess statistical heterogeneity by visual inspection of the forest plots using the Chi2 test with a significance level of P value less than 0.1 and the I2 statistic. We planned to base our interpretation of the I2 statistic results on those suggested in The Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity; and
75% to 100% may represent very substantial ('considerable') heterogeneity.
Assessment of reporting biases
To reduce the risk of reporting bias, we searched multiple sources to identify published and unpublished results, and requested unpublished data from study authors. We also screened clinical trials registers for protocols and registration documents of included studies that were prospectively published; we planned to use evidence of prospective registration or published protocols to judge whether studies were at risk of selective reporting bias. If there were sufficient numbers of studies in a meta‐analysis (at least 10), we planned to generate a funnel plot to assess the risk of publication bias (Deeks 2021).
Data synthesis
We pooled results of comparable studies using random‐effects models. The choice of the model was made by careful consideration of the extent of possible clinical and methodological heterogeneity between the studies. We used 95% CIs. We considered not pooling data where there was considerable heterogeneity (I2 statistic value greater than 75%) that could not be explained by the diversity of methodological or clinical features among trials; if data were not pooled, we planned to present trial data in the analyses or tables for illustrative purposes and report these in the text.
Subgroup analysis and investigation of heterogeneity
We found insufficient studies to conduct subgroup analysis.
If sufficient data were available, we planned the following subgroup analyses.
Type of soft tissue reconstruction: pedicled versus free flaps, muscle versus fasciocutaneous flaps.
Upper versus lower limb fractures.
Age (up to and including 60 years versus over 60 years).
Type of skeletal fixation: for example, intramedullary versus extramedullary devices; internal fixation versus definitive external fixation.
Injury severity, in terms of whether flap coverage was or was not needed.
Time to surgical reconstruction (threshold 72 hours; NICE 2016) for comparison three (timing of debridement)). The relative importance of the timing of wound debridement and time to definitive wound closure is unclear, but we assumed that early wound debridement is beneficial irrespective of the timing of surgical reconstruction; we had proposed to explore and use subgroup analysis to explore this assertion.
We planned to use the test for subgroup differences in Review Manager 5 to determine whether there was evidence for a difference in treatment effect between subgroups (Review Manager 2020).
Sensitivity analysis
We found insufficient studies to conduct meaningful sensitivity analysis.
If sufficient data were available, we planned to assess the robustness of our findings by conducting sensitivity analyses. These would have included testing the following.
Inclusion of quasi‐randomised trials or other trials at high or unclear risk of selection bias due to inadequate concealment of allocation.
Inclusion of trials at high or unclear risk of attrition bias due to incomplete outcome data.
-
For missing data in individual trials:
excluding trials at high risk of attrition bias;
where appropriate, replacing denominators with numbers randomised for all outcomes.
Inclusion of trials using inadequate definitions of outcome measures, such as that for non‐union.
Inclusion of trials and data with clear or probable unit‐of‐analysis issues.
The choice of statistical model for pooling (fixed‐effect versus random‐effects).
In the protocol, we specified a number of thresholds for timing (Chan 2020). These thresholds may vary between countries as well as over time within countries. We wished to explore the impact of changing these thresholds on the findings of any meta‐analysis.
Timing of antibiotic administration (comparison two). We planned to explore how varying the one‐hour threshold alters the findings. We hypothesised that early antibiotic administration is beneficial irrespective of the timing of surgery. To test this, we planned to also perform a sensitivity analysis to assess how sensitive the results are to a change in this assumption. Firstly, we would have included all eligible studies. Secondly, we would only have included participants who met the NICE criteria with regard to the timings of wound debridement (within 12 to 24 hours, depending on injury characteristics) and wound closure or soft tissue reconstruction (within 72 hours) (NICE 2016).
Timing of wound debridement (comparison three). Currently, NICE guidance (NICE 2016) and the British Orthopaedic Association 2017 recommend surgical debridement within 12 hours of the injury for high‐energy open fractures that are not highly contaminated. Previously, the standards recommended a 24‐hour window. Therefore, we planned to investigate whether the evidence supports either of these recommendations. Furthermore, many clinicians have previously used six hours as a standard. Therefore, we adopted an exploratory approach to this analysis, depending on the data available, and remained mindful of these prespecified thresholds.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of evidence for all clinical outcomes (Schünemann 2021). The certainty rating ‘high’ was reserved for a body of evidence‐based on RCTs. We downgraded the quality rating to 'moderate', 'low’, or 'very low' depending on the presence and extent of five factors: study limitations, inconsistency of effect, imprecision, indirectness, and publication bias. We prepared a summary of findings table for each of the main comparisons for the following outcomes:
limb function, using recognised PROMs at 12 months;
health‐related quality of life, using PROMs at 12 months;
deep SSI;
delayed or non‐union;
unplanned operations at 12 months;
counts of adverse events, as described in Types of outcome measures;
return to work by 12 months.
As described in Types of outcome measures, we recognised that the selection of many of the time thresholds and cut‐offs is arbitrary. We reflected any changes to these definitions in our summary of findings tables and described the differences to the protocol where relevant.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We screened a total of 3127 records from the following databases: CENTRAL (307), MEDLINE (736), Embase (902), BIOSIS Citation Index (567), NTIS (308), Open Grey (68), Grey Literature Report (5), the WHO International Clinical Trials Registry Platform (107) and ClinicalTrials.gov (127). We also screened references identified from searches of other resources and found one potentially eligible study from a search of a personal database of the Cochrane Bone, Joint and Muscle Trauma Co‐ordinating Editor.
The search identified a total of 16 reports for potential inclusion, for which we obtained full reports where possible. Upon further inspection, we selected three studies (with four records) for inclusion in the review (Benson 1983; Ondari 2016; Russell 2005), and we excluded nine studies (with 12 records) (Bergman 1982; Braun 1987; Carsenti‐Etesse 1999; Mahajan 2016; Moehring 2000; NCT04678154; Pape 2007; Patzakis 1996; Saveli 2013). We found no eligible ongoing studies.
A flow diagram summarising the study selection process in shown in Figure 2.
2.
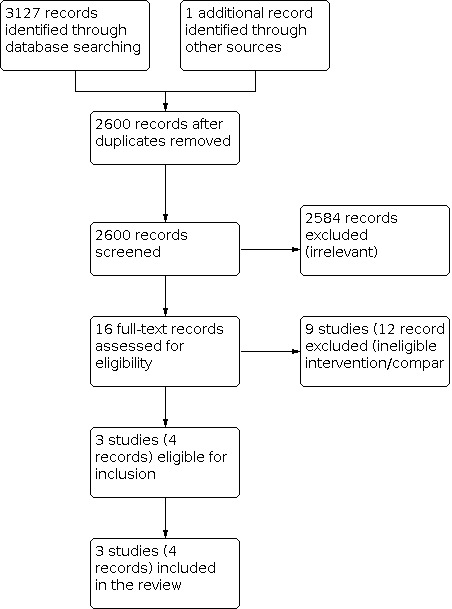
PRISMA Study flow diagram.
Included studies
We included three RCTs in the review (Benson 1983; Ondari 2016; Russell 2005). We found no cluster‐randomised or cross‐over studies. We were only able to collect limited study characteristics for Russell 2005, which was reported as an abstract. See Characteristics of included studies.
Benson 1983 and Ondari 2016 were both single centre studies, conducted in the USA and Kenya, respectively. Russell 2005 was a multicentre study conducted in 30 trauma centres in the USA. In total, studies included 613 randomised participants with 617 open fractures. The participants were adults in Benson 1983 and Ondari 2016; although the age of participants was not reported in Russell 2005, we expected that most (or all) of the participants were adults. Where reported, there was a higher proportion of men and the mean age was between 30 and 34 years of age. The fractures were in both upper and lower limbs in Benson 1983, and limited to tibial fractures in the other studies; where reported, these were a result of high‐energy trauma such as road traffic accidents. The grade of injury was not reported in Benson 1983Ondari 2016 included Gustilo II fractures, and Russell 2005 included grade II or IIIa fractures. Apart from the essential detail of the intervention in the study reports, we could not always ascertain the additional detail about the management steps for treatment of fractures (for example, the timing of antibiotic administration or wound debridement and whether a single‐stage or multi‐stage surgical approach was adopted).
Ondari 2016 evaluated the duration of antibiotic treatment (24 hours or five days). The other studies both evaluated the timing of wound closure (immediate or delayed). In Benson 1983 the mean time of delayed wound closure was 5.9 days, but the delayed time was not reported in Russell 2005.
All studies reported infection data. However, study authors did not always clearly describe infections as deep or superficial, and we made judgements based on other information in the study reports about this distinction. Using these judgements, we included data as superficial infection in Ondari 2016, and deep infection in Russell 2005. Timing of outcome assessment was not always clear. Ondari 2016 specified that infection data were collected at two, five, and 14 days after injury, but follow‐up time was not reported in Benson 1983 Although mean follow‐up in Russell 2005 was at 296 days, we could not be certain whether all or some of the outcomes were measured at the end of follow‐up. The short follow‐up time in Ondari 2016 prohibits the collection of longer‐term outcomes that better inform the full course of recovery for people with these injuries, including both clinical outcomes (such as delayed union) and PROMS (i.e. limb‐function and health‐related quality of life).
Benson 1983 was supported by grants from pharmaceutical companies, whereas funding sources were not reported in the other studies; we note, however, that Ondari 2016 declared no conflicts of interest.
Excluded studies
We excluded nine studies after assessment of the full‐text records (see Characteristics of excluded studies). These studies all compared interventions and comparisons in open fractures that were ineligible for this review. Six studies investigated antibiotic use: different types of antibiotics (Bergman 1982), different routes of antibiotics (Moehring 2000; NCT04678154), single or combined antibiotic treatment (Patzakis 1996; Saveli 2013), or antibiotics compared to a placebo (Braun 1987). Carsenti‐Etesse 1999 investigated different types of antibiotics, which were given for different durations, but this study was primarily about bacterial cultures using outdated methodology of swab collection, which fails to obtain representative organisms causing deep infection (Høiby 2015). Mahajan 2016 compared a conventional protocol to an enhanced protocol which included differences in antibiotic drugs as well as routes of administration and additional wound cleaning solutions. Pape 2007 compared different timing of internal fixation; this was a retrospective study and only 25% were open fractures.
Risk of bias in included studies
We have summarised the risk of bias judgements for the individual trials in Figure 3 and Figure 4. We described them in the risk of bias tables in Characteristics of included studies.
3.

Risk of bias summary
4.

Risk of bias graph
Allocation
We judged Ondari 2016 to have low risk of selection bias for sequence generation because this study described use of computer‐generated randomisation. However, this was unclear in the other studies, in which no information was reported, and methods of allocation concealment were unclear in all studies.
Blinding
We judged all three studies to be at high risk of performance and detection bias. It was not possible to blind clinicians to the interventions in this review and we could not account for possible changes in clinical management to individual participants because of the interventions. Similarly, we believed that outcome assessment of infections and delayed union was subjective and open to the possibility of bias when the intervention is not masked.
Incomplete outcome data
In Russell 2005, we noted a large number of losses which were not explained, and we could not determine if they were balanced between groups because numbers randomised to each group were not reported; we judged attrition bias to be high risk. In Benson 1983, we noted a small number of lost participants from outcome data. Additionally, study authors used different units in their presentation of outcome data (number of participants or number of wounds) which were not always clearly labelled such that we could not easily determine the extent of attrition; we judged the risk to be unclear. In Ondari 2016, most participants were included in the analysis, and we judged the risk of attrition bias to be low.
Selective reporting
Ondari 2016 was retrospectively registered with a clinical trials register, and it was not feasible to judge the risk of selective reporting bias from these retrospectively published documents. The remaining studies were not registered with a clinical trials register and did not report a prospectively published protocol; it was therefore only feasible for us to judge the risk of selective reporting bias as being unclear.
Other potential sources of bias
We considered whether clinical management protocols were identical for each intervention group within a study. No studies explicitly stated differences in their management protocols, and we therefore assumed that these studies did not include risk of bias because of this.
We did not identify any other sources of bias in Benson 1983 and Ondari 2016, but judged Russell 2005 to be at high risk of other bias because we assumed that this abstract report was not peer‐reviewed.
Effects of interventions
We report the results for the comparisons made by the included trials below. Findings are also reported in Table 1 and Table 2. We did not conduct subgroup analyses or sensitivity analyses because we had insufficient studies.
None of the studies randomised participants according to the timing of the start of prophylactic antibiotic treatment or the timing of wound debridement following injury. For the comparison group 'reconstructive surgery', no studies evaluated single‐stage and multi‐stage surgery or one‐stage 'fix‐and‐flap' versus two‐stage 'fix‐then‐flap'.
Duration of prophylactic antibiotic treatment
In this comparison, no studies reported data for limb function, HRQoL, deep surgical site infection, delayed or non‐union, or resource‐related outcomes.
Adverse events
Superficial wound infection
One study reported data for infections (Ondari 2016). Although study authors did not explicitly define these infection data as superficial, infections were measured at 14 days using a score of more than 20 on the ASEPSIS (Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay) wound scoring system (Wilson 1986), and we judged that these were most likely to be superficial infections. We are uncertain whether antibiotics given for 24 hours or five days affect superficial infections; the effect estimate was imprecise (RR 1.19, 95% CI 0.49 to 2.87, favours 5‐day treatment; 1 study, 77 participants; Analysis 1.1). We judged the certainty of this evidence to be very low, downgrading by one level for serious risk of bias and two levels for very serious imprecision because the effect estimate was derived from few participants and the wide confidence interval indicated possible benefits as well as harms.
1.1. Analysis.

Comparison 1: Duration of prophylactic antibiotic treatment, Outcome 1: Adverse events: superficial infection
No other adverse events were reported.
Reconstructive surgery: timing of the stages of surgery
Studies in this comparison evaluated the timing of wound closure. No studies reported data for limb function, HRQoL, or resource‐related outcomes.
Deep surgical site infection
Two studies reported deep infections (Benson 1983; Russell 2005). Although Russell 2005 did not explicitly define these infection data as deep, mean study follow‐up was at 296 days, and we judged that these were most likely to be deep infections. We are uncertain whether closing the wound immediately or with a delay affects deep infections; the estimate was imprecise (RR 0.82, 95% CI 0.37 to 1.80; I2 = 5%; favours immediate closure; 2 studies, 458 participants; Analysis 2.1). We note that the time of delayed wound closure was not defined in Russell 2005. We judged the certainty of the evidence to be very low, downgrading by two levels for very serious risk of bias and one level for serious imprecision because the effect estimate indicated possible benefits as well as harms.
2.1. Analysis.

Comparison 2: Timing of wound closure, Outcome 1: Deep infection
Delayed or non‐union
One study reported the number of participants who developed delayed union or non‐union, as well as the mean to time to union (Russell 2005). Similarly, we are uncertain of the effect of timing on non‐union; this effect estimate was also imprecise (RR 1.13, 95% CI 0.83 to 1.55, favours delayed closure; 1 study, 387 participants; Analysis 2.2). We judged the certainty of the evidence to be very low, downgrading by two levels for very serious risk of bias and one level for serious imprecision because the effect estimate indicated possible benefits as well as harms.
2.2. Analysis.

Comparison 2: Timing of wound closure, Outcome 2: Delayed union or non‐union
We did not calculate a mean difference for the time to union data, as these were reported without distribution values. The mean time to union with immediate wound closure was 177 days, and the mean time when closure was delayed was 173 days.
Adverse events
Superficial wound infection
Only one study reported the number of people with superficial infections (Benson 1983), and we are uncertain whether the timing of wound closure affects this outcome (RR 6.45, 95% CI 0.35 to 120.43, favours delayed closure; 1 study, 71 participants; Analysis 2.3).
2.3. Analysis.

Comparison 2: Timing of wound closure, Outcome 3: Adverse events: superficial infection
No other adverse events were reported. We judged the certainty of the evidence to be very low, downgrading by two levels for very serious risk of bias and two levels for very serious imprecision because the effect estimate indicated possible benefits as well as harms and sample size was very small.
Discussion
Summary of main results
We included three RCTs with 613 adults who had 617 open long bone fractures. Two studies were exclusively of tibial fractures and the other study included upper and lower limb fractures.
One study compared the duration of prophylactic antibiotics (24 hours or five days of antibiotic treatment). We found no evidence of any differences between groups in superficial infections, but we judged the certainty of this evidence to be very low. This study did not report limb function, health‐related quality of life (HRQoL), delayed or non‐union, other adverse events, or any resource‐related outcomes.
Two studies compared the time of wound closure (immediate or delayed). We found no evidence of any differences in deep infection, superficial infection and delayed or non‐union, but again the certainty of this evidence was very low. Neither of these studies reported limb function, HRQoL, other adverse events, or any resource‐related outcomes.
We found no studies comparing the timing of the start of prophylactic antibiotic treatment or the timing of wound debridement following injury. We also did not find any studies comparing single‐stage surgery with multi‐stage surgery, or one‐stage 'fix‐and‐flap' surgery with two stage 'fix‐and‐flap' surgery.
Overall completeness and applicability of evidence
The studies in this review included participants with open long‐bone fractures. Where reported, the mechanism of injury was typical of high energy trauma, with a higher proportion of men and a mean age between 30 and 34 years old.
We only found evidence for two comparisons in this review. We could not always determine from the study reports what the full management protocols used by the trauma units were. For example, study authors did not explicitly state whether surgery was completed as a single or multi‐stage procedure. In addition, we note that one study was published in 1983 (Benson 1983); we anticipate that changes in management of open fractures in the last thirty years mean that we cannot confidently state the findings are generalisable to a modern trauma setting. It is also important to view this lack of evidence in the context of the vast heterogeneity of trauma delivery in different countries and healthcare systems. This is particularly relevant in open fractures, the outcomes of which are time‐sensitive, particularly for deep infection rates. For example, in the UK, there is a major trauma network system and national guidance and standards for managing open fractures. Such a system makes it possible to achieve early debridement and skeletal and soft tissue reconstruction, which may not be feasible in other healthcare systems.
Studies reported few outcomes, and in particular did not report participant‐relevant outcomes such as limb function and quality of life. One study had such a short follow‐up period that it prohibited the collection of outcomes to inform a full course of recovery for people with these injuries. Furthermore, while we note that the outcomes of deep SSI and delayed or non‐union are often reported separately, there is likely a large overlap between them as subacute deep SSI is a likely cause of delayed or non‐union. While the lack of outcome reporting is not unexpected, it is disappointing that we are unable to present a broader picture, beyond commonly‐reported clinical outcomes, of the benefits and harms of treatment options.
Quality of the evidence
The certainty of the evidence for all outcomes in this review was very low. Lack of information in the study reports meant that we could not always make judgements (other than 'unclear') for common risk of bias domains and all studies were at high risk of performance and detection bias because of a lack of blinding, which was mostly unavoidable. None of the studies reported prospective clinical trial registration, typical of older studies which predate a requirement for this. Russell 2005 provided the largest data set for this review, with the most participants and outcome data. However, this study was reported only as an abstract and we were unsuccessful at finding a full study report, or in attempts to contact study authors for more information. We judged that including this abstract (which we presumed was not a peer‐reviewed report) in the review increased the risk of other bias. We therefore downgraded the certainty of the evidence for this reason.
We also downgraded most of the evidence for imprecision because we noted effect estimates that included possible benefits as well as harms.
Whist the studies provided direct evidence for this review (eligible treatments for adults with open long bone fractures), we had insufficient studies to effectively evaluate inconsistency or publication bias.
Potential biases in the review process
We conducted a thorough search and independently assessed study eligibility, extracted data, and assessed risk of bias in the included studies before reaching consensus together or with one other review author.
Studies all reported infection rates that were variably defined, none using the preferred CDC definition of a deep SSI (Horan 2008). The follow‐up study periods were also inadequate. Ondari 2016 reported infection rates at day 14 following injury, while Russell 2005 reported a mean follow‐up of 296 days; Benson 1983 did not report follow‐up periods. Russell 2005 also reported on delayed or non‐union rates, time to union and wound problems, but we were unable to obtain further information beyond what was available in the conference abstract. No studies adequately described the staging of treatment (e.g. single‐stage or multi‐stage). Our attempts to contact study authors for more information were unsuccessful, meaning that we made changes to our outcome definitions which were subjective and based only on the limited in the information in study reports. We cannot be certain whether these judgements were accurate.
Agreements and disagreements with other studies or reviews
In 2016, the National Institute of Health and Care Excellence (UK) published Guideline NG37 on the assessment and management of complex fractures, including open fractures (NICE 2016). The guideline development process undertook systematic reviews addressing specific review questions; including:
What is the optimum time to administer prophylactic antibiotics for suspected open fractures? No RCTs were retrieved, and cohort studies were included. The conclusion was based on very low‐quality clinical evidence suggesting that prophylactic antibiotics given after one hour in highly contaminated tibial shaft fractures increases deep surgical site infection rate.
What is the optimal timing of initial debridement of open fractures? No RCTs were retrieved, and cohort studies were included.There was very low‐quality evidence regarding the deep surgical site infection rate, and early debridement may or may not reduce this.
Is the use of initial definitive fixation and cover more clinically and cost‐effective in the management of open fractures compared with staged fixation and cover? One RCT and cohort studies were included. There was very low‐quality evidence to demonstrate that definitive fixation and the immediate cover was associated with better outcomes than definitive fixation and staged cover. There was also very low‐quality evidence to suggest that definitive fixation and the immediate cover was associated with better outcomes than staged fixation and staged cover.
What is the most clinical and cost‐effective time to achieve definitive soft tissue cover in open fractures? One RCT and cohort studies were retrieved. Very poor‐quality evidence suggests that early cover is associated with reduced rates of deep infection.
While there are non‐randomised observational studies on interventions for the treatment of open fractures, lack of up‐to‐date evidence from RCTs precludes our ability to increase the certainty of the evidence in this guideline.
Authors' conclusions
Implications for practice.
There is a lack of evidence from randomised controlled trials to inform decisions regarding the different aspects of surgical and antibiotic management of open fractures. This review includes only three studies of two comparisons, and the certainty of its evidence is very low.
Implications for research.
While there are non‐randomised observational studies on interventions for the treatment of open fractures, we did not find high‐certainty evidence from RCTs on different practices. Given difficulties in study design in this field, it may be appropriate to include well‐designed non‐randomised studies in future updates of the review. Improvements in patient outcomes have been attributed to the development of trauma networks, earlier debridement and closure of wounds, and timely delivery of prophylactic antibiotic treatment. However, well‐designed randomised trials with adequate power are needed to guide surgical and antibiotic treatment of open fractures. Future studies should also be of sufficient duration to enable the long‐term measurement of patient‐relevant outcomes, including limb function (e.g. Lower Extremity Functional Scale) and quality of life (e.g. EuroQol‐Five Dimensions‐5L). We found no ongoing studies in our search of clinical trials registers, and this remains an important research topic.
History
Protocol first published: Issue 3, 2020
| Date | Event | Description |
|---|---|---|
| 16 March 2020 | Amended | Amended affiliation details for James K‐K Chan |
Acknowledgements
We are grateful to Joanne Elliott for editorial support on the review and Maria Clarke for her help with the search. We thank Rachel Richardson for her comments and help at editorial review. We also thank Michael Kelly (clinical referee) and Janet Wale (consumer referee) for their helpful feedback.
This review was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma (BJMT) Group. JR is an NIHR Postdoctoral Fellow. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Editorial and peer‐reviewer contributions
Cochrane BJMT supported the authors in the development of this review. Sharon Lewis and Xavier Griffin are members of the Cochrane BJMT editorial base, but were not involved in the editorial process or decision‐making for this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Toby Lasserson, Deputy Editor in Chief, Cochrane
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joanne Elliott, Managing Editor, Cochrane Bone, Joint and Muscle Trauma Group
Methodological Editor (advised on methodology and review content): Rachel Richardson, Associate Editor, Cochrane
Copy Editor (copy‐editing and production): Andrea Takeda (Copy Edit Support)
Peer‐reviewers (provided comments and recommended an editorial decision): Michael Kelly (clinical reviewer), Janet Wale (consumer reviewer)
Appendices
Appendix 1. Search strategies
The Cochrane Central Register of Controlled Trials (CENTRAL CRS Web)
The CENTRAL search was run in two stages: the first search was run in April 2020 and a second top‐up search was run in February 2021.
Search 1
1. MESH DESCRIPTOR lower extremity EXPLODE ALL AND CENTRAL:TARGET (6678) 2. MESH DESCRIPTOR upper extremity EXPLODE ALL AND CENTRAL:TARGET (6984) 3. MESH DESCRIPTOR bones of lower extremity EXPLODE ALL AND CENTRAL:TARGET (1650) 4. MESH DESCRIPTOR bones of upper extremity EXPLODE ALL AND CENTRAL:TARGET (720) 5. MESH DESCRIPTOR Arm Bones EXPLODE ALL AND CENTRAL:TARGET (286) 6. MESH DESCRIPTOR Femur EXPLODE ALL AND CENTRAL:TARGET (15) 7. MESH DESCRIPTOR Leg bones AND CENTRAL:TARGET (886) 8. MESH DESCRIPTOR Fibula AND CENTRAL:TARGET (72) 9. MESH DESCRIPTOR Tibia AND CENTRAL:TARGET (578) 10. (femur or femoral or tibia* or fibula* or humor* or radius or radii or radial or ulna*): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (27558) 11. (leg* or foot or feet or hip* or knee* or ankle): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (90854) 12. (arm* or hand* or elbow* or shoulder*): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (155535) 13. (lower extremit* or lower limb or upper extremit* or upper limb or forelimb* or forearm*): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (25787) 14. MESH DESCRIPTOR Radius fractures AND CENTRAL:TARGET (488) 15. MESH DESCRIPTOR Humeral fractures AND CENTRAL:TARGET (151) 16. MESH DESCRIPTOR Femoral fractures AND CENTRAL:TARGET (306) 17. MESH DESCRIPTOR Tibial fractures AND CENTRAL:TARGET (328) 18. MESH DESCRIPTOR Ulna fractures EXPLODE ALL AND CENTRAL:TARGET (67) 19. MESH DESCRIPTOR Ankle fracturesAND CENTRAL:TARGET (140) 20. MESH DESCRIPTOR Shoulder fracturesAND CENTRAL:TARGET (106) 21. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 AND CENTRAL:TARGET (259850) 22. MESH DESCRIPTOR Fractures, Open AND CENTRAL:TARGET (123) 23. (plateau ankle or Weber or Monteggia or Galeazzi or Hoffa or Pilon or Schatzker): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (323) 24. ((open or compound or trauma* or Gustilo or Tscherne or high energy) NEAR3 fracture): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (822) 25. 22 or 23 or 24 AND CENTRAL:TARGET (1173) 26. 21 and 25 AND CENTRAL:TARGET (808) 27. MESH DESCRIPTOR Debridement AND CENTRAL:TARGET (658) 28. MESH DESCRIPTOR Wound Infection WITH QUALIFIER PC EXPLODE ALL AND CENTRAL:TARGET (1) 29. MESH DESCRIPTOR surgical wound dehiscence AND CENTRAL:TARGET (454) 30. MESH DESCRIPTOR surgical wound infection AND CENTRAL:TARGET (3418) 31. (debrid*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (3126) 32. MESH DESCRIPTOR Surgery, Plastic AND CENTRAL:TARGET (114) 33. MESH DESCRIPTOR Reconstructive Surgical Procedures AND CENTRAL:TARGET (754) 34. MESH DESCRIPTOR Fracture Fixation EXPLODE ALL AND CENTRAL:TARGET (1664) 35. MESH DESCRIPTOR Orthopedic Fixation Devices EXPLODE ALL AND CENTRAL:TARGET (2631) 36. MESH DESCRIPTOR Surgical Flaps EXPLODE ALL AND CENTRAL:TARGET (1387) 37. (wound* or cover* or flap* or reconstruct* or closure): AB,EH,KW,KY,MC,MH,TI,TO (61359) 38.MESH DESCRIPTOR Anti‐Bacterial Agents EXPLODE ALL AND CENTRAL:TARGET (19892) 39. (antibiotic* or antimicrob* or anti bacteria* or antibacteria*): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (40619) 40. (coamoxiclav or amoxicillin or cephalosporin or gentamicin or teicoplanin or cefuroxime or cefazolin or ceftriazone or penicillin or clindaymycin or aminoglycosides or vancomycin or levofloxacin or metronidazole or tazocin or piperacillin or tazobactam or linezolid or flucloxacillin or ampicillin): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (20332) 41. 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 AND CENTRAL:TARGET (114515) 42. 41 and 26 AND CENTRAL:TARGET (292)
Search 2
43. 01/04/2020_TO_04/02/2021:CRSCREATED AND CENTRAL:TARGET (117722) 44. #42 AND #43 (15)
MEDLINE (Ovid Online)
The MEDLINE search was run in two stages: the first search was run in April 2020 and a second top‐up search was run in February 2021.
Search 1
1 exp lower extremity/ or exp upper extremity/ or exp "bones of lower extremity"/ or exp "bones of upper extremity"/ (466379) 2 exp Arm Bones/ (22773) 3 Leg bones/ or exp Femur/ or Fibula/ or Tibia/ (87437) 4 (femur or femoral or tibia* or fibula* or humor* or radius or radii or radial or ulna*).tw. (384735) 5 (leg* or foot or feet or hip* or knee* or ankle).tw. (854297) 6 (arm* or hand* or elbow* or shoulder*).tw. (891988) 7 (lower extremit* or lower limb or upper extremit* or upper limb or forelimb* or forearm*).tw. (164389) 8 Radius fractures/ or Humeral fractures/ (16000) 9 Femoral fractures/ or Tibial fractures/ or exp Ulna fractures/ (32240) 10 Ankle fractures/ or Shoulder fractures/ (4725) 11 or/1‐10 (2200617) 12 Fractures, Open/ (5367) 13 (plateau ankle or Weber or Monteggia or Galeazzi or Hoffa or Pilon or Schatzker).tw. (7532) 14 ((open or compound or trauma* or Gustilo or Tscherne or high energy) adj3 fracture).tw. (6376) 15 or/12‐14 (18108) 16 11 and 15 (9119) 17 Debridement/ (16066) 18 exp Wound Infection/pc (16061) 19 surgical wound dehiscence/ or surgical wound infection/ (41731) 20 debrid*.tw. (26704) 21 Surgery, Plastic/ (26143) 22 Reconstructive Surgical Procedures/ (49465) 23 exp Fracture Fixation/ or exp Orthopedic Fixation Devices/ (108906) 24 exp Surgical Flaps/ (60732) 25 (wound* or cover* or flap* or reconstruct* or closure).tw. (936164) 26 exp Anti‐Bacterial Agents/ (720155) 27 (antibiotic* or antimicrob* or anti bacteria* or antibacteria*).tw. (489360) 28 (coamoxiclav or amoxicillin or cephalosporin or gentamicin or teicoplanin or cefuroxime or cefazolin or ceftriazone or penicillin or clindaymycin or aminoglycosides or vancomycin or levofloxacin or metronidazole or tazocin or piperacillin or tazobactam or linezolid or flucloxacillin or ampicillin).tw. (142291) 29 or/17‐28 (2031635) 30 Randomized Controlled Trial.pt. (503318) 31 Controlled Clinical Trial.pt. (93602) 32 randomized.ab. (475360) 33 placebo.ab. (206664) 34 drug therapy.fs. (2192418) 35 randomly.ab. (330644) 36 trial.ab. (500803) 37 groups.ab. (2030386) 38 or/30‐37 (4672916) 39 exp Animals/ not Humans.sh. (4686353) 40 38 not 39 (4050902) 41 16 and 29 and 40 (672)
Search 2
42 (202004* or 202005* or 202006* or 202007* or 202008* or 202009* or 202010* or 202011* or 202012* or 2021*).ed,dt. (1900359) 43 41 and 42 (64)
Embase (Ovid Online)
The Embase search was run in two stages: the first search was run in April 2020 and a second top‐up search was run in February 2021.
Search 1
1 exp Lower limb/ or exp "bones of the leg and foot"/ (450347) 2 exp arm bone/ (28290) 3 leg bone/ or exp femur/ or fibula/ or tibia/ (91786) 4 (femur or femoral or tibia* or fibula* or humor* or radius or radii or radial or ulna*).tw. (454316) 5 (leg* or foot or feet or hip* or knee* or ankle).tw. (1050310) 6 (arm* or hand* or elbow* or shoulder*).tw. (1149997) 7 (lower extremit* or lower limb or upper extremit* or upper limb or forelimb* or forearm*).tw. (212843) 8 radius fracture/ or humerus fracture/ (15749) 9 femur fracture/ or tibia fracture/ or exp ulna fracture/ (30949) 10 ankle fracture/ or shoulder fracture/ (5427) 11 or/1‐10 (2632965) 12 open fracture/ (5462) 13 (plateau ankle or Weber or Monteggia or Galeazzi or Hoffa or Pilon or Schatzker).tw. (9332) 14 ((open or compound or trauma* or Gustilo or Tscherne or high energy) adj3 fracture).tw. (7639) 15 or/12‐14 (20604) 16 11 and 15 (10900) 17 debridement/ (38624) 18 exp wound Infection/pc (3343) 19 wound dehiscence/ or surgical infection/ (57627) 20 debrid*.tw. (32650) 21 plastic surgery/ (56920) 22 reconstructive surgery/ (8876) 23 fracture fixation/ or exp orthopedic fixation device/ (94668) 24 surgical flaps/ (6029) 25 (wound* or cover* or flap* or reconstruct* or closure).tw. (1141069) 26 antiinfective agent/ (166221) 27 (antibiotic* or antimicrob* or anti bacteria* or antibacteria*).tw. (612200) 28 (coamoxiclav or amoxicillin or cephalosporin or gentamicin or teicoplanin or cefuroxime or cefazolin or ceftriazone or penicillin or clindaymycin or aminoglycosides or vancomycin or levofloxacin or metronidazole or tazocin or piperacillin or tazobactam or linezolid or flucloxacillin or ampicillin).tw. (183924) 29 or/17‐28 (1995990) 30 Randomized controlled trial/ (593991) 31 Controlled clinical study/ (463848) 32 Random*.ti,ab. (1505365) 33 randomization/ (86346) 34 intermethod comparison/ (258522) 35 placebo.ti,ab. (297508) 36 (compare or compared or comparison).ti. (483777) 37 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2072561) 38 (open adj label).ti,ab. (78166) 39 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (222479) 40 double blind procedure/ (168096) 41 parallel group*1.ti,ab. (25162) 42 (crossover or cross over).ti,ab. (101324) 43 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (322408) 44 (assigned or allocated).ti,ab. (379673) 45 (controlled adj7 (study or design or trial)).ti,ab. (339873) 46 (volunteer or volunteers).ti,ab. (237652) 47 trial.ti. (289564) 48 or/30‐47 (4548570) 49 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5942953) 50 48 not 49 (3927766) 51 16 and 29 and 50 (794)
Search 2
52 (2020* or 2021*).dc,yr. (2450928) 53 51 and 52 (108)
BIOSIS Citation Index (Web of Science)
#1 TS=((femur or femoral or tibia* or fibula* or humor* or radius or radii or radial or ulna*) NEAR/5 (fracture*)) Indexes=BCI Timespan=All years (16,395) #2 TS=((leg* or foot or feet or hip* or knee* or ankle) NEAR/5 (fracture*)) Indexes=BCI Timespan=All years (12,560) #3 TS=((arm* or hand* or elbow* or shoulder*) NEAR/5 (fracture*)) Indexes=BCI Timespan=All years (1,558) #4 TS=((extremit* or limb or forelimb* or forearm*) NEAR/5 (fracture*)) Indexes=BCI Timespan=All years (2,968) #5 TS=(plateau ankle or Weber or Monteggia or Galeazzi or Hoffa or Pilon or Schatzker) Indexes=BCI Timespan=All years (7,195) #6 TS=((open or compound or trauma* or Gustilo or Tscherne or “high energy”) NEAR/5 (fracture*)) Indexes=BCI Timespan=All years (6,786) #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 Indexes=BCI Timespan=All years (40,248) #8 TS=(debrid*) Indexes=BCI Timespan=All years (8,991) #9 TS=(wound* or cover* or flap* or reconstruct* or closure) Indexes=BCI Timespan=All years (755,692) #10 TS=(antibiotic* or antimicrob* or anti bacteria* or antibacteria*) Indexes=BCI Timespan=All years (794,696) #11 TS=(coamoxiclav or amoxicillin or cephalosporin or gentamicin or teicoplanin or cefuroxime or cefazolin or ceftriazone or penicillin or clindaymycin or aminoglycosides or vancomycin or levofloxacin or metronidazole or tazocin or piperacillin or tazobactam or linezolid or flucloxacillin or ampicillin) Indexes=BCI Timespan=All years (174,198) #12 #8 OR #9 OR #10 OR #11 Indexes=BCI Timespan=All years (1,555,579) #13 #7 AND #12 Indexes=BCI Timespan=All years (4,029) #14 TS=( random* or factorial* or crossover* or "cross‐over*" or placebo* or "doubl* blind*" or "singl* blind*" or assign* or allocat* or volunteer* or "trial" or "groups" or "controlled") Indexes=BCI Timespan=All years (3,033,546 #15 #13 AND #14 Indexes=BCI Timespan=All years (631) #16 TI=(RAT OR RATS OR MOUSE OR MOUSE OR DOG OR DOGS OR RABBIT OR RABBITS OR PIG OR PIGS OR SWINE OR PORCINE) Indexes=BCI Timespan=All years (2,080,685) #17 #15 NOT #16 Indexes=BCI Timespan=All years (567)
National Technical Information Service (NTIS) Title: Open Fracture OR Keyword: Open Fracture (308)
Open Grey Open Fracture (68)
Grey Literature Report Fracture (5)
Clinicaltrials.gov Open Fracture (127)
The WHO International Clinical Trials Registry Platform Open Fractures (107)
Appendix 2. Template data extraction form
| Methods |
Study design: Review comparison group: |
| Participants |
Total number of randomised participants: Inclusion criteria: Exclusion criteria: Setting: Baseline characteristics Group 1 (specify intervention)
Group 2 (specify intervention)
|
| Interventions |
General details (if not included as baseline characteristics, include details of antibiotics, duration of antibiotic treatment, wound debridement, staging and timing of reconstructive surgery, type of soft tissue reconstruction, type of skeletal fixation): Group 1
Group 2
|
| Outcomes |
Outcomes reported by study authors: Outcomes relevant to the review: Notes: |
| Notes |
Study dates: Funding/sponsorship source: Declarations of interest by study authors: |
Appendix 3. Assessment of risk of bias tool
| Domain | Consider whether there was a risk of: | Author’s risk of bias judgement (low/unclear/high) | Support for judgement (quote/comment) | Notes |
|
Random sequence generation (selection bias) |
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | ||
| Allocation concealment (selection bias) | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. In particular this might apply where blocked randomisation is used in that the balancing of assignment within blocks could result in some prediction of sequence. |
||
|
Blinding of participants and personnel
(performance bias) Subjective outcomes: e.g. limb function and HRQoL using PROMs, physical function, pain |
Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | ||
|
Blinding of participants and personnel
(performance bias) Objective outcomes: e.g. deep SSI, amputation, unplanned operations, flap failure, wound infection, wound dehiscence, primary wound related infections, thromboembolic events, pressure sores |
Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | As above | ||
|
Blinding of outcome assessment
(detection bias) Subjective outcomes: e.g. limb function and HRQoL using PROMs, physical function, pain |
Detection bias due to knowledge of the allocated interventions by outcome assessors. | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | ||
|
Blinding of outcome assessment
(detection bias) Objective outcomes: e.g. deep SSI, amputation, unplanned operations, flap failure, wound infection, wound dehiscence, primary wound related infections, thromboembolic events, pressure sores |
Detection bias due to knowledge of the allocated interventions by outcome assessors. | As above | ||
|
Incomplete outcome data
(attrition bias) Subjective outcomes: e.g. limb function and HRQoL using PROMs, physical function, pain |
Attrition bias due to amount, nature or handling of incomplete outcome data. | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | ||
|
Incomplete outcome data
(attrition bias) Objective outcomes: e.g. deep SSI, amputation, unplanned operations, flap failure, wound infection, wound dehiscence, primary wound related infections, thromboembolic events, pressure sores |
Attrition bias due to amount, nature or handling of incomplete outcome data. | As above | ||
|
Selective reporting
(reporting bias) |
Reporting bias due to selective outcome reporting. | We will record if the study was prospectively registered with a trial registry and whether there was a prospectively published protocol. Where a trial registry entry or protocol was available prospectively we will compare the planned outcome reporting with the final trial report. | ||
|
Other bias
(other performance bias) Performance bias: for instance, provision of other interventions that should be comparable in both groups; or clear differences in personal characteristics of lead clinicians. |
Bias due to the provision of other interventions that should be comparable in both groups; or clear differences in personal characteristics of lead clinicians. | This is incompletely covered in the ‘blinding (performance bias)’. Describe the measures taken to avoid performance bias, such as comparable training in the interventions. | ||
| Other bias | Bias due to other issues that we did not anticipate | Describe the details of the other bias. |
Footnotes
HRQoL: health‐related quality of life PROM: patient‐reported outcome measure SSI: surgical site infection
Data and analyses
Comparison 1. Duration of prophylactic antibiotic treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adverse events: superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Timing of wound closure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Deep infection | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.80] |
| 2.2 Delayed union or non‐union | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Adverse events: superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benson 1983.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 x 2 factorial design Review comparison group: reconstructive surgery: staging and timing ('fix‐then‐flap'; wound closure immediate or delayed) Note:
|
|
| Participants |
Total number of randomised participants: 78 (82 fractures) Inclusion criteria: adults with severe orthopaedic trauma (open fractures) Exclusion criteria: wounds open for > 24 hours prior to admission; marine or river contaminated wounds; lawnmower wounds; high‐velocity gunshot wounds; where wound closure was deemed physically impossible Setting: single centre; University of California, Davis Medical Center, Sacramento, USA Baseline characteristics Group 1 (immediate closure)
Group 2 (delayed closure)
Overall:
Note:
|
|
| Interventions |
General details: cefazolin or clindamycin IV over 10 to 60‐minute period every 6 hours, started when debridement was initiated, and continued for a minimum of 5 days. Although not explicitly stated, we interpreted that skeletal fixation treatment, if necessary, would be done at this first operation. Group 1 (immediate)
Group 2 (delayed)
Note:
|
|
| Outcomes |
Outcomes reported by study authors: deep infection and superficial infection Outcomes relevant to the review: deep infection and superficial infection Note:
|
|
| Notes |
Study dates: not reported Funding/sponsorship sources: funding from Smith Kline and French and Upjohn Laboratories Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patient sample was divided into four groups by use of random selection of numbers" Comment: we could not determine from this information whether the sequence generation was truly random. |
| Allocation concealment (selection bias) | Unclear risk | No details given of the methods used for allocation concealment. |
| Blinding of participants and personnel (performance bias) Test | High risk | Because of the nature of the intervention groups, it was not feasible to blind participants or clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding of outcome assessors. In this study, it is highly unlikely that blinding of outcome assessors took place if this was not reported. Assessment of the outcome was likely to have been influenced by knowledge of intervention received. The diagnosis of the main outcome of interest, i.e. infection, was based on clinical picture, which is subjective (if "the tissues around the wound were red, swollen, or tender", "Wounds were not considered infected when there were ...delayed or nonunions, and negative cultures of drainage"). Delayed and non‐unions are often caused by deep fracture site infection and culture negative drainage is subject to a high false negative rate. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The unit of analysis was variable throughout the report; some participants had more than one wound and data were reported either by number of wounds or number of participants. There appeared to be unexplained loss of participants in some outcome data tables, meaning that we could not always be certain of attrition. |
| Selective reporting (reporting bias) | Unclear risk | We found no protocol for this study, and the study was published prior to clinical trials register provision. We could not effectively assess risk of reporting bias without these documents. |
| Other bias | Low risk | Other performance bias: although not explicitly stated, we assumed that the provision of other interventions was comparable between groups. Other bias: we identified no other sources of bias |
Ondari 2016.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel design Review comparison group: duration of prophylactic antibiotic treatment |
|
| Participants |
Total number of randomised participants: 84 Inclusion criteria: isolated Gustilo grade II open tibial fractures; aged between 18 and 80 years Exclusion criteria: paediatric patients; Gustilo II open tibial fractures whose wounds could not be closed primarily after debridement; fractures not debrided within 24 hours of injury; non–traumatic open tibia fractures such as pathological fractures; cigarette smokers; people with diabetes mellitus, HIV/AIDS, psychosis or chronic renal failure; people on corticosteroids or chemotherapy; referred from other medical facilities where they had already received any form of treatment; haemodynamically unstable; refused to give consent Setting: single centre; national referral and teaching hospital in Nairobi, Kenya Baseline characteristics (only reported for analysed participants) Group 1 (short duration)
Group 2 (long duration)
Note:
|
|
| Interventions |
General details: participants assessed and managed according to ATLS protocol; temporary splint, wound covered, antibiotic administration, then X‐ray and baseline blood tests; 240 mg gentamycin and 1.5 g cefuroxime administered IV; standard debridement protocol; fracture stabilisation according to surgeon preference and implant availability; primary wound closure without tension; if casting was used for stabilisation then wound closed prior to casting (cast window created after 24 hours at wound site); clinical evaluation of wounds at day 2, 5, and 14 after debridement Group 1
Group 2
Note:
|
|
| Outcomes |
Outcomes reported by study authors: wound infection with ASEPSIS score > 20 (scale 0 to 65 with scores 0 to 20 indicating normal wound healing) collected at day 2, 5, and 14 Outcomes relevant to the review: wound infection at day 14 Notes:
|
|
| Notes |
Study dates: September 2014 to March 2015 Funding/sponsorship source: Kenyatta National Hospital Declarations of interest: study authors declare no conflicts of interest Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used to allocate participants to one of two parallel arms of treatment using a computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Test | High risk | Because of the nature of the intervention groups, it was not feasible to blind participants or clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. Assessment was likely to have been influenced by knowledge of intervention received, particularly as diagnosis of interest using the ASEPSIS score necessitates a subjective element. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 7 participants, but these were clearly reported. |
| Selective reporting (reporting bias) | Unclear risk | Study authors reported retrospective clinical trials registration. We could not feasibly use the trials register documents to effectively assess risk of selective reporting bias. |
| Other bias | Low risk | Other performance bias: although not explicitly stated, we assumed that the provision of other interventions was comparable between groups. Other bias: we identified no other sources of bias. |
Russell 2005.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel group Review comparison group: reconstructive surgery: staging and timing ('fix‐then‐flap'; wound closure immediate or delayed) |
|
| Participants |
Total number of randomised participants: 451 Inclusion criteria: grade II & IIIa tibial diaphyseal fractures Exclusion criteria: not reported Setting: multicentre; 30 trauma centres, USA Baseline characteristics: study authors stated that "the demographics and smoking rates were similar for the groups". Note:
|
|
| Interventions |
General details: participants treated with standardised debridement, antibiotic and reamed locked intramedullary nail protocol; mean follow‐up of 296 days Group 1
Group 2
Note:
|
|
| Outcomes |
Outcomes reported by study authors: rate of infections, wound complications, rehospitalisations, time to union and number with delayed union/non‐union Outcomes relevant to the review: infection, time to union and number with delayed union/non‐union Notes:
|
|
| Notes |
Study dates: not reported Funding: not reported Declarations of interest by study authors: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as a randomised study, but no details given about methods used to randomise participants to groups. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Test | High risk | Because of the nature of the intervention groups, it was not feasible to blind participants or clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although not explicitly stated, we assumed that outcome assessors were not blinded to the intervention groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We noted loss of 64 participants and reasons for these losses were not explained. Because study authors did not report the number of participants randomised to each group, it was not possible to ascertain if these losses were balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report clinical trials registration or a prospectively published protocol. It was not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | High risk | Other performance bias: although not explicitly stated, we assumed that the provision of other interventions was comparable between groups (unclear). Other bias: study was only published as an abstract, which we expected was not peer‐reviewed (high). |
ASEPSIS: scoring method for postoperative wound infection (Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay); ATLS: Advanced Trauma Life Support; IV: intravenous(ly); M/F: male/female; RCT: randomised controlled trial; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergman 1982 | RCT, participants with open and closed fractures (some data were reported separately for open fractures). We excluded this study because it compared two types of antibiotics. |
| Braun 1987 | RCT, participants with open fractures. We excluded this study because it compared a 10‐day course of prophylactic cloxacillin with a placebo. |
| Carsenti‐Etesse 1999 | RCT, comparing different antibiotic agents in open leg fractures. Although antibiotics were given at different times in the two groups (single dose of pefloxacin IV compared with a 2‐day course of cefazlin IV followed by a 3‐day course of oral oxacillin), the primary aim of the study was to assess the correlation between organisms grown from preoperative and perioperative cultures and those grown from infected sites. The collection of swab material was not consistent with current standards. |
| Mahajan 2016 | RCT, participants with open fractures. We excluded this study as it compared a standard treatment protocol with an enhanced protocol (differences included different antibiotics with different routes of administration, as well as additional wound cleaning solutions). |
| Moehring 2000 | RCT, participants with open fractures. We excluded this study because it compared two different routes of antibiotic administration (beads versus IV). |
| NCT04678154 | RCT, participants with open fractures. We excluded this study as it compared different routes of antibiotic administration. |
| Pape 2007 | Retrospective cohort study comparing different times for internal fixation of fractures; only 25% were open fractures |
| Patzakis 1996 | RCT, participants with open fractures. We excluded this study because it compared a single antibiotic agent with a combined antibiotic treatment. |
| Saveli 2013 | RCT, participants with open fractures. We excluded this study because it compared a single antibiotic agent with a combined antibiotic treatment. |
IV: intravenous(ly); RCT: randomised controlled trial
Differences between protocol and review
Review information
Review authors: one new review author joined the review team (SL)
Title: following consumer feedback, we edited the review title from 'Timing and staging of antibiotic administration and surgery for open long bone fractures of the upper and lower limbs' to 'Timing of antibiotic administration, wound debridement, and stages of reconstructive surgery for open long bone fractures of the upper and lower limbs'.
Objectives
We made a minor change to the objectives to reflect edits made to the title.
Methods
Criteria for considering studies for this review
Types of interventions: for 'duration of prophylactic antibiotic treatment', we expanded the comparative time points to include any short versus long course of antibiotic treatment. This was consistent with other comparisons, in which other thresholds were considered in otherwise eligible studies. We removed some text from this section related to co‐interventions (about negative pressure wound therapy) and added this to the Data extraction and management section. We also removed some text related to operations (for flap loss, wound dehiscence); this is already included in the Types of outcome measures (adverse events) .
Types of outcome measures: we found that eligible studies did not use our protocol definitions to describe infection. Therefore, we expanded the definition criteria for infection by explaining that we made judgements about the type of infection from other information in the study report. We explained what we did in the event that time points did not match those described in the list of outcomes, or time points were not defined.
Search methods for identification of studies
Searching other resources: we did not conduct forward citations searching for included studies (in Web or Science or Scopus), searches of orthopaedic‐specific sources or reference lists of evidence‐based guidelines. We judged the search of electronic databases in this review to be sufficiently broad.
Data collection and analysis
Selection of studies: we specified using Covidence to screen titles and abstracts.
Data extraction and management: we included a template data extraction in Appendix 2. We also clarified that we made judgements about clinical management in the event that contact with study authors was unsuccessful.
Assessment of risk of bias in included studies: we did not consider baseline imbalances as 'other bias' in this review; this imbalance would indicate possible selection bias and we therefore considered this when judging risk of selection bias (sequence generation). We removed this row from the table in Appendix 3, and we added an additional row for 'other bias' to account for biases which we had not anticipated.
Assessment of reporting bias: we explained that we also used clinical trials register searches in an attempt to assess the risk of selective reporting bias.
Data synthesis: we explained the decision to use a random‐effects model. In this review, we combined data from two studies for only one outcome measure. Lack of information in the studies meant that we could be certain of differences in clinical management; we used a random‐effects model to account for these possible differences.
Summary of findings tables: we were unable to ascertain the time points of collection of some outcomes (deep infection and surgical infection) and therefore we have removed the 90‐day time point from the Methods section for the summary of findings tables.
Contributions of authors
JC (content expert): sifted and identified included studies, extracted study data, interpreted the findings, and drafted the review. AA (content expert): sifted and identified included studies, extracted study data, interpreted the findings, reviewed and approved the final review. SL (systematic reviewer): extracted study data, interpreted the findings, and drafted the review. JR (content expert): reviewed and approved the final review. XG (content expert and guarantor for the review): reviewed and approved the final review. JN (content expert and guarantor for the review): reviewed and approved the final review.
Sources of support
Internal sources
-
National Institute for Health Research, UK
Clinician Scientist Award funds XG full time over a five‐year fellowship
-
Oxford National Institute for Health Research Biomedical Research Centre, UK
The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC)
-
National Institute for Health Research, UK
Postdoctoral Fellowship Award funds JR part time over a five‐year fellowship
External sources
No sources of support provided
Declarations of interest
JC: Cochrane UK Fellow 2017 to 2018. AA: none SL: none. SL was not involved in the editorial process. JR: funded by the NIHR. This represents independent research funded by the NIHR. The views expressed are the author's own, and are not necessarily those of the NIHR, NHS or the Department of Health and Social Care. XG: works within a research group that has been funded for several trials of negative pressure dressings and has published trials that may be relevant to this review. He has ongoing expert consultancy with several companies. XG is fully funded by the NIHR. The views expressed are the author's own and are not necessarily those of the NIHR, NHS or the Department of Health and Social Care. XG was not involved in the editorial process. JN: co‐applicant on an NIHR‐funded trial for negative pressure wound therapy for closed incisions in people with major trauma of the lower limb ‐ Wound Healing in Surgery for Trauma (WHIST). He has also lectured on courses on the management of open fractures, sponsored by orthopaedic implant manufacturers. JN is member of the NICE Guidance Development Group for Complex and Non‐Complex Fractures. JN is an expert witness providing medico‐legal reports including on open fractures.
New
References
References to studies included in this review
Benson 1983 {published data only}
- Benson DR, Riggins RS, Lawrence RM, Hoeprich PD, Huston AC, Harrison JA.Treatment of open fractures: a prospective study. Journal of Trauma 1983;23(1):25-30. [DOI] [PubMed] [Google Scholar]
Ondari 2016 {published data only}
- Ondari JN, Masika MM, Ombachi RB, Ating'a JE.Unblinded randomized control trial on prophylactic antibiotic use in Gustilo II open tibia fractures at Kenyatta National Hospital, Kenya. Injury 2016;47(10):2288-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- PACTR201606001689132.Unblinded randomized control trial on prophylactic antibiotic use in Gustilo II open tibia fractures at Kenyatta National Hospital, Kenya. pactr.samrc.ac.za/Search.aspx (first received 21 June 2016). [DOI] [PubMed]
Russell 2005 {published data only (unpublished sought but not used)}
- Russell G, Laurent S, Sasser H, OTA Open Fracture Study Group.Immediate versus delayed closure of grade II and IIIA tibial fractures: a prospective, randomized, multicenter study. In: OTA Abstracts Session XI - Tibia Paper # 47. ota.org/education/meetings-and-courses/abstracts/immediate-versus-delayed-closure-grade-ii-and-iiia-tibial. 22 October 2005.
References to studies excluded from this review
Bergman 1982 {published data only}
- Bergman BR.Antibiotic prophylaxis in open and closed fractures: a controlled clinical trial. Acta Orthopaedica Scandinavica 1982;53(299):57-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Braun 1987 {published data only}
- Braun R, Enzler MA, Rittmann WW.A double-blind clinical trial of prophylactic cloxacillin in open fractures. Journal of Orthopaedic Trauma 1987;1(1):12-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carsenti‐Etesse 1999 {published data only}
- Carsenti-Etesse H, Doyon F, Desplaces N, Gagey O, Tancrede C, Pradier C, et al.Epidemiology of bacterial infection during management of open leg fractures. European Journal of Clinical Microbiology & Infectious Diseases 1999;18(5):315-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gagey O, Doyon F, Dellamonica P, Carsenti-Etesse H, Desplaces N, Tancrede C, et al.Infection prophylaxis in open leg fractures. Comparison of a dose of pefloxacin and 5 days of cefazolin-oxacillin. A randomized study of 616 cases. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur 1999;85(4):328-36. [PMID: ] [PubMed] [Google Scholar]
- Gagey O, Doyon F, Desplaces N, la Monica P, Tancrede C, Delavault P, et al.A controlled study of prophylactic antibiotic treatment of open fractures of the tibia (single dose of Pefloxacine versus five days of Cephazoline and Oxacillin): a series of 616 patients. Journal of Bone and Joint Surgery. British Volume 1997;79(Suppl 1):63. [Google Scholar]
Mahajan 2016 {published data only}
- Mahajan K, Verma V, Singh GK, Kumar S, Avasthi S.A randomized controlled study to compare conventional and evidence based treatment protocols in fresh compound fractures. Journal of Clinical and Diagnostic Research 2016;10(9):RC01-05. [DOI: 10.7860/JCDR/2016/19234.8405] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moehring 2000 {published data only}
- Moehring HD, Gravel C, Chapman MW, Olson SA.Comparison of antibiotic beads and intravenous antibiotics in open fractures. Clinical Orthopaedics and Related Research 2000;372(372):254-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT04678154 {published data only}
- NCT04678154.Evaluation of a new strategy for protocolized antibiotic care for severe open fractures: SEXTANT. clinicaltrials.gov/ct2/show/NCT04678154 (first received 21 December 2020).
Pape 2007 {published data only}
- Pape HC, Rixen D, Morley J, Husebye EE, Mueller M, Dumont C, et al, Epoff Study Group.Impact of the method of initial stabilization for femoral shaft fractures in patients with multiple injuries at risk for complications (borderline patients). Annals of Surgery 2007;246(3):491-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patzakis 1996 {published data only}
- Patzakis MJ, Bains RS, Lee J, Shepherd L, Holtom P, Harvey F.A prospective randomized double blind study comparing single agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. Orthopaedic Transactions 1996;20(1):25. [DOI] [PubMed] [Google Scholar]
- Patzakis MJ, Bains RS, Lee J, Shepherd L, Singer G, Ressler R, et al.Prospective, randomized, double-blind study comparing single-agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. Journal of Orthopaedic Trauma 2000;14(8):529-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Saveli 2013 {published data only}
- Saveli CC, Morgan SJ, Belknap RW, Ross E, Stahel PF, Chaus GW, et al.Prophylactic antibiotics in open fractures: a pilot randomized clinical safety study. Journal of Orthopaedic Trauma 2013;27(10):552-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Audige 2005
- Audige L, Griffin D, Bhandari M, Kellam J, Ruedi TP.Path analysis of factors for delayed healing and nonunion in 416 operatively treated tibial shaft fractures. Clinical Orthopaedics and Related Research 2005;438:221-32. [DOI] [PubMed] [Google Scholar]
Bergner 1981
- Bergner M, Bobbitt RA, Carter WB, Gilson BS.The Sickness Impact Profile: development and final revision of a health status measure. Medical Care 1981;19(8):787-805. [DOI] [PubMed] [Google Scholar]
Binkley 1999
- Binkley JM, Stratford PW, Lott SA, Riddle DL.The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Physical Therapy 1999;79(4):371-83. [PubMed] [Google Scholar]
Bondurant 1988
- Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD.The medical and economic impact of severely injured lower extremities. Journal of Trauma and Acute Care Surgery 1988;28(8):1270-3. [DOI] [PubMed] [Google Scholar]
Brazier 2002
- Brazier J, Roberts J, Deverill M.The estimation of a preference-based measure of health from the SF-36. Journal of Health Economics 2002;21(2):271-92. [DOI] [PubMed] [Google Scholar]
British Orthopaedic Association 2017
- British Orthopaedic Association, British Association of Plastic, Reconstructive and Aesthetic Surgeons.Open fractures; 2017. Available at www.boa.ac.uk/static/3b91ad0a-9081-4253-92f7d90e8df0fb2c/29bf80f1-1cb6-46b7-afc761119341447f/open%20fractures.pdf.
Brooks 1996
- Brooks R.EuroQol: the current state of play. Health Policy 1996;37(1):53-72. [DOI] [PubMed] [Google Scholar]
Chan 2012
- Chan JK, Harry L, Williams G, Nanchahal J.Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plastic Reconstructive Surgery 2012;130(2):e284-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2019
- Chang Y, Bhandari M, Zhu KL, Mirza RD, Ren M, Kennedy SA, et al.Antibiotic prophylaxis in the management of open fractures: a systematic survey of current practice and recommendations. JBJS Reviews 2019;7(2):e1. [DOI] [PubMed] [Google Scholar]
Chen 2017
- Chen W, Lv H, Liu S, Liu B, Zhu Y, Chen X, et al.National incidence of traumatic fractures in China: a retrospective survey of 512,187 individuals. Lancet Global Health 2017;5(8):e807-17. [DOI] [PubMed] [Google Scholar]
Costa 2018
- Costa ML, Achten J, Bruce J, Tutton E, Petrou S, Lamb SE, et al.Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb: the WOLLF randomized clinical trial. Journal of the American Medical Association 2018;319(22):2280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Court‐Brown 2012
- Court-Brown CM, Bugler KE, Clement ND, Duckworth AD, McQueen MM.The epidemiology of open fractures in adults. A 15-year review. Injury 2012;43(6):891-7. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence systematic review software.Version accessed on 12 November 2020. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
de Bruin 1994
- Bruin AF, Diederiks JP, Witte LP, Stevens FC, Philipsen H.The development of a short generic version of the Sickness Impact Profile. Journal of Clinical Epidemiology 1994;47(4):407-18. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Dolan 1997
- Dolan P.Modeling valuations for EuroQol health states. Medical Care 1997;35(11):1095-108. [DOI] [PubMed] [Google Scholar]
Eccles 2020
- Eccles S, Handley B, Khan U, McFadyen, Nanchahal J, Nayagam S, editor(s).Standards for the management of open fractures. oxfordmedicine.com/view/10.1093/med/9780198849360.001.0001/med-9780198849360 (accessed 1 February 2020). [DOI: 10.1093/med/9780198849360.001.0001] [DOI]
Enneking 1993
- Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ.A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clinical Orthopaedics and Related Research 1993;286:241-6. [PubMed] [Google Scholar]
Gillespie 2010
- Gillespie WJ, Walenkamp GH.Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD000244. [DOI: 10.1002/14651858.CD000244.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glass 2011
- Glass GE, Barrett SP, Sanderson F, Pearse MF, Nanchahal J.The microbiological basis for a revised antibiotic regimen in high-energy tibial fractures: preventing deep infections by nosocomial organisms. Journal of Plastic Reconstructive and Aesthetic Surgery 2011;64(3):375-80. [DOI] [PubMed] [Google Scholar]
Godina 1986
- Godina M.Early microsurgical reconstruction of complex trauma of the extremities. Plastic Reconstructive Surgery 1986;78(3):285-92. [DOI] [PubMed] [Google Scholar]
Gopal 2000
- Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM.Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. Journal of Bone and Joint Surgery. British Volume 2000;82(7):959-66. [DOI] [PubMed] [Google Scholar]
Gopal 2004
- Gopal S, Giannoudis PV, Murray A, Matthews SJ, Smith RM.The functional outcome of severe, open tibial fractures managed with early fixation and flap coverage. Journal of Bone and Joint Surgery. British Volume 2004;86(6):861-7. [DOI] [PubMed] [Google Scholar]
Gosselin 2004
- Gosselin RA, Roberts I, Gillespie WJ.Antibiotics for preventing infection in open limb fractures. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD003764. [DOI: 10.1002/14651858.CD003764.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustilo 1976
- Gustilo RB, Anderson JT.Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. Journal of Bone and Joint Surgery. American Volume 1976;58(4):453-8. [PubMed] [Google Scholar]
Gustilo 1984
- Gustilo RB, Mendoza RM, Williams DN.Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. Journal of Trauma 1984;24(8):742-6. [DOI] [PubMed] [Google Scholar]
Harris 2009
- Harris AM, Althausen PL, Kellam J, Bosse MJ, Castillo R, Lower Extremity Assessment Project Study Group.Complications following limb-threatening lower extremity trauma. Journal of Orthopaedic Trauma 2009;23(1):1-6. [DOI] [PubMed] [Google Scholar]
Hedges 1982
- Hedges LV.Fitting continuous models to effect size data. Journal of Educational and Behavioral Statistics 1982;7(4):245-70. [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Horan 2008
- Horan TC, Andrus M, Dudeck MA.CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309-32. [DOI] [PubMed] [Google Scholar]
Høiby 2015
- Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, et al, ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli.ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clinical Microbiology and Infection 2015;21(Suppl 1):S1-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Iheozor‐Ejiofor 2018
- Iheozor-Ejiofor Z, Newton K, Dumville JC, Costa ML, Norman G, Bruce J.Negative pressure wound therapy for open traumatic wounds. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD012522. [DOI: 10.1002/14651858.CD012522.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkinson 1999
- Jenkinson C, Stewart-Brown S, Petersen S, Paice C.Assessment of the SF-36 version 2 in the United Kingdom. Journal of Epidemiology & Community Health 1999;53(1):46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keating 2000
- Keating JF, Blachut PA, O'Brien PJ, Court-Brown CM.Reamed nailing of Gustilo grade-IIIB tibial fractures. Journal of Bone and Joint Surgery British Volume 2000;82(8):1113-6. [DOI] [PubMed] [Google Scholar]
Kuripla 2021
- Kuripla C, Tornetta P 3rd, Foote CJ, Koh J, Sems A, Shamaa T, et al.Timing of flap coverage with respect to definitive fixation in open tibia fractures. Journal of Orthopaedic Trauma 2021;35(8):430-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane 2021. Available www.training.cochrane.org/handbook.
Liu 2012
- Liu DS, Sofiadellis F, Ashton M, MacGill K, Webb A.Early soft tissue coverage and negative pressure wound therapy optimises patient outcomes in lower limb trauma. Injury 2012;43(6):772-8. [DOI] [PubMed] [Google Scholar]
Mathews 2015
- Mathews JA, Ward J, Chapman TW, Khan UM, Kelly MB.Single-stage orthoplastic reconstruction of Gustilo-Anderson Grade III open tibial fractures greatly reduces infection rates. Injury 2015;46(11):2263-6. [DOI] [PubMed] [Google Scholar]
NICE 2016
- NICE.Fractures (complex): assessment and management NG37; 2016. www.nice.org.uk/guidance/ng37.
Parker 2018
- Parker B, Petrou S, Masters JP, Achana F, Costa ML.Economic outcomes associated with deep surgical site infection in patients with an open fracture of the lower limb. The Bone & Joint Journal 2018;100-B(11):1506-10. [DOI] [PubMed] [Google Scholar]
Pollak 2000
- Pollak AN, McCarthy ML, Burgess AR.Short-term wound complications after application of flaps for coverage of traumatic soft-tissue defects about the tibia. The Lower Extremity Assessment Project (LEAP) Study Group. Journal of Bone and Joint Surgery. American Volume 2000;82-A(12):1681-91. [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Salen 1994
- Salen BA, Spangfort EV, Nygren AL, Nordemar R.The Disability Rating Index: an instrument for the assessment of disability in clinical settings. Journal of Clinical Epidemiology 1994;47(12):1423-35. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Seekamp 1996
- Seekamp A, Regel G, Tscherne H.Rehabilitation and reintegration of multiply injured patients: an outcome study with special reference to multiple lower limb fractures. Injury 1996;27(2):133-8. [DOI] [PubMed] [Google Scholar]
Sheehy 2010
- Sheehy SH, Atkins BA, Bejon P, Byren I, Wyllie D, Athanasou NA, et al.The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. Journal of Infection 2010;60(5):338-43. [DOI] [PubMed] [Google Scholar]
Whitehouse 2017
- Whitehouse MR, McDaid C, Kelly MB, Moran CG, Costa ML.The effect of timing of antibiotic delivery on infection rates related to open limb fractures: a systematic review. Emergency Medicine Journal 2017;34:613-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilson 1986
- Wilson AP, Treasure T, Sturridge MF, Grüneberg RN.A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1986;1(8476):311-3. [DOI] [PubMed] [Google Scholar]
Young 2019
- Young K, Aquilina A, Chesser TJ, Costa ML, Hettiaratchy S, Kelly MB, et al.Open tibial fractures in major trauma centres: a national prospective cohort study of current practice. Injury 2019;50(2):497-502. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chan 2020
- Chan JK-K, Aquilina AL, Rodrigues JN, Griffin XL, Nanchahal J.Timing and staging of antibiotic administration and surgery for open long bone fractures of the upper and lower limbs. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013555. [DOI: 10.1002/14651858.CD013555] [DOI] [PMC free article] [PubMed] [Google Scholar]


