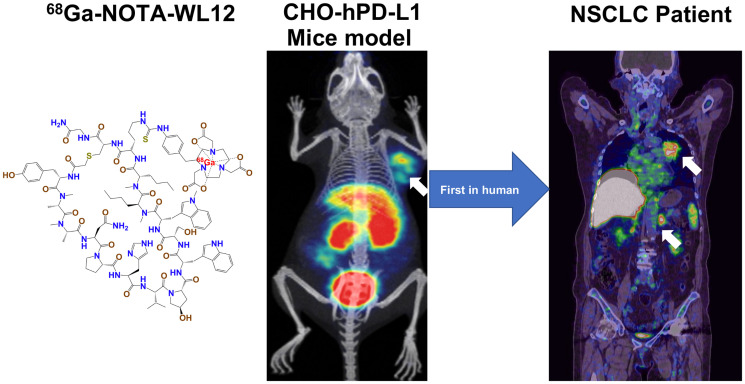
Visual Abstract
Keywords: PD-(L)1, PET/CT, immune checkpoint inhibitor, non–small cell lung cancer, pembrolizumab
Abstract
68Ga-NOTA-WL12 is a peptide-based PET imaging agent. We conducted a first-in-human study of 68Ga-NOTA-WL12 for PET to study the in vivo biodistribution, metabolism, radiation dosimetry, safety, and potential for quantifying programmed death ligand-1 (PD-L1) expression levels in patients with advanced non–small cell lung cancer (NSCLC). Methods: In vitro assessment of the PD-L1 expression and cellular uptake of 68Ga-NOTA-WL12 was performed, followed by in vivo evaluation of 68Ga-NOTA-WL12 uptake in mouse models with tumors. Nine patients with NSCLC with lesions expressing PD-L1 were enrolled and monitored for adverse events during the study. 68Ga-NOTA-WL12 and paired 18F-FDG PET/CT imaging were performed. Uptake (SUV, SUL [SUVlean], and kBq/mL) values of tumors and normal organs were obtained. Radiopharmaceutical biodistribution, radiation dosimetry, and the relationship of tumor uptake to PD-L1 expression were evaluated. Follow-up 18F-FDG PET/CT was performed in patients who had undergone treatment with a combination of pembrolizumab with chemotherapy. Results: 68Ga-NOTA-WL12 exhibited PD-L1–specific uptake in vitro and in PD-L1–positive tumors in vivo. 68Ga-NOTA-WL12 PET imaging proved safe with acceptable radiation dosimetry. Physiologic tracer uptake was mainly visible in the liver, spleen, small intestine, and kidney. Tumors were clearly visible, particularly in the lungs, with a tumor-to-lung ratio of 4.45 ± 1.89 at 1 h. One hour was a suitable time point for image acquisition because no significant differences were noted in tumor-to-background ratios between 1 and 2 h. A strong, positive correlation was found between tumor uptake (SUVpeak) and PD-L1 immunohistochemistry results (r = 0.9349; P = 0.002). 68Ga-NOTA-WL12 and 18F-FDG PET studies suggest that PD-L1 PET before therapy may indicate the therapeutic efficacy of pembrolizumab plus chemotherapy combination treatment. Conclusion: Our first-in-human findings demonstrate the safety and feasibility of 68Ga-NOTA-WL12 for noninvasive, in vivo detection of tumor PD-L1 expression levels, indicating potential benefits for clinical PD-L1 therapy.
Treatment of non–small cell lung cancer (NSCLC) has advanced considerably over the past 40 y. In addition to the advent of molecularly targeted therapies, inhibition of immune checkpoints using anti–programmed cell death (ligand)-1 (PD-[L]1) monoclonal antibodies has revolutionized the management of patients with advanced NSCLC (1–6).
Therapeutics targeting the PD-(L)1 axis are now a first-line option for advanced NSCLC without genetic aberrations (7). Numerous clinical studies have shown that PD-L1 expression identified NSCLC patients who are most likely to respond to immunotherapy, such as pembrolizumab (7,8). U.S. Food and Drug Administration–approved PD-L1 assessment using immunohistochemistry and its interpretation is often based on a single biopsy or several small biopsies, which poorly represent the heterogeneity of PD-L1 expression within and between patients (9,10). Additionally, tumor biopsy is not always practical when the lesion site is inaccessible. These limitations indicate a need for tools to detect PD-L1 levels in the whole body to improve our understanding of the response of NSCLC to therapies targeting the PD-(L)1 axis.
PET enables quantitative, real-time, noninvasive assessment of target expression levels and dynamics in the whole body (11,12). Recently, several studies have investigated molecular imaging of PD-L1 expression. Biologics, such as radiolabeled antibodies and adnectin-derived small protein radiotracers, have shown promise in early phase clinical trials (13–18). High-affinity, low-molecular-weight radiotracers labeled with 64Cu, 68Ga, and 18F have been developed and shown to detect graded levels of PD-L1 expression in vivo in preclinical models of several cancer types, including NSCLC (13–15,19,20). One of those agents, WL12, is a high-affinity PD-L1–binding small peptide labeled with 68Ga. 68Ga-WL12 proved a suitable scaffold for imaging PD-L1 expression in preclinical studies with PET (19). The tractable pharmacokinetics and high-contrast PD-L1–specific images exhibited by 68Ga-WL12 within 60 min of injection indicate the potential for further evaluation and clinical translation. Here, we report the first-in-humans evaluation of a peptide-based PET imaging agent derived from WL12, 68Ga-NOTA-WL12 (Supplemental Fig. 1A; supplemental materials are available at http://jnm.snmjournals.org), as well as its safety, radiation dosimetry, and imaging characteristics, to compare PET imaging with immunohistochemistry and therapy evaluation in patients with advanced NSCLC.
MATERIALS AND METHODS
General
This was a prospective, phase I, open-label, nonrandomized, diagnostic imaging study in advanced NSCLC patients (n = 9) between March 2020 and September 2020 (trial registration ID NCT04304066). The Medical Ethics Committee of Peking University Cancer Hospital (2019 KT62) approved this study. Oral and written informed consent was obtained from all the participants.
NOTA-WL12 was custom synthesized and provided by Chinapeptides. Briefly, to radiolabel NOTA-WL12, 68GaCl3 (925–1,110 MBq) was mixed with 195 μL of 1 M (pH 8.5) sodium acetate buffer and reacted with NOTA-WL12 (30 μg) at 60°C for 15 min. The final product was purified using a Sep-Pak (Waters) and obtained in >99% radiochemical purity by high-performance liquid chromatography. Details regarding the production, quality control, and murine radiotoxicity of 68Ga-NOTA-WL12 can be found in the supplemental materials (Supplemental Table 1).
Patient inclusion criteria were being clinically diagnosed with NSCLC, with lesions expressing positive PD-L1; and having an Eastern Cooperative Oncology Group performance score of 0–2. Exclusion criteria were severe liver or kidney dysfunction and chemoradiotherapy or targeted therapy before PET/CT scans. PD-L1 expression in available lesions was evaluated by immunohistochemistry using antibody clone 22C3 (Dako Denmark A/S; catalog M3653). Dako PD-L1 immunohistochemistry 22C3 pharmDx is an approved screening criterion for pembrolizumab application in NSCLC (21,22). Positive PD-L1 expression was defined as a tumor proportion score (TPS) ≥ 1%, whereas high PD-L1 expression was defined as a TPS ≥ 50%.
Flow Cytometric Analysis and Small-Animal PET
The Chinese hamster ovary (CHO) cell line was obtained from American Type Culture Collection. A CHO cell line with constitutive PD-L1 expression (CHO-hPD-L1) was generated in our laboratory and described previously (23). Details of the cell culture, flow cytometry analysis for PD-L1 expression, and in vitro assays are provided in the supplemental materials.
For PET imaging, nonobese diabetic (NOD)/severe combined immunodeficient (SCID) mice bearing CHO-hPD-L1 or CHO tumors were intravenously injected with approximately 7.4 MBq (∼0.17 μg) of 68Ga-NOTA-WL12 in 200 μL of saline, and PET images were acquired at 30, 60, and 120 min. To establish in vivo specificity, animals were coinjected with 50 μg of unlabeled WL12. Image analysis and PD-L1 immunohistochemistry of the CHO-hPD-L1 and CHO tumors are described in the supplemental materials.
PET/CT
Patients were injected intravenously with 68Ga-NOTA-WL12 (1.9–3.7 MBq/kg). To determine the optimal peptide dose required to obtain high-contrast images, an escalated nonradiolabeled WL12 mass dose was coadministered with the imaging agent. The first 2 patients received 0 μg, the next 3 received 5 ug, then 60 ug, and the final group of 3 received 120 μg. Because patients coadministered with 120 μg of WL12 showed lower uptake in the liver, the last 4 patients were coinjected with 120 μg of WL12. The first patient underwent a dynamic scan at 6 time points in 1 h. The remaining 8 patients underwent PET/CT at 1 h after injection, and 6 of those were also scanned at 2 h after injection (2 patients were unable to tolerate the full 2-h examination). Imaging was performed using a Biograph mCT Flow 64 scanner (Siemens) (120 kV; 146 mAs; slice: 3 mm; matrix: 200 × 200; iterations:2; subsets: 11; filter: 5 mm gaussian), continuously moving the patient bed at a speed of 1.5 mm/s to cover the entire body, from the top of the skull to the middle of the femur. Images were reconstructed with ordered-subset expectation maximization. CT reconstruction used a standard method with a 512 × 512 matrix and a layer thickness of 3–5 mm. CT data were used to correct the PET images for attenuation. Vital signs, laboratory studies, and electrocardiograms were obtained before injection, during the screening period, and 2 d after PET/CT. All patients underwent paired 18F-FDG PET/CT scans (1 h after injection) within a week after 68Ga-NOTA-WL12 PET/CT using the same imaging system. Three patients repeated the 18F-FDG PET/CT examination within 3 wk after combination therapy including pembrolizumab and chemotherapy.
Radiation Dosimetry
Data from 4 patients with 120 μg of coinjected WL12 were used for 68Ga-NOTA-WL12 dosimetry analysis, and the heart contents, lungs, liver, spleen, pancreas, kidneys, uterus, urinary bladder contents, and body remainder were selected as source organs. The volumes of the source organs manually drawn on CT images were calculated, and their mean counts/mL (kBq/mL) were determined from PET images at 1 and 2 h time points. Dosimetry was estimated using OLINDA/EXM software (version 2.0; Hermes Medical Solutions AB). Detailed procedures are provided in the supplemental materials.
Image Analysis
Images were analyzed by 2 experienced nuclear medicine physicians. The uptake parameters of major organs, tissues, and lesions were obtained to determine the organ biodistribution. SUVlean (SUL) was obtained by standardizing SUV to the body mass, which is less dependent on body habitus (24). No significant difference was observed in the coefficients of variation between SULmean and SUVmean (Supplemental Fig; 2A; Supplemental Table 2). Accordingly, we used SULmean and percentage of injected activity to describe radiopharmaceutical uptake in normal organs. To compare the uptake differences between the liver, spleen, small intestine (SI), and kidney among patients coadministered with different doses of WL12, multisite measurement was applied, and the uptake was calculated as an average of the number of patients corresponding to different dose administrations separately (Supplemental Fig. 3). To analyze normal organ uptake at different time points, 6 patients with 1- and 2-h imaging were involved. The maximum, peak, and mean values of SUV and SUL in biopsied lesions were obtained and used to correlate with PD-L1 TPS. Tumor uptake of 68Ga-NOTA-WL12 higher than that of blood pool (BP) was considered positive. Evaluation of the therapeutic response was based on PERCIST (24) (for 18F-FDG PET/CT evaluations) and RECIST 1.1 (25) (for CT evaluations) standards.
Statistics
Differences and correlations among parameters were tested using the Wilcoxon signed-rank test, Mann–Whitney U test, and Spearman correlation using IBM SPSS Statistics (version 24; IBM Corp.) software. P values < 0.05 were considered statistically significant.
RESULTS
Safety Assessment
68Ga-NOTA-WL12 was produced with >99% purity with a specific activity of 18.5–296 GBq/μmol (Supplemental Fig. 1B). The safety parameters were measured. Murine radiotoxicity indicated that 167–200 MBq of 68Ga-NOTA-WL12 were safe for humans (Supplemental Fig. 4). Details can be found in the Supplemental Materials.
In Vitro Cellular Studies and Small-Animal PET Imaging
Flow cytometry showed that the mean fluorescence intensity values of CHO-hPD-L1 were higher than those of CHO, as was cellular uptake extent (Supplemental Fig. 5).
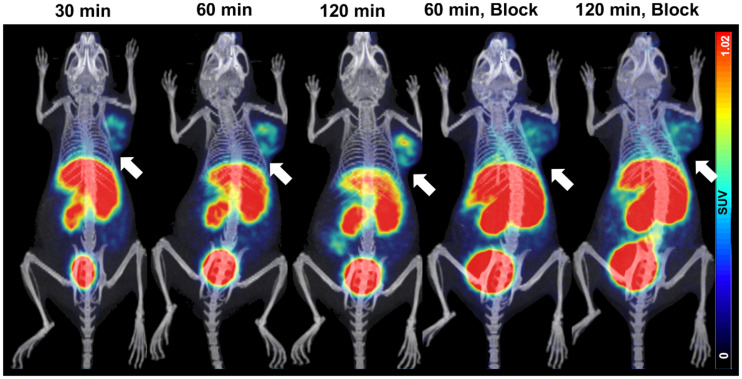
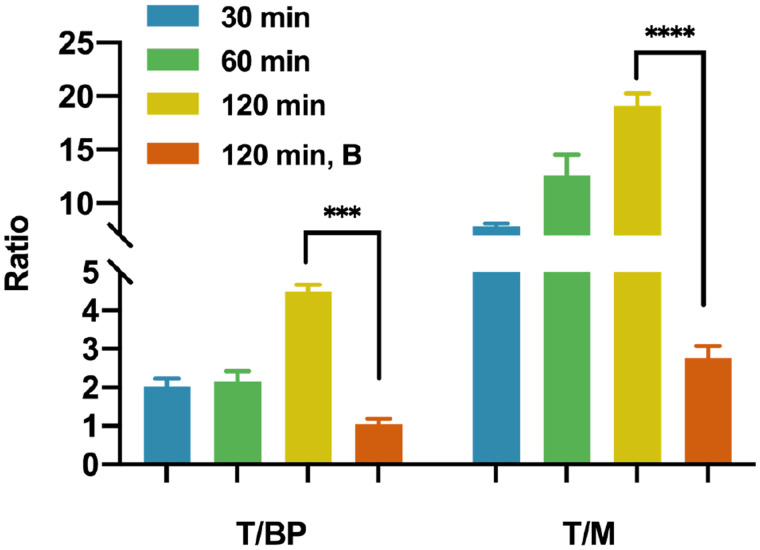
NOD/SCID mice bearing CHO-hPD-L1 tumors showed intense uptake of 68Ga-NOTA-WL12 in the tumors by 120 min (tumor–to–blood-pool uptake [T/BP]: 4.5 ± 0.2; tumor-to-muscle uptake [T/M]: 19.1 ± 1.2) (Fig. 1), whereas negative control CHO tumors showed minimal uptake (Supplemental Fig. 6A). Additionally, tumor uptake decreased with the coinjection of NOTA-WL12, indicating in vivo specificity (Fig. 2). Immunohistochemistry analysis showed high PD-L1 expression in CHO-hPD-L1 tumors (Supplemental Fig. 6B).
FIGURE 1.
PET/CT images of NOD/SCID mice with CHO-hPD-L1 tumors at different time points after 68Ga-NOTA-WL12 injection and of mice receiving 50 μg amount of blocking dose.
FIGURE 2.
Ratios of T/BP and T/M in mice receiving 68Ga-NOTA-WL12 with and without blocking dose at different time points.
Patient Characteristics
Nine patients (8 men and 1 woman; median age, 68 y; age range, 47–80 y) with histopathologically proven NSCLC (5 adenocarcinoma, 4 squamous cell carcinoma) were included. Clinical stage, therapeutic regimen, PD-L1 expression and other characteristics are summarized in Table 1. The mean administered radioactivity of 68Ga-NOTA-WL12 was 195 ± 30 MBq (range, 167–270 MBq).
TABLE 1.
Patient Characteristics
| Addition of Wl12 (ΜG) | Patient | Sex | Age (y) | ECOG score | Tumor type | PD-L1 expression* | Clinical stage | Tumor size (cm) | Therapy regimen |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | M | 47 | 1 | LUSC | 8% | cT4N2M1a IVa | 7.2 × 6.1 | Nab-paclitaxel + carboplatin + pembrolizumab |
| 0 | 2 | M | 72 | 0 | LUSC | 35% | cT2N3M0 IIIb | 4 × 3.4 | Paclitaxel + cisplatin |
| 5 | 3 | M | 68 | 0 | LUSC | 8% | cT4N1M1a IVa | 5.8 × 4.7 | Nab-paclitaxel + carboplatin + pembrolizumab |
| 60 | 4 | M | 68 | 1 | LUAC | 25% | cT4N1M0 IIIa | 5.1 × 4.0 | Nab-paclitaxel + carboplatin |
| 120 | 5 | M | 80 | 2 | LUAC | 40% | cT4N3M1c IVb | 5.7 × 3.9 | toripalimab |
| 6 | M | 58 | 0 | LUAC | 30% | cT2aN2M1c IVb | 3.1 × 2.3 | Pemetrexed + carboplatin + pembrolizumab | |
| 7 | M | 63 | 1 | LUSC | 25% | cT2bN2M1b IVa | 4.2 × 4.1 | pembrolizumab | |
| 8 | M | 53 | 1 | LUAC | 35% | cT2bN2M1b IVa | 3.1 × 1.9 | Pemetrexed + carboplatin + sintilimab | |
| 9 | F | 80 | 2 | LUAC | 80% | cT3N3M1c IVb | 5.9 × 5.3 | Pembrolizumab |
ECOG = Eastern Cooperative Oncology Group; LUSC = lung squamous cell carcinoma; LUAC = lung adenocarcinoma.
Safety
Nine 68Ga-NOTA-WL12 PET/CT examinations were performed, with no adverse or clinically detectable pharmacologic effects. No significant changes were observed in vital signs, results of laboratory studies, or electrocardiograms.
Biodistribution
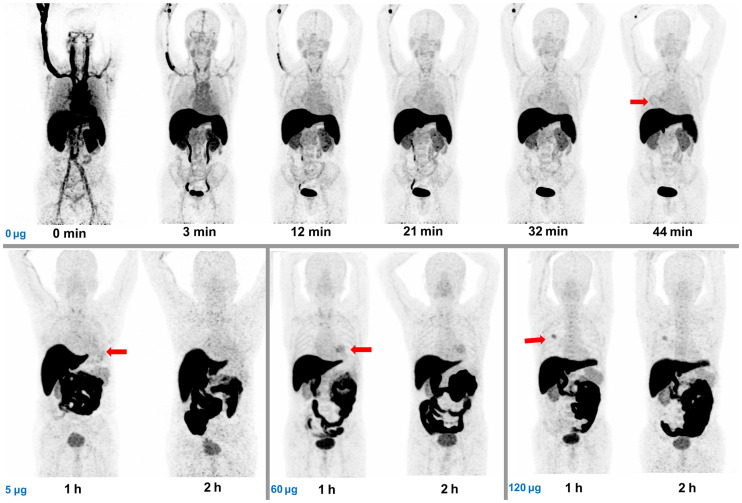
Uptake of 68Ga-NOTA-WL12 was mainly observed in the SI and liver, followed by moderate-to-low uptake in the kidneys, tumor, and spleen, and low uptake in the lungs and bone marrow (Fig. 3). This uptake pattern was similar to that observed in preclinical studies (19,20,26,27). With an increasing precursor dose coadministered with 68Ga-NOTA-WL12, we observed decreased and increased uptake in the liver and SI, respectively, as well as a gradual decrease in the spleen uptake. Radioactivity uptake in the kidneys and BP at 1 h remained similar irrespective of the mass of WL12 used (Fig. 3; Supplemental Figs. 7A and 7B). Uptake observed in the liver, SI, and kidneys indicates that clearance of 68Ga-NOTA-WL12 was primarily through the hepatobiliary system and secondarily through renal excretion. Except for the significant increase in uptake in the SI and the significant decrease in uptake in the liver from 1 to 2 h, other normal organs showed a downward trend with no significant differences (Supplemental Figs. 7C and 7D).
FIGURE 3.
Maximum-intensity-projection imaging for biodistribution and organ uptake of 68Ga-NOTA-WL12 at different time points after injection administered without and with increasing precursor doses (5 μg, 60 μg, 120 μg). Primary lesions are indicated by red arrows.
Radiation Dosimetry
Table 2 summarizes the individual organ doses and effective doses of patients administered 68Ga-NOTA-WL12 with120 μg of WL12 (n = 4) (administered dose: 224 ± 37 [range, 192–270 MBq]). The SI (3.47E–01 mGy/MBq) received the highest dose, indicating that WL12 is metabolized by hepatobiliary clearance, a characteristic observed with lipophilic agents. The intestinal absorbed dose remained well below the threshold for the human intestinal acute dose (6 Gy) (28). Radiation dosimetry was acceptable at 1.85E–02 ± 4.07E–03 mSv/MBq (4.1 mSv per patient), which is lower than the radiation dose of conventional 18F-FDG PET/CT (7.0–14 mSv) (29).
TABLE 2.
Organ Observed Doses and Whole-Body Effective Doses
| Organ/tissue | Absorbed dose (mGy/MBq) |
|---|---|
| Adrenals | 2.60e–02 |
| Brain | 8.80e–04 |
| Esophagus | 7.43e–03 |
| Eyes | 8.88e–04 |
| Gallbladder wall | 3.21e–02 |
| Left colon | 1.52e–02 |
| SI | 3.47e–01 |
| Stomach wall | 8.41e–03 |
| Right colon | 1.26e–02 |
| Rectum | 6.83e–03 |
| Heart wall | 1.45e–02 |
| Kidneys | 3.42e–02 |
| Liver | 1.92e–01 |
| Lungs | 1.55e–02 |
| Pancreas | 2.13e–02 |
| Prostate | 4.39e–03 |
| Salivary glands | 1.09e–03 |
| Red marrow | 6.10e–03 |
| Osteogenic cells | 4.05e–03 |
| Spleen | 2.89e–02 |
| Testes | 1.16e–03 |
| Thymus | 3.85e–03 |
| Thyroid | 2.15e–03 |
| Urinary bladder wall | 1.16e–02 |
| Total body | 1.04e–02 |
| Effective dose (mSv/MBq) | 1.85e–02 |
Tumor Uptake and Correlation with Immunohistochemistry
Rapid clearance of radioactivity was observed from the BP, resulting in high T/BP (and muscle) ratios. Thus, the T/BP and T/M ratios were 1.48 ± 0.42 and 1.56 ± 0.49 and 5.31 ± 1.99 and 4.87 ± 1.28 at 1 and 2 h, respectively. High contrast was also observed in the lungs with tumor-to-lung ratios of 4.45 ± 1.89 and 5.18 ± 2.27 at 1 and 2 h, respectively. No significant differences were noted in the tumor-to-background ratios (BP, lungs, and M) at 1 and 2 h (Supplemental Fig. 2B), indicating that 1 h after radiotracer administration is a suitable time point for image acquisition.
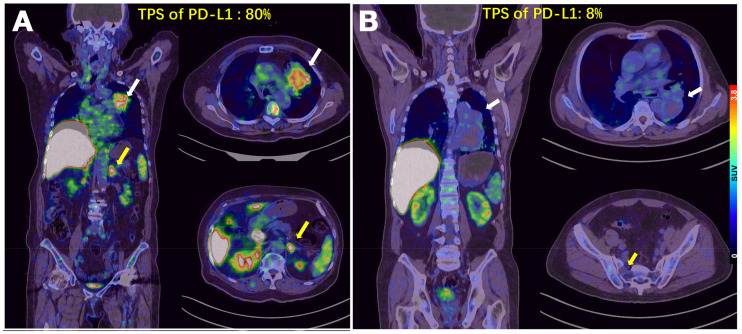
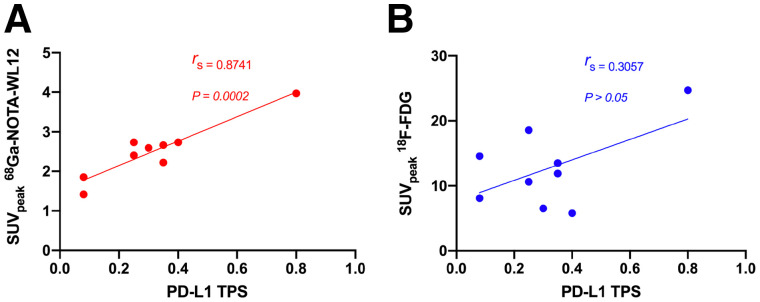
In patients with high PD-L1 expression, tumor uptake of 68Ga-NOTA-WL12 (TPS: 80%; SUVmax: 4.87) was higher than that in patients with low PD-L1 expression (TPS: 8%; SUVmax: 1.84) (Fig. 4). All calculated PET parameters of 68Ga-NOTA-WL12, except for the ratios of tumor uptake to BP, correlated with the corresponding PD-L1 TPS on immunohistochemistry (Supplemental Table 3) (SUVpeak [r = 0.9349, rs = 0.8741; P = 0.002] [Fig. 5A]). In contrast to 68Ga-NOTA-WL12, uptake of 18F-FDG in the lesions did not correlate with PD-L1 expression, with a tenuous relationship to SUVpeak (r = 0.5529, rs = 0.3057; P = 0.1226) (Fig. 5B).
FIGURE 4.
(A) Patient 9, an 80-y-old woman with advanced NSCLC and a PD-L1 TPS of 80%. SUVmax of primary tumor was 4.87 (white arrow) and that of left adrenal metastasis was 5.47 (yellow arrow) on 68Ga-NOTA-WL12 PET. (B) Patient 3, a 68-y-old man with a PD-L1 TPS of 8%. SUVmax of primary tumor in left lung (white arrow) and right sacral metastasis (yellow arrow) were 1.84 and 0.8, respectively, on 68Ga-NOTA-WL12 PET.
FIGURE 5.
Relationship between PD-L1 expression detected by immunohistochemistry and tumor uptake (SUVpeak) of 68Ga-NOTA-WL12 (rs = 0.8741, P < 0.0005) (A) and 18F-FDG (rs = 0.3057, P > 0.05) (B).
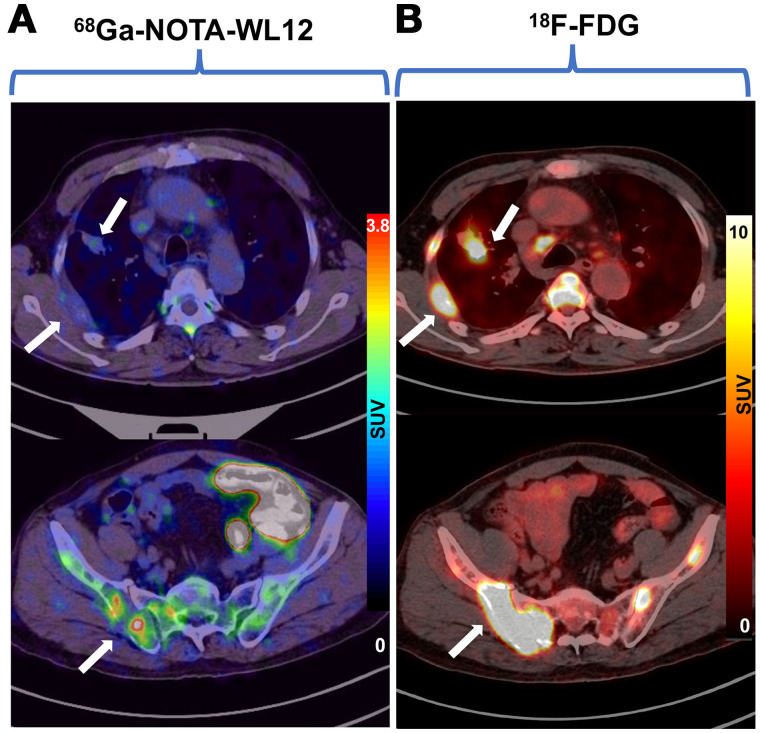
We also noted the intra- and intertumoral heterogeneity of 68Ga-NOTA-WL12 uptake in some patients, reflecting the heterogeneity of PD-L1 expression reported with other PD-L1 imaging agents (Fig. 6) (13,16). The uptake of 18F-FDG was intense in the tumors regardless of PD-L1 expression levels, with no significant heterogeneity among or within lesions.
FIGURE 6.
For patient 8, a 68-y-old man with lung adenocarcinoma, images showed inhomogeneous intra- and intertumoral uptake on 68Ga-NOTA-WL12 PET/CT (A) and high homogeneous uptake in tumors (white arrow) on 18F-FDG PET/CT (B).
Relationship of 68Ga-NOTA-WL12 Uptake to Therapy
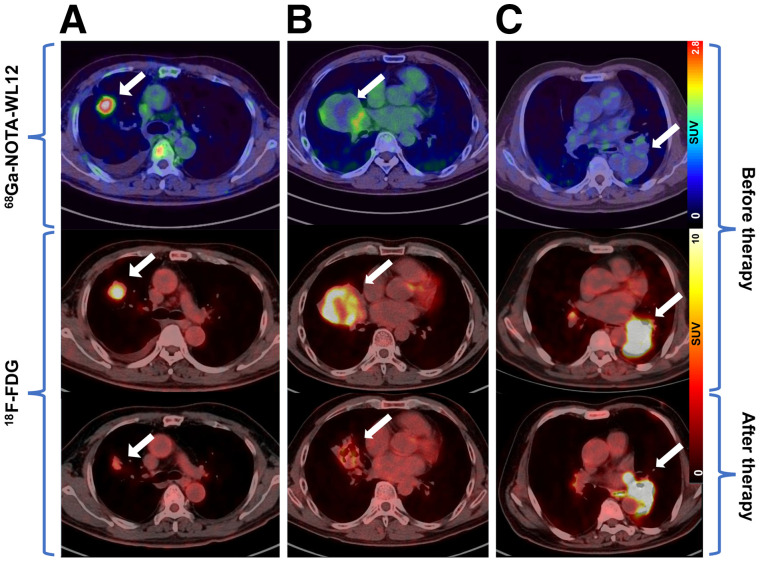
Three patients underwent 18F-FDG PET/CT after combination (pembrolizumab plus chemotherapy) treatment. In patients 1 and 6, with PD-L1 TPS values of 8% and 30%, respectively, 68Ga-NOTA-WL12 uptake in tumors before treatment showed an SUVmax of 2.21 and 3.05 (Figs. 7A and 7B; Supplemental Table 4), respectively. These 2 patients were rated as partial metabolic responses (PMR, PERCIST (24)) and stable disease (RECIST 1.1 (25)).
FIGURE 7.
(A) Patient 6, a 58-y-old man with adenocarcinoma and a PD-L1 TPS of 30%. SUVmax of 68Ga-NOTA-WL12 was 3.05 and that of 18F-FDG decreased from 8.03 to 3.10. (B) Patient 1, a 47-y-old man with squamous cell carcinoma and a TPS of 8%. SUVmax of 68Ga-NOTA-WL12 was 2.21 and that of 18F-FDG decreased from 9.13 to 3.54. (C) Patient 3, a 68-y-old man with squamous cell carcinoma and a TPS of 8%. SUVmax of 68Ga-NOTA-WL12 was1.84 and that of 18F-FDG increased from 16.55 to 21.38. All lesions were indicated by white arrows.
Patient 3, with a PD-L1 TPS value of 8%, showed negative 68Ga-NOTA-WL12 uptake before therapy (SUVmax of tumor: 1.84; SUVmax of BP: 1.78). This patient showed increased uptake of 18F-FDG in the primary tumor and a new brain metastasis on the posttherapy scan, and he was further rated as progressive disease (PD, PERCIST and RECIST) (Fig. 7C; Supplemental Table 4). Thus, patients with PMR/stable disease exhibited positive uptake of 68Ga-NOTA-WL12 before therapy.
DISCUSSION
We describe the first-in-humans evaluation of 68Ga-NOTA-WL12, a peptide-based PD-L1 imaging agent, in patients with NSCLC. We demonstrated that 68Ga-NOTA-WL12 is safe and effective for PET imaging of PD-L1 expression.
Inhibition of the PD-(L)1 axis has proved a remarkable success in treating patients with NSCLC (1–4). PD-L1 expression, determined by needle biopsy, is currently the only validated biomarker used as a companion diagnostic test for NSCLC patient selection for pembrolizumab therapy (22). Several studies have demonstrated variations in PD-L1 expression within patients and within tumors, due to heterogeneity of target expression (30). PD-L1 heterogeneity may still confound a single positive biopsy result, leading to inappropriate administration of therapy, contributing to the moderate correlation between PD-L1 status and survival rates (30). However, nuances in PD-L1 heterogeneity and its relevance to response are emerging. Patients with PD-L1 TPS values ≥ 50% can now be treated with pembrolizumab as a first-line option, indicating that increased PD-L1 expression is related to improved clinical outcomes (31). Patients with PD-L1 expression > 75% and > 90% have benefitted more than those with 50%–75%, suggesting that analysis and correlation of outcome data using PD-L1 expression on a continuous 0%–100% scale rather than using predefined cutoffs (≥50%) would be more accurate. These studies indicate that PD-L1 heterogeneity is an underappreciated aspect in assessing and guiding immune checkpoint therapies. Furthermore, these issues are compounded in advanced-stage NSCLC patients because a biopsy of every lesion is not feasible. Noninvasive quantification of PD-L1 levels could provide complementary information to address those challenges, as shown herein and by other groups (13,16).
Many radiolabeled probes targeting PD-L1 have been validated in preclinical models, such as antibodies, antibody fragments, small proteins, and peptides (13–17). Correspondingly, the preclinical experiments in this study confirmed that the uptake of 68Ga-NOTA-WL12 was highly correlated to PD-L1 expression. The accumulation of 89Zr-atezolizumab, a PD-L1 antibody, in patients with breast, lung, and bladder cancers showed a higher predictive value than immunohistochemistry or genomic sequencing for therapeutic response (16). Additionally, the accumulation of 89Zr-atezolizumab and another PD-L1 PET imaging agent, 18F-BMS-986192, was found to be heterogeneous between and within patients (13,16). Several studies revealed that the addition of nonradiolabeled peptide precursor reduced liver uptake through competitive metabolism; however, the excretion of 68Ga-NOTA-WL12 to the SI was elevated with the increase in the peptide dose. That finding differed from other saturation antibody studies (32), suggesting that the mass of 68Ga-NOTA-WL12 in circulation was relatively stable, and the addition of peptide would not likely affect tumor uptake. Therefore, patients with different mass doses of peptide precursor were enrolled. The correlation between uptake and therapeutic outcome, together with the inter- and intratumor heterogeneity observed with 68Ga-NOTA-WL12 uptake, indicated the potential to predict the effectiveness of immune therapy.
Radiolabeled antibodies such as 89Zr-atezolizumab and 89Zr-nivolumab, because of their long physical half-lives and circulation times, may encounter difficulties in delineating the changes in PD-L1 expression (13,16). Additionally, PET measures of radiolabeled antibodies do not accurately measure target expression; they are a combination of tracer exposure and target expression (16). Small-molecule radiotracers such as 68Ga-NOTA-WL12 provide a direct measure of the PD-L1 status within hours of radiotracer administration, likely because of better tissue penetration. Determining the characteristics of WL12 peptide binding to PD-L1 also presents opportunities to quantify the pharmacologic activity of PD-L1 antibodies within the tumor bed in a manner agnostic to the antibody type, as recently shown in preclinical models (20).
Therapeutic evaluation based on 18F-FDG PET/CT for lung cancer is widely recognized by physicians (24,33). Three patients in this study who received immunotherapy underwent 18F-FDG PET/CT for therapeutic evaluation. Patients with positive uptake of 68Ga-NOTA-WL12 were rated as having a PMR, and patients with a negative uptake of 68Ga-NOTA-WL12 were rated as having PD. Although 2 of the 3 patients shared the same PD-L1 expression level (TPS: 8%), the outcomes (PMR and PD) were quite different. These results suggest that higher 68Ga-NOTA-WL12 uptake in lesions may indicate a better prognosis than lower 68Ga-NOTA-WL12 uptake, regardless of the level of PD-L1 expression determined by immunohistochemistry. Those observations in a small number of patients merit further validation in larger patient cohorts.
A potential limitation of our study is the small number of patients involved. Nonetheless, our study is similar to other first-in-humans phase 1 studies of radiopharmaceuticals and has sufficient power to assess safety, suitable imaging time points, radiation dosimetry, and preliminary correlation of 68Ga-NOTA-WL12 uptake with immunohistochemistry. Another limitation of the study is that immunohistochemistry was performed only on index lesions, limiting our ability to quantify intralesional variation within a given patient.
CONCLUSION
This first-in-humans study of 68Ga-NOTA-WL12, a low-molecular-weight peptide-derived imaging agent, demonstrates the feasibility and potential of quantifying PD-L1 levels in NSCLC with PET within a clinically viable time frame. 68Ga-NOTA-WL12 proved safe, with favorable biodistribution and radiation dose estimates similar to those of other radiopharmaceuticals. 68Ga-NOTA-WL12 uptake measures correlated with PD-L1 levels detected by immunohistochemistry, suggesting its suitability as a complementary diagnostic to immunohistochemistry to quantify PD-L1 levels for patient selection and therapeutic monitoring in anti-PD-L1 therapy.
DISCLOSURE
Financial support for this study was provided by the National Science and Technology Major Project (no. 2020ZX09201023), National Natural Science Foundation of China (81671733, 81871386, and 81871387), and Beijing Excellent Talents Funding (2017000021223ZK33). Sridhar Nimmagadda and Martin G. Pomper are supported by NIH 1R01CA236616 and NIH P41EB024495; they are coinventors on a pending U.S. patent covering WL12 and, as such, are entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. Sridhar Nimmagadda and Martin G. Pomper are consultants for Precision Molecular Inc., which has licensed the patent covering WL12 from Johns Hopkins University, and own equity in D&D Pharmatech, the parent company of Precision Molecular, Inc. No other potential conflict of interest relevant to this article was reported.
KEY POINTS.
QUESTION: Can the peptide-based radiotracer 68Ga-NOTA-WL12 detect and represent the expression levels of PD-L1 in patients with NSCLC?
PERTINENT FINDINGS: We demonstrated the feasibility and potential of 68Ga-NOTA-WL12 to quantify PD-L1 levels in preclinical models and in patients with NSCLC, with a strong relationship between tumor uptake and PD-L1 immunohistochemistry.
IMPLICATIONS FOR PATIENT CARE: 68Ga-NOTA-WL12 can be used to image PD-L1 in vivo, indicating its potential as a complementary diagnostic to immunohistochemistry to quantify PD-L1 levels for patient selection and therapeutic monitoring in anti-PD-L1 therapy.
REFERENCES
- 1. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 5. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20:1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Remon J, Passiglia F, Ahn MJ, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15:914–947. [DOI] [PubMed] [Google Scholar]
- 7. Rosner S, Reuss JE, Forde PM. PD-1 blockade in early-stage lung cancer. Annu Rev Med. 2019;70:425–435. [DOI] [PubMed] [Google Scholar]
- 8. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 9. Kluger HM, Zito CR, Turcu G, et al. PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res. 2017;23:4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi H, Longmire MR, Ogawa M, Choyke PL, Kawamoto S. Multiplexed imaging in cancer diagnosis: applications and future advances. Lancet Oncol. 2010;11:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9:4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xing Y, Chand G, Liu C, et al. Early phase I study of a 99mTc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer. J Nucl Med. 2019;60:1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donnelly DJ, Smith RA, Morin P, et al. Synthesis and biologic evaluation of a novel 18F-labeled adnectin as a PET radioligand for imaging PD-L1 expression. J Nucl Med. 2018;59:529–535. [DOI] [PubMed] [Google Scholar]
- 16. Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–1858. [DOI] [PubMed] [Google Scholar]
- 17. Heskamp S, Hobo W, Molkenboer-Kuenen JD, et al. Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies. Cancer Res. 2015;75:2928–2936. [DOI] [PubMed] [Google Scholar]
- 18. Huisman MC, Niemeijer AN, Windhorst AD, et al. Quantification of PD-L1 expression with 18F-BMS-986192 PET/CT in patients with advanced-stage non-small cell lung cancer. J Nucl Med. 2020;61:1455–1460. [DOI] [PubMed] [Google Scholar]
- 19. De Silva RA, Kumar D, Lisok A, et al. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol Pharm. 2018;15:3946–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar D, Lisok A, Dahmane E, et al. Peptide-based PET quantifies target engagement of PD-L1 therapeutics. J Clin Invest. 2019;129:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Food and Drug Administration. Dako PD-L1 IHC 22C3 pharmDx. September 2015. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013c.pdf. Accessed January 19, 2022.
- 22. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 23. Chatterjee S, Lesniak WG, Gabrielson M, et al. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget. 2016;7:10215–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 26. Lesniak WG, Mease RC, Chatterjee S, et al. Development of [18F]FPy-WL12 as a PD-L1 specific PET imaging peptide. Mol Imaging. 2019;18:1536012119852189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatterjee S, Lesniak WG, Miller MS, et al. Rapid PD-L1 detection in tumors with PET using a highly specific peptide. Biochem Biophys Res Commun. 2017;483:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects. Ann ICRP. 2012;41:1–322. [DOI] [PubMed] [Google Scholar]
- 29. Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–895. [PubMed] [Google Scholar]
- 30. Hofman P. PD-L1 immunohistochemistry for non-small cell lung carcinoma: which strategy should be adopted? Expert Rev Mol Diagn. 2017;17:1097–1108. [DOI] [PubMed] [Google Scholar]
- 31. Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019;30:1653–1659. [DOI] [PubMed] [Google Scholar]
- 32. Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics. 2016;6:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beer L, Hochmair M, Haug AR, et al. Comparison of RECIST, iRECIST, and PERCIST for the evaluation of response to PD-1/PD-L1 blockade therapy in patients with non-small cell lung cancer. Clin Nucl Med. 2019;44:535–543. [DOI] [PubMed] [Google Scholar]