Abstract
The ability of cecropin A to permeabilize and depolarize the membranes of Escherichia coli ML-35p bacteria has been compared to its bactericidal activity in an extension of earlier studies performed on synthetic lipid vesicle membranes (L. Silvestro, K. Gupta, J. H. Weiser, and P. H. Axelsen, Biochemistry 36:11452–11460, 1997). Our results indicate that differences in the concentration dependences of membrane permeabilization and depolarization seen in synthetic vesicles are not manifested in whole bacteria. The concentration dependences of both phenomena roughly correlate with bactericidal activity, suggesting that the bactericidal mechanism of cecropin A is related to membrane permeabilization.
Cecropin A is one of the most extensively studied antimicrobial polypeptides among the many that are produced by insects as components of their host defense systems against bacterial infection (6, 7, 14). While there is a broad consensus that the site of antibacterial action of these polypeptides is the plasma membrane, the precise mechanism—and the way in which they discriminate between bacterial and host cell membranes—remains unclear.
Studies of cecropin A and related peptides have shown that cecropin A is a linear 37-residue polypeptide composed entirely of ordinary l-amino acids (21). It is unstructured in aqueous solution but has the potential to form α-helices in partially organic solvent (8, 19). Early studies of their bactericidal mechanism suggested that cecropins bind to negatively charged membrane lipids and form a closely packed layer (20) or “carpet” of peptide (5, 15) which renders the membranes permeable. Later studies demonstrated that cecropins form partially selective ion channels (2). Evidence against a mechanism involving specific chiral receptors was provided by studies showing that analogues composed entirely of d-amino acids retained full activity (22). Models have been proposed for the ion channel formed by cecropins (3), and evidence is available indicating that they aggregate and assume a transbilayer orientation in membranes (12, 13). We have recently shown that the action of cecropin A on synthetic lipid vesicles is concentration dependent, forming ion channels at low peptide/lipid ratios and “pores” large enough to pass various probe molecules at higher peptide/lipid ratios (16). In that set of experiments we demonstrated that leakage of water-soluble probe molecules was “all or nothing,” supporting the idea that the pores through which the probes escape are stable, water-filled transmembrane conduits.
One limitation of many studies of antimicrobial peptide action is that they have been performed on synthetic lipid vesicles rather than bacteria. This is necessary in many cases to make particular types of studies feasible or interpretable. However, vesicle studies leave important questions open about the true nature of antimicrobial peptide action in a vastly more complex chemical milieu. Therefore, we have extended our earlier studies of cecropin A action on phospholipid vesicles to whole bacteria.
MATERIALS AND METHODS
Materials.
Cecropin A, with the sequence +KWKLFKKIEK VGQNIRDGII KAGPAVAVVG QATQIAK-CONH, was synthesized by standard solid-phase methods at the Mayo Peptide Core Facility (Rochester, Minn.). After purification by reversed-phase high-performance liquid chromatography (LC), it was confirmed to have a molecular weight of 4005 by mass spectrometry (MS). DiSC3(5) (3,3′-dipropylthiodicarbocyanine) was obtained from Molecular Probes (Eugene, Oreg.) and prepared as a stock solution of 2 mM in ethanol. EDTA and valinomycin were obtained from Fluka (Uppsala, Sweden). Melittin, ONPG (o-nitrophenyl-β-d-galactopyranoside), and Tris were obtained from Sigma (St. Louis, Mo.). Melittin was prepared as a 1-mg/ml stock solution in 10 mM Tris buffer. ONPG was prepared as a 25 mM stock in the same buffer and shielded from light during storage. Peptide concentrations were determined by a bicinchoninic acid assay (Pierce, Rockford, Ill.) with an albumin standard. Escherichia coli strain ML-35 (lacI lacY lacZ+) transformed with plasmid pBR322 (to yield ML-35p) (11) was a gift from Robert I. Lehrer (University of California—Los Angeles).
Bactericidal activity.
Bacteria were grown to mid-log phase at 37°C in Luria-Bertani medium, centrifuged (1,000 × g; 10 min), resuspended in buffer (150 mM Tris, pH 7), washed twice in 10 mM Tris, pH 7, and brought to 108 CFU/ml (assuming an optical density at 620 μm [OD620] of 0.35 corresponded to 108 CFU/ml). They were then diluted 10 times in 135 μl of 10 mM Tris buffer containing cecropin A (0 to 5 μM). After 10-min incubations at 25°C, the suspensions were placed on ice and diluted 10- to 10,000-fold in 10 mM Tris buffer, and 10-μl aliquots were spotted on Luria-Bertani agar plates for overnight culture at 30°C and manual counting of CFU with the assistance of a dissecting microscope.
Inner membrane integrity.
E. coli ML-35p constitutively expresses cytoplasmic β-galactosidase and periplasmic β-lactamase and is ampicillin resistant and lactose permease deficient (10, 11). The expression of β-lactamase is not relevant to these experiments, but the presence of β-galactosidase in the cytoplasm enables one to assess inner membrane integrity by immersing the cells in a solution of a chromogenic substrate. The organisms were grown to stationary phase at 37°C, centrifuged, and washed as described above and then diluted to an OD620 of 0.35 in 10 mM Tris buffer, pH 7. A 15-μl aliquot of the washed suspension was further diluted in 135 μl of 10 mM Tris buffer containing 2.5 mM ONPG and between 0 and 20 μM cecropin A in 96-well microtiter plates for a final volume of 150 μl per well. The hydrolysis of ONPG to o-nitrophenol (ONP) in each well was followed by measuring the absorbance at 415 nm at 1-min intervals for 60 min at 25°C with a Bio-Rad 550 plate reader. For positive and negative controls, melittin (23 μM) or DiSC3(5) (12 μM) was substituted for cecropin A.
Membrane depolarization.
Membrane depolarization measurements were performed with DiSC3(5), a lipophilic potentiometric dye that changes its fluorescence intensity in response to changes in transmembrane potential. Bacteria in mid-log phase were centrifuged (1,000 × g; 10 min), washed in 150 mM Tris, incubated with 1 mM EDTA in 150 mM Tris buffer (pH 7) for 15 min at 25°C, washed twice with 10 mM Tris (pH 7), and finally diluted to an OD620 of 0.35 in 10 mM Tris buffer (pH 7). A 100-μl aliquot of the bacterial suspension was added to 900 μl of the dilution buffer, followed by 1 μl of DiSC3(5) stock (17). The steady-state fluorescence intensity at 668 ± 1.7 nm was measured in a 4- by 10-mm stirred cell with a Fluorolog 2 spectrofluorimeter SPEX (Edison, N.J.) in a right-angle configuration and with excitation at 606 ± 1.7 nm. After allowing the dye signal to stabilize for about 4.5 min, 5 μl of a solution of cecropin A in 10 mM Tris at pH 7 was added. The concentration of the peptide solution added was adjusted to yield final concentrations between 0 and 5.0 μM. In experiments similar to those previously conducted with liposomes, we demonstrated that light scattering due to bacteria was negligible under these conditions (data not shown) (16). The level of ethanol from the DiSC3(5) stock solution did not exceed 0.1% in any experiment. Fluorescence intensities after various additions were volume corrected, and the results are expressed as a percentage change in signal intensity.
LC-MS.
To measure the concentration of cecropin A remaining free in solution during the membrane depolarization experiments (i.e., peptide that had not adsorbed to bacteria), a bacterial suspension with 2.5 μM cecropin A prepared as for a membrane depolarization experiment was centrifuged to produce a bacterium-free supernatant. A 10-μl aliquot of the supernatant along with a set of cecropin A standards was spiked with an internal standard (substance P) and separated via high-performance LC on a 1.0-mm by 25-cm Phenomenex (Torrance, Calif.) Primesphere C18 column operated at a flow rate of 400 μl/min, with a mobile phase of 0.05% trifluoroacetic acid in water and an acetonitrile gradient increasing from 18 to 45% over 30 min. The separation was monitored by a mass spectrometer (Quattro II; Micromass Inc., Beverly, Mass.) equipped with a coaxial electrospray probe and triple-quadrupole analyzer. The sampling-cone voltage was set to 40 V, the capillary voltage was set to 3.5 kV, and the source temperature was set to 65°C. The mass analyzer was set in single-ion recording mode for the analyte and internal-standard ions.
RESULTS
Bactericidal activity.
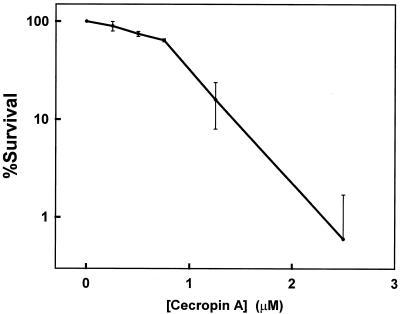
Cecropin A killed E. coli ML-35p quickly and at low concentrations. We observed 50 and 90% killing after 10-min incubations in 0.9 and 1.7 μM cecropin A, respectively (Fig. 1), and >99% killing after 10 min at 2.5 μM. These concentrations are comparable to the antimicrobial activity we reported previously for cecropin A against K-1 and K-12 strains of E. coli (16). Control experiments showed that 23 μM melittin was >99.9% lethal but that 12 μM DiSC3(5) was not toxic.
FIG. 1.
Viability of E. coli ML-35p bacteria after a 10-min incubation with cecropin A. The error bars represent ± 1 standard deviation for an n of 3.
Inner membrane integrity.
Permeabilization of the inner membrane of E. coli strain ML-35p was followed by adding a suspension of bacteria to a buffer containing ONPG and cecropin A and measuring the absorbance of ONP at 415 nm versus time. ONP is produced by the action of cytoplasmic β-galactosidase on ONPG. ONPG does not penetrate the inner membranes of intact bacteria and remains extracellular because E. coli strain ML-35p is deficient in lactose permease. Agents that compromise inner membrane integrity permit ONPG to diffuse into the bacterial cell, where it is hydrolyzed to ONP.
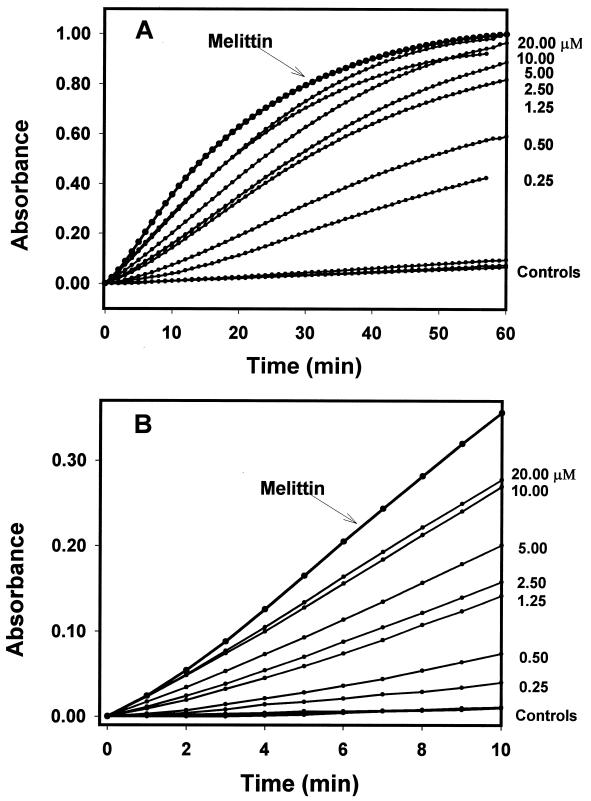
The addition of bacterial suspensions to a solution of cecropin A and ONPG caused [ONP] (i.e., the concentration of ONP) to rise and plateau (Fig. 2A). This plateau represents substrate depletion, because the same amount of ONPG substrate was present in each experiment. The addition of bacteria to 23 μM melittin caused this plateau to be reached faster than the highest concentration of cecropin A used (20 μM), while control substances [including DiSC3(5)] did not accelerate ONP production beyond baseline levels. Centrifugation of a bacterial suspension treated for 20 min with 2.5 or 5.0 μM cecropin A, or 23 μM melittin, resulted in a supernatant devoid of measurable β-galactosidase activity, indicating that these treatments did not release β-galactosidase from bacterial cells into the medium. Neither valinomycin nor DiSC3(5) (used as described below for membrane depolarization studies) permeabilized the membrane enough to result in measurable ONP production.
FIG. 2.
ONP production kinetics. Each datum point represents an average of three independent trials. Experimental conditions were chosen so that complete hydrolysis of ONPG yielded an absorbance of 1.0. Valinomycin and DiSC3(5) resulted in ONP levels indistinguishable from those of the buffer control; melittin produced ONP faster than 20 μM cecropin A. (A) Results for 60 min. (B) Results for the first 10 min.
We analyzed these data assuming that there are three basic processes influencing d[ONP]/dt, the rate of ONP production at any given concentration of cecropin A. First, simple hydrolysis of ONPG produces ONP at a constant rate, i.e., d2[ONP]/dt2 = 0. Second, permeabilization increases ONP production only insofar as it is a rate-limiting process. As the membrane is permeabilized, the opportunity for β-galactosidase to act on ONPG increases, and d2[ONP]/dt2 > 0. Third, substrate depletion decreases the rate of ONP production and yields a d2[ONP]/dt2 value of <0.
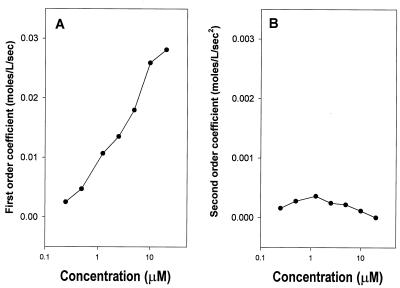
To simplify our analysis, we analyzed only the first 10 min (after a 1-min mixing period) to eliminate the influence of substrate depletion on the rate of ONP production (Fig. 2B). The concentration of ONP over this interval, which is proportional to its absorbance [A(t)], is well described by a second-order polynomial: [ONP]t = k · A(t)415 = at2 + bt + c, where k is a scaling constant. The rate of ONP production is therefore given by the equation d[ONP]/dt = 2at + b, and changes in rate are given by the equation d2[ONP]/dt2 = 2a. Plots of the second- and first-order coefficients (a and b) recovered versus cecropin A concentration are shown in Fig. 3.
FIG. 3.
First- (A) and second (B)-order coefficients for polynomials describing the rate of ONP production shown in Fig. 2B. The vertical axes are scaled so that points at identical positions in both graphs would make equal quantitative contributions to the amount of ONP produced at 10 min.
The second-order coefficient a (Fig. 3B) and the constant c (not shown) are virtually 0 over the range of cecropin A concentrations examined. This indicates that membrane permeabilization is fast on the time scale of these measurements and is not a rate-limiting process over this time period. The rate of ONP production appears to be a first-order process described by the coefficient b, and this coefficient is linearly related to the log of the peptide concentration. This relationship between b and peptide concentration suggests that a process other than peptide-induced permeability limits the rate of ONPG hydrolysis, e.g., substrate diffusion. If b was an exponential function of [cecropin A], this would suggest that several cecropin A molecules were required to form a structure that permeabilized the membrane. This may be the case at lower concentrations and over shorter times. On the time and concentration scales of these experiments, however, the linear dependence of b on the logarithm of [cecropin A] indicates that progressively smaller fractions of cecropin A are involved in permeabilizing the membrane as [cecropin A] increases.
Membrane depolarization.
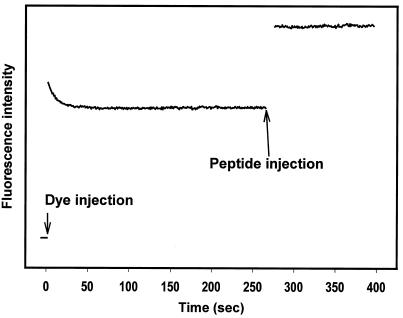
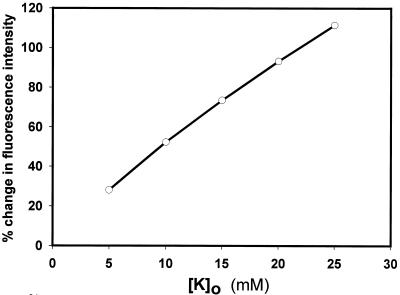
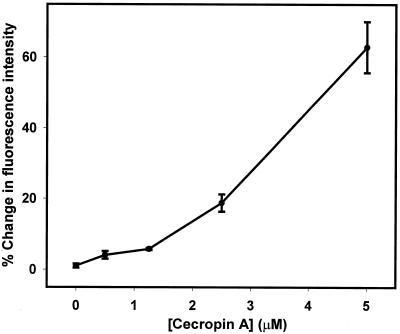
Cecropin A-induced changes in the bacterial membrane potential were measured with the potentiometric dye DiSC3(5). Upon addition to a suspension of bacterial cells, DiSC3(5) partitions into the cell membranes and equilibrates within 20 s (Fig. 4). Cecropin A was added approximately 4 min after the fluorescence emission intensity stabilized. This caused an immediate increase in fluorescence intensity, indicating rapid membrane depolarization (Fig. 4). The dye response in whole bacteria was further characterized by adjusting external potassium ion concentrations in the presence of valinomycin. This demonstrated that some depolarization occurred with 5 mM external potassium (implying that the internal potassium concentration is somewhat lower) and that 25 mM potassium could be expected to cause a greater-than-100% increase in fluorescence intensity (Fig. 5). In response to cecropin A, depolarization was minimal at concentrations of ≤1.25 μM, but 5 μM peptide caused a >60% increase (Fig. 6).
FIG. 4.
Fluorescence intensity of DiSC3(5) in E. coli. Increases and decreases in fluorescence intensity represent membrane depolarization and hyperpolarization, respectively. After the addition of DiSC3(5) to the bacterial suspension, equilibration is nearly complete within 30 s. Data collection was stopped for 10 s while dye and cecropin A were added so that injection artifacts would not be seen.
FIG. 5.
Fluorescence intensity changes of DiSC3(5) in E. coli in the presence of valinomycin and various external potassium ion concentrations, showing that depolarization by elevated external potassium can result in a fluorescence intensity increase of over 100%.
FIG. 6.
Fluorescence intensity changes of DiSC3(5) in E. coli upon exposure to cecropin A. The error bars represent standard deviations.
Experimental constraints required that cecropin A be added as a small volume of concentrated stock solution to a much larger volume of bacterial suspension. This transiently exposed some bacteria in the suspension to concentrations much higher than the nominal concentration, and those bacteria may have absorbed enough peptide to make the actual concentration of free peptide lower than the nominal concentration. Therefore, bacterial suspensions prepared as for a membrane depolarization experiment with a nominal peptide concentration of 2.5 μM were centrifuged for 10 min at 1,000 × g. The supernatant was assayed for cecropin A by LC-MS with substance P as an internal standard and a set of cecropin A solutions in buffer as calibration and retention time standards. This experiment found 1.4 μM cecropin A remaining in the supernatant—a concentration sufficient to kill at least 80% of the bacteria present.
DISCUSSION
Two technical considerations bear critically on the interpretation of these results. First, we measured the bactericidal activity of cecropin A by using a survival assay in liquid medium. This was the most appropriate measurement because our purpose was to compare antimicrobial activity to the concentrations required to permeabilize the inner bacterial membrane. Because permeabilization is most likely a lethal rather than inhibitory event, MICs derived from growth inhibition assays were not suitable for our purpose. Furthermore, published methods for determining MICs for cecropin A appear to depend on assumptions about peptide diffusion in agar or agarose (9), and the results vary with the technique and materials used (1).
Second, inner membrane integrity was measured by adding aliquots of a bacterial suspension to a known concentration of peptide. This approach avoided the exposure of some bacteria to high concentrations of peptide that occurs when aliquots of a concentrated peptide solution are added to a bacterial suspension. The same approach was used when measuring bactericidal activity, but for technical reasons this approach was not possible when measuring changes in membrane potential.
These technical considerations are relevant to a comparison of the bactericidal and the permeabilizing activities of cecropin A (Fig. 1 and 3). The threshold concentrations for the onset of both activities are similar and on the order of 0.25 μM. By itself, this suggests that the bactericidal activity of cecropin A is related to its permeabilizing activity. However, bactericidal activity was maximal at 2.5 μM peptide, while this concentration yielded only a half-maximal rate of ONP production. Thus, permeabilizing activity lagged behind bactericidal activity as the peptide concentration was increased.
The simplest explanation for this lag is that any permeabilization of the membrane is lethal but that the hydrolytic capacity of cellular β-galactosidase exceeded the amount of ONPG that entered the cells through the membrane permeabilized by 2.5 μM peptide. The enzyme was unlikely to be saturated with substrate, since its extracellular concentration (0.7 μM) was the same as the Km of β-galactosidase at low magnesium concentrations (18). Thus, higher concentrations of peptide can increase the rate of ONPG hydrolysis by increasing the extent to which the membrane is permeabilized. They would not, however, be any more effective at killing bacteria. It remains entirely possible that bactericidal activity and permeabilizing activity are not directly related (as demonstrated recently for another cationic antimicrobial peptide [4]), but a close relationship between these activities is suggested by their similar thresholds of activity. This contrasts sharply with our earlier studies of lipid vesicles, which indicated that much higher concentrations of cecropin A were needed to cause permeability changes (16).
We could not measure the depolarizing activity of cecropin A by the same technique used to measure bactericidal or permeabilizing activity because the intensity of DiSC3(5) depends on its partitioning across a membrane. As a consequence, injecting DiSC3(5)-labeled bacteria into a known concentration of peptide (as we did to measure bactericidal and permeabilizing activities) dilutes extracellular DiSC3(5) and causes changes in its signal intensity that are not related in a simple way to dilution or changes in membrane potential. For this reason, we elected to add small volumes of concentrated cecropin A to a bacterial suspension equilibrated with DiSC3(5).
Therefore, the results of these membrane depolarization studies must be interpreted cautiously. They are most remarkable for what did not happen: a nominal peptide concentration of 1.4 μM is >80% effective in a bactericidal assay (Fig. 1), but a free concentration of 1.4 μM did not even come close to fully depolarizing bacterial membranes. While a rigorous quantitative comparison cannot be made because of differences in technique, Fig. 3, 5, and 6 suggest that the amount of depolarization is roughly comparable to the limited level of ONP production expected at the same concentration. This again contrasts sharply with our earlier lipid vesicle experiments, in which permeability changes required much higher concentrations of peptide than did membrane potential changes (16).
Our ability to establish the bactericidal mechanism of cecropin A by comparing the time dependences of various phenomena is limited because the time dependences of the three assays we have employed in this study (survival, permeability, and potential change) are all fundamentally different. The effect of cecropin A on inner membrane integrity over a 10-min period may not reflect peptide-induced lethal effects that persist after the bacteria have been exposed to peptide for a 10-min period. On the other hand, the concentration dependence of depolarization and permeabilization in whole bacteria is roughly similar to the concentration dependence of bactericidal activity, suggesting that both bactericidal activity and membrane depolarization may be consequences of membrane permeabilization. These results are in sharp contrast to the concentration dependence we had previously observed in lipid vesicles (16), and they underscore the difficulties of drawing conclusions about bactericidal mechanisms from studies of synthetic membranes.
In other antimicrobial peptides it has been possible to manipulate antimicrobial activity by means of experimental conditions and to show that it is independent of membrane-permeabilizing activity, leading some investigators to the critical suggestion that the site of action may not be the cytoplasmic membrane (23, 24). This may eventually prove to be the case with cecropin A as well, although our data neither support nor refute this possibility. This conclusion awaits a demonstration that the antimicrobial and membrane-permeabilizing activities of cecropin A can be manipulated separately by means of experimental conditions. In the meantime, the thrust of the results presented here indicates that these activities exhibit similar concentration dependences in whole bacteria and that differences in the concentration dependences of membrane permeabilization and depolarization observed in synthetic vesicles are not manifested in whole bacteria.
ACKNOWLEDGMENTS
P.H.A. is supported by NIH grant GM54617 and a grant-in-aid from the American Heart Association.
We are grateful to Elena Lysenko, Misha Shchepetov, Miki Kapoor, Jane Gould, Jean Kim, and Nina Pan for assistance with bacterial cell culture; to Robert I. Lehrer and Yoon Cho for E. coli ML-35p bacteria; and to participants in the Second Gordon Research Conference on Antimicrobial Peptides for helpful discussions.
REFERENCES
- 1.Boman H G, Wade D, Boman I A, Wahlin B, Merrifield R B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 2.Christensen B, Fink J, Merrifield R B, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durell S R, Raghunathan G, Guy H R. Modeling the ion channel structure of cecropin. Biophys J. 1992;63:1623–1631. doi: 10.1016/S0006-3495(92)81730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedrich C, Scott M G, Karunaratne N, Yan H, Hancock R E W. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob Agents Chemother. 1999;43:1542–1548. doi: 10.1128/aac.43.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazit E, Boman A, Boman H G, Shai Y. Interaction of mammalian antibacterial cecropin P1 with phospholipid vesicles. Biochemistry. 1995;34:11479–11488. doi: 10.1021/bi00036a021. [DOI] [PubMed] [Google Scholar]
- 6.Hancock R E W, Chapple D S. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann J A. Innate immunity of insects. Curr Opin Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 8.Holak T A, Engstrom A, Kraulis P J, Lindeberg G, Bennich H, Jones T A, Gronenborn A M, Clore G M. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988;27:7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- 9.Hultmark D, Engström Å, Andersson K, Steiner H, Bennich H, Boman H G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2:571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrer R I, Barton A, Daher K A, Harwig S S L, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli. J Clin Investig. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehrer R I, Barton A, Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple- wavelength spectrophotometry. J Immunol Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- 12.Mchaourab H S, Hyde J S, Feix J B. Aggregation state of spin-labeled cecropin AD in solution. Biochemistry. 1993;32:11895–11902. doi: 10.1021/bi00095a019. [DOI] [PubMed] [Google Scholar]
- 13.Mchaourab H S, Hyde J S, Feix J B. Binding and state of aggregation of spin-labeled cecropin AD in phospholipid bilayers: effects of surface charge and fatty acyl chain length. Biochemistry. 1994;33:6691–6699. doi: 10.1021/bi00187a040. [DOI] [PubMed] [Google Scholar]
- 14.Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47:451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry. 1992;31:12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- 16.Silvestro L, Gupta K, Weiser J N, Axelsen P H. The concentration-dependent membrane activity of cecropin A. Biochemistry. 1997;36:11452–11460. doi: 10.1021/bi9630826. [DOI] [PubMed] [Google Scholar]
- 17.Sims P J, Waggoner A S, Wang C H, Hoffman J F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- 18.Sinnott M L, Withers S G. The necessity of magnesium cation for acid assistance aglycone departure in catalysis by Escherichia coli (lacZ) beta-galactosidase. Biochem J. 1978;175:539–546. doi: 10.1042/bj1750539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner H. Secondary structure of the cecropins: antibacterial peptides from the moth Hyalophora cecropia. FEBS Lett. 1982;137:283–287. doi: 10.1016/0014-5793(82)80368-2. [DOI] [PubMed] [Google Scholar]
- 20.Steiner H, Andreu D, Merrifield R B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988;939:260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- 21.Steiner H, Hultmark D, Engström Å, Bennich H, Boman H G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 22.Wade D, Boman A, Wåhlin B, Drain C M, Andreu D, Boman H G, Merrifield R B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci USA. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M H, Hancock R E W. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Wu M H, Maier E, Benz R, Hancock R E W. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]