Abstract
Background:
The advent of direct-acting antiviral therapy for Hepatitis C (HCV) has made using HCV-viremic donors a viable strategy to address the donor shortage in heart transplantation. We employed a large-scale simulation to evaluate the impact and cost-effectiveness of using Hepatitis C-viremic donors for heart transplant.
Methods:
We simulated detailed histories from time of listing until death for the real-world cohort of all adults listed for heart transplant in the United States from July 2014 to June 2019 (n = 19,346). These populations were imputed using historical data and capture “real-world” heterogeneity in geographic and clinical characteristics. We estimated the impact of an intervention in which all candidates accept HCV+ potential donors (n = 472) on transplant volume, waitlist outcomes, and lifetime costs and quality-adjusted life years (QALYs).
Results:
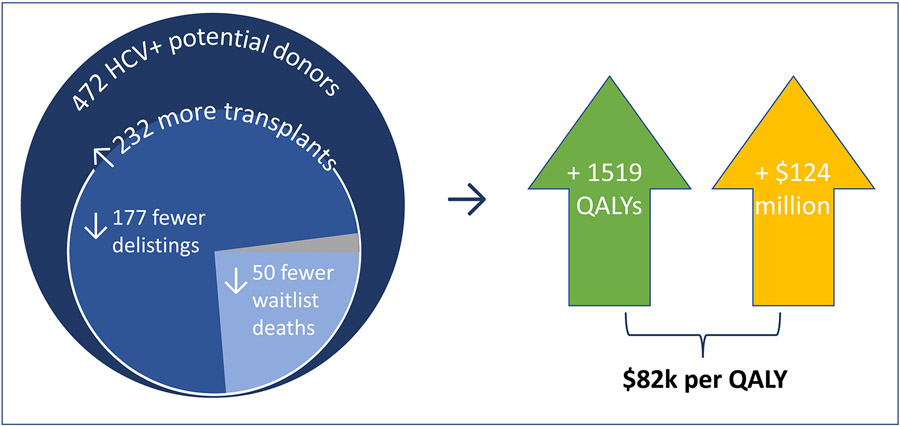
The intervention produced 232 more transplants, 132 fewer delistings due to deterioration, and 50 fewer waitlist deaths within this five-year cohort and reduced wait times by 3-11% (varying by priority status). The intervention was cost-effective, adding an average of 0.08 QALYs per patient at a cost of $124 million ($81,892 per QALY). DAA therapy and HCV care combined account for 11% this cost, with the remainder due to higher costs of transplant procedures and routine post-transplant care. The impact on transplant volume varied by blood type and region and was correlated with donor-to-candidate ratio (ρ = 0.71).
Conclusions:
Transplanting HCV+ donor hearts is likely to be cost-effective and improve waitlist outcomes, particularly in regions and subgroups experiencing high donor scarcity.
Introduction
Demand for heart transplant in the United States has outpaced the supply of donors, prompting various proposed strategies to expand the donor pool.1-4 Direct acting antiviral (DAA) therapies against Hepatitis C have cure rates of ~95%,5 which makes transplanting hearts from HCV-viremic (HCV+) donors into HCV-negative recipients a tenable option. Single-center trials suggest this strategy is safe and effective,6-11 but it involves a trade-off; a patient willing to accept an HCV+ donor may receive transplant sooner, but with the potential costs and morbidity associated with DAA therapy and chronic HCV infection.
A prior study estimates that, from the standpoint of an individual patient, these benefits outweigh the costs and HCV+ donor heart transplant is cost-effective.12 HCV+ donors are increasingly utilized nationwide, but with significant variation by region and program.13,14 Before recommending universal adoption of this strategy, one must also consider the impact on a system-wide scale - noting that this impact would extend far beyond the minority of individuals who receive an HCV+ heart. Increased utilization of HCV+ donor hearts may lead to decreased organ scarcity and overall shorter wait times.11 The extent to which this “indirect” benefit augments the impact and cost-effectiveness of HCV+ donor utilization is unknown.
Our first aim was to assess the impact of universal acceptance of HCV+ donor hearts on waitlist outcomes and overall survival in the US. Second, we estimated the cost-effectiveness of universal HCV+ donor acceptance, accounting for both “direct” and “indirect” benefits as characterized above. Third, we assessed the cost-effectiveness and impact of intermediate strategies in which only patient subgroups utilize HCV+ donors.
Methods
Overview
We used data from the Scientific Registry of Transplant Recipients (SRTR) to characterize our analytic cohort, which consists of adults (> 18 years at time of listing) newly listed for heart transplant in the US from July 2014 through June 2019. Patients listed for multiple organ transplant were excluded. HCV+ donor utilization strategies were evaluated using a modified version of the Thoracic Simulated Allocation Model (TSAM), which has been described and validated previously.15,16 The model and study population are further described in Supplementary Appendix (SI, S2) and Figure 1.
Figure 1. Model schematic showing simulated patient history from time of listing until death on waitlist, delisting, or transplant.
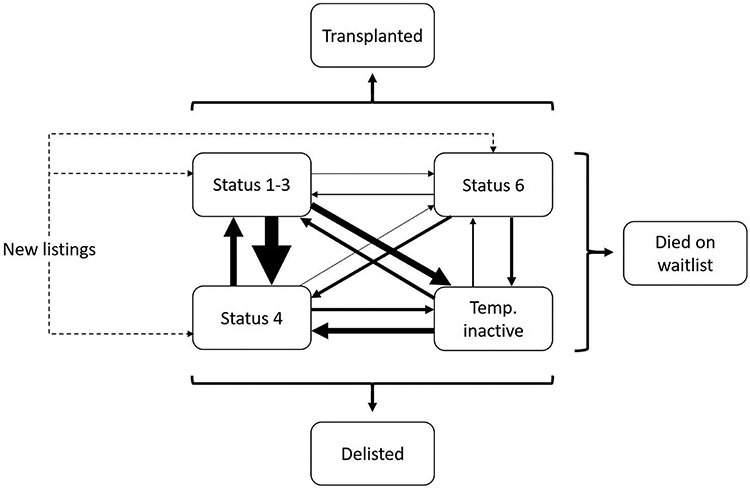
The caliber of solid lines connecting waitlist states (Status 1-3, Status 4, Status 6, and temporarily inactive) is proportional to the corresponding transition probabilities. Concurrently, patients in any waitlist state can transition to the terminal states “delisted” or “died on waitlist”, with probabilities varying by waitlist state. Transitions to "transplanted" are possible for all waitlist states except for “temporarily inactive”. The probability of transitioning to “transplanted” is endogenous to the model and will vary by waitlist state, by individual patient characteristics, and by the availability of acceptable donors (which in turn varies by intervention/policy scenario). Transition probabilities between states are detailed in Table S1.
Analyses
We compared a “primary intervention” in which all patients on the waitlist are willing to accept HCV+ donor hearts with a “control” scenario in which none are willing to accept HCV+ donors. We also evaluated intermediate strategies in which 1) only patients of higher priority status at listing (Status 1-3) accept HCV+ donors and 2) only type O candidates – who face the longest wait times (median 17 months) (2) - accept HCV+ donors. We performed multiple complete simulations for each scenario and resulting outcome estimates represent an average across all iterations. The number of simulations was determined using a pre-specified “stopping rule” detailed in Supplementary Appendix (S9).
We tested the sensitivity of the cost-effectiveness estimates for the primary intervention to assumptions for all input parameters. The plausible range for each was informed by prior studies as detailed in Table S5. We performed a probabilistic sensitivity analysis in which all input parameters varied simultaneously. We also evaluated the cost-effectiveness of the primary intervention under alternative scenarios in which, compared to base case assumptions, waitlist outcomes are improved, the uptake of the intervention is incomplete, and recipients of HCV+ donors experience an 50% absolute increase in the probability of acute cellular rejection, as suggested by a prior study.17 These and other sensitivity analyses are further detailed in Supplementary Appendix (S9).
Outcomes
For the analytic cohort, we calculated transplant rates, expected wait times, and numbers of waitlist deaths, delistings, and transplants, stratifying each by status at listing. Additional metrics included projected waitlist size (on June 30, 2024) and number of new chronic HCV infections, defined as the presence of donor-derived HCV viremia beyond 6 months post-transplant, despite treatment with initial and salvage courses of DAA therapy. We attached health utilities to each disease state and estimated quality-adjusted life years (QALYs) and costs (from time of listing until death) for patients in the analytic cohort. We also stratified QALYs and costs by disease state and setting.
Incremental cost-effectiveness ratios (ICERs) were calculated to compare policy strategies. Costs (in 2018 US dollars) and QALYs were discounted at a rate of 3% per year. We defined cost-effective or “efficient” strategies as those with an ICER of less than $100,000 per QALY.18
Results
Population characteristics
Characteristics of the patient and donor populations are detailed in Table 2. Our analytic cohort included 19,346 candidates newly listed for transplant from July 2014 through June 2019 (an average of 3869 per year). HCV-viremia prevalence was 2.8% (n=472) over the first five years of the model (July 2014 – June 2019), increasing from 0.7% in year 1 to 5.8% in year 5; these figures reflect observed prevalence (by nucleic acid amplification testing) among potential donors in SRTR over the same time period. By assumption, HCV-viremia prevalence remained constant at 4.8% thereafter (July 2019 – June 2024). HCV-viremia prevalence among potential donors by blood type was lowest for type AB (2.3%), highest for type O (3.1%), and near average for type A (2.6%) and type B (2.8%). UNOS regions varied significantly in terms of HCV prevalence (0.9% to 7.7%) and the ratio of candidates to potential donor hearts (0.9 to 2.1), as detailed in Figure S1. HCV+ donors were more likely than average to have age under 50, white race, and history of smoking or cocaine use.
Table 2.
Characteristics of the potential donor and heart transplant candidate populations and intervention impact by subgroup (July 2014 - June 2019)
| Variable | All potential donors* (n= 16520) |
All HCV- viremic potential donors* (n= 472) |
All newly listed HT candidates** (n= 19346) |
% increase in transplants in intervention vs. control scenario by subgroup† |
|---|---|---|---|---|
| Age > 50 years (%) | 6.2% | 2.3% | 65.2% | 1.9% |
| Race (%) | ||||
| White | 62.6% | 83.1% | 62.8% | 1.9% |
| Black | 17.0% | 7.2% | 23.7% | 1.9% |
| Hispanic | 16.3% | 7.4% | 8.9% | 1.6% |
| Asian / Pacific Islander | 2.0% | 0.2% | 3.8% | 1.2% |
| Other | 2.1% | 2.1% | 0.7% | 4.1% |
| Female (%) | 27.3% | 29.9% | 26.5% | 1.6% |
| Blood Type (%) | ||||
| A | 35.7% | 32.6% | 36.9% | 1.3% |
| B | 11.1% | 10.8% | 14.4% | 1.3% |
| AB | 2.4% | 1.9% | 4.7% | 0.8% |
| O | 50.8% | 54.7% | 43.9% | 2.8% |
| UNOS region (%) | ||||
| 1 | 3.6% | 9.7% | 4.7% | 6.1% |
| 2 | 11.2% | 22.5% | 10.9% | 2.5% |
| 3 | 15.2% | 13.1% | 11.9% | 1.1% |
| 4 | 11.0% | 3.8% | 11.0% | 1.2% |
| 5 | 16.3% | 8.7% | 15.3% | 0.5% |
| 6 | 4.0% | 3.2% | 3.2% | 0.8% |
| 7 | 7.5% | 3.0% | 9.2% | 2.3% |
| 8 | 7.3% | 2.3% | 6.1% | 0.6% |
| 9 | 3.7% | 3.2% | 6.6% | 4.3% |
| 10 | 8.6% | 12.1% | 7.8% | 1.8% |
| 11 | 11.4% | 18.4% | 12.0% | 2.6% |
| Status at listing (%) | ||||
| 1-3 | 27.4% | 1.6% | ||
| 4 | 43.2% | 1.8% | ||
| 6 | 26.0% | 2.4% | ||
| Temporarily inactive | 3.4% | 2.2% | ||
| Donor risk factors | ||||
| Diabetes Mellitus | 3.2% | 2.1% | ||
| Smoking (past or current) | 9.2% | 17.2% | ||
| Cocaine use (past or current0 | 20.6% | 55.3% | ||
| CMV-seropositive | 59.2% | 57.0% | ||
| Cause of death: Anoxia | 37.6% | 73.3% | ||
| Cause of death: Cerebrovascular | 14.6% | 6.4% | ||
| Positive Hepatitis B core antibody | 1.6% | 5.7% | ||
| CDC “increased risk”‡ | 27.8% | 87.9% |
Includes potential donors available from July 2014 through June 2019, defined as those used for transplant in real-life and unused donors with the absence of selected comorbidities (further detailed in Supplementary Appendix (S2)). Applying these exclusion criteria resulted in a potential donor sample with very low (<1%) rates of other comorbidities not listed above (e.g. prior myocardial infarction, reduced left ventricular ejection fraction) and none with HIV infection or Hepatitis B viremia.
Includes patients newly listed for transplant from July 2014 through June 2019
Intervention scenario: all patients are willing to accept HCV+ donors; control scenario: no patients are willing to accept HCV+ donors (see Methods for details). Calculated by comparing average transplant volume across all model runs of the intervention scenario with average transplant volume across model runs of the control scenario.
Per Centers for Disease Control (CDC) criteria, defined by the presence of one or more social behaviors (e.g. intravenous drug use) designated as high-risk for HIV
Bold/italics indicates a significant (p < 0.05) difference as assessed by z-score, when comparing 1) % increase in transplants by subgroup vs. % increase across the entire recipient population or 2) prevalence of each variable among HCV-viremic donors with prevalence across the entire donor population.
CDC: Centers for Disease Control; CMV: cytomegalovirus; HCV: Hepatitis C; HIV: Human Immunodeficiency Virus; HT: Heart Transplant; UNOS: United Network for Organ Sharing
System-wide impact
We performed 10 model runs for all intervention and control scenarios, per pre-specified stopping rules. The primary intervention (all patients accept HCV+ donors) resulted in an additional 232 transplants (a 1.8% increase) among patients in our five-year analytic cohort compared to control. This net increase was composed of 325 additional transplants using HCV+ donors (2.5% of total) and 103 fewer transplants using HCV- donors. The intervention produced an estimated 0.65 new chronic HCV infections per year.
Waitlist deaths, delistings due to deterioration, and total delistings (due to deterioration or improvement) each decreased by 3-4% in the intervention (vs. control) scenario. Expected waiting times decreased by one month among patients at Status 4 and by 7.2 months in Status 6, with only a marginal change (0.1 months) in Status 1-3. Among the (on average) 3869 patients added to the waitlist annually from July 2014 through June 2024, 2904 remained on the waitlist at the end of this period in the intervention scenario – a 7.5% decrease compared to the control scenario (n = 3138). System-wide impact metrics are summarized in Figure 2 and detailed in Table 3.
Figure 2. Summary of outcomes in primary intervention (all patients accept HCV+ donors) compared to control (no patients accept HCV+ donors) scenarios.
Outcomes and costs include those accrued by all patients listed for transplant during a five-year period (July 2014 – June 2019). The shaded grey area represents five fewer patients remaining on the heart transplant waitlist at the end of the model’s time horizon (June 2024).
Table 3. System-wide impact metrics in the primary intervention (all accept HCV+ donors) vs. control scenario (none accept HCV+ donors).
Each outcome represents the number of each event occurring among patients listed during a five-year year period (July 2014 – June 2019) averaged across 10 model runs.
| Outcome | Intervention (all accept HCV+ donors) |
Control (none accept HCV+ donors) |
Difference |
|---|---|---|---|
| Transplants | |||
| Status 1-3 | 6,344 | 6,354 | −10 |
| Status 4 | 6,197 | 5,997 | 200 |
| Status 6 | 452 | 411 | 42 |
| Total | 12,994 | 12,762 | 232 |
| # HCV+ donor transplants | 325 | - | 325 |
| Transplant rate per 100 waitlist years (Expected wait time, months) * | |||
| Status 1-3 | 463 (2.6) | 451 (2.7) | 11.4 (0.1) |
| Status 4 | 86 (14.1) | 81 (15.1) | 5.5 (1.0) |
| Status 6 | 20 (62.1) | 18 (69.3) | 2.0 (7.2) |
| Status 1-6 (combined) | 120 (10.2) | 114 (10.7) | 5.4 (0.5) |
| Waitlist deaths | 1,478 | 1,528 | −50 |
| Delistings | 4,823 | 5,000 | −177 |
| Delistings due to deterioration | 3,174 | 3,306 | −132 |
| Waitlist deaths + delistings due to deterioration | 4,652 | 4,834 | −182 |
Transplant rate calculated as: (# of transplants among patients at status x) divided by (aggregate # of patient years spent at status x). Expected wait time at status x represents the expected time to transplant for a hypothetical patient who is fixed at status x.
HCV: Hepatitis C
The impact of the intervention – measured as a percentage increase in transplant volume – is shown in Table 2. It varied significantly by blood type and region, but not significantly by priority status at listing or demographic characteristics. The impact was above average for patients of blood type O and below average for other blood types. Impact ranged from 0.5% in UNOS Region 5 to 6.1% in Region 1 and was correlated with region-specific HCV+ prevalence (ρ = 0.78) and candidate-to-donor ratio (ρ = 0.71).
Cost-effectiveness
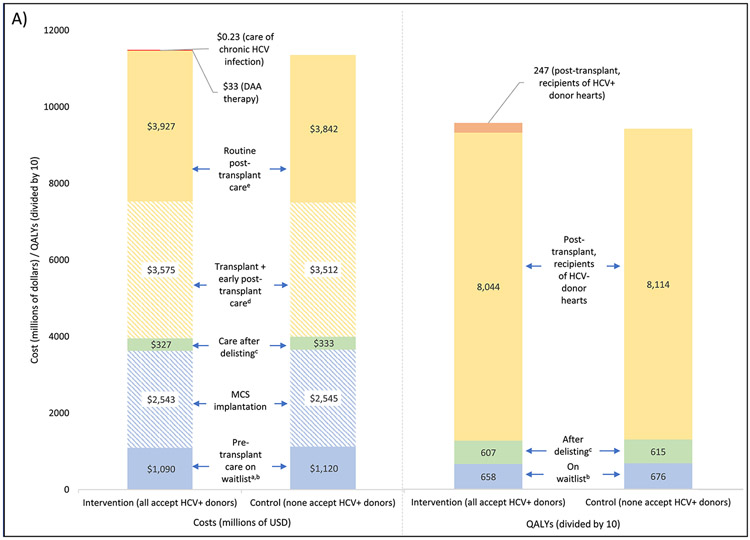
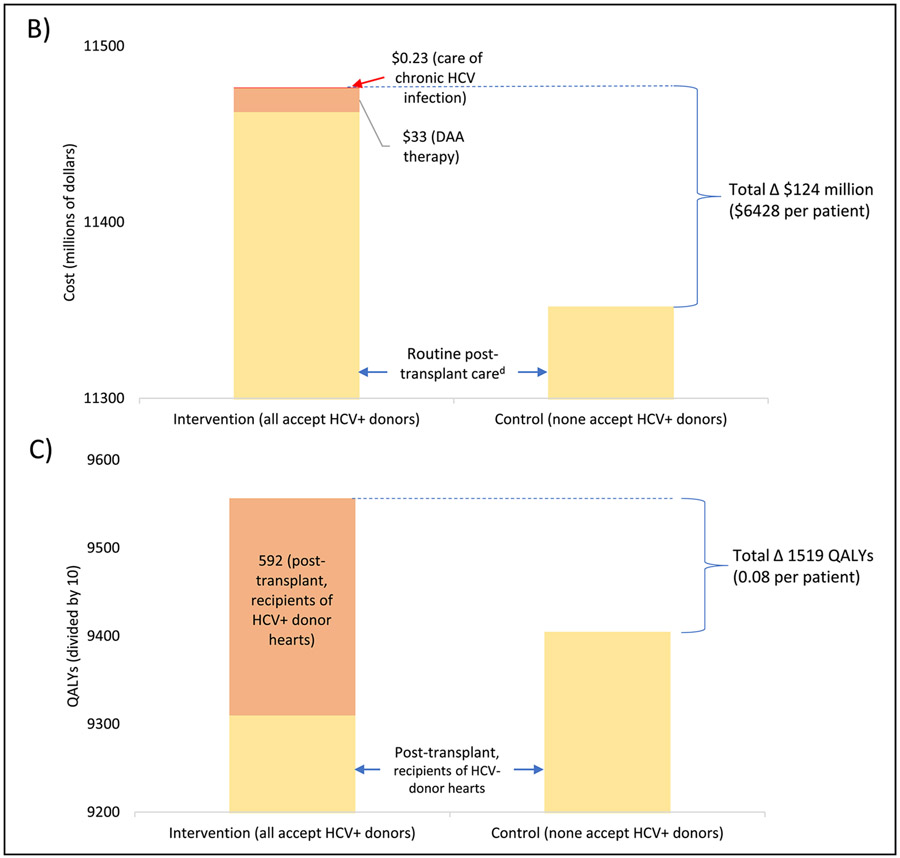
A breakdown of total costs and QALYs by disease state and setting is shown in Figure 3. Within the five-year analytic cohort, total costs (from listing until death) were $11.48 billion in the primary intervention and $11.35 billion in the control scenario. The difference ($124 million) was mainly due to higher expenditures on transplant (increased by $63 million) and routine post-transplant care (increased by $85 million) unrelated to HCV. These differences were partly offset by reductions in the costs of pre-transplant care (decreased by $30 million), MCS implantation (decreased by $2.3 million), and care after delisting (decreased by $5.8 million). Costs attributable to HCV (only present in the intervention scenario) included $13.7 million for DAA therapy and $229,834 for chronic HCV care; together these accounted for just 0.12% of total costs and 11.2% of the difference between intervention and control.
Figure 3. Distribution of total costs and quality-adjusted life years (QALYs) in intervention (all accept HCV+ donors) and control (none accept HCV+ donors) scenarios.
Costs are expressed in millions of US dollars and QALYs are expressed in tens of QALYs (to allow for a consistent axis for both charts). Cost and QALYs estimates are based on a cohort of patients listed for transplant between July 2014 and June 2019 and discounted at a rate of 3% per year (see Methods for details); both are tabulated from the date of listing until death. Complete costs/QALYs across all categories are shown in panel (A). A smaller segment of this chart is shown in panels (B) and (C) to better detail differences between the two scenarios.
a excludes one-time cost of MCS implantation
b includes patients who are temporarily delisted
c refers to patients who are delisted without undergoing transplant
d excludes cost of DAA therapy
e excludes care associated with HCV infection and its sequelae
DAA: direct acting antiviral; HCV: Hepatitis C; MCS: mechanical circulatory support; QALY: quality-adjusted life year
Total QALYs were 95,565 in the primary intervention and 94,047 in the control scenario, a difference of 1519. Recipients of HCV+ donors contributed 3.0% of post-transplant QALYs. On an average per patient basis, the primary intervention led to 0.08 additional QALYs at a cost of $6428, with a resulting ICER of $81,892 per QALY.
Comparison of policy strategies
We compared the primary intervention (“all accept”) with intermediate strategies in which HCV+ donor utilization is limited to status 1-3 candidates (“status 1-3 only”) and blood type O candidates (“type O only”); total costs and QALYs for each are displayed in Figure 4. Both were cost-effective when compared to control scenario, with ICERs of $85,482 and $82,235 per QALY, respectively. Moving from the lowest cost intervention ( “type O only”) to the second-lowest (“all accept”) adds 485 QALYs at an ICER of $81,162 per QALY. The “Status 1-3 only” strategy is dominated, offering slightly fewer QALYs than “all accept” at slightly higher cost. However, neither the difference in QALYs (45) nor costs ($1.5 million) was statistically significant (p > 0.05), as assessed using the two sample t-test. Of note, “Status 1-3 only” resulted in 108 more non-local transplants than “all accept” (where organ recovery and transplant occurred in different donor service areas).
Figure 4. Cost-effectiveness frontier comparing the primary intervention (all accept HCV+ donors) and selected intermediate HCV+ donor acceptance strategies.
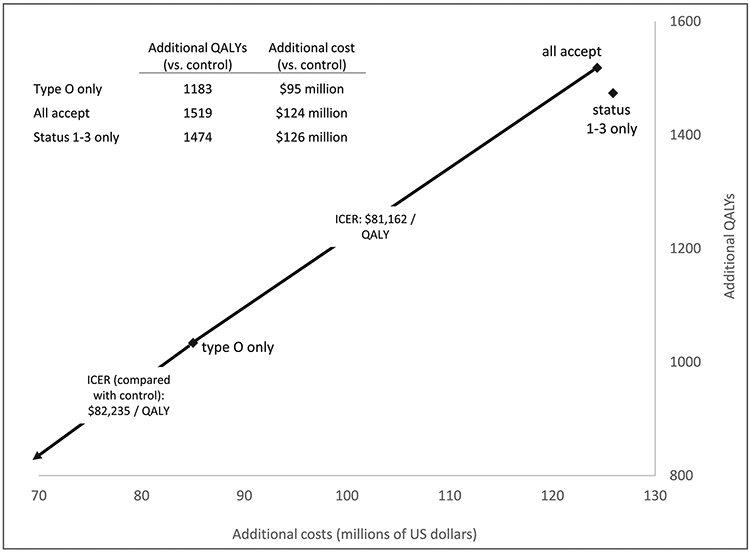
Intermediate strategies are those in which HCV+ donor acceptance is limited to a specific blood type (“Type O only”) or priority strata (“Status 1-3 only”) at listing. "Additional" costs and QALYs represent the difference between a selected intervention strategy and the control scenario (none accept HCV+ donors). Each scenario’s outcomes represent the average across ten model iterations. Overlaying the figure are incremental cost-effectiveness ratios (ICERs; calculated as difference in costs divided by difference in QALYs) comparing neighboring strategies on the cost-effectiveness frontier. On average, the “status 1-3 only” strategy is dominated by “all accept” (conferring fewer QALYs at higher total costs). However, the differences in both costs and QALYs between the “all accept” and “status 1-3 only” scenarios were not statistically significant (p > 0.05 in two sample t-test).
HCV: Hepatitis C; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year
Sensitivity analyses
Eight of the model’s 31 input parameters (listed in Table 1) exhibited a range of greater than $5000 per QALY in corresponding ICERs (comparing primary intervention with control). The range in ICERs for each is displayed in Figure 5. Increasing DAA cost by 50% ($21k) resulted in an ICER of $86,024 per QALY. Imposing a 10% decrement in survival among patients who receive HCV+ donor hearts (and do not acquire chronic HCV infection) resulted in an ICER of $88,205 per QALY. Other HCV-related parameters each produced less than $5k per QALY variation in ICER across their plausible range. The alternative scenarios in which waitlist outcomes are improved, the uptake of the intervention is incomplete, and HCV+ donors confer increased risk of acute cellular rejection (further detailed in Supplementary Appendix (S9)) produced ICERs of $62,228, $81,336, and $88,257 per QALY, respectively. Among 10,000 trials in our probabilistic sensitivity analysis, the ICER was less than $100k per QALY in 76.8% of trials with a standard deviation of $20,517 per QALY.
Table 1. Model parameterization for base case and sensitivity analyses.
A detailed rationale for each assumption is provided in Table S5.
| Parameter | Base case value (plausible range*) |
|---|---|
| HCV-related parameters | |
| Probability of sustained virologic response after DAA therapy | 99% (97% - 99%) |
| Survival reduction post-transplant for recipients of HCV+ heart with sustained virologic response | 0% (0% - 10%) |
| Survival reduction post-transplant for recipients of HCV+ heart without sustained virologic response | 20% (10% - 40%) |
| Decrement in quality of life weight due to chronic HCV infection | 30% (20% - 40%) |
| Other epidemiologic parameters | |
| % of Status 1-3 patients admitted to the ICU | 30% (20% - 40%) |
| % of Status 1-4 patients listed who receive MCS** | 51% (26% - 76%) |
| Survival after delisting due to improvement (years) | 15.8 (11.8 - 19.6) |
| Survival after delisting due to deterioration (months) | 3 (1 - 6) |
| % delisted due to deterioration, by highest status prior to delisting: | |
| Status 1-3 | 76% (68% - 83%) |
| Status 4 | 72% (65% - 80%) |
| Status 6 | 46% (42% - 51%) |
| temporarily inactive | 83% (75% - 91%) |
| Quality of life weights | |
| HT waitlist, Status 1-3 | 0.4 (0.3 - 0.5) |
| HT waitlist, Status 4 | 0.6 (0.53 to 0.74) |
| HT waitlist, Status 6 | 0.74 (0.56 - 0.93) |
| HT waitlist, temporarily inactive | 0.57 (0.43 - 0.71) |
| Post- heart transplant | 0.76 (0.67 - 0.85) |
| Delisted due to deterioration | 0.53 (0.4 to 0.6) |
| Delisted due to improvement | 0.78 (0.74 to 0.94) |
| Cost parameters * | |
| Fixed (one-time) costs of care/interventions | |
| DAA therapy, per HCV+ donor transplant | $42,083 |
| MCS** implantation | $340,727† |
| Heart transplant | $303,190† |
| End-of-life care, patients delisted for deterioration | $64,251 |
| Annual costs of care by disease state | |
| HT waitlist, Status 1-3 | $140,347 |
| HT waitlist, Status 4 | $67,963 |
| HT waitlist, Status 6 | $31,466 |
| HT waitlist, temporarily inactive | $31,466 |
| Post- heart transplant | $35,985 |
| Delisted due to improvement | $27,000 |
| Cost per day of ICU care (while on waitlist)‡ | $2,728 |
| Additional annual cost of care, post-transplant patients with chronic HCV infection | $8,730 |
All cost parameters were assumed to have a plausible range of +/− 50% in sensitivity analyses
MCS represents all forms of mechanical circulatory support including temporary and durable ventricular assist devices, intra-aortic balloon pump, and extracorporeal membrane oxygenation.
Fixed costs of transplant and MCS incorporate the costs of early post-HT and post-MCS care, as detailed in Table S5
Cost of ICU care is specifically tabulated only during the time on the waitlist; for post-transplant patients, the costs of ICU care are built into the fixed cost of transplant (which includes early post-HT care, as described above).
DAA Direct-acting antiviral; HCV: Hepatitis C; HT: Heart transplant; ICU: Intensive care unit; MCS: Mechanical circulatory support
Figure 5. Tornado diagram of the results of sensitivity analyses.
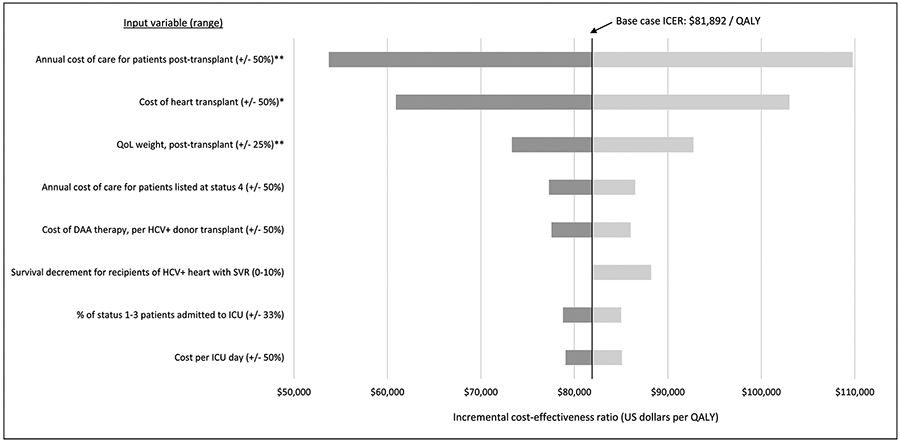
For plausible ranges of each input variable (shown in parentheses), the range of ICER estimates for the primary intervention (all accept HCV+ donors) versus control scenario (none accept HCV+ donors) is shown. The chart includes all input variables for which the corresponding range in ICERs is greater than $5000 per QALY.
* Includes the cost of early post-transplant care as detailed in Table S5
** Applies to post-transplant patients without chronic HCV infection
DAA: direct acting antiviral; HCV: Hepatitis C; ICER: incremental cost-effectiveness ratio; ICU: intensive care unit; QALY: quality-adjusted life year
Discussion
Modeling a historical real-world cohort of transplant candidates and potential donors (July 2014 – June 2019), we found that universal HCV+ donor heart acceptance increased the potential donor pool by 2.9% (n = 472) and overall transplant volume by 1.8% (n = 232), with this impact varying by demographic, blood type, and region. There were larger effects on adverse waitlist outcomes (3-4% decrease), expected wait times (4.5% decrease), and the projected size of the waitlist (7.5% decrease), indicating that the benefits of HCV+ donor utilization extend beyond the small fraction of patients who actually receive HCV+ organs. Universal HCV+ donor utilization was cost-effective (ICER: $82k per QALY) across a wide range of parameter assumptions. The additional cost ($124 million) in the primary intervention scenario is mainly attributable to that of additional transplant procedures and post-transplant care unrelated to HCV. Costs and morbidity associated with chronic HCV infection had minimal impact on cost-effectiveness and DAA cost was influential, but much less so than other cost parameters.
This finding - that costs related to HCV itself have little impact on the cost-effectiveness of using HCV+ donors – may seem unintuitive. It is better understood by considering the following: 1) DAA costs ($42k per patient) pale in comparison to other transplant-related costs, including $303k for the transplant itself and $36k per year of routine post-transplant care; 2) the rarity of chronic HCV (~3 new cases over 5 years) means that related costs are negligible on aggregate relative to other transplant costs; and 3) pre-transplant care and associated complications are expensive, particularly for patients at the highest priority status ($140k per year), in the ICU ($2728 per day), and/or requiring MCS ($341k per implant); thus the payoff to transplanting more patients before they reach this point (by using HCV+ donors) largely offsets the costs of HCV therapy. From this initially unintuitive finding, it follows that the cost-effectiveness of HCV+ donor transplants is similar to that of HCV- donor transplants.
Our findings support the hypothesis that subgroups facing higher donor scarcity and longer wait times experience the most benefit from HCV+ donor utilization. This includes patients of blood type O (2.8% increase in transplants) and in the Northeast (2.5 - 6.1% increase in transplants in UNOS regions 1, 2, and 9). However, the benefits of HCV+ donor utilization are not exclusive to these groups. Even where potential donors outnumber transplant candidates (regions 3, 6, and 8), the intervention produced a ~1% increase in transplants - suggesting that patients with “difficult-to-match” characteristics (e.g. large size, geographic remoteness) experience an effective scarcity of organs and benefit from an expanded donor pool. One would expect the intervention’s impact to depend on the availability of nearby compatible HCV+ donors; consistent with this, both donor HCV prevalence and intervention impact were highest for blood type O and for region 1.
We compared our primary intervention (all patients accept HCV+ donors) to alternative policy strategies in which only selected subgroups accept HCV+ organs and found no evidence to support the latter. For example, limiting HCV+ organs to type O recipients produced an ICER (compared to control) of $82k per QALY. Limiting HCV+ organs to the highest urgency patients (Status 1-3 only) did not significantly differ from the primary intervention in terms of total QALYs or costs. However, a potential disadvantage of “Status 1-3 only” is that it entails more “long-distance” transplants of HCV+ organs to Status 1-3 candidates (in lieu of local Status 4 patients). That “long-distance” transplants entail higher organ procurement costs, longer organ ischemic times, and potentially worse outcomes is supported by prior analyses.19,20 Had our model captured these disadvantages, “Status 1-3 only” would have appeared less efficient.
Policy Implications
Our study is the first to employ a large-scale simulation of heterogenous “real-world” donor and patient populations to estimate the system-wide impact and cost-effectiveness of using HCV+ donors for transplant. Our findings complement a prior study which, by performing 1000 simulations and calculating costs and QALYs for a typical patient in isolation, answers the question: “From the standpoint of an individual patient, is use of HCV+ donors cost-effective?”.12 Our study answers a different question, more relevant for policymakers: “From the standpoint of the entire transplant system, is use of HCV+ donors cost-effective?”. Viewed in this context, it is not surprising that our reported ICER ($82k per QALY) compares favorably with that previously reported ($86k per QALY).12 This difference in ICERs may reflect the “indirect” benefits accrued by the rest of the waitlist population (in the form of increased organ availability) when a single patient accepts an HCV+ donor heart.
Notably, our reported ICER also compares favorably with the most recent estimates for destination left ventricular assist device therapy ($103k per QALY),21 a potential alternative to transplant for much of our population of interest.22 This comparator may carry more practical weight for policymakers than the abstract “$100k per QALY” standard, which remains subject to debate.18
Our analysis evaluates the costs of HCV+ donor utilization on a healthcare system perspective, it does not address the critical question of who bears these costs. For DAA therapy, the answer currently depends on payor and context and out-of-pocket costs are likely to be prohibitively high for some patients.23,24 Unless insurers provide adequate DAA coverage, then widespread adoption of this high-value, lifesaving intervention will be limited and inequitable.
Study Limitations
Our model’s population and assumptions are based on a historical cohort of transplant candidates and donors. Accordingly, our findings answer the question, “what would have happened if HCV+ donors were utilized from 2014 to 2019?”. Indeed the more policy-relevant question is “how would HCV+ donor utilization would affect future cohorts?”. However, any effort to explicitly forecast the effects of HCV+ donor utilization in a future cohort would be prohibitively uncertain, due to unforeseeable changes in allocation policy and the composition of the waitlist population. We could have addressed the latter issue and improved our generalizability by repeating all analyses for a range of “pseudo-populations”, but the model’s computational time made this infeasible.
Our sensitivity analyses show that HCV+ donor utilization remains cost-effective allowing for foreseeable changes including 1) improving waitlist outcomes (e.g. following the 2018 allocation policy change25) 2) decreasing DAA prices, 3) increasing MCS utilization, and 4) incomplete intervention uptake. We also show that the intervention remains cost-effective even if DAA efficacy is lower than previously reported and if HCV+ donors confer higher risk of acute cellular rejection or marginally (−10%) worse long-term survival. While quality of life weights and future costs are uncertain, the wide range for each input used in our sensitivity analyses renders our findings robust to this uncertainty. Beyond DAA therapy, there are HCV therapy-related activities that our model does not capture (e.g. viral load monitoring). That the intervention remains cost-effective even allowing for a $21k increase in DAA costs suggests that a more granular accounting of these activities would not change our results.
We use registry data to characterize the potential donor population, in which HCV+ prevalence is 1.3% during the first three years of the model and 4.8% thereafter. If the latter persists, the future impact of HCV+ donor utilization would exceed that reported in our study. Conversely, increased use of DAA therapy to treat hepatitis C in the general population could reduce the size of the HCV+ donor pool and thus the intervention’s aggregate impact.26 Disparities by region in donor scarcity and HCV prevalence will likely change over time, affecting regional variation in the impact of using HCV+ donors. Yet while the costs and benefits of the intervention will depend on unpredictable geographic and temporal variation in the composition of the donor pool, our sensitivity analyses suggest that its cost-effectiveness is robust to such variation.
In conclusion, our findings suggest that transplanting HCV+ donor hearts is cost-effective, improves population-level outcomes, and should thus be part of a multi-faceted strategy to address the donor shortage in heart transplantation.
Supplementary Material
Figure S1. Comparison of donor scarcity by UNOS region and the impact of HCV+ donor utilization on regional transplant volume. The impact of HCV+ donor utilization on transplant volume is expressed as the percentage increase in the number of transplants under the primary intervention (i.e. all patients accept HCV+ hearts) compared to the control scenario (none accept HCV+ hearts) within our analytic cohort (patients listed for transplant between July 2014 and June 2019). Donor scarcity is expressed as the ratio of newly listed transplant candidates to potential donor hearts over the same five-year period. Both metrics were calculated for each of the 11 UNOS regions (abbreviated as 1, 2, etc.) and plotted above. The diameter of points is proportional to the prevalence of HCV-viremia among donors in each region in the above five-year period. A map of UNOS regions is shown overlaying the figure.
Impact by region is correlated with organ scarcity (ρ = 0.71) and donor HCV prevalence (ρ = 0.78). Of note, impact in regions 7 and 9 (2.3% and 4.3%, respectively) is significantly higher the prevalence of HCV in the donor poor of each region (1.1% and 2.4%, respectively). This is possible because donor organs are not confined by regional borders; regions 7 and 9 neighbor (and thus can draw organs from) a region with higher HCV prevalence (regions 10 and 1, respectively).
HCV: Hepatitis C; UNOS: United Network for Organ Sharing
Figure S2. Probabilistic sensitivity analysis results. Shown is the distribution of incremental cost-effectiveness ratio (ICER) of the primary intervention (all accept HCV+ donors) across 10,000 simulation runs.
HCV: Hepatitis C; ICER: incremental cost-effectiveness ratio; UNOS: United Network for Organ Sharing; QALY: quality-adjusted life year
Acknowledgements:
The authors thank Dr. Paul Kwo (Professor of Medicine, Stanford University School of Medicine) for his valuable input to this manuscript.
Sources of Funding:
This work is supported by grants R01 HL125303 entitled “Evidence Based Evaluation and Acceptance of Donor Hearts for Transplantation and T32 HL094274-10 entitled “Research Training in Myocardial Biology at Stanford” from the National Institute of Health, Bethesda, MD.
List of abbreviations:
- DAA
direct-acting antiviral
- HCV
Hepatitis C
- HT
heart transplant
- ICER
incremental cost effectiveness ratio
- ICU
intensive care unit
- MCS
mechanical circulatory support
- QALY
quality-adjusted life year
- SRTR
Scientific Registry of Transplant Recipients
- TSAM
Thoracic Simulated Allocation Model
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Conflict of Interest Statement: Dr. Teuteberg has relationships with Abbott (consulting), Abiomed (advisory board), Medtronic (speaking, advisory board), CareDx (speaking, advisory board), Paragonix (speaking). Other authors have no disclosures.
References
- 1.Cowger JA Addressing the Growing U.S. Donor Heart Shortage: Waiting for Godot or a Transplant? J Am Coll Cardiol 69, 1715–1717 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Colvin M et al. OPTN/SRTR 2017 Annual Data Report: Heart. Am J Transplant 19 Suppl 2, 323–403 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Thomas SS & D’Alessandro DA Traumatic Brains and Broken Hearts: Mending the Donor Shortage in Cardiac Transplantation. J Am Coll Cardiol 70, 1259–1261 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Hsich EM Matching the Market for Heart Transplantation. Circ Heart Fail 9, e002679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asselah T, Marcellin P & Schinazi RF Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 38 Suppl 1, 7–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moayedi Y, Gulamhusein AF, Ross HJ, Teuteberg JJ & Khush KK Accepting hepatitis C virus-infected donor hearts for transplantation: Multistep consent, unrealized opportunity, and the Stanford experience. Clin Transplant 32, e13308 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Aslam S, Yumul I, Mariski M, Pretorius V & Adler E Outcomes of heart transplantation from hepatitis C virus-positive donors. J. Heart Lung Transplant 38, 1259–1267 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Woolley AE et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med 380, 1606–1617 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlendorf KH et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J. Heart Lung Transplant 37, 763–769 (2018). [DOI] [PubMed] [Google Scholar]
- 10.McLean RC et al. Transplanting hepatitis C virus-infected hearts into uninfected recipients: A single-arm trial. Am. J. Transplant 19, 2533–2542 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Gernhofer YK et al. The impact of using hepatitis c virus nucleic acid test-positive donor hearts on heart transplant waitlist time and transplant rate. J Heart Lung Transplant 38, 1178–1188 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Logan C et al. Cost-effectiveness of using hepatitis C viremic hearts for transplantation into HCV-negative recipients. Am. J. Transplant (2020) doi: 10.1111/ajt.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SS et al. Trends in the use of hepatitis C viremic donor hearts. J Thorac Cardiovasc Surg S0022-5223(20)32640–4 (2020) doi: 10.1016/j.jtcvs.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Madan S et al. Utilization rates and clinical outcomes of hepatitis C positive donor hearts in the contemporary era. J Heart Lung Transplant 38, 907–917 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Lehr CJ, Skeans M & Valapour M Validating thoracic simulated allocation model predictions for impact of broader geographic sharing of donor lungs on transplant waitlist outcomes. J Heart Lung Transplant 39, 433–440 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Thompson D et al. Simulating the allocation of organs for transplantation. Health Care Manag Sci 7, 331–338 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Gidea CG et al. Increased early acute cellular rejection events in hepatitis C-positive heart transplantation. J Heart Lung Transplant S1053-2498(20)31624–7 (2020) doi: 10.1016/j.healun.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Neumann PJ, Cohen JT & Weinstein MC Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N. Engl. J. Med 371, 796–797 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Crawford TC et al. The Paradoxical Relationship Between Donor Distance and Survival After Heart Transplantation. Ann Thorac Surg 103, 1384–1391 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Weiss ES et al. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant 31, 266–273 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Silvestry SC et al. Cost-Effectiveness of a Small Intrapericardial Centrifugal Left Ventricular Assist Device. ASAIO J 66, 862–870 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayes-Genis A et al. Destination therapy with left ventricular assist devices in non-transplant centres: the time is right. Eur Cardiol 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bova S et al. Access to direct-acting antivirals for hepatitis C-negative transplant recipients receiving organs from hepatitis C-viremic donors. Am J Health Syst Pharm zxab207 (2021) doi: 10.1093/ajhp/zxab207. [DOI] [PubMed] [Google Scholar]
- 24.Wong RJ et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol 113, 1329–1338 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Kilic A et al. Evolving Trends in Adult Heart Transplant With the 2018 Heart Allocation Policy Change. JAMA Cardiol 6, 159–167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardi A et al. Hepatitis C: Is eradication possible?. Liver Int 39, 416–426 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Bostic R & Slaughter MS Health-Economic Aspects of MCS Therapy. in Mechanical Circulatory Support in End-Stage Heart Failure: A Practical Manual (eds. Montalto A, Loforte A, Musumeci F, Krabatsch T & Slaughter MS) 595–603 (Springer International Publishing, 2017). doi: 10.1007/978-3-319-43383-7_57. [DOI] [Google Scholar]
- 28.Söderlund C et al. Acute cellular rejection the first year after heart transplantation and its impact on survival: a single-centre retrospective study at Skåne University Hospital in Lund 1988-2010. Transpl Int 27, 482–492 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Saab S et al. Use of hepatitis C-positive grafts in hepatitis C-negative liver transplant recipients is cost effective. Clin Transplant 32, e13383 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Scott N et al. Cost-effectiveness of transplanting lungs and kidneys from donors with potential hepatitis C exposure or infection. Sci Rep 10, 1459 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckman MH, Woodle ES, Thakar CV, Alloway RR & Sherman KE Cost-effectiveness of Using Kidneys From HCV-Viremic Donors for Transplantation Into HCV-Uninfected Recipients. Am. J. Kidney Dis 75, 857–867 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Kadatz M, Klarenbach S, Gill J & Gill JS Cost-effectiveness of using kidneys from hepatitis C nucleic acid test-positive donors for transplantation in hepatitis C-negative recipients. Am. J. Transplant 18, 2457–2464 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Gupta G, Zhang Y, Carroll NV & Sterling RK Cost-effectiveness of hepatitis C-positive donor kidney transplantation for hepatitis C-negative recipients with concomitant direct-acting antiviral therapy. Am. J. Transplant 18, 2496–2505 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Akuta N et al. Retreatment efficacy and predictors of ledipasvir plus sofosbuvir to HCV genotype 1 in Japan. J. Med. Virol 89, 284–290 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Omland LH et al. Increased mortality among persons infected with hepatitis C virus. Clin. Gastroenterol. Hepatol 9, 71–78 (2011). [DOI] [PubMed] [Google Scholar]
- 36.VanderPluym C et al. Survival in patients removed from the heart transplant waiting list before receiving a transplant. J. Heart Lung Transplant 33, 261–269 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Grady KL et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J. Heart Lung Transplant 33, 412–421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirklin JK et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J. Heart Lung Transplant 32, 141–156 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Göhler A et al. Utility estimates for decision-analytic modeling in chronic heart failure--health states based on New York Heart Association classes and number of rehospitalizations. Value Health 12, 185–187 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Emin A, Rogers CA, Banner NR, & Steering Group, UK Cardiothoracic Transplant Audit. Quality of life of advanced chronic heart failure: medical care, mechanical circulatory support and transplantation. Eur J Cardiothorac Surg 50, 269–273 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Sharples LD et al. Cost-effectiveness of ventricular assist device use in the United Kingdom: results from the evaluation of ventricular assist device programme in the UK (EVAD-UK). J. Heart Lung Transplant 25, 1336–1343 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Saeed I, Rogers C, Murday A, & Steering Group of the UK Cardiothoracic Transplant Audit. Health-related quality of life after cardiac transplantation: results of a UK National Survey with Norm-based Comparisons. J. Heart Lung Transplant 27, 675–681 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Buendía F et al. Relationship between functional capacity and quality of life in heart transplant patients. Transplant Proc 43, 2251–2252 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Long EF, Swain GW & Mangi AA Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail 7, 470–478 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Haddad H et al. Canadian Real-World Experience of Using Sacubitril/Valsartan in Patients With Heart Failure With Reduced Ejection Fraction: Insight From the PARASAIL Study. CJC open 2, 344–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers JG et al. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail 5, 10–16 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Voigt J et al. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol 37, 312–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gershengorn HB, Garland A & Gong MN Patterns of Daily Costs Differ for Medical and Surgical Intensive Care Unit Patients. Ann Am Thorac Soc 12, 1831–1836 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Gordon SC et al. Prevalence of cirrhosis in hepatitis C patients in the Chronic Hepatitis Cohort Study (CHeCS): a retrospective and prospective observational study. Am. J. Gastroenterol 110, 1169–1177; quiz 1178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trinchet J-C, Ganne-Carrié N, Nahon P, N’kontchou G & Beaugrand M Hepatocellular carcinoma in patients with hepatitis C virus-related chronic liver disease. World J. Gastroenterol 13, 2455–2460 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of donor scarcity by UNOS region and the impact of HCV+ donor utilization on regional transplant volume. The impact of HCV+ donor utilization on transplant volume is expressed as the percentage increase in the number of transplants under the primary intervention (i.e. all patients accept HCV+ hearts) compared to the control scenario (none accept HCV+ hearts) within our analytic cohort (patients listed for transplant between July 2014 and June 2019). Donor scarcity is expressed as the ratio of newly listed transplant candidates to potential donor hearts over the same five-year period. Both metrics were calculated for each of the 11 UNOS regions (abbreviated as 1, 2, etc.) and plotted above. The diameter of points is proportional to the prevalence of HCV-viremia among donors in each region in the above five-year period. A map of UNOS regions is shown overlaying the figure.
Impact by region is correlated with organ scarcity (ρ = 0.71) and donor HCV prevalence (ρ = 0.78). Of note, impact in regions 7 and 9 (2.3% and 4.3%, respectively) is significantly higher the prevalence of HCV in the donor poor of each region (1.1% and 2.4%, respectively). This is possible because donor organs are not confined by regional borders; regions 7 and 9 neighbor (and thus can draw organs from) a region with higher HCV prevalence (regions 10 and 1, respectively).
HCV: Hepatitis C; UNOS: United Network for Organ Sharing
Figure S2. Probabilistic sensitivity analysis results. Shown is the distribution of incremental cost-effectiveness ratio (ICER) of the primary intervention (all accept HCV+ donors) across 10,000 simulation runs.
HCV: Hepatitis C; ICER: incremental cost-effectiveness ratio; UNOS: United Network for Organ Sharing; QALY: quality-adjusted life year